Abstract
Immunosuppression with B7 antagonists might have 2 opposite effects: reducing T-cell costimulation through CD28 but also preventing CTLA-4 from transmitting its negative regulatory signal. We therefore hypothesized that a selective blockade of CD28 might be qualitatively different from blocking B7. It was previously reported that CD28 modulation prolongs allograft survival in the rat and reverses induction of experimental autoimmune encephalomyelitis in mice. However, whether CD28 or B7 blockade results in similar immunosuppression on alloimmune and self-restricted responses to soluble antigens has not yet been investigated. Here, we addressed this issue in vitro with antagonist anti-CD28 Fab fragments and in vivo using the modulating anti-rat JJ319 monoclonal antibody. As in the inhibition of B7 with CTLA4 immunoglobulin, anti-CD28 Fab fragments inhibited allogenic T-cell proliferation in mixed cultures. In vivo modulation of CD28 blocked the expansion of alloreactive T cells and promoted their apoptosis. In contrast, selective blockade of CD28 did not modify T-cell proliferative responses and antibody production to soluble antigens, whereas blocking B7 with CTLA4 immunoglobulin did. Our data show that blocking CD28, while leaving CTLA4-B7 interactions undisturbed, inhibits alloreactive CD4+ T-cell expansion but does not modify the response to nominal antigens presented in the context of a self-major histocompatibility complex. That B7 engagement is needed for self-restricted responses whereas engagement of CD28 is not essential adds to the suggestion that another unidentified ligand of B7 might deliver a costimulatory signal in the absence of CD28.
Introduction
Complete activation of T cells requires the delivery of at least 2 signals by antigen-presenting cells (APCs). The first is provided through T-cell receptor (TCR) engagement of the peptide–major histocompatibility complex (MHC) on the APC, and the second is provided by the interaction of accessory receptors on T cells with their ligands on APCs and is referred to as costimulation. The CD28 molecule on T cells binds to B7-1 or B7-2 on APCs, thereby providing signal 2 for the initiation of naive T-cell responses. In conjunction with a TCR stimulus, it allows high-level interleukin-2 (IL-2) production and provides an essential survival signal for T cells,1 thereby preventing apoptosis or the induction of anergy that may occur in response to signal 1 alone. CD28 regulates T-cell cycle entry and progression through the G1 phase in an IL-2–independent manner, resulting in the activation of cyclins.2 After activation, T cells up-regulate the surface expression of CTLA-4, a homologue of CD28, that binds the same ligands with higher affinity and serves as a negative regulator of T-cell activation. In addition to its competition with CD28 for binding to B7 molecules, CTLA-4 recruits phosphatases that dephosphorylate activated molecules in the CD3 complex, and it reduces nuclear translocation of RelA, which leads to a suppression of the production of multiple cytokines produced by Th1 and Th2 cells, including IL-2, interferon (IFN)–γ, IL-3, IL-4, and IL-10.3 Actually, direct antagonism of the TCR-mediated signal by CTLA-4, independent of CD28, has been shown to be sufficient to explain its inhibitory effect.4
Despite evidence that CTLA-4 is a regulator of T-cell responses, some observations indicate that CTLA-4 may be of relatively minor importance for the down-regulation of primary immune responses.5 In addition, though lymphoproliferative disease is observed in CTLA-4–deficient mice, arguing for a role of CTLA-4 in inhibiting T-cell expansion, it is not observed in chimeric recipients when wild-type and CTLA-4−/− T cells coexist.6Moreover, it was reported that a CTLA-4–B7 interaction can also costimulate T-cell clonal expansion and production of cytolytic T cells.7,8 In vivo studies with B7 antagonists have demonstrated a central role for B7-mediated costimulation in antibody response,9 autoimmune disease,10-13 and graft rejection,14 15 but the respective participation of CTLA-4 and CD28 in these responses is not completely defined.
In vitro studies with murine and human T-cell clones have indicated that antigenic stimulation in the absence of costimulation signaling through B7/CD28 causes the T cells to enter a state of long-term hyporesponsiveness, known as anergy, connected to the failure to induce IL-2 gene transcription.16 Immunosuppression with B7 antagonists, however, might have 2 opposite effects—reducing T-cell costimulation through CD28 and preventing CTLA-4 from transmitting its negative regulatory signal. Therefore, we hypothesized that selective blockade of CD28 could be more effective at inducing immunosuppression and anergy than B7 blockade. Expected molecular consequences of the selective blockade of CD28 with an unmodified CTLA-4–B7 interaction would be a block in the transition from G0 to G1 and an induction of the antiapoptotic factor Bcl-xL in resting CD4+ T cells,17 whereas it would induce Fas-independent cell death in activated CD4+ T cells.18 Indeed, selective blockade of CD28 molecules was shown to reduce the reactivity of autoreactive cells in experimental autoimmune encephalomyelitis19 and in experimental autoimmune uveoretinitis,11 and it was more immunosuppressive than blocking the ligands for CD28 and CTLA-4 in graft-versus-host disease (GVHD).20 Selective blockade of CD28 also delayed allograft rejection in the rat.21 This selective immunosuppression, however, did not induce long-term tolerance.11,21 The extent to which blocking CD28 would modify antibody responses depends on the type of immunization, because responses to viral infection22 or immunization with cardiomyosin23 are impaired in CD28−/− mice whereas responses to keyhole limpet hemocyanin (KLH) are normal.24
Here, we added to these studies a comparison between the effects of antagonizing B7 versus CD28 on immune responses to nominal antigens and to direct allorecognition. Using either Fab fragments from an anti-human CD28 antibody25 in vitro or the modulating anti-rat CD28 JJ319 antibody21 in vivo, we demonstrated that though, as expected, B7 inhibition reduces direct pathway activation of the growth of T cells subjected to allostimulation and immune responses to soluble antigens, selective inhibition of CD28 does not modify the responses of T cells activated against self-MHC–restricted determinants. No modification of antibody responses, antigen-triggered cell proliferation, or cytokine induction was noted. In contrast, it was effective in reducing the proliferation of T cells stimulated through the direct allorecognition pathway in vitro and in vivo. These data indicate that blocking CD28 results in a qualitatively different effect from blocking B7. In addition, our results match recent data suggesting additional yet unidentified ligands for B7 on T cells.
Materials and methods
Animals and cells
Inbred adult rats (200-250 g) from the Lewis (LEW).1A (RT1a) and the LEW.1W (RT1u) congenic strains were purchased from Janvier (Savigny/Orge, France). All animals were 5- to 7-week-old males and were maintained in our animal facility under EOPS conditions according to institutional guidelines. T cells were purified with the rat T-cell enrichment columns (R&D Systems, Minneapolis, MN). Human peripheral CD4+ T cells were obtained from healthy, unrelated volunteers and were prepared with the Lympho Kwick Th system (Ingen, Rungis, France), according to the manufacturer's instructions.
Antibodies and reagents
Anti–rat CD28 JJ319 hybridoma was obtained from T. J. Dengler.21 Isotypic control mouse immunoglobulin IgG1 3G8 was from American Type Culture Collection (Manassas, VA). Anti–human CD28 CD28.3 hybridoma was from INSERM U 119.25 Antibodies were purified from cell culture supernatant on protein G–Sepharose. Fab fragments were prepared using the Immunopure IgG1 Fab preparation kit (Pierce, Rockford, IL). Affinities of CD28.3 mAb and their Fab fragments were compared by biosensor analysis (Biacore) and were found to be 4.3 × 10−9 and 1.7 × 10−9, respectively. Purified CTLA4 immunoglobulin was obtained from R. Peach (Bristol-Myers Squibb, Stamford, CT). Keyhole limpet hemocyanin was from Sigma (St Louis, MO), and dinytrophenol-conjugated ovalbumin was a gift from F. Nisol (University of Louvain, Belgium). Antibodies were given intraperitoneally at a dose of 1 mg/injection. CTLA4 immunoglobulin was given intraperitoneally at a dose of 0.5 mg/injection.
Proliferation assays
Human peripheral blood mononuclear cells (PBMCs) or CD4+ T cells, or rat T cells were seeded in triplicate at a final concentration of 105 cells/well in RPMI 1640 (Sigma), glutamine, nonessential amino acids, sodium pyruvate, and antibiotics, and 10% heat-inactivated autologous serum (plus 5 × 10−5 M 2-βME for rat cells) and were cultured for 5 days with 2 × 104 allogeneic dendritic cells (for rat) or 105 PBMCs (human) irradiated at 30 Gy. Proliferation was measured by 1 μCi (37 Bq) 3H incorporation after 16-hour incubation. Results expressed are cpm per triplicate or proliferation indexes: (cpm MLR−cpm stimulating cells alone)/(cpm responding cells alone).
Immunizations and self-restricted responses
LEW.1A rats were immunized in the footpad with 50 μg KLH in complete Freund adjuvant. Serum and draining lymph nodes were harvested 11 days after injection. Triplicate 0.2-mL cultures of draining lymph node cells (3 × 105/well) were cultured in RPMI 1640 (Sigma) supplemented with 2-ME, glutamine, nonessential amino acids, sodium pyruvate, antibiotics, and 0 to 50 μg/mL KLH. Cultures were incubated for 72 hours and were pulsed with [3H] thymidine (1.0 μCi (37 Bq)/10μL per well) for the last 16 hours. Data are presented as stimulation indexes (average counts per minute in cultures with stimulus divided by those in control cultures without stimulus) or Δcpm (Δcpm = mean cpm in cultures with stimulus − mean cpm in control cultures without stimulus).
Assay for DNP- and KLH-specific antibody production
Serum levels of anti-dinytrophenol (DNP) and anti-KLH IgG1, IgG2a, IgG2b, IgG2c, and IgM subclasses were determined by enzyme-linked immunosorbent assay, as described previously.26 Briefly, 96-well microtiter plates (Immulon, Chantilly, VA) were coated with bovine γ globulin–DNP or with KLH at 5 μg/mL. After blocking the plates with gelatin (Sigma) and incubating serum serial dilutions in 0.1% phosphate-buffered saline–Tween 20 for 2 hours at 37°C, plates were developed using horseradish peroxidase–conjugated mouse monoclonal anti–rat individual isotypes (IgG1, MARG1-2; IgG2a, MARG2a-1; IgG2b, MARG2b-3; IgG2c, MARG2c-5; IgM, MARM-4; generous gift from H. Bazin, University of Louvain, Belgium). The concentration of anti-DNP antibody was estimated using standard curves generated by incubating the DNP-coated plates with anti-DNP rat monoclonal antibody (IgG1, LODNP-1; IgG2a, LODNP-16; IgG2b, LODNP11; IgM, LODNP34; generous gift from H. Bazin). Titer comparisons were performed in the linear range of the assay. Titers of anti-DNP IgG2c responses and anti-KLH antibody responses are represented as the serum dilution needed to reach an OD405of 0.5 in the assay.
Graft-versus-host disease induction and monitoring of cell proliferation in vivo
A 1:1 mixture of mononuclear cells from rat spleen and mesenteric lymph nodes was resuspended at 5 × 107cells/mL in RPMI containing 5 μM 5-6-carboxy fluorescein diacetate succinimidyl ester (CFDASE; Molecular Probes, Eugene, OR) and was incubated at 37°C for 20 minutes, as previously described.27 Unbound CFDASE or the deacetylated form, CFSE, was quenched by 3 washes in complete medium. CFSE-labeled cells (2 × 108) were injected intravenously in congeneic or allogeneic rats treated 24 hours earlier with whole-body sublethal irradiation of 8 Gy using a cobalt Co 60 source. Recipients were killed after 3 days for analysis of spleen and mesenteric lymph node cells. Sensitivity of FL1 detection was adjusted so that residual recipient T cells could be distinguished from CFSE-labeled cells that divided up to 9 times.
Flow cytometry
At the time of harvest, spleen and mesenteric lymph node cells were washed in cold phosphate-buffered saline containing 1% bovine serum albumin and 0.1% sodium azide. At least 2 × 105cells per sample were stained with biotin-conjugated monoclonal antibody specific for CD4 (W3/25), CD8 (ox8), CD25 (ox39), and CD62L (ox85) or with biotin-conjugated Annexin-V (Immunotech, Marseille, France) followed by PC5-conjugated streptavidin (Immunotech). Cytometry was performed on a Becton Dickinson FACSCalibur single-laser cytometer using standard Cell Quest acquisition analysis software, and fluorescence compensation was achieved using the appropriate single-fluorochrome–labeled samples. Twenty thousand events in the lymphocyte acquisition gate were collected. Analysis of cell division (CFSE–fluorescence profile) was restricted to the CD4+subset of CFSE-labeled cells.
Quantitation of cytokine mRNA
Cells (4 × 106) from mixed lymphocyte reactions were collected after 5 days of culture. Cells were lysed in guanidinium isothiocyanate, and total RNA was extracted as previously described.28 Total RNA (10 μg) was treated with DNAse (Promega, Charbonnières, France) and was retro-transcribed using 100 μM oligo dT (Gibco BRL), 10 mM dithiothreitol (Promega), 0.5 mM each of 4 dNTPs, 40 U RNAse OUT, and 200 U M-MLV reverse transcriptase. Quantitative polymerase chain reaction was carried out in a 7700 Sequence Detector TaqMan (Perkin Elmer) using the following primers : IL-2, 5′-AAACACAGCTACAACTGGAGCA-3′ and 5′-GCTGATTAAGTCCCTGGGTCTT-3′; IFN-γ, 5′-TGTCCAACGCAAAGCAATACA-3′ and 5′-TTCGCTTCCCTGTTTTAGCTG-3′; IL-4, 5′-CACCGAGTTGACCGTAACAGAC-3′ and 5′-TACTCTGGTTGGCTTCCTTCAC-3′; IL-5, 5′-TGTATGCCATCCCCACAGAA-3′ and 5′-TTTCCACAGTACCCCCTTGC-3′; HPRT, 5′-ATTGACACTGGCAAAACAATGCA-3′ and 5′-TCCAACACTTCGTGGGGTCC-3′.
Results
Selective blockade of CD28 reduces T-cell proliferation in primary and secondary mixed lymphocyte reaction
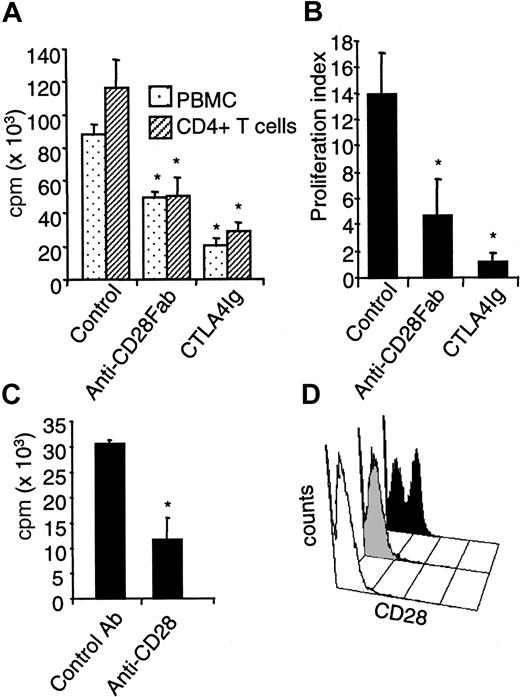
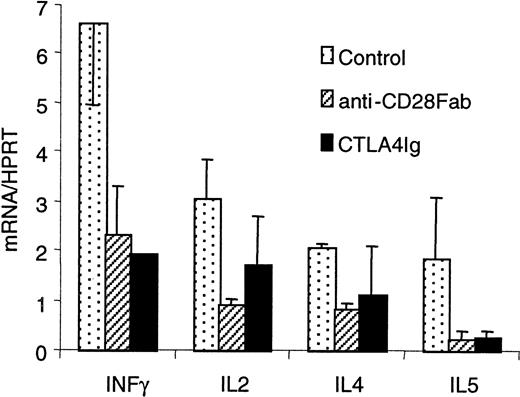
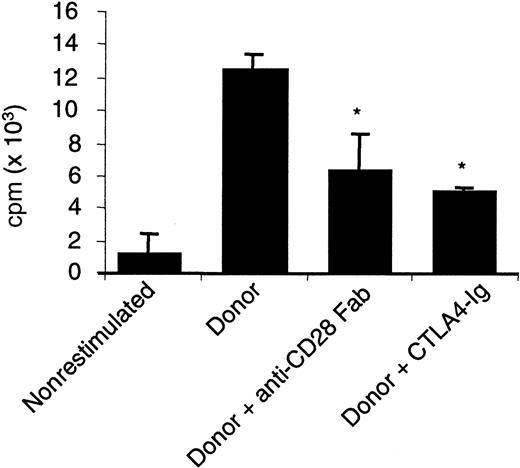
To compare a selective blockade of CD28 with a blockade of B7, we first assayed the effect of monovalent Fab fragments from a blocking anti-CD28 antibody with that of CTLA4 immunoglobulin on mixed lymphocyte reactions (MLRs) using pure T cells as proliferating responders. The direct presentation pathway accounts for 90% of the measured proliferation in this setting.29 Allogenic proliferation of PBMCs or CD4+ T cells was typically reduced by 60% in the presence of optimal doses of anti-CD28 Fab fragments (10 μg/mL), whereas CTLA4 immunoglobulin (5 μg/mL) reduced proliferation to 75% in human MLR and nearly completely in rat MLR (Figure 1A-B). Proliferation was reduced to the same extent with anti-CD86 antibodies (data not shown). A similar reduction of proliferation was obtained ex vivo using CD4+CD28− T cells from rats treated with the modulating JJ319 anti-CD28 antibody, stimulated with allogenic APCs (Figure 1C-D). The observed reduction of T-cell proliferation with CD28 or B7 blockade was paralleled by a reduction in the production of IFN-γ and IL-2 in human MLR (Figure 2). Up-regulation of messenger RNA for IL-4 and IL-5 was also inhibited by anti-CD28 Fab and CTLA4 immunoglobulin. In secondary stimulation in vitro with donor APC, the addition of anti-CD28 Fab or CTLA4 immunoglobulin reduced proliferation by 40% to 50% (Figure3), showing that the inhibition of CD28 or B7 also blocked the proliferation of primed alloimmune cells to a similar extent.
Specific CD28 blockade reduces human and rat T-cell proliferation in primary MLR.
(A) Human PBMCs or CD4+ T cells (105) were stimulated with 105 allogenic irradiated PBMCs with 10 μg/mL control IgG, anti–human CD28 Fab fragments, or 5 μg/mL CTLA4 immunoglobulin, and proliferation was measured after 5 days. Mean ± SD of triplicates from 1 of 8 representative experiments is shown. (B) Purified LEW.1A rat T cells (105) from spleen were stimulated with 2 × 104 irradiated LEW.1W rat dendritic cells, incubated with 5 μg/mL control IgG, anti–rat CD28 Fab fragments, or CTLA4 immunoglobulin and processed as in panel A. Data are mean ± SD of 3 experiments. (C) LEW.1A rats received 1 mg modulating anti–rat CD28 JJ319 intraperitoneally at days 0 and 2, and spleens were collected at day 3; 105 purified T cells were then challenged with 2 × 104 irradiated allogenic dendritic cells without further addition of antibody in vitro. (D) Expression of CD28 on spleen cells from rat treated with modulating anti-CD28 mAb. The white histogram indicates the background level of fluorescence with secondary antibody alone; shaded histogram, spleen lymphocytes at day 3 from a rat treated with 1 mg anti-CD28 mAb at days 0 and 2; and black histogram, spleen lymphocytes at day 3 from a rat treated with 1 mg control IgG at days 0 and 2. *indicates significative at P < .05.
Specific CD28 blockade reduces human and rat T-cell proliferation in primary MLR.
(A) Human PBMCs or CD4+ T cells (105) were stimulated with 105 allogenic irradiated PBMCs with 10 μg/mL control IgG, anti–human CD28 Fab fragments, or 5 μg/mL CTLA4 immunoglobulin, and proliferation was measured after 5 days. Mean ± SD of triplicates from 1 of 8 representative experiments is shown. (B) Purified LEW.1A rat T cells (105) from spleen were stimulated with 2 × 104 irradiated LEW.1W rat dendritic cells, incubated with 5 μg/mL control IgG, anti–rat CD28 Fab fragments, or CTLA4 immunoglobulin and processed as in panel A. Data are mean ± SD of 3 experiments. (C) LEW.1A rats received 1 mg modulating anti–rat CD28 JJ319 intraperitoneally at days 0 and 2, and spleens were collected at day 3; 105 purified T cells were then challenged with 2 × 104 irradiated allogenic dendritic cells without further addition of antibody in vitro. (D) Expression of CD28 on spleen cells from rat treated with modulating anti-CD28 mAb. The white histogram indicates the background level of fluorescence with secondary antibody alone; shaded histogram, spleen lymphocytes at day 3 from a rat treated with 1 mg anti-CD28 mAb at days 0 and 2; and black histogram, spleen lymphocytes at day 3 from a rat treated with 1 mg control IgG at days 0 and 2. *indicates significative at P < .05.
Specific CD28 blockade reduces cytokine production in primary MLR.
Human PBMCs (105) were stimulated with 105allogenic irradiated PBMCs with 10 μg/mL control IgG, anti–human CD28 Fab fragments, or 10 μg/mL CTLA4 immunoglobulin. Total mRNA was extracted from 48 wells after 5 days, retrotranscribed, and analyzed by quantitative polymerase chain reaction. Results (mean ± SD of 3 measurements) are relative numbers of transcripts reported to HPRT.
Specific CD28 blockade reduces cytokine production in primary MLR.
Human PBMCs (105) were stimulated with 105allogenic irradiated PBMCs with 10 μg/mL control IgG, anti–human CD28 Fab fragments, or 10 μg/mL CTLA4 immunoglobulin. Total mRNA was extracted from 48 wells after 5 days, retrotranscribed, and analyzed by quantitative polymerase chain reaction. Results (mean ± SD of 3 measurements) are relative numbers of transcripts reported to HPRT.
Specific CD28 blockade reduces proliferation in secondary MLR.
Human PBMCs (107) were stimulated with 107allogenic irradiated PBMCs. After 7 days, cells were washed and cultivated for 3 days. Then 2 × 104 primed cells were restimulated with 2 × 104 donor-irradiated PBMCs with 10 μg/mL control IgG, anti–human CD28 Fab fragments, or 5 μg/mL CTLA4 immunoglobulin. Proliferation was measured after 3 days. Mean ± SD of triplicate results from 1 of 4 representative experiments is shown. *indicates significative at P < .05.
Specific CD28 blockade reduces proliferation in secondary MLR.
Human PBMCs (107) were stimulated with 107allogenic irradiated PBMCs. After 7 days, cells were washed and cultivated for 3 days. Then 2 × 104 primed cells were restimulated with 2 × 104 donor-irradiated PBMCs with 10 μg/mL control IgG, anti–human CD28 Fab fragments, or 5 μg/mL CTLA4 immunoglobulin. Proliferation was measured after 3 days. Mean ± SD of triplicate results from 1 of 4 representative experiments is shown. *indicates significative at P < .05.
Down-modulation of CD28 in vivo inhibits donor T-cell expansion and development of graft-versus-host disease
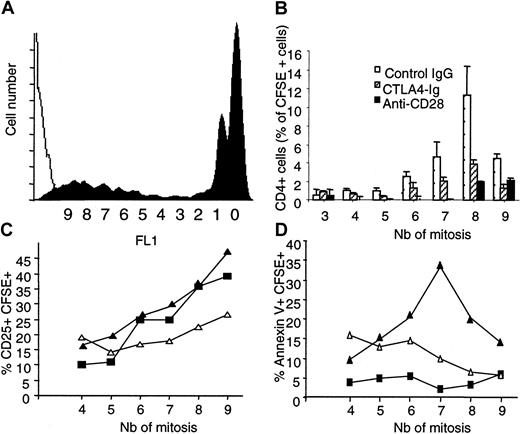
To investigate whether the reduced proliferation of allogeneic T cells by selective CD28 blockade we observed in vitro could also be evidenced in vivo, we followed up the fate and function of T cells that recognize recipient alloantigens after in vivo transfer. We used a model in which CFSE-labeled spleen cells from LEW.1A rats were transplanted into sublethally irradiated allogenic LEW.1W recipients. In this model, donor CD4+ T cells engraft, expand, and represent more than 95% of proliferating cells, resulting in lethal GVHD within 13 days. Sublethally irradiated recipients were treated with modulating anti-CD28 mAb, CTLA4 immunoglobulin, or mouse IgG. On day 3, CFSE+ donor cells in recipient spleen and mesenteric lymph nodes were analyzed for CFSE fluorescence intensity, expression of CD25, and binding of Annexin V on donor CD4+ T cells. Fifty percent of CFSE-labeled CD4+ T cells recovered after 3 days either had undergone no division (ie, their fluorescence was maximal) or had undertaken a single mitosis. Because halving of CFSE intensity was also found in syngenic controls on 50% of the cells, we considered one division as not related to allogenic stimulation. Homeostatic growth (more than one division) of syngenic CFSE-labeled CD4+ T cells in irradiated recipients was significant (10% or 15% of transplanted cells in mesenteric lymph nodes or spleen, respectively) but was weaker than the proliferating allogenic CD4+ T cells, which represented 35% or 40% of mononuclear cells in MLN (not shown) and spleen (Figure4A), respectively. Most (80%) of proliferating allogenic T cells underwent between 6 and 9 mitoses after 3 days.
Modulation of CD28 in vivo reduces allogenic T-cell proliferation in GVHD.
Spleen mononuclear cells from LEW.1A rats (3 × 108) were labeled with CFSE and transplanted intravenously into irradiated LEW.1W. Recipients received intraperitoneally 1 mg control IgG or modulating anti–rat CD28 JJ319 or 0.5 mg CTLA4 immunoglobulin at days 0 and 2. At 3 days, spleen lymphocytes were harvested and analyzed by FACS after gating on CD4+ cells. (A) The black histogram indicates the CFSE profile of control-treated recipients gated on CD4+ T cells; and the white histogram, FL1 signal of recipient CD4+ T cells from nontransplanted control. Y axis shows cell number per fluorescence channel. X axis shows halving of fluorescence after each mitosis. (B) The histogram indicates the measurement of alloreactive CD4+ T cells according to their division status. Data are mean ± SD from 3 animals. (C, D) Expression of CD25 and binding of Annexin V on proliferating CFSE+ cells. (▪) Recipients treated with control IgG. (▵) Recipients treated with CTLA4 immunoglobulin. (▴) Recipients treated with anti-CD28. Nb indicates the number of mitosis according to fluorescence intensity halving.
Modulation of CD28 in vivo reduces allogenic T-cell proliferation in GVHD.
Spleen mononuclear cells from LEW.1A rats (3 × 108) were labeled with CFSE and transplanted intravenously into irradiated LEW.1W. Recipients received intraperitoneally 1 mg control IgG or modulating anti–rat CD28 JJ319 or 0.5 mg CTLA4 immunoglobulin at days 0 and 2. At 3 days, spleen lymphocytes were harvested and analyzed by FACS after gating on CD4+ cells. (A) The black histogram indicates the CFSE profile of control-treated recipients gated on CD4+ T cells; and the white histogram, FL1 signal of recipient CD4+ T cells from nontransplanted control. Y axis shows cell number per fluorescence channel. X axis shows halving of fluorescence after each mitosis. (B) The histogram indicates the measurement of alloreactive CD4+ T cells according to their division status. Data are mean ± SD from 3 animals. (C, D) Expression of CD25 and binding of Annexin V on proliferating CFSE+ cells. (▪) Recipients treated with control IgG. (▵) Recipients treated with CTLA4 immunoglobulin. (▴) Recipients treated with anti-CD28. Nb indicates the number of mitosis according to fluorescence intensity halving.
Treatment of recipients with CTLA4 immunoglobulin reduced recovery of proliferating cells by 60% (10% of allospecific growth instead of 25% in control IgG-treated animals). Treatment of recipients with modulating anti-CD28 mAb was even more efficient and reduced recovery of proliferating cells by more than 80% (5% of allospecific growth instead of 25% in control IgG-treated animals) (Figure 4B). CD25 was expressed on 12% of host (CFSE−) mononuclear cells and of donor allogenic cells before 4 mitoses. The proportion of responding CD25+ cells increased with cycling. Treatment of recipients with CTLA4 immunoglobulin reduced the proportion of CD25-expressing cells by 40%, whereas treatment with anti-CD28 mAb did not (Figure4C). These data indicate that alloresponsive T cells are activated after several cycles of division and that a selective blockade of CD28 reduces the accumulation of proliferating cells but does not prevent the activation of the few cells that still divide. In contrast B7 blockade was less efficient at inhibiting the accumulation of dividing cells, but it reduced the activation of responding cells. Annexin V bound to less than 5% of host CFSE− cells in spleen or MLN. Binding to proliferating (CFSE weak) alloresponsive cells was similarly low. Treatment of recipients with CTLA4 immunoglobulin induced Annexin V binding on 10% to 15% of cells that underwent up to 6 divisions, and this level decreased to 5% after 8 divisions. In contrast, treatment with anti-CD28 mAb induced Annexin V binding on a proportion of cells increasing with proliferation. After 7 division cycles, 35% of cells were entering apoptosis. Interestingly, after 8 and 9 cycles of division (most proliferating allogenic cells were found at this stage), the percentage of Annexin V+ cells was reduced to reach 20% to 15% (Figure 4D). The effect of anti-CD28 treatment on allospecific growth was also evidenced by the partial prevention of GVHD lethality (data not shown). In conclusion, we found that the modulation of CD28 commits to apoptosis allogenic CD4+ T cells stimulated in vivo through the direct presentation pathway and that it reduces their proliferation.
In contrast to alloreactive responses, antibody responses to nominal antigens were not modified by selective down-modulation of CD28
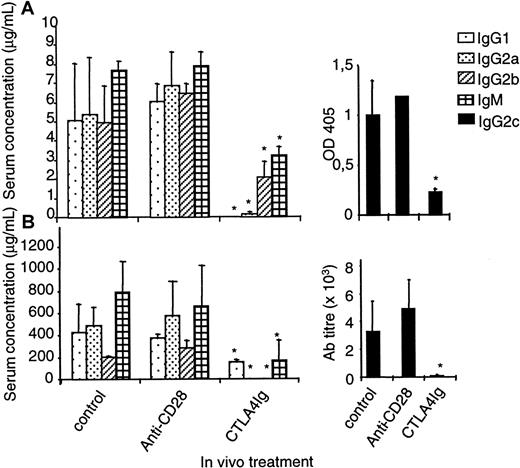
To compare the effect obtained using B7 antagonists with CD28 antagonists on antibody responses, we immunized rats intraperitoneally with 10 μg DNP-OVA without adjuvant or with 50 μg KLH subcutaneously in CFA and treated them with control IgG, CTLA4 immunoglobulin, or modulating anti-CD28 mAb every 2 days from day 0 until day 6 after immunization. Blood sampling at day 6 of animals treated with anti-CD28 mAb confirmed that CD28 had been fully modulated on PBMCs. Animals treated in parallel and killed on day 6 confirmed that a full modulation of CD28 expression was also obtained on spleen and draining lymph node T cells. Treatment with CTLA4 immunoglobulin inhibited antibody responses of all subclasses measured after 2 weeks, regardless of whether adjuvant was used in immunizations. In sharp contrast, no reduction, in any subclass, could be observed after treatment with anti-CD28 mAb, even when antigen was given without adjuvant (Figure 5A-B). Thus, the antibody response in the rat with fully modulated CD28 was normal.
Modulation of CD28 in vivo does not modify antibody responses.
Rats were immunized intraperitoneally with 10 μg DNP-OVA without adjuvant (A) or subcutaneously with 50 μg KLH in CFA (B). Recipients received intraperitoneally 1 mg control IgG or modulating anti–rat CD28 JJ319 or 0.5 mg CTLA4 immunoglobulin at days 0, 2, 4, and 6. Isotyping of the specific antibody responses was performed by enzyme-linked immunosorbent assay at day 12 (A) or 18 (B). *indicates significative at P < .05.
Modulation of CD28 in vivo does not modify antibody responses.
Rats were immunized intraperitoneally with 10 μg DNP-OVA without adjuvant (A) or subcutaneously with 50 μg KLH in CFA (B). Recipients received intraperitoneally 1 mg control IgG or modulating anti–rat CD28 JJ319 or 0.5 mg CTLA4 immunoglobulin at days 0, 2, 4, and 6. Isotyping of the specific antibody responses was performed by enzyme-linked immunosorbent assay at day 12 (A) or 18 (B). *indicates significative at P < .05.
Proliferative responses to KLH immunization are unmodified by specific CD28 down-modulation
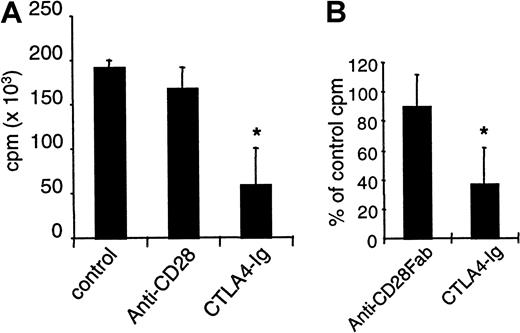
Draining lymph node cells from rats immunized with KLH in Complete Freund Adjuvant (CFA) were restimulated in vitro with KLH, and we measured proliferation after 3 days. Cells from rats treated with control IgG or with anti-CD28 mAb fully responded to this secondary stimulation, whereas proliferation was reduced by 75% if CTLA4 immunoglobulin was infused (Figure 6A). Moreover, the addition of anti-CD28 Fab fragments in vitro in KLH-restimulated cells from immunized untreated animals did not reduce proliferation, whereas CTLA4 immunoglobulin was effective in this setting (Figure 6B). Thus, proliferative responses to soluble antigens can be inhibited by B7 blockade but not by CD28 blockade.
Inhibition of CD28 does not modify proliferative responses to KLH immunization.
Rats were immunized subcutaneously with 50 μg KLH in CFA. Recipients received intraperitoneally 1 mg control IgG or modulating anti–rat CD28 JJ319 or 0.5 mg CTLA4 immunoglobulin, as indicated, at days 0, 2, 4, and 6. Draining lymph node cells were collected after 2 weeks and were restimulated in vitro with 2.5 μg/mL KLH. Proliferation was measured after 3 days. (A) Mean proliferation of nonrestimulated cells was 5000 cpm. (B) In cultures from immunized but control-treated animals, control IgG, anti-CD28 Fab (10 μg/mL), or CTLA4 immunoglobulin (10 μg/mL) was added, and proliferation was measured after 3 days. *indicates significative at P < .05.
Inhibition of CD28 does not modify proliferative responses to KLH immunization.
Rats were immunized subcutaneously with 50 μg KLH in CFA. Recipients received intraperitoneally 1 mg control IgG or modulating anti–rat CD28 JJ319 or 0.5 mg CTLA4 immunoglobulin, as indicated, at days 0, 2, 4, and 6. Draining lymph node cells were collected after 2 weeks and were restimulated in vitro with 2.5 μg/mL KLH. Proliferation was measured after 3 days. (A) Mean proliferation of nonrestimulated cells was 5000 cpm. (B) In cultures from immunized but control-treated animals, control IgG, anti-CD28 Fab (10 μg/mL), or CTLA4 immunoglobulin (10 μg/mL) was added, and proliferation was measured after 3 days. *indicates significative at P < .05.
Discussion
In this study, we investigated the role of CD28 in T-cell responses to alloantigens elicited by the “direct” presentation pathway and to nominal antigens presented in the context of self-MHC molecules. We compared the blockade of CD28 (using Fab fragments from antagonist antibodies or modulating mAb) with that of B7 (using CTLA4 immunoglobulin). Fab fragments do not induce signal transduction, yet they remain capable of efficient blockade of B7 binding.19
We show first that anti-CD28 Fab reduced the proliferation of CD4+ T cells in vitro elicited by the direct pathway of allorecognition. CD28 and B7 blockade reduced the production of Th1 and Th2 cytokines in primary MLR. A reduction in TH2 development after CD28 blockade is consistent with the role of CD28 in increasing IL-4 receptor sensitivity, which drastically promotes Th2 generation through the IL-4–mediated pathway.30 CD28 and B7 blockade were also effective in secondary stimulation (Figure 3), indicating that naive and primed alloreactive cells equally necessitate costimulation through CD28. In vivo, in a model of GVHD in which mitosis of alloreactive cells can be monitored, the administration of modulating anti-CD28 mAb is effective in the inhibition of allogenic T-cell proliferation. Proliferating cells had an activated phenotype: they expressed high levels of CD25 (and low levels of CD62L, not shown), regardless of whether modulating anti-CD28 mAb was given. Administration of CTLA4 immunoglobulin consistently reduced by 40% the percentage of CD25+ cells in proliferating cells (and increased in proportion the percentage of CD62L+cells, not shown). That B7 blockade and not CD28 blockade inhibits activation of alloreactive T cells, whereas CD28 blockade inhibits proliferation more strongly, suggests that B7 delivers an activation signal through a molecule other than CD28. Reports showing that CTLA-4 can promote costimulation in vivo7 8 suggest that a signal through CTLA-4 might promote activation in GVHD. Alternatively, a B7 ligand other than CTLA-4 and CD28, the existence of which was functionally demonstrated recently, might promote activation.
In contrast with data previously reported in mice,31proliferating rat T cells did not commit to apoptotic cell death in untreated, allogenic, irradiated recipients (Figure 4D). In anti-CD28–treated rats, however, it was clear that T cells became increasingly susceptible to apoptosis with each cycle of cell division. Above 8 divisions this susceptibility to apoptosis decreased and was correlated with a higher recovery of alloreactive cells. This commitment to apoptotic cell death with proliferation was not present with B7 blockade (Figure 4), suggesting that the difference lies in the unaltered CTLA-4–B7 interaction when anti-CD28 mAb, but not CTLA4 immunoglobulin, is used, which might promote the inhibition of cell division and cell death. Maximal apoptotic events were measured only after 7 divisions, which refers to reports showing that cross-linking of CTLA-4 on resting CD4+ T cells blocks transition from G0 to G1 and induces the antiapoptotic factor Bcl-xL,17whereas cross-linking CTLA-4 on activated CD4+ T cells induces Fas-independent cell death.18 However because of the complex and yet not fully clarified mechanisms of action of CTLA-4 in vivo that have been reported so far, ranging from costimulation7,8 to inhibition,32 a role for a free CTLA-4–B7 interaction that may inhibit allogeneic T-cell growth in our GVHD model remains speculative. Collectively, these data show that blocking costimulation through CD28 or B7 results in a reduction of alloreactive T-cell proliferation but that the mechanisms are different.
Soluble antigens are processed by self-APCs that expose antigenic peptides in association with self-MHC class 2 molecules. APCs then signal CD4+ T cells that proliferate and provide help for effector T-cell responses and antibody responses. Molecules stimulated by B7 appear essential for the development of the immune response to self-restricted presentation because CTLA4 immunoglobulin completely blocks the induction of T-dependent alloantibodies.15 Here, we found that a selective blockade of CD28 in the rat has no effect on self-restricted responses measured in vivo or ex vivo, whereas B7 blockade with CTLA4 immunoglobulin clearly inhibits this type of response in primary or secondary stimulation. The strength of immunization does not appear to determine whether CD28 is required as similar results were obtained in a weak (low dose without adjuvant) and in a strong (high dose in CFA) immunization protocol. This suggests at least that B7 molecules are instrumental in self-restricted responses to soluble antigens, independent of CD28, and it would mean that either B7–CTLA-4 interaction is paradoxically required for self-restricted responses to occur or that another, yet unidentified, ligand for B7 on T cells is necessary. The first hypothesis is refuted by numerous studies evidencing suppression of the production of multiple cytokines3 and cyclins33 after engagement of CTLA-4, which is clearly identified as a CD28 antagonist. However, other reports have shown that CTLA-4 can actually costimulate T-cell clonal expansion and the production of cytolytic T cells.7,8 The second hypothesis pertains to the data by Mandelbrot et al34 showing that B7-dependent costimulation can be evidenced in CD28–CTLA4 double-knockout mice.
Autoreactive T cells that serve as a pool of potentially pathogenic cells are positively selected in the thymus for their low affinity for peptides bound to self-MHC molecules expressed on cortical epithelial cells. Their low affinity is likely caused by the physico-chemical characteristics of the TCR-α CDR3 region, which tends to be cross-reactive and “sticky.”35 It is, therefore, tempting to attribute the effect of CD28 blockade on several autoimmune disorders reported in mice19,20,36,37 to the relatively low affinity of the interactions involved. Indeed, Teh and Teh38 recently reported that the dependence on CD28 for the efficient deletion of self-specific thymocytes is determined by the concentration, affinity/avidity, and length of exposure to the deleting ligand. In transplantation, a proportion of naive T cells can be activated by the recognition of either intact donor MHC–peptide complexes on allogenic APCs (direct pathway) or by peptides derived from allogenic molecules presented on self-MHC (indirect pathway). As much as 20% of peripheral T cells appear able to be contacted in such a direct way with allogenic APCs, but most of the TCR–MHC interactions do not reach an affinity threshold sufficient to result in full activation and proliferation.39 An interaction of substantially higher affinity is likely to drive alloreactive T-cell proliferation after direct contact with allogenic APCs. In this study, we show that CD28 blockade appears sufficient to modulate direct allogenic stimulation of T cells. Stimulation by superantigen, which mimics MHC framework–dependent allorecognition, can also be inhibited.40 Anti-CD28 treatment21 or use of CD28−/− recipients,41 however, prolongs survival but does not allow for long-term survival of allogenic grafts, confirming the idea that CD28 blockade is principally active on the direct pathway responsible for the initiation of rejection.
In conclusion, our data demonstrate that CD28 costimulation is required for full responses of CD4+ T cells to stimulation by allogenic APCs but that it is not required for responses to soluble antigens, whereas both types of responses require B7 molecules. Selectively blocking CD28 might be relevant for clinical transplantation because the direct presentation pathway of alloantigens can lead to acute rejection, but the induction of immune tolerance is thought to require a self-restricted response and a free CTLA-4–B7 interaction.
We thank C. Usal and S. Iyer for excellent technical assistance, G. Boulday and J. M. Heslan for technical advice, and R. Peach for purified CTLA4 immunoglobulin. We also thank P. Vusiau for biosensor analysis, F. Nisol and H. Bazin for anti–rat immunoglobulin mAb and DNP-OVA, and M. Brunet for irradiations.
Supported in part by the Fondation Progreffe and by the postgenome program, grant 109, of the French government (MENRT).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jean-Paul Soulillou, ITERT, INSERM U437, CHU Hotel Dieu, 30 Bld Jean Monnet, 44093 Nantes, France; e-mail address:bvanhove@nantes.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal