Fusions of cancer cells and dendritic cells (DCs) are effective in the treatment of animal tumor models and patients with metastatic renal carcinoma. In this study, we have fused DCs with mouse 4TOO plasmacytoma cells. The results demonstrate that vaccination of mice with the fusion cells (FC/4TOO) is associated with induction of antitumor humoral and cytotoxic T lymphocyte (CTL) responses. Immunization with FC/4TOO cells protected mice against tumor challenge. In addition, treatment of established multiple myeloma with FC/4TOO cells was associated with prolongation of survival but not with eradication of disease. As interleukin (IL)-12 potentiates the induction of immune responses, recombinant mouse IL-12 was administered with the FC/4TOO vaccine. Treatment of mice with FC/4TOO and IL-12 was associated with increased CTL activity and T-cell proliferation responses. Treatment with FC/4TOO and IL-12 also resulted in eradication of established disease. These findings demonstrate that immunization with FC/4TOO fusion cells and IL-12 potentiates antitumor immunity and the treatment of murine multiple myeloma.
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) that initiate primary immune responses.1,2 DCs are distinguished from B lymphocytes and macrophages by their abundant expression of major histocompatibility complex (MHC) class I, class II, costimulatory molecules, and adhesion molecules, which provide secondary signals for the stimulation of naive T-cell populations.3-5 In addition, DCs have the ability to efficiently prime both CD8+ and CD4+immunity.6 On the basis of these findings, antitumor vaccines have been developed with DCs that have been pulsed, transfected, or transduced to present peptides derived from tumor antigens.7-12 These approaches require a known tumor antigen, and few at present have been identified for human tumors.
As an approach to exploit both known and unidentified tumor epitopes, DCs have been fused to cancer cells to generate heterokaryons that combine the expression of molecules needed for immune stimulation with presentation of a potential repertoire of tumor antigens.13 Vaccination with fusions of murine tumor cells and syngeneic DCs has been shown to eliminate established metastatic disease in diverse models.13-17 This approach has also been extended to the fusion of human tumor cells with autologous and allogeneic DCs.18,19 The human fusions were effective in stimulating cytotoxic T-lymphocyte (CTL) responses against autologous tumors.18,19 Moreover, vaccination with tumor-DC fusions has been successful in the treatment of patients with metastatic renal cell carcinoma.20
Interleukin 12 (IL-12) is a heterodimeric cytokine that up-regulates DC expression of costimulatory molecules.21,22 IL-12 induces stimulation of Th1 reactivity.23 Other studies have shown that IL-12 inhibits induction of anergy, reverses immunologic unresponsiveness to tumor antigens, and expands antigen-specific CD8+ T cells.12,24-26 In concert with these findings, IL-12 was found to be necessary for the rejection of tumors in mice and to improve the efficacy of antitumor vaccines using peptide-pulsed DCs27,28 and RNA-transfected DCs.12
In the present study, we describe a fusion cell vaccine for the treatment of a mouse model of multiple myeloma. The results demonstrate that the vaccine is effective in prolonging the survival of mice with established disease. We also show that, when combined with IL-12, the fusion cell vaccine is effective in the long-term eradication of multiple myeloma.
Materials and methods
Cell culture and fusion
Murine Balb/c plasmacytoma 4TOO,29 murine Balb/c MPC-11, and human SKO-007 myeloma cells (American Type Culture Collection [ATCC], Manassas, VA) were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Syngeneic DCs were generated from the bone marrow of Balb/c mice by culture in 20 ng/mL granulocyte-macrophage colony-stimulating factor (Sigma, St Louis, MO) for 5 days as described.13,30 The DCs were fused with 4TOO cells (FC/4TOO) at a 10:1 ratio in the presence of 50% polyethylene glycol (Sigma) as described.13 30 The FC/4TOO cells were then selected in hypoxanthine-aminopterin-thymidine (HAT) medium (Sigma) for 5 to 8 days.
Flow cytometry
To quantitate the DC/4TOO fusion cells, 4TOO cells were stained with the red fluorescence dye PKH-26 (Sigma), washed with phosphate-buffered saline (PBS), and then fused with DCs. DCs, PKH-26–labeled 4TOO cells, and FC/4TOO cells were incubated with monoclonal antibody (MAb) M1/42/3.9.8 (anti-MHC class I; ATCC), MAb anti-M5/114 (anti-MHC class II), MAb 16-10A1 (anti-CD80), or MAb GL1 (anti-CD86) (Pharmingen, San Diego, CA) for 30 minutes on ice. After washing with PBS, the cells were incubated with the appropriate fluorescein isothiocyanate–conjugated antihamster, antirat, or antimouse immunoglobulin G (IgG) for another 30 minutes on ice. Samples were then washed, fixed, and analyzed by FACScan (Becton Dickinson, Bedford, MA). For dual staining, cells were incubated with anti–Syndecan-1 (anti-CD138; Pharmingen) for 30 minutes at 4°C and then with fluorescein isothiocyanate–conjugated goat antimouse IgG. The cells were washed and then incubated with phycoerythrin-conjugated anti-MHC class II for 30 minutes at 4°C. After washing again, cells were fixed with 2% paraformaldehyde and subjected to bidimensional FACScan and analysis using Cellquest software.
Mixed lymphocyte reactions
DCs, 4TOO cells, and FC/4TOO cells were irradiated (30 Gy) and incubated at the indicated ratios with allogeneic (C57BL/6) T cells in 96-well U-bottom plates for 5 days. The T cells were prepared as described.13 Proliferation of T cells was assessed by pulsing with 0.037 MBq (1 μCi)/well [3H]-thymidine (New England Nuclear, Boston, MA) for 12 hours and monitoring for tritium incorporation.
Humoral immune responses
Sera were obtained from mice at 7 days after the second immunization. Microtiter plates were precoated with 4TOO, MPC-11, or SKO-007 cell lysate by incubation overnight at 4°C. The wells were washed and incubated with a 4-fold dilution of mouse sera for 1 hour at 4°C. After washing and incubation with goat antimouse IgG conjugated to horseradish peroxidase (Amersham, Piscataway, NJ), the antibody complexes were detected by development with o-phenylenediamine (Sigma) and measured in an enzyme-linked immunosorbent assay microplate autoreader EL 310 at 490 nm.
Cytotoxicity assays
4TOO, MPC-11, and SKO-007 cell targets were labeled with51Cr for 60 minutes at 37°C, added to 96-well v-bottom plates, and incubated with splenocytes from the immunized mice for 5 hours at 37°C. The culture supernatants were assayed in a gamma counter. Spontaneous release of 51Cr was assessed by incubation of targets in the absence of splenocytes. Maximum or total51Cr release was determined by incubation of targets in 0.1% Triton X-100. Percentage of specific 51Cr release was determined by the equation: percentage of specific release = [(experimental − spontaneous)/(maximum − spontaneous)] × 100.
T-cell proliferation assay
Lymph node cells (LNCs) and splenocytes from immunized mice were prepared as single cell suspensions. Erythrocytes and dead cells were removed by centrifugation into a Ficoll-Hypaque gradient. LNCs and splenocytes were washed and resuspended in RPMI-1640 medium supplemented with 15 mM HEPES (pH 7.4), 5% HI-FCS, 2 mM L-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 × 105β-mercaptoethanol. Cells were incubated with irradiated FC/4TOO cells at a 10:1 ratio in 96-well, U-bottom plates for 5 days. Cell proliferation was assessed by [3H]thymidine incorporation after an additional 12-hour incubation.
Mouse IL-12 production and assay
Recombinant mouse IL-12 (rmIL-12) was prepared from the culture supernatants of SP2/O cells transfected to stably express the p35 and p40 subunits (kindly provided by Dr Knight, Centocor). Production of IL-10 and IL-12 by DCs, 4TOO cells, and FC/4TOO cells was measured by the mouse IL-10 and IL-12 OptEIA enzyme-linked immunosorbent assay kits (Pharmingen).
Antitumor studies in vivo
Groups of 10 Balb/c mice were immunized twice subcutaneously with 106 DCs; irradiated 4TOO, DCs, and 4TOO that had been coincubated for 24 hours (not fused); or FC/4TOO cells on days 0 and 7. On day 14, the mice were challenged intravenously with 2 × 105 4TOO plasmacytoma cells. In the treatment studies, Balb/c mice were injected intravenously with 2 × 105 4TOO plasmacytoma cells on day 0. On days 2, 6, and 10, the mice were treated intravenously with 1 × 10,6 2 × 106, or 5 × 106 FC/4TOO cells. rmIL-12 was administered intraperitoneally. Irradiated 4TOO cells were used as controls.
Results
Fusion of 4TOO plasmacytoma cells and DCs
Murine Balb/c plasmacytoma 4TOO cells were fused to syngeneic bone marrow–derived DCs in the presence of polyethylene glycol. To assess the formation of heterokaryons, the cells were cultured for 7 days and then analyzed for expression of surface markers selective for the fusion cell population. Red fluorescence from staining with PKH-26 was detectable on the surface of 4TOO cells but not on DCs (Figure1A). By contrast, MHC class II, CD80, and CD86 were expressed by DCs and not by 4TOO cells (Figure 1A). MHC class I was detectable on both 4TOO cells and DCs (Figure 1A). In concert with the formation of heterokaryons, MHC class II, CD80, and CD86 were detectable on the FC/4TOO fusion cells (Figure 1A). Moreover, detection of red fluorescence and MHC class II, CD80, and CD86 confirmed the formation of heterokaryons (Figure 1A). On the basis of the findings, the fusion efficiency was between 30% and 50%. To confirm these findings, 4TOO, DCs, and FC/400 cells were analyzed by bidimensional flow cytometry for reactivity with antibodies against MHC class II and Syndecan, an antigen expressed by myeloma cells.31 The results demonstrate that, in contrast to DCs and 4TOO cells, more than 50% of the FC/4TOO cell population expressed both MHC class II and Syndecan (Figure 1B). To characterize in part the function of FC/4TOO cells, we compared their activity with 4TOO cells or DCs as stimulators in primary allogeneic mixed lymphocyte reactions. The 4TOO cells had little effect on T-cell proliferation (Figure 1C). By contrast, FC/4TOO cells, like DCs, stimulated proliferation of the allogeneic T cells (Figure 1C). These findings demonstrate that the FC/4TOO cells, which express MHC and costimulatory molecules, are functional in mixed lymphocyte reactions.
Phenotype and function of FC/4TOO fusion cells.
(A) DCs, 4TOO plasmacytoma, and FC/4TOO fusion cells were analyzed by bidimensional flow cytometry for PKH-26 fluorescence and expression of the indicated antigens. (B) DCs, 4TOO cells, and FC/4TOO fusion cells were analyzed by bidimensional flow cytometry for the expression of MHC class II and Syndecan. (C) DCs (●), 4TOO plasmacytoma (○), FC/4TOO fusion cells (▴), and medium alone (□) were cocultured with C57BL/6 T cells at different ratios for 5 days. [3H]-thymidine uptake was measured at 12 hours after a pulse of 0.037 MBq (1 μCi)/well. The stimulation index is expressed as the mean ± SE of 3 experiments each performed in triplicate.
Phenotype and function of FC/4TOO fusion cells.
(A) DCs, 4TOO plasmacytoma, and FC/4TOO fusion cells were analyzed by bidimensional flow cytometry for PKH-26 fluorescence and expression of the indicated antigens. (B) DCs, 4TOO cells, and FC/4TOO fusion cells were analyzed by bidimensional flow cytometry for the expression of MHC class II and Syndecan. (C) DCs (●), 4TOO plasmacytoma (○), FC/4TOO fusion cells (▴), and medium alone (□) were cocultured with C57BL/6 T cells at different ratios for 5 days. [3H]-thymidine uptake was measured at 12 hours after a pulse of 0.037 MBq (1 μCi)/well. The stimulation index is expressed as the mean ± SE of 3 experiments each performed in triplicate.
Induction of immunity with FC/4TOO cells
To assess whether immunization with FC/4TOO cells induces an anti-4TOO immune response, mice were vaccinated subcutaneously twice with 1 × 106 irradiated DCs, 4TOO cells, or FC/4TOO cells. In contrast to DCs, immunization with 4TOO or FC/4TOO cells resulted in induction of anti-4TOO antibody responses (Figure2A). Immunization with FC/4TOO was also associated with induction of an antibody response against murine MPC-11 myeloma cells, but to a lesser extent than that obtained against 4TOO cells (Figure 2B). As a control, there was no detectable antibody response against human SKO-007 myeloma cells (Figure 2B). To assess cell-mediated immunity, splenocytes from the immunized mice were analyzed for CTL activity against 4TOO cell targets. There was little, if any, lysis of 4TOO cells in the presence of splenocytes from mice immunized with DCs (Figure 2C). CTL activity was detectable in mice immunized with irradiated 4TOO cells, and this response was more pronounced in mice vaccinated with FC/4TOO cells (Figure 2C). As controls, CTL activity was detectable against MPC-11 but not SKO-007 cells (Figure 2D). These findings indicate that, like irradiated 4TOO cells, immunization with FC/4TOO is associated with induction of humoral and cell-mediated immune responses.
Induction of antitumor humoral and cellular responses by 4TOO and FC/4TOO fusion cell immunization.
(A) Balb/c mice (6 per group) were immunized twice subcutaneously with 1 × 106 irradiated 4TOO plasmacytoma cells (■), FC/4TOO cells (●), or DCs (□). PBS (○) was used as a control. Serum was collected on day 7 after the last immunization and was examined for reactivity with 4TOO cell lysates by enzyme-linked immunosorbent assay. (B) Balb/c mice (3 per group) were twice immunized with FC/4TOO. The sera were collected and added to plates coated with lysates from 4TOO (●), MPC-11 (▴), or SKO-007 (□) cells. (C) Splenocytes were isolated 7 days after vaccinating twice with 1 × 106 irradiated 4TOO cells (■), FC/4TOO cells (●), DCs (□), or PBS (○). The T cells were purified by nylon wool columns and incubated with 4TOO targets at the indicated ratios. (D) CTLs isolated from mice twice immunized with FC/4TOO were analyzed: CTL activity against 4TOO (●), MPC-11 (▴), or SKO-007 (□) targets. CTL activity was determined by the 51Cr release assay. The CTL results are expressed as the mean ± SD of 3 replicates.
Induction of antitumor humoral and cellular responses by 4TOO and FC/4TOO fusion cell immunization.
(A) Balb/c mice (6 per group) were immunized twice subcutaneously with 1 × 106 irradiated 4TOO plasmacytoma cells (■), FC/4TOO cells (●), or DCs (□). PBS (○) was used as a control. Serum was collected on day 7 after the last immunization and was examined for reactivity with 4TOO cell lysates by enzyme-linked immunosorbent assay. (B) Balb/c mice (3 per group) were twice immunized with FC/4TOO. The sera were collected and added to plates coated with lysates from 4TOO (●), MPC-11 (▴), or SKO-007 (□) cells. (C) Splenocytes were isolated 7 days after vaccinating twice with 1 × 106 irradiated 4TOO cells (■), FC/4TOO cells (●), DCs (□), or PBS (○). The T cells were purified by nylon wool columns and incubated with 4TOO targets at the indicated ratios. (D) CTLs isolated from mice twice immunized with FC/4TOO were analyzed: CTL activity against 4TOO (●), MPC-11 (▴), or SKO-007 (□) targets. CTL activity was determined by the 51Cr release assay. The CTL results are expressed as the mean ± SD of 3 replicates.
FC/4TOO cells induce antitumor activity
To assess the induction of antitumor immunity, mice were immunized with irradiated DCs, 4TOO cells, or FC/4TOO cells and then challenged intravenously with 2 × 105 viable 4TOO cells. Mice vaccinated with DCs, 4TOO cells, or DCs coincubated with 4TOO cells rapidly succumbed to their disease at a rate similar to that found in control mice given PBS (Figure 3A). By contrast, 80% of the mice immunized with FC/4TOO cells were protected against tumor challenge (Figure 3A). In studies to assess treatment of established disease, mice were first given 2 × 105 4TOO cells intravenously on day 0 and then treated with different doses of irradiated FC/4TOO cells on days 2, 6, and 10. Compared with mice immunized with 1 × 106 irradiated 4TOO cells or DCs alone, those mice given 2 × 105 FC/4TOO cells survived longer (Figure 3B). Moreover, treatment with higher doses of FC/4TOO cells was associated with progressively longer durations of survival (Figure 3B). CTL activity against 4TOO cells reached approximately 20% as a consequence of FC/4TOO vaccination and then decreased to background levels, as the mice succumbed to their disease. These results indicate that the antitumor immune response induced by FC/4TOO vaccination corresponds with disease course. Nonetheless, all mice treated with FC/4TOO succumbed to the tumor (Figure 3B). To determine whether a lower tumor burden can be eradicated, mice given 1 × 105 tumor cells were treated with 2 × 106 irradiated FC/4TOO cells. By using these experimental conditions, survival was extended, but all of the animals died of progressive disease (Figure 3C). These findings indicated that FC/4TOO immunization is effective only in part in inducing antitumor activity.
Antitumor immunity induced by immunization with FC/4TOO cells.
(A) Balb/c mice (10 per group) were immunized twice subcutaneously with 1 × 106 irradiated 4TOO cells (■), DCs alone (□), FC/4TOO cells (●), or DCs mixed with 4TOO cells (▴). PBS (○) was used as a control. On day 14, the mice were challenged intravenously with 2 × 105 4TOO cells. (B) Balb/c mice (10 per group) were injected intravenously with 2 × 105 4TOO plasmacytoma cells on day 0 and then treated intravenously with irradiated 4TOO cells (□), 1 × 106 FC/4TOO (▴), 2 × 106 FC/4TOO (■), and 5 × 106FC/4TOO (●) on days 2, 6, and 10. (C) Balb/c mice (10 per group) were injected intravenously with 1 × 105 4TOO cells on day 0 and then treated with 2 × 106 FC/4TOO cells (●) on days 2, 6, 10, 35, and 50. Irradiated 4TOO cells were used as a control (□). The mice were monitored for survival. Similar results were obtained in 3 separate experiments.
Antitumor immunity induced by immunization with FC/4TOO cells.
(A) Balb/c mice (10 per group) were immunized twice subcutaneously with 1 × 106 irradiated 4TOO cells (■), DCs alone (□), FC/4TOO cells (●), or DCs mixed with 4TOO cells (▴). PBS (○) was used as a control. On day 14, the mice were challenged intravenously with 2 × 105 4TOO cells. (B) Balb/c mice (10 per group) were injected intravenously with 2 × 105 4TOO plasmacytoma cells on day 0 and then treated intravenously with irradiated 4TOO cells (□), 1 × 106 FC/4TOO (▴), 2 × 106 FC/4TOO (■), and 5 × 106FC/4TOO (●) on days 2, 6, and 10. (C) Balb/c mice (10 per group) were injected intravenously with 1 × 105 4TOO cells on day 0 and then treated with 2 × 106 FC/4TOO cells (●) on days 2, 6, 10, 35, and 50. Irradiated 4TOO cells were used as a control (□). The mice were monitored for survival. Similar results were obtained in 3 separate experiments.
IL-12 potentiates FC/4TOO-induced T-cell responses
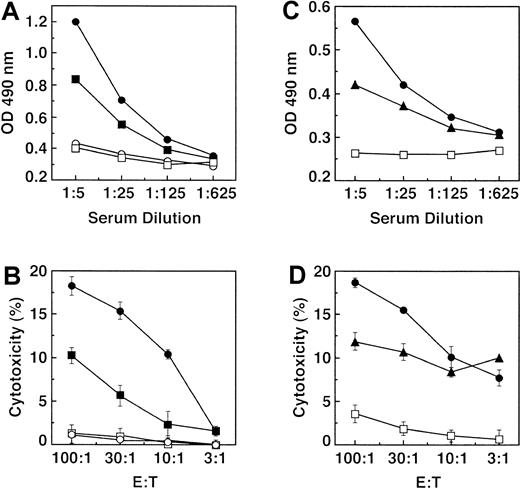
Whereas IL-12 improves the effectiveness of DC-based antitumor vaccines,12,27 28 we added IL-12 to immunizations with FC/4TOO cells (Figure4). IL-12 (500 ng/mouse) was administered intraperitoneally after each vaccination to potentiate FC/4TOO-induced activation of T cells (Figure 4). Cells from draining lymph nodes and splenocytes were assayed for proliferation in response to FC/4TOO cells. The results demonstrate that proliferation of LNCs from mice immunized with FC/4TOO + IL-12 was stimulated to a greater extent than that obtained with FC/4TOO-immunized mice (Figure 4A). As controls, lower levels of proliferation were found with LNCs from mice immunized with DCs or 4TOO cells (Figure 4A). Similar results were obtained with splenocytes from the immunized mice (Figure 4B). To assess the effects of IL-12 on cell-mediated immunity, LNCs and splenocytes were assayed for CTL activity against 4TOO cell targets. The results show that lysis of 4TOO cells by LNCs from FC/4TOO + IL-12–immunized mice was higher than that obtained with LNCs from mice immunized with FC/4TOO cells alone (Figure 4C). LNCs from control mice immunized with DCs or 4TOO cells exhibited a lower level of CTL activity (Figure 4C). Similar findings were obtained when splenocytes were used as a source of CTLs (Figure 4D). These results demonstrate that IL-12 potentiates FC/4TOO-induced T-cell responses.
IL-12 potentiates FC/4TOO-induced T-cell responses.
Mice (3 per group) were immunized twice subcutaneously with 1 × 106 4TOO (○), DCs (◊, FC/4TOO (▵), or FC/4TOO combined with intraperitoneal IL-12 (200 ng/mouse) (▴) as depicted in the schema. LNCs (A) and splenocytes (B) were harvested on day 15. The indicated number of cells was incubated with irradiated FC/4TOO cells at a ratio of 10:1 for 5 days. Uptake of [3H]thymidine was measured at 12 hours after a pulse of 0.037 MBq (1 μCi)/well. The stimulation index is presented as the mean ± SD of 3 replicates. T cells from the LNCs (C) and splenocytes (D) were purified through nylon wool and incubated with 4TOO cells at the indicated ratios. CTL activity was determined by the 51Cr release assay.
IL-12 potentiates FC/4TOO-induced T-cell responses.
Mice (3 per group) were immunized twice subcutaneously with 1 × 106 4TOO (○), DCs (◊, FC/4TOO (▵), or FC/4TOO combined with intraperitoneal IL-12 (200 ng/mouse) (▴) as depicted in the schema. LNCs (A) and splenocytes (B) were harvested on day 15. The indicated number of cells was incubated with irradiated FC/4TOO cells at a ratio of 10:1 for 5 days. Uptake of [3H]thymidine was measured at 12 hours after a pulse of 0.037 MBq (1 μCi)/well. The stimulation index is presented as the mean ± SD of 3 replicates. T cells from the LNCs (C) and splenocytes (D) were purified through nylon wool and incubated with 4TOO cells at the indicated ratios. CTL activity was determined by the 51Cr release assay.
One potential explanation for IL-12–induced potentiation of FC/4TOO vaccination is that production of IL-12 by FC/4TOO is decreased as a consequence of fusing DCs with tumor cells. In this context, IL-10 is produced by certain tumors, and IL-10 suppresses IL-12 expression.32 33 To determine whether IL-10 affects IL-12 production by FC/4TOO cells, IL-10 and IL-12 levels were measured in culture supernatants. The results demonstrate that IL-10 levels are substantially higher than IL-12 levels in supernatants from 4TOO cells (Table 1). By contrast, DCs expressed lower levels of IL-10 and higher levels of IL-12 (Table 1). Compared with 4TOO cells, IL-10 levels in the supernatants of FC/4TOO cells were also relatively low, and IL-12 levels were similar to those obtained with DCs (Table 1). These findings and those obtained in vivo indicate that IL-12 potentiation of FC/4TOO-induced immunity is not a consequence of decreased IL-12 production by the fusion cells.
Production of interleukin 10 and interleukin 12
| Cell . | mIL-10 . | mIL-12 . |
|---|---|---|
| 4TOO | 275.12 ± 7.15 | 101.44 ± 4.15 |
| DC | 16.31 ± 2.33 | 123.52 ± 5.16 |
| FC/4TOO | 23.52 ± 6.29 | 101.63 ± 5.25 |
| Cell . | mIL-10 . | mIL-12 . |
|---|---|---|
| 4TOO | 275.12 ± 7.15 | 101.44 ± 4.15 |
| DC | 16.31 ± 2.33 | 123.52 ± 5.16 |
| FC/4TOO | 23.52 ± 6.29 | 101.63 ± 5.25 |
The results (pg/mL) represent the mean + SE of 3 determinations performed on supernatants obtained after culturing for 8 days. mIL, murine interleukin.
IL-12 potentiates the FC/4TOO vaccine
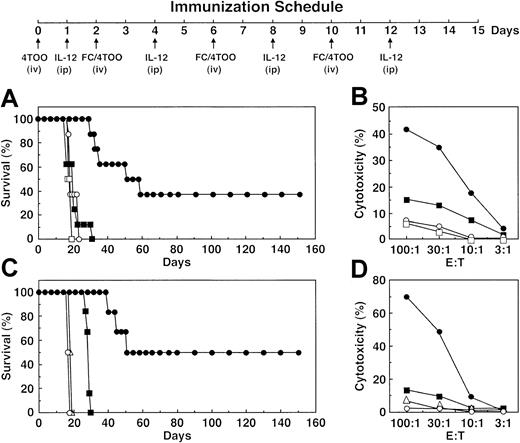
To assess the role of IL-12 in the treatment of established disease, other experiments were performed in which 1 × 106 4TOO cells were administered intravenously on day 0, and the mice were treated with FC/4TOO cells on days 2, 6, and 10. IL-12 (500 ng/mouse) was administered intraperitoneally after each vaccination to potentiate FC/4TOO-induced activation of T cells (Figure5A). The results demonstrate that, at this high tumor innoculum, immunization with FC/4TOO cells had no effect on survival (Figure 5A). By contrast, treatment with FC/4TOO cells and 500 ng IL-12 resulted in 40% of the mice surviving for 300 days when the experiment was terminated (Figure 5A and data not shown). The killed animals showed no evidence of disease. Other cohorts were killed on day 16 after immunization for analysis of CTL activity. Mice given IL-12 alone showed no evidence for induction of anti-4TOO activity (Figure 5B). Significantly, and in concert with the antitumor data, vaccination with FC/4TOO and IL-12 was more effective in inducing CTL activity as compared with that found with FC/4TOO alone (Figure5B). To confirm these findings, treatment studies were performed in which FC/4TOO cells were administered with 200 ng IL-12/mouse. The results demonstrate that, under these experimental conditions, 50% of the mice survived 160 days when the experiment was terminated (Figure5C). None of the surviving mice had evidence of disease. Other cohorts treated in this manner were killed for analysis of CTL activity. The results confirm that IL-12 potentiates the effects of FC/4TOO immunization on the induction of a CTL response against 4TOO cells (Figure 5D).
Treatment of 4TOO plasmacytoma with FC/4TOO vaccination and IL-12.
Immunization schedule for the intravenous injection of 4TOO and FC/4TOO cells combined with intraperitoneal administration of IL-12. (A) Balb/c mice (10 per group) were injected intravenously with 1 × 106 4TOO cells on day 0 and then treated with IL-12 (500 ng/dose) and FC/4TOO cells (●) as outlined in the schedule. The mice were also treated with 1 × 106 irradiated 4TOO cells (□), FC/4TOO (■), or IL-12 (○; 500 ng/dose) alone. (B) Splenocytes from mice treated as described in (A) were harvested on day 16. The T cells were purified by nylon wool column and incubated with 4TOO targets at the indicated ratios. CTL activity was determined by the 51Cr release assay. (C) Balb/c mice (10 per group) were injected with 1 × 106 4TOO cells on day 0 and then treated with IL-12 (200 μg/dose) and FC/4TOO cells (●). Mice treated with 1 × 106 irradiated 4TOO (▵), FC/4TOO cells (■), or IL-12 alone (○; 200 ng/dose) were used as controls. (D) Splenocytes from mice treated as described in (C) were isolated on day 16 and incubated with 4TOO targets at the indicated ratios. CTL activity was determined by the 51Cr release assay. Similar results were obtained in 2 separate experiments.
Treatment of 4TOO plasmacytoma with FC/4TOO vaccination and IL-12.
Immunization schedule for the intravenous injection of 4TOO and FC/4TOO cells combined with intraperitoneal administration of IL-12. (A) Balb/c mice (10 per group) were injected intravenously with 1 × 106 4TOO cells on day 0 and then treated with IL-12 (500 ng/dose) and FC/4TOO cells (●) as outlined in the schedule. The mice were also treated with 1 × 106 irradiated 4TOO cells (□), FC/4TOO (■), or IL-12 (○; 500 ng/dose) alone. (B) Splenocytes from mice treated as described in (A) were harvested on day 16. The T cells were purified by nylon wool column and incubated with 4TOO targets at the indicated ratios. CTL activity was determined by the 51Cr release assay. (C) Balb/c mice (10 per group) were injected with 1 × 106 4TOO cells on day 0 and then treated with IL-12 (200 μg/dose) and FC/4TOO cells (●). Mice treated with 1 × 106 irradiated 4TOO (▵), FC/4TOO cells (■), or IL-12 alone (○; 200 ng/dose) were used as controls. (D) Splenocytes from mice treated as described in (C) were isolated on day 16 and incubated with 4TOO targets at the indicated ratios. CTL activity was determined by the 51Cr release assay. Similar results were obtained in 2 separate experiments.
Discussion
Idiotype (Id) structures on the surface immunoglobulin of malignant clonal B cells represent potential tumor-specific antigens. Studies in animal models of multiple myeloma have identified both antibodies and T cells that exhibit specific recognition of the idiotypic antigen. Immunization with soluble Id + immunoglobulin protects mice against subsequent challenge with Id+, but not Id−, tumor cells.34 Vaccine studies in patients with myeloma have also demonstrated the induction of anti-Id responses.35-39 Thus, although Id epitopes represent myeloma-specific antigens, given the genomic instability associated with tumor progression, there are conceivably other epitopes that are unique to malignant B-cell clones. In the present studies, we have used a plasmacytoma-DC fusion cell vaccine to target unique epitopes and thereby the induction of a tumor-specific immune response. The results demonstrate the generation of heterokaryons that are functional as a vaccine in the stimulation of a humoral immune response against epitopes expressed by the plasmacytoma cells. The results also demonstrate that immunization with the FC/4TOO cells induce antitumor T-cell proliferative and CTL responses. The demonstration that immunization with FC/4TOO induces partial cross-reactivity with MPC-11 myeloma cells supports the recognition of shared antigens that are probably not idiotypic. These findings thus support the induction of antimyeloma immune responses in the absence of a defined tumor antigen.
Tumor-DC fusion cell vaccines have been found to be effective in the treatment of established carcinomas, lymphomas, and melanomas in mice.13-17 These findings have recently been extended to the treatment and long-term survival of patients with metastatic renal cell carcinoma.20 The present results demonstrate that fusions of myeloma cells and DCs are effective in protecting mice against tumor challenge. In addition, treatment of established myeloma with the FC/4TOO cell vaccine was effective in prolonging survival. However, in contrast to the results obtained in other mouse tumor models, mice with established myeloma failed to respond to the vaccine with long-term survival. One potential explanation for these findings is that induction of a cell-mediated antitumor response, although detectable after immunization with the fusion cell vaccine, was insufficient to completely suppress growth of the malignant cells. In this context, fusions of certain types of tumor cells with DCs may result in heterokaryons that express the necessary MHC, costimulatory, and adhesion molecules, but not the requisite levels of cytokines needed for an optimal CTL response.
Previous work has demonstrated that IL-12 functions as a third signal, along with the T-cell receptor and costimulatory molecules, in reversing tolerance and expanding antigen-specific CD8+cells.25,26 Immunization with the FC/4TOO cell vaccine alone was effective, only in part, in extending survival of mice with established myeloma, whereas administration of FC/4TOO cells and IL-12 resulted in eradication of the disease. Antitumor CTL and T-cell proliferative responses induced by the FC/4TOO vaccine were also potentiated by coadministration of IL-12. Fusion cells track to paracortical areas of lymph nodes and form clusters with T cells.33 The IL-12 was administered 2 days after each of 3 FC/4TOO vaccinations to increase IL-12 levels at a time when the fusion cells would potentially be activating T cells in regional lymph nodes. As activation and expansion of antigen-specific T cells is in part dependent on IL-12,25 26 the level of IL-12 secretion by the fusion cells could define the magnitude of the CTL response. Our in vitro data indicate that FC/4TOO cells produce IL-12 at a level comparable to that of DCs. These findings, however, do not exclude the possibility that production of IL-12 by FC/4TOO cells in vivo is insufficient for optimal induction of an antitumor immune response. Indeed, the finding that systemic administration of IL-12 stimulates the effects of the FC/4TOO vaccine on induction of both CTL and antitumor activities provides support for insufficient local production of IL-12 by the fusion cells.
Multiple myeloma is typically incurable, even with myeloablative chemotherapy and stem cell transplantation. The findings of the present study present the first evidence for a vaccine that is effective in the treatment of established myeloma in the mouse. Given the limitations of animal models, immunization with myeloma-DC fusions may represent an effective approach for the treatment of myeloma in patients. In this context, fusions of human myeloma and autologous DCs have been prepared that are functional in the activation of autologous T cells.40 The findings of the present work also support an immunization strategy in clinical trials that includes coadministration of IL-12.
We thank Dr Michael Kuehl for providing the 4TOO cell line.
Supported by grant CA78378 from the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Donald Kufe, Dana-Farber Cancer Institute, Harvard Medical School, 44 Binney Street, Boston, MA 02115; e-mail:donald_kufe@dfci.harvard.edu.

![Fig. 1. Phenotype and function of FC/4TOO fusion cells. / (A) DCs, 4TOO plasmacytoma, and FC/4TOO fusion cells were analyzed by bidimensional flow cytometry for PKH-26 fluorescence and expression of the indicated antigens. (B) DCs, 4TOO cells, and FC/4TOO fusion cells were analyzed by bidimensional flow cytometry for the expression of MHC class II and Syndecan. (C) DCs (●), 4TOO plasmacytoma (○), FC/4TOO fusion cells (▴), and medium alone (□) were cocultured with C57BL/6 T cells at different ratios for 5 days. [3H]-thymidine uptake was measured at 12 hours after a pulse of 0.037 MBq (1 μCi)/well. The stimulation index is expressed as the mean ± SE of 3 experiments each performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/7/10.1182_blood.v99.7.2512/6/m_h80722348001.jpeg?Expires=1765033043&Signature=Enn95VHyssxvN9sorrSrThdfoyQqP3ceprNwxR-4Sa1rIGcm~Fn1EizNQAfVt4CDxtxTbld91UHF1LYkLnu1vSPxm-4kHxDIuLIkHT5Bv4ZIra8K5odTWO~dBIPosoR42b8~VJEYqJC5DLDRgy6NcHEJiAAgWbqOLz-fgDEhnNGpg-9syYsvWNtTs60j0stJ5M07FfXTdaWN~~-cQqiusiPpDsZXkgQBp2OekiE8-X9vTo04KRVRQYteoiQNU1P7gGF~oNoMjBmkpASAA5RrQe~zL2UnGYETi6fJrlyppS~Y7Ix9JEN0hRL5aEky~rl3oy1TgcX~uiCMkndkkyvSHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. IL-12 potentiates FC/4TOO-induced T-cell responses. / Mice (3 per group) were immunized twice subcutaneously with 1 × 106 4TOO (○), DCs (◊, FC/4TOO (▵), or FC/4TOO combined with intraperitoneal IL-12 (200 ng/mouse) (▴) as depicted in the schema. LNCs (A) and splenocytes (B) were harvested on day 15. The indicated number of cells was incubated with irradiated FC/4TOO cells at a ratio of 10:1 for 5 days. Uptake of [3H]thymidine was measured at 12 hours after a pulse of 0.037 MBq (1 μCi)/well. The stimulation index is presented as the mean ± SD of 3 replicates. T cells from the LNCs (C) and splenocytes (D) were purified through nylon wool and incubated with 4TOO cells at the indicated ratios. CTL activity was determined by the 51Cr release assay.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/7/10.1182_blood.v99.7.2512/6/m_h80722348004.jpeg?Expires=1765033043&Signature=D2hiEZIgR4HRt1J3npl5sPnyRBWESIu1VCO8Gd5~ozQpuVvh-q~Me6QtBuE7hfBJaGTGRkDPffWuu-6ockC-dhZzdps19l8-kuLROmbHMh7RbRafadieUNL51s4CD4IA5m-ZbQOQ-xVtMoFE37XZdjRVDGeNwkk63gbgzRb~4X1uFfZSQFjOMnOsQK~jCsSBHlm~XWTpuiNaQFP-hvzWpcG26HNSYcIIsoHsOJB~pXN~AfpmXW~9l-RLLQJ-IaWzxJaKz2u8QOqzDE7DkkGFKhn~k2I1T9kmV95YV6NW7BLoVChA167WPTHmr-pax8uOcACDZMlUlWVti7nHED4vDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal