Abstract
Although CD34 cell dose is known to influence outcome of peripheral stem cell– and/or T-cell–depleted transplantation, such data on unmanipulated marrow transplantation are scarce. To study the influence of CD34+ cell dose on hematopoietic reconstitution and incidence of infections after bone marrow transplantation, we retrospectively analyzed 212 patients from January 1994 to August 1999 who received a transplant of an unmanipulated graft from an HLA-identical sibling donor. Median age was 31 years; 176 patients had hematologic malignancies. Acute graft-versus-host disease prophylaxis consisted mainly in cyclosporin associated with methotrexate (n = 174). Median number of bone marrow nucleated cells and CD34+ cells infused were 2.4 × 108/kg and 3.7 × 106/kg, respectively. A CD34+ cell dose of 3 × 106/kg or more significantly influenced neutrophil (hazard ratio [HR] = 1.37, P = .04), monocyte (HR = 1.47, P = .02), lymphocyte (HR = 1.70, P = .003), erythrocyte (HR = 1.77, P = .0002), and platelet (HR = 1.98,P = .00008) recoveries. CD34+ cell dose also influenced the incidence of secondary neutropenia (HR = 0.60,P = .05). Bacterial and viral infections were not influenced by CD34 cell dose, whereas it influenced the incidence of fungal infections (HR = 0.41, P = .008). Estimated 180-day transplantation-related mortality (TRM) and 5-year survival were 25% and 56%, respectively, and both were highly affected by CD34+ cell dose (HR = 0.55, P = .006 and HR = 0.54, P = .03, respectively). Five-year survival and 180-day TRM were, respectively, 64% and 19% for patients receiving a CD34+ cell dose of 3 × 106/kg or more and 40% and 37% for the remainders. In conclusion a CD34+ cell dose of 3 × 106/kg or more improved all hematopoietic recoveries, decreased the incidence of fungal infections and TRM, and improved overall survival.
Introduction
Allogeneic bone marrow transplantation (BMT) has been widely used to treat different types of malignant and nonmalignant hematologic disorders as well as congenital metabolic and immunodeficiency diseases. Although peripheral blood stem cells (PBSCs) have been increasingly used since 1994, bone marrow (BM) remains the main source of allogeneic hematopoietic stem cells.1Patient-, disease-, and transplantation-related factors (such as age, disease status, type of donor, etc) have been extensively studied and have shown significant influence on outcome after BMT. It has been realized that the total number of nucleated cells (NCs) infused is a significant prognostic factor after allogeneic stem cell transplantation for different outcomes: A low dose of NCs has been associated with an increased risk of rejection,2,3 with a slower engraftment rate,3 an increased transplantation-related mortality (TRM),4-6 and a lower disease-free survival,4-7 independently of the type of donor (related or unrelated) and the source of stem cell transplanted (BM, peripheral stem cell, or cord blood cells).8 9
CD34 designates a surface membrane molecule present in all committed and noncommitted hematopoietic progenitor cells and has been used as a surrogate marker of the hematopoietic stem cell content mainly in mobilized peripheral stem cell (autologous or allogeneic)10-13 and in BM14-17transplantations. Studies dealing with allogeneic stem cell transplantation have already showed that CD34+ cell dose is a significant prognostic factor for survival and TRM. However, the reason of such influence is not clear because it seems that neutrophil recovery is not influenced by CD34+ cell dose in the allogeneic BMT setting.14 18 To understand why CD34+ cell dose has an effect on TRM and survival after allogeneic BMT, we addressed the question whether a better recovery of other parameters of hematopoietic reconstitution (ie, secondary neutropenia and recovery of monocytes, lymphocytes, erythrocytes, and platelets) as well as its effect on infections could explain a decreased TRM and better survival after BMT.
Patients, materials, and methods
Patient, donor, and transplantation characteristics
Between January 1, 1994, and August 31, 1999, a total of 214 consecutive patients underwent HLA-identical sibling BMT at the bone marrow transplant unit of Hôpital Saint Louis, Paris, France. Two patients were excluded because of insufficient data collected (n = 1) and absence of CD34+ quantification (n = 1). Table1 summarizes the patient, disease, donor, and transplantation characteristics of the 212 analyzed patients.
Patient, disease, donor, and transplantation characteristics of the 212 enrolled patients
| Characteristics . | Median (range) N (%) . |
|---|---|
| Patient | |
| Age, y | 31 (3.3-55.8) |
| Weight, kg | 64 (13.6-100) |
| Female | 82 (38.7%) |
| Positive CMV serology | 139 (65.6%) |
| Underlying diagnosis | |
| Chronic leukemia* | 56 (26.4%) |
| Acute leukemia† | 87 (41.0%) |
| Other malignancies‡ | 33 (15.6%) |
| Non-malignancies1-153 | 36 (17.0%) |
| Disease stage (for malignancies) | |
| Advanced1-155 | 27 (12.7%) |
| Donor | |
| Age, y | 30 (1.2-64.8) |
| Female | 88 (41.5%) |
| Sex match | 96 (45.3%) |
| Donor F recipient M | 61 (28.8%) |
| ABO match | 149 (70.3%) |
| ABO major mismatch | 37 (17.4%) |
| Positive CMV serology | 108 (50.9%) |
| Transplantation | |
| GVH prophylaxis | |
| Cyclosporin + methotrexate | 174 (82.2%) |
| Cyclosporin + methotrexate + other | 23 (10.8%) |
| Cyclosporin ± corticosteroids | 15 (7.1%) |
| Conditioning | |
| Irradiation based | 86 (40.6%) |
| TBI + Cy ± others | 38 |
| TBI + Mel ± others | 42 |
| TAI + low-dose Cy | 6 |
| Chemotherapy based | 126 (59.4%) |
| Bu + Cy | 76 |
| Bu + Cy + VP16 | 24 |
| Cy | 21 |
| Bu + others | 5 |
| Laboratory values | |
| NC, 108/kg | 2.4 (0.28-5.97) |
| CD34, 106/kg | 3.7 (0.07-18.7) |
| CD3, 106/kg, n = 48 | 2.2 (0.3-5.4) |
| NC ≥ 2.4 × 106/kg | 111 (52.4%) |
| CD34 ≥ 3.0 × 106/kg | 142 (67.0%) |
| Use of prophylactic hematopoietic growth factors (until day + 7) | 13 (6.1%) |
| Characteristics . | Median (range) N (%) . |
|---|---|
| Patient | |
| Age, y | 31 (3.3-55.8) |
| Weight, kg | 64 (13.6-100) |
| Female | 82 (38.7%) |
| Positive CMV serology | 139 (65.6%) |
| Underlying diagnosis | |
| Chronic leukemia* | 56 (26.4%) |
| Acute leukemia† | 87 (41.0%) |
| Other malignancies‡ | 33 (15.6%) |
| Non-malignancies1-153 | 36 (17.0%) |
| Disease stage (for malignancies) | |
| Advanced1-155 | 27 (12.7%) |
| Donor | |
| Age, y | 30 (1.2-64.8) |
| Female | 88 (41.5%) |
| Sex match | 96 (45.3%) |
| Donor F recipient M | 61 (28.8%) |
| ABO match | 149 (70.3%) |
| ABO major mismatch | 37 (17.4%) |
| Positive CMV serology | 108 (50.9%) |
| Transplantation | |
| GVH prophylaxis | |
| Cyclosporin + methotrexate | 174 (82.2%) |
| Cyclosporin + methotrexate + other | 23 (10.8%) |
| Cyclosporin ± corticosteroids | 15 (7.1%) |
| Conditioning | |
| Irradiation based | 86 (40.6%) |
| TBI + Cy ± others | 38 |
| TBI + Mel ± others | 42 |
| TAI + low-dose Cy | 6 |
| Chemotherapy based | 126 (59.4%) |
| Bu + Cy | 76 |
| Bu + Cy + VP16 | 24 |
| Cy | 21 |
| Bu + others | 5 |
| Laboratory values | |
| NC, 108/kg | 2.4 (0.28-5.97) |
| CD34, 106/kg | 3.7 (0.07-18.7) |
| CD3, 106/kg, n = 48 | 2.2 (0.3-5.4) |
| NC ≥ 2.4 × 106/kg | 111 (52.4%) |
| CD34 ≥ 3.0 × 106/kg | 142 (67.0%) |
| Use of prophylactic hematopoietic growth factors (until day + 7) | 13 (6.1%) |
CMV indicates cytomegalovirus; GVH, graft-versus-host; TBI, total body irradiation; TAI, thoracoabdominal irradiation; Cy, cyclophosphamide; Mel, melphalan; Bu, busulfan; VP16, etoposide; NC, nucleated cells.
55 chronic myeloid leukemia (CML): 37 in first chronic phase (CP), 5 in second CP, 11 in accelerated phase, and 2 in blastic crisis; 1 chronic lymphocytic leukemia.
45 acute lymphoblastic leukemia (ALL): 27 in first complete remission (CR1), 6 in CR2, 3 in CR3+, and 9 in relapse/refractory (REL) disease; 42 acute myeloblastic leukemia (AML): 31 in CR1, 7 in CR2, and 4 in REL disease.
14 Non-Hodgkin lymphoma (NHL), 12 myelodysplastic syndrome (MDS), 7 other myeloproliferative syndromes.
23 Severe aplastic anemia, 7 Fanconi anemia, 4 paroxysmal nocturnal hemoglobinuria, 1 congenital dyserythropoietic anemia, and 1 Glanzmann thrombasthenia.
Advanced stage: CML in blastic crisis, AML/ALL in relapse or refractory disease, NHL in resistant or untreated relapse, MDS classified as refractory anemia with excess of blast or with excess of blast in transformation and secondary acute leukemia (modified from IBMTR classification).
Graft-versus-host disease prophylaxis, conditioning regimen, and supportive therapy
Prophylaxis for acute graft-versus-host disease (GVHD) consisted of the standard combination of cyclosporine and methotrexate in 174 (82.1%) patients. All patients received an unmanipulated BMT.
Conditioning for transplantation varied according to diagnosis. Eighty-six (40.6%) patients received an irradiation-containing regimen. Eighty patients received fractionated total body irradiation (12 Gy in 6 fractions over 3 days), and 6 patients with Fanconi anemia received a specific regimen associating thoracoabdominal irradiation (4.5 Gy single dose) with low-dose cyclophosphamide. One hundred twenty-six (59.4%) patients received a chemotherapy-based conditioning. Twenty-four (11.3%) patients with BM failure syndromes received intravenous antithymocyte globulin before the transplant.
All patients were isolated in laminar airflow rooms. Irradiated and leukocyte-depleted blood products were used for all patients. Patients were transfused with red blood cells (RBCs) or platelets when hemoglobin was less than 80 g/L (8 g/dL) and platelet count was less than 20 × 109/L, respectively. Selective gut decontamination with oral antibiotics and viral/fungal/parasitic prophylaxis were performed according to local policy, which remained constant during the 6-year period of the study. A preemptive treatment with ganciclovir or foscarnet for cytomegalovirus (CMV) reactivation based on CMV antigenemia screening was used from 1994. Hematopoietic growth factors were not routinely given, and only 13 (6.1%) patients received hematopoietic growth factor because of patients' inclusion in a randomized clinical trial, conditioning toxicity, or severe infection.
Graft collection, manipulation, and stem cell content (NCs and CD34) quantification
BM was harvested from both posterior iliac crests, under general anesthesia. Marrow was aspirated with plastic syringes in aliquots of 2 to 10 mL and diluted with heparinized tissue culture medium RPMI 1640 (for BM collected between 1994 and 1995) or acid-citrate dextrose (for collections between 1995 and 1999). Routine processing of BM consisted in the preparation of a buffy-coat, by centrifugation on Cobe 2991 at 3000 rpm for 5 minutes. Buffy-coat cells were resuspended in human serum albumin and infused to the patient. This method yields approximately 85% of the starting nucleated and CD34+cells and allows concentration to 10% of the original volume, by elimination of supernatant and part of the erythrocytes. In situations of major donor-recipient ABO incompatibility, RBCs were eliminated by centrifugation on Cobe 2991. Briefly, 450 mL diluted buffy-coat cells, with hematocrit adjusted to less than 20%, were layered on 150 mL Ficoll-Hypaque and centrifuged for 15 minutes at 400g. After Ficoll washing, mononuclear cells were resuspended in human serum albumin and infused to the patient. This process allows recovery of 20% of the initial NCs, of which 75% are mononucleated cells, and of 56% of the CD34+ cells with less than 0.1% of the original red cells.
Automated cell counts and CD34+ cell quantification were performed both before and after processing, but only the latter was taken into account in this study. CD34+ cell quantification was performed as previously described by fluorescence analysis.18 Briefly, one million BM cells at initial and final steps of the procedure were incubated for 10 minutes at room temperature with 20 μL HPCA2-phycoerythrin and anti-CD45–fluorescein isothiocyanate monoclonal antibodies (Becton Dickinson, France). Immunofluorescence analysis was performed by using a 5-parameter FACSscan (Becton Dickinson Immunocytometry Systems, San Jose, CA). The total number of CD34+ cells obtained at the end of processing corresponds to the number infused to the patient.
End-point definitions and statistical analysis
Hematopoietic reconstitution.
Neutrophil recovery was defined as the first of 3 consecutive days with neutrophils more than 0.5 × 109/L during the first 60 days after transplantation. Secondary neutropenia was defined as neutrophil engraftment followed by a decrease of neutrophils below 0.5 × 109/L for at least 3 days. Monocyte recovery was defined as the first day after transplantation with monocyte counts more than 0.3 × 109/L during the first 60 days after transplantation. Lymphocyte recovery was defined as the first day with lymphocyte counts more than 0.5 × 109/L during the first 180 days after transplantation. Platelet recovery was defined as the first of 7 days of unsupported platelets more than 20 × 109/L during the first 180 days after transplantation. Erythrocyte recovery was defined as the first of 10 days of unsupported hemoglobin more than 80 g/L during the first 100 days after transplantation.
Infection definitions.
The date of the first episode of severe bacterial, viral, and invasive fungal infections of each patient was analyzed. We included CMV infection in the severe viral infection group for statistical purposes. Definitions follow.
Severe viral infections. CMV disease was diagnosed according to previously published criteria.19 Herpes simplex virus infection was defined as a respiratory, digestive, or neurologic disease with isolation of herpes simplex virus in culture. Adenovirus infection was diagnosed if adenovirus was present in one site whatever technique was used, except immunohistochemistry on biopsy. Probable adenovirus disease was defined as the presence of adenovirus in 2 or more sites whatever technique, except immunohistochemistry on biopsy, and definitive adenovirus disease as histochemistry on biopsy or positive culture from biopsy (except gastrointestinal) or positive sample of cerebral spinal fluid. Other viral infections were considered as severe when a virus was isolated from the site of disease and necessitated antiviral treatment. CMV infection was defined by a positive antigenemia (presence of 2 or more positive nuclei per 200 000 leukocytes).
Severe bacterial infections. We considered severe bacterial infections when sepsis, pneumonia, or septic shock was diagnosed according to previously published criteria.20 21 Pneumonia was also diagnosed when clinical/radiologic signs of pneumonia improved after empiric antibacterials but not antifungals in the absence of positive blood and/or bronchoalveolar lavage culture.
Invasive fungal infections. (These definitions were adapted from Lortholary et al.22) Candidemia was defined by one or more positive blood culture for Candida sp. Disseminated candidosis was defined by clinical and/or radiologic signs of fungal infection with one or more positive blood culture forCandida sp. Proven invasive aspergillosis was defined by histopathology/cytopathology evidence of Aspergillus sp from a needle aspiration or biopsy with evidence of associated damaged tissue or positive culture obtained by a sterile procedure with clinical or radiologic signs consistent with infection. We considered clinical and radiologic signs of invasive aspergillosis with a positive antigenemia (but without microbiologic identification) as a probable invasive aspergillosis. Proven invasive fungal infection (IFI) was defined by histopathology/cytopathology evidence of fungi (other thanAspergillus sp) from a needle aspiration or biopsy with evidence of associated damaged tissue or positive culture obtained by a sterile procedure with clinical or radiologic signs consistent with infection.
Other outcomes.
Acute and chronic GVHDs were diagnosed and graded according to published criteria.23-25 All patients were considered evaluable for acute GVHD at day +1 after transplantation. Occurrence of chronic GVHD was evaluated among patients who survived with sustained engraftment from day +100 after transplantation.
Survival was calculated from transplantation to death from any cause. TRM was calculated from transplantation to death related to transplantation and not to relapse until day 180.
Statistical analysis
The reference date of March 1, 2000, was used. Statistical analyses were independently performed for each end point, ie, neutrophil, monocyte, lymphocyte, hemoglobin, and platelet recoveries; time to secondary neutropenia; and time to bacterial, viral, and fungal infection.
Incidences of each event were nonparametrically estimated. Then, each prognostic analysis was based on the same procedure as described below. First, univariable regression models were fitted with estimated hazard ratio (HR) and 95% confidence interval (CI). The predictive effect of each of the following variables was assessed: recipient and donor ages, recipient and donor genders, recipient weight, recipient and donor CMV serologies, gender match, ABO compatibility, ABO major mismatch, female donor to a male recipient, diagnosis of malignancy, diagnosis of chronic leukemia, diagnosis of acute leukemia, diagnosis of malignancy other than leukemia, advanced stage of disease, use of a radiation-based conditioning, use of busulfan + cyclophosphamide, and NC and CD34+ marrow cell doses. CD34+ cell dose (3 × 106/kg) was introduced after dichotomization according to a cutoff of clinical significance, defined according to a previous publication by the National Institutes of Health group.16 Multivariable regression models were then fitted in which all covariates previously selected as having prognostic value at the 10% level were introduced simultaneously with the CD34+ cell dose (Table 2).
Variables included in multivariable analysis for each end point
| End points . | Variables . |
|---|---|
| Neutrophil engraftment | BuCy, CD34 ≥ 3.0 × 106/kg, donor female to recipient male, female recipient, irradiation-based regimen, NC ≥ 2.4 × 108/kg |
| Secondary neutropenia | Advanced stage, BuCy, CD34 ≥ 3.0 × 106/kg, female recipient, NC ≥ 2.4 × 108/kg, gender match |
| Monocyte recovery | BuCy, CD34 ≥ 3.0 × 106/kg, female recipient, irradiation-based regimen, weight |
| Lymphocyte recovery | Acute leukemia, age, age of donor, CD34 ≥ 3.0 × 106/kg, chronic leukemia, female donor to a male recipient, NC ≥ 2.4 × 108/kg, positive CMV serology, weight |
| Hemoglobin recovery | CD34 ≥ 3.0 × 106/kg, chronic leukemia, NC ≥ 2.4 × 108/kg, gender match |
| Platelet engraftment | CD34 ≥ 3 × 106/kg, female recipient, advanced stage, irradiation-based regimen, malignancy, NC ≥ 2.4 × 108/kg |
| Invasive fungal infection | Acute leukemia, advanced stage, age, CD34 ≥ 3 × 106/kg, donor age, NC ≥ 2.4 × 108/kg, other malignancies |
| Transplantation-related mortality | ABO major incompatibility, advanced stage, age, CD34 ≥ 3 × 106/kg, donor age, donor sex, irradiation-based regimen, NC ≥ 2.4 × 108/kg, weight |
| Overall survival | ABO major incompatibility, advanced stage, age, BuCy, CD34 > 3 × 106/kg, positive CMV donor serology, donor age, female recipient, irradiation-based regimen, malignancy, NC ≥ 2.4 × 108/kg, other malignancies, weight |
| End points . | Variables . |
|---|---|
| Neutrophil engraftment | BuCy, CD34 ≥ 3.0 × 106/kg, donor female to recipient male, female recipient, irradiation-based regimen, NC ≥ 2.4 × 108/kg |
| Secondary neutropenia | Advanced stage, BuCy, CD34 ≥ 3.0 × 106/kg, female recipient, NC ≥ 2.4 × 108/kg, gender match |
| Monocyte recovery | BuCy, CD34 ≥ 3.0 × 106/kg, female recipient, irradiation-based regimen, weight |
| Lymphocyte recovery | Acute leukemia, age, age of donor, CD34 ≥ 3.0 × 106/kg, chronic leukemia, female donor to a male recipient, NC ≥ 2.4 × 108/kg, positive CMV serology, weight |
| Hemoglobin recovery | CD34 ≥ 3.0 × 106/kg, chronic leukemia, NC ≥ 2.4 × 108/kg, gender match |
| Platelet engraftment | CD34 ≥ 3 × 106/kg, female recipient, advanced stage, irradiation-based regimen, malignancy, NC ≥ 2.4 × 108/kg |
| Invasive fungal infection | Acute leukemia, advanced stage, age, CD34 ≥ 3 × 106/kg, donor age, NC ≥ 2.4 × 108/kg, other malignancies |
| Transplantation-related mortality | ABO major incompatibility, advanced stage, age, CD34 ≥ 3 × 106/kg, donor age, donor sex, irradiation-based regimen, NC ≥ 2.4 × 108/kg, weight |
| Overall survival | ABO major incompatibility, advanced stage, age, BuCy, CD34 > 3 × 106/kg, positive CMV donor serology, donor age, female recipient, irradiation-based regimen, malignancy, NC ≥ 2.4 × 108/kg, other malignancies, weight |
These variables were previously selected as having prognostic value at 10% level.
BuCy indicates busulfan associated to cyclophosphamide for the preparative regimen; NC, nucleated cells infused; CMV, cytomegalovirus.
Statistical tools used to estimate incidences and to assess the influence of each factor on either outcome, either individually or jointly, were the Kaplan-Meier estimator and the Cox regression model. However, because the recovery of cell subsets or hemoglobin and the development of infection were events that compete with patient death, estimations of incidence of these events relied on the nonparametric estimator of cumulative incidence curves, whereas predictive analyses were based on the proportional hazards model for these subdistributions of competing risks.26
Correlation between NC and CD34+ cell doses was tested by the Pearson correlation test. The Mann-Whitney test was used to compare differences of NC and CD34+ cell doses between ABO match/minor mismatch and ABO major mismatch groups.
All statistical tests were 2-sided, with P ≤ .05 indicating statistical significance. Statistical analyses were performed with SAS 8.1 (SAS, Cary, NC) and Splus2000 (MathSoft, Seattle, WA) software packages.
Results
Graft composition and infusion
The median dose of NCs and CD34+ cells infused was 2.4 × 108/kg (range, 0.28-5.97) and 3.7 × 106/kg (range, 0.07-18.7), respectively. There was a moderate, although significant, correlation between the number of NCs and CD34+ cells infused (R = 0.58,P < .0001). As we concentrated the graft in major ABO incompatibility, the median dose of NCs per kilogram was significantly greater in patients with an ABO matched/minor mismatched donor than in patients with an ABO major mismatched donor (2.5 × 108/kg [range, 0.43-5.97] versus 0.8 × 108/kg [range, 0.28-3.40], respectively,P < .001). The median dose of CD34+ cells per kilogram was also greater in patients with an ABO matched/minor mismatched donor than in patients with an ABO major mismatched donor: 3.9 × 108/kg (range, 0.07-18.70) and 2.9 × 108/kg (range, 0.87-14.00), respectively, (P = .03).
Hematopoietic recovery
Neutrophil recovery.
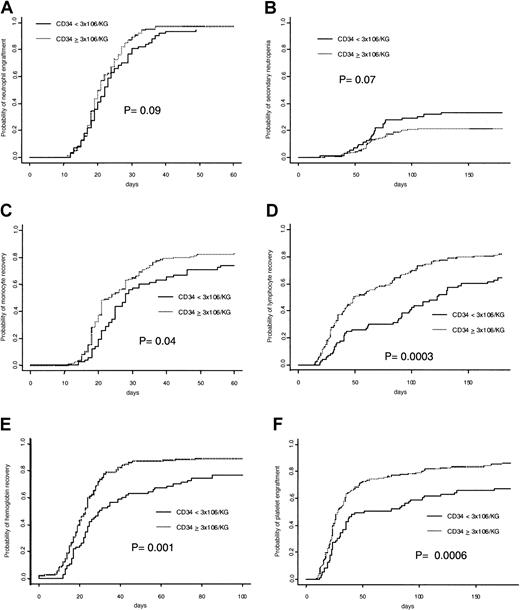
Six patients died during the first 28 days after transplantation without neutrophil recovery and 2 patients had nonengraftment. A total of 204 (96.2%) patients achieved an absolute neutrophil count more than 0.5 × 109/L. The cumulative incidence rate of recovery at day 60 was 96.2% overall, with specific incidence of 97.1% in patients receiving 3 × 106/kg or more CD34+ cells and 93.1% in patients receiving less than 3 × 106/kg CD34+ cells (Figure1A). Results of multivariable analysis (Table 3) showed that infusion of more than 3 × 106/kg CD34+ (HR = 1.37; 95% CI = 1.01-1.85, P = .04) was associated with faster neutrophil engraftment.
Cumulative incidence of hematopoietic recoveries according to CD34 cell dose.
The panels show (A) neutrophil, (B) secondary neutropenia, (C) monocyte, (D) lymphocyte, (E) hemoglobin, and (F) platelet recoveries.
Cumulative incidence of hematopoietic recoveries according to CD34 cell dose.
The panels show (A) neutrophil, (B) secondary neutropenia, (C) monocyte, (D) lymphocyte, (E) hemoglobin, and (F) platelet recoveries.
Multivariable analyses of hematopoietic recovery, fungal infection, transplantation-related mortality, and overall survival
| . | Hazard ratio (95% confidence interval) . | P . |
|---|---|---|
| Neutrophil engraftment | ||
| Female recipient | 1.72 (1.25-2.37) | .01 |
| CD34 ≥ 3.0 × 106/kg | 1.37 (1.01-1.85) | .04 |
| Secondary neutropenia | ||
| Advanced stage of disease | 2.12 (1.09-4.12) | .03 |
| Female recipient | 0.51 (0.28-0.94) | .03 |
| CD34 ≥ 3.0 × 106/kg | 0.60 (0.35-1.00) | .05 |
| Monocyte recovery | ||
| Female recipient | 1.46 (1.05-2.03) | .03 |
| CD34 ≥ 3.0 × 106/kg | 1.47 (1.07-2.03) | .02 |
| Lymphocyte recovery | ||
| Age of donor3-150 | 0.98 (0.97-1.00) | .01 |
| Positive CMV serology | 1.62 (1.15-2.28) | .006 |
| CD34 ≥ 3.0 × 106/kg | 1.70 (1.20-2.41) | .003 |
| Hemoglobin recovery | ||
| Chronic leukemia | 1.43 (1.05-1.96) | .02 |
| CD34 ≥ 3.0 × 106/kg | 1.77 (1.31-2.39) | .0002 |
| Gender match | 0.74 (0.55-0.98) | .04 |
| Platelet engraftment | ||
| Advanced stage of disease | 0.51 (0.30-0.86) | .01 |
| Female recipient | 1.37 (1.01-1.87) | .01 |
| CD34+ ≥ 3 × 106/kg | 1.98 (1.41-2.77) | .00008 |
| Invasive fungal infection | ||
| Advanced stage of disease | 2.49 (1.12-5.57) | .03 |
| CD34+ ≥ 3 × 106/kg | 0.41 (0.21-0.79) | .008 |
| Transplantation-related mortality | ||
| Age of recipient3-150 | 1.04 (1.02-1.06) | .0004 |
| Advanced stage of disease | 2.61 (1.37-4.98) | .0043-150 |
| Female donor | 0.52 (0.29-0.95) | .03 |
| CD34+ ≥ 3 × 106/kg | 0.54 (0.32-0.94) | .03 |
| Overall survival | ||
| Age of recipient3-150 | 1.03 (1.02-1.04) | .001 |
| Advanced stage of disease | 2.53 (1.60-4.25) | .0005 |
| Female | 0.57 (0.36-0.89) | .01 |
| CD34+ ≥ 3 × 106/kg | 0.55 (0.36-0.85) | .006 |
| . | Hazard ratio (95% confidence interval) . | P . |
|---|---|---|
| Neutrophil engraftment | ||
| Female recipient | 1.72 (1.25-2.37) | .01 |
| CD34 ≥ 3.0 × 106/kg | 1.37 (1.01-1.85) | .04 |
| Secondary neutropenia | ||
| Advanced stage of disease | 2.12 (1.09-4.12) | .03 |
| Female recipient | 0.51 (0.28-0.94) | .03 |
| CD34 ≥ 3.0 × 106/kg | 0.60 (0.35-1.00) | .05 |
| Monocyte recovery | ||
| Female recipient | 1.46 (1.05-2.03) | .03 |
| CD34 ≥ 3.0 × 106/kg | 1.47 (1.07-2.03) | .02 |
| Lymphocyte recovery | ||
| Age of donor3-150 | 0.98 (0.97-1.00) | .01 |
| Positive CMV serology | 1.62 (1.15-2.28) | .006 |
| CD34 ≥ 3.0 × 106/kg | 1.70 (1.20-2.41) | .003 |
| Hemoglobin recovery | ||
| Chronic leukemia | 1.43 (1.05-1.96) | .02 |
| CD34 ≥ 3.0 × 106/kg | 1.77 (1.31-2.39) | .0002 |
| Gender match | 0.74 (0.55-0.98) | .04 |
| Platelet engraftment | ||
| Advanced stage of disease | 0.51 (0.30-0.86) | .01 |
| Female recipient | 1.37 (1.01-1.87) | .01 |
| CD34+ ≥ 3 × 106/kg | 1.98 (1.41-2.77) | .00008 |
| Invasive fungal infection | ||
| Advanced stage of disease | 2.49 (1.12-5.57) | .03 |
| CD34+ ≥ 3 × 106/kg | 0.41 (0.21-0.79) | .008 |
| Transplantation-related mortality | ||
| Age of recipient3-150 | 1.04 (1.02-1.06) | .0004 |
| Advanced stage of disease | 2.61 (1.37-4.98) | .0043-150 |
| Female donor | 0.52 (0.29-0.95) | .03 |
| CD34+ ≥ 3 × 106/kg | 0.54 (0.32-0.94) | .03 |
| Overall survival | ||
| Age of recipient3-150 | 1.03 (1.02-1.04) | .001 |
| Advanced stage of disease | 2.53 (1.60-4.25) | .0005 |
| Female | 0.57 (0.36-0.89) | .01 |
| CD34+ ≥ 3 × 106/kg | 0.55 (0.36-0.85) | .006 |
CMV, cytomegalovirus.
Continuous variable.
Secondary neutropenia.
Neutropenia of less than 0.5 × 109 neutrophil/L after initial engraftment was found in 54 (25.5%) patients, with a cumulative estimated rate at day 180 of 25.6%. The incidence of secondary neutropenia was lower in patients receiving a CD34+ cell dose of 3 × 106/kg or more than in patients receiving less than 3 × 106/kg (21.6% versus 33.3%, P = .07; Figure 1B). In multivariable analysis a CD34+ cell dose of at least 3 × 106/kg (HR = 0.60; 95% CI = 0.35-1.00,P = .05) was associated with a lower incidence of secondary neutropenia.
Monocyte recovery.
A total of 169 (79.7%) patients achieved monocyte recovery, with an estimated rate of 79.2% at day 60. The 60-day cumulative estimated rate of monocyte recovery in patients receiving CD34+ cell dose 3 × 106/kg or more was 82.0% versus 74.0% in those receiving less (P = .004; Figure 1C). Multivariable analysis (Table 3) showed that a CD34+ cell dose more than 3 × 106/kg (HR = 1.47; 95% CI = 1.07-2.03,P = .02) was associated with faster monocyte recovery.
Lymphocyte recovery.
At day 180, the cumulative estimate rate of lymphocyte recovery was 75.9%, with 161 (75.9%) patients achieving a lymphocyte count of 0.5 × 109/L or more. The time to lymphocyte recovery was significantly faster for patients receiving 3 × 106/kg or more CD34+ cells (ie, 82.0%) than for those receiving less (64.4%; P = .0003; Figure 1D). In univariable analyses, age and weight of recipient, diagnosis of chronic or acute leukemia, donor age, NC dose more than 2.4 × 108/kg, and CD34+ cell dose more than 3 × 106/kg significantly affected lymphocyte recovery. In multivariable analysis (Table 2), CD34+ cell dose more than 3 × 106/kg (HR = 1.70; 95% CI = 1.20-2.41,P = .003) was retained as being positively associated with the outcome, jointly with donor age and positive CMV serology.
Erythrocyte recovery.
The cumulative estimated incidence of hemoglobin recovery by day 100 was 84.9%. Time to hemoglobin of 80 g/L or more in patients receiving a CD34+ cell dose at least 3 × 106/kg was significantly faster than in remainders (89.2% versus 76.7%, respectively; P = .001; Figure 1E). Results of multivariable analysis showed that CD34+ cell dose more than 3 × 106/kg (HR = 1.77; 95% CI = 1.31-2.39,P = .0002) was associated with faster hemoglobin recovery (Table 3).
Platelet recovery.
Cumulative estimated incidence of platelet recovery by day 180 was 79.7%. Cumulative estimated rate of platelet recovery was 86.3% versus 67.1% in patients receiving more or less than 3 × 106/kg CD34+ cells, respectively (Figure1F). Female recipients (P = .025), NC dose of more than 2.4 × 108/kg (P = .017), and a CD34+ cell dose of more than 3 × 106/kg (P = .0006) were significantly associated with a faster platelet engraftment in univariable analyses. However, a diagnosis of malignancy (P = .10) and an advanced stage of disease (P = .023) were associated with slower platelet engraftment. By applying a multivariable model (Table 3), the most favorable factor affecting platelet engraftment was infusion of a CD34+ cell dose of 3 × 106/kg or more (HR = 1.98; 95% CI = 1.41-2.77, P = .00008).
Infections
At day 180 after transplantation, 133 (62.7%) patients had presented at least one episode of microbiologically and/or clinically documented severe bacterial, viral, or fungal infection. Infection was directly responsible for 39 (52.0%) of 75 transplantation-related deaths in the whole period of followup. The cumulative incidence rate of infected patients by day 180 was 62.7%.
A bacterial episode of infection occurred in 60 (28.3%) patients. Cumulative incidence of patients with at least one bacterial infection was 28.4% at day 180. Similarly, the cumulative incidence of patients who experienced at least one viral infection was 42.0% at day 180, with a total of 91 events (mainly CMV infection). CD34+cell dose did not influence incidence of bacterial or viral infection (P = .40 and P = .26, respectively).
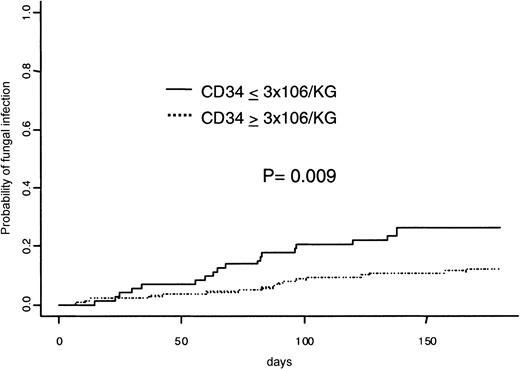
The estimated probability of experiencing at least one IFI at 180 days was 17.0%. Figure 2 shows the difference of incidence of IFI according to the number of CD34+ cells infused, ie, 12.2% in patients with a CD34+ cell dose of 3 × 106/kg or more and 26.3% in those with a CD34+ cell dose less than 3 × 106/kg;P = .009. There were 26 aspergillosis (7 microbiologically not proven), 7 candidemia, and 3 other disseminated fungal infections (2 episodes of disseminated candidiasis and 1 of disseminatedMalassezia furfur). In multivariable analysis, a dose of CD34+ cells of 3 × 106/kg or more still decreased the probability of IFI (HR = 0.41; 95% CI = 0.21-0.79,P = .008) when adjusting for confounding variables.
Graft-versus-host disease
Acute GVHD of grade II or more occurred in 101 (47.6%) patients with a cumulative rate of 49% at day 100. Acute GVHD of grade III or IV developed in 36 (17.0%) patients with an estimated incidence of 17% at day 100. Chronic GVHD developed in 86 (49.1%) of 175 patients at risk, of which 42 patients had limited disease and 44 had extensive disease. The 5-year cumulative incidence of chronic GVHD was 54%. CD34+ cell dose did not affect the occurrence of acute GVHD (grade II-IV; P = .84), acute GVHD (grade III-IV;P = .48), or chronic GVHD (P = .39).
TRM and survival
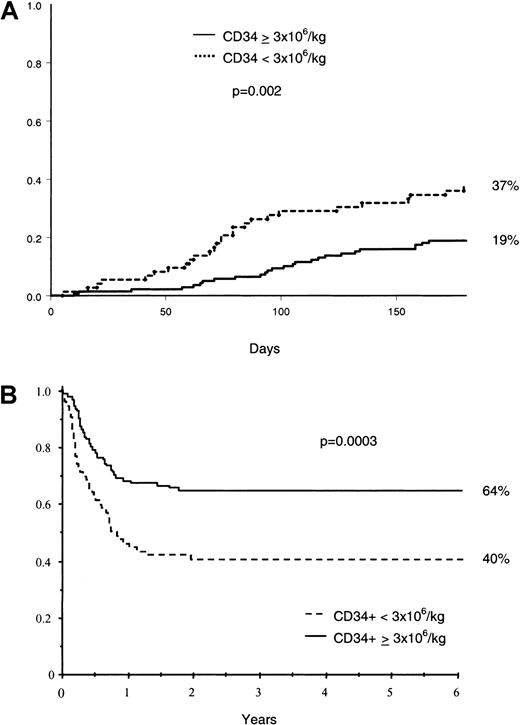
Fifty-two (24.5%) patients died of transplantation-related complications during the first 180 days after transplantation. The main causes of TRM were IFI (n = 15), GVHD (n = 11), bacterial infection (n = 7), and acute respiratory distress syndrome (n = 7). Kaplan-Meier estimate of 180-day TRM was 37% in patients receiving a CD34+ cell dose less than 3 × 106 and 19% in remainders (Figure 3). In the Cox multivariable analysis, infusion of 3 × 106 or more CD34+ cells per kilogram (HR = 0.54; 95% CI = 0.32-0.94, P = .03) was associated with a reduction in TRM. Mortality related to infection during the first 180 days after transplantation was greater among patients receiving less than 3 × 106 CD34+ cells (16.4% versus 8.6% for those receiving a higher dose).
TRM and survival.
Cumulative incidence of TRM at day 180 (A), and Kaplan-Meier estimate of overall survival (B) according to CD34+ cell dose.
TRM and survival.
Cumulative incidence of TRM at day 180 (A), and Kaplan-Meier estimate of overall survival (B) according to CD34+ cell dose.
With a median follow-up of 3.4 years (range, 0.6-6.1), 121 (57.1%) patients were alive on March 1, 2000. Four patients were lost to follow-up. The 5-year estimate of survival for the whole population was 56%. Figure 3 shows survival curves according to infused CD34+ cell dose. Ninety-one (42.9%) patients died: 14 (6.6%) after relapse, 75 (35.4%) of transplantation-related complications, and 2 of other causes. Multivariable analysis showed that a CD34+ cell dose of 3.0 × 106/kg or more (HR = 0.55; 95% CI = 0.36-0.85, P = .006) was associated with a better survival rate.
Discussion
The importance of CD34+ cell quantification has been clearly demonstrated in autologous or allogeneic peripheral blood hematopoietic stem cell transplantations.10-13 Studies of CD34+ cell dose in the setting of allogeneic BMT have been mainly restricted to T-cell–depleted related or unmanipulated unrelated BMT. Mavroudis et al14 were the first to suggest in only 28 patients who received a T-cell–depleted BM graft that CD34+ cell dose predicted survival, posttransplantation morbidity, and rate of hematologic recovery. More recently, the same group showed that a CD34+ cell dose greater than 3 × 106/kg correlated with a better survival rate, lower TRM, and less relapses.16 Besides this study, data on the influence of CD34+ cell graft content on survival, TRM, and platelet recovery has only been reported in unrelated unmanipulated BMT.15 However, in this study, as well as in another,17 no correlation between CD34+ cell dose and neutrophil engraftment has been shown.
In our retrospective unicentric cohort study, we were able to show that CD34+ cell dose significantly influenced hematopoietic reconstitution. Indeed, our data confirm and extend previous findings that a higher CD34+ cell dose improves hematopoietic recovery.14
Most previous studies in allogeneic BM and PBSC transplantations failed to show a correlation between CD34+ cell dose and neutrophil engraftment.14,15,18,27,28 However, we found a significant relation between a dose of BM CD34+ cell dose of 3 × 106/kg or more and faster neutrophil engraftment in agreement with the findings of Singhal et al.29 Also of importance is our finding that the incidence of secondary neutropenia correlates with CD34+ cell dose. Patients receiving a CD34+ cell dose of less than 3 × 106/kg had a greater risk of secondary neutropenia. Short-term engraftment of neutrophils may depend on CD34+ progenitor cells committed to granulocyte lineage, even if other accessory cells, such as T lymphocytes, also play a role on engraftment.30,31 Studies on secondary neutropenia after allogeneic BMT are scarce. Neutropenia occurring after ganciclovir prophylaxis for CMV disease after BMT correlates better with low marrow cellularity at day +21 posttransplantation than with other markers of engraftment such as time to neutrophil or platelet engraftment.32 Sierra et al6 have shown that a lower NC dose correlates with a greater incidence of neutropenia less than 0.5 × 109/L during the initial 15 weeks after transplantation. The National Institutes of Health group showed that a dose of CD34+cells more than 2 × 106/kg was correlated with the needs for significantly less granulocyte colony-stimulating factor (G-CSF) to maintain neutrophil counts during ganciclovir treatment.14
In addition, we demonstrated that faster monocyte recovery occurred with an infused CD34+ cell dose more than 3 × 106/kg. Lymphocyte reconstitution was also affected by the CD34+ cell dose. Patients receiving more than 3 × 106/kg CD34+ cells had a median time to recovery of total lymphocytes (> 0.5 × 109/L) 2 months shorter than patients receiving a lower dose. We have described in 67 patients that recovery of CD3+, CD8+, and B-cell lymphocytes were influenced by CD34+ cell dose.33
Regarding erythrocyte reconstitution, CD34+ cell dose was also correlated with faster hemoglobin recovery. Previous studies on autologous PBSCs and also on allogeneic BMT had suggested a lower RBC transfusion requirement and a faster RBC transfusion independence with higher doses of CD34+ cells.14,34 Platelet recovery also correlates with CD34+ cell dose as previously reported by other groups.11,14,15,17,27 35 A more complete and sustained erythrocyte/megakaryocytic reconstitution may be achieved when a higher CD34+ cell dose is used, allowing patients to be transfusion independent earlier.
As previously reported, our data confirm that CD34+ cell dose significantly influences TRM and overall survival.14-17 Slower or inadequate immunologic recovery is associated with high infection rates after allogeneic BMT. Storek et al36 have shown that low B-cell and monocyte counts on day 80 were associated with a greater incidence of severe infections (mainly viral and fungal) in allogeneic BMT.36 Secondary neutropenia after ganciclovir prophylaxis for CMV infections was associated with higher rate of bacteremia and fungal infections.32 We hypothesize that, because CD34+ cell dose significantly influences hematopoietic recovery, a faster and more robust immunologic recovery occurs with higher doses and, consequently, diminishes the risk of infection-related death and TRM. Indeed, this is the first study which described that CD34+ cell dose significantly influenced fungal infection, a rather late event after allogeneic BMT and a leading cause of TRM.37,38 Although this finding clearly warrants further confirmation, this association is of obvious clinical importance. Furthermore, our data showed that monocyte and lymphocyte recovery were faster with higher doses of CD34+ cells. CD34+ cell dose influenced neutrophil-macrophage-monocyte reconstitution that plays an essential role for protection from fungal infections.39 40 As invasive fungal infection occurs later after BMT, secondary neutropenia, rather than early neutrophil engraftment, is an important risk factor that might also explain the higher fungal infection rate in patients receiving a lower CD34+ cell dose. Finally, the main cause of transplantation-associated death was infection (24 of 53 deaths during the first 180 days after transplantation), with a higher infection-associated mortality rate in patients receiving a lower CD34+ cell dose.
One of the advantages of this study is that it comes from a single institution, in which 212 consecutive patients, who underwent allogeneic BMT, had been grafted with the same BM collection technique and treated with the same supportive therapy. CD34+ cell quantification techniques did not change during the duration of the study. A potential disadvantage of our study is that our threshold for CD34+ cell dose might not be useful for other institutions because CD34 quantification can vary between centers. It is known that CD34+ cell quantification is a better surrogate marker for the stem cell content. Quantification for granulocyte-macrophage colony-forming units has been used, but it is time-consuming and results are only available after 2 weeks. NC dose has been widely used because it is easy to measure and it has been shown to correlate with neutrophil, lymphocyte, and platelet recoveries.3,5,7However, little is known about NC dose and other hematopoietic parameters and long-term engraftment. In multivariate analysis, we found that the CD34+ cell dose was a better predictor of hematopoietic recovery. Unfortunately, we did not quantify other cell subsets in the BM graft that could influence outcomes. Of course, one question raised with our study is how to increase the CD34+cell dose. Several ways of achieving this goal can be proposed: G-CSF–stimulated PBSCs can provide a higher CD34+ cell graft content compared with steady-state BM.17 Controversy remains about the possible higher incidence of chronic GVHD after allogeneic PBSC transplantation.41-43 Estimation of CD34+ cell dose at the beginning of harvesting, as suggested, could be an opportunity to optimize BMT outcomes.44 Use of hematopoietic growth factors (rhuG-CSF or rhuGM-CSF) in selected cases after engraftment could also be an option to be tested.
In conclusion, our results show that HLA-identical related unmanipulated BM CD34+ cell dose more than 3 × 106/kg improves hematopoietic recovery and significantly reduces the risk of secondary neutropenia, resulting in reduction of fungal infection rate, TRM, and, consequently, improvement of survival.
Supported by the Association de Recherche sur les Transplantations Médullaires (H.B., V.R., and F.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eliane Gluckman, Service d'Hématologie-Greffe de Moelle, Hôpital Saint-Louis, 1, avenue Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail:eliane.gluckman@sls.ap-hop-paris.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal