Abstract
Adenovirus infection of hematopoietic cells frequently requires high virus concentrations and long incubation times to obtain moderate infection levels because these cells have low levels of Coxsackie and adenovirus receptor (CAR) and αv integrin. The effect of treatment with FR901228 (depsipeptide), a histone deacetylase inhibitor in phase 2 clinical trials, was studied in K562 cells, granulocyte–colony-stimulating factor–mobilized peripheral blood mononuclear cells (PBMCs), and CD34+ peripheral blood stem cells (PBSCs). FR901228 increased CAR and αvintegrin RNA levels and histone H3 acetylation. FR901228 treatment before adenovirus infection was associated with at least a 10-fold increase in transgene expression from a β-galactosidase–expressing adenoviral vector. More than 80% of the PBMCs or CD34+ PBSCs from 7 different donors were β-galactosidase–positive after adenovirus infection with a multiplicity of infection of 10 for 60 minutes. Increased CAR, αv integrin, and acetylated histone H3 levels were observed in PBMCs from a patient treated with FR901228. These studies suggest that FR901228 can increase the efficiency of adenoviral infection in hematopoietic cells.
Introduction
One of the major limitations in using adenoviral vectors for gene therapy is inefficient infection of hematopoietic cells. In fact, this phenomenon has been used to purge bone marrow of contaminating tumor cells.1,2 The reason for poor infectivity of hematopoietic cells is the low level of receptors required for adenovirus infection.3 To infect cells efficiently, adenovirus serotypes 2 and 5 require Coxsackie and adenovirus receptor (CAR) for attachment and αv integrin for internalization.4-6 Although CAR and αvintegrin facilitate infection, adenovirus can infect cells through other less efficient means.7 The use of inefficient entry pathways by adenovirus may explain why high levels of adenovirus infection of hematopoietic cells require high viral titers (multiplicity of infection [MOI], 500 or greater) and long incubation times (12-24 hours).8-14 More efficient adenovirus transduction has been obtained by the addition of polycations or cationic lipids.15,16 Increased adenovirus infection efficiency has also been obtained using infection through non-CAR–mediated mechanisms.17-19 An alternative approach is to up-regulate CAR or αv integrin levels.20 In this study we describe the use of the histone deacetylase inhibitor FR901228,21 22 a drug currently in clinical trials, as a therapeutic tool to increase both CAR and αv integrin RNA levels before adenovirus infection. This increase in receptor levels is associated with an increase in transgene expression after adenovirus infection in hematopoietic cells.
Study design
FR901228
FR901228 was isolated by the Fujisawa Company.21This drug is currently undergoing phase 2 evaluation in acute and chronic leukemias, T-cell lymphomas, and ovarian and renal cancer. FR901228 was obtained from the Pharmaceutical Management Branch, Cancer Therapy Evaluation Program, National Cancer Institute (Bethesda, MD).
Cell culture
K562 is a cell line derived from a human chronic myeloid leukemia in erythroid blast crisis (ATCC, Manassas, VA). Granulocyte–colony-stimulating factor–mobilized peripheral blood mononuclear cells (PBMCs) and CD34+ selected peripheral blood stem cells (PBSCs) were obtained from Poietic Technologies (Gaithersburg, MD). Cells were cultured in 20% fetal bovine serum, 10 ng/mL thrombopoietin, 10 ng/mL stem cell factor, and 30 ng/mL FLT-3 ligand (Research Diagnostics, Flanders, NJ).
Adenovirus
The Ad5.CMV-LacZ is an E1 and E3 gene-deleted, replication-defective type 5 adenovirus obtained from Qbiogene (Carlsbad, CA). The AdCMVβgal virus was grown and purified, and the titer was determined by the TCID50 assay as described by the manufacturer.
Results and discussion
The addition of histone deacetylase inhibitors after adenoviral infection is known to increase the expression of viral proteins and transgene expression.23,24 Studies performed in our laboratory with cell lines from solid tumors showed that treatment with the histone deacetylase inhibitor FR901228 caused an increase in CAR and αv integrin RNA levels. FR901228 treatment of cell lines before, but not during or after, adenovirus infection was associated with a 4- to 10-fold increase in transgene expression after adenoviral vector infection.25 In this study we investigated whether FR901228-treated hematopoietic cells exhibit a similar pattern.
Cytotoxicity studies were performed to determine a minimally cytotoxic FR901228 concentration for K562 cells and granulocyte–colony-stimulating factor–mobilized PBMCs and CD34+ PBSCs. The drug concentration showing no or minimal cytotoxicity that was selected for these studies was 1 ng/mL FR901228 for K562 cells, whereas normal hematopoietic cells were exposed to 0.3 ng/mL or less (data not shown).
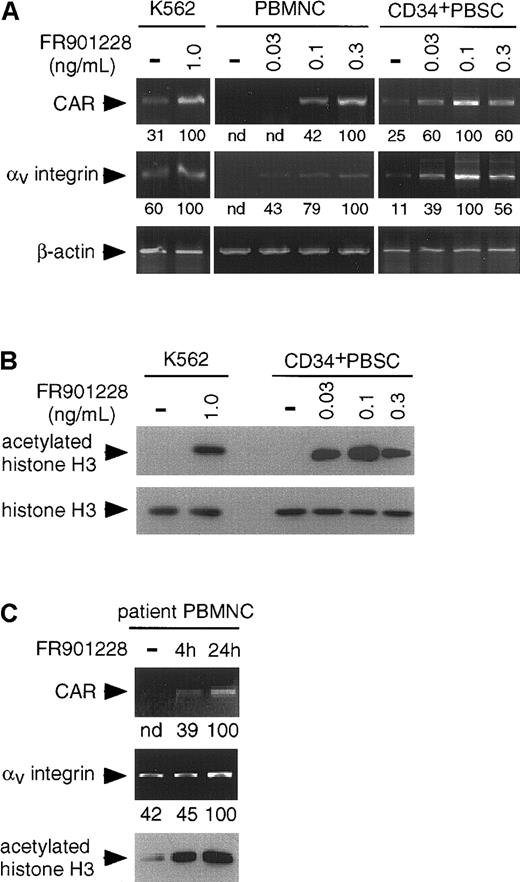
Figure 1A shows CAR and αv integrin RNA levels determined by reverse transcription–polymerase chain reaction (RT-PCR) analysis. CAR expression was detectable in K562 cells before the administration of FR901228 and was increased approximately 3 times after FR901228 administration. In treated PBMCs and CD34+ PBSCs, CAR expression reached levels comparable to those in treated K562 cells. Similar results were found when αv integrin expression was examined. Thus, in PBMCs and CD34+ PBSCs, noncytotoxic concentrations of FR901228 were able to induce CAR and αvintegrin expression significantly.
Effects of FR901228 treatment on hematopoietic cells by RT-PCR and Western blot analyses.
(A) RT-PCR analysis of CAR and αv integrin RNA levels in FR901228-treated cells. K562 cells were incubated without (−) and with the indicated concentrations of FR901228 for 72 hours before the isolation of RNA. Frozen PBMCs and CD34+ PBSCs were thawed and expanded in medium for 4 days and then treated without (−) and with the indicated concentrations of FR901228 for 24 hours before isolation of RNA. RNA was isolated, and RT-PCR was performed as previously described25 using β-actin as the loading control. For quantitation, which is shown below the photographs, the density of the bands was determined by densitometry, the brightest was set to a value of 100, and the lighter bands were normalized to it. nd indicates not detectable. (B) Western blot analysis of FR901228-treated cells. K562 cells or CD34+ PBSCs were incubated without (−) or with the indicated concentrations of FR901228. K562 cells were treated for 48 hours, and CD34+ PBSCs were treated for 24 hours. Total cellular protein was isolated, and Western blot analysis was performed as previously described.25 Histone H3 functions as the loading control. (C) RT-PCR and Western blot analyses of PBMCs from a patient treated with FR901228. RNA and protein were isolated from PBMCs from a patient receiving FR901228 on our phase 1 trial. Time points are before the start of drug infusion (−), at the end of the 4-hour infusion, and after 24 hours. Quantitation, which is shown below the photographs, is as described above. nd indicates not detectable
Effects of FR901228 treatment on hematopoietic cells by RT-PCR and Western blot analyses.
(A) RT-PCR analysis of CAR and αv integrin RNA levels in FR901228-treated cells. K562 cells were incubated without (−) and with the indicated concentrations of FR901228 for 72 hours before the isolation of RNA. Frozen PBMCs and CD34+ PBSCs were thawed and expanded in medium for 4 days and then treated without (−) and with the indicated concentrations of FR901228 for 24 hours before isolation of RNA. RNA was isolated, and RT-PCR was performed as previously described25 using β-actin as the loading control. For quantitation, which is shown below the photographs, the density of the bands was determined by densitometry, the brightest was set to a value of 100, and the lighter bands were normalized to it. nd indicates not detectable. (B) Western blot analysis of FR901228-treated cells. K562 cells or CD34+ PBSCs were incubated without (−) or with the indicated concentrations of FR901228. K562 cells were treated for 48 hours, and CD34+ PBSCs were treated for 24 hours. Total cellular protein was isolated, and Western blot analysis was performed as previously described.25 Histone H3 functions as the loading control. (C) RT-PCR and Western blot analyses of PBMCs from a patient treated with FR901228. RNA and protein were isolated from PBMCs from a patient receiving FR901228 on our phase 1 trial. Time points are before the start of drug infusion (−), at the end of the 4-hour infusion, and after 24 hours. Quantitation, which is shown below the photographs, is as described above. nd indicates not detectable
Because FR901228 is a histone deacetylase inhibitor, we sought to determine whether histone deacetylase inhibition might be the mechanism responsible for this phenomenon. Support for this mechanism comes from our studies in solid tumor cell lines that showed that the histone deacetylase inhibitors sodium butryate26 and trichostatin A27 also increased CAR and αv integrin RNA levels.25 Up-regulation of CAR by sodium butyrate has been reported in bladder cancer cell lines.28 Figure 1B shows Western blot analysis of K562 cells and CD34+ PBSCs. Incubation in FR901228 resulted in a marked increase in acetylated histone H3 while causing no significant change in total histone H3 levels. These results demonstrate that the concentrations of FR901228 used to induce CAR and αv integrin can inhibit histone deacetylase.
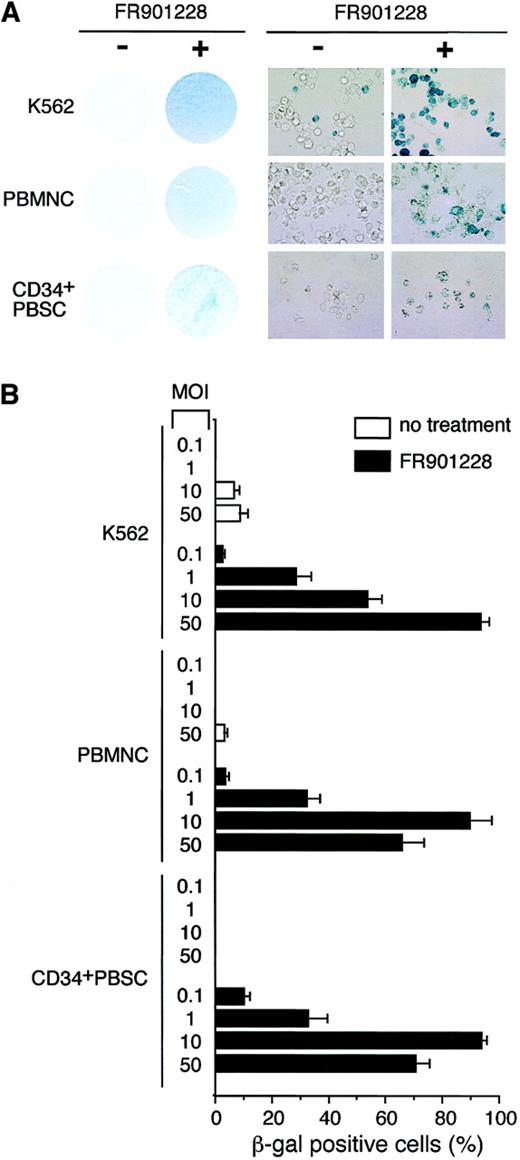
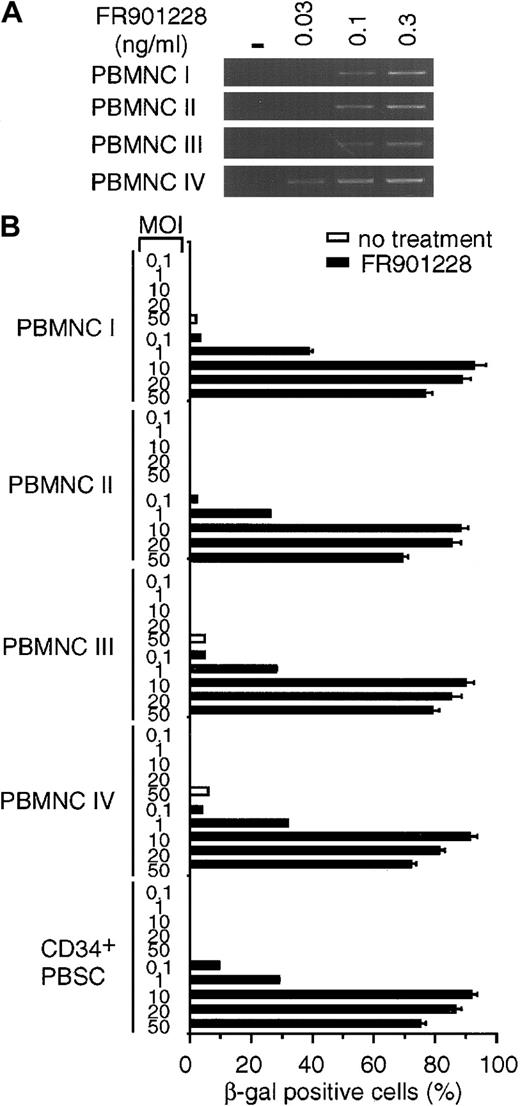
We next sought to determine whether the increases in CAR and αv integrin levels could enhance adenoviral infectivity as determined by transgene expression after infection. K562 cells, PBMCs, and CD34+ PBSCs were incubated with or without FR901228. At the end of the incubation period, FR901228 was removed, and the cells were infected with an adenoviral vector carrying the β-galactosidase gene under the direction of the cytomegalovirus promoter. As shown in Figure 2A, all FR901228-treated cells demonstrated a marked increase in blue color as a result of transgene expression in a higher percentage of cells. The quantitation shown in Figure 2B was achieved by examining the cells under high-power magnification. In all cases, transgene expression in more than 80% of cells was achieved with a low viral titer for PBMCs and CD34+ PBSCs (MOI = 10) and for K562 (MOI = 50). Because the transduction efficiencies and gene expression levels can vary among donors, samples from additional donors were analyzed. Figure3B shows the data from adenovirus infection of 4 additional PBMC donors and one additional CD34+ PBSC donor. These donors also showed an increase in CAR RNA expression and a greater than 80% adenoviral infection rate after FR901228 treatment. These results demonstrate that pretreatment of normal hematopoietic cells with low concentrations of FR901228 can markedly increase the number of hematopoietic cells infected by low adenovirus titers (MOI ≤ 10) with short incubation times (1 hour). Our results contrast sharply with previous studies that found it necessary to use high concentrations of virus (MOI ≥ 500) for long incubation times (12-24 hours) to achieve a moderate level of infected cells.8-12
Expression of β-galactosidase transgene after adenovirus infection of FR901228-treated cells.
K562 cells were treated for 48 hours with 1 ng/mL FR901228. Frozen PBMCs and CD34+ PBSCs were thawed and expanded in medium for 4 days and then treated with 0.1 ng/mL FR901228 for 24 hours. Cells were infected with AdCMVβgal for 1 hour in medium without serum. Infected cells were maintained in media with serum for an additional 72 hours before staining for β-galactosidase activity using the β-Gal Staining Kit (Invitrogen, Carlsbad, CA). (A) Photographs of cells infected with adenovirus and stained for β-galactoside activity. On the left are photographs of cells incubated without (−) and with (+) FR901228 before they were infected with AdCMVβgal. K562 cells were infected at an MOI of 50, whereas PBMCs and CD34+ PBSCs were infected with an MOI of 10. Cells are shown at a higher magnification on the right. (B) Quantitation of β-galactosidase–positive cells after adenovirus infection. β-galactosidase–positive cells were counted from 3 nonoverlapping fields of cells treated with FR901228 or control cells infected with AdCMVβgal at the indicated MOI.
Expression of β-galactosidase transgene after adenovirus infection of FR901228-treated cells.
K562 cells were treated for 48 hours with 1 ng/mL FR901228. Frozen PBMCs and CD34+ PBSCs were thawed and expanded in medium for 4 days and then treated with 0.1 ng/mL FR901228 for 24 hours. Cells were infected with AdCMVβgal for 1 hour in medium without serum. Infected cells were maintained in media with serum for an additional 72 hours before staining for β-galactosidase activity using the β-Gal Staining Kit (Invitrogen, Carlsbad, CA). (A) Photographs of cells infected with adenovirus and stained for β-galactoside activity. On the left are photographs of cells incubated without (−) and with (+) FR901228 before they were infected with AdCMVβgal. K562 cells were infected at an MOI of 50, whereas PBMCs and CD34+ PBSCs were infected with an MOI of 10. Cells are shown at a higher magnification on the right. (B) Quantitation of β-galactosidase–positive cells after adenovirus infection. β-galactosidase–positive cells were counted from 3 nonoverlapping fields of cells treated with FR901228 or control cells infected with AdCMVβgal at the indicated MOI.
Rapid induction of β-galactosidase transgene expression after adenovirus infection of FR901228-treated cells.
Frozen PBMCs and CD34+ PBSCs from different donors were thawed and incubated in medium overnight. FR901228 was added to the treated cells, and all cells were incubated for 24 hours. (A) RT-PCR analysis of CAR RNA in FR901228-treated PBMCs. PBMCs were treated without (−) or with the indicated concentration of FR901228. Samples were prepared as described in the legend to Figure 1. (B) Quantitation of β-galactosidase– positive cells after adenovirus infection. Untreated cells or cells treated with 0.1 ng/mL FR901228 were infected with the indicated MOI of ADCMVβgal for 1 hour in the absence of serum. Serum was added to the medium. Cells were incubated for 24 hours and were analyzed for β-galactosidase activity as described in the legend to Figure 2. The viability of the PBMCs, as determined by trypan blue exclusion, was at least 94%. The viability of CD34+PBSCs, as determined by propidium iodide exclusion, was 95%. In addition, 92% of these cells showed expression of the CD34 epitope at the conclusion of the experiment.
Rapid induction of β-galactosidase transgene expression after adenovirus infection of FR901228-treated cells.
Frozen PBMCs and CD34+ PBSCs from different donors were thawed and incubated in medium overnight. FR901228 was added to the treated cells, and all cells were incubated for 24 hours. (A) RT-PCR analysis of CAR RNA in FR901228-treated PBMCs. PBMCs were treated without (−) or with the indicated concentration of FR901228. Samples were prepared as described in the legend to Figure 1. (B) Quantitation of β-galactosidase– positive cells after adenovirus infection. Untreated cells or cells treated with 0.1 ng/mL FR901228 were infected with the indicated MOI of ADCMVβgal for 1 hour in the absence of serum. Serum was added to the medium. Cells were incubated for 24 hours and were analyzed for β-galactosidase activity as described in the legend to Figure 2. The viability of the PBMCs, as determined by trypan blue exclusion, was at least 94%. The viability of CD34+PBSCs, as determined by propidium iodide exclusion, was 95%. In addition, 92% of these cells showed expression of the CD34 epitope at the conclusion of the experiment.
Finally, we sought preliminary evidence that increased CAR and αv integrin levels might occur in vivo by examining PBMCs obtained from a patient enrolled in our phase 1 FR901228 study. The effective drug concentration used in our in vitro studies is well within the range currently administered to patients.29 Figure 1C demonstrates that CAR expression in RNA isolated from patient PBMCs was not detected by RT-PCR before the start of FR901228 administration. However, after completion of a 4-hour FR901228 infusion, CAR expression increased, and this was further increased at 24 hours. Coincident with this, the level of acetylated histone H3 also increased after the administration of FR901228.
In summary, we have demonstrated that noncytotoxic doses of FR901228, a histone deacetylase inhibitor, can result in marked increases in the expression of CAR and αv integrin RNA in hematopoietic cells. This increase mediates enhanced transgene expression after adenovirus infection. These studies suggest a simple, clinically practical method for increasing the sensitivity of hematopoietic cells to adenoviral gene therapy vectors. By avoiding the need for high adenoviral titers, the likelihood of viral toxicity is reduced. These studies may lead to new approaches for the treatment of neoplastic, inflammatory, immunologic, and inherited hematologic disorders.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tito Fojo, Cancer Therapeutics Branch, Center for Cancer Research, National Cancer Institute, Bldg 10, Rm 12C103, MSC 1910, 9000 Rockville Pike, Bethesda, MD 20892; e-mail:tfojo@helix.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal