Natural killer (NK) cells are characterized by the ability to kill cells that lack HLA class I molecules while sparing autologous normal (HLA class I+) cells. However, patients with transporter-associated antigen processing (TAP) deficiency, though displaying strong reductions of HLA class I surface expression, in most instances do not experience NK-mediated autoimmune phenomena. A possible mechanism by which TAP−/− NK cells avoid autoreactivity against autologous HLA class I–deficient cells could be based on either quantitative or qualitative defects of surface receptors involved in NK cell triggering. In this study we show that NK cells derived from 2 patients with TAP2−/− express normal levels of all known triggering receptors. As revealed by the analysis of polyclonal and clonal NK cells, these receptors display normal functional capabilities and allow the killing of a panel of NK-susceptible targets, including autologous B-LCLs. On the other hand, TAP2−/− NK cells were unable to kill either allogeneic (HLA class I+) or autologous (HLA class I− ) phytohemagglutinin (PHA) blasts even in the presence of anti-HLA class I monoclonal antibody. These data suggest that TAP2−/− NK cells express still unknown inhibitory receptor(s) capable of down-regulating the NK cell cytotoxicity on binding to surface ligand(s) expressed by T cell blasts. Functional analyses, both at the polyclonal and at the clonal level, are consistent with the concept that the putative inhibitory receptor is expressed by virtually all TAP2−/− NK cells, whereas it is present only in rare NK cells from healthy persons. Another possibility would be that TAP2−/− NK cells are missing a still unidentified triggering receptor involved in NK cell-mediated killing of PHA blasts.
Introduction
The peptide transporter–associated antigen processing (TAP)1,2 is a heterodimer (formed by TAP-1 and TAP-2 subunits) that imports into the lumen of the endoplasmic reticulum the peptides required for a correct assembly of HLA class I molecules. Thus, cells derived from patients displaying defective expression of either of the TAP subunits are characterized by a strong reduction of mature HLA class I molecules at the cell surface.3 It is well known that impaired HLA class I expression renders target cells susceptible to NK-mediated cytotoxicity.4-9 Therefore, in patients with TAP deficiency, NK-mediated autoimmune reactions could occur unless unknown fail-safe mechanisms prevent an attack against autologous normal cells expressing insufficient amounts of HLA class I molecules. In agreement with this concept, freshly isolated TAP2−/− NK cells were unable to kill autologous, HLA class I–negative, B-lymphoblastoid cell lines (B-LCLs).10 However, this tolerance may be broken in cases of inflammation. Indeed TAP−/− patients have been reported to have type 1 bare lymphocyte syndrome that is accompanied in childhood by sinusitis and recurrent bronchitis and in adulthood by chronic lung inflammation and bronchiectasia.3,10 In a recent report, some TAP-deficient adult patients have been described with necrotizing granulomatous lesions in the upper respiratory tract and in the skin, with infiltrating, activated NK or T cell receptor γδ+ cells.11 Thus, at least in these patients, a sustained activation of NK cells, which is likely to occur in the context of recurrent infections and chronic inflammation, may lead to the disruption of self-tolerance by NK cells and, consequently, to autoimmune manifestations. In this context, after culture in the presence of interleukin (IL)-2, TAP2−/− NK cells have been reported to acquire the ability to kill autologous Epstein-Barr virus (EBV)–transformed B-LCLs10 or autologous fibroblasts.12 On the other hand, though some evidence exists for the occurrence of autoimmune phenomena, it is conceivable that TAP−/− NK cells adapt to the surrounding HLA class I–negative microenvironment to avoid inappropriate attacks on otherwise normal cells. A possible mechanism that could allow TAP−/− NK cells to spare autologous normal cells would be based on the defective expression of one or another NK cell–triggering receptor.
In this context, although NK cells from TAP2−/− patients have been shown to express a normally diversified repertoire of HLA class I–specific KIRs,10 no data are available on the expression and function of the recently identified Natural Cytotoxicity Receptors (NCRs).13 In healthy persons, these receptors (including NKp46, NKp30, and NKp44)14-16 are responsible for the induction of NK cell–mediated cytotoxicity against tumor cells and normal allogeneic cells such as phytohemagglutinin (PHA) blasts.17 In the current study, we show that NCRs are expressed at normal levels in NK cells from 2 TAP2−/−patients. More important, in activated TAP2−/− NK cells, NCRs allow killing of a wide range of target cells including allogeneic tumor cell lines and autologous B-LCLs. However, TAP2−/−NK cells did not lyse autologous PHA-induced T cell blasts in spite of the lack of HLA class I expression. Our data also provide suggestive evidence for the existence of receptor–ligand interactions that prevent NK cell–mediated cytotoxicity against autologous normal cells.
Materials and methods
TAP2−/− patients
The 2 patients analyzed (E.M.O. and E.F.A.) were previously described.3 10 E.M.O. and E.F.A. are siblings (12 and 21 years old, respectively) homozygous for a stop mutation in theTAP-2 gene. As a consequence, their cells express less than 3% of HLA class I molecules compared with normal cells. They experience recurrent bacterial sinobronchial infections but have no history of viral infections.
Approval was obtained from the Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki. The use of initials was approved by informed consent by both the patients and the healthy donor.
Purification of peripheral blood lymphocytes and generation of polyclonal or clonal NK populations from patients and from healthy donors
Peripheral blood lymphocytes (PBLs) were derived from healthy donors or from patients by Ficoll-Hypaque gradients and depletion of plastic-adherent cells. To obtain enriched NK cells, PBLs were incubated with anti-CD3 (JT3A), anti-CD4 (HP2.6), and anti–HLA-DR (D1.12) monoclonal antibodies (mAbs) (30 minutes at 4°C) followed by goat antimouse-coated Dynabeads (Dynal, Oslo, Norway) (30 minutes at 4°C) and immunomagnetic depletion.14,16CD3−4−DR− cells were cultured on irradiated feeder cells in the presence of 100 U/mL recombinant IL-2 (Proleukin; Chiron, Emeryville, CA) and 1.5 ng/mL PHA (Gibco, Paisley, Scotland) to obtain polyclonal NK cell populations or, after limiting dilution, NK cell clones. To obtain polyclonal T cell populations, PBL were cultured on irradiated feeder cells in the presence of 100 U/mL rIL-2 and 1.5 ng/mL PHA.18
Monoclonal antibodies
The following mAbs were produced in our laboratory: JT3A (immunoglobulin [Ig]G2a, anti-CD3), BAB281,14 and KL24719 (IgG1 and IgM, respectively, anti-NKp46), Z23116 and KS3820 (IgG1 and IgM, respectively, anti-NKp44), AZ2015 (IgG1, anti-NKp30), MA15221(IgG1, anti-NKp80), PP3519 (IgG1, anti-h2B4), BAT22122 and ECM217 (IgG1 and IgG2b respectively, anti-NKG2-D), QA7923 (IgG1, anti-p75-AIRM-1), E59/12624 (IgG1, anti-IRP60), 1F1 (IgG1, anti-LAIR-1), c127 (IgG1, anti-CD16), c218 and A6/220 (IgG1 and IgM, respectively, anti-CD56), A6-1366 (IgM, anti-HLA class I).
D1.12 (IgG2a, anti-HLA-DR) mAb was provided by Dr R. S. Accolla (Pavia, Italy). MCA531 (IgM, anti-CD20) mAb was purchased from Serotec. HP2.6 (IgG2a, anti-CD4) mAb was provided by Dr P. Sanchez-Madrid (Madrid, Spain).
Flow cytofluorometric analysis
Cells were stained with the appropriate mAb followed by phycoerythrin- or fluorescein isothiocyanate–conjugated, isotype-specific, goat antimouse second reagent (Southern Biotechnology, Birmingham, AL). Samples were analyzed by 1- or 2-color cytofluorometric analysis (FACScan Becton Dickinson, Mountain View, CA) as previously described.14 16
Cell lines, cytolytic assays, and interferon-γ production
Targets used in the cytolytic assays were the following: MEL15 (MEL15392, human melanoma)9; M14 (human melanoma)25; SMMC (human hepatocarcinoma)26; BW1502 (murine thymoma); 721.221 (HLA class I–negative B-lymphoblastoid cell line); LM EBV (B-lymphoblastoid cell line derived from a healthy donor); ST-EMO EBV and ST-EFA EBV (B-lymphoblastoid cell lines derived from the patients). The FcγR-positive P815 (murine mastocytoma) target cell line was used for redirected killing experiments.14-16 Cells were tested for cytolytic activity in a 4-hour chromium Cr 51 release assay in the absence or in the presence of various mAbs. Concentrations of the various mAbs were 10 μg/mL for the masking experiments and 0.5 μg/mL for the redirected killing experiments. E/T ratios are indicated in the text.
For the interferon (IFN)-γ assay, 8 × 105 cultured NK cells derived from each patient or from 2 healthy donors were stimulated overnight in plastic wells precoated or not with each of the following mAbs: c218 (IgG1, anti-CD56, 3 μg/mL), BAB281 (IgG1, anti-NKp46, 3 μg/mL), or c127 (IgG1, anti-CD16 0.5 μg/mL). Cell-free supernatants were harvested and analyzed for IFN-γ by an enzyme immunoassay purchased from Biosource Europe s.a. Nivelles (Belgium).
Results
Cell surface expression of NK cell triggering receptors in TAP2−/− patients
Previous studies indicated that the cytolytic activity of normal NK cells correlated with the surface density of NKp46.26In view of these data, we analyzed whether a defective expression of NKp46 could be responsible for the inability of TAP2−/−NK cells to kill autologous cells. Thus, fresh or cultured NK cells isolated from patients E.M.O. and E.F.A.,3 10 both TAP2−/−, or from healthy donors were assessed by cytofluorometric analysis for the surface expression of various NK cell markers including NKp46.
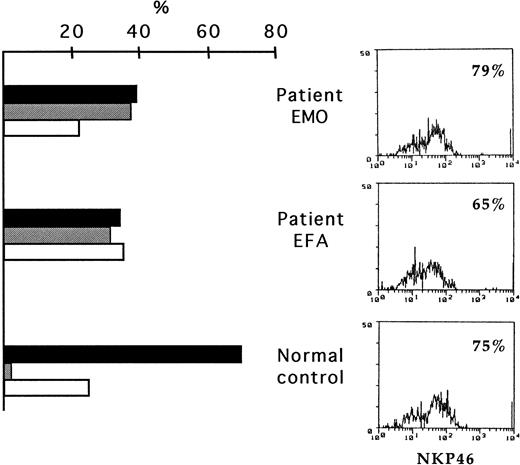
Most (approximately 70%) of fresh TAP2−/− NK cells expressed high surface density of NKp46 (NKp46bright). Thus, their surface phenotype did not significantly differ from those of most healthy persons14 (Figure1). Accordingly, the low levels of cytolytic activity previously detected in fresh NK cells from these 2 patients10 did not appear to correlate with a defective expression of NKp46. In line with the results on the surface expression of NKp46, that of NKp3015 also did not reveal substantial differences in comparison with healthy donors (not shown). However, analysis of the surface expression of CD56 and CD16 revealed the existence of high percentages of CD56++CD16−in TAP2−/− NK cells (30%-40%). Moreover, most cells expressing this phenotype were NKp46bright (Figure 1).
Expression of NKp46 in freshly derived NK cells from TAP2−/− patients.
PBMC from the patients and from a healthy control donor were depleted of CD3+, HLA-DR+, and adherent cells and were analyzed by double fluorescence for the expression of NKp46, CD56, and CD16. This analysis led to the identification of different NK cell subsets. Histograms on the left show the percentages of the CD56+CD16+NKp46bright cells (black bars), CD56brightCD16−NKp46brightcells (gray bars), and CD56+CD16+NKp46dull cells (white bars) in the 2 patients and in the healthy donor. Cytofluorometric profiles on the right show the overall surface expression of NKp46 in the same donors.
Expression of NKp46 in freshly derived NK cells from TAP2−/− patients.
PBMC from the patients and from a healthy control donor were depleted of CD3+, HLA-DR+, and adherent cells and were analyzed by double fluorescence for the expression of NKp46, CD56, and CD16. This analysis led to the identification of different NK cell subsets. Histograms on the left show the percentages of the CD56+CD16+NKp46bright cells (black bars), CD56brightCD16−NKp46brightcells (gray bars), and CD56+CD16+NKp46dull cells (white bars) in the 2 patients and in the healthy donor. Cytofluorometric profiles on the right show the overall surface expression of NKp46 in the same donors.
Next, polyclonal and clonal TAP2−/− NK cell populations, expanded in vitro in the presence of IL-2 for 15 days, were analyzed for the expression of various NK cell markers. Our data indicate that more than 80% of polyclonal NK cells were represented by classical CD56+ CD16+ NKp30 brightNKp46bright cells (Figure 2). This suggests that the CD56++ CD16− subset detected in fresh NK cells may be characterized by a limited capability to undergo proliferation in vitro. As in healthy controls, most NK cells undergoing proliferation also expressed high levels of NKp44 (Figure 2), (expressed only on in vitro culture of NK cells).16 Polyclonal NK cell populations also expressed normal levels of 2B4 and NKG2-D, whereas the expression of NKp80 was decreased (Figure 2) compared with that in most healthy donors.21
Expression of various triggering receptors in cultured TAP2−/− NK cells.
NK cells from patient E.M.O. were cultured in the presence of IL-2 for 15 days and were analyzed by cytofluorometry. (upper panels) Expression of CD16 and NCRs (NKp46, NKp30, and NKp44). (lower panels) Expression of 2B4, NKG2-D, and NKp80. Cells were analyzed by double fluorescence for the expression of NKp46 in combination with CD16 or by single fluorescence for the expression of the other indicated molecules. Comparable results were obtained with NK cells from E.F.A.
Expression of various triggering receptors in cultured TAP2−/− NK cells.
NK cells from patient E.M.O. were cultured in the presence of IL-2 for 15 days and were analyzed by cytofluorometry. (upper panels) Expression of CD16 and NCRs (NKp46, NKp30, and NKp44). (lower panels) Expression of 2B4, NKG2-D, and NKp80. Cells were analyzed by double fluorescence for the expression of NKp46 in combination with CD16 or by single fluorescence for the expression of the other indicated molecules. Comparable results were obtained with NK cells from E.F.A.
NK cell clones derived from the same TAP2−/− patients were further analyzed. In line with previous data indicating that TAP2−/− NK cells display poor proliferative capacity,10 NK cells in E.M.O. and E.F.A. were characterized by low clonal efficiency. However, sufficient numbers of clones were obtained to allow further phenotypic and functional analyses. In agreement with data on polyclonal cell populations, most (approximately 80%) NK cell clones expressed the CD16+NKp46bright surface phenotype. The remaining clones were either CD16+ NKp46dull (approximately 17%) or CD16− NKp46bright (3%).
TAP2−/− NK cell clones were further studied for the surface expression of various receptors and coreceptors known to be involved in the regulation of the NK-mediated cytolytic activity.17 Thus, in addition to NKp30 and NKp44, we also analyzed 2B4, NKp80, and NKG2-D. All NK cell clones displayed a normal 2B4+ NKG2-D+ phenotype, whereas the levels of surface expression of NKp30 and NKp44 correlated with that of NKp46, as previously established in healthy donors.26 Similarly, expression levels of NKp80 appeared to correlate, at least in part, with those of NCR (not shown) though we could observe some clones expressing an NCRbright phenotype that were NKp80dull.
Analysis of the triggering capability of different receptors in TAP2−/− NK cells
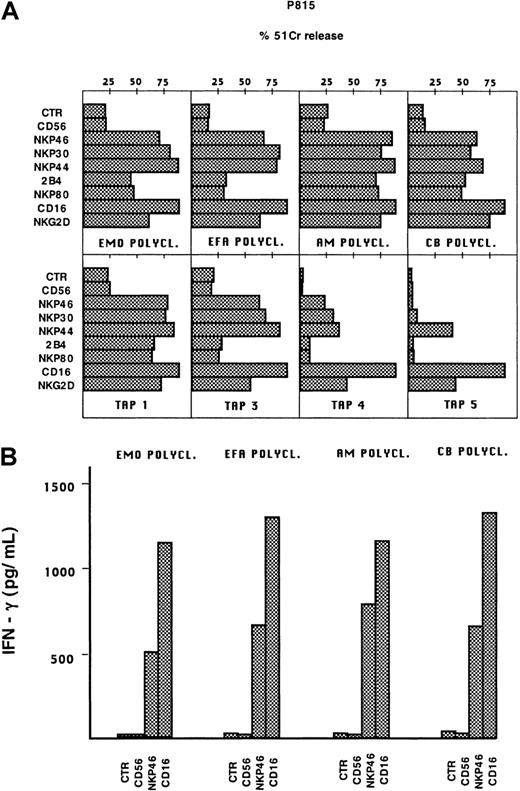
We next assessed whether the various triggering receptors expressed on TAP2−/− NK cells were functionally competent and could induce NK cell activation. To this end, polyclonal NK cell populations or clones derived from patients or from healthy controls were analyzed in a redirected killing assay using P815 target cells14-16 (Figure 3A). These experiments were performed in the absence or in the presence of mAbs specific for various triggering receptors, including NKp46, NKp44, NKp30, CD16, NKp80, 2B4, and NKG2-D. In the presence of mAb directed to NCR, NKG2-D, or CD16, the cytolytic responses of TAP2−/−polyclonal NK cells were similar to those elicited by normal NK cells, whereas lower responses were elicited by anti-2B4 or anti-NKp80 mAbs. As revealed by clonal analysis, TAP2−/− NK cell clones characterized by the NKp46bright phenotype were strongly cytolytic in response to anti-CD16, anti-NKp44, anti-NKp30, or anti-NKG2-D mAbs. At variance with healthy donors, some clones expressing the NCRbright phenotype gave poor responses to anti-2B4 or anti-NKp80 mAbs (see clone TAP3). As expected, NCRdull clones were poorly responsive to most stimuli with the exception of anti-CD16 and anti-NKG2-D mAbs.19,22 26
Cytolytic activity and IFN-γ production by TAP2−/− NK cells after stimulation with mAbs against triggering receptors.
(A) Effector cells were analyzed in a redirected killing assay against the FcγR+ P815 target cells in the absence (CTR) or in the presence of the following mAbs: BAB281 (IgG1, anti-NKp46); AZ20 (IgG1, anti-NKp30); Z231 (IgG1, anti-NKp44); PP35 (IgG1, anti-2B4); MA152 (IgG1, anti-NKp80); c127 (IgG1, anti-CD16); BAT221 (IgG1, anti-NKG2-D), and c218 (IgG1, anti-CD56). In this representative experiment, the polyclonal NK cell populations derived from the 2 patients (E.M.O. and E.F.A.) and 2 healthy donors (A.M. and C.B.) and 4 clones from E.M.O. (TAP1, TAP3, TAP4, TAP5) are shown (similar results could be obtained with E.F.A. clones). NK clones displayed the following phenotypes: TAP1 (NCRbright 2B4+NKp80+), TAP3 (NCRbright 2B4+NKp80+), TAP4 (NCRdull 2B4+NKp80+), TAP5 (NCRdull 2B4+NKp80dull). The E:T ratio used in this experiment was 4:1. (B) The same polyclonal NK cell populations were assessed for IFN-γ production either in the absence of stimulation (CTR) or after stimulation with each of the following mAb: c218 (IgG1, anti-CD56, 3μg/mL), BAB281 (IgG1, anti-NKp46, 3 μg/mL), c127 (IgG1, anti-CD16 0.5 μg/mL).
Cytolytic activity and IFN-γ production by TAP2−/− NK cells after stimulation with mAbs against triggering receptors.
(A) Effector cells were analyzed in a redirected killing assay against the FcγR+ P815 target cells in the absence (CTR) or in the presence of the following mAbs: BAB281 (IgG1, anti-NKp46); AZ20 (IgG1, anti-NKp30); Z231 (IgG1, anti-NKp44); PP35 (IgG1, anti-2B4); MA152 (IgG1, anti-NKp80); c127 (IgG1, anti-CD16); BAT221 (IgG1, anti-NKG2-D), and c218 (IgG1, anti-CD56). In this representative experiment, the polyclonal NK cell populations derived from the 2 patients (E.M.O. and E.F.A.) and 2 healthy donors (A.M. and C.B.) and 4 clones from E.M.O. (TAP1, TAP3, TAP4, TAP5) are shown (similar results could be obtained with E.F.A. clones). NK clones displayed the following phenotypes: TAP1 (NCRbright 2B4+NKp80+), TAP3 (NCRbright 2B4+NKp80+), TAP4 (NCRdull 2B4+NKp80+), TAP5 (NCRdull 2B4+NKp80dull). The E:T ratio used in this experiment was 4:1. (B) The same polyclonal NK cell populations were assessed for IFN-γ production either in the absence of stimulation (CTR) or after stimulation with each of the following mAb: c218 (IgG1, anti-CD56, 3μg/mL), BAB281 (IgG1, anti-NKp46, 3 μg/mL), c127 (IgG1, anti-CD16 0.5 μg/mL).
We next assessed whether the cytolytic activity of TAP2−/− NK cells paralleled the ability to produce cytokines (ie, IFN-γ). As shown in Figure 3B, polyclonal NK cells from E.M.O. and E.F.A., after stimulation with anti-NKp46 or anti-CD16 mAbs, were able to produce levels of IFN-γ comparable to those of healthy donors.
TAP2−/− NK cells efficiently kill tumor or EBV-transformed target cells
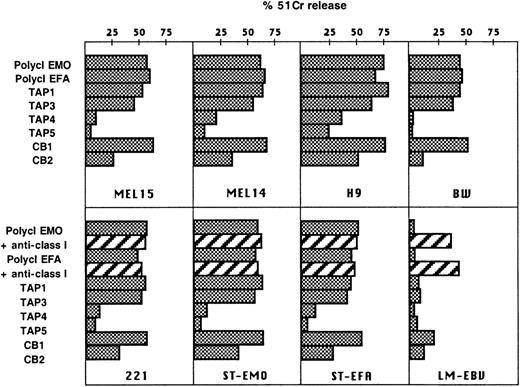
TAP2−/− NK cells were analyzed for their spontaneous cytotoxicity against a panel of NK-susceptible target cell lines.9,25,26 These included the MEL14 and MEL15 melanomas (Figure 4), the SMMC hepatocarcinoma (not shown), the H9 T cell lymphoma, the BW1502 murine thymoma, and the HLA class I–negative EBV-LCL 221 (Figure 4). Polyclonal NK cell populations derived from TAP2−/− patients did not display significant differences in their ability to lyse these targets compared with healthy controls. Similar results were obtained with TAP2−/− NK cell clones. In particular, the NCRbright NK cell clones (see the representative TAP1 and TAP3 clones) were highly cytolytic, whereas the NCRdullclones (eg, TAP 4 and TAP 5 clones) displayed poor cytolytic activity against the same targets (Figure 4). Remarkably, similar results were also obtained when TAP2−/− NK clones were analyzed against autologous B-LCLs (Figure 4). These data clearly imply that allogeneic tumors and autologous B-LCLs express ligands recognized by the triggering receptors expressed by TAP2−/− NK cells. This was further confirmed by the finding that mAb-mediated masking of the various NCR (using KL247, KS38, and AZ20 mAbs) could strongly inhibit cytotoxicity against the above targets (not shown). Consistent with previous results, killing of autologous B-LCLs was not modified by the addition of anti-HLA class I mAb.10 This would mean that the low levels of HLA class I molecules3 expressed by TAP2−/− B-LCLs, possibly associated with TAP-independent viral peptides,27 do not affect the lysis of these targets mediated by autologous NK cells. On the other hand, lysis of allogeneic HLA class I+ B-LCLs (LM EBV) by TAP2−/− NK cells was increased in the presence of anti-HLA class I mAb (Figure 4). This result is consistent with our previous observation that TAP2−/− NK cells express functional KIR.10
Cytolytic activity of TAP2−/− NK cells against tumor or EBV-transformed targets.
TAP2−/− polyclonal NK cell populations and clones (TAP1, TAP3, TAP4, TAP5) were assessed for cytotoxicity in comparison with 2 representative clones derived from a healthy donor: CB1 (NCRbright) and CB2 (NCRdull). (upper panels) Effectors cells were analyzed in a cytolytic assay against the indicated tumor target cells (E:T ratio, 4:1). (lower panels) The same effector cells were analyzed against different EBV-transformed B-LCLs, including the HLA class I-negative 721.221 LCL, ST-EMO LCL, ST-EFA LCL, and LM-EBV LCL (derived from a healthy donor) (E:T ratio, 4:1). The TAP2−/− polyclonal NK cells were assessed for cytotoxicity in the absence or in the presence of anti-HLA class I mAb (A6/136 IgM).
Cytolytic activity of TAP2−/− NK cells against tumor or EBV-transformed targets.
TAP2−/− polyclonal NK cell populations and clones (TAP1, TAP3, TAP4, TAP5) were assessed for cytotoxicity in comparison with 2 representative clones derived from a healthy donor: CB1 (NCRbright) and CB2 (NCRdull). (upper panels) Effectors cells were analyzed in a cytolytic assay against the indicated tumor target cells (E:T ratio, 4:1). (lower panels) The same effector cells were analyzed against different EBV-transformed B-LCLs, including the HLA class I-negative 721.221 LCL, ST-EMO LCL, ST-EFA LCL, and LM-EBV LCL (derived from a healthy donor) (E:T ratio, 4:1). The TAP2−/− polyclonal NK cells were assessed for cytotoxicity in the absence or in the presence of anti-HLA class I mAb (A6/136 IgM).
TAP2−/− NK cells are unable to kill autologous PHA blasts
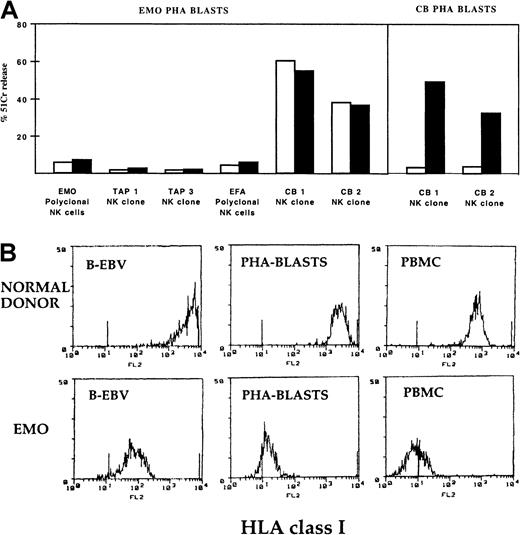
Taken together, the data above suggest that fully competent and potentially harmful NK cells do exist in TAP2−/−patients. In view of these findings, we further analyzed whether such potentially autoreactive TAP2−/− NK cells were also capable of killing normal, nonvirally infected (HLA class I negative) autologous target cells. To this end, cultured polyclonal TAP2−/− NK cells were assessed for their ability to lyse autologous PHA-induced T cell blasts. As shown in Figure5A, though these target cells were highly susceptible to cytotoxicity mediated by selected normal allogeneic NK cells from different donors (note 2 representative clones, CB 1 and CB 2, derived from donor CB in Figure 5A), they were not killed by TAP2−/− polyclonal or clonal autologous NK cells. Thus, contrary to EBV-infected cells (B-LCL), normal PHA blasts resulted fully protected from autologous TAP2−/− NK cells. As shown in Figure 5B, TAP2−/− PHA blasts expressed an HLA class I surface density lower than that of TAP2−/−B-EBV–transformed cells, thus suggesting that HLA class I molecules are unlikely to be important in the resistance of TAP2−/−PHA blasts to autologous NK cells. Indeed, the addition of anti-HLA class I mAbs had no effect on the lysis of TAP2−/− PHA blasts mediated by either normal or TAP2−/− NK cells. On the other hand (as expected), in healthy donors (eg, donor C.B.) lysis of PHA blasts by autologous NK cells occurred in the presence of anti-HLA class I mAb.
TAP2−/− NK cells are unable to kill autologous PHA blasts.
(A) Polyclonal NK cell populations derived from E.M.O. or from E.F.A. and 2 clones from E.M.O. displaying the NCRbrightphenotype were assessed for cytolytic activity against E.M.O. PHA blasts either in the absence (white bars) or in the presence (black bars) of anti-HLA class I mAb. Controls were represented by 2 clones (CB1 and CB2) derived from a healthy donor that were analyzed against E.M.O. PHA blasts and against autologous PHA blasts (CB PHA blasts). The E:T ratio used in this representative experiment was 10:1. (B) HLA class I surface density on the indicated cell populations derived from E.M.O. and from a healthy control donor. The different cell types were stained with anti-HLA class I mAb (A6/136 IgM) and then were analyzed by cytofluorometry. HLA class I expression levels in cells derived from E.F.A. were comparable to those detected in E.M.O.
TAP2−/− NK cells are unable to kill autologous PHA blasts.
(A) Polyclonal NK cell populations derived from E.M.O. or from E.F.A. and 2 clones from E.M.O. displaying the NCRbrightphenotype were assessed for cytolytic activity against E.M.O. PHA blasts either in the absence (white bars) or in the presence (black bars) of anti-HLA class I mAb. Controls were represented by 2 clones (CB1 and CB2) derived from a healthy donor that were analyzed against E.M.O. PHA blasts and against autologous PHA blasts (CB PHA blasts). The E:T ratio used in this representative experiment was 10:1. (B) HLA class I surface density on the indicated cell populations derived from E.M.O. and from a healthy control donor. The different cell types were stained with anti-HLA class I mAb (A6/136 IgM) and then were analyzed by cytofluorometry. HLA class I expression levels in cells derived from E.F.A. were comparable to those detected in E.M.O.
As shown above, most TAP2−/− NK cell clones were characterized by reduced responses to anti-2B4 or anti-NKp80 mAbs in redirected killing assays. The inability to kill autologous PHA blasts could be related to the low triggering capability of these surface molecules. This possibility, however, was ruled out by the finding that some selected TAP2−/− NK cell clones displayed normal responses to anti-2B4 or anti-NKp80 mAbs, whereas they still failed to lyse autologous PHA blasts (see clone TAP 1 in Figure 5). These data suggest that PHA-induced T cell blasts (and possibly other normal tissues) from TAP2−/− patients are protected from NK cells. This protection could reflect the expression of a still unknown cell surface ligand that may be lost (or modified) in EBV-transformed cells. We investigated whether such putative protective ligand was selectively expressed by TAP2−/− PHA blasts. Cultured polyclonal NK cells derived from patient E.M.O. were analyzed for their ability to lyse a panel of normal allogeneic PHA blasts (including CB PHA blasts). These experiments were performed in the absence or in the presence of anti-HLA class I mAbs (to avoid interferences by KIRs expressed by TAP2−/− NK cells) (Figure6). Remarkably, all the allogeneic PHA blasts analyzed were resistant to lysis by TAP2−/− NK cells, thus suggesting that the expression of the putative protective ligand is not restricted to TAP2−/− PHA blasts. The presence of a ligand that protects PHA blasts from lysis by TAP2−/− NK cells implies that these cells express an inhibitory receptor specific for this ligand. Is this inhibitory receptor expressed also by NK cells from healthy controls? To answer this question, NK cell clones derived from healthy donors were assessed for their ability to lyse TAP2−/− PHA blasts (Figure7, left panel). Most such clones were highly cytolytic; however, a minor subset (less than 10%) failed to kill TAP2−/− PHA blasts. Similar results were obtained against autologous or allogeneic PHA blasts (in this case, in the presence of anti-HLA class I mAb). It is of note that these rare NK cell clones from healthy donors were strongly cytolytic against TAP2−/− EBV-infected target cells (Figure 7, right panel) or against the various tumor cells analyzed above (not shown). In most instances, these clones expressed the NCRbright phenotype. Taken together, these data suggest that the putative inhibitory receptor that allows TAP2−/− NK cells to spare normal autologous cells (PHA blasts) is expressed also by a minor subpopulation of normal NK cells.
Cytolytic activity mediated by TAP2−/− NK cells against a panel of allogeneic PHA blasts.
Polyclonal NK cells derived from E.M.O. or from a healthy donor (C.B.) were assessed for cytolytic activity against PHA blasts derived from different donors in the absence (white bars) or in the presence (black bars) of anti-HLA class I mAb. The E:T ratio was 10:1.
Cytolytic activity mediated by TAP2−/− NK cells against a panel of allogeneic PHA blasts.
Polyclonal NK cells derived from E.M.O. or from a healthy donor (C.B.) were assessed for cytolytic activity against PHA blasts derived from different donors in the absence (white bars) or in the presence (black bars) of anti-HLA class I mAb. The E:T ratio was 10:1.
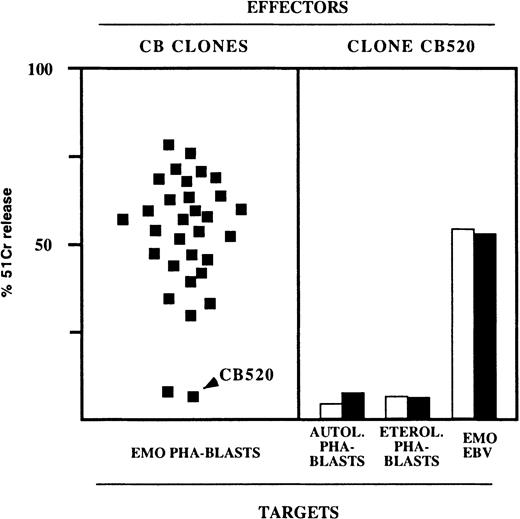
Rare NK clones derived from healthy donors are unable to kill TAP2−/− PHA blasts.
(left panel) Representative experiment in which a large number of NK clones derived from a healthy donor (C.B.) were assessed for cytolytic activity against E.M.O. PHA blasts in the presence of anti-HLA class I mAb (each square represents the cytotoxicity of an individual clone). (right panel) NK clones displaying no cytolytic activity against E.M.O. PHA blasts were also unable to kill autologous or heterologous PHA blasts but killed B-EBV target cells. In this experiment, a representative clone (CB 520) was assessed for cytotoxicity against the indicated target in the absence (white bars) or in the presence (black bars) of anti-HLA class I mAb. The E:T ratio was 10:1 (PHA blasts) or 4:1 (B-EBV targets).
Rare NK clones derived from healthy donors are unable to kill TAP2−/− PHA blasts.
(left panel) Representative experiment in which a large number of NK clones derived from a healthy donor (C.B.) were assessed for cytolytic activity against E.M.O. PHA blasts in the presence of anti-HLA class I mAb (each square represents the cytotoxicity of an individual clone). (right panel) NK clones displaying no cytolytic activity against E.M.O. PHA blasts were also unable to kill autologous or heterologous PHA blasts but killed B-EBV target cells. In this experiment, a representative clone (CB 520) was assessed for cytotoxicity against the indicated target in the absence (white bars) or in the presence (black bars) of anti-HLA class I mAb. The E:T ratio was 10:1 (PHA blasts) or 4:1 (B-EBV targets).
Discussion
In this study we provide evidence that the NK cells isolated from TAP2−/− patients do not present abnormalities in the level of expression and function of various triggering receptors involved in natural cytotoxicity. The finding that TAP2−/− NK cells can efficiently lyse tumor cells or B-LCL while sparing normal PHA blasts is likely to reflect the expression of a protective ligand on these normal cells. Moreover, functional data indicate that this ligand interacts with a putative inhibitory receptor expressed by all TAP2−/− NK cells but only by a minor fraction (less than 10%) of NK cells from healthy donors.
A mechanism by which NK cells from TAP2−/− patients could spare autologous cells is the lack or the down-regulation of triggering receptors responsible for NK cell activation in the lysis of HLA class I–negative target cells.17 Although in healthy persons the loss of HLA class I occurs in cells undergoing tumor transformation or viral infection,28 in TAP2−/− patients the lack of expression of HLA class I is a feature common to all tissues. Thus, in TAP2−/− patients, to avoid the NK-mediated attack to self-cells, the NK cell receptor repertoire could be shaped toward the selection of NK cells with impaired cytolytic activity. To analyze this possibility we first performed phenotypic analysis of NK cells freshly isolated from 2 TAP2−/−patients.3,10 These studies revealed a significantly higher proportion of CD56++CD16− NK cells in TAP2−/− patients (30%-40%) than in healthy controls (less than 10%). NK cells characterized by this phenotype have been reported to be poorly cytolytic and are thought to represent relatively immature NK cells.29-31 In this context, the tolerance of resting TAP2−/− NK cells toward autologous cells could be, at least in part, the result of a block of NK cell maturation. However, this cannot fully explain why fresh NK cells are poorly cytolytic against HLA class I–negative targets (such as K562) or autologous (TAP2−/−) B-LCL.10 An additional explanation could be the absence or low level of expression of triggering NK receptors. As established by previous studies,26 in freshly isolated NK cells a strict correlation exists between the level of surface expression of NKp46 (bright vs dull) and the magnitude of NK-mediated cytotoxicity against HLA class I–negative target cells such as K562. Thus, we analyzed the NKp46 phenotype of resting TAP2−/− peripheral NK cells. NKp46 was expressed at high density in most NK cells. Thus, it appears that, in TAP2−/− patients, no direct correlation exists between low levels of cytotoxicity of fresh NK cells and their NKp46 phenotype. Because approximately 40% of the NKp46bright NK cells from TAP2−/− donors expressed the CD56++CD16− phenotype, it is conceivable that the low cytolytic activity of resting TAP2−/− NK cells may be explained on the basis of their stage of maturation.
It has been suggested that autoreactive phenomena described in some TAP−/− patients with chronic inflammation are mediated by activated NK cells.11 Thus, we analyzed in more detail the expression and the function of various triggering NK receptors in activated NK cells derived from TAP2−/− patients. Polyclonal NK cell populations and NK cell clones cultured in the presence of IL-2 have been analyzed. These studies revealed normal levels of expression and function of the natural cytotoxicity receptors (including NKp46, NKp30, and NKp44)13 and of NKG2-D.22,32 On the other hand, in some instances a defective functional response to stimuli acting through 2B419,33-36 and NKp8021 could be observed. Remarkably, most of the cultured TAP2−/− NK cells or clones expressed CD16. This implies that the “immature” CD56++CD16− subset in fresh NK cells underwent poor proliferation in vitro (or underwent maturation?). The cytolytic activity of TAP2−/− NK cell clones against NK-susceptible tumor target cells was comparable to that of normal NK clones. This would mean that triggering receptors expressed by cultured TAP2−/− NCRbright NK cells can induce NK cell activation on interaction with tumors or virally infected cells (eg, autologous B-LCLs). Although not shown, TAP2−/− NK cells could kill autologous fibroblasts, thus confirming our previous report.12 This capability was restricted to NKp46bright NK cells, and lysis was inhibited by mAb-mediated masking of NKp46. These data raise the question of whether the NKp46-dependent killing of autologous fibroblasts may be involved in the pathogenesis of the autoimmune manifestations in some HLA class I–deficient patients. In this context, it will be interesting to test whether the development of vasculitis or granuloma in TAP-deficient patients correlates with higher proportions of NKp46brightcells.
The potential autoreactivity of TAP2−/− NK cells was further evaluated against a different source of normal autologous cells represented by PHA-induced T cell blasts. Significantly, TAP2−/− PHA blasts were resistant to autologous NK cells, yet they were highly susceptible to lysis by NK cells from healthy donors. This strongly suggests that TAP2−/− PHA blasts express ligands recognized by triggering NK receptors. In agreement with this concept, mAb-mediated masking of NCRs prevented the killing of TAP2−/− PHA blasts by normal NK cells (as shown by previous studies, NCRs are required for the NK-mediated lysis of PHA blasts).15 Thus, our current data on TAP2−/−NK cells are clearly reminiscent of previous data obtained in β2-microglobulin–deficient or TAP-1 knockout mice.37 38These studies showed that β2-m− NK cells, while sparing autologous concanavalin A blasts and bone marrow cells, could lyse allogeneic major histocompatibility complex class I−tumors. In view of our current finding that TAP2−/− NK cells express functional NCR, it is unlikely that they fail to kill PHA blasts because of the lack of still undefined triggering receptor(s). We favor the hypothesis that the observed phenomenon may reflect the occurrence of an interaction between a still undefined inhibitory receptor expressed on TAP2−/− NK cells and its ligand on PHA blasts. This explanation is also supported by a series of additional data: (1) lysis of PHA blasts could not be restored by the addition of anti-HLA class I mAbs (Figures 5, 6) or by the mAb-mediated masking of one or another HLA class I–specific inhibitory receptor (data not shown); (2) TAP2−/− NK cells failed to lyse autologous and allogeneic PHA blasts, thus suggesting that the putative protective ligand(s) is not confined to TAP2−/− cells; (3) NK cells that fail to kill PHA blasts are present also in healthy donors, but they are scarcely represented within the total NK cell pool; (4) in healthy persons, these rare NK cells display an NCRbright phenotype, indicating that the lack of cytolytic activity against PHA blasts is not the result of a general inability to lyse but rather of the occurrence of an inhibitory interaction (this is also supported by the finding that NK cells displayed a strong cytolytic activity against various NK-susceptible tumor target cells).
Taken together, our data indicate that to avoid attacks on normal cells, shaping of the NK cell receptor repertoire in TAP2−/− patients is not based on the down-regulation of triggering receptors. Rather, it appears to be skewed toward the selection of NK cells expressing a still undefined inhibitory receptor. Remarkably, in healthy persons, this putative receptor is expressed on a minor fraction of NK cells. The restricted expression of this inhibitory receptor rules out the possible involvement of previously identified, non-HLA–specific inhibitory receptors including LAIR-1,39,40 p75/AIRM1,23 and IRp60,24 because they are expressed by virtually all normal NK cells. That they are not involved in this phenomenon was also directly demonstrated by experiments in which mAb-mediated masking of these receptors did not allow TAP2−/− NK cells to lyse autologous PHA blasts (not shown).
Regarding the nature of the putative ligand(s) for the inhibitory receptor, it does not seem to be related to classical HLA class I molecules. Indeed, it is expressed on TAP2−/− cells, and lysis of target cells could not be reversed by the addition of conventional anti-class I mAbs. Notably, its expression seems to be altered in B-LCLs (because these cells are lysed by TAP2−/− NK cells). This may suggest down-regulation of the ligand as a consequence of viral infection, or it may suggest that its expression is constitutively different in T cells and B cells. Future studies should focus on the identification and molecular characterization of receptor(s) and ligand(s) involved in this novel inhibitory interaction.
We thank Tiziana Baffi for secretarial assistance.
Supported by grants from Fondazione Italiana per la Ricerca sul Cancro, Associazione Italiana per la Ricerca sul Cancro, Istituto Superiore di Sanità, Ministero della Sanità, and Ministero dell'Università e della Ricerca Scientifica e Tecnologica, Consiglio Nazionale delle Ricerche, Progetto Finalizzato Biotecnologie, MURST—CNR 5% CNR Biotechnology program 95/95, Telethon-Italy (grant E.0892), Etablissement Francaise du Sang-Alsace, and Fondation Touraine.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alessandro Moretta, Dipartimento di Medicina Sperimentale, Sezione di Istologia, Via G.B. Marsano 10, 16132 Genova, Italy; e-mail: alemoret@unige.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal