Abstract
Between April 1988 and July 1998, 510 patients with myelodysplastic syndromes (MDS) underwent unrelated donor bone marrow transplantation (BMT) facilitated by the National Marrow Donor Program. Median age was 38 years (range, <1-62 years). Several conditioning regimens and graft-versus-host disease (GVHD) prophylaxis methods were used, and T-cell depletion was used in 121 patients. Donors were serologically matched for HLA-A, -B, and -DRB1 antigens for 74% of patients. Of 437 patients evaluable for engraftment, 24 (5% cumulative incidence, with 95% confidence interval [CI] of 3%-7%) failed to engraft, and an additional 33 (8% cumulative incidence; 95% CI, 6%-10%) had late graft failure. Grades II to IV GVHD developed in 47% of patients (95% CI, 43%-49%), and limited and extensive chronic GVHD developed at 2 years in 27% (95% CI, 24%-30%). The incidence of relapse at 2 years was 14% (95% CI, 11%-17%). Greater relapse was independently associated with advanced MDS subtype and no acute GVHD. The estimated probability of disease-free survival (DFS) at 2 years was 29% (95% CI, 25%-33%). Improved DFS was independently associated with less advanced MDS subtype, higher cell dose, recipient cytomegalovirus (CMV) seronegativity, shorter interval from diagnosis to transplantation, and transplantation in recent years. Common causes of death were treatment-related complications accounting for 82% of fatalities. The 2-year cumulative incidence of treatment-related mortality (TRM) was 54% (95% CI, 53%-61%). Sixty-nine percent of TRM occurred within the first 100 days, and 93% occurred within the first year of transplantation. Higher TRM was independently associated with older recipient and donor age, HLA mismatch, and recipient CMV seropositivity. This study demonstrates that unrelated donor BMT cures a significant proportion of patients with MDS. TRM is the major problem limiting the success of unrelated donor BMT in MDS. The observations made in this study should facilitate the design of prospective trials aimed at improving the results of unrelated donor stem cell transplantation for MDS.
Introduction
Allogeneic hematopoietic stem cell transplantation is the only curative therapy available for patients with myelodysplastic syndrome (MDS). Single-center experience1-7and registry data8,9 have clearly shown that bone marrow transplantation (BMT) from HLA-matched siblings can cure 40% to 50% of patients with MDS. The main obstacles limiting the success of BMT in these patients have been disease relapse and treatment-related mortality (TRM).1-9 Outcomes after related donor stem cell transplantation have been extensively studied. Disease-free survival is strongly correlated with patient age, MDS subtype, prognostic score, and chromosomal abnormalities.1,2,5,6,8,9 Relapse is associated with MDS subtype, prognostic score, chromosomal abnormalities, and disease duration.1-9 TRM is strongly correlated with disease duration, patient age, and HLA matching.1-9
Unfortunately, approximately only one third of patients with MDS eligible for allogeneic BMT have a suitable HLA-matched related donor. Therefore, unrelated donor stem cell transplantation has the potential for use in large numbers of patients with MDS. Only a few studies, generally including small numbers of patients receiving transplants from unrelated donors, have been published.10-17 The impact of patient, disease, transplant, and donor characteristics on outcome after unrelated donor BMT for MDS is not well known. We report here the posttransplantation outcomes of the first 510 unrelated donor BMTs performed as therapy for MDS using marrow from volunteer donors recruited through the National Marrow Donor Program (NMDP).
Patients and methods
Patients
The study population consisted of 510 patients with MDS who received marrow transplants at 87 NMDP-approved transplantation centers (76 in the United States and 11 in Canada, Europe, and Israel) between April 1988 and July 1998. During this time, an additional 24 patients underwent unrelated donor transplantation but were not included in this study because they had undergone prior stem cell transplantation (20 autologous, 4 allogeneic). In 4 of the 510 patients, information on relapse status was unavailable.
The diagnosis of MDS and MDS subtypes was established by the centers according to the well-established criteria of the French-American-British classification system.18 Diagnoses were confirmed or adjusted, if indicated, by the NMDP data center based on reported hematologic features. Patients with hypocellular MDS and less than 5% marrow blasts and MDS patients with neutropenia, thrombocytopenia, or both but without anemia were classified as having refractory anemia (RA; this term was used as an equivalent of refractory cytopenia). Patients with MDS with myelofibrosis, patients whose blood and marrow pictures could not fit the French-American-British criteria (unclassifiable MDS), and patients in whom the MDS subtype at diagnosis was not known were also included in this study. Children with juvenile myelomonocytic leukemia and adults with the myeloproliferative form of chronic myelomonocytic leukemia (CMML)19 were excluded. One hundred forty-seven patients with MDS whose disease had evolved to acute leukemia before transplantation were included. Forty-seven of these 147 patients underwent chemotherapy and achieved remission before starting conditioning. Transformation to acute leukemia was defined by blood or marrow blast count greater than 30%.20
The distribution of MDS subtypes at the time of diagnosis and before transplantation is summarized in Table 1. For analyses of transplantation outcomes, patients with refractory anemia with ringed sideroblasts (RARS), CMML (myelodysplastic form), MDS with myelofibrosis, and unknown or unclassifiable MDS were grouped together because of small numbers. Seventy-four patients with advanced MDS (5% or more blasts) or in blastic transformation who achieved hematologic remission (marrow blast count less than 5% and normal peripheral counts) or a second RA phase (marrow blast count less than 5% and persistently subnormal blood counts after chemotherapy) were also analyzed together because they had similar outcomes. Fifty-eight patients with advanced MDS or in blastic transformation whose disease did not change or changed to a different subtype without achieving hematologic remission after chemotherapy were analyzed in their original group. For example, a patient in blastic phase with more than 30% blasts who had a marrow blast count between 5% and 30% after chemotherapy was included for analysis in the acute myelocytic leukemia (AML) group.
MDS subtypes at diagnosis and before transplantation
| MDS subtype . | At diagnosis . | Before transplantation . |
|---|---|---|
| RA | 189 | 116 |
| RARS | 11 | 9 |
| RAEB | 161 | 85 |
| RAEB-t | 106 | 105 |
| CMML (nonproliferative form) | 19 | 11 |
| Myelofibrotic MDS | 12 | 8 |
| Unclassifiable or unknown MDS subtype | 12 | 2 |
| Acute myelocytic leukemia | 0 | 100 |
| In complete remission or in second RA phase | 0 | 74 |
| MDS subtype . | At diagnosis . | Before transplantation . |
|---|---|---|
| RA | 189 | 116 |
| RARS | 11 | 9 |
| RAEB | 161 | 85 |
| RAEB-t | 106 | 105 |
| CMML (nonproliferative form) | 19 | 11 |
| Myelofibrotic MDS | 12 | 8 |
| Unclassifiable or unknown MDS subtype | 12 | 2 |
| Acute myelocytic leukemia | 0 | 100 |
| In complete remission or in second RA phase | 0 | 74 |
RARS indicates refractory anemia with ringed sideroblasts.
Classification of patients according to their risk category using a recently published International Prognostic Scoring System21 was not possible because most data collection occurred before publication of the scoring system. All patients or their guardians signed informed consent for transplantation and for submission of data to the NMDP. Informed consent was approved by the local transplant center Institutional Review Board or its equivalent.
Pretransplantation and transplantation characteristics of all patients are summarized in Table 2. The most common preparative regimen was fractionated or single-dose total body irradiation (TBI) plus cyclophosphamide (CY), alone or in combination with other chemotherapeutic agents in 335 (66%) patients, or chemotherapy alone, usually busulfan (BU) plus CY alone or in combination with other chemotherapeutic agents in 128 (25%) patients. Graft-versus-host disease (GVHD) prophylaxis included a variety of in vivo pharmacologic and ex vivo T-cell–depletion methods. One hundred twenty-one patients received T-cell–depleted marrow grafts, and 389 patients received T-cell–replete marrow grafts. Most recipients of T-cell–depleted marrow grafts also underwent posttransplantation in vivo GVHD prophylaxis. The most common pharmacologic in vivo GVHD prophylaxis was a cyclosporine-based regimen, which was used in 326 (64%) patients.
Patient and donor characteristics
| Characteristics . | No. patients . |
|---|---|
| Total patients in study | 510 |
| Median recipient age, y (range) | 38 (< 1-62) |
| Recipient age distribution, y | |
| 20 or younger | 144 |
| 21-30 | 63 |
| 31-40 | 85 |
| 41-50 | 144 |
| 51 or older | 74 |
| Sex, donor:recipient | |
| M:F | 112 |
| M:M | 190 |
| F:M | 101 |
| F:F | 107 |
| Median time from diagnosis to transplantation, mo (range) | 9 (< 1-246) |
| Etiology | |
| Idiopathic | 398 |
| Postradiation, chemotherapy, or both | 70 |
| Postaplastic anemia | 26 |
| Post-MPD (polycythemia vera, essential thrombocythemia) | 3 |
| Other disease | 4 |
| Unknown | 9 |
| Cytogenetics | |
| Normal | 126 |
| Abnormal | 238 |
| Failure/unknown | 146 |
| Recipient CMV serology | |
| Positive | 272 |
| Negative | 234 |
| Unknown | 4 |
| HLA-A, -B, -DRB1 matching | |
| HLA-A, -B, -DR identical | 376 |
| Allele potential identical | 24 |
| Mismatch | 110 |
| Preparative regimen | |
| TBI/CY ± other | 317 |
| BU/CY ± other | 128 |
| TBI/BU/CY | 18 |
| Other | 47 |
| GVHD prophylaxis | |
| T-cell depletion | 121 |
| CSA ± other | 326 |
| Other | 63 |
| Median cell dose (range) | |
| Non–T-cell depletion (× 108 nucleated cells/kg) | 2.9 (0.07-11.9) |
| T-cell depletion (× 108 nucleated cells/kg) | 0.8 (0.02-3.5) |
| Donor | |
| Median age, y (range) | 37 (19-59) |
| No. males | 302 |
| CMV seropositivity | 186 |
| Characteristics . | No. patients . |
|---|---|
| Total patients in study | 510 |
| Median recipient age, y (range) | 38 (< 1-62) |
| Recipient age distribution, y | |
| 20 or younger | 144 |
| 21-30 | 63 |
| 31-40 | 85 |
| 41-50 | 144 |
| 51 or older | 74 |
| Sex, donor:recipient | |
| M:F | 112 |
| M:M | 190 |
| F:M | 101 |
| F:F | 107 |
| Median time from diagnosis to transplantation, mo (range) | 9 (< 1-246) |
| Etiology | |
| Idiopathic | 398 |
| Postradiation, chemotherapy, or both | 70 |
| Postaplastic anemia | 26 |
| Post-MPD (polycythemia vera, essential thrombocythemia) | 3 |
| Other disease | 4 |
| Unknown | 9 |
| Cytogenetics | |
| Normal | 126 |
| Abnormal | 238 |
| Failure/unknown | 146 |
| Recipient CMV serology | |
| Positive | 272 |
| Negative | 234 |
| Unknown | 4 |
| HLA-A, -B, -DRB1 matching | |
| HLA-A, -B, -DR identical | 376 |
| Allele potential identical | 24 |
| Mismatch | 110 |
| Preparative regimen | |
| TBI/CY ± other | 317 |
| BU/CY ± other | 128 |
| TBI/BU/CY | 18 |
| Other | 47 |
| GVHD prophylaxis | |
| T-cell depletion | 121 |
| CSA ± other | 326 |
| Other | 63 |
| Median cell dose (range) | |
| Non–T-cell depletion (× 108 nucleated cells/kg) | 2.9 (0.07-11.9) |
| T-cell depletion (× 108 nucleated cells/kg) | 0.8 (0.02-3.5) |
| Donor | |
| Median age, y (range) | 37 (19-59) |
| No. males | 302 |
| CMV seropositivity | 186 |
Donor–recipient HLA matching
The technology of HLA typing has significantly evolved in the 10-year study period. Donor and recipient HLA typing was performed by the standard serologic microcytotoxicity method (100% of HLA-A and -B and 10% of HLA-DR) and low- to high-resolution DNA-based methods, primarily sequence-specific oligonucleotide probe typing (90% of HLA-DR). Unrelated donor selection was based on matching at the level of resolution available at the time of transplantation. To accommodate the resultant variability in HLA typing data, HLA match between recipient and donor was defined as one of these categories: (1) known match—donor and recipient typing were at the same level of resolution and matched perfectly (eg, DRB1-0101 vs DRB1*0101); (2) potential match—donor and recipient typing were reported at different levels of resolution (eg, DRB1*03 vs DRB1*0301)—so that at the lowest level of resolution (DRB1*03), the pair was defined as a potential match, but it could not be determined whether a match existed at the higher level of resolution (DRB1*0301); (3) known mismatch—donor and recipient typing, regardless of level of resolution reported, could not possibly match (eg, DRB1*0101 vs DR4).
Among the 510 patients, 376 (74%) received marrow from a donor who was a known match at HLA-A, -B, and -DR, 110 (22%) received marrow from a donor who was a known mismatch at HLA-A, -B, and -DR, and 24 (5%) received marrow with a potential match at HLA-A, -B, and -DR. Allele-level HLA-DRB1 typing using several DNA-based methods was available for 463 patients. It revealed a known allele mismatch at the DRB1 locus in 30 (6%) of pairs identified as HLA-A and -B match and as HLA-DR potential match.
Marrow donors and marrow collection
Unrelated donors were identified through the NMDP based in Minneapolis, Minnesota. The median time interval between initiation of the preliminary search for a donor and identification of a donor was 5 months (range, 1-100 months). This interval has decreased over time. Before donation, each volunteer donor underwent complete medical evaluation, received extensive counseling about marrow donation, and signed an intent-to-donate form at the NMDP-approved donor center. Donor characteristics are shown in Table 2. Marrows were collected according to standards established by the NMDP22 at 95 NMDP-approved collection centers. Harvested marrow was placed in sterile plastic bags and transported to the NMDP-approved transplant centers by courier. Transported marrow was infused without any manipulation in 188 patients and after manipulation to remove ABO-incompatible red cells, plasma, or both in 201 patients and to remove T cells in 121 patients.
Data collection and outcome definition
Data collection methods have been well standardized by the NMDP and have been described in detail.17,22 23 Donor information, harvest procedure, and recipient information were collected using standardized forms provided by the NMDP. These forms captured baseline information and pretransplantation, transplantation, and posttransplantation events. Follow-up information was reported at 100 days, 6 months, 1 year, and then annually after transplantation. Patient outcome was analyzed to date of last reported follow-up or to date of death. Data reporting for analysis was updated as of September 1999. Median follow-up of the surviving patients was 24 months (range, 3-97 months).
Engraftment was defined as having occurred on the first of 3 consecutive posttransplantation days on which the absolute neutrophil count (ANC) exceeded 500/μL. Patients who did not achieve this level of ANC at any time after transplantation were considered to have primary graft failure. Patients with initial engraftment in whom severely hypocellular marrow and ANC of less than 500/μL recurred for more than 3 days were considered to have secondary or late graft failure. Patients who survived at least 28 days were considered evaluable for engraftment.
Stage of involvement of the skin, liver, and intestinal tract by acute GVHD was determined according to established criteria,24,25 and an overall grade was assigned on the basis of the sum of individual stages as described.17Chronic GVHD was assessed as limited (mild skin involvement only) or extensive (skin, liver, and intestinal tract involvement).26
Relapse was defined as the recurrence of hematologic abnormalities. Causes of death other than relapse were considered to represent competing risk. Patients who were alive without relapse were censored at last follow-up. TRM was defined as death with no evidence of hematologic relapse. Relapse was considered a competing risk, and patients who were alive without relapse were censored at last follow-up. Disease-free survival (DFS) was defined as survival without morphologic evidence of recurrent MDS.
Statistical analysis
Estimates of the probability of engraftment, acute GVHD (grades II-IV), chronic GVHD, relapse, and TRM were expressed as cumulative incidence.27 Survival curves were calculated using the Kaplan-Meier product limit method.28 The log-rank test29 was used to test the effect of variables on survival and overall DFS. Variables considered for inclusion in multivariate analysis were those displaying significant association with a transplantation outcome in univariate analysis or for which prior studies suggested a possible prognostic value. Proportional hazards Cox regression model30 was then used to assess the independent effect of recipient, donor, or transplant characteristics (recipient age, recipient sex, recipient CMV status, donor age, donor sex, donor CMV status, time from diagnosis to transplantation, year of infusion, donor–recipient HLA matching, cell dose, use of TBI in the conditioning regimen, T-cell depletion, and MDS subtypes) on transplantation outcomes. Additional variables were included in some analyses—eg, history of acute GVHD in the analysis of chronic GVHD and chronic GVHD in the analysis of functional status.
Results
Engraftment and graft failure
Seventy-three (14%) patients died within the first 27 days of transplantation without evidence of engraftment or GVHD. Of the 437 patients evaluable for engraftment, 380 (87%) achieved sustained donor engraftment and 57 (13%) had graft failure. Twenty-four patients had primary engraftment failure, and 33 had late graft failure. The cumulative incidence of engraftment before day 100 was 84% (95% CI, 82%-86%). Median time to engraftment was 18 days (range, 8-40 days). Recipients of T-cell–depleted grafts engrafted faster than recipient of non–T-cell–depleted grafts—15 days (range, 9-37 days) versus 19 days (range, 8-40 days) (P = .02). However, there was no difference in the overall engraftment rate between T-cell– and non–T-cell–depleted grafts at 100 days—83% (range, 78%-88%) versus 85% (range, 83%-87%) (P = .66).
According to the results of multivariate analysis, higher cell dose, female sex of the patient, male sex of the donor (there was no significant interaction between the sexes of patients and donors), and transplantation between 1994 and 1998 were independently associated with higher rates of engraftment (Table3). Donor–recipient HLA matching and conditioning regimen (TBI containing vs non-TBI containing) did not influence the probability of engraftment in this series of patients. T-cell depletion was removed from the multivariate model for engraftment because T-cell depletion does not obey the proportional hazards model assumption.
Multivariate proportional hazards regression analysis of transplant outcomes in 510 patients with MDS treated with unrelated donor marrow transplantation
| . | RR (95% CI) . | P . | Favorable . |
|---|---|---|---|
| Neutrophil engraftment | |||
| Cell dose | 1.10 (1.03-1.18) | .006 | Higher |
| Donor sex (female) | 0.76 (0.60-0.93) | .01 | Male |
| Recipient sex (female) | 1.24 (1.00-1.53) | .05 | Female |
| Year of infusion (1988-1993) | 0.67 (0.48-0.94) | .02 | Recent transplantation |
| Acute GVHD | |||
| Year of infusion (1988-1993) | 1.91 (1.21-3.02) | .0006 | Recent transplantation |
| HLA-A, -B, -DRB1 match | 0.65 (0.45-0.94) | .02 | Match |
| Preparative regimen | 1.73 (1.08-2.76) | .02 | No TBI |
| Relapse | |||
| RAEB-t | 4.92 (1.96-12.4) | .0007 | RA |
| AML | 7.67 (3.03-19.4) | .0001 | RA |
| Acute GVHD | 0.52 (0.29-0.95) | .03 | Grades II-IV |
| Treatment-related mortality | |||
| Acute GVHD | 2.52 (1.90-3.34) | .0001 | Grades 0-I |
| HLA-A, -B, -DRB1 match | 0.66 (0.50-0.88) | .004 | Match |
| Recipient CMV serology | 1.46 (1.13-1.89) | .004 | Negative |
| Recipient age | 1.13 (1.04-1.22) | .005 | Younger |
| Donor age | 1.20 (1.04-1.39) | .01 | Younger |
| Overall survival | |||
| Acute GVHD | 2.13 (1.66-2.74) | .0001 | Grades 0-I |
| AML | 2.08 (1.46-2.97) | .0001 | RA |
| RAEB-t | 1.66 (1.18-2.35) | .004 | RA |
| Cell dose | 0.89 (0.83-0.96) | .003 | Higher |
| Recipient CMV serology | 1.44 (1.14-1.81) | .002 | Negative |
| Time to transplantation (< 9 mo) | 0.76 (0.61-0.96) | .02 | Early transplantation |
| Year of infusion (1988-1993) | 1.45 (1.02-2.06) | .04 | Recent transplantation |
| HLA-A, -B, -DRB1 match | 0.78 (0.60-1.00) | .05 | Match |
| Disease-free survival | |||
| Acute GVHD | 1.93 (1.50-2.47) | .0001 | Grades 0-I |
| AML | 2.04 (1.43-2.91) | .0001 | RA |
| RAEB-t | 1.71 (1.22-2.40) | .002 | RA |
| Cell dose | 0.89 (0.83-0.96) | .003 | Higher |
| Recipient CMV serology | 1.46 (1.16-1.84) | .001 | Negative |
| Time to transplantation (< 9 mo) | 0.78 (0.62-0.98) | .03 | Early transplantation |
| Year of infusion (1988-1993) | 1.43 (1.01-2.01) | .03 | Recent transplantation |
| . | RR (95% CI) . | P . | Favorable . |
|---|---|---|---|
| Neutrophil engraftment | |||
| Cell dose | 1.10 (1.03-1.18) | .006 | Higher |
| Donor sex (female) | 0.76 (0.60-0.93) | .01 | Male |
| Recipient sex (female) | 1.24 (1.00-1.53) | .05 | Female |
| Year of infusion (1988-1993) | 0.67 (0.48-0.94) | .02 | Recent transplantation |
| Acute GVHD | |||
| Year of infusion (1988-1993) | 1.91 (1.21-3.02) | .0006 | Recent transplantation |
| HLA-A, -B, -DRB1 match | 0.65 (0.45-0.94) | .02 | Match |
| Preparative regimen | 1.73 (1.08-2.76) | .02 | No TBI |
| Relapse | |||
| RAEB-t | 4.92 (1.96-12.4) | .0007 | RA |
| AML | 7.67 (3.03-19.4) | .0001 | RA |
| Acute GVHD | 0.52 (0.29-0.95) | .03 | Grades II-IV |
| Treatment-related mortality | |||
| Acute GVHD | 2.52 (1.90-3.34) | .0001 | Grades 0-I |
| HLA-A, -B, -DRB1 match | 0.66 (0.50-0.88) | .004 | Match |
| Recipient CMV serology | 1.46 (1.13-1.89) | .004 | Negative |
| Recipient age | 1.13 (1.04-1.22) | .005 | Younger |
| Donor age | 1.20 (1.04-1.39) | .01 | Younger |
| Overall survival | |||
| Acute GVHD | 2.13 (1.66-2.74) | .0001 | Grades 0-I |
| AML | 2.08 (1.46-2.97) | .0001 | RA |
| RAEB-t | 1.66 (1.18-2.35) | .004 | RA |
| Cell dose | 0.89 (0.83-0.96) | .003 | Higher |
| Recipient CMV serology | 1.44 (1.14-1.81) | .002 | Negative |
| Time to transplantation (< 9 mo) | 0.76 (0.61-0.96) | .02 | Early transplantation |
| Year of infusion (1988-1993) | 1.45 (1.02-2.06) | .04 | Recent transplantation |
| HLA-A, -B, -DRB1 match | 0.78 (0.60-1.00) | .05 | Match |
| Disease-free survival | |||
| Acute GVHD | 1.93 (1.50-2.47) | .0001 | Grades 0-I |
| AML | 2.04 (1.43-2.91) | .0001 | RA |
| RAEB-t | 1.71 (1.22-2.40) | .002 | RA |
| Cell dose | 0.89 (0.83-0.96) | .003 | Higher |
| Recipient CMV serology | 1.46 (1.16-1.84) | .001 | Negative |
| Time to transplantation (< 9 mo) | 0.78 (0.62-0.98) | .03 | Early transplantation |
| Year of infusion (1988-1993) | 1.43 (1.01-2.01) | .03 | Recent transplantation |
Cumulative incidence rates were as follows: overall graft failure, 13% (95% CI, 10%-16%); primary graft failure, 5% (95% CI, 3%-7%); late graft failure, 8% (95% CI, 6%-10%). Relapse or residual disease was noted in 8 patients who had primary graft failure and in 10 patients who had late graft failure. In multivariate regression analysis, late graft failure was closely associated with lower cell dose (P = .001). MDS subtype, recipient sex, recipient age, CMV status, donor–recipient HLA matching, year of infusion, use of TBI-containing preparative regimens, and time to transplantation were not associated with a significant difference in the risk for late graft failure.
Nine of the 24 patients with primary graft failure underwent second marrow transplantation, and 2 (8%) were alive at the date of analysis. The other 15 patients died of complications related to pancytopenia before they could undergo second transplantation.
Seven of the 33 patients with late graft failure underwent second transplantation, and only one was alive at the time of analysis. Fourteen of the remaining 26 patients recovered marrow function (no chimerism studies are available), and 3 were alive at the date of analysis. Thus, only 4 (12%) of the 33 patients who had late graft failure were long-term survivors.
Graft-versus-host disease
Of 387 patients with sustained engraftment who survived at least 28 days and who were evaluable for the development of acute GVHD, 211 had grades II to IV acute GVHD. Of these 211 patients, 138 had grade III or IV acute GVHD. The cumulative incidence rate at 100 days of grades II to IV acute GVHD was 47% (95% CI, 43%-51%), and of grades III and IV acute GVHD, it was 32% (95% CI, 28%-36%). In univariate analysis, younger recipient age, T-cell depletion, and lower cell dose were associated with a lower incidence of acute GVHD. Multivariate analysis revealed that donor–recipient HLA match, transplantation performed between 1994 and 1998, and use of preparative regimens (not including TBI) were independently associated with a lower incidence of grades III and IV acute GVHD (Table 3).
The cumulative incidence of limited and extensive chronic GVHD at 2 years after transplantation was 27% (95% CI, 24%-30%). In univariate analysis, patients with a history of grades II to IV acute GVHD, older recipients, patients with refractory anemia (RA), patients receiving T-cell–replete marrow grafts and those who received higher cell doses had higher incidences of chronic GVHD. However, in multivariate analysis, only history of grades II to IV acute GVHD was associated with a higher incidence of chronic GVHD after controlling for the transplant center and MDS subtype effects (P < .0001). Donor–recipient HLA matching was not correlated with chronic GVHD.
Relapse
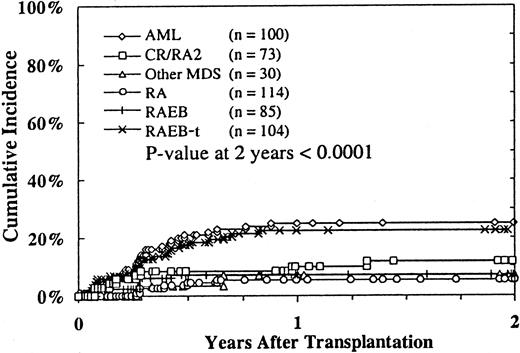
Disease recurred after transplantation in 71 (14%) of 506 patients (in 4 patients, the relapse status was unknown). The 2-year cumulative incidence of relapse was 14% (95% CI, 11%-17%). Multivariate analysis demonstrated that relapse was independently associated with MDS subtype (Table 3; Figure1) and acute GVHD. Patients with RA with excess blasts in transformation (RAEB-t) and patients with MDS in whom AML already developed had higher incidences of relapse than patients with RA. Patients with grades II to IV acute GVHD had lower incidences of relapse. Time to transplantation, donor–recipient HLA matching, use of TBI preparative regimen, T-cell depletion, and chronic GVHD were not independently associated with altered risk for relapse after controlling for the transplant center and MDS subtype effects.
Incidence of relapse according to MDS subtype before transplantation.
Relapse status was unavailable for 4 patients.
Incidence of relapse according to MDS subtype before transplantation.
Relapse status was unavailable for 4 patients.
At the time of analysis, of the 71 MDS patients who had relapse after transplantation, only 4 (6%) were alive with active disease. Six patients died after undergoing second transplantation. Fifteen of the 70 patients with relapsed disease (donor lymphocyte infusion [DLI] data missing for one patient) received infusions of nonirradiated lymphocytes from the original marrow donor. Two patients achieved hematologic remission and subsequently died of other complications.
Disease-free survival
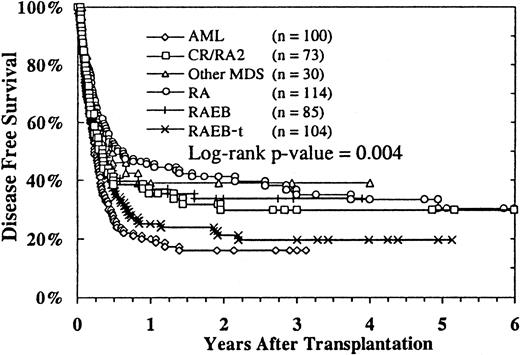
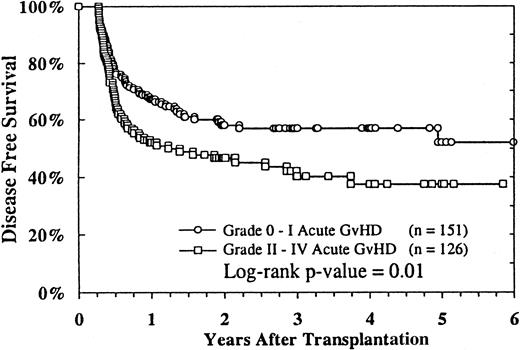
Of the 506 patients whose survival and relapse status were known, 147 were alive in remission with a median follow-up of 24 months (range, 3-97 months). The estimated probability of disease-free survival (DFS) at 2 years was 29% (95% CI, 25%-33%), and at 4 years it was 26% (95% CI, 22%-30%). DFS according to MDS subtype is shown in Figure 2. Patients with RA and RAEB and patients with advanced MDS who were in remission or in a second RA phase had better DFS rates than patients with RAEB-t and MDS-related AML. In multivariate analysis, the diagnosis of RA, higher cell dose, recipient CMV seronegativity, transplantation less than 9 months after diagnosis, grade 0 or I acute GVHD (Figure3), and transplantation from 1994 to 1998 were independently associated with better DFS (Table 3). Recipient age, donor age, donor–recipient HLA matching, use of TBI-containing preparative regimens, T-cell depletion, chronic GVHD, and cell dose were not independently associated with any difference in DFS.
Probability of DFS according to MDS subtypes.
Relapse status was unavailable for 4 patients.
Probability of DFS according to MDS subtypes.
Relapse status was unavailable for 4 patients.
Analysis of cell dose in recipients of non–T-cell–depleted marrow grafts revealed that transplantation of a marrow cell dose above the median value of 2.9 × 108 nucleated cells/kg was associated with faster neutrophil engraftment and better DFS (P = .0001 and .003, respectively).
Analysis of DFS according to etiology, be it idiopathic (n = 396), therapy-related (n = 70), or postaplastic anemia (n = 26), revealed that patients in the latter group did better that those in the 2 other groups (P = .02). There was a higher number of patients with RA in the postaplastic anemia group (P < .001). DFS rates at 2 years for these groups were 29%, 24%, and 59%, respectively. However, though the DFS remained constant for 4 years after transplantation in the idiopathic and therapy-related groups, it declined and reached a plateau of 34% at approximately 4 years in the postaplastic anemia group.
Analysis of DFS according to cytogenetic findings in 361 patients in whom the results of chromosome studies and relapse status were available revealed no significant differences between the 126 patients with normal karyotype and the 235 patients with one or more chromosome abnormalities. DFS rates at 2 years were 34% and 27%, respectively.
Analysis of DFS according to the preparative regimen revealed that patients conditioned with busulfan and cyclophosphamide had better overall survival (OS) and DFS rates and lower relapse rates than patients who were not prepared with this regimen (P = .03, .01, and .01, respectively; log rank test for OS and DFS and χ2 analysis for relapse). In contrast, there were no significant differences in OS, DFS, and relapse rates between patients conditioned with TBI and cyclophosphamide and patients prepared with other preparative regimens.
Survival and functional status
Of the 510 patients, 152 (30%) were alive 3 months to 8 years after transplantation; 147 were free of disease, 4 had relapsed disease, and 1 had unknown disease status. The probability of survival at 2 years was 30% (95% CI, 26%-34%), and at 4 years it was 26% (95% CI, 22%-30%). The probability of survival was higher for patients who did not have grades II to IV acute GVHD (P = .0009) and for patients with RA (P = .008). In multivariate analysis, the diagnosis of RA, higher cell dose, recipient CMV seronegativity, transplantation less than 9 months after diagnosis, transplantation between 1994 and 1998, donor–recipient HLA matching, and grade 0 or I acute GVHD were independently associated with better OS (Table 3).
The functional status of 86 patients surviving 2 years after transplantation was assessed using the Karnosfky (KPS) or Lansky (LPS) activity scores. Sixty-five (76%) patients had normal or near normal activity scores (KPS/LPS, 90%-100%), and 21 (24%) had significant ongoing limitations (KPS/LPS, 50%-80%). In multivariate analysis, the only parameters associated with inferior KPS/LPS were the use of TBI in the preparative regimen, after controlling for the transplant center and disease status effects, and chronic GVHD. Patients who underwent TBI were less likely to have a KPS/LPS of 90% to 100% than patients who did not undergo TBI (P = .03).
Causes of death and treatment-related mortality
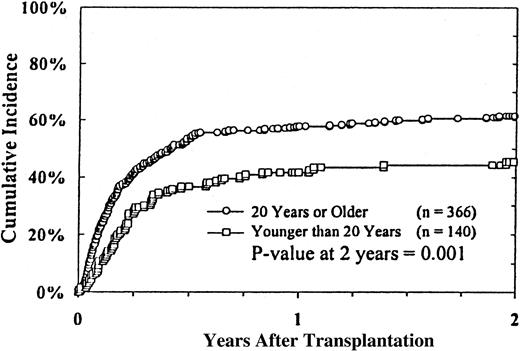
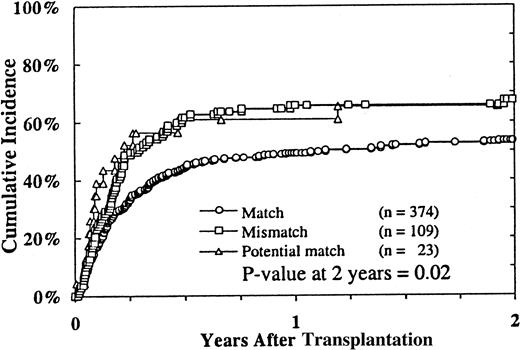
The primary causes of death in 358 (70%) patients are listed in Table 4. Disease relapse was the primary cause of death in 66 (18%) patients. The most common causes of death were treatment-related complications, which occurred in 292 (82%) of the 358 patients. Sixty-nine percent of the treatment-related deaths occurred within the first 100 days and 93% within the first year after transplantation. The 2-year cumulative incidence of TRM was 54% (95% CI, 53%-61%). Infections (28%), acute and chronic GVHD (17%), and regimen-related toxicity (17%) were the most frequent complications leading to death. Fungal infections accounted for 42% of the infectious deaths, whereas bacterial infections accounted for 25% and viral infections for approximately 11% of infectious deaths. Pulmonary (21 of 59 patients) and hepatic (16 of 59 patients) toxicities were the most common causes of mortality from regimen-related toxicity. In multivariate analysis, TRM was closely associated with recipient age (Figure4), donor–recipient HLA mismatching (Figure 5), donor age, patient CMV seropositivity, and grades II to IV acute GVHD (Table 3).
Primary causes of death after unrelated donor marrow transplantation in 358 patients with MDS
| Cause . | No. patients (%) . |
|---|---|
| Graft failure | 8 (2) |
| Regimen-related toxicity | 59 (17) |
| Acute and chronic GVHD | 60 (17) |
| Infections | 101 (28) |
| Other | 64 (18) |
| Relapse | 66 (18) |
| Cause . | No. patients (%) . |
|---|---|
| Graft failure | 8 (2) |
| Regimen-related toxicity | 59 (17) |
| Acute and chronic GVHD | 60 (17) |
| Infections | 101 (28) |
| Other | 64 (18) |
| Relapse | 66 (18) |
Discussion
This study of a large series of patients with MDS confirms earlier NMDP analysis results in 32 patients that marrow transplants derived from histocompatible, unrelated donors can cure a significant proportion of patients with MDS.17 The 2-year DFS of 29% was comparable to those reported by the European (28%, n = 118 patients) and Japanese (23%, n = 33 patients) registries14-16 and was slightly lower than the 2-year DFS of 38% reported from the largest American single institution series of 52 patients.12 The main obstacle to the success of unrelated donor marrow transplantation in this and other series of unrelated donor BMT for MDS12 14-16 has been the high incidence of fatal transplant-related complications. Comparison of unrelated donor BMT results with those of HLA-matched related BMT clearly shows that the main difference is the higher TRM rate and, consequently, lower DFS rate associated with unrelated donor BMT. When donor–recipient relationships were not considered, other patient, disease, transplant, and donor characteristics seemed similar in their influence on the outcomes of either unrelated or related donor BMT. The most significant factors determining a successful outcome in this series of unrelated donor BMT for MDS were disease status before transplantation, level of histocompatibility between donor and recipient, interval from diagnosis to BMT, patient age, patient CMV serologic status, and cell dose.
An association of increased risk for graft failure with HLA disparity between donor and recipient was not found in this study. One limitation of the current analysis was that HLA typing was not at the same level of resolution for all patients. However, in the overall series of 5246 patients who received marrow transplants from unrelated donors facilitated by the NMDP, an association between similarly defined HLA disparities and graft failure was demonstrated.31 HLA disparity has been shown to have a significant correlation with graft failure in related and unrelated donor BMT, though this complication occurs more frequently with unrelated donor transplants.17,32-35 The basis for the higher incidence of graft failure after unrelated donor transplantation is the greater degree of genetic disparity between unrelated and related donor–recipient pairs. This has been demonstrated in several studies in which high-resolution DNA-based techniques have been used for the typing of class 1 and class 2 HLA antigens.36-40 Why there was an absence of such an association in the subgroup of patients who underwent transplantation for MDS is unclear.
The most important variable correlated with the high incidence of acute GVHD in this series was HLA disparity, as shown in other studies.17,41-44 The higher incidence of acute GVHD in unrelated donor BMT than in related donor BMT for the same level of serologic match is attributed largely to molecular differences in HLA-A, -B, and -DRB1 antigens, which cannot be distinguished by serologic methods but can now be defined by more sensitive high-resolution DNA typing methods.36-40 Moreover, recent data suggest that mismatches at other class I and class II HLA alleles, particularly HLA-C and HLA-DQB1, which were not taken into consideration for donor selection in this series, also play a role in the development of acute GVHD in recipients of unrelated donor transplants.34,39 43-46
HLA disparities between donor and recipient also predicted high TRM in this study. This was certainly related to the high incidence of acute and chronic GVHD and associated complications, as shown in this and other series of unrelated donor BMT for MDS12,14-16 and unrelated donor BMT for aplastic anemia47 and leukemia.32,43,48 Other parameters predicting high TRM included increasing recipient age, recipient CMV seropositivity, and donor age. The association of patient age with TRM has been well documented in related donor BMT for MDS1-9 and in the European14,15 and the Seattle12 series of unrelated donor BMT for MDS. Older patients have higher incidences of GVHD and regimen-related complications. The association of CMV seropositivity of the recipient with a high incidence of CMV disease and TRM after unrelated donor transplantation is also well documented.49 50
Posttransplantation relapse rates in this series of unrelated donor transplantation were considerably lower than the reported relapse rates after HLA-matched related marrow grafts.1-9 MDS subtype before transplantation ranked as the single most important prognostic variable affecting relapse risk after either type of transplant. Although patients with more advanced forms of MDS—particularly RAEB-t and AML after MDS—experienced a similarly high relapse rate after receiving unrelated donor marrow grafts, intermediate forms of MDS, notably RAEB, appeared to have lower relapse rates than those reported in series of related donor BMT.1-9 This observation suggests that transplants from unrelated donor applied to more indolent forms of MDS have an enhanced graft-versus-leukemia effect. This is consistent with results reported in other series of unrelated donor transplants for MDS,12 CML,43and advanced AML.48 The reduced incidence of relapse observed among patients with acute GVHD observed in this and the European series14 may reflect the sensitivity of these forms of MDS to alloreactive T cells, a finding also consistent with the observed sensitivity of relapsed MDS to adoptive transfer of donor lymphocytes from HLA-matched related donors.51 52
We could not demonstrate a DFS advantage for T-cell depletion in this heterogenous series of patients who underwent transplantation with marrow grafts depleted of T cells by a variety of methods and achieving different degrees of depletion. Similar results were obtained in the analysis of the European series of unrelated donor BMT for MDS.14 An ongoing prospective trial by the NMDP comparing unmodified to T-cell–depleted BMT may clarify the benefits or lack of benefits of T-cell depletion in unrelated donor stem cell transplantation.
The current study has identified parameters that influence DFS and that may assist in the timing of transplantation and in determining the quality of the marrow allograft. As in BMT for MDS from HLA-matched siblings1-9 and unrelated donor BMT for CML,32,43 patients in this and in the European series14,15 of unrelated donor BMT for MDS fared better if transplantation was performed soon after diagnosis and at an early stage of disease. Therefore, patients with MDS who are candidates for unrelated donor BMT should initiate an unrelated donor search after the diagnosis is confirmed and should proceed with transplantation without delay once a donor has been identified. This analysis also showed that the use of a BU/CY conditioning regimen was associated with improved DFS and a lower incidence of severe acute GVHD and relapse. Similar encouraging findings with a BU/CY regimen have been reported in a single institution study.53 Although potential unrecognized selection bias cannot be excluded in a retrospective analysis, these findings suggest that a busulfan-based conditioning regimen may favorably impact the incidence of acute GVHD, TRM, and DFS without compromising the risk for disease recurrence in patients with MDS.
Another variable predicting better DFS in this study was higher marrow cell dose. The importance of cell dose in determining successful transplant outcomes has been shown in syngeneic BMT for leukemia,54 HLA-matched related donor BMT for leukemia,55-57 and unrelated donor BMT for CML32 and advanced AML.48 The mechanisms involved in this survival benefit are only partly understood. Recent studies58 59 have shown that one reason is the higher number of hematopoietic stem cells, as measured by CD34+cell content, which correlates with higher engraftment, decreased TRM, and increased DFS rates. Our data confirmed the relationship between cell dose and engraftment, but they did not show a clear correlation of cell dose with TRM. The use of peripheral blood stem cell transplants, which contain higher numbers of stem cells and lymphocytes than marrow transplants, may improve outcomes in patients with MDS.
Another finding of our study was that modification of disease status before transplantation resulted in higher DFS because of lower relapse rates. Patients with advanced MDS, particularly RAEB-t and AML after MDS, who had been induced into complete remission or were in a second RA phase before undergoing conditioning, had better DFS and lower relapse rates than patients who underwent transplantation without a change in disease status. The latter group, however, included patients who underwent chemotherapy but did not achieve remission. The beneficial effect of induction of remission before transplantation has been shown in patients with primary or therapy-related MDS who were treated with HLA-matched related BMT.8,60 However, Anderson et al61 could not demonstrate an advantage of remission induction in a series of patients with advanced MDS. The issue of pretransplantation chemotherapy is still controversial, and prospective trials are needed to better assess the benefit of this approach.
Recent studies62 report that some patients with advanced MDS can benefit from autologous stem cell transplantation provided they achieve remission with chemotherapy and have an adequate autologous stem cell graft. De Witte et al15 recently updated the European experience in 126 patients and showed a DFS rate of 33% at 3 years, which is comparable to the DFS rate of 28% observed in this series of patients. Wattel et al63 report a DFS rate of 42% at 4 years in a prospective trial of autologous stem cell transplantation in MDS. The high incidence of relapse in autologous transplantation is counterbalanced by the high TRM rate in unrelated donor BMT. It is of note, however, that only those patients who achieve sustained remission and who have sufficient numbers of hematopoietic precursor cells can proceed to autologous transplantation. Autologous stem cell transplantation is a valid option for the patient who fulfills these criteria and does not have access to a suitable unrelated donor. Because these conditions occur only in a small fraction of patients with advanced MDS and because unrelated donors are found for more that 50% of patients, unrelated donor stem cell transplantation offers a chance of cure for patients who otherwise have limited long-term survival, even after they achieve remission.64 65
In summary, the NMDP experience clearly demonstrates that unrelated donor BMT is a valid therapeutic option for patients with MDS and that the best results are achieved in the early stages of disease and within the first year of diagnosis. Although outcome has improved in the past 10 years as a result of improvements in transplantation methodologies and supportive care measures, high TRM rates are still the main factor limiting the success of unrelated donor BMT in MDS. A number of parameters predicting outcomes have been identified in this study, which should facilitate the design of prospective trials exploring new approaches to improve the results of unrelated donor BMT for MDS. The systematic use of high-resolution DNA-based methods for HLA typing and selection of molecularly matched donors, of less toxic preparative regimens, of better GVHD prophylaxis methods, and of higher cell dose peripheral blood stem cell transplantation should result in improved outcome in the next few years.
We thank the members of the MDS Research Study Group—Drs Douglas Adkins, Joseph H. Antin, Nancy Bunin, James Casper, William Drobyski, Roger Herzig, Alan F. List, Philip MacCarthy, Margaret O'Donnell, George Selby, Mary Territo, Phyllis Warkentin, and John R. Wingard—for their valuable and critical comments, and we thank Darlene Kitajima and Maria Matlack of the NMDP staff for their invaluable help at different stages of this study.
Supported by the MDS Foundation (H.C.-M.), National Institutes of Health grant HL 36444 (H.J.D.), the National Marrow Donor Program, the Health Resources and Services Administration (grant 240-97-0036), and the Office of Naval Research (N00014-93-0658) to the National Marrow Donor Program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hugo Castro-Malaspina, Bone Marrow Transplant Service, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021; e-mail: castro-h@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal