Abstract
In a prospective cohort study, we assessed the incidence of spontaneous and risk period–related venous thromboembolism (VTE) in asymptomatic family members of patients who experienced VTE and had the factor V Leiden mutation. In all, 561 family members of 131 probands were included, 313 of whom were carriers (299 heterozygous and 14 homozygous) and 248 of whom were noncarriers of the factor V Leiden mutation. Average follow-up was 4 years (range, 4 months-6 years). There were 1255 and 984 observation-years of follow-up in carriers and noncarriers, respectively. Eight episodes of VTE occurred in heterozygous carriers, resulting in an annual incidence of 0.67% (95% confidence interval [CI], 0.29-1.33). Two events occurred in the absence of associated risk factors, determining an annual incidence of spontaneous VTE of 0.17% (95% CI, 0.02-0.6). Only one VTE (risk period–related) occurred in noncarriers, with an annual incidence of 0.1% (95% CI, 0.003-0.56). Relative risk for VTE in heterozygous carriers compared with noncarriers of the factor V Leiden mutation was 6.6 (95% CI, 1.1-39.8). Risk period–related VTE occurred with an incidence of 18% and 5% per risk period in heterozygous carriers and in noncarriers, respectively. Thus, the low rate of VTE in asymptomatic family members carrying the mutation did not justify continuous anticoagulant prophylaxis. Screening families of symptomatic probands with the factor V Leiden mutation has the potential to identify those asymptomatic carriers who might benefit from thromboprophylaxis during risk periods.
Introduction
Factor V Leiden mutation (factor V Arg506→Gln) is the most common genetic defect associated with an increased risk for venous thromboembolism (VTE).1-3 Its prevalence in the white population is approximately 5% and is as high as 20% to 40% in patients with documented VTE, depending on selection criteria. Case-control studies have shown that heterozygous carriers of this mutation exhibit a 3- to 7-fold increased risk for VTE than healthy controls.1,4-6 The association of factor V Leiden with other coagulation abnormalities predisposing to thrombosis, including deficiencies of antithrombin, protein C, protein S, hyperhomocystinemia, and the prothrombin variant G20210A may further increase such risk.7-10 Most persons identified as carriers of the factor V Leiden mutation as a result of family studies, however, do not exhibit other associated coagulation defects.
Benefits from an early identification of carriership status among asymptomatic subjects in terms of appropriate prophylactic measures should be balanced against the disadvantage of potential bleeding complications secondary to preventive treatment and the inconvenience of labeling otherwise healthy people as having a disease. Thus, valid estimates of the absolute risk for spontaneous and risk period–related VTE in asymptomatic family members who are carriers of the factor V Leiden mutation are crucial for clinical decision making. According to the results of 2 recent retrospective cohort studies involving a large number of families with the factor V Leiden mutation,11,12the overall annual incidence of VTE in carriers of this mutation was found to be approximately 0.50% in persons older than 15 years of age. This rate is approximately 5 times higher than that reported for the general population.13
In this prospective follow-up study, we assessed the absolute risk for spontaneous and risk period–related VTE in a wide number of asymptomatic family members of probands who were carriers of factor V Leiden and had experienced a thrombotic episode.
Patients and methods
Identification of cohorts
All consecutive patients treated at our Thrombosis Center at Padua University-Hospital with a documented episode of VTE were screened for deficiencies of antithrombin, protein C, protein S, and the presence of factor V Leiden mutation. Patients with a single identified factor V Leiden mutation served as index patients for this study. Because prothrombin variant 20210A was not yet known at the time of study planning, this test was not routinely performed in our study population. However, assessment of this abnormality was systematically performed in all study patients with symptomatic events. If it was confirmed, identified patients were removed from the analysis.
All consenting family members of index patients older than 15 years were eligible for this investigation. Detailed medical history was obtained, with specific attention paid to the occurrence of prior VTE. Patients with a history of previously documented VTE were excluded from the study, as were those with active cancer. Eligible patients underwent compression ultrasonography (CUS) of the legs, and subjects with positive test results were also excluded. In the remaining subjects, factor V Leiden determination was performed as indicated elsewhere.12 According to laboratory results, subjects were classified as carriers or noncarriers of the factor V mutation. Patients in whom cancer developed during follow-up were removed from the analysis. Recruitment into the study started in June 1994 and ended in June 2000. Follow-up of recruited subjects was completed in October 2000. Informed consent was obtained from all study participants in accordance with the Helsinki protocol.
Study design
In this prospective cohort study, no continuous anticoagulant prophylaxis was given to the included subjects. During risk periods for VTE, the use of anticoagulant prophylaxis was suggested, but any decision concerning its use was left to the discretion of the treating physician. Risk periods for VTE were surgery, trauma, prolonged (more than 7 days) immobilization, pregnancy, and postpartum. The use of oral contraceptives was discouraged, but not prohibited, in the study groups.
Recruited subjects were asked to return to our institution for clinical evaluation every 6 months or earlier if clinical symptoms suggestive of deep vein thrombosis (DVT) or pulmonary embolism (PE) developed. At each visit they were interviewed, and special attention was paid to risk periods for thrombosis. Objective tests (CUS or phlebography in patients with suspected DVT, ventilation or perfusion lung scanning or spiral computed tomography followed by pulmonary angiography when needed in patients with suspected PE) were systematically used to diagnose or rule out a thromboembolic event in every symptomatic patient.14 15 All study subjects and their physicians received written information concerning the implications of the observed factor V Leiden mutation and advice to consider anticoagulant prophylaxis during risk periods. Our institutional review board approved the study protocol.
Laboratory assay
Blood samples were collected into plastic syringes containing 3.8% (wt/vol) sodium citrate in a ratio of 1:9 (vol/vol) anticoagulant to blood. Assays for antithrombin, protein C, and protein S and DNA analysis for the factor V Leiden mutation were performed as previously described.12,16 DNA analysis for prothrombin variant 20210A, tests for antiphospholipid antibodies, and determination of fasting plasma homocysteine levels were performed only in patients who became symptomatic for VTE during the follow-up. The methods used for these determinations have been described.17-19
Outcomes
The primary outcome was the occurrence of objectively documented VTE. This was defined as DVT of the leg or the arm, PE, or objectively documented venous thrombosis in other sites. A venous thromboembolic event was categorized as either spontaneous or secondary to a risk period. Spontaneous thromboembolism was defined as an event occurring without a predisposing risk period. Secondary VTE was defined as an event occurring during or within 3 months of a risk period.
Statistical analysis
The annual incidence of the first episode of spontaneous or risk period–related VTE, and its 95% confidence intervals (CIs) were calculated in the carrier and noncarrier groups and were expressed as number of events per 100 patient-years. Risk period–related VTE was related to the type of risk period and to anticoagulant prophylaxis administration. Relative risk for the development of total, spontaneous, and risk period–related VTE was calculated by dividing the incidence rate in carriers by the incidence rate in noncarrier family members. The 95% CIs and P values were calculated according to the normal approximation of the binomial distribution or by using the exact procedure as appropriate. Thrombosis-free survival in heterozygous carriers and in noncarriers was analyzed according to the Kaplan-Meier method. Both curves were compared using the log-rank test.
Results
Study populations
One hundred thirty-one unrelated patients with objectively documented VTE and factor V Leiden mutation served as index patients in this study. Mean age of index patients was 48 years (range, 16 to 93 years), and 35% of their first thrombotic events occurred spontaneously. From these 131 patients, 656 family members were identified as potentially eligible for participation in the study. Of these, 53 refused to participate. Of the remaining 603 subjects who were interviewed and underwent a baseline CUS, 42 were excluded because of the previous VTE (36) or the positive CUS (6). Thus 561 subjects were enrolled into the study; 313 (145 males) were found to be carriers of factor V Leiden (299 heterozygous, 14 homozygous) and 248 (125 males) noncarriers. Mean age of the subjects at the time of their inclusion in the study was 44 years (range, 15 to 95 years) in carriers and 43 years (range, 16 to 89 years) in noncarriers.
Follow-up
There were 1255 observation years in carriers (68 years in homozygous, 1187 in heterozygous) and 984 observation years in noncarriers of factor V Leiden. Average duration of observation was 4 years in each group (range, 4 months-6 years). At least 1 year of follow-up was available in 521 subjects. No subject died or was lost to follow-up.
Incidence of venous thromboembolism
Thromboembolic events during follow-up were recorded in 8 (2%) carriers of factor V Leiden, all of them belonging to the group of heterozygous subjects, accounting for an overall annual incidence of VTE of 0.67% (95% CI, 0.29-1.33). Two events were spontaneous (annual incidence of spontaneous VTE, 0.17%; 95% CI, 0.02-0.6), and the remaining 6 were risk period–related (Table1). All events affected the proximal veins of either leg and were associated with symptomatic PE in one patient. Four events occurred in patients younger than 45, and the remaining occurred in patients older than 45 years. Observation-years of follow-up were similar in both age categories.
Incidence of risk period–related venous thromboembolism in 32 heterozygous family members with the factor V Leiden mutation and in 19 subjects belonging to the control group
| Type of risk period . | Factor V Leiden + . | Factor Leiden V − . | ||
|---|---|---|---|---|
| No. . | VTE . | No. . | VTE . | |
| Trauma or surgery | 19 (6) | 4 (1) | 13 | 1 |
| Pregnancy/postpartum | 12 (5*) | 2 | 3 | 0 |
| Oral contraceptives | 3 | 0 | 5 | 0 |
| Total | 34 (11) | 6 (1) | 21 | 1 |
| Type of risk period . | Factor V Leiden + . | Factor Leiden V − . | ||
|---|---|---|---|---|
| No. . | VTE . | No. . | VTE . | |
| Trauma or surgery | 19 (6) | 4 (1) | 13 | 1 |
| Pregnancy/postpartum | 12 (5*) | 2 | 3 | 0 |
| Oral contraceptives | 3 | 0 | 5 | 0 |
| Total | 34 (11) | 6 (1) | 21 | 1 |
Numbers in parentheses represent the patients who received prophylaxis or VTE events that occurred under prophylaxis.
Prophylaxis only in the postpartum period.
Of the patients in the noncarrier group in whom VTE symptoms developed, a 60-year-old woman was retrospectively excluded from the analysis because she was found to be a heterozygous carrier of the G20210A prothrombin variant. In addition, metastatic breast cancer developed 2 months after the thrombotic event. The only evaluable event in the noncarrier group was a proximal DVT arising in association with a triggering factor (Table 1). The annual incidence of VTE in this group was 0.1% (95% CI, 0.003-0.56). Relative risk for overall VTE in carriers of factor V Leiden compared with noncarriers was 6.6 (95% CI, 1.1-39.8).
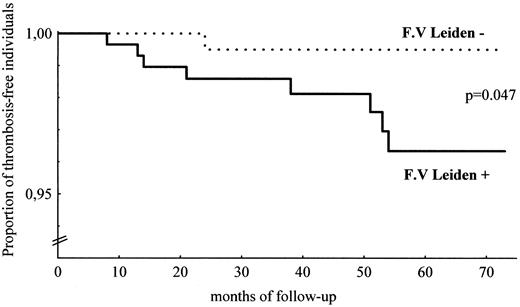
Thrombosis-free survival curves in carriers and noncarriers of factor V Leiden are plotted in Figure 1 as a function of the duration of follow-up. Among carriers, only those who were heterozygous were considered for Kaplan-Meier analysis. The difference between the curves appears to be statistically significant (log-rank test: P = .047).
Venous thromboembolism-free survival in the cohort (Kaplan-Meier method).
Thrombosis-free survival curves in 299 heterozygous carriers of factor V Leiden (solid line) and in 248 noncarriers (dotted line) are plotted as a function of the duration of follow-up. Homozygous carriers are not considered in this analysis. F.V., factor V.
Venous thromboembolism-free survival in the cohort (Kaplan-Meier method).
Thrombosis-free survival curves in 299 heterozygous carriers of factor V Leiden (solid line) and in 248 noncarriers (dotted line) are plotted as a function of the duration of follow-up. Homozygous carriers are not considered in this analysis. F.V., factor V.
As shown in Table 2, 34 risk periods were experienced by 32 heterozygous carriers of factor V Leiden, complicated by thromboembolism in 6 patients and accounting for an incidence of 17.6% per risk period (95% CI, 8.3-33.5). Three pregnancies occurred during follow-up in 2 homozygous women. They received low-dose low molecular weight heparin (LMWH) during postpartum periods only and remained asymptomatic. Among noncarriers, 21 risk periods were experienced by 19 persons, complicated by VTE in 1 case, accounting for an incidence of 4.8% per risk period (95% CI, 0.8-22.7).
Characteristics of the subjects who experienced VTE during the study
| Subject no. . | Sex/age, y* . | Defect . | Risk period . | Location VTE . |
|---|---|---|---|---|
| 1 | F/41 | Factor V Leiden† | Postpartum | Proximal DVT |
| 2 | M/66 | Factor V Leiden† | Trauma and surgery | Proximal DVT + PE |
| 3 | M/21 | Factor V Leiden† | Trauma | Proximal DVT |
| 4 | M/27 | Factor V Leiden† | Trauma and immobilization | Proximal DVT |
| 5 | F/69 | Factor V Leiden† | None | Proximal DVT |
| 6 | F/75 | Factor V Leiden† | None | Proximal DVT |
| 7 | F/59 | Factor V Leiden† | Trauma and immobilization | Proximal DVT |
| 8 | F/34 | Factor V Leiden† | Postpartum | Proximal DVT |
| 9 | M/55 | None | Hip surgery | Proximal DVT |
| 10 | F/60 | Prothrombin 20210A† | None (metastatic breast cancer‡) | Proximal DVT |
| Subject no. . | Sex/age, y* . | Defect . | Risk period . | Location VTE . |
|---|---|---|---|---|
| 1 | F/41 | Factor V Leiden† | Postpartum | Proximal DVT |
| 2 | M/66 | Factor V Leiden† | Trauma and surgery | Proximal DVT + PE |
| 3 | M/21 | Factor V Leiden† | Trauma | Proximal DVT |
| 4 | M/27 | Factor V Leiden† | Trauma and immobilization | Proximal DVT |
| 5 | F/69 | Factor V Leiden† | None | Proximal DVT |
| 6 | F/75 | Factor V Leiden† | None | Proximal DVT |
| 7 | F/59 | Factor V Leiden† | Trauma and immobilization | Proximal DVT |
| 8 | F/34 | Factor V Leiden† | Postpartum | Proximal DVT |
| 9 | M/55 | None | Hip surgery | Proximal DVT |
| 10 | F/60 | Prothrombin 20210A† | None (metastatic breast cancer‡) | Proximal DVT |
Age at the time of VTE.
Heterozygous defect.
Diagnosis made 2 months after VTE event.
In 11 of 34 risk periods experienced by factor V Leiden carriers in which anticoagulant prophylaxis was given (low-dose LMWH in 8 cases, high-dose LMWH in 1, low-dose unfractionated heparin in 2), 1 VTE occurred after trauma and resulted in an incidence of 9.1% per risk period. Five VTE events occurred during the 23 risk periods in which no anticoagulant prophylaxis was given, with an incidence of 21.7% per risk period.
Discussion
In full agreement with data from recent retrospective studies,11,12 the annual incidence of venous thromboembolic events occurring in asymptomatic carriers of factor V Leiden during a 6-year prospective follow-up was low. Spontaneous VTE occurred in carriers with an incidence of 0.17% patient-years as previously observed,20 whereas no spontaneous VTE occurred in the noncarriers. Accordingly, it should be expected that a spontaneous VTE event will occur during 1 year of follow-up for every 600 asymptomatic family members who are heterozygous carriers of the factor V Leiden mutation. This rate is considerably lower than that found in deficiencies of antithrombin, protein C, and protein S (approximately 0.8% patient-years).16 In addition, though limited observation-years are available, all 14 homozygous carriers of factor V Leiden remained asymptomatic during follow-up. Taken together, these observations indicate that factor V Leiden mutation is a mild risk factor for VTE. It is noteworthy, however, that in the current study the annual incidence of overall VTE in heterozygous carriers (0.67% patient-years) differed significantly from that found in family members with a normal genotype (0.1% patient-years); the latter is fully consistent with estimates available in the general population.13,21 However, because the risk for serious bleeding during the use of oral anticoagulants is much higher, ranging from 2% to 4% patient-years,22-24 there is likely no expected benefit from long-term anticoagulant prophylaxis in asymptomatic carriers of factor V Leiden. The question arises whether routinely screening family members of symptomatic carriers of a single identified factor V Leiden mutation is justified. In this prospective study, the risk for overall VTE was about 6.6 times higher in heterozygous carriers than in noncarriers, suggesting that family screening has the potential to identify a group of persons at higher risk for VTE. This increased risk is comparable to that found in a previous case-control study of consecutive patients with objectively documented VTE. 1
The increased risk for VTE found in heterozygous carriers during the follow-up is more pronounced during risk periods. Although the relatively small number of risk periods and the nonuniform approach concerning anticoagulant prophylaxis preclude definitive conclusions, it is interesting that the incidence of risk period–related VTE in carriers was 9.1% per risk period and 21.7% per risk period in the presence or the absence of anticoagulant prophylaxis, respectively. These figures are similar to those found in a recent prospective study with a similar study design addressing carriers of antithrombin, protein C, and protein S deficiencies,16 in whom the incidence of risk period–related VTE was 4.5% and 16.7% per risk period depending on whether prophylaxis was or was not given, respectively.
Major trauma or surgery was complicated by VTE in 21% and 7.6% in carriers and noncarriers of factor V Leiden, respectively, suggesting that under these situations an increased risk for VTE indeed exists. Therefore, family members might take advantage of screening for factor V Leiden, assuming that the administration or optimization, or both, of prophylaxis has the potential to prevent, at least in part, these VTE events. This must be further addressed in proper studies.
Pregnancy outcome may be influenced by the presence of factor V Leiden mutation, as reported in several studies.12,25 26 The postpartum period is considered a particularly high risk time for VTE in factor V Leiden carriers, and prophylaxis is usually given to women under these circumstances. In our cohort, almost 28% of postpartum periods were complicated by VTE in women who did not receive prophylaxis. No VTE events occurred in heterozygous or homozygous women who received prophylaxis during postpartum periods. This finding suggests a potential benefit from screening female family members of fertile age because of the possibility of preventing postpartum-related thrombosis in carriers of factor V Leiden with the use of prophylaxis.
In carriers of factor V Leiden, no thromboembolism was observed during long-term hormonal therapy. This finding, which contrasts with most available information,27,28 might be accounted for by the low number of investigated risk periods, partly resulting from the tendency of young women who were conscious of their thrombophilic status or of thrombotic problems in their families to refrain from taking potentially dangerous drugs. In addition, even homozygous carriers of factor V Leiden mutation remain asymptomatic for VTE during long-term oral contraceptive treatment.29 Thus, additional studies are needed to establish whether women of fertile age who are considering the use of oral contraceptives or who intend to become pregnant might benefit from the routine screening of this mutation. Costs of such an approach might be affordable because of the selection of persons to be screened (asymptomatic women in fertile age who are family members of a symptomatic [VTE] carrier of a single identified factor V Leiden mutation), which undoubtedly would reduce the high cost–benefit ratio of unselected population screening.
We believe that our results provide a valid estimate of the annual frequency of thromboembolic events in asymptomatic carriers of a single identified heterozygous factor V Leiden mutation. All identified subjects were prospectively followed up at the study center. All episodes of clinically suspected DVT or PE were investigated with properly validated objective tests. Moreover, confounding factors were minimized by the exclusion from our cohort of patients who had a history of thromboembolism and other known thrombophilic conditions. Although our conclusions stem from a moderate-sized sample of patients followed-up for an average of 6 years, it is unlikely that a different estimate of the risk for spontaneous venous thromboembolism will be obtained by a prolongation of the follow-up period. Finally, because of the limited number of homozygous carriers of the factor V Leiden mutation included in this study, no conclusion regarding the risk for VTE can be drawn for this condition.
In conclusion, asymptomatic persons who are daily identified in coagulation laboratories through the screening of families with factor V Leiden exhibit a low risk for venous thromboembolism. Systematic screening for this abnormality in family members of symptomatic patients with a single identified factor V Leiden mutation has the potential to identify those persons who might benefit from prophylaxis for VTE in high-risk situations. However, accurate evaluation of the cost–benefit ratio of screening selected family members should be performed in properly designed studies before this is recommended as a routine approach.
We thank Sonia Luni and Mariangela Fadin for excellent technical assistance.
Supported in part by a grant from Regione del Veneto, Ricerca Sanitaria Finalizzata no. 783/01/97 (P.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paolo Simioni, Department of Medical and Surgical Sciences, Via Ospedale, 105 35128-Padua, Italy; e-mail:paolo.simioni@unipd.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal