CD38, a surface glycoprotein of unrestricted lineage, is an ectoenzyme (adenosine diphosphate [ADP] ribosyl cyclase/cyclic ADP-ribose hydrolase) that regulates cytoplasmic calcium. The molecule also performs as a receptor, modulating cell-cell interactions and delivering transmembrane signals, despite showing a structural ineptitude to the scope. CD38 ligation by agonistic monoclonal antibodies induced signals leading to activation of the lytic machinery of natural killer (NK) cells from adults; similar signals could not be reproduced in YT and NKL, 2 CD16− human NK-like lines. It was hypothesized that CD38 establishes a functional cooperation with professional signaling molecules of the NK cell surface. The present work answers the question about the molecule exploited by CD38 for signaling in NK cells, using as a model CD16− NK lines genetically corrected for CD16 expression. Our results indicate that a functional CD16 molecule is a necessary and sufficient requisite for CD38 to control an activation pathway, which includes calcium fluxes, tyrosine phosphorylation of ZAP70 and mitogen-activated protein kinase, secretion of interferon-γ, and cytotoxic responses. Fluorescence resonance energy transfer and cocapping experiments also showed a surface proximity between CD38 and CD16. These results were confirmed by using the NKL cell line, in which CD16+ and CD16− variants were obtained without genetic manipulation. Together, our findings show CD38 to be a unique receptor molecule that cannot signal by itself but whose receptor function is rescued by functional and physical associations with a professional signaling structure that varies according to lineage and environment. This molecule is CD16 in NK cells.
Introduction
Human CD38 is the prototype of a family of proteins that share structural similarities and ectoenzymatic activities involved in the production of calcium-mobilizing compounds.1-3 Aside from its ectoenzymatic activities and, apparently with independent modalities, CD38 may perform as a receptor, ruling adhesion and signaling in T4 and B lymphocytes,5 monocytes,6 and natural killer (NK) cells.7,8 The receptor functions of CD38 are regulated through interaction with a counterreceptor, identified as CD31.9 The signaling events initiated by interactions between CD38 and CD31 (and fully mimicked by agonistic anti-CD38 monoclonal antibodies [mAbs]) were initially studied in the dynamic context of circulating CD38+ T lymphocytes adhering to CD31+ endothelial cells.10 Use of this model allowed definition of some of the events that take place after the interaction and that include calcium (Ca++) mobilization from cytosolic stores, tyrosine phosphorylation of selected substrates, activation of nuclear factors, and secretion of cytokines.11
It is generally agreed that CD38 controls a specific signaling pathway in T cells, B cells, NK cells, and monocytes. In spite of this evidence, the modalities through which the signal is initiated remain elusive. The molecule has neither the canonical structure of a receptor nor the hallmark domains. Indeed, the cytoplasmic tail is short and lacks docking sites and it is not tyrosine phosphorylated on activation.12,13 Such negative characteristics are even more evident in CD157, the other member of the protein family, whose signaling features are known, notwithstanding a glycophosphatidylinositol linkage to the cell membrane.14 15
Some clues can be extrapolated from cocapping experiments, which show that CD38 associates on the cell membrane with professional signaling receptors such as the T-cell receptor (TCR)-CD3 complex in T cells, the B-cell receptor (BCR) in B cells, and CD16 in NK cells.16 A hypothesis to explain the signaling properties of CD38 is that the molecule exploits the signaling machinery of professional receptors to deliver its own independent signals. This idea was first supported by experiments using CD38+ T-cell lines deficient in components of the signaling apparatus of the TCR-CD3 complex.17,18 The inability of CD38 to signal in these cells was overcome by reconstituting a complete TCR-CD3 complex, thereby indicating that CD38 signaling depends on the presence of a functional TCR. These observations were recently expanded by studies using T lymphocytes purified from the intestinal lamina propria as a model in which the TCR complex is physiologically impaired.19 A comparative analysis of circulating versus residential T lymphocytes from the same individuals indicated that CD38 signaling is sensitive to the operational environment and seems to proceed through distinct pathways, even within the same cell lineage. The features of CD38 signaling in lamina propria T cells impaired in TCR-mediated signalings were clearly different from those of circulating T cells and derived line models with a functional TCR (eg, lack of Ca++ mobilization and phosphorylation of the phospholipase C γ protein in the former population). The inference is that in conditions in where the TCR is not fully operative, CD38 simply switches to other receptors. Similar observations were made in studies using murine B cells, although murine and human CD38 may not be fully comparable.20
The question behind this paper is whether the molecular parasitism exerted by CD38 in T cells may also occur in other cell lineages and serves to defines a novel type of coreceptor molecule. Here, we report results obtained in the NK lineage by using NK-like lines either expressing or deficient in membrane CD16. The results show conclusively that the presence of CD16 is a necessary requisite for CD38 to control a complex signaling pathway that includes cytoplasmic and nuclear events, release of cytokines, and activation of cytolytic functions.
Materials and methods
Cells
The NK-like cell line YT was established from samples obtained from a patient with acute lymphoblastic lymphoma (ALL) and thymoma.21 The NKL cell line, from a patient with large granular lymphocyte leukemia,22 was obtained from R. Galandrini (Rome, Italy). The cell lines used in the cytotoxicity experiments were BV-173 and NALM-6 (pre-B), Raji and Daudi (Burkitt lymphoma), Karpas 707, RPMI 8226, ARH 77 and LP-1 (myelomalike), Kasumi-1, HL-60, NB-4, U937, Mono-Mac-6 (acute myelogenous leukemia), Jurkat, HPB-ALL, and SUPT-1 (T-cell ALL). P815 (murine mastocytoma)23 and K562 (human erythroleukemia),24 which are both positive for FcγR, were used in the redirected lysis experiments. CD31 transfectants were obtained by transfecting specific complementary DNAs (cDNAs) into murine L fibroblasts.10
Cells were cultured in RPMI-1640 medium (Sigma, Milan, Italy) with 10% heat- inactivated fetal-calf serum (FCS; Seromed, Berlin, Germany), 50 μg/mL gentamicin, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Sigma). The NKL cells required addition of human recombinant interleukin (IL) 2 (200 IU/mL; Chiron, Emeryville, CA).
Antibodies
The anti-CD38 mAb IB4 (IgG2a) was selected because of its agonistic properties and used as such or as F(ab′)2. Other anti-CD38 reagents were OKT10 (IgG1), SUN-4B7 (IgG1), and IB6 (IgG1). CB16 (anti-CD16, IgG1), CB71 (anti-CD71, IgG1), O1.65 (anti-HLA class I, IgG1), and JAS (anti-gp120, IgG2airrelevant isotype-matched control) were locally produced and purified.
Fluorescein isothiocyanate (FITC)–conjugated anti-CD25 and phycoerythrin (PE)–conjugated anti-CD69 mAbs were from BD Bioscience (Milan, Italy). FITC-conjugated goat antimouse immunoglobulin (GaMIg; Caltag, Burlingame, CA) was used in indirect immunofluorescence studies. Tetrarhodamine isothiocyanate (TRITC)–conjugated GaMIg and FITC-streptavidin were both from Dako (Glostrup, Denmark). IB4 and CB16 mAbs were conjugated to biotin (Bio-Spa, Milan, Italy). An affinity-purified F(ab′)2 preparation of rabbit immunoglobulin (Ig) to mouse IgG (RaMIg; locally produced) and an F(ab′)2 donkey antimouse IgG (DaMIgG; Jackson ImmunoResearch Laboratories, West Grove, PA) were used as cross-linkers.
The recombinant antiphosphotyrosine (anti-pTyr) antibody coupled to RC20-horseradish peroxidase (HRP) was from BD Biosciences. E10 mAb, an anti–phospho-p44/p42 mitogen-activated protein kinase (MAPK; T202/Y204), was from New England Biolabs (Beverly, MA). The affinity-purified rabbit polyclonal anti–extracellular-regulated kinase (anti-ERK) 2 and anti-ZAP70 (LR) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-ZAP70 (Zap-4) serum was from S.C. Ley (London, United Kingdom). HRP-conjugated, affinity-purified goat antirabbit IgG (GaRIgG [Fc]) and goat antimouse IgG (GaMIgG) were from Promega (Madison, WI). GaMIgG-coated magnetic beads (Dynal, Oslo, Norway) were used to select CD16+ cells in cultures of YT and NKL lines.
Constructs
CD16 cDNA in a pMX plasmid (L.L. Lanier, San Francisco, CA)25 was amplified by polymerase chain reaction (PCR), with the primers designed according to the published CD16 sequence (GeneBank accession no. M24854). PCR amplification was done by using an automated DNA thermal cycler (Perkin Elmer, Boston, MA) for 30 cycles after an initial denaturation for 5 minutes at 94°C. The reaction product was visualized by electrophoresis on a 1.5% agarose gel containing Tris-borate-EDTA buffer and ethidium bromide (0.5 μg/mL).
An aliquot (1 μL) of the PCR product was ligated to a pcDNA3.1 expression vector by using the TA cloning system, and transformation was carried out on Escherichia coli TOP10 cells (all from Invitrogen, Carlsbad, CA). Positive transformants were analyzed for the presence and correct orientation of CD16 cDNA both by PCR (using a combination of the T7 forward primer and a specific reverse primer that bound to the inner sequence of CD16 cDNA) and by digestion with theKpnI (10 U/μg) restriction enzymes (New England Biolabs). The selected transformant, CD16-pcDNA3.1, was analyzed by sequencing, grown in LB medium, and purified by using a Quantum Prep plasmid kit (Bio-Rad, Hercules, CA).
Transfection
CD16-pcDNA3.1 (20 μg) was linearized by treatment withScaI restriction enzyme (20 IU/μg, New England Biolabs), purified with ethanol, checked for purity and concentration, and used to transfect YT cells by electroporation (250 V/0.4 cm and 960 μF). After a 2-week incubation in medium containing 1 mg/mL G418 (Sigma), neomycin-resistant colonies were isolated, recloned by using serial dilution, and referred to as YT CD16+. YT cells were similarly transfected with the empty pcDNA3.1 vector, selected by using G418, and referred to as YT mock cells.
Ca++ fluxes
Intracellular Ca++ concentrations were measured by flow cytometry after loading the cells with Fluo 3-acetoxymethyl (Fluo 3-AM; Sigma), a Ca++-sensitive fluorescent dye. YT CD16+ or YT mock cells and CD16+ and CD16− NKL cells were washed twice in RPMI-1640 medium with 5% FCS and incubated (106 cells/mL for 1 hour at 37°C) with 5 μM Fluo 3-AM in the presence of 0.01% pluronic F127 (Sigma). Cells were then washed, incubated for 10 minutes at room temperature with the selected mAb (10 μg/mL), and washed again. Cross-linking RaMIg (20 μg/mL) was added 10 seconds after starting a continuous fluorescence-activated cell-sorter scanning (FACSort; BD Biosciences) analysis at 37°C. Changes in Ca++concentrations were monitored by plotting the shift in the Fluo 3-AM fluorescence during 540 seconds and presented as changes in Fluo 3-AM intensity over time.19 An irrelevant isotype-matched mAb was included as the control, and efficient loading of the cells was verified by adding the A23187 ionophore (Sigma).
Tyrosine phosphorylation
YT CD16+ and YT mock cells were starved for 12 hours in RPMI-1640 medium with 0.5% FCS at 37°C in a 5% carbon dioxide (CO2) incubator, collected, and incubated for 10 minutes on ice with an F(ab′)2 preparation of the IB4 mAb (10 μg/106 cells), anti-CD16 mAb (5 μg/106cells). An F(ab′)2 preparation of the isotype-matched irrelevant JAS mAb (10 μg/106 cells) was used for the control condition. The unbound mAb was eliminated by washing with cold medium, and the cells were then incubated for 10 minutes on ice with an F(ab′)2 fragment of DaMIgG at a concentration of 4 μg/106 cells. The cells were subsequently allowed to react with the relevant mAb at 37°C for 1 minute, and lysis was obtained by using 1% NP-40 lysis buffer (20 mM HEPES [pH 7.6], 150 mM sodium chloride [NaCl], 50 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM ethyleneglycotetraacetic acid, 50 μM phenylarsine oxide, 10 mM iodoacetamide, 1 mM phenylmethyl sulfonyl fluoride, and 2 μg/mL each of antipain, chymostatin, leupeptin, and pepstatin) for 20 minutes on ice.26 After removal of nuclei by centrifugation, an aliquot of the lysates was diluted in Laemmli sample buffer, boiled for 5 minutes, and stored at −80°C before being subjected to sodium dodecyl sulfate–polyacrylamide electrophoresis (SDS-PAGE). Immunoprecipitation and Western blotting were done as described previously.26 27
The gel was then transferred to a polyvinylidene difluoride (PVDF) membrane with a semidry transfer apparatus (Hoefer, Pharmacia Biotech, San Francisco, CA) in Tris-glycine buffer containing 20% methanol and 0.035% SDS at 0.8 mA/cm.2 To ensure proper recovery of all migrated proteins, transfer efficiency was confirmed by Ponceau red staining. The membrane was blocked in 1% bovine serum albumin (BSA); washed in 10 mM Tris (pH 7.4), 100 mM NaCl, and 0.1% Tween 20; and allowed to react with HRP-conjugated RC20 anti-pTyr mAb for 2 hours. For reblotting, the filter was subsequently stripped by washing in a buffer containing 150 mM NaCl and 10 mM Tris–hydrochloric acid (pH 2.2), blocked again in 2.5% nonfat dry milk, and incubated for 1 hour with the primary antibody of interest diluted in 1% milk. After washing, HRP-conjugated GaRIgG was added and the membrane was washed and developed again by using electrogenerated chemiluminescence reagents (Amersham, Little Chalfont, United Kingdom).
Cytokine release
YT CD16+ and YT mock cells were plated (106 cells/well) in 24-well plates (BD Biosciences) coated with RaMIg (20 μg/mL) in RPMI-1640 medium with 5% FCS in the presence of anti-CD38, anti-CD16 (both at a concentration of 10 μg/mL), or both.4 To determine whether CD31-CD38 cognate interactions were inducing interferon γ (IFN-γ) release, YT CD16+ and YT mock cells were cocultured for 24 hours at 37°C in a 5% CO2 incubator with CD31+transfectants, with the mock-transfected cells used as controls. The amounts of IFN-γ produced were determined by using an immunoenzymatic kit (R&D Systems, Minneapolis, MN).
Cell-mediated cytotoxicity
Tumor cell lines were labeled with chromium 51 (51Cr; NEN, Cologno Monzese, Italy; 100 μCi (3700 Bq)/106 cells) for 1 hour at 37°C, washed, and used as targets. The cytotoxic activity of the YT and NKL cell lines was measured in standard 4-hour 51Cr release assays.28 For redirected cytotoxicity studies, P815 and K562 targets were labeled with 51Cr, washed, and incubated with a rabbit antimouse IgG (RaMIgG; 5 μg/mL) for 20 minutes at 4°C. The unbound mAb was removed by washing, and the cells (5 × 104 in 100 μl) were added to each well. The effector cells were incubated for 20 minutes at room temperature with the selected mAbs (5 μg/mL), washed, and serially diluted (final volume, 100 μL). After 4 hours of incubation at 37°C, 100 μL was collected from each sample and the radioactivity measured with a γ-counter.8
The percentage of specific lysis was calculated as follows: [(experimental counts per minute − spontaneous counts per minute)/(maximal counts per minute − spontaneous counts per minute)] × 100. The spontaneous counts per minute represented the radioactivity released by the target cells alone, whereas the values for maximal counts per minute were obtained by adding 1% Triton X-100 to the cells. Spontaneous release from both treated and untreated tumor cells was ≤ 10% of the maximum release for all valid assays.
Fluorescence resonance energy transfer studies
The OKT10 mAb was conjugated to Cy3 dye with a FluoroLink-Ab Cy3 labeling kit (Amersham), and conjugations were verified by spectrophotometric and spectrofluorometric measurements. Cells were washed with ice-cold phosphate-buffered saline (PBS) with 5% FCS and 0.1% sodium azide, incubated on ice for 1 hour with the FITC-conjugated mAb (the donor fluorophore) and the Cy3-conjugated OKT10 (the accepting fluorophore), washed, and analyzed immediately with a FACSort instrument to determine energy transfer between FITC and Cy3-labeled proteins on the cell surface. Fluorescence resonance energy transfer (FRET) to Cy3 was detected by using standard methods.29 FITC was excited at 488 nm and Cy3 emissions were collected at greater than 600 nm. The median linear channel of fluorescence was used as an indicator of the presence (a positive shift over background level) or absence (no shift or negative shift) of energy transfer. The Wilcoxon matched pair, signed rank test was used to determine the significance of results.
Cocapping experiments
YT CD16+ cells (0.5 × 106) were incubated with the selected biotinylated primary mAb for 30 minutes on ice, washed, and allowed to react with FITC-conjugated streptavidin for 20 minutes on ice. Samples were then moved to 37°C for 40 minutes to induce capping, and ice-cold PBS with 0.5% BSA and 0.1% azide were added.30 Counterstaining was done with unlabeled mAbs and TRITC-conjugated GaMIg. After washing, cells were fixed, placed on poly-L-lysine–coated (Sigma) coverslips, and analyzed with a C-VIEW-12-BUND camera fitted to an Olympus 1 × 70 microscope (Milan, Italy). The images were collected by using ANALYSIS software.
Results
Establishment of a CD16+ YT line and selection of CD16+ and CD16− sublines of NKL cells
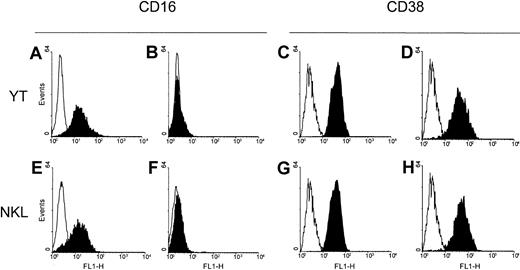
CD38 functions as a signaling receptor in NK cells, but NK-like lines lacking CD16 are apparently refractory to CD38 signaling.8 To obtain evidence functionally linking CD38 to CD16 in the NK lineage, the human CD16 gene was transfected into CD38+ CD16− YT cells, which represent an accepted model of continuous in vitro NK lineage. Transfection by electroporation of a pcDNA3.1 expression vector enclosing the full-length human CD16 gene in YT cells resulted in selection of clones expressing CD16 (Figure 1A). Transfection with the empty plasmid had no effect (Figure 1B). The CD16+ cells were further subcloned by limiting dilution, and selection was maintained by use of immunomagnetic beads and CD16 mAb separation. The CD38+ NKL line, which originally expressed CD16 at a low density in a 40% subset of cells, was split into CD16+ and CD16− subsets by extensive cloning using limiting dilution (Figure 1E,F). The CD16+NKL subline was maintained under positive selection by using the same methods employed for the YT CD16+ transfectants. The homogeneity and stability of the CD16− NKL subline was ensured by cycles of cloning. These procedures did not affect CD38 expression, which remained at comparable levels in the YT CD16+ cells and the YT mock cells (Figure 1C,D) and in the CD16+ and CD16− NKL cells (Figure 1G,H).
Construction of CD16+ clones of the YT line by transfection and selection of CD16+ and CD16− variants of the NKL line.
(A) Stable transfection by electroporation of a plasmid containing the full-length human CD16 gene resulted in production of YT clones homogeneously expressing CD16. (B) Control YT cells transfected with the empty plasmid (YT mock cells) did not have detectable membrane CD16. YT CD16+ cells (C) and YT mock cells (D) expressed comparable levels of CD38. NKL cells were cloned by limiting dilution and selected as CD16+ (E) and CD16− (F) sublines. Both sublines were stable over time, and they expressed comparable molecular densities of CD38 (G,H). Empty histograms show the staining obtained with an irrelevant isotype-matched control.
Construction of CD16+ clones of the YT line by transfection and selection of CD16+ and CD16− variants of the NKL line.
(A) Stable transfection by electroporation of a plasmid containing the full-length human CD16 gene resulted in production of YT clones homogeneously expressing CD16. (B) Control YT cells transfected with the empty plasmid (YT mock cells) did not have detectable membrane CD16. YT CD16+ cells (C) and YT mock cells (D) expressed comparable levels of CD38. NKL cells were cloned by limiting dilution and selected as CD16+ (E) and CD16− (F) sublines. Both sublines were stable over time, and they expressed comparable molecular densities of CD38 (G,H). Empty histograms show the staining obtained with an irrelevant isotype-matched control.
Ca++ mobilization
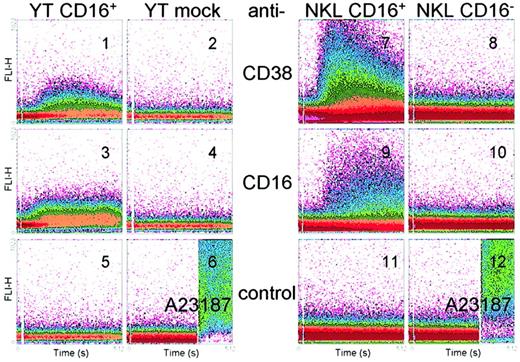
Although expressed by wild-type YT cells, CD38 cannot by itself mobilize Ca++. Experiments were devised to determine whether the presence of surface CD16 confers on (or restores to) CD38 the ability to signal. The first observation was that the transfected CD16 molecule was an efficient receptor and that engagement with an agonistic mAb was followed by Ca++ mobilization (Figure2, panel 3). The second observation was that CD38 ligation in YT CD16+ cells induced cytoplasmic Ca++ currents, thereby indicating that the presence of CD16 was a necessary and sufficient requisite responsible for the newly acquired feature of CD38 (Figure 2, panel 1). The profile was characterized by a rapid rise in intracytoplasmic Ca++levels, stably maintained for more than 200 seconds and then declining slowly during ∼ 100 to 150 seconds. The wave was similar in amplitude and kinetics to that obtained with CD16, although the latter was slightly more sustained (Figure 2, panel 3). None of the mAbs induced Ca++ mobilization without cross-linking by RaMIg, suggesting that engagement of more than 2 receptor molecules is required. Use of an F(ab′)2 preparation of the IB4 mAb yielded the same Ca++ profiles, thereby ruling out the presence of Fc receptor (FcR)–mediated or background-mediated effects (data not shown). Furthermore, addition of the nonagonistic anti-CD38 mAbs OKT10, SUN-4B7, and IB6 (data not shown) as well as of an isotype-matched irrelevant IgG2a mAb (Figure 2, panel 5), or RaMIg alone (Figure 2, panel 6, left), did not yield detectable effects. The absence of Ca++ fluxes in the YT mock cells could not be referred to impaired cell labeling, since the ionophore A23187 induced the expected maximal Ca++ mobilization (Figure 2, panel 6, right).
The mAb ligation of CD38 and CD16 induces Ca++ currents in CD16+ YT and NKL cells.
YT and NKL cells were loaded with the fluorescent indicator Fluo 3-AM and preincubated for 10 minutes at room temperature with agonistic anti-CD38 (panels 1, 2, 7, and 8), anti-CD16 (panels 3, 4, 9, and 10), and anti-gp120 (panels 5 and 11). The cells were then washed and analyzed continuously at 37°C by using a FACSort instrument. RaMIg was added 10 seconds after the analysis began and was by itself unable to mobilize Ca++ (panels 6 and 12, left). Appropriate dye loading by the cells was verified by adding the ionophore A23187 (panels 6 and 12, right). Data are presented as a density plot of the shift in the Fluo 3-AM fluorescence (y-axis) during 512 seconds (x-axis) and are from 6 experiments.
The mAb ligation of CD38 and CD16 induces Ca++ currents in CD16+ YT and NKL cells.
YT and NKL cells were loaded with the fluorescent indicator Fluo 3-AM and preincubated for 10 minutes at room temperature with agonistic anti-CD38 (panels 1, 2, 7, and 8), anti-CD16 (panels 3, 4, 9, and 10), and anti-gp120 (panels 5 and 11). The cells were then washed and analyzed continuously at 37°C by using a FACSort instrument. RaMIg was added 10 seconds after the analysis began and was by itself unable to mobilize Ca++ (panels 6 and 12, left). Appropriate dye loading by the cells was verified by adding the ionophore A23187 (panels 6 and 12, right). Data are presented as a density plot of the shift in the Fluo 3-AM fluorescence (y-axis) during 512 seconds (x-axis) and are from 6 experiments.
Similar experiments were done with NKL sublines to confirm that the observed effects were not restricted to a genetically modified line. When ligated with an anti-CD38 mAb and then cross-linked by RaMIg, NKL CD16+ cells gave rise to prominent Ca++ fluxes (Figure 2, panel 7). The resulting wave was different from that observed with the YT CD16+ cells: the spike was steeper and higher, with a declining phase beginning after ∼ 50 seconds, similar to the profile observed in normal human NK cells or T lymphocytes freshly purified from blood.8 19 CD16 behavior in these cells paralleled that observed in the YT model (Figure 2, panel 9), with Ca++ mobilization starting ∼ 50 seconds after addition of RaMIg and peaking after ∼ 200 seconds, with stable levels maintained until the end of the recording time. The isotype-matched mAb was ineffective, as was RaMIg alone (Figure 2, panels 11 and 12, left). No Ca++ mobilization was observed after exposure of the NKL CD16− cells to anti-CD38 or control anti-CD16 mAbs (Figure 2, panels 8 and 10). Appropriate dye loading by the cells was confirmed by adding the ionophore A23187 (Figure 2, panels 6 and 12, right).
Tyrosine phosphorylation of cytoplasmic substrates
After it was determined that the YT CD16+ cells became responsive to CD38 signaling, the main steps in the pathway were identified by conducting the following experiments. The cytoplasmic substrates acquiring tyrosine phosphorylation on signaling by CD38 and CD16 were analyzed in YT CD16+ cells and control YT mock cells. Both cell populations were treated for 1 minute at 37°C with the F(ab′)2 fragment of the anti-CD38 mAb IB4 or the anti-CD16 mAb CB16 and further cross-linked by addition of the F(ab′)2 preparation of a donkey antimouse Ig (DaMIg). The F(ab′)2 fragment of an irrelevant isotype-matched mAb was used as the control.
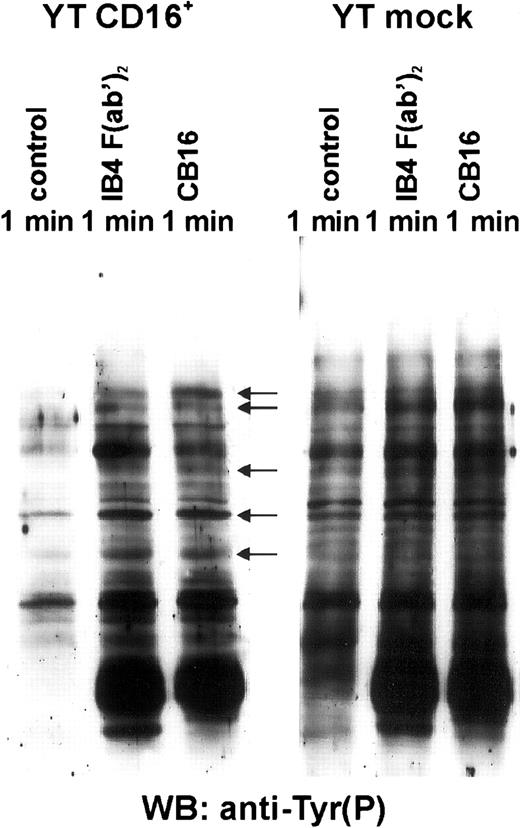
Anti-pTyr Western blot assays done on cell lysates clearly showed a differential phosphorylation of discrete cytoplasmic substrates in YT CD16+ cells incubated with F(ab′)2 IB4 mAb or CB16 (Figure 3, lanes 2 and 3). The most relevant bands with increased intensity compared with that of control-stimulated cells (Figure 3, lane 1) were at about 72, 70, 52, 44, 42, 38, and 36 kd. In contrast, neither CD38 nor CD16 engagement by specific mAbs induced any significant increase in protein tyrosine phosphorylation in YT mock cells (Figure 3, lanes 5 and 6). These results indicate that CD16 expression is required for efficient CD38-mediated protein tyrosine kinase activation.
Surface expression of CD16 is required for CD38-mediated increases in protein tyrosine phosphorylation.
YT CD16+ and YT mock cell lines were resuspended in serum-free RPMI-1640 medium and incubated with the appropriate mAb. Cells were then lysed, subjected to SDS-PAGE (10% gel under reducing conditions), transferred to PVDF membranes, and immunoblotted with RC20-HRP, an antiphosphotyrosine mAb. The same number of cell equivalents was loaded in each well. Molecular mass markers are indicated in kilodaltons. Data are from 3 independent experiments.
Surface expression of CD16 is required for CD38-mediated increases in protein tyrosine phosphorylation.
YT CD16+ and YT mock cell lines were resuspended in serum-free RPMI-1640 medium and incubated with the appropriate mAb. Cells were then lysed, subjected to SDS-PAGE (10% gel under reducing conditions), transferred to PVDF membranes, and immunoblotted with RC20-HRP, an antiphosphotyrosine mAb. The same number of cell equivalents was loaded in each well. Molecular mass markers are indicated in kilodaltons. Data are from 3 independent experiments.
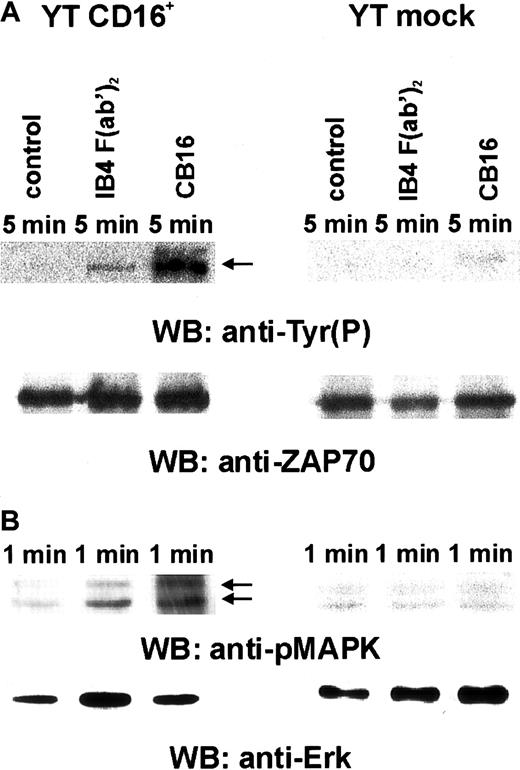
ZAP70 was selected as a target because it is tyrosine phosphorylated on CD38 activation in normal human NK cells. CD38 ligation in YT CD16+ cells resulted in a significant increase in ZAP70 (Figure 4A), as determined by immunoprecipitation and immunoblotting with anti-pTyr. No effects were observed in YT mock cells (Figure 4, upper panel, lane 2 versus lane 5). CD16 triggering induced a prominent tyrosine phosphorylation in YT CD16+ cells but not in YT mock cells, as expected (Figure4, upper panel, lane 3 versus lane 6). All samples had comparable amounts of protein (Figure 4A, lower panel).
CD38-mediated ZAP70 and MAPK activation depends on CD16 expression.
YT CD16+ and YT mock cells were prepared and incubated as described in the legend for Figure 3. ZAP70 activation was determined by immunoprecipitating the lysates with an affinity-purified rabbit polyclonal anti-ZAP70 antibody and by immunoblotting with the anti-pTyr RC20-HRP mAb (A, upper panel) and then by reprobing with rabbit anti-ZAP70 (Zap-4) serum (A, lower panel). ERK activation was determined by immunoblotting with an anti–diphospho-ERK mAb (B, upper panel). The filter was then stripped and reprobed with an antitotal ERK-2 antibody (B, lower panel). Data are from 3 independent experiments.
CD38-mediated ZAP70 and MAPK activation depends on CD16 expression.
YT CD16+ and YT mock cells were prepared and incubated as described in the legend for Figure 3. ZAP70 activation was determined by immunoprecipitating the lysates with an affinity-purified rabbit polyclonal anti-ZAP70 antibody and by immunoblotting with the anti-pTyr RC20-HRP mAb (A, upper panel) and then by reprobing with rabbit anti-ZAP70 (Zap-4) serum (A, lower panel). ERK activation was determined by immunoblotting with an anti–diphospho-ERK mAb (B, upper panel). The filter was then stripped and reprobed with an antitotal ERK-2 antibody (B, lower panel). Data are from 3 independent experiments.
CD38-mediated ERK activation in T cells depends on surface expression of a functional TCR-CD3 complex.27 The requirements for CD38-mediated ERK activation were tested by immunoblotting total cell lysates from both YT CD16+ and YT mock cells with anti–diphospho-ERK antibodies before and after CD38 or CD16 ligation (Figure 4B). The blots were stripped and reprobed with total ERK–specific antibodies to ensure that the individual lanes were loaded with an equivalent amount of proteins. CD38 or CD16 triggering in YT CD16+ cells caused a significant activation of ERK-1 and ERK-2 (Figure 4B, upper panel, lanes 2 [IB4 F(ab′)2] and 3 [CB16]) compared with results in control-treated cells (Figure4B, lane 1). In contrast, anti-CD38 or anti-CD16 ligation failed to induce ERK activation in CD16− YT mock cells (Figure 4B, lanes 5 and 6). Together, these findings indicate that CD16 is required for activation of the CD38-mediated signaling pathway.
CD38 signaling in YT CD16+ cells triggers secretion of IFN-γ
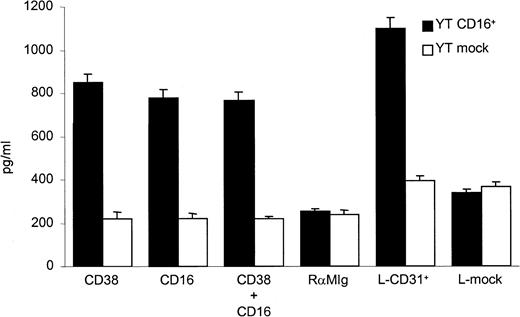
Analysis of cytokine release was used to provide additional confirmation of the ability of CD38 to transduce biologically relevant signals in YT CD16+ cells. The secretion of cytokines in culture medium witnesses successful delivery of a signal to the nucleus and implementation of genetic programs controlling the proteins under evaluation. Moreover, IFN-γ secretion is one of the events controlled by MAPK activation in NK cells and a sensitive indicator of activation of YT cells.31 We found that CD16+ YT cells cultured for 20 hours in the presence of the IB4 mAb—used as either a full molecule or an F(ab′)2 fragment—and cross-linked with RaMIg secreted IFN-γ in the culture medium in amounts consistently greater than those secreted by controls. Treatment with anti-CD16 mAb and cross-linking with RaMIg also promoted IFN-γ secretion. No synergy was observed when the cells were treated with both mAbs under the experimental conditions used (Figure5).
YT CD16+ cells secrete IFN-γ in response to CD38 signaling.
YT CD16+ cells were incubated with anti-CD38, anti-CD16, or both; washed; and cultured for 20 hours in 24-well plates coated with RaMIg. YT mock cells did not secrete IFN-γ under the experimental conditions used. The same activatory signals were recorded with cocultivation of YT CD16+ cells and CD31+transfectants. Data are mean ± SD (vertical bars) results from 4 independent experiments
YT CD16+ cells secrete IFN-γ in response to CD38 signaling.
YT CD16+ cells were incubated with anti-CD38, anti-CD16, or both; washed; and cultured for 20 hours in 24-well plates coated with RaMIg. YT mock cells did not secrete IFN-γ under the experimental conditions used. The same activatory signals were recorded with cocultivation of YT CD16+ cells and CD31+transfectants. Data are mean ± SD (vertical bars) results from 4 independent experiments
To strengthen the biologic relevance of the signal highlighted by using agonistic mAbs, cytokine assays were reproduced with CD31 used as a physiologic trigger for CD38. CD31+ transfectants were cocultivated with YT CD16+ cells for 24 hours and mock-transfected cells served as controls. The amounts of IFN-γ in the culture medium increased, and the quantities were greater than those induced by agonistic mAbs (Figure 5).
CD38 becomes a receptor modulating cytotoxicity in YT CD16+ cells
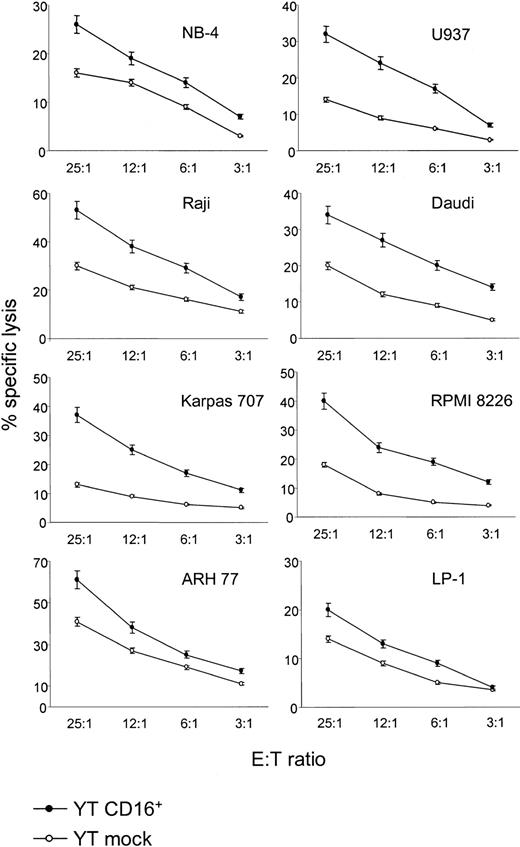
Although lacking CD16, YT cells were reported to kill target tumor cells in conventional cytotoxicity assays, a key element of their representability of the NK lineage. The CD11a, CD28, and 2B4 molecule are some of the active killer receptors identified so far.32-34 Transfection of CD16 into these cells produces profound changes in the lytic potential of YT cells. Indeed, the presence of CD16 resulted in an increased ability to kill selected B (Raji, Daudi, RPMI 8226, Karpas 707, ARH 77, and LP-1) and myeloid targets (NB-4 and U937), but it had no effect in other cell lines of the same lineages (Namalwa, NALM-6, WT18, Kasumi-1, HL-60, Mono-Mac-6, and K562) or any of the T-cell targets assayed (Figure6). These results could be interpreted in the light of recent findings pointing to the existence of a cell-bound ligand for CD16.35 On the other hand, they could indicate a direct contribution of the CD38-CD31 system, since U937 and NB-4 (CD31+ myeloid cells) show increased death on incubation with YT CD16+ cells.
CD16 transfection into YT cells confers an increase in lytic power against selected tumor targets.
YT/CD16+ cells showed increased cytotoxicity toward B-cell lines (Raji, Daudi, RPMI 8226, Karpas 707, ARH 77, and LP-1) and myeloid cell lines (NB-4 and U937). Data are mean ± SD (vertical bars) results from at least 3 independent experiments.
CD16 transfection into YT cells confers an increase in lytic power against selected tumor targets.
YT/CD16+ cells showed increased cytotoxicity toward B-cell lines (Raji, Daudi, RPMI 8226, Karpas 707, ARH 77, and LP-1) and myeloid cell lines (NB-4 and U937). Data are mean ± SD (vertical bars) results from at least 3 independent experiments.
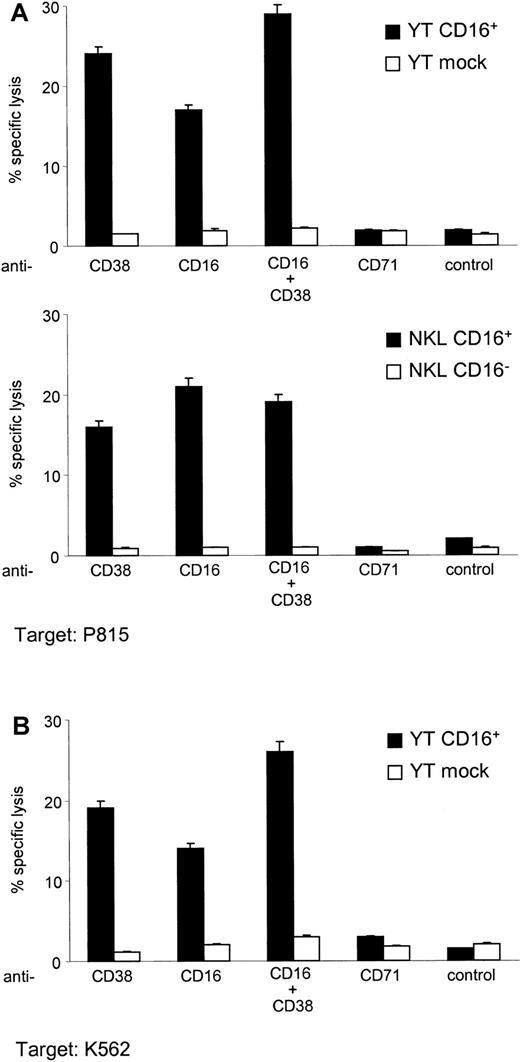
Formal proof of the involvement of CD38 in activation of lytic programs was provided by the results of redirected cytotoxicity assays. The conclusions of these experiments, performed using the murine P815 and the human K562 FcRγ+ target cells, indicate that CD38 ligation in YT CD16+ cells is followed by a significant release of lytic granules, whereas ligation is completely uneventful in mock-transfected or wild-type cells. These observations were confirmed by using NKL cells (Figure 7).
CD38 is a cytotoxic trigger in YT CD16+ and NKL CD16+ cells.
Target cells used in the redirected cytotoxicity experiments were the murine mastocytoma P815 (A) and the human erythroleukemia K562 (B) lines, selected because both express FcRγ. The cells were labeled with 51Cr, washed, and incubated with RaMIg (5 μg/mL) for 30 minutes at 4°C. Anti-CD16 and anti-CD71 mAbs and the medium alone were used as relevant and irrelevant controls, respectively. Solid bars show the percentage of cytotoxicity on triggering of CD16+YT cells and the CD16+ variant of the NKL cells. YT mock cells and the NKL CD16− variants (open bars) were also included. The untransfected wild-type YT cell line showed the same behavior as the YT mock cells. Vertical bars represent the mean ± SD results from 6 independent experiments.
CD38 is a cytotoxic trigger in YT CD16+ and NKL CD16+ cells.
Target cells used in the redirected cytotoxicity experiments were the murine mastocytoma P815 (A) and the human erythroleukemia K562 (B) lines, selected because both express FcRγ. The cells were labeled with 51Cr, washed, and incubated with RaMIg (5 μg/mL) for 30 minutes at 4°C. Anti-CD16 and anti-CD71 mAbs and the medium alone were used as relevant and irrelevant controls, respectively. Solid bars show the percentage of cytotoxicity on triggering of CD16+YT cells and the CD16+ variant of the NKL cells. YT mock cells and the NKL CD16− variants (open bars) were also included. The untransfected wild-type YT cell line showed the same behavior as the YT mock cells. Vertical bars represent the mean ± SD results from 6 independent experiments.
CD38 is laterally associated with CD16
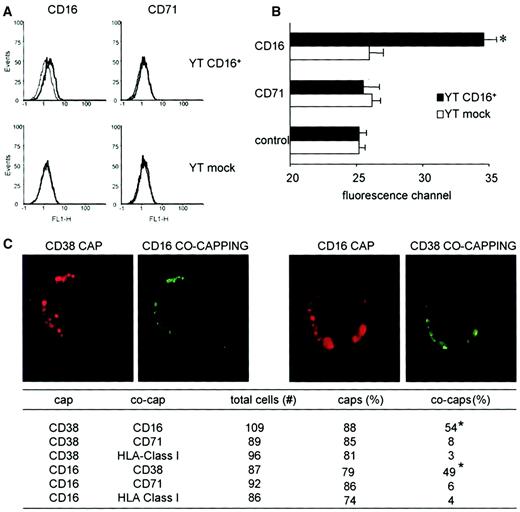
The unique ability of CD38 to associate functionally with TCR and BCR relies on close proximity as an indispensable requisite for CD38 to exploit the signaling machinery of professional surface receptors. This need was confirmed by analyzing the lateral associations between CD38 and CD16 in YT CD16+ cells as evaluated by FRET. The results obtained indicate that CD38 and the de novo expressed CD16 are physically associated in YT cells. The association is significant and specific, as shown by the lack of energy transfer with CD71, the transferrin receptor, which was expressed at a similar density by these cells (Figure 8). Confirmation of these findings was provided by the cocapping experiments. Antibody-mediated capping is an energy-dependent redistribution of cell-surface molecules to a single pole of the cell. In general, only molecules bound by the antibody will be redistributed to the area of the cap unless they have a particular association with other structures that are in turn induced to undergo cocapping to the same area. We found that CD38 capping was followed by CD16 cocapping and vice versa (Figure 8C). No cocapping was observed with anti-HLA class I mAb, used as an isotype-matched control. In addition, a summary of data acquired from a large number of cells is presented in Figure 8C.
CD38 is laterally associated with CD16 on the membrane of YT CD16+ cells.
(A) Cells were stained with Cy3-conjugated CD38 mAb and the indicated FITC-conjugated mAb. FITC was excited at 488 nm and Cy3 emissions were collected at more than 600 nm. The cytofluorographic profiles show representative data from 3 independent experiments. Each quadrant shows Cy3 emissions at more than 600 nm in the absence and presence of the indicated FITC-conjugated mAb. A right shift of the curve indicates FRET. (B) Bar graph shows the mean ± SD for the median fluorescence intensities on the membrane of YT CD16+ cells, expressed as median fluorescent channels derived from the results of 3 independent experiments. The asterisk indicates the CD16 FRET that is significantly higher than the CD71 FRET in the same cell line (P < .05; Wilcoxon matched pair, signed rank test). (C) Capping of the CD38 molecules induces cocapping of CD16 but not of HLA class I molecules. The same result was obtained by reverting the order of the mAbs, ie, by capping with CD16 and cocapping with CD38. The table shows cumulative data from several experiments. The numbers of caps and cocaps is presented as a percentage of cells analyzed. Cells showing partial redistribution of the surface molecule detected by the primary capping antibody were excluded from the analysis. The asterisks indicate a significant difference. Original magnification × 50.
CD38 is laterally associated with CD16 on the membrane of YT CD16+ cells.
(A) Cells were stained with Cy3-conjugated CD38 mAb and the indicated FITC-conjugated mAb. FITC was excited at 488 nm and Cy3 emissions were collected at more than 600 nm. The cytofluorographic profiles show representative data from 3 independent experiments. Each quadrant shows Cy3 emissions at more than 600 nm in the absence and presence of the indicated FITC-conjugated mAb. A right shift of the curve indicates FRET. (B) Bar graph shows the mean ± SD for the median fluorescence intensities on the membrane of YT CD16+ cells, expressed as median fluorescent channels derived from the results of 3 independent experiments. The asterisk indicates the CD16 FRET that is significantly higher than the CD71 FRET in the same cell line (P < .05; Wilcoxon matched pair, signed rank test). (C) Capping of the CD38 molecules induces cocapping of CD16 but not of HLA class I molecules. The same result was obtained by reverting the order of the mAbs, ie, by capping with CD16 and cocapping with CD38. The table shows cumulative data from several experiments. The numbers of caps and cocaps is presented as a percentage of cells analyzed. Cells showing partial redistribution of the surface molecule detected by the primary capping antibody were excluded from the analysis. The asterisks indicate a significant difference. Original magnification × 50.
Discussion
Cytotoxicity not restricted by major histocompatibility complex (MHC) results from a cooperative interaction among an array of monomorphic adhesion receptors.36,37 It was previously reported that engagement of CD38 on normal human NK cells elicits cytoplasmic Ca++ currents, phosphorylation of the CD3-ζ and FcRIII-γ chains, ZAP70, and c-Cbl. Long-terms events include the synthesis and secretion of cytokines and activation of cytolytic functions.7,8 Thus, CD38 may be considered a member of the family of adhesion molecules that function as cytotoxic triggers on NK cells, a feature shared with CD2, CD44, CD69, and other molecules.38-42
The uniqueness of CD38 relies on its unsuitable intracellular tail and consequent need to depend on other functional receptors to elicit signals, as previously demonstrated in T- and B-lymphocyte models.5,17,18 The working hypothesis behind this paper is that CD38 relies on CD16 to deliver signals in NK cells. Preliminary indirect evidence comes from the analysis of the NK-like CD16− YT line, where CD38 is apparently refractory to mobilize Ca++. Therefore, YT cells were selected as a model for studying the mechanisms behind CD38-mediated signal transduction in NK cells. YT cells are considered to be NK cells on the basis of their morphologic features, lack of TCR rearrangement, constitutive expression of intermediate affinity IL-2 receptor, and ability to mediate MHC-unrestricted cytotoxicity.21Furthermore, YT cells are characterized by the presence of cytoplasmic CD3ζ-ζ homodimers, and they transcribe CD3ε but not CD3γ or CD3δ.33
The approach selected was to transfect the human CD16 gene into YT cells and compare the effects resulting from CD38 ligation in the 2 cell populations. The first finding was that transfection of CD16 into YT cells produces expression of a functional receptor able to mobilize Ca++ and induces tyrosine phosphorylation of several substrates. This was not unexpected, since YT cells have the necessary intracellular mediators and adaptor proteins. The second finding was that CD16 transfection confers signaling properties on CD38, as witnessed by the appearance of clear-cut cytoplasmic Ca++currents and a complex profile of phosphorylated proteins.
Some of the substrates were identified and included ZAP70 and ERK 1/2. The pathway depicted so far apparently overlaps, in many instances, that elicited by CD38 in normal NK cells and envisages sequential activation of the ZAP70 and of ERK1/2 proteins, serine-threonine kinases belonging to the MAPK family. Such a signaling cascade ultimately converges on the nucleus, resulting in changes in gene expression that control activation of the NK lytic machinery.
The downstream events include production and secretion of IFN-γ. To rule out any pitfall coming from the use of mAbs, this experiment was repeated by interacting the CD38 receptor with the CD31 ligand, as expressed by murine fibroblasts. The results indicated clearly that only YT CD16+ cells can produce IFN-γ when cocultivated with CD31+ transfectants, showing that agonistic mAbs replace the natural ligand.
Standard and redirected cytotoxicity studies found that CD16 expression was itself sufficient to change the lytic propensities of the cells. CD16 transfection was followed by an increased ability to kill selected B and myeloid targets, whereas it had no effect in T-cell lines. These results are in line with published data from independent groups and point to the possibility of a cell-surface ligand for CD16.35 Formal confirmation was provided by redirected lysis experiments using P815 (a murine mastocytoma cell line) and K562 (a human erythroleukemia line) to highlight the contribution of the CD38 pathway to lysis. The results show conclusively that CD38 is an active killing receptor, provided that CD16 is expressed on the surface of YT cells. Because cytotoxicity is the most relevant biologic characteristic of NK cells, these data were confirmed by using the NKL cell line, in which CD16+ and CD16− sublines were obtained without genetic manipulation.
The functional association between CD16 and CD38 is likely to take place at the plasma membrane level, since YT cells lack CD16 but have its intracellular signaling machinery. Functional synergy in leukocytes is usually indicated by close contiguity or location in specialized areas of the cell surface.43 In this study, the existence of a physical proximity between the 2 structures was confirmed by FRET and cocapping experiments done in the YT CD16+ cell line. These findings provide formal and instrumental backup results to those of cocapping experiments done using NK cells from peripheral blood.16 Furthermore, the size, adhesive properties, and localization in membrane rafts44 indicate CD38 as a candidate member of the immunologic synapse.
The results concur to portray a molecule that is unable by itself to signal but that is rescued as a receptor by association with a professional signaling structure. This molecule is CD16 in NK cells. In this case, CD38 would be a unique coreceptor molecule, enabled to function by the dominant receptor itself. Crucial questions that remain to be answered concern induction of the system. Is it a ligand-induced or a substrate-induced conformational change? What is the role of the interaction between CD16 and its putative cell-surface–bound ligand in the activation of CD38?
Finally, YT cells are not representative of the majority of mature NK cells in the peripheral blood of adults; for one thing, NK cells in adults do not express CD28, which is detectable on YT cells at a substantial density. In this respect, and also because the YT line was established from samples from a child with ALL and thymoma, YT cells are more likely representative of fetal NK cells, which lack CD16 and express CD28. Analysis of CD38 expression and function in fetal NK cells is likely to add insight into the biologic features of this unique receptor molecule.
We thank Lewis L. Lanier (San Francisco, CA) for providing reagents and suggestions, R. Galandrini (Rome, Italy) for providing cells, and Francesca Urbani (Rome, Italy) for assistance.
Supported by grants from AIRC, Special Projects AIDS (Istituto Superiore di Sanità), Biotechnology (CNR/MURST), and Cofinanziamento (MURST) to F.M.; from Comisión Interministerial de Ciencia y Tecnologı́a SAF99-0024 to J.S.; and from Instituto de Salud Carlos III-FIS, Ministerio de Sanidad y Consumo, Programa Nacional de Salud (01/1073) to M.Z. Financial contributions were provided by the Compagnia di SanPaolo, Cariverona, and Ghirotti Foundations, and Regione Piemonte. S.D. is a student of the Postgraduate School of Medical Oncology, University of Torino Medical School, Torino, Italy. M.Z. is supported by Comitato de Investigadores, Programa Nacional de Salud, Ministerio de Sanidad y Consumo, Spain.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Fabio Malavasi, Laboratory of Immunogenetics, University of Torino Medical School, Via Santena, 19, 10126 Torino, Italy; e-mail: fabio.malavasi@unito.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal