Techniques for the quantitation of virus-specific and alloantigen-reactive T cells vary in their measurement of clinically relevant T-cell effector populations, their sensitivity and quantitative accuracy, and the time required to obtain measurable results. We compared frequencies of Epstein-Barr virus (EBV)–specific and major alloantigen-reactive T cells as measured by flow cytometric analysis of responding T cells producing intracellular interferon-γ (IFN-γ) and by limiting-dilution analysis (LDA) of cytotoxic T-cell precursors (CTLp) at sequential time points during the generation of EBV-specific T-cell lines. The expansion of EBV-specific T lymphocytes and the depletion of alloreactive T cells in cultures of T cells sensitized with autologous EBV-transformed targets followed similar kinetics when measured by either method. Frequencies of EBV- specific T cells generating intracellular IFN-γ exceeded by 25- to 90-fold the frequencies of responding CTLp at each stage of expansion, whereas the frequencies of alloreactive T cells generating intracellular IFN-γ exceeded by 30- to 220-fold those detected by LDA. The assay that quantitated T cells producing IFN-γ yielded more reproducible and precise results than LDA. Furthermore, frequencies detected by the enumeration of T cells responding to immunodominant EBNA 3a and EBNA 3c peptides by IFN-γ production or their capacity to bind peptide-HLA tetramers were strikingly similar and represented significant fractions of T cells generating IFN-γ in response to autologous EBV B lymphoblastoid cell line. Functional analysis of responding viable T cells, fractionated on the basis of their secretion of IFN-γ, demonstrated that EBV-specific and alloantigen cytotoxic T cells were predominately or exclusively detected in the CD8+IFN-γ+ fraction of T cells. Strikingly, the CD4+IFN-γ+ cell fractions were not cytotoxic against EBV-transformed or allogeneic targets.
Introduction
Adoptive immunotherapy with donor leukocytes or Epstein-Barr virus (EBV)–specific T-cell infusions is effective in inducing durable remissions of monoclonal EBV-lymphoproliferations complicating allogeneic bone marrow transplantation.1,2However, infusions of peripheral blood mononuclear cells (PBMCs) or donor-derived EBV-sensitized T-cell lines early in their development may also contain various numbers of alloreactive T cells capable of inducing severe graft-versus-host disease (GVHD). Determination of antigen-specific and alloreactive T-cell frequencies in these cell fractions before adoptive transfer can provide a means for estimating the likelihood of a subsequent tumor response and of the risk for GVHD.1-4
Limiting-dilution analysis (LDA) is a standard method for the determination of cytotoxic T-cell precursor (CTLp) frequencies.5,6 Previous reports by Lucas et al7 and others8 have illustrated the feasibility and usefulness of monitoring CTLp frequencies to EBV after allogeneic marrow transplantation and after adoptive T-cell therapy of EBV-lymphoproliferative disorders. However, LDA is labor intensive. Twelve days are needed to generate results, and LDA underestimates the frequencies of antigen-reactive T cells detectable by other methods.9 10 Recently, techniques using Brefeldin A (Sigma, St Louis, MO) to inhibit cytokine secretion and anti–interferon-γ (IFN-γ) monoclonal antibodies to stain intracellular IFN-γ after T-cell fixation and permeabilization have permitted quantitation of IFN-γ+ cells in specific T-cell subpopulations by fluorescence-activated cell sorter (FACS) analysis within 2 days of test initiation. In this study, we have compared the EBV-specific and alloreactive T-cell frequencies detected by intracellular cytokine staining or by LDA at serial time points during the generation of EBV-specific T-cell lines from healthy seropositive donors. We have also compared the reproducibility and the precision of the 2 assays. In addition, we determined the cytotoxic potential of purified viable IFN-γ–secreting CD8+ and CD4+ antigen-specific T cells and compared this activity with that of isolated IFN-γ− subpopulations.
Materials and methods
Generation of EBV-specific T cells
EBV-specific cytotoxic T lymphocytes (CTLs) were generated from healthy EBV-seropositive donors as previously described.4 In brief, T cells were isolated from blood by using Ficoll-Hypaque gradient separation of peripheral blood mononuclear cells and then by using negative selection of CD3+ T cells by depletion of CD20+ B cells, CD14+ monocytes, and CD56+ NK cells with monoclonal antibody-coated immunomagnetic beads (Miltenyi Biotec, Auburn, CA). Thereafter, aliquots of the T-cell–enriched population at 1 × 106 cells/mL were stimulated with 2.5 × 104/mL 60 Gy-irradiated autologous EBV-transformed B lymphoblastoid cell line (BLCL) in Iscoves modified Dulbecco medium supplemented with 10% heat-inactivated human AB serum (Gemini, Calabasas, CA), for 7 days in 25-cm2 flasks. Cells were restimulated weekly at an effector-stimulator ratio of 5:1 and were recultured at a concentration of 1 × 106/mL. Interleukin-2 (5 IU; Collaborative Biomedical Products, Bedford, MA) was added to the cultures on day 10 and 2 to 3 times a week thereafter.
Alloantigen-reactive T cells were generated using the same culture conditions, but by stimulating PBMCs with EBV BLCL generated from an HLA-A– and -B–disparate unrelated healthy donor. Quantitation of EBV-reactive and alloresponsive T cells by LDA and by measurements of IFN-γ–producing T cells were performed at days 0, 7, 14, 21, and 28 of culture.
Quantitation of EBV-specific and alloreactive IFN-γ–producing T cells
At the onset and at several points in the development of each EBV-specific T-cell line, donor T lymphocytes at a concentration of 1 × 106/mL were mixed with autologous or allogeneic EBV BLCL at an effector-stimulator cell ratio of 5:1. Control tubes containing effector cells or stimulators only were incubated separately until the staining procedure. Brefeldin A was added to nonstimulated and stimulated samples at a concentration of 10 μg/mL cells. Tubes were incubated overnight for 16 hours in a humidified 5% CO2 incubator at 37°C.
Immediately before fixation and permeabilization, BLCL and effector cells of the nonstimulated control tubes were combined. Aliquots of the bulk nonstimulated and of the stimulated cultures were transferred to tubes for staining with monoclonal antibodies. Cells were stained with 5 μL monoclonal anti-CD3 labeled with allophycocyanin (APC) and 10 μL anti-CD8 peridin chlorophyll protein (PerCP) or anti-CD4 PerCP (BD Biosciences, San Jose, CA) and were incubated for 20 minutes at room temperature in the dark. Cells were washed with 2 mL phosphate-buffered saline (PBS)–bovine serum albumin (BSA)–azide (AZ) (PBS + 0.5% BSA + 0.1% AZ). Cells were centrifuged, supernatant discarded, and 100 μL reagent A (Fix & Perm Cell Permeabilization Reagents A & B; Caltag Laboratories, Burlingame, CA) was added to each tube to fix the cells. These cells were then incubated for 15 minutes. Cells were washed with PBS + BSA + AZ, and 100 μL reagent B (Caltag Laboratories) was added for permeabilization. Intracellular staining was performed by adding 10 μL phycoerythrin (PE)–labeled mouse immunoglobulin G1 (IgG1) isotype control PE or CD69 PE (BD Biosciences) and 10 μL mouse IgG1 isotype control fluorescein isothiocyanate (FITC) or IFN-γ FITC (BD PharMingen, San Diego, CA) monoclonal antibody. Cells were incubated for 20 minutes at room temperature, in the dark, washed twice, and further fixed in 1% formalin.
Stained and fixed cells were subsequently analyzed and quantitated using a FACSCalibur flow cytometer with dual lasers for 4-color capability (BD Biosciences), using CELLQuest software. Cells were first identified by forward and side light scatter and then by gating the CD3+ cells in a CD3 APC versus side scatter dot plot. Ten thousand events were acquired in the combined gate. For further identification of the cells, gating on the CD3+CD8+ or CD3+CD4+cells was performed. Quadrant markers were established based on analysis of the nonstimulated control and isotype control tubes.
Limiting-dilution analysis
To evaluate CTLp frequencies in EBV-BLCL–activated T cells, LDA was performed on days 0, 7, 14, 21, and 28 of cultured T-cell lines according to a modification of the methods of Bourgault et al5 and Langhorne and Lindahl,6 as described by Lucas et al.7 Briefly, the T cells were seeded in final volumes of 200 μL in 24 replicate wells per dilution of T cells in 96-well round-bottomed microplates. Decreasing effector cell concentrations were stimulated with 1 × 104 6000 cGy-irradiated autologous BLCL. Autologous PBMCs (1 × 104) irradiated with 3000 cGy were added as feeders. On days 0, 3, and 7 of each assay, 10 IU/mL IL-2 was added to the cultures. On day 10, the plates were split and tested against autologous BLCL and allogeneic targets. Wells were scored positive when chromium Cr 51 release exceeded the average plus 3 SD of control wells. CTLp frequencies were calculated by the method of Taswell11using a computer program provided by Dr Y. Kawanishi (Medical College of Wisconsin, Milwaukee).
Comparisons of sensitivity and precision of assays
To measure the precision of this assay and compare it with the assay measuring IFN-γ–producing cells, 5 LDAs were performed simultaneously on replicate samples of T cells obtained at the same time from the same cultured T-cell line. To assess and compare the sensitivity of the LDA with the sensitivity of the flow cytometric analysis of producing IFN-γ in response to antigen, simultaneous assays were performed on samples from the same EBV-specific T-cell line diluted 1:2, 1:4, 1:8, 1:16, and 1:160 of the number of effector cells plated in the standard assays.
Determination of peptide-specific CD8+ T cells
HLA-A2 and HLA-B8 tetrameric complexes were generated with human β2-microglobulin and an HLA-A2 binding EBNA 3c (LLDFVRFMGV) or an HLA-B8 binding peptide of EBNA 3a (QAKWRLQTL) as previously described and provided by the MSKCC Tetramer Core Facility.12 13 T cells from an HLA-A*0201+ donor and an HLA-B*0801+ donor, sensitized for 3 weeks against autologous EBV BLCLs, were prepared for FACS analyses by staining the cells with 0.25 mg/mL PE-labeled tetrameric complex, 5 μL monoclonal anti-CD3 APC, 10 μL anti-CD8 PerCP, and 10 μL anti-CD69L FITC (BD Bioscience). Cells were incubated for 20 minutes, washed, and analyzed with a FACSCalibur flow cytometer as described above.
To compare the results of quantitation of antigen-reactive T cells by tetramer assays or IFN-γ–producing cells, donor PBMCs at 1 × 106 cells/mL were incubated with 2.5 μg peptide (Research Genetics, Huntsville, AL) of EBNA 3c (LLDFVRFMGV) or EBNA 3a (QAKWRLQTL) in Iscoves modified Dulbecco medium for 3 hours at room temperature before the stimulation of aliquots of the same T cells. The peptide NLVPMVATV from the CMV pp65 antigen was also included as a control. Staining and analyses of IFN-γ–producing CD8+ cells were then performed as described above.
Purification of viable IFN-γ–secreting CD8+ and CD4+ T cells
EBV-sensitized IFN-γ–secreting T cells were isolated using the IFN-γ secretion assay (Miltenyi Biotec), according to the principles and instructions of the manufacturer.14 15 In brief, EBV-sensitized T cells were stimulated for 14 hours with autologous EBV BLCLs at an effector-stimulator ratio of 5:1. Alloantigen-sensitized T cells were stimulated identically with the allogeneic EBV BLCLs. Cells were washed in cold buffer (PBS containing 0.5% BSA and 2 mM EDTA) and were resuspended in cold medium, and 20 μL IFN-γ catch reagent per 107 cells was added for 5 minutes on ice. Cells were diluted in warm medium (37°C) at a concentration of 1 × 106/mL for 45 minutes. After washing in cold buffer, 20 μL IFN-γ detection antibody (PE) per 107 cells was added. In addition, CD8 FITC or CD4 FITC was added, and cells were incubated for 15 minutes on ice. Cells were washed, supernatant was completely removed, and CD8+IFN-γ+ and CD8+IFN-γ− populations or CD4+IFN-γ+ and CD4+IFN-γ− cells were purified using a MoFlo cell sorter (Cytomation, Fort Collins, CO). Dual-color cell staining with anti-CD8 or anti-CD4 and anti–IFN-γ monoclonal antibodies allowed purification of double-color positive cells (CD8+IFN-γ+ or CD4+IFN-γ+) and single-color CD8 or CD4 cells (CD8+IFN-γ− or CD4+IFN-γ−) to high purity (greater than 95%). Cell fractions were subsequently used for cytotoxicity assays as described below.
Cytotoxicity assay
Cytolytic activity of purified CD8+IFN-γ+ and CD8+IFN-γ− or CD4+IFN-γ+ and CD4+IFN-γ− effector cells was assayed against 51Cr-labeled targets in standard 4-hour release assays. Target cells included autologous BLCLs and HLA class I mismatched allogeneic BLCLs. Briefly, 1 × 106 target cells were incubated with 100 μCi (3.7 MBq) 51Cr for 1 hour, washed 3 times, and plated in 96 wells. Cytotoxicity was analyzed using 4 × 104, 2 × 104, and 1 × 104 effector cells to 4 × 103 target cells per well in a total volume of 200 μL. All targets were plated in triplicate.
After an incubation of 4 hours, supernatants were harvested, and the specific cytotoxicity was determined using a microplate scintillation counter (Packard Instruments, Downers Grove, IL). Percentage specific lysis was calculated as 100% × (experimental release − spontaneous release)/(maximum release × spontaneous release). Maximum release was obtained by adding 100 μL 5% Triton X-100 to the 100 μL medium-containing target cells. Spontaneous release was consistently below 15% of maximum release in all assays.
Statistical analysis
The number of EBV-specific T cells per 105 T lymphocytes was recorded using intracellular IFN-γ and LDA assays. Coefficient of variation, defined as the standard deviation divided by the mean, was used to measure the precision of 5 replicates of these positive-value variables.
Data for both assays were recorded at different titration levels. The intracellular IFN-γ assay was computed at undiluted concentrations of the T cells and at 1:2, 1:4, 1:8, 1:16, and 1:160 of the stimulated T cells. Data at titration levels of the undiluted concentration, 1:2 and 1:4 only, were recorded for the LDA assay because there were no demonstrable CTLp frequencies at titration levels from 1:8 to 1:160. The adequacy of a straight-line fit through the origin was examined for each of these assays. Two points determined straight line—the value of an assay at the undiluted concentration and the origin. The statistic used to assess the closeness of the assay values to this straight line was R2, which measures the proportion of variability in the assay about the origin explained by the line through the origin. An R2 value of 1 means the points fit the line exactly. Given that the recorded assay value at the undiluted value is subject to variability, a range ofR2 values was computed based on the range of undiluted values to assess the sensitivity of theR2 statistic.
Results
Determination of antigen-specific T cells by intracellular IFN-γ production of CD8+ T cells
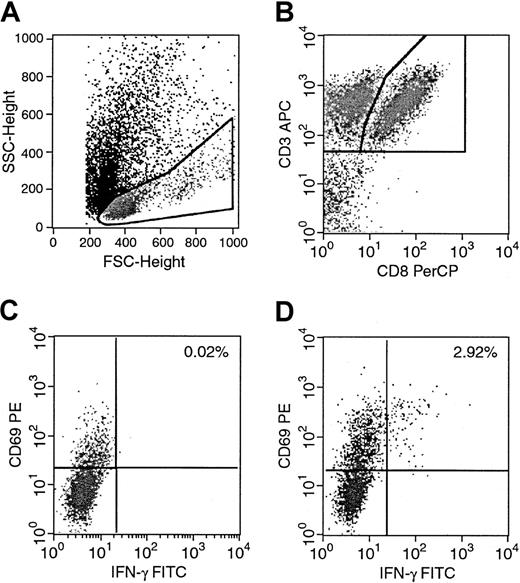
We performed 4-color staining to determine the percentage of IFN-γ–producing cells in specific T-cell subpopulations detectable by FACS analysis. An example, in which a culture was used after one restimulation with autologous EBV BLCL is demonstrated in Figure1. Forward scatter and side scatter were used for gating the lymphocytes. Staining with CD3 APC and CD8 PerCP allowed a second gate on the double-positive CD3+CD8+ lymphocytes. Subsequent analysis of the CD3+CD8+ cell fraction with CD69 PE, an activation marker, and IFN-γ FITC was used to determine the percentage of IFN-γ–producing CD3+CD8+lymphocytes activated in response to stimulation with EBV BLCL. In all our experiments, we used nonstimulated controls to determine background staining and subtracted the obtained percentage from the results of the stimulated samples. Figure 1C shows a background of 0.02% in the nonstimulated controls, which was subtracted from the 2.94% initially obtained in the culture. As a result, Figure 1D thus shows 2.92% of IFN-γ–producing activated CD3+CD8+lymphocytes in this example.
Determination of intracellular IFN-γ–producing antigen-specific T cells by 4-color FACS analysis.
Donor T cells were stimulated with autologous or allogeneic EBV BLCL overnight for 16 hours. Nonstimulated control tubes contained effector cells and stimulators mixed immediately before staining. Stimulated and nonstimulated tubes were then prepared simultaneously. (A) Lymphocytes were identified and gated. (B) A second gate including the CD3+CD8+ T lymphocytes was set, and the CD69+IFN-γ+ cells of the nonstimulated and stimulated samples were determined. (C) Nonstimulated control with background activity of 0.02%. (D) Stimulated cultures with 2.92% of CD8+CD69+IFN-γ+ reduced by background.
Determination of intracellular IFN-γ–producing antigen-specific T cells by 4-color FACS analysis.
Donor T cells were stimulated with autologous or allogeneic EBV BLCL overnight for 16 hours. Nonstimulated control tubes contained effector cells and stimulators mixed immediately before staining. Stimulated and nonstimulated tubes were then prepared simultaneously. (A) Lymphocytes were identified and gated. (B) A second gate including the CD3+CD8+ T lymphocytes was set, and the CD69+IFN-γ+ cells of the nonstimulated and stimulated samples were determined. (C) Nonstimulated control with background activity of 0.02%. (D) Stimulated cultures with 2.92% of CD8+CD69+IFN-γ+ reduced by background.
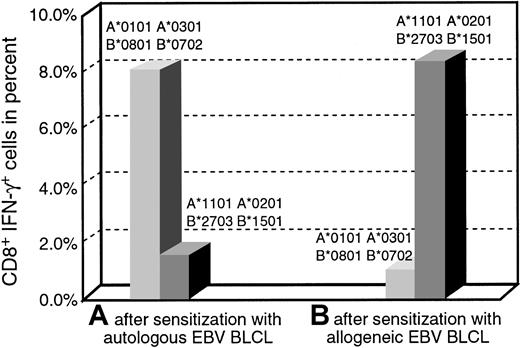
To examine the specificity of IFN-γ production, we sensitized PBMCs from the same donor with autologous EBV BLCL or HLA-mismatched allogeneic EBV BLCL for 14 days and determined the percentage of IFN-γ–producing activated CD3+CD8+lymphocytes in response to the autologous or the allogeneic target. Figure 2 shows that 8% of CD3+CD8+ lymphocytes sensitized with autologous BLCL produced intracellular IFN-γ in response to the autologous EBV BLCL cells, whereas only 1.7% of the cells produced IFN-γ when challenged with allogeneic EBV BLCL. In contrast, only 0.9% of the of CD3+CD8+ lymphocytes sensitized with allogeneic EBV BLCL for 2 weeks responded with IFN-γ production to the autologous EBV BLCL stimulus, but 8.2% of the same T cells responded to stimulation with allogeneic EBV BLCL. These results demonstrate the capacity of the assay to detect and quantitate CD3+CD8+ T cells exhibiting antigen-specific intracellular IFN-γ production after sensitization in vitro, and they suggest that the fraction of HLA-unrestricted EBV-reactive T cells generated when allogeneic EBV BLCL are used for sensitization is extremely small.
Quantitation of CD8+IFN-γ+cells after sensitization with autologous or allogeneic EBV BLCL.
An EBV-specific and an alloreactive T-cell line of the same donor were generated, and the IFN-γ–producing CD8+ T cells in response to autologous or allogeneic BLCL were determined on day 12 of culture. Bars on the left illustrate the responses of T cells sensitized with autologous BLCL, and bars on the right illustrate the responses of T cells sensitized with allogeneic BLCL. Both T-cell lines predominantly produced IFN-γ specifically in response to their sensitizing BLCL.
Quantitation of CD8+IFN-γ+cells after sensitization with autologous or allogeneic EBV BLCL.
An EBV-specific and an alloreactive T-cell line of the same donor were generated, and the IFN-γ–producing CD8+ T cells in response to autologous or allogeneic BLCL were determined on day 12 of culture. Bars on the left illustrate the responses of T cells sensitized with autologous BLCL, and bars on the right illustrate the responses of T cells sensitized with allogeneic BLCL. Both T-cell lines predominantly produced IFN-γ specifically in response to their sensitizing BLCL.
Kinetics of EBV-specific and alloreactive CD8+IFN-γ+ T lymphocytes
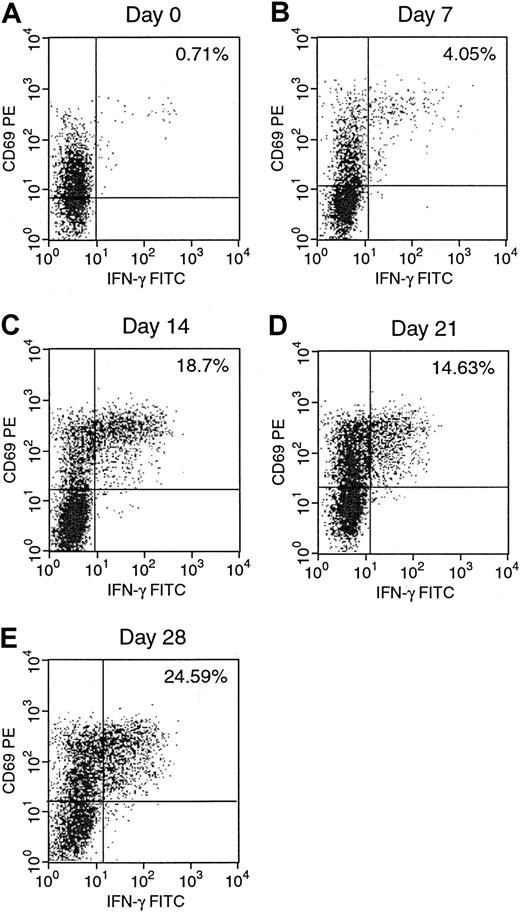
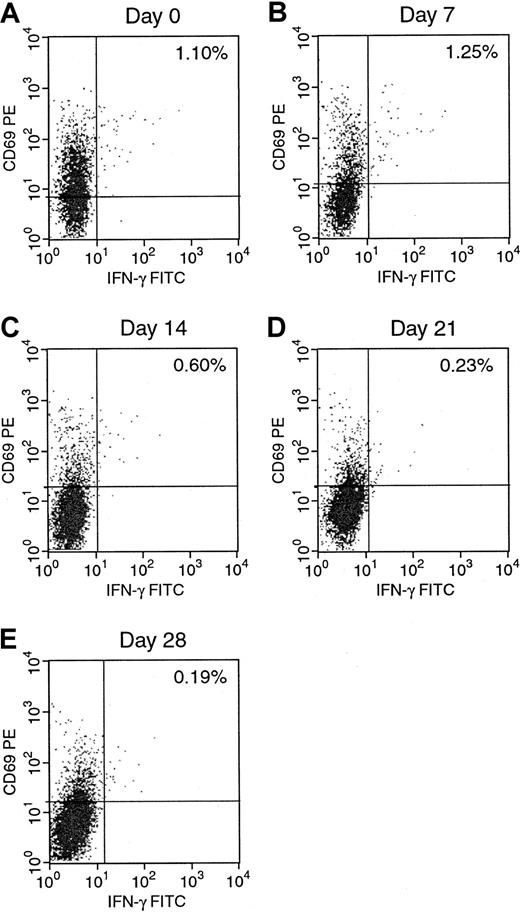
We next examined the development of EBV-reactive T cells during the generation of EBV-specific T-cell lines. The results for donor A are presented in Figures 3 and4. Figure 3 shows a gradual increase of IFN-γ–producing activated EBV-specific CD3+CD8+ T cells from 0.71% on day 0 to 24.59% after 4 stimulations with autologous EBV BLCL on day 28. As can be seen in Figure 4, the percentage of alloreactive CD3+CD8+ T cells in the same culture decreased gradually from 1.10% on day 0 to 0.19% on day 28 over the same 4-week period. These figures clearly demonstrate an increase in the number of EBV-reactive T cells and a concurrent, but less dramatic, reduction in the number of alloreactive T cells in the autologous EBV BLCL sensitized T-cell cultures over time when analyzed by intracellular cytokine staining. In the unsensitized culture on day 0, the number of alloreactive T cells in this donor was slightly higher than the number of EBV-reactive T cells, with a ratio of anti-EBV T cells to antialloreactive T cells of 0.6. This ratio increased in the 4-week period to 129, achieved by day 28 of culture, by which time the proportion of EBV-reactive T cells increased 34-fold while the proportion of alloreactive T cells decreased 5.7-fold.
Serial quantitation of EBV-specific CD8+IFN-γ+ in an EBV-specific T-cell line.
IFN-γ–producing CD8+ cells in response to autologous EBV BLCL were determined weekly from day 0 to day 28 of culture.
Serial quantitation of EBV-specific CD8+IFN-γ+ in an EBV-specific T-cell line.
IFN-γ–producing CD8+ cells in response to autologous EBV BLCL were determined weekly from day 0 to day 28 of culture.
Serial quantitation of alloreactive CD8+IFN-γ+ in the EBV-specific T-cell line.
IFN-γ–producing CD8+ cells in response to allogeneic EBV BLCL were determined weekly from day 0 to day 28 of culture.
Serial quantitation of alloreactive CD8+IFN-γ+ in the EBV-specific T-cell line.
IFN-γ–producing CD8+ cells in response to allogeneic EBV BLCL were determined weekly from day 0 to day 28 of culture.
Comparison of EBV-specific and alloreactive T cells by intracellular cytokines and LDA
Table 1 presents the anti-EBV and antialloreactive frequencies, determined by LDA and by flow cytometric quantitation of IFN-γ–producing CD3+ cells of T cells from 2 healthy seropositive donors generated over the course of 28 days in response to autologous EBV BLCL or allogeneic BLCL. Results are normalized to the number of T cells detected by each assay per 105 cells analyzed. As can be seen in Table 1 (donor A), anti-EBV CTLp frequencies as measured by LDA rose from 28 per 105 cells on day 0 to 333 per 105 cells on day 28 of culture, whereas frequencies of alloreactive CTLp decreased steadily from 36 per 105 cells on day 0 to 0.9 per 105 cells on day 28. Frequencies detected by flow cytometric quantitation of CD3+ T cells exhibiting intracellular IFN-γ staining were 700 per 105 cells for anti-EBV and 1100 per 105 cells for alloreactive cells on day 0, and they were 23 100 per 105 cells for anti-EBV and 200 per 105 cells for alloreactive T cells on day 28 of culture. Results of assays performed during the generation of EBV-specific T cells from donor B are also presented in Table 1. The anti-EBV frequency of 10 per 105 cells and the antialloreactive frequency of 8 per 105 cells on day 0, assessed by LDA, compares with 900 per 105 cells and 600 per 105 cells of anti-EBV and antialloreactive CD3+ T cells, respectively, on day 0 obtained using the intracellular cytokine staining assay. This demonstrates a 90-fold and a 75-fold higher frequency of EBV-specific and alloreactive T cells when assessed by staining for intracellular IFN-γ. The anti-EBV CTLp frequency of 132 per 105 T cells on day 28 of culture that corresponds to a frequency of 5200 per 105 CD3+T cells detected by intracellular IFN-γ production again demonstrates a 39-fold higher frequency of anti-EBV reactive T cells when assessed by cytokine staining. The frequency of alloreactive CD3+ T cells assessed by detection of intracellular IFN-γ production was 0.0% at this time point and could not be compared with the results obtained by LDA.
Quantitation of absolute numbers of EBV-specific and alloreactive T cells
| . | Day 0 . | Day 7 . | Day 14 . | Day 21 . | Day 28 . |
|---|---|---|---|---|---|
| Donor A | |||||
| Intracellular IFN-γ production* | |||||
| Anti-EBV CD3+ | 700 | 3 100 | 15 600 | 13 500 | 23 100 |
| Anti-allo CD3+ | 1 100 | 500 | 600 | 400 | 200 |
| Anti-EBV CD3+CD8+ | 315 | 1 880 | 11 407 | 9 928 | 16 660 |
| Anti-allo CD3+CD8+ | 495 | 564 | 366 | 204 | 136 |
| Anti-EBV CD3+CD4+ | 80 | 810 | 2 520 | 4 536 | 5 848 |
| Anti-allo CD3+CD4+ | ND | 54 | 42 | 72 | 170 |
| Limiting-dilution analysis† | |||||
| Anti-EBV CD3+ | 28 | 35 | 274 | 300 | 333 |
| Anti-allo CD3+ | 36 | 6.6 | 4.1 | 1.4 | 0.9 |
| Donor B | |||||
| Intracellular IFN-γ production* | |||||
| Anti-EBV CD3+ | 900 | 1 000 | 5 100 | 6 700 | 5 200 |
| Anti-allo CD3+ | 600 | 400 | 200 | 100 | ND |
| Anti-EBV CD3+CD8+ | 448 | 580 | 2 117 | 6 298 | 7 200 |
| Anti-allo CD3+CD8+ | 288 | 435 | 232 | 67 | ND |
| Anti-EBV CD3+CD4+ | 657 | 420 | 2 982 | 1 800 | 561 |
| Anti-allo CD3+CD4+ | 365 | 70 | 71 | ND | ND |
| Limiting-dilution analysis† | |||||
| Anti-EBV CD3+ | 10 | 22 | 38 | 70 | 132 |
| Anti-allo CD3+ | 8 | 23 | 3 | 1 | 0.8 |
| . | Day 0 . | Day 7 . | Day 14 . | Day 21 . | Day 28 . |
|---|---|---|---|---|---|
| Donor A | |||||
| Intracellular IFN-γ production* | |||||
| Anti-EBV CD3+ | 700 | 3 100 | 15 600 | 13 500 | 23 100 |
| Anti-allo CD3+ | 1 100 | 500 | 600 | 400 | 200 |
| Anti-EBV CD3+CD8+ | 315 | 1 880 | 11 407 | 9 928 | 16 660 |
| Anti-allo CD3+CD8+ | 495 | 564 | 366 | 204 | 136 |
| Anti-EBV CD3+CD4+ | 80 | 810 | 2 520 | 4 536 | 5 848 |
| Anti-allo CD3+CD4+ | ND | 54 | 42 | 72 | 170 |
| Limiting-dilution analysis† | |||||
| Anti-EBV CD3+ | 28 | 35 | 274 | 300 | 333 |
| Anti-allo CD3+ | 36 | 6.6 | 4.1 | 1.4 | 0.9 |
| Donor B | |||||
| Intracellular IFN-γ production* | |||||
| Anti-EBV CD3+ | 900 | 1 000 | 5 100 | 6 700 | 5 200 |
| Anti-allo CD3+ | 600 | 400 | 200 | 100 | ND |
| Anti-EBV CD3+CD8+ | 448 | 580 | 2 117 | 6 298 | 7 200 |
| Anti-allo CD3+CD8+ | 288 | 435 | 232 | 67 | ND |
| Anti-EBV CD3+CD4+ | 657 | 420 | 2 982 | 1 800 | 561 |
| Anti-allo CD3+CD4+ | 365 | 70 | 71 | ND | ND |
| Limiting-dilution analysis† | |||||
| Anti-EBV CD3+ | 10 | 22 | 38 | 70 | 132 |
| Anti-allo CD3+ | 8 | 23 | 3 | 1 | 0.8 |
Absolute numbers were determined per 105 T lymphocytes by LDA and intracellular IFN-γ production. EBV-specific and alloreactive CD3+ cells per 105 T lymphocytes were determined by LDA at weekly incremental time points from day 0 to day 28 during the generation of EBV-specific T-cell lines of donors A and B. Results were compared to EBV-specific and alloreactive CD3+ cells producing intracellular IFN-γ per 105 T lymphocytes. FACS analysis also determined the CD8+IFN-γ+ and CD4+IFN-γ+ subpopulations per 105T cells.
ND indicates not detectable.
Absolute numbers of EBV-specific and alloreactive T cells per 105 T lymphocytes by intracellular IFN-γ production.
Absolute numbers of EBV-specific and alloreactive T cells per 105 T lymphocytes obtained by LDA.
These results thus demonstrate that at each stage of culture, the frequencies of both EBV-specific and alloreactive T cells are 25- to 220-fold higher by quantitation of intracellular IFN-γ–producing CD3+ T cells than by LDA.
We also examined the frequencies of EBV-specific and alloreactive CD8+IFN-γ+ and CD4+IFN-γ+ T cells in these cultures at the same time intervals (Table 1). In both cultures, the proportional representation of CD8+ T cells increased markedly over the course of the culture. The proportional increase in frequencies of EBV-reactive T cells and the decrease in frequencies of alloreactive T cells in the CD4+IFN-γ+ T-cell fraction from donor A closely paralleled those of the CD8+IFN-γ+ cells. However, among the T cells generated from donor B, the population of EBV-reactive CD4+IFN-γ+ T cells decreased after 14 days of culture, whereas the CD8+IFN-γ+EBV-specific T-cell frequency continued to increase.
Limiting-dilution assay measures clonogenic cells of either CD4+ or CD8+ type, which likely represent only a small fraction of the antigen-reactive T cells capable of generating cytokines at the initiation of culture. Because CD8+EBV-specific T cells tend to increase disproportionally in the course of development of a T-cell line, however, we were interested to determine whether the disparities in T-cell frequencies detected by the 2 techniques reflected differences in the subpopulations of T cells expanded after in vitro sensitization over the course of culture. As shown in Figure 5A-D, alterations in frequencies of EBV-specific and alloreactive CD3+IFN-γ+ T cells and the CD8+IFN-γ+ fraction thereof largely paralleled alterations in the frequencies of EBV-specific T cells detected by LDA throughout the course of culture. In contrast, alterations in EBV-specific and alloreactive CD4+IFN-γ+ T-cell frequencies did not regularly parallel those detected by LDA or by assays of total CD3+IFN-γ+ or CD8+IFN-γ+ T cells.
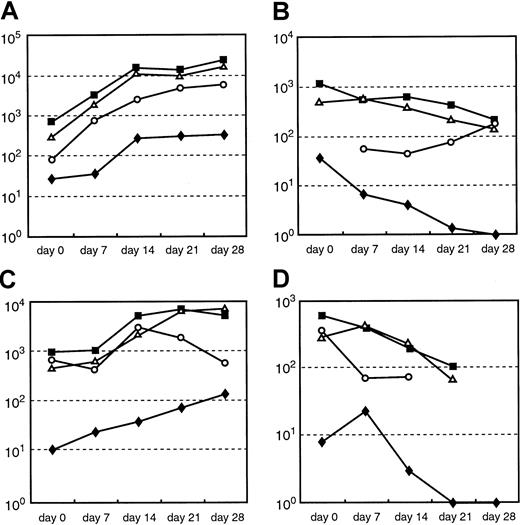
Comparison of quantitation of the absolute numbers per 105 T cells of anti-EBV and alloreactive T cells by LDA and by intracellular IFN-γ staining over the course of culture.
(A, C) Absolute numbers of anti-EBV–reactive CD3+ cells per 105 T cells by LDA (♦) and absolute numbers of intracellular IFN-γ–producing CD3+ (▪), CD8+ (▵), and CD4+ (○) per 105T cells in response to stimulation with autologous EBV BLCL of donor A and donor B, respectively. (B, D) Absolute numbers of anti-alloreactive CD3+ cells per 105 T cells by LDA (♦) and the absolute numbers of intracellular IFN-γ–producing CD3+(▪), CD8+ (▵), and CD4+ (○) per 105 T cells in response to stimulation with allogeneic EBV BLCL of donor A and donor B, respectively. Despite the 25- to 90-fold higher quantitation of EBV-specific and the 30- to 220-fold higher quantitation of alloreactive T-cell numbers obtained by determination of intracellular IFN-γ staining, the alterations in frequencies detectable by both assays largely paralleled each other over the course of culture.
Comparison of quantitation of the absolute numbers per 105 T cells of anti-EBV and alloreactive T cells by LDA and by intracellular IFN-γ staining over the course of culture.
(A, C) Absolute numbers of anti-EBV–reactive CD3+ cells per 105 T cells by LDA (♦) and absolute numbers of intracellular IFN-γ–producing CD3+ (▪), CD8+ (▵), and CD4+ (○) per 105T cells in response to stimulation with autologous EBV BLCL of donor A and donor B, respectively. (B, D) Absolute numbers of anti-alloreactive CD3+ cells per 105 T cells by LDA (♦) and the absolute numbers of intracellular IFN-γ–producing CD3+(▪), CD8+ (▵), and CD4+ (○) per 105 T cells in response to stimulation with allogeneic EBV BLCL of donor A and donor B, respectively. Despite the 25- to 90-fold higher quantitation of EBV-specific and the 30- to 220-fold higher quantitation of alloreactive T-cell numbers obtained by determination of intracellular IFN-γ staining, the alterations in frequencies detectable by both assays largely paralleled each other over the course of culture.
Comparison of precision and sensitivity of EBV-specific T cells by intracellular cytokines and LDA
To determine and to compare precision of the quantitation of T cells generating IFN-γ by intracellular cytokine staining and frequencies detected by LDA, we generated an autologous EBV-specific T-cell line for 13 days and performed each of the assays 5 times independently using the same T-cell line. The results, shown in Table2, present the absolute numbers of intracellular IFN-γ–producing CD3+ T cells per 105 T lymphocytes obtained by the intracellular cytokine assays, from 3220 to 3700 CD3+IFN-γ+ cells per 105 T lymphocytes of the EBV-reactive T-cell line. T cells of the same culture were used to perform 5 LDAs simultaneously. As demonstrated in Table 2, the results of CTLp frequencies obtained varied between 1.7 and 11 per 105 T lymphocytes. To assess the precision of intracellular IFN-γ assay and the LDA, the 5 independent samples of the number of EBV-specific T cells per 105 T lymphocytes were recorded as the coefficient of variation for the intracellular IFN-γ assay and LDA and were 0.06 and 0.6, respectively, demonstrating the greater precision for the intracellular IFN-γ assay.
Precision and sensitivity of intracellular IFN-γ assay and LDA
| Replicate 1 | Replicate 2 | Replicate 3 | Replicate 4 | Replicate 5 | Average of the replicates | |
| Flow cytometric intracellular IFN-γ assay | ||||||
| Precision* | 3700 | 3570 | 3570 | 3220 | 3220 | 3456 |
| Sensitivity† | ||||||
| Concentration | 1:2 | 1:4 | 1:8 | 1:16 | 1:160 | |
| Results | 1840 | 1470 | 700 | 460 | 40 | |
| 1780 | 1320 | 820 | 380 | 90 | ||
| 2000 | 1240 | 830 | 360 | 60 | ||
| Average of the triplicates | 1870 | 1340 | 780 | 400 | 60 | |
| Replicate 1 | Replicate 2 | Replicate 3 | Replicate 4 | Replicate 5 | Average of the replicates | |
| Limiting-dilution analysis | ||||||
| Precision‡ | 11 | 4 | 10 | 6 | 1.7 | 4.4 |
| Sensitivity2-153 | ||||||
| Concentration | 1:2 | 1:4 | 1:8 | 1:16 | 1:160 | |
| Results | 1.2 | 0.8 | ND | ND | ND | |
| Replicate 1 | Replicate 2 | Replicate 3 | Replicate 4 | Replicate 5 | Average of the replicates | |
| Flow cytometric intracellular IFN-γ assay | ||||||
| Precision* | 3700 | 3570 | 3570 | 3220 | 3220 | 3456 |
| Sensitivity† | ||||||
| Concentration | 1:2 | 1:4 | 1:8 | 1:16 | 1:160 | |
| Results | 1840 | 1470 | 700 | 460 | 40 | |
| 1780 | 1320 | 820 | 380 | 90 | ||
| 2000 | 1240 | 830 | 360 | 60 | ||
| Average of the triplicates | 1870 | 1340 | 780 | 400 | 60 | |
| Replicate 1 | Replicate 2 | Replicate 3 | Replicate 4 | Replicate 5 | Average of the replicates | |
| Limiting-dilution analysis | ||||||
| Precision‡ | 11 | 4 | 10 | 6 | 1.7 | 4.4 |
| Sensitivity2-153 | ||||||
| Concentration | 1:2 | 1:4 | 1:8 | 1:16 | 1:160 | |
| Results | 1.2 | 0.8 | ND | ND | ND | |
Five independent samples were analyzed to determine the precision of the intracellular IFN-γ assay and LDA, as described in “Materials and methods.” Sensitivity was determined by analyzing samples after serial dilution of the T cells, as described in “Materials and methods.” Results represent absolute numbers of EBV-specific T cells per 105 T lymphocytes.
ND indicates not detectable.
Results of 5 replicates of the undiluted concentration are shown.
The results and average of triplicates for the titrations 1:2 to 1:160 are shown.
Results of 5 replicates of the undiluted concentration are shown.
The results for the titrations 1:2 to 1:160 are shown.
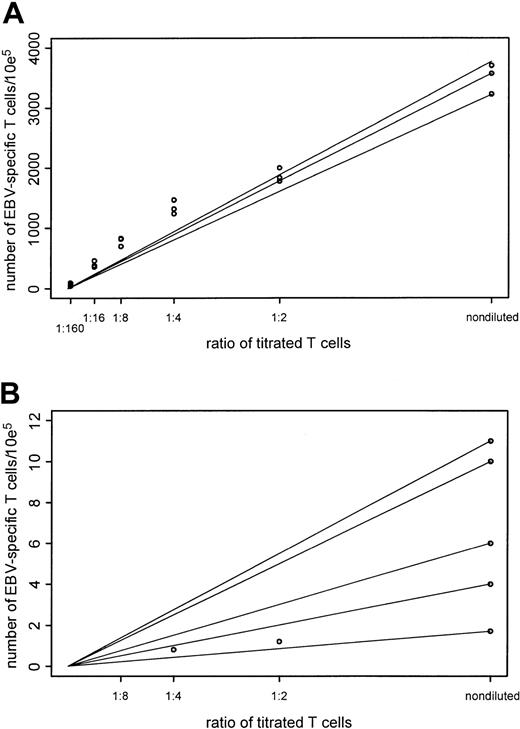
We were further interested in evaluating the sensitivity of the assays. For this reason we performed linear titrations of effector cells, without changing any other variable, and we performed the assays using the same T-cell culture with reduced numbers of effector cells. To evaluate the percentage of IFN-γ–producing CD3+ cells, we mixed EBV BLCL-activated T cells at decreasing numbers (50% of the previous sample) with increasing numbers of nonactivated T cells. Therefore, we anticipated a decrease of IFN-γ–producing cells by 50% in each subsequent sample (Table 2). The straight line shown in Figure 6A connects the values of the assay at the undiluted concentrations with the origin, whereas the dots show the actual data values. Allowing the undiluted value to vary from 3220 to 3700 EBV-specific T cells per 105 T cells resulted in a range of R2 values of 0.968 to 0.979. These results suggest that the intracellular IFN-γ assay is well predicted by a straight line through the origin and is robust to the choice of the undiluted values. In this experiment, the detection level of this assay is also reflected by the 60 IFN-γ–producing CD3+ T cells per 105 T lymphocytes (6 events per 104events acquired) obtained with the last dilution. Similar detection levels were confirmed in subsequent experiments using 2 different donor EBV-specific T-cell lines, in which detection levels of 0.03% (30 CD3+IFN-γ+ cells per 105 T lymphocytes) and 0.02% (20 CD3+IFN-γ+ cells per 105 T lymphocytes) were demonstrated (data not shown). Thus, this analysis also demonstrates that frequencies of CD3+IFN-γ+ can be precisely quantitated by intracellular cytokine staining in the range of 0.05% (5 events per 104 events acquired), corresponding to a frequency of 1 per 2000.
Comparison of precision and sensitivity of the intracellular IFN-γ and the LDA assays.
Adequacy of a straight-line fit through the origin was examined for each of the assays. Two points determined the straight line—a value of the assay at the undiluted concentration and the origin. Data values for the intracellular IFN-γ assay were recorded at undiluted concentration levels and at titrations of 1:2, 1:4, 1:8, 1:16, and 1:160 of stimulated T cells. Data values for the LDA assay were recorded at the undiluted concentration, at 1:2 and 1:4 titration levels. To assess the closeness of the assay values to this straight line, the R2 statistic was used. AnR2 value of 1 resulted when the points fit exactly. Because the recorded assay value at the undiluted value was subject to variability, a range of R2values was computed to assess the sensitivity of theR2 statistic. (A) Results for the intracellular IFN-γ assay. Range of values at the undiluted values to create a straight line through the origin, 3220-3700. Coefficient of variation, 0.06. Range of R2 values, 0.968-0.979. (B) Results for the LDA assay. Range of values at the undiluted values to create a straight line through the origin, 1.7-11. Coefficient of variation, 0.6. Range ofR2 values, 0.0-0.96.
Comparison of precision and sensitivity of the intracellular IFN-γ and the LDA assays.
Adequacy of a straight-line fit through the origin was examined for each of the assays. Two points determined the straight line—a value of the assay at the undiluted concentration and the origin. Data values for the intracellular IFN-γ assay were recorded at undiluted concentration levels and at titrations of 1:2, 1:4, 1:8, 1:16, and 1:160 of stimulated T cells. Data values for the LDA assay were recorded at the undiluted concentration, at 1:2 and 1:4 titration levels. To assess the closeness of the assay values to this straight line, the R2 statistic was used. AnR2 value of 1 resulted when the points fit exactly. Because the recorded assay value at the undiluted value was subject to variability, a range of R2values was computed to assess the sensitivity of theR2 statistic. (A) Results for the intracellular IFN-γ assay. Range of values at the undiluted values to create a straight line through the origin, 3220-3700. Coefficient of variation, 0.06. Range of R2 values, 0.968-0.979. (B) Results for the LDA assay. Range of values at the undiluted values to create a straight line through the origin, 1.7-11. Coefficient of variation, 0.6. Range ofR2 values, 0.0-0.96.
As shown in Table 2, when the serially titrated T cells were examined by LDA, the CTLp frequencies at the first 3 dilutions were 4.4, 1.2, and 0.8 EBV-specific T cells per 105 T lymphocytes and could not be determined for the last 3 concentrations because there was no demonstrable cytotoxicity at the lower titration levels. For this reason the results for the LDA assay, shown in Figure 6B, were based on the 3 values of undiluted, 1:2 and 1:4 concentrations of the titrated T cells. Varying the undiluted value between 1.7 and 11 EBV-specific T cells per 105 T cells produced an R2range between 0.0 and 0.96. In comparison, the range ofR2 values based on the same 3 titrations for the intracellular IFN-γ assay was 0.977 to 0.986. Thus, the intracellular IFN-γ assay is more robust than LDA regarding the effects of variability in the undiluted value. High variability in the LDA assay reduces the effectiveness of the goodness-of-fit comparison.
Evaluation of the EBV-specific cytotoxic activity of CD8+ and CD4+ IFN-γ+ and IFN-γ− T lymphocytes
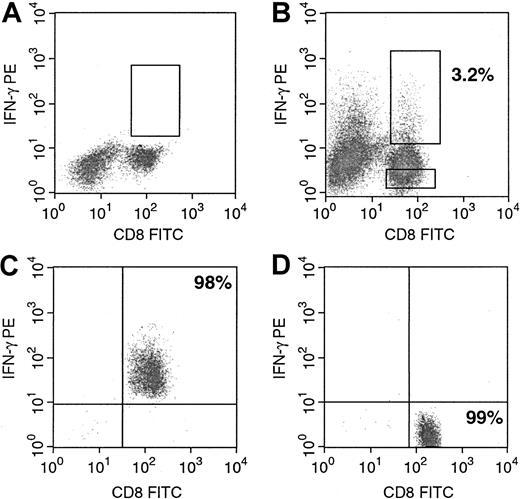
Differences in frequencies detected by LDA and by quantitation of T cells generating IFN-γ could in part reflect differences in the clonogenic potential of IFN-γ–producing T cells and variations in the capacity of cytokine-producing T cells to lyse susceptible targets. To evaluate the function of the IFN-γ–secreting and –nonsecreting CD8+ and CD4+ lymphocytes, we used the IFN-γ secretion assay, which allows labeling of surface IFN-γ with a PE-conjugated anti–IFN-γ monoclonal antibody. Staining with a second FITC-labeled anti-CD8 or anti-CD4 monoclonal antibody allows purification of CD8+IFN-γ+ and CD8+IFN-γ− or CD4+IFN-γ+ and CD4+IFN-γ− T lymphocytes by FACS analysis. As shown in Figure 7, before sorting, 3.2% of the CD8+ T cells also stained for anti–IFN-γ PE (Figure 7B) after they were stimulated overnight with autologous EBV BLCL, whereas among the nonstimulated control cells IFN-γ–secreting T cells could not be detected (Figure 7A). Stimulated T cells could thereafter be sorted into CD8+IFN-γ+ (purity, 98%) and CD8+IFN-γ− (purity, 99%) fractions (Figure 7C-D). We then performed a standard 51Cr release assay to analyze the cytotoxic activity of the separated CD8+IFN-γ+ and CD8+IFN-γ− or CD4+IFN-γ+ and CD4+IFN-γ− T lymphocytes. Figure8 presents results obtained with purified CD8+IFN-γ+ and CD8+IFN-γ− subpopulations of donor A. Figure9 presents the results acquired with the sorted CD8+IFN-γ+ and CD8+IFN-γ− and the sorted CD4+IFN-γ+ and CD4+IFN-γ− populations of donor B. In both experiments, the CD8+IFN-γ+ T cells induced significant lysis of the autologous EBV BLCL at low effector–target cell ratios, whereas these cells did not display lysis of the allogeneic targets. The CD8+IFN-γ−subpopulations of donor A did not induce significant lysis of the autologous or the allogeneic targets, whereas those from donor B exhibited a low level of cytotoxicity against the autologous targets. Of importance, neither the CD4+IFN-γ+ nor the CD4+IFN-γ− cells induced significant lysis of the autologous EBV BLCL of donor B. These results indicate that the IFN-γ–secreting CD8+ T-cell fraction is enriched for virus-specific cytotoxic effector cells, whereas the CD8+IFN-γ− and the CD4+subpopulations are largely depleted of cytotoxic effectors.
Purification of viable IFN-γ–secreting CD8+ T cells by FACSort.
Cells were prepared and stained as described in “Materials and methods.” (A) Nonstimulated control cells. (B) CD8+IFN-γ+ and CD8+IFN-γ− cell subpopulations before sorting. (C) Purified CD8+IFN-γ+ and (D) purified CD8+IFN-γ− cell populations. Both subpopulations were separately used for subsequent cytotoxicity assays. CD4+IFN-γ+ and CD4+IFN-γ− cell subpopulations were purified accordingly.
Purification of viable IFN-γ–secreting CD8+ T cells by FACSort.
Cells were prepared and stained as described in “Materials and methods.” (A) Nonstimulated control cells. (B) CD8+IFN-γ+ and CD8+IFN-γ− cell subpopulations before sorting. (C) Purified CD8+IFN-γ+ and (D) purified CD8+IFN-γ− cell populations. Both subpopulations were separately used for subsequent cytotoxicity assays. CD4+IFN-γ+ and CD4+IFN-γ− cell subpopulations were purified accordingly.
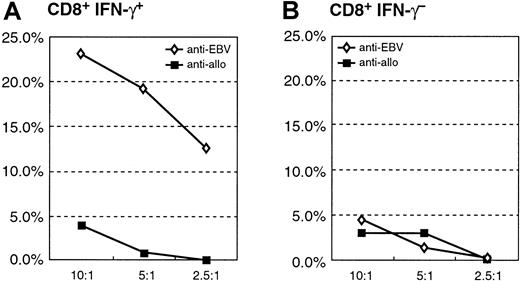
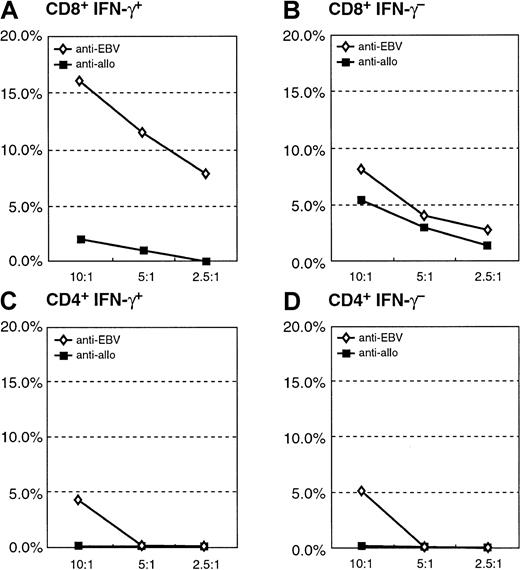
Cytotoxicity assay of the purified CD8+IFN-γ+ and CD8+IFN-γ− after sensitization with autologous EBV BLCL of donor A.
(A) Specific cytotoxic potential of the autologous target of the CD8+IFN-γ+ cells; allogeneic BLCL are not recognized. (B) Little anti-EBV or anti-alloreactive cytolytic activity of the CD8+IFN-γ− subpopulation.
Cytotoxicity assay of the purified CD8+IFN-γ+ and CD8+IFN-γ− after sensitization with autologous EBV BLCL of donor A.
(A) Specific cytotoxic potential of the autologous target of the CD8+IFN-γ+ cells; allogeneic BLCL are not recognized. (B) Little anti-EBV or anti-alloreactive cytolytic activity of the CD8+IFN-γ− subpopulation.
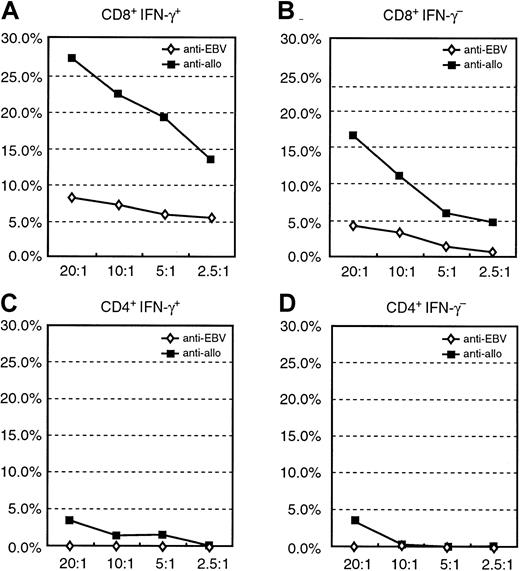
Cytotoxicity assay of the purified CD8+IFN-γ+ and CD8+IFN-γ− or CD4+IFN-γ+ and CD4+IFN-γ− after sensitization with autologous EBV BLCL of donor B.
(A) Specific cytolytic potential of the autologous and allogeneic EBV BLCL of the CD8+IFN-γ+. (B) No significant cytolytic activity of the CD8+IFN-γ−subpopulation against autologous or allogeneic EBV BLCL. (C, D) No cytolytic activity of the CD4+IFN-γ+ or CD4+IFN-γ− subpopulations against autologous and allogeneic EBV-transformed B-cell targets.
Cytotoxicity assay of the purified CD8+IFN-γ+ and CD8+IFN-γ− or CD4+IFN-γ+ and CD4+IFN-γ− after sensitization with autologous EBV BLCL of donor B.
(A) Specific cytolytic potential of the autologous and allogeneic EBV BLCL of the CD8+IFN-γ+. (B) No significant cytolytic activity of the CD8+IFN-γ−subpopulation against autologous or allogeneic EBV BLCL. (C, D) No cytolytic activity of the CD4+IFN-γ+ or CD4+IFN-γ− subpopulations against autologous and allogeneic EBV-transformed B-cell targets.
In a separate experiment, T cells from donor B were sensitized to allogeneic EBV BLCL, and the CD4+ and CD8+fractions were again separated and sorted into IFN-γ+ and IFN-γ− fractions. As shown in Figure10, the CD8+IFN-γ+ and CD8+IFN-γ− T-cell fractions exhibited specific cytotoxicity against the allogeneic target. However, the level of cytotoxicity was greater in the CD8+IFN-γ+fraction at all E/T ratios tested. Strikingly, though more than 5% of the CD4+ T cells were IFN-γ+, neither the CD4+IFN-γ+ T cells nor the CD4+IFN-γ− T cells induced significant lysis of the allogeneic target cells.
Cytotoxicity assay of the purified CD8+IFN-γ+ and CD8+IFN-γ− or CD4+IFN-γ+ and CD4+IFN-γ− after sensitization with allogeneic EBV BLCL of donor B.
(A) Sensitization of donor T cells with allogeneic EBV BLCL permits the generation CD8+IFN-γ+ T cells, capable of lysing the stimulating allogeneic target cells. (B) Cytolytic activity of the CD8+IFN-γ− cells. (C, D) No cytotoxic potential of the CD4+IFN-γ+ or CD4+IFN-γ− subpopulations.
Cytotoxicity assay of the purified CD8+IFN-γ+ and CD8+IFN-γ− or CD4+IFN-γ+ and CD4+IFN-γ− after sensitization with allogeneic EBV BLCL of donor B.
(A) Sensitization of donor T cells with allogeneic EBV BLCL permits the generation CD8+IFN-γ+ T cells, capable of lysing the stimulating allogeneic target cells. (B) Cytolytic activity of the CD8+IFN-γ− cells. (C, D) No cytotoxic potential of the CD4+IFN-γ+ or CD4+IFN-γ− subpopulations.
Comparison of EBV peptide-specific T cells by intracellular cytokines and tetramer analysis
We were also interested in determining the relative frequencies of T cells reactive against immunodominant peptides of EBV in the T cells sensitized to EBV BLCL over 3 weeks of culture. Accordingly, the sensitized T cells from 2 donors, one expressing HLA-A*0201, the other HLA-B*0801, were evaluated for CD8+ T cells binding tetramers of HLA-A*0201 bearing the EBNA 3c immunodominant peptide, LLDFVRFMGV, or HLA-B*0801 bearing the immunodominant EBNA 3a peptide, QAKWRLQTL. At the same time, these T cells were stimulated overnight with autologous irradiated PBMCs pulsed with these peptides or with autologous EBV BLCL and thereafter were analyzed for CD8+IFNγ+ T cells. As shown in Figure11A, 4.3% of the CD8+ T cells from the HLA-A*0201 donor bound the HLA-A*0201-EBNA 3c peptide tetramers, with most (3.44%) of these cells CD62L low (Figure11B). Only 0.05% of these cells bound the HLA-B*0801-EBNA 3a peptide tetramers (Figure 11C-D). Similarly, as shown in Figure 11E-F, 4% of the CD8+ T cells produced IFN-γ after secondary stimulation with EBNA 3c peptide-pulsed PBMCs compared with 0.05% of T cells secondarily stimulated with the EBNA 3a peptide-pulsed PBMCs. In studies of lymphocytes from a second HLA-A*0201+ donor sensitized to autologous EBV BLCL for 2 weeks, 0.4% of the T cells bound the EBNA 3c tetramer (Figure12A). Similarly, 0.3% generated IFN-γ after a secondary sensitization with the EBNA 3c peptide (Figure 12B). In contrast, only 0.02% of the T cells generated IFN-γ in response to the EBNA 3a peptide that binds the HLA-B*0801 allele not present on the T cells (Figure 12C) or in response to another HLA-A*0201 binding peptide, a peptide from the CMV pp65 antigen, NLVPMVATV (Figure 12D). In comparison with the 0.4% of T cells responding to the EBNA 3c peptide, 3.9% generated IFN-γ in response to secondary stimulation with autologous EBV BLCL.
EBNA 3c-specific T cells determined by tetramer staining and by intracellular IFN-γ production of an EBV-reactive T-cell line of an HLA-A*0201+ donor.
(A) Cells were triple stained with anti-CD3, anti-CD8, and HLA tetramer (EBNA3c [LLDFVRFMGV]). (B) Cells were additionally stained with anti-CD62L. (C, D) Cells were stained with an unrelated tetramer–peptide complex (EBNA 3a [QAKWRLQTL]) as a control. (E) Cells were stained with anti-CD3, anti-CD8, and anti–IFN-γ after secondary stimulation with EBNA3c [LLDFVRFMGV] peptide-pulsed autologous PBMCs. (F) Cells were additionally stained with anti-CD69. (G, H) Cells were stained after secondary stimulation with irrelevant EBNA 3a [QAKWRLQTL] peptide-pulsed autologous PBMCs as a control. Each plot is gated on live CD3+CD8+ T cells stained with the respective tetramer or anti-IFN-γ antibody. Percentage of peptide-specific CD8+ cells is indicated in the upper quadrants of each plot.
EBNA 3c-specific T cells determined by tetramer staining and by intracellular IFN-γ production of an EBV-reactive T-cell line of an HLA-A*0201+ donor.
(A) Cells were triple stained with anti-CD3, anti-CD8, and HLA tetramer (EBNA3c [LLDFVRFMGV]). (B) Cells were additionally stained with anti-CD62L. (C, D) Cells were stained with an unrelated tetramer–peptide complex (EBNA 3a [QAKWRLQTL]) as a control. (E) Cells were stained with anti-CD3, anti-CD8, and anti–IFN-γ after secondary stimulation with EBNA3c [LLDFVRFMGV] peptide-pulsed autologous PBMCs. (F) Cells were additionally stained with anti-CD69. (G, H) Cells were stained after secondary stimulation with irrelevant EBNA 3a [QAKWRLQTL] peptide-pulsed autologous PBMCs as a control. Each plot is gated on live CD3+CD8+ T cells stained with the respective tetramer or anti-IFN-γ antibody. Percentage of peptide-specific CD8+ cells is indicated in the upper quadrants of each plot.
EBNA 3c [LLDFVRFMGV] peptide-specific IFN-γ production of HLA-A*0201+ EBV-reactive T cells.
An EBV-reactive T-cell line of an HLA-A*0201+donor was generated for 2 weeks, and the peptide-specific CD3+CD8+ T cells were determined by tetramer analysis or the intracellular IFN-γ assay. (A) Demonstrates that 0.4% of the CD3+CD8+ bound the HLA tetramer (EBNA3c [LLDFVRFMGV]). (B) Similarly, 0.3% of the CD3+CD8+ cells responded with IFN-γ production after secondary stimulation with EBNA3c (LLDFVRFMGV) peptide-pulsed PBMCs. (C, D) Only 0.02% of aliquots of the same T cells responded with IFN-γ production after secondary stimulation with autologous PBMCs, pulsed with EBNA 3a [QAKWRLQTL], which binds the HLA-B*0801 allele not expressed by this donor, or in response to another HLA-A*0201-binding peptide, the NLVPMVATV peptide from the CMV pp65 antigen, respectively. (E) Demonstrates that 3.9% of the CD3+CD8+ cells responded with intracellular IFN-γ production after secondary stimulation with autologous EBV BLCL.
EBNA 3c [LLDFVRFMGV] peptide-specific IFN-γ production of HLA-A*0201+ EBV-reactive T cells.
An EBV-reactive T-cell line of an HLA-A*0201+donor was generated for 2 weeks, and the peptide-specific CD3+CD8+ T cells were determined by tetramer analysis or the intracellular IFN-γ assay. (A) Demonstrates that 0.4% of the CD3+CD8+ bound the HLA tetramer (EBNA3c [LLDFVRFMGV]). (B) Similarly, 0.3% of the CD3+CD8+ cells responded with IFN-γ production after secondary stimulation with EBNA3c (LLDFVRFMGV) peptide-pulsed PBMCs. (C, D) Only 0.02% of aliquots of the same T cells responded with IFN-γ production after secondary stimulation with autologous PBMCs, pulsed with EBNA 3a [QAKWRLQTL], which binds the HLA-B*0801 allele not expressed by this donor, or in response to another HLA-A*0201-binding peptide, the NLVPMVATV peptide from the CMV pp65 antigen, respectively. (E) Demonstrates that 3.9% of the CD3+CD8+ cells responded with intracellular IFN-γ production after secondary stimulation with autologous EBV BLCL.
When the HLA-B*0801+ donor EBV-sensitized T cells were tested, 1.46% of the CD8+ T cells bound the HLA-B*0801-EBNA 3a peptide tetramers (Figure13A-B), whereas only 0.15% bound the HLA-A*0201-EBNA 3c tetramers (data not shown). Similarly, 1.49% of the CD8+ T cells produced IFN-γ after stimulation with EBNA 3a peptide-pulsed PBMCs (Figure 13C-D), and 0.01% produced IFN-γ in response to the EBNA 3c peptide-pulsed PBMCs (data not shown). On the other hand, when the T cells were restimulated overnight with autologous EBV BLCL, 4% of the CD8+ T cells were IFN-γ+ (Figure 13E-F). These findings indicate a close correlation between the proportions of immunodominant peptide-specific CD8+ T cells detected by tetramers and by intracellular cytokine production. They further suggest that CD8+ T cells responding to an immunodominant peptide, such as the B*0801 binding EBNA 3a peptide, may contribute most of the cytokine-producing EBV-specific T cells generated after stimulation with autologous EBV BLCL.
EBNA 3a-specific T cells determined by tetramer analysis and by IFN-γ production of an HLA-B*0801+ donor.
(A) Cells were triple stained with anti-CD3, anti-CD8, and HLA tetramer (EBNA 3a [QAKWRLQTL]). (B) Cells were additionally stained with anti-CD62L. (C) Cells were stained with anti-CD3, anti-CD8, and anti–IFN-γ after secondary sensitization with EBNA 3a (QAKWRLQTL) peptide-pulsed autologous PBMCs. (D) Cells were additionally stained with anti-CD69. (E, F) Cells were stained after stimulation with autologous EBV BLCL. Each plot is gated on live CD3+CD8+ T cells stained with the respective tetramer or anti–IFN-γ antibody. Percentage of specific CD8+ cells is indicated in the upper quadrant of each plot.
EBNA 3a-specific T cells determined by tetramer analysis and by IFN-γ production of an HLA-B*0801+ donor.
(A) Cells were triple stained with anti-CD3, anti-CD8, and HLA tetramer (EBNA 3a [QAKWRLQTL]). (B) Cells were additionally stained with anti-CD62L. (C) Cells were stained with anti-CD3, anti-CD8, and anti–IFN-γ after secondary sensitization with EBNA 3a (QAKWRLQTL) peptide-pulsed autologous PBMCs. (D) Cells were additionally stained with anti-CD69. (E, F) Cells were stained after stimulation with autologous EBV BLCL. Each plot is gated on live CD3+CD8+ T cells stained with the respective tetramer or anti–IFN-γ antibody. Percentage of specific CD8+ cells is indicated in the upper quadrant of each plot.
Discussion
This study was conducted to compare assays quantitating EBV-specific and allospecific T cells by LDA of cytolytic T-cell precursors or by measurement of T cells generating intracellular IFN-γ regarding their sensitivity and reproducibility across the wide range of specific T-cell frequencies detected in the course of establishing EBV-specific T-cell lines. Our results demonstrate that before and at each stage in the development of an EBV-specific T-cell line, frequencies of specific T cells detected by LDA were 25- to 90-fold lower than those detected by quantitating CD3+ T cells producing IFN-γ and 11- to 55-fold lower than the frequencies of IFN-γ+ T cells in the CD3+CD8+T-cell fraction that contained the entire population of functionally cytotoxic T cells. These results are in accord with the recent studies of Kuzushima et al,16 who compared EBV-specific T-cell frequencies by each method at one time point in T-cell cultures stimulated with autologous EBV BLCL. In addition, though LDAs permit quantitation of clonogenic effector cells with antigen-specific cytolytic activity, our results indicate that the reproducibility and precision of the LDA assay for estimating antigen-specific T cells in sensitized populations is strikingly inferior to the assay quantitating antigen-specific IFN-γ+ T cells.
Clonogenic EBV-specific cytotoxic T-cell precursors measured by LDA might be expected to represent functionally distinct populations of effector cells from antigen-responsive, cytokine-producing CD4+ and CD8+ T cells. Furthermore, we anticipated that the relative representation of such populations in antigen-stimulated cultures might diverge from that of cytokine-producing cells during the development of an antigen-specific T-cell line. The flow cytometric assay used to quantitate IFN-γ+ T cells allowed us to evaluate the expansion of CD4+ and CD8+ antigen-specific T cells in comparison with LDA. Our results demonstrate a comparable increase in the frequencies of EBV-specific CTLp and EBV-specific IFN-γ–producing CD8+ T cells over the course of 28 days of culture. Concurrent analysis of the frequencies of alloreactive CD8+ T cells remaining in these T-cell lines also demonstrated relatively parallel decrements in the frequencies of alloreactive T cells detected by these assays. CD4+IFN-γ+ EBV-reactive T cells also increased proportionally over the course of the in vitro expansion. However, though the frequencies of CD4+IFN-γ+alloreactive T cells decreased in donor B to nondetectable levels, in donor A they increased during the course of expansion. The basis for this increase is unclear, but it might reflect the expansion of T cells reactive against self-class 2 HLA complexes with EBV peptides mimicking an alloantigen presented by the third-party cells used in the assay. In this regard, the capacity of the EBV antigen presented by certain HLA alleles to mimic a different HLA allele has been described by Burrows et al.17
Functional studies of separated CD8+IFN-γ+and CD8+IFN-γ− effector cells also demonstrate that the CD8+ IFN-γ+ cells indeed include almost all EBV-specific CD8+ cytotoxic T cells in the sensitized T-cell population. In contrast, the CD8+IFN-γ− effector cells showed little lysis of the autologous or the allogeneic EBV BLCL targets. A significant fraction of CD4+IFN-γ+EBV-specific T cells was also generated from each donor. Conversely, CD4+IFN-γ+ T cells, sensitized with autologous or allogeneic EBV-transformed B cells, exhibited no significant cytolytic activity. The functions of isolated CD4+IFN-γ+ EBV-specific and allospecific T cells are as yet poorly defined. It is possible that these cells represent classic Th1 T cells. We are evaluating whether and to what degree these cells augment the proliferation, cytokine production, or cytolytic activity of the EBV-specific CD8+cytotoxic T cells. Whether these cells can inhibit the growth of clonogenic EBV-transformed BLCL also remains to be determined.
Our studies suggest that the frequencies of T cells with detectable intracellular IFN-γ can be precisely quantitated to a lower limit of approximately 0.05% or 1 of 2000. Accurate estimates of lower frequencies are difficult with this methodology. Although the sensitivity of the assay is excellent for quantitating T cells reactive against highly immunogenic pathogens such as EBV or against major alloantigens, it may be less useful for quantitating T cells reactive against minor alloantigens or relatively nonimmunogenic tissue antigens, unless the T cells are expanded after exposure to the antigen in vivo after adoptive transfer or after extended in vitro sensitization. A variation of this assay, the ELISPOT assay of cytokine-producing T cells, may provide advantages for detection of such antigen-specific T cells at lower frequencies.18 19This approach requires purification of lymphocyte subpopulations before quantitation of antigen-reactive T cells in each subset. Accuracy of the results may be limited if the cytokine measured is also produced by cells presenting the antigen, such as EBV-transformed B cells or macrophages.
Measuring T cells binding peptide–HLA tetramers provides a precise and sensitive approach for, in turn, measuring antigen-specific T cells, provided the immunogenic peptides and restricting HLA alleles are known.20,21 However, this approach has been used to quantitate EBV-peptide–specific T-cell responses in seropositive healthy controls and in recipients of marrow allograft and to measure responses against minor alloantigens in sensitized healthy hosts and in patients in whom GVHD develops after allogeneic transplantation.12 The donors evaluated in this study each expressed a common HLA allele, HLA-A*0201 in one and HLA-B*0801 in the other. Because T-cell responses to latent EBV infection are predominantly directed against the nuclear antigens EBNA 3a and 3c and because immunodominant peptides binding HLA-A*0201 and HLA-B*0801 have been defined,20 we quantitatively compared T cells responding to these peptides in lines initially sensitized with EBV BLCLs as measured, on the one hand, by T cells binding EBNA 3c peptide HLA-A*0201 tetramers and EBNA 3a peptide HLA-B*0801 tetramers and, on the other hand, by T cells generating IFN-γ in response to overnight stimulation with the peptide. These studies demonstrated a striking concordance between the 2 assays when measuring responses to the peptides. Furthermore, in the T-cell cultures studied, the proportion of T cells generating IFN-γ in response to EBNA 3a or EBNA 3c peptide represented 10% to 50% of the T cells responding to rechallenge with autologous EBV BLCL. We are evaluating whether and to what degree there is concordance and equivalent sensitivity between these assays when testing responses in fresh samples from seropositive donors or when testing responses to subdominant or weakly immunogenic viral peptides or to minor alloantigens.
Previous studies have shown that EBV-specific T-cell lines generated over periods of 28 to 42 days are enriched for EBV-reactive CD8+ T cells and are relatively depleted of alloreactive T cells detectable in classic cytotoxic assays.4 8 The results presented in Table 1 illustrate that substantial numbers of alloreactive T cells are still detectable by each assay at days 14 and 21 of culture. In a 28-day period of culture with intermittent specific sensitization, the frequency of EBV-reactive T cells increased 16- to 34-fold, whereas the frequency of T cells reactive against major alloantigens decreased by 4- to 6-fold. T cells derived from such EBV-specific cell lines have been administered at doses ranging from 3 to 5 × 105 T cells per kilogram to treat patients with EBV lymphoma. Major alloantigen-reactive T cells provided by such doses are estimated to range from 3 to 10 per kilogram as assayed by LDA and as many as 100 to 200 T cells per kilogram as measured by IFN-γ–producing T cells. Because such infusions have not been complicated by GVHD in HLA-disparate recipients, these findings, if confirmed in analyses of larger series, may provide a useful estimate of the doses of alloreactive T cells that can be safely administered in HLA-disparate donor–recipient pairs.
In summary, we demonstrate that antigen-specific and alloreactive T-cell frequencies can be rapidly and precisely determined by FACS analysis. Four-color staining allows the determination of intracellular IFN-γ production of activated lymphocyte subpopulations in response to antigenic stimulus. We also show that the CD8+ T cells exhibiting virus-specific cytotoxic activity are almost exclusively contained within the CD8+IFN-γ+ T-cell population. This method may thus permit the development of new strategies for the selection and expansion of these effector cells for application in adoptive immunotherapy approaches.
We thank Ingrid Leiner of the Tetramer Core Facility for tetramer production; Sylvie Wiener-Fedus and Donna M. Weinstein of the Cellular Immunology Laboratory for excellent technical assistance; and Patrick Anderson and Tom Delohery of the Flow Cytometry Core Facility for their support in performing the FACSorts.
Supported in part by National Cancer Institute grants CA59350, CA23766; National Heart, Lung, and Blood Institute grant HL53752; The Larry H. Smead Fund, The Aubrey Fund for Pediatric Cancer Research, and The Vincent Astor Chair Clinical Research Fund. G.K. is the recipient of a Translational Research Grant from the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Guenther Koehne, Bone Marrow Transplantation Service, Memorial Sloan-Kettering Cancer Center, Rm K-312 B, 1275 York Ave, New York, NY 10021; koehneg@mskcc.org.

![Fig. 11. EBNA 3c-specific T cells determined by tetramer staining and by intracellular IFN-γ production of an EBV-reactive T-cell line of an HLA-A*0201+ donor. / (A) Cells were triple stained with anti-CD3, anti-CD8, and HLA tetramer (EBNA3c [LLDFVRFMGV]). (B) Cells were additionally stained with anti-CD62L. (C, D) Cells were stained with an unrelated tetramer–peptide complex (EBNA 3a [QAKWRLQTL]) as a control. (E) Cells were stained with anti-CD3, anti-CD8, and anti–IFN-γ after secondary stimulation with EBNA3c [LLDFVRFMGV] peptide-pulsed autologous PBMCs. (F) Cells were additionally stained with anti-CD69. (G, H) Cells were stained after secondary stimulation with irrelevant EBNA 3a [QAKWRLQTL] peptide-pulsed autologous PBMCs as a control. Each plot is gated on live CD3+CD8+ T cells stained with the respective tetramer or anti-IFN-γ antibody. Percentage of peptide-specific CD8+ cells is indicated in the upper quadrants of each plot.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/5/10.1182_blood.v99.5.1730/5/m_h80522213011.jpeg?Expires=1769854356&Signature=wgthzlP613H8jk4-Fwciav-xtDEZMACU3d-3HtOGxyCVWTbhfomw1BLW1uDMcPubwFLGYn8UmB22SkMbVxuP5NWRai5O8zULXl2r7muTtAa6eGbXDjjE-eEdpE-WLHAk~FkFDC~BFW74s0Z8Nlj64ps3UXFd7~qhRA7p8Wd~SCDrprU89Ag72MajBJkbP9A2Kfety2umo1lkk3hw5V13Ib88Te76Tny16-EkV-cTaG6MwjUdeQCmxKJFs~JCT1eyn9pi6JE3jgfLbtdVQssKwoZYNEf0FEuKJWiniw4w5FUPEuGO9Bbqv~ObQ3ugEZG2-CPvjakUoeHTpgz2v4r1Aw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 12. EBNA 3c [LLDFVRFMGV] peptide-specific IFN-γ production of HLA-A*0201+ EBV-reactive T cells. / An EBV-reactive T-cell line of an HLA-A*0201+donor was generated for 2 weeks, and the peptide-specific CD3+CD8+ T cells were determined by tetramer analysis or the intracellular IFN-γ assay. (A) Demonstrates that 0.4% of the CD3+CD8+ bound the HLA tetramer (EBNA3c [LLDFVRFMGV]). (B) Similarly, 0.3% of the CD3+CD8+ cells responded with IFN-γ production after secondary stimulation with EBNA3c (LLDFVRFMGV) peptide-pulsed PBMCs. (C, D) Only 0.02% of aliquots of the same T cells responded with IFN-γ production after secondary stimulation with autologous PBMCs, pulsed with EBNA 3a [QAKWRLQTL], which binds the HLA-B*0801 allele not expressed by this donor, or in response to another HLA-A*0201-binding peptide, the NLVPMVATV peptide from the CMV pp65 antigen, respectively. (E) Demonstrates that 3.9% of the CD3+CD8+ cells responded with intracellular IFN-γ production after secondary stimulation with autologous EBV BLCL.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/5/10.1182_blood.v99.5.1730/5/m_h80522213012.jpeg?Expires=1769854356&Signature=SQd8JWChJStIWRGDY0--wSQPtIs~CfKLpq3WX7AlB05PNoJX7Ew4meZQi9MJwdjaGbwpk1gw-rwrLkJipYLs3epAV2gEzBZyicLnVztHuk3F8kdY1gbdBZe2DA9-UFyeNivj2zWsHOYzfE7eTaCxuVWmZTAIWEOFnZIHist4FxepN6w1L2JSnw98nOGxuO1hsuOAz-nd0Io5xJ9mVwctX-bG-onLh-el8JTLLM5yY-MMrMQyOrhauL6x9M~iwtI3F-IngBTM3jbeY65ckFLkNFOQcCcRvm6DGlWgtaMnG7ZmO9rvsWHl9lXtFTds7u4k42Szx2Ih2I3c0sYaKnyLMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 13. EBNA 3a-specific T cells determined by tetramer analysis and by IFN-γ production of an HLA-B*0801+ donor. / (A) Cells were triple stained with anti-CD3, anti-CD8, and HLA tetramer (EBNA 3a [QAKWRLQTL]). (B) Cells were additionally stained with anti-CD62L. (C) Cells were stained with anti-CD3, anti-CD8, and anti–IFN-γ after secondary sensitization with EBNA 3a (QAKWRLQTL) peptide-pulsed autologous PBMCs. (D) Cells were additionally stained with anti-CD69. (E, F) Cells were stained after stimulation with autologous EBV BLCL. Each plot is gated on live CD3+CD8+ T cells stained with the respective tetramer or anti–IFN-γ antibody. Percentage of specific CD8+ cells is indicated in the upper quadrant of each plot.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/5/10.1182_blood.v99.5.1730/5/m_h80522213013.jpeg?Expires=1769854356&Signature=sbQee2vi4cf4YLf0xbZLcVROYqRZNSNeZcPchthq2gdXTvVGwWIiEk2BbV54ciq5RNvHj1lX7fvyTrfnd0fIfizg1FRX8DT2qiTjPnwoOAbxXLxZQECYgbB7To6UNxYqEdNUKO7SJ3s3RcKXUva1XA-PIMTCHUaPZZMrtposbzs-rIkMM4Gj5a96aQ4Oqj6CPQB~I6bM~K3CSFE2nUkiI-5CRIVXvFUsLDgdaAAUVOQO2j~K0YLwSBrD2m9jMb8sQVRzQf~cB0C259zzAgzYTIY~0XqWBDQUvCSAQVobRiSzTJA1f8dcLTOH0mWv6TZVsnNmevzvcNzTR1zdQ1INYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal