Abstract
Plasma cells (PCs) are the final B-cell differentiation stage. Recent evidence reveals relevant functional differences within the PC compartment. In rodents, early PCs formed in secondary lymphoid tissues show enhanced apoptosis and short life span, whereas PCs present in a final destination organ, such as the bone marrow (BM), have reached a stable prolonged survival state. BM PCs arrive at this organ as a circulating precursor whose cellular nature remains uncertain. An initial aim of this study was to characterize this circulating cell. We hypothesized that antibody-secreting cells detectable in the human blood after immunization might be a candidate precursor. These cells were obtained from the blood of volunteers immunized 6 days earlier with tetanus toxoid (tet), and they were unambiguously identified as PCs, as demonstrated by their expression of the CD38h phenotype, by morphology, by immunoglobulin (Ig) intracytoplasmic staining, and by IgG-tet–secreting capacity in vitro. In addition, by using the common CD38h feature, human PCs from tonsil (as a possible source of early PCs), from blood from tet-immunized donors (as the putative precursors of BM PCs), and from BM (as a deposit organ) have been purified and their phenotypes compared. The results show that a variety of differentiation molecules, proteins involved in the control of apoptosis, the B-cell transcription factors, positive regulatory domain I-binding factor 1/B lymphocyte-induced maturation protein 1 and B cell–specific activating protein and, at least partially, the chemokine receptor CXCR4 were expressed by human PCs following a gradient of increasing maturity in the direction: tonsil→blood→BM. However, PCs from these different organs showed a local pattern of adhesion molecule expression. These observations are discussed in light of the complex physiology of the human PC compartment.
Introduction
Plasma cells (PCs) are the morphologically well-defined cellular end point of the B-lymphocyte differentiation sequence, and, as such, they exhibit biochemical and structural features indicative of their full commitment to the synthesis and secretion of antibody (Ab). Therefore, PCs are ultimately responsible for the humoral immune response. Experiments in rodents have revealed that, soon after antigen (Ag) entry, PCs are formed in inductive territories of the secondary lymphoid tissue, initially within Ag-activated foci occurring in the T-cell areas of draining lymphoid tissue and, if the Ag persists, they are also generated in germinal centers (GCs).1-3 After this early phase, the number of PCs falls drastically in these areas and they start accumulating in final deposit locations, namely the bone marrow (BM) and the lamina propria (LP), for systemic and mucosal humoral responses, respectively.4-6 PCs present in the deposit organs are not formed in situ, but they derive from close precursors generated in distant lymphoid organs as a result of Ag stimulation, which migrate into these areas through the circulation.7,8 The nature of this PC precursor has not been fully clarified. It is well established that PCs present in the deposit organs are the cells responsible for the majority of Ig and Ab formation in vivo.4,6 Despite their morphologic and functional similarities, PCs contained in different organs show marked differences in several respects. Thus, experiments of 3H-thymidine incorporation in rats indicate that Ig-secreting cells from inductive areas (lymph nodes, spleen) show a life span of less than 3 days, whereas the life span of BM PCs is more than 3 weeks.9 More recently, experiments in mice using bromodeoxyuridine staining demonstrated the existence of long-lived (several months after immunization) Ab-secreting PCs found almost exclusively in the BM, whereas the majority of early formed Ab-secreting PCs present in peripheral lymphoid tissues exhibited a very short life span,10,11 and died soon after formation, apparently by apoptosis.5 In addition, differences in the number and quality of the IGVgene somatic mutations within the PC pool have been documented, indicative of a progressive enrichment in PCs that synthesize Ab of higher affinity in the deposit organ.12 13 Collectively, these data indicate that PC functional and maturational levels are not homogeneous, and that their capabilities increase from the early PC stage occurring in lymphoid secondary tissues, to the BM PCs. The mechanisms that determine these differences remain largely unclear.
Less is known about the formation and maturation of PCs in humans. Low numbers of PCs are present in similar inductive areas of the secondary lymphoid organs, and it is thought that they are the result of ongoing Ag activation.14 In addition, human BM becomes the major reservoir of Ig-secreting PCs in the systemic humoral immune response, and, as in animal models, human BM PCs are not generated in situ, but seem to be formed in inductive areas, and then reach the BM as circulating PC precursors.15 In this regard, following immunization to a large variety of Ags, specific Ab-forming cells generated in the local lymphoid tissues can be transiently detected in the human circulation.16-19 It is conceivable that these circulating Ab-secreting cells are the precursors of BM PCs. Furthermore, human Ig–forming cells from secondary lymphoid organs such as tonsil and lymph nodes exhibit short Ig secretion kinetics (3 days) in culture, whereas BM and LP PCs secrete Ig in vitro for more prolonged periods.16-23 In addition, human tonsillar PCs, as well as Ag-induced circulating Ab-forming cells or blood pre-PCs observed in reactive plasmacytosis, can undergo apoptosis either spontaneously or induced by CD95 cross-linking.24-26 In contrast, human BM PCs are not susceptible to this regulatory mechanism.25 Taken together, these data indicate the existence of relevant differences within the human PC compartment. Although their significance remains uncertain, these observations suggest the possibility that human PCs undergo their maturational progression in a way similar to that demonstrated for PCs in rodent models.
Terminal differentiation of B lymphocytes into the PC stage is under the control of several transcription factors, some of which have recently been identified. First, B cell–specific activating protein (BSAP), the product of the pax 5 gene, is a transcription factor expressed only by B-lymphoid cells, in which it plays an essential role in the B-cell lineage commitment,27 as well as in B-cell growth and survival during the ontogenic development and in the initial activation and differentiation of mature B lymphocytes.28 In fact, the capacity to express BSAP is lost only in the latter stages of B-cell differentiation.29 In contrast, the transcription factor positive regulatory domain I–binding factor 1 (PRDI-BF1), the murine homolog of which is known as Blimp-1 (B lymphocyte–induced maturation protein 1), is expressed only in the later B-cell differentiation stages as documented in both mouse and human systems,30-32where this factor appears to control a variety of aspects of the maturation program of PCs.30
A common feature of human PCs is the expression of high levels of CD38 molecules on their surface (CD38h cells),14and this marker has allowed their identification and, to a certain extent, their phenotypic characterization and isolation.21,24 33-35 In the present study, circulating Ag-induced Ab-secreting cells have been unambiguously identified as CD38h cells as well as PCs, and the common CD38h expression on human PCs has been used to perform a comparative and extensive phenotype analysis of: (1) PCs obtained from an inductive secondary lymphoid organ such as the tonsil, as a possible source of early PCs; (2) PCs released to the circulation after Ag immunization, as a possible transitional phase; and (3) normal BM PCs, as an example of PCs from a final destination organ. In addition, the expression of BSAP and PRDI-BF1/Blimp-1 messenger RNA (mRNA) by purified PCs from these 3 locations has been determined by reverse transcription–polymerase chain reaction (RT-PCR). The results of the phenotyping reveal that, within the PC compartment, a gradient of increasing maturity was observed in the direction: tonsil→blood→BM. In addition, PRDI-BF1/Blimp-1 was expressed in PCs from the 3 territories, but only PCs from inductive areas additionally expressed BSAP. Finally, PCs from these different organs exhibited distinctive patterns of adhesion molecule expression. These findings are discussed in light of the complex biology of the human PC.
Materials and methods
Materials
Cycloheximide was purchased from Sigma (St Louis, MO). Fluorescein isothiocyanate (FITC)–labeled monoclonal antibody (mAb) against CD11a, CD19, CD20, CD44, and CD45, phycoerythrin (PE)–labeled mAb against CD19, CD20, CD21, CD22, CD31, CD38, CD49d, CD54, CD62L, CD95, and HLA-DR, purified mAb against CD19 and Cy-Chrome (CyC)–labeled mAb against CD38 and the corresponding isotypic negative controls were provided by Becton Dickinson (San Jose, CA). EDTA was purchased from Pharmacia Biotech (Uppsala, Sweden). FITC-labeled mAb against CD9, PE-labeled mAb against CD40, CD50, and CD138, and purified mAb against CD31 and CD138 were provided by Coulter (Miami, FL). PE-labeled mAbs against the chemokine receptors CXCR4 and CXCR5 were purchased from R & D Systems (Minneapolis, MN). Goat anti–mouse IgG and goat anti-FITC magnetic microbeads, selection columns of MS+ and VS+ type and miniMACS and midiMACS magnets were obtained from Miltenyi Biotec (Auburn, CA). FITC-conjugated mAb against Bcl-2 and p63 (VS38c clone), FITC-conjugated rabbit polyclonal Ab against human Igs, and intrastain kit were from Dako (Glostrup, Denmark).
Purification of CD38h and B cells from different tissues
Tonsils were obtained from patients undergoing tonsillectomy for chronic tonsillitis. The tonsillar tissue was chopped into small pieces and the resulting cell suspension was washed in culture medium and separated into T- and non–T-cell populations by a previously reported rosette technique.16,22 The non–T-cell population consisted mostly of B cells (> 97% CD19+ cells) and was termed the B-cell fraction. CD31+ cells were purified from the B-cell fractions by an immunomagnetic activated cell selection (MACS) technique to enrich the CD38h cell subset, as previously reported.36 This CD31+ cell fraction was used as the starting point to achieve the final purification of CD38h cells by fluorescence-activated cell sorting (FACS). Heparinized blood was obtained from volunteers 6 days after tetanus toxoid (tet) booster immunization (5 LF) and peripheral blood mononuclear cells (PBMCs) were prepared by density centrifugation on Ficoll-Hypaque.22 B cells from this fraction (including CD38h cells) were purified by a MACS technique that used anti CD19-FITC mAb and goat anti-FITC magnetic microbeads. The resulting CD19+ cell fraction was used to achieve the final purification of CD38h cells by FACS. Bone marrow mononuclear cells (BMMCs) were prepared as reported,20,21 and CD38h cells were isolated by treatment with anti-CD138 mAb and 2 rounds of CD138+cell selection by MACS.26 Blood CD19+ cells from nonimmunized individuals were isolated by MACS (as for immunized volunteers). Approval was obtained from the institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Cell staining and flow cytometry analysis
Cells (200 μL cells at 0.5-5 × 106 cell/mL) were incubated with optimal concentrations of mAb for 20 minutes in the dark at 4°C. For Bcl-2 and VS38c, an intrastaining kit was used following the manufacturer's instructions. After 2 washes, FACS analysis was performed on a FACScalibur cytometer (Becton Dickinson) equipped with an air-cooled argon ion laser emitting 15 mW at 488 nm. The instrument was equipped with 3 fluorescence detector photomultiplier tubes, with green fluorescence (FITC) being collected through a 530/30-nm bandpass, red/orange (PE) through a 585/42-nm bandpass, and red (CyC) through a 650-nm longpass filter. Cell analysis was performed with CELLQUEST software (Becton Dickinson). Light scatter signals were recorded in linear mode and fluorescence signals in logarithmic mode. CD38h cells were gated on a CD20/CD38 dot-plot of tonsillar CD31+ cell fraction and on a CD19/CD38 dot-plot of blood CD19+ cell fraction and BMMCs. The third fluorescence was used to explore the phenotype of these PCs for differentiation markers (CD19, CD20, CD22, CD40, CD45, CD138, VS38c, and HLA-DR), survival factors (CD95 and Bcl-2), chemokine receptors CXCR4 and CXCR5, and adhesion molecules (CD9, CD11a, CD21, CD31, CD44, CD49d, CD50, CD54, and CD62L). Data from 2000 to 5000 CD38h cells/sample were collected, and the percentage as well as the mean fluorescence intensity (MFI) of the CD38hcells positive for each analyzed molecule were monitored. The results are expressed as the mean ± SEM of a variety of experiments. Statistical analysis was carried out by using the Student ttest. Differences were considered significant whenP < .05.
FACS separation and PC identification
Sorting of CD38h cells was performed on a FACStar-Plus cytometer (Becton Dickinson) equipped with an air-cooled argon ion laser emitting 100 mW at 488 nm. Fluorescence detector photomultiplier tubes and filters were similar to those described above. Tonsil CD31+ and blood CD19+ cells were labeled for CD20 (FITC) and CD38 (PE). CD38h cells were gated with CELLQUEST software and sorted in C-normal mode. To determine the nature of purified cells, sorted CD38h cells (0.1 × 106 cells in 100 μL phosphate-buffered saline) were cytocentrifuged on slides and stained by the Giemsa technique. The percentage of cells with PC morphology was determined by optical microscopy. Cells containing intracytoplasmic Igs were detected on cytospin cell preparations by a direct immunofluorescence technique. Positive cells were identified by fluorescence microscopy. Blood CD38h sorted cells and the remaining non-CD38hcells were also cultured at 5 × 103 cells in 0.2 mL, and secreted IgG-tet was determined in the culture supernatants by an enzyme-linked immunosorbent assay technique, as previously described.37 Tonsil GC and follicular mantle (FM) B-cell fractions were also purified by sorting of tonsil B cells contained in the CD38+ CD20high gate (GC B cells) and CD38−CD20+ gate (FM B cells), as reported elsewhere.23
Detection of the B-cell transcription factors PRDI-BF1 and BSAP by RT-PCR
The presence of transcripts for PRDI-BF1/Blimp-1 and BSAP was investigated in highly purified human B-cell fractions, including blood CD19 cells from nonimmunized healthy individuals, tonsil GC and FM B-cell fractions, and isolated PCs from tonsil, blood, and BM. To this end, total RNA from each cellular fraction was purified using the acid-guanidine-thiocyanate-phenol-chloroform method. After a DNAse I treatment, first-strand complementary DNA (cDNA) copies were synthesized by using Moloney leukemia virus reverse transcriptase (Roche Diagnostics, Barcelona, Spain) with oligo-dT as a primer in a total volume of 100 μL and then PCR was performed. The following oligonucleotide primers were used for PCR: for PRDI-BF1/Blimp-1 sense primer 5′-ATGCGGATATGACTCTGTGGA-3′ and antisense primer 5′-CTCGGTTGCTTTAGACTGCTC-3′; for BSAP (sense) 5′-CAGCATAGTGTCCACTGGCT-3′ and (antisense) 5′-CCTGTCAGCGTCGGTGCTGA-3′. cDNA (3 μL) was amplified in a PCR reaction (PTC-100 MJ Thermocycler, MJ-Research, Waltham, MA) using each primer and Taq DNA polymerase (Bioline, London, United Kingdom). The cycler conditions for PRDI-BF1/Blimp-1 and BSAP were a denaturing step at 94°C for 1 minute, an annealing step at 65°C for 1 minute, and an amplification step at 72°C for 1 minute, for 35 cycles, followed by a final additional amplification step at 72°C for 7 minutes. The amplified products were analyzed on a 1.2% agarose gel containing ethidium bromide and visualized by UV light illumination. The amounts of β-actin cDNA were evaluated, with sense primer 5′-TACCACTGGCATCGTGATGGACT-3′ and antisense primer 5′-CGTCACACTTCATGATGGAG-3′, as cDNA internal control. The cycler conditions for β-actin were as above, except that the annealing temperature was 62°C and only 30 cycles were performed.
Results
Identification of in vivo–induced IgG-tet–secreting cells present in the human blood as CD38h cells as well as PCs
It is well established that, a few days after in vivo immunization to a variety of Ag, specific Ab-forming cells are transiently detected in the human circulation.16-19 This Ab-secreting cell has not yet been clearly identified. The CD38hphenotype is a feature exhibited by human Ab-secreting cells14 and, consequently, the proportion of CD38h cells was monitored in the blood of healthy volunteers before and 6 days after a conventional tet booster, when IgG-tet–secreting cells are maximally found in the circulation.16 Blood samples obtained before the booster showed either undetectable or very low numbers of CD38hcells (< 0.03% of the blood mononuclear cells), whereas in the samples taken after the booster (postboost), a clear increase of this population was detected (0.60% ± 0.1%; mean ± SEM; n = 11). Initial phenotypic analysis of these postboost CD38h cells showed that they were also CD19+. As a consequence, a protocol was designed to isolate blood postboost CD38h cells that included a first pre-enrichment step using immunomagnetic selection of CD19+ cells, followed by a FACS for CD38h cells. Postboost CD19+-selected cells exhibited a marked increase in the proportion of CD38hcells (22.4% ± 9.1%; mean ± SEM; n = 6). FACS selection of CD38h cells in the latter population yielded an extremely enriched population of CD38h cells. Figure1 (middle panel) shows an example of this isolation method. As can be seen, CD38h cells isolated from the blood of immunized individuals exhibited intense staining for intracytoplasmic Ig and most of them (96.5% ± 1%; mean ± SEM; n = 4) showed a clear PC morphology, as determined by Wright-Giemsa staining of cytocentrifugal preparations. To test the relationship between the CD38h cells isolated from the blood and the tet immunization, purified PCs and the rest of the blood cells (devoid of CD38h cells) were cultured and their capacity to produce IgG-tet was evaluated in the supernatants. IgG-tet secretion was detected only in the supernatant of cultured PCs (12 and 66 ng/mL versus < 0.05 and < 0.05 ng/mL, for cultured purified CD38h cells versus non-CD38h cells, respectively, in 2 different experiments).
Purification of human PCs from different territories.
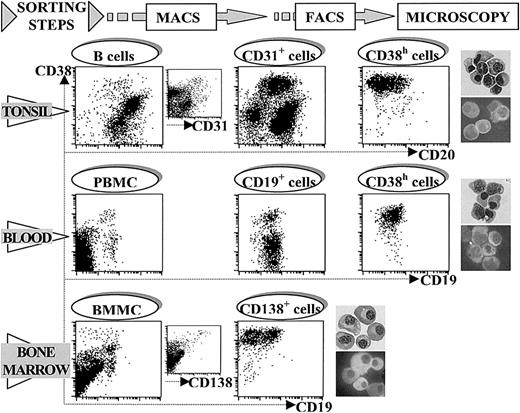
The figure shows a representative example of the consecutive steps followed in the protocol used for the purification of PCs from tonsil (CD20/CD38 staining, upper panel), blood (CD19/CD38 staining, middle panel), and BM (CD19/CD38 staining, lower panel). Tonsil CD38h cells were pre-enriched by treatment with anti-CD31 mAb and MACS, and this cell fraction was the starting point in isolating tonsil CD38h cells by FACS. PBMC from tet-immunized volunteers were used in a first step of blood CD38h cell pre-enrichment by treatment with anti-CD19 mAb and MACS. Blood CD38h cells were finally purified from this CD19+ cell fraction by FACS. BM CD38h cells were isolated from BMMCs in a single step by treatment with anti-CD138 mAb and MACS. In the 3 territories CD38h cells were identified as PCs by Giemsa staining as well as intracytoplasmic Ig staining of cytospin preparations of purified CD38h cells (upper and lower photograph, respectively), in each territory. Original magnification × 300.
Purification of human PCs from different territories.
The figure shows a representative example of the consecutive steps followed in the protocol used for the purification of PCs from tonsil (CD20/CD38 staining, upper panel), blood (CD19/CD38 staining, middle panel), and BM (CD19/CD38 staining, lower panel). Tonsil CD38h cells were pre-enriched by treatment with anti-CD31 mAb and MACS, and this cell fraction was the starting point in isolating tonsil CD38h cells by FACS. PBMC from tet-immunized volunteers were used in a first step of blood CD38h cell pre-enrichment by treatment with anti-CD19 mAb and MACS. Blood CD38h cells were finally purified from this CD19+ cell fraction by FACS. BM CD38h cells were isolated from BMMCs in a single step by treatment with anti-CD138 mAb and MACS. In the 3 territories CD38h cells were identified as PCs by Giemsa staining as well as intracytoplasmic Ig staining of cytospin preparations of purified CD38h cells (upper and lower photograph, respectively), in each territory. Original magnification × 300.
Figure 1 also shows the procedure used for the purification of tonsil and BM PCs. The upper panel shows that tonsil PCs could be isolated by using a previously reported technique that included a first pre-enrichment step using immunomagnetic selection of CD31+cells (from 1.67 ± 0.3 to 14.2 ± 1.4; mean ± SEM; n = 14), followed by FACS of CD38h cells.35With this method, a high purification of tonsil PCs, as determined by morphologic criteria, was obtained (96.7% ± 0.9%; mean ± SEM; n = 4). Figure 1, lower panel, shows the technique used to isolate BM PCs. The initial proportion of CD38h cells in the BMMC fraction was 0.44% ± 0.1% (mean ± SEM; n = 12). BM CD38h cells also expressed high levels of CD138. After 2 rounds of MACS selection of CD138+ cells, the proportion of PCs as defined by morphologic criteria reached 97.7% ± 0.9% (mean ± SEM; n = 4). Morphologically, the lymphoplasmocytic type of PC showing a denser nucleus and less developed cytoplasm was more frequent in purified PCs from tonsil and blood. In contrast, typical PCs, with chromatin peripherally condensed and prominent arcoplasm, were the major population in purified BM PCs. In the 3 territories, the proportion of PCs contained in the non-CD38h cell populations remained below 0.1%.
Comparative analysis of differentiation and survival marker expression by human PCs from different organs
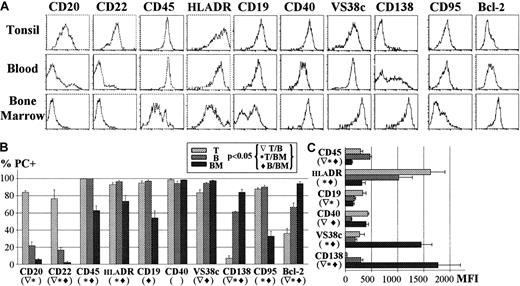
Once the correspondence between CD38h phenotype and PC morphology had been demonstrated for the 3 organs under study, the former feature was used for a comparative analysis of the expression of a wide variety of differentiation and survival markers on PCs from tonsil, blood, and BM. To obtain a clear resolution of CD38h/PCs, these experiments were carried out using tonsil CD31+ cells, blood CD19+ cells, and BMMC fractions. A representative example of this comparison is shown in Figure 2 (upper panel). The results representing the percentage of positive PCs and MFI of expression for a series of experiments are summarized in the lower panel of Figure 2(left and right graphics, respectively). As can be seen, the expression of the B-cell markers CD20 and CD22 was positive in tonsil PCs, but disappeared in blood and BM PCs. CD45 and DR also showed a decreasing pattern of expression, although a clear reduction was observed only in BM PCs. CD19 was expressed by all tonsil and blood PCs, but was negative in one half of the BM PCs. CD40 was exhibited by PCs from the 3 organs, although a slightly reduced level of expression was observed in the blood PCs. VS38c mAb recognizes p63 protein of the endoplasmic reticulum and has been previously described as a good marker for PCs.38 This molecule was similarly expressed by tonsil and blood PCs, but its expression was enhanced by an average of 6 fold in BM PCs. The expression of CD138 (syndecan 1), a molecule clearly associated with the acquisition of the PC stage,26,29 was low in tonsil PCs and intermediate in blood PCs, and showed an increase of up to 8 times in BM PCs. Finally, 2 molecules that regulate cell survival, the antiapoptotic protein bcl-239 and the death receptor CD95,40 were also examined. Tonsil and blood PCs exhibited low but detectable levels of CD95, whereas most BM PCs failed to express CD95, as previously reported.25 In contrast, the expression of bcl-2 was lower for tonsil PCs, intermediate for blood PCs, and higher for BM PCs.
Compared differentiation and survival molecule expression by tonsil, blood, and BM PCs.
(A) The figure shows a representative example of tonsil (T), blood (B), and BM PC expression of several differentiation molecules (CD20, CD22, CD45, HLA-DR, CD19, CD40, VS38c, CD138) and survival-associated molecules (CD95, Bcl-2). The mean ± SEM of several experiments (n ≥ 5) for each marker expressed as the percentage is shown in part B and the MFI of positive PCs is shown in part C.
Compared differentiation and survival molecule expression by tonsil, blood, and BM PCs.
(A) The figure shows a representative example of tonsil (T), blood (B), and BM PC expression of several differentiation molecules (CD20, CD22, CD45, HLA-DR, CD19, CD40, VS38c, CD138) and survival-associated molecules (CD95, Bcl-2). The mean ± SEM of several experiments (n ≥ 5) for each marker expressed as the percentage is shown in part B and the MFI of positive PCs is shown in part C.
Expression of the transcription factors BSAP and PRDI-BF1/Blimp-1 by PCs isolated from tonsil, blood, and BM
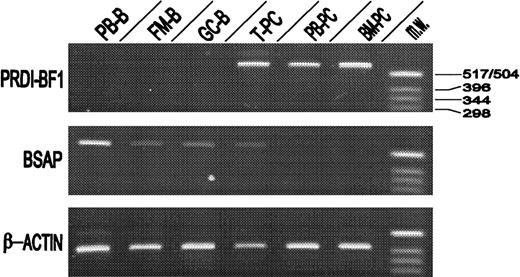
The presence of transcripts for BSAP and PRDI-BF1/Blimp-1 transcription factors was investigated by RT-PCR. To this end, tonsil, blood, and BM PCs were highly purified by the method described in Figure 1. As can be seen in Figure 3, isolated peripheral blood B lymphocytes, FM B cells, and GC B cells clearly expressed BSAP but did not contain PRDI-BF1/Blimp-1. In contrast, the 3 PC populations clearly expressed PRDI-BF1/Blimp-1, but only tonsil PCs additionally expressed BSAP. Identical results were obtained in 3 separate experiments.
Messenger RNA for PRDI-BF1/Blimp-1 and BSAP expression levels in different B-cell fractions.
Relative amounts of PRDI-BF1/Blimp-1 and BSAP mRNAs were determined by RT-PCR from highly purified peripheral blood B lymphocytes (PB-B), follicular mantle B cells (FM-B), germinal center B cells (GC-B), tonsil plasma cells (T-PC), peripheral blood plasma cells (PB-PC), and bone marrow plasma cells (BM-PC). RT-PCR using β-actin mRNA sequences served as an internal standard. As molecular weight marker aHinfI digestion of pBR322 was used (MW). Data are representative of the results of 3 experiments using different donors.
Messenger RNA for PRDI-BF1/Blimp-1 and BSAP expression levels in different B-cell fractions.
Relative amounts of PRDI-BF1/Blimp-1 and BSAP mRNAs were determined by RT-PCR from highly purified peripheral blood B lymphocytes (PB-B), follicular mantle B cells (FM-B), germinal center B cells (GC-B), tonsil plasma cells (T-PC), peripheral blood plasma cells (PB-PC), and bone marrow plasma cells (BM-PC). RT-PCR using β-actin mRNA sequences served as an internal standard. As molecular weight marker aHinfI digestion of pBR322 was used (MW). Data are representative of the results of 3 experiments using different donors.
Comparative analysis of the pattern of expression of adhesion molecules and chemokine receptors by PCs from tonsil, blood, and BM
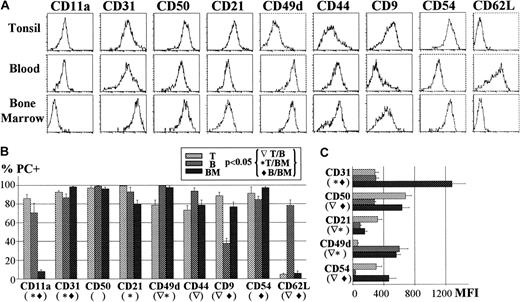
In the last series of experiments, the pattern of adhesion molecule expression exhibited by PCs from the 3 different locations was determined as in Figure 2. A representative example of this comparative study is depicted in the upper panel of Figure 4. Results representing the percentage and MFI of positive PCs in several experiments are summarized in the lower panel of Figure 4 (left and right graphic, respectively). As can be seen, CD11a was expressed, although at low level, by tonsil and blood PCs, and was virtually absent from BM PCs. CD21 was positive in the PCs from the 3 tissues, but its level of expression was higher for tonsil PCs. Very high expression of CD31 was observed on PCs from the 3 territories, although BM PCs showed a 4-fold increase in the expression level of this molecule. Likewise, CD49d, although positive in all PCs, was maximally expressed by the blood and BM populations. A different pattern of expression was found for CD9, CD50, and CD54, which were equally detected in tonsil and BM PCs, but their expression decreased in blood PCs. In contrast, CD62L was absent in tonsil and BM PCs, but it was present on most blood PCs. Finally, the expression of the chemokine receptors CXCR4 and CXCR5 was also explored in human PCs. Table 1 shows that CXCR4 expression was up-regulated in increasing proportions of PCs, according to the axis tonsil→blood→BM. Nevertheless, this phenomenon appeared to be partial because only a fraction of the BM PCs were positive for this receptor. In contrast, only a marginal expression of CXCR5 was observed in PCs from the 3 tissues.
Compared adhesion molecule expression by tonsil, blood, and BM PCs.
(A) The figure shows a representative example of tonsil (T), blood (B), and BM PC expression of several adhesion molecules (CD11a, CD31, CD50, CD21, CD49d, CD44, CD9, CD54, CD62L). The mean ± SEM of several experiments (n ≥ 5) for each marker expressed as the percentage as shown in part B and as the MFI of positive PCs as shown in part C.
Compared adhesion molecule expression by tonsil, blood, and BM PCs.
(A) The figure shows a representative example of tonsil (T), blood (B), and BM PC expression of several adhesion molecules (CD11a, CD31, CD50, CD21, CD49d, CD44, CD9, CD54, CD62L). The mean ± SEM of several experiments (n ≥ 5) for each marker expressed as the percentage as shown in part B and as the MFI of positive PCs as shown in part C.
CXCR4 and CXCR5 expression by human PC (CD38hcells) from tonsil, blood, and BM*
| . | . | Tonsil . | Blood . | BM . | P . | ||
|---|---|---|---|---|---|---|---|
| T/B . | B/BM . | T/BM . | |||||
| No. | 4 | 5 | 5 | ||||
| CXCR4 | % | 11 (2.7)† | 21 (1.5) | 40 (12) | NS | .007 | .013 |
| MFI | 92 (16) | 92 (53) | 121 (42) | NS | NS | NS | |
| CXCR5 | % | 11 (5) | 12 (1) | 9 (3) | NS | NS | NS |
| . | . | Tonsil . | Blood . | BM . | P . | ||
|---|---|---|---|---|---|---|---|
| T/B . | B/BM . | T/BM . | |||||
| No. | 4 | 5 | 5 | ||||
| CXCR4 | % | 11 (2.7)† | 21 (1.5) | 40 (12) | NS | .007 | .013 |
| MFI | 92 (16) | 92 (53) | 121 (42) | NS | NS | NS | |
| CXCR5 | % | 11 (5) | 12 (1) | 9 (3) | NS | NS | NS |
T indicates tonsil; B, blood; BM, bone marrow; NS, not significant.
Mean (SEM).
Discussion
Plasma cells have been generally considered the final and homogenous stage of B-lymphocyte differentiation. However, experimental data accumulated in recent years from both rodents and humans demonstrate that PCs are a complex cell compartment comprising cell subsets differing in several significant respects. In rodent systems, increasing evidence indicates that early PCs formed in inductive areas of secondary lymphoid organs show an enhanced tendency to undergo apoptosis and, as a consequence, are short-lived, whereas BM PCs, which originate from undetermined circulating precursors, exhibit a markedly prolonged life span and improved Ab affinity.5,9-11Therefore, it is reasonable to think that human PCs might follow a similar maturational sequence. In fact, the differences observed in the capacity to undergo apoptosis and in the kinetics of Ig secretion by human PCs from different tissues16-22,25 also support this view. In this context, the circulating precursor of BM PCs might represent a transitional stage between early PCs and BM PCs. Because the nature of these precursors has not been fully clarified, we hypothesized that human Ab-secreting cells that are transiently present in the blood after immunization16-19 might have reached the PC stage and might be the precursors of BM PCs. Accordingly, the initial aim of the present study was to characterize this cell population. Thus, prior to a conventional tet immunization, the frequency of blood cells exhibiting the CD38h phenotype, a feature ascribed to human PCs in other organs,14 was extremely low, whereas their number increased by at least 20 times in the sample obtained 6 days after the tet booster, when the presence of IgG-tet–secreting cells is maximally detected.16 These tet-induced CD38hcells were isolated using a 2-step protocol that combined immunomagnetic selection of CD19+ cells followed by FACS of CD38h cells. These highly purified cells could be clearly identified as PCs by both morphologic criteria and analysis of intracytoplasmic Ig content. Furthermore, IgG-tet secretion was confined to this PC population, indicating their relationship with the ongoing antitet response in vivo. This is the first time that Ab-secreting cells that transiently circulate after in vivo Ag stimulation have been purified and clearly identified as PCs. The tet-induced increase of blood CD38h cells fell back to prebooster values by day 21 after the immunization (data not shown), a time frame that is concordant with the kinetics of PC appearance in the BM in animal models of humoral response10,12 13 and, accordingly, supports the view that the Ag-induced circulating PCs might be the BM PC precursor.
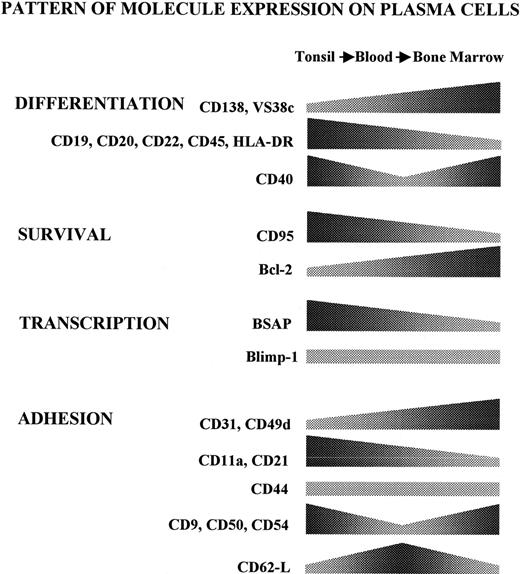
In parallel experiments, PCs were also isolated from a secondary lymphoid organ such as the tonsil, as well as from a deposit organ such as the BM, by previously reported procedures.26,36 The common CD38h feature demonstrated for the PC obtained from tonsil, blood, and BM allowed an assessment of a variety of molecules expressed by these cell subsets. Although studies of this kind have been previously reported for individual organs,24 33-35 a broad comparative analysis of PCs from these 3 locations has not been consistently performed before. In addition, the inclusion of Ag-induced circulating PCs in this analysis made it possible to identify a putative transitional stage between the early PCs formed in inductive organs (tonsil) and those PCs that have reached the BM. The comparison of B-cell differentiation molecules revealed the existence of important differences among PCs from the 3 tissues under study. Figure 5 gives a schematic summary of all these changes. Thus, tonsil PCs were CD19+CD20low CD22+ CD45+ DR+VS38c+ CD138−/low. This profile of differentiation marker expression was also observed in PC populations obtained from 2 lymph node and spleen samples (data not shown), which suggested a common stage for early PCs formed in secondary lymphoid tissues. On the other hand, Ag-induced circulating PCs showed the profile CD19+ CD20− CD22−CD45+ DR+ VS38c+CD138−/+, and the BM PCs exhibited the phenotype CD19+/− CD20− CD22−CD45+/− DR+/− VS38chighCD138high. According to the data, there appears to be a pattern of gradual decrease in the expression of several markers, including CD20, CD22, CD45, DR, and CD19, suggesting a possible process of transition of PCs in their passage from the tonsil, via the blood, to the BM. This transition process is also indicated by the changes detected in the expression of VS38c and CD138, 2 well-established maturational markers of PCs, which showed a gradual increase in the direction: tonsil→blood→BM.
Compared expression of the studied molecules in human PCs.
The figure shows a schematic representation of the different phenotypic patterns displayed by PCs along the axis tonsil→blood→BM. The various trends have been simplified, combining percentage and MFI data of positive cells.
Compared expression of the studied molecules in human PCs.
The figure shows a schematic representation of the different phenotypic patterns displayed by PCs along the axis tonsil→blood→BM. The various trends have been simplified, combining percentage and MFI data of positive cells.
The comparison of the PC expression of molecules involved in the regulation of cell survival also revealed a maturational transition within the PC compartment (Figure 5). Thus, early PCs obtained from tonsil, as well as tet-induced circulating PCs, expressed low but detectable levels of the death receptor CD95, a molecule almost totally absent from the BM PCs. In addition, the expression of the antiapoptotic protein bcl-2 was lower in tonsil PCs, intermediate in blood PCs, and maximal in normal BM PCs. Thus, early PCs obtained from inductive organs appear to express molecules that confer propensity to undergo apoptosis, whereas PCs that home to the BM seem to express a nonapoptotic phenotype. These data are in good agreement with previous reports that demonstrate an increased tendency to develop cell death by early and reactive PCs occurring in secondary lymphoid tissues and in the circulation, but not by BM PCs.24-26 Furthermore, experimental models of humoral responses induced in mice revealed the occurrence of massive cell death by apoptosis in early PCs.5 Therefore, the acquisition of a nonapoptotic phenotype appears to be an important hallmark in PC maturation, and such a state seems to be largely confined to those PCs that reach deposit organs such as the BM. It is of interest that, in Ag-stimulated mice, only a residual number of long-lived PCs remain in the spleen,10,41 and this state has been connected with the existence in the PC vicinity of auxiliary dendritic cells apparently required for PC survival, and whose number is limited in this nondeposit organ.41,42 In this regard, the apoptosis of human tonsil and blood PCs can be delayed by coculturing these cells with BM stromal cells.24 35 Further work will be needed to clarify the significance of extensive cell death by early PCs as well as the selective mechanisms involved in the transition into the nonapoptotic and long-living PC compartment.
The transcription factor PRDI-BF1/Blimp-1 is expressed in B lymphocytes at their latest stages of maturation in humans and mice,30-32 and its presence in this phase determines many features typical of the PC differentiation program.30 In contrast, the ectopic expression of this factor before they reach this maturational stage drives apoptotic signals to B lymphocytes.43 The present results demonstrate the presence of apparently similar quantities of mRNA for this factor in human PCs purified from the 3 territories under study, which is in agreement with previous immunohistochemical data.32Differentiation to PCs has also been connected with the disappearance of BSAP. The relevant role of the down-regulation of BSAP during PC maturation is emphasized by the observation that its enforced expression blocked the transition to PCs.44 Thus, the progression into the PC stage seems to be regulated by the opposing balance of these 2 factors. The observation that purified tonsil PCs still retained detectable quantities of mRNA for BSAP reinforces the notion that early PCs formed in inductive areas of secondary lymphoid tissues are immature and suggests that the complete loss of BSAP might be a requisite for the triggering of the terminal PC maturation program.
Collectively, the results strongly indicate the existence of distinguishable PC stages that predominate in each of the 3 different organs under study, suggesting that the human PC differentiation program requires the transition through successive maturational and migratory steps. Despite this finding, certain levels of heterogeneity do exist in the 3 PC populations analyzed. For example, BM PCs consist of CD19+ and CD19− cell subsets and comprise cells that exhibit a very heterogeneous labeling for CD45 or DR. The same is also true for blood PC CD138 expression. These observations indicate that PC populations are not totally homogenous and that, to some degree, mixed cell subsets might be present in these organs.
The present study was also focused on the pattern of adhesion molecule expression exhibited by human PCs. Although this issue has been explored previously (for reviews, see Helfrich et al45 and Thomas and Witzing46), a broad comparative analysis of normal PCs from different territories has not been as yet carried out. Therefore, in an attempt to delineate their distinctive migratory and homing capacities, the presence of these molecules on human tonsil, blood, and BM PCs was compared. An initial conclusion is that these cells largely differed in the type and quantity of adhesion molecules expressed on their surface (changes summarized in the scheme of Figure5). Thus, at one extreme, tonsil PCs exhibited the profile CD11a+ CD21+ CD31+ and CD49dlow, whereas, at the other extreme, the BM PC profile was CD11a− CD21low CD31high and CD49dhigh, with blood PCs showing an intermediate phenotype, a finding suggestive of the latter cells being a transitional PC stage. In addition, tonsil and BM PCs shared high expression of CD9, CD50, and CD54, 3 molecules that were absent or very slightly expressed by circulating PCs. This observation probably relates these 3 molecules with the common sessile status of tonsil and BM PCs. Thus, in addition to these 3 molecules (CD9, CD50, and CD54), CD11a, CD21 and, perhaps, CD22 appear to be important for tonsil PC localization, whereas CD49d, CD31, and CD138 seem to be involved in the specific PC attachment to the BM microenvironment. CD44 was the only adhesion molecule that showed a constant level of expression. CD62L was uniquely expressed in the circulating stage of PCs. It is unlikely that this finding connects blood PCs to a migratory pathway into lymph nodes through high endothelial venules, apparently a major role of this selectin,47 because experiments in rodents have demonstrated that Ag-induced PCs migrate to BM, but not to distant lymph nodes.48 Accordingly, CD62L on circulating PCs would contribute to the homing of these cells to the BM, by recognizing some as yet undetermined ligands. In this regard, CD62E is a known ligand for CD62L molecules expressed on certain cells,49,50 and CD62E is a molecule constitutively expressed by human BM endothelial cells.51 Therefore, this could be a possible ligand for the CD62L present on circulating PCs. Collectively, all these data revealed that the adhesion protein pattern exhibited by PCs is markedly different depending on the territory from where they originate, which must be of relevance to understanding how PCs become attached to different microenvironments. This is of special interest because these cells appear to undergo, concomitantly with their maturational progression, a migratory pathway through the circulation, from the inductive organs into the final destination organs. In this regard, the relevant role that chemokines and their receptors play in the localization of lymphoid cells is now clear.52 Namely, it has been recently reported that, compared with their B-cell precursors, mouse PCs down-regulate CXCR5 and increase their sensitivity to CXCL12, the ligand for CXCR4. Moreover, PCs from chimeric mice reconstituted with CXCR4-deficient immune system accumulate in peripheral lymphoid organs and in the blood, but do not home at the BM.53These findings indicate that CXCR4 plays a role in enabling PC localization into the BM in mice. Accordingly, the expression of these 2 chemokine receptors was examined in human PCs. Present results indicate that CXCR5 is also down-regulated in the human PC compartment. In addition, the expression of CXCR4 by human PCs increased following the direction tonsil→blood→BM, although only a fraction (40%) of the human BM PCs expressed this receptor. These results do not allow a clear role to be established for CXCR4 in the homing of human BM PC, because several possibilities could be invoked to explain this observation: (1) CXCR4 might be required only in the initial steps (endothelium recognition, diapadesis into a PC-specific microenvironmental niche), but this receptor might be lost after homing (CXCR4− cell subset); (2) there might exist 2 different mechanisms of BM PC homing, one dependent on CXCR4, the other not; (3) CXCR4− PCs might comprise a pool of recirculating PCs; and (4) the possibility that CXCR4 is not required for human BM PC homing is less probable. Therefore, further work will be required to ascertain the role of these distinctive molecule expression profiles in the human PC migration physiology.
The authors thank J. Martorell for his help in morphologic plasma cell identification and E Roldán for fruitful discussion.
Supported by grants 94/0498, 96/2116, and 01/1590 from Fondo de Investigaciones Sanitarias of Spain.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
José A. Brieva, Servicio de Inmunologı́a, Hospital Universitario Puerta del Mar, Avenida Ana de Viya 21, 11009 Cádiz, Spain; e-mail:jabrieva@hpm.sas.junta-andalucia.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal