In haploidentical transplantation, the mismatched haplotype of the donor can originate from either of the parents. We refer to such mismatched haplotypes as noninherited maternal antigens (NIMA haplotype) or noninherited paternal antigens (NIPA haplotype). To determine whether exposure to maternal HLA antigens benefits patients undergoing bone marrow transplantation, we analyzed graft failure and graft-versus-host disease (GVHD) after transplantations from parental or haploidentical sibling donors. We studied 269 patients receiving 1 or 2 HLA-A, -B, -DR antigen-mismatched sibling or parental non–T-cell–depleted bone marrow transplants for acute myelogenous leukemia, acute lymphoblastic leukemia, or chronic myelogenous leukemia between 1985 and 1997 that were reported to the International Bone Marrow Transplant Registry. Included were 121 (45%) NIMA-mismatched and 148 (55%) NIPA-mismatched transplantations. Sixty-three (52%) of the NIMA-mismatched transplants and 69 (47%) of the NIPA-mismatched transplants were from haploidentical sibling donors. Sibling transplantations mismatched for NIMA had similar rates of graft failure but lower rates of acute GVHD (P < .02) than NIPA-mismatched sibling transplantations. In the first 4 months after transplantation, mother-to-child transplantations involved significantly less chronic GVHD than father-to-child transplantations (P < .02). Treatment-related mortality (TRM) was significantly higher after parental transplantations (P = .009 for mother; P = .03 for father) than after haploidentical sibling transplantations mismatched for the NIMA. Non–T-cell–depleted bone marrow transplants donated by haploidentical siblings to recipients mismatched for NIPA and transplants donated by parents caused more acute and chronic GVHD and TRM than transplants donated by haploidentical siblings mismatched for NIMA.
Introduction
Allogeneic bone marrow transplantation can cure several hematologic malignancies. The outcome of this procedure depends to a large extent on the degree of human leukocyte antigen (HLA) identity between donor and recipient. Unfortunately, only 25% to 30% of transplant candidates have a genotypically HLA-identical sibling donor.1 Bone marrow from haploidentical family members is also used for transplantation.2 If the unshared HLA haplotype is partially matched with the recipient, transplantation outcome is acceptable though significantly inferior to HLA-identical sibling transplantation. The important influence of the degree of HLA matching on bone marrow and organ transplantation outcome is documented in many publications. Few studies address why a sizable percentage of HLA-mismatched stem cell or organ grafts function as well as HLA-identical ones.3 4
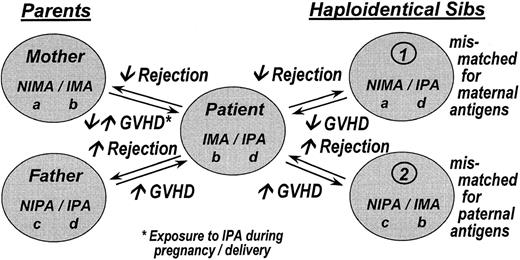
It is possible that exposure, during in utero development or nursing, to maternal HLA antigens has a lifelong influence on the immune system. Two separate studies demonstrate that approximately 50% of patients with antibodies against a large number of HLA antigens have not formed antibodies against maternal HLA antigens that the patient did not inherit, the noninherited maternal antigens (NIMA) (Figure1).5 6 Reactivity against noninherited paternal antigens (NIPA) is significantly higher.
Proposed scheme to designate the haplotypes of a family in which one of the siblings (patient) is a bone marrow transplant recipient with a prior prediction of clinical outcome after hematopoietic stem cell transplantation.
Parents or siblings who share a haplotype with the recipient but differ for the other are potential donors. The patient has inherited the IMA haplotype from the mother and the IPA haplotype from the father. The NIMA haplotype carries the noninherited maternal antigens, and the NIPA haplotype carries the noninherited paternal antigens. Potential donors who are genotypically identical are not shown. IMA, inherited maternal antigens; IPA, inherited paternal antigens.
Proposed scheme to designate the haplotypes of a family in which one of the siblings (patient) is a bone marrow transplant recipient with a prior prediction of clinical outcome after hematopoietic stem cell transplantation.
Parents or siblings who share a haplotype with the recipient but differ for the other are potential donors. The patient has inherited the IMA haplotype from the mother and the IPA haplotype from the father. The NIMA haplotype carries the noninherited maternal antigens, and the NIPA haplotype carries the noninherited paternal antigens. Potential donors who are genotypically identical are not shown. IMA, inherited maternal antigens; IPA, inherited paternal antigens.
It was speculated that exposure to NIMA in utero or while nursing induces (partial) tolerance to NIMA in approximately 50% of patients. It was expected, on the basis of these findings and of corroborating evidence from the literature,7-9 that kidney allografts donated by the mother would have superior graft survival than those donated by the father. This turned out not to be true.10However, a collaborative study of 9 transplantation centers showed that graft survival of kidneys donated by haploidentical siblings mismatched (like the mother) for the NIMA haplotype was similar to that of kidneys donated by an HLA-identical sibling.4 Why a kidney donated by a haploidentical sibling mismatched for NIMA would fare better than a similarly NIMA-mismatched maternal graft was unclear.
In another study, transplanted kidneys donated by unrelated donors mismatched for 1 or 2 HLA-A antigens identical to the HLA-A NIMA of the recipient had graft survival rates significantly superior to those of other HLA-A–mismatched grafts.11 No such differences were observed for HLA-B and -DR–mismatched grafts.
In bone marrow transplantation, the situation is more complex (Figure1). If durable engraftment can be compared to the survival of an organ allograft, the expectation is that there will be less graft rejection if the donor is mismatched for the NIMA of the recipient than if the donor is mismatched for the NIPA. Similarly, there should be less graft-versus-host disease (GVHD) with NIMA- than with NIPA-mismatched transplantations. Because graft failure and GVHD contribute to transplantation success, treatment-related mortality (TRM) and overall survival might also differ between NIMA and NIPA mismatches. We tested these assumptions in 269 recipients of non–T-cell–depleted parental or haploidentical sibling bone marrow transplantations for acute myelogenous leukemia (AML), acute lymphoblastic lymphoma (ALL), and chronic myelogenous leukemia (CML) reported to the International Bone Marrow Transplant Registry (IBMTR) between 1985 and 1997. The study compares graft failure, acute and chronic GVHD, TRM, and survival with NIMA- versus NIPA-mismatched transplantations.
Patients and methods
Data collection
The IBMTR is a working group of more than 400 transplant teams worldwide that contribute detailed data concerning their allogeneic hematopoietic stem cell transplantations to a statistical center located at the Health Policy Institute of the Medical College of Wisconsin.12 IBMTR participants are required to register all consecutive transplantations; compliance is monitored by on-site audits. Patients are observed longitudinally with yearly follow-up. Computerized error checks, physician review of submitted data, and on-site audits of participating centers ensure data quality.
Inclusion criteria
The study includes 269 patients who underwent non–T-cell–depleted 1 or 2 HLA-A, -B, or -DR antigen-mismatched sibling or parental bone marrow transplantation between 1985 and 1997 for AML, ALL, or CML and who had adequate donor, recipient, and parental HLA serologic typing to allow classification as NIMA mismatched or NIPA mismatched. One hundred twenty-one (45%) donor-recipient pairs were NIMA mismatched, and 148 (55%) were NIPA mismatched. Twenty-nine additional NIMA-mismatched and 21 additional NIPA-mismatched recipients who met other eligibility criteria but received T-cell–depleted grafts were identified in the IBMTR database. These patients were excluded from multivariate analysis because of inadequate numbers to adjust for confounding by T-cell depletion, which has substantial effects on engraftment and GVHD. Data were reported by 122 transplant centers in 30 countries on 6 continents. Median follow-up was 54 months (range, 7-135 months), with 75% of the recipients followed for 25 or more months. Five hundred ninety patients meeting other eligibility criteria for the study were reported to the IBMTR but did not have adequate HLA typing information for parents and, hence, were not included in this report. Comparison of the 2 populations showed that those who did not have adequate HLA information were older, had higher rates of CML, and underwent transplantation earlier. Sex distribution and disease stages were similar for both populations. Engraftment, chronic GVHD, and overall survival rates were similar, but acute GVHD was more frequent in those with adequate HLA data (54% versus 44%).
End points
The study considered graft failure, acute and chronic GVHD, TRM, and overall survival. Graft failure was defined as failure to achieve a neutrophil count greater than 0.5 × 109/L or the achievement of a neutrophil count greater than 0.5 × 109/L followed by a decrease to less than 0.5 × 109/L. Incidence and time to development of grades 2 to 4 acute GVHD were evaluated in patients surviving 21 days with evidence of engraftment.13 Time to occurrence of any chronic GVHD was evaluated in patients surviving 90 days after transplantation with engraftment.14 TRM was defined as death in continuous complete remission.15 The end point for survival analyses was death from any cause.
Statistical analysis
Patient-, disease-, and transplant-related variables between groups were compared using the χ2 statistic for categorical variables and the Mann-Whitney U test for continuous variables. Probabilities of survival were calculated using the Kaplan-Meier estimator, censored at the last day of contact; the log-rank test was used for univariate comparisons of survival probabilities. Cumulative incidence curves were used to calculate probabilities of graft failure, acute GVHD, chronic GVHD, and TRM.16 Death was used as the competing risk for graft failure, acute GVHD, and chronic GVHD. Hematologic relapse was used as the competing risk for TRM.
Differences in outcomes of NIMA- versus NIPA-mismatched transplantation were evaluated in multivariate analyses using Cox proportional hazards regression to adjust for other potentially confounding differences between the cohorts.17 Variables considered were recipient age and sex, Karnofsky performance score at diagnosis, disease type, disease stage and duration at transplantation, donor age and sex, conditioning regimen, GVHD prophylaxis, calendar year of transplantation, and number and class of HLA-antigen mismatches. Only factors significantly (P < .05) associated with outcome were retained in the final models.
For each variable and outcome, the assumption of proportional hazards was tested using a time-dependent covariate. When this indicated differential effects over time (nonproportional hazards), models were constructed breaking the posttransplantation course into 2 time periods, using the maximized partial likelihood method to find the most appropriate breakpoint. NIPA-mismatched sibling donor for the end point of time to acute GVHD and paternal donor for the end point of time to chronic GVHD were 2 variables with nonproportional hazards. Forward stepwise variable selection at a .05 significance level was used to identify covariates other than HLA mismatch that were associated with outcome. In each step of the analysis, the covariate for NIMA versus NIPA mismatch was included in the model. First-order interactions between NIMA versus NIPA mismatch and all significant covariates were considered. Overall covariate effects were analyzed using the Wald test. Examination for center effects used a random effects model or a frailty model18; there was no evidence of confounding of main effects by center effects. All Pvalues were 2-sided.
Results
Patients
Donor–recipient relationship, sex match, and histocompatibility are listed in Table 1. One hundred thirty-two transplant donors were haploidentical siblings, and 137 transplant donors were parents.
Patients, disease, and transplant-related characteristics
| Variable . | Donor . | |||
|---|---|---|---|---|
| Sibling . | Parent . | |||
| NIMA mismatched . | NIPA mismatched . | Mother . | Father . | |
| N | 63 | 69 | 79 | 58 |
| Age (y), median (range) | 17 (2-50) | 20 (2-62) | 15 (1-40) | 17 (1-43) |
| Male sex (%) | 42 (67) | 39 (56) | 48 (61) | 33 (57) |
| Pretransplantation Karnofsky performance score less than 90% | 14 (24%) | 17 (27%) | 17 (24%) | 33 (65%) |
| Disease type | ||||
| AML (%) | 26 (41) | 14 (20) | 27 (34) | 20 (34) |
| ALL (%) | 23 (37) | 30 (44) | 36 (46) | 19 (33) |
| CML (%) | 14 (22) | 25 (36) | 16 (20) | 19 (33) |
| Disease stage at transplantation* | ||||
| Early (%) | 22 (35) | 23 (34) | 22 (28) | 14 (25) |
| Intermediate (%) | 21 (34) | 23 (34) | 26 (33) | 24 (44) |
| Advanced (%) | 19 (31) | 21 (32) | 30 (39) | 17 (31) |
| Disease duration longer than 12 mo (%) | 38 (60) | 29 (42) | 45 (57) | 33 (57) |
| Donor-recipient sex-match | ||||
| M-M (%) | 20 (32) | 19 (28) | — | 33 (57) |
| F-M (%) | 22 (35) | 20 (29) | 48 (60) | — |
| M-F (%) | 13 (20) | 16 (23) | — | 25 (43) |
| F-F (%) | 8 (13) | 14 (20) | 31 (40) | — |
| HLA-antigen class mismatched | ||||
| Class 1 (%) | 46 (73) | 43 (62) | 46 (58) | 35 (61) |
| Class 2 (%) | 13 (21) | 19 (28) | 17 (22) | 13 (22) |
| Classes 1 and 2 (%) | 4 (6) | 7 (10) | 16 (20) | 10 (17) |
| Number of HLA-antigen mismatched | ||||
| 1 Ag mismatch (%) | 59 (94) | 60 (87) | 26 (33) | 23 (40) |
| 2 Ag mismatch (%) | 4 (6) | 9 (13) | 53 (67) | 35 (60) |
| Conditioning regimen | ||||
| TBI and Cy with or without other (%) | 32 (51) | 35 (51) | 42 (53) | 31 (53) |
| TBI and other (%) | 5 (8) | 10 (14) | 8 (10) | 7 (12) |
| BuCy with or without other (%) | 22 (35) | 19 (28) | 19 (24) | 11 (19) |
| Other (%) | 4 (6) | 5 (7) | 10 (13) | 9 (16) |
| GVHD prophylaxis | ||||
| CsA with or without other (%) | 14 (22) | 17 (25) | 18 (23) | 10 (17) |
| MTX with or without other (%) | 6 (10) | 3 (4) | 5 (6) | 6 (10) |
| CsA and MTX with or without other (%) | 43 (68) | 49 (71) | 56 (71) | 42 (73) |
| Year of transplantation | ||||
| 1985 to 1989 (%) | 22 (35) | 23 (33) | 30 (38) | 20 (34) |
| 1990 to 1993 (%) | 22 (35) | 25 (36) | 24 (30) | 19 (33) |
| 1994 to 1997 (%) | 19 (30) | 21 (31) | 25 (32) | 19 (33) |
| Variable . | Donor . | |||
|---|---|---|---|---|
| Sibling . | Parent . | |||
| NIMA mismatched . | NIPA mismatched . | Mother . | Father . | |
| N | 63 | 69 | 79 | 58 |
| Age (y), median (range) | 17 (2-50) | 20 (2-62) | 15 (1-40) | 17 (1-43) |
| Male sex (%) | 42 (67) | 39 (56) | 48 (61) | 33 (57) |
| Pretransplantation Karnofsky performance score less than 90% | 14 (24%) | 17 (27%) | 17 (24%) | 33 (65%) |
| Disease type | ||||
| AML (%) | 26 (41) | 14 (20) | 27 (34) | 20 (34) |
| ALL (%) | 23 (37) | 30 (44) | 36 (46) | 19 (33) |
| CML (%) | 14 (22) | 25 (36) | 16 (20) | 19 (33) |
| Disease stage at transplantation* | ||||
| Early (%) | 22 (35) | 23 (34) | 22 (28) | 14 (25) |
| Intermediate (%) | 21 (34) | 23 (34) | 26 (33) | 24 (44) |
| Advanced (%) | 19 (31) | 21 (32) | 30 (39) | 17 (31) |
| Disease duration longer than 12 mo (%) | 38 (60) | 29 (42) | 45 (57) | 33 (57) |
| Donor-recipient sex-match | ||||
| M-M (%) | 20 (32) | 19 (28) | — | 33 (57) |
| F-M (%) | 22 (35) | 20 (29) | 48 (60) | — |
| M-F (%) | 13 (20) | 16 (23) | — | 25 (43) |
| F-F (%) | 8 (13) | 14 (20) | 31 (40) | — |
| HLA-antigen class mismatched | ||||
| Class 1 (%) | 46 (73) | 43 (62) | 46 (58) | 35 (61) |
| Class 2 (%) | 13 (21) | 19 (28) | 17 (22) | 13 (22) |
| Classes 1 and 2 (%) | 4 (6) | 7 (10) | 16 (20) | 10 (17) |
| Number of HLA-antigen mismatched | ||||
| 1 Ag mismatch (%) | 59 (94) | 60 (87) | 26 (33) | 23 (40) |
| 2 Ag mismatch (%) | 4 (6) | 9 (13) | 53 (67) | 35 (60) |
| Conditioning regimen | ||||
| TBI and Cy with or without other (%) | 32 (51) | 35 (51) | 42 (53) | 31 (53) |
| TBI and other (%) | 5 (8) | 10 (14) | 8 (10) | 7 (12) |
| BuCy with or without other (%) | 22 (35) | 19 (28) | 19 (24) | 11 (19) |
| Other (%) | 4 (6) | 5 (7) | 10 (13) | 9 (16) |
| GVHD prophylaxis | ||||
| CsA with or without other (%) | 14 (22) | 17 (25) | 18 (23) | 10 (17) |
| MTX with or without other (%) | 6 (10) | 3 (4) | 5 (6) | 6 (10) |
| CsA and MTX with or without other (%) | 43 (68) | 49 (71) | 56 (71) | 42 (73) |
| Year of transplantation | ||||
| 1985 to 1989 (%) | 22 (35) | 23 (33) | 30 (38) | 20 (34) |
| 1990 to 1993 (%) | 22 (35) | 25 (36) | 24 (30) | 19 (33) |
| 1994 to 1997 (%) | 19 (30) | 21 (31) | 25 (32) | 19 (33) |
Data are for patients after acute or chronic leukemia who received 1 or 2 antigen-mismatched sibling or parental transplants from 1985 to 1997, as reported to the IBMTR. TBI indicates total body irradiation; Cy, cyclophosphamide; Bu, busulfan; CsA, cyclosporine; MTX, methotrexate.
Early leukemia, first complete remission or first chronic phase; intermediate leukemia, greater than or equal to second complete remission; greater than or equal to second chronic phase or accelerated phase; advanced leukemia, patients never in remission, in any relapse or blast phase.
Patient and disease characteristics are listed in Table 1. The only statistically significant difference between the NIMA- and NIPA-mismatched sibling transplant recipients was disease duration before transplantation, which was longer in patients mismatched for NIMA (P < .03).
Transplantation characteristics are listed in Table 1. No statistically significant differences were observed between NIMA- and NIPA-mismatched sibling transplantations. Of note, parental donors were older and parental grafts had higher degrees of HLA mismatch than sibling grafts, but characteristics of paternal and maternal grafts were similar.
Univariate outcomes
Unadjusted probabilities of graft failure, GVHD, TRM, and survival by donor type are given in Table2.
Probabilities of graft failure, GVHD, TRM, and death by donor type
| Outcome . | Donor . | |||
|---|---|---|---|---|
| Sibling . | Parent . | |||
| NIMA mismatched . | NIPA mismatched . | Mother . | Father . | |
| 2-y probability of graft failure (%) | 18 (10-29) | 13 (6-24) | 20 (12-29) | 29 (17-42) |
| 100-d probability of grades 2-4 acute GVHD (%) | 41 (30-53) | 55 (44-66) | 58 (48-68) | 63 (51-75) |
| 2-y probability of chronic GVHD (%) | 28 (17-40) | 29 (19-40) | 31 (21-41) | 28 (18-40) |
| 2-y probability of TRM (%) | 36 (24-48) | 36 (25-48) | 52 (40-63) | 45 (32-59) |
| 2-y probability of survival (%) | 45 (33-57) | 44 (32-56) | 30 (20-40) | 24 (14-36) |
| Outcome . | Donor . | |||
|---|---|---|---|---|
| Sibling . | Parent . | |||
| NIMA mismatched . | NIPA mismatched . | Mother . | Father . | |
| 2-y probability of graft failure (%) | 18 (10-29) | 13 (6-24) | 20 (12-29) | 29 (17-42) |
| 100-d probability of grades 2-4 acute GVHD (%) | 41 (30-53) | 55 (44-66) | 58 (48-68) | 63 (51-75) |
| 2-y probability of chronic GVHD (%) | 28 (17-40) | 29 (19-40) | 31 (21-41) | 28 (18-40) |
| 2-y probability of TRM (%) | 36 (24-48) | 36 (25-48) | 52 (40-63) | 45 (32-59) |
| 2-y probability of survival (%) | 45 (33-57) | 44 (32-56) | 30 (20-40) | 24 (14-36) |
Multivariate analyses
Table 3 shows relative risks of transplantation outcomes by donor type, adjusting for other factors associated with transplantation outcome, including degree of donor–recipient histocompatibility.
Results of multivariate analyses comparing graft failure, GVHD, and TRM according to donor-recipient mismatch patterns for non–T-cell–depleted sibling or parent transplantation
| Outcome . | Patients at risk . | Combined model sibling–parent RR (95% CI) . | P . | Parents-only model RR (95% CI) . |
|---|---|---|---|---|
| Graft failure3-150,3-151 | ||||
| NIMA (sibling donor) | 63 | 1.0 | — | — |
| NIPA (sibling donor) | 67 | 0.61 (0.25-1.53) | .30 | — |
| Mother donor | 79 | 0.86 (0.39-1.89) | .70 | 1.0 |
| Father donor | 55 | 1.12 (0.50-2.50) | .78 | 0.38 (0.09-1.59) |
| Acute grades 2 to 4 GVHD3-152,3-153 | ||||
| NIMA (sibling donor) | 63 | 1.0 | — | — |
| NIPA (sibling donor) up to 7 d | 69 | 0.21 (0.03-1.61) | .13 | — |
| NIPA (sibling donor) more than 7 d | — | 1.86 (1.15-3.05) | .02 | — |
| Mother donor | 79 | 1.88 (1.15-3.05) | .01 | 1.0 |
| Father donor | 57 | 2.20 (1.32-3.66) | .003 | 1.17 (0.75-1.81) |
| Chronic GVHD3-154,3-159 | ||||
| NIMA (sibling donor) | 63 | 1.0 | — | — |
| NIPA (sibling donor) | 69 | 1.76 (0.90-3.44) | .10 | — |
| Mother donor | 79 | 2.32 (1.22-4.42) | .01 | 1.0 |
| Father donor up to 4 mo | 57 | 4.11 (1.89-8.95) | .0004 | 2.44 (1.12-5.34) |
| Father donor more than 4 mo | — | 0.74 (0.16-3.28) | .69 | 0.40 (0.08-1.70) |
| TRM3-160 | ||||
| NIMA (sibling donor) | 63 | 1.0 | — | — |
| NIPA (sibling donor) | 69 | 1.30 (0.73-2.31) | .38 | — |
| Mother donor | 79 | 2.02 (1.19-3.43) | .009 | 1.0 |
| Father donor | 57 | 1.92 (1.07-3.45) | .03 | 0.95 (0.56-1.56) |
| Outcome . | Patients at risk . | Combined model sibling–parent RR (95% CI) . | P . | Parents-only model RR (95% CI) . |
|---|---|---|---|---|
| Graft failure3-150,3-151 | ||||
| NIMA (sibling donor) | 63 | 1.0 | — | — |
| NIPA (sibling donor) | 67 | 0.61 (0.25-1.53) | .30 | — |
| Mother donor | 79 | 0.86 (0.39-1.89) | .70 | 1.0 |
| Father donor | 55 | 1.12 (0.50-2.50) | .78 | 0.38 (0.09-1.59) |
| Acute grades 2 to 4 GVHD3-152,3-153 | ||||
| NIMA (sibling donor) | 63 | 1.0 | — | — |
| NIPA (sibling donor) up to 7 d | 69 | 0.21 (0.03-1.61) | .13 | — |
| NIPA (sibling donor) more than 7 d | — | 1.86 (1.15-3.05) | .02 | — |
| Mother donor | 79 | 1.88 (1.15-3.05) | .01 | 1.0 |
| Father donor | 57 | 2.20 (1.32-3.66) | .003 | 1.17 (0.75-1.81) |
| Chronic GVHD3-154,3-159 | ||||
| NIMA (sibling donor) | 63 | 1.0 | — | — |
| NIPA (sibling donor) | 69 | 1.76 (0.90-3.44) | .10 | — |
| Mother donor | 79 | 2.32 (1.22-4.42) | .01 | 1.0 |
| Father donor up to 4 mo | 57 | 4.11 (1.89-8.95) | .0004 | 2.44 (1.12-5.34) |
| Father donor more than 4 mo | — | 0.74 (0.16-3.28) | .69 | 0.40 (0.08-1.70) |
| TRM3-160 | ||||
| NIMA (sibling donor) | 63 | 1.0 | — | — |
| NIPA (sibling donor) | 69 | 1.30 (0.73-2.31) | .38 | — |
| Mother donor | 79 | 2.02 (1.19-3.43) | .009 | 1.0 |
| Father donor | 57 | 1.92 (1.07-3.45) | .03 | 0.95 (0.56-1.56) |
Bu indicates busulfan; Cy, cyclophosphamide; TBI, total body irradiation; MTX, methotrexate; RR, relative risk; CI, confidence interval.
Model stratified by use of methotrexate for GVHD prophylaxis.
Other significant variables include: use of BuCy vs CyTBI for conditioning (RR, 2.44; 95% CI, 1.22-4.91; P = .01); Karnofsky performance score < 90% vs ≥ 90% (RR, 0.50; 95% CI, 0.31-2.29; P = .04).
Model stratified by disease stage before transplantation. Time-dependent covariate, such that the relative risk of AGVHD differs between NIMA and NIPA sibling before and after 7 days after transplantation.
Other significant variable, includes use of BuCy vs CyTBI for conditioning (RR, 0.47; 95% CI, 0.25-0.89; P = .02).
Pairwise comparison: NIPA sibling (early) vs mother donor (P = .03); NIPA sibling (late) vs mother (P = .96); NIPA sibling early vs father (P = .02); NIPA sibling late vs father (P = .48).
Time-dependent covariate, such that the relative risk of chronic GVHD differs between NIMA and father as donor before and after 4 months after transplantation. Other significant variables include class 1 antigen mismatch vs class 1 and 2 mismatch (RR, 0.44; 95% CI, 0.24-0.84; P = .01); class 2 antigen mismatch vs class 1 and 2 mismatch (RR, 0.32; 95% CI, 0.13-0.80; P = .02).
Pairwise comparison: NIPA sibling vs mother donor (P = .05); NIPA sibling vs father donor early (P = .001); NIPA sibling vs father donor late (P = .18).
Other significant variables include donor age ≥ 35 y (RR, 2.47; 95% CI, 1.36-4.50; P = .003); advanced disease stage at transplantation (RR, 1.89; 95% CI, 1.18-3.02;P = .008); transplantation year 1989 or later (RR, 0.59; 95% CI, 0.40-0.87; P = .009).
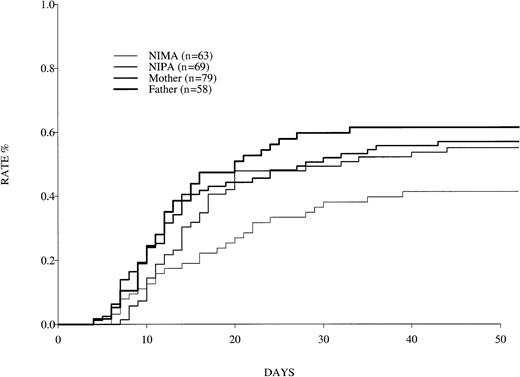
Relative risks of graft failure and GVHD after NIPA-mismatched sibling transplantations, maternal transplantations, and paternal transplantations versus NIMA-mismatched sibling transplantations are shown in Table 3. NIPA-mismatched sibling transplantations involved significantly more acute grades 2 to 4 GVHD than NIMA-mismatched sibling transplantations, starting 7 days after transplantation (P = .02) (Figure 2). A similar but not statistically significant trend was observed for chronic GVHD.
Occurrence of grades 2 to 4 GVHD in patients who received bone marrow from haploidentical donors.
Data were reported to the IBMTR for 1985 to 1997.
Occurrence of grades 2 to 4 GVHD in patients who received bone marrow from haploidentical donors.
Data were reported to the IBMTR for 1985 to 1997.
Maternal and paternal transplantations were associated with significantly more acute and chronic GVHD and TRM than NIMA-mismatched but not NIPA-mismatched sibling transplantations (Table3). Graft failure and survival did not differ by donor type.
Table 3 also shows results of separate multivariate analyses comparing maternal and parental transplantations. Although graft failure appeared to occur less often with maternal versus than with paternal donors, this did not reach statistical significance. In the first 4 months after transplantation, the risk for chronic GVHD was significantly higher in patients who received paternal grafts than in those who received maternal grafts. Acute GVHD, TRM, and overall survival rates were similar.
Discussion
This study provides evidence that exposure to maternal HLA antigens during the prenatal and the perinatal periods can have lifelong significance, at least with respect to GVHD risk. Transplantation from a haploidentical sibling donor to a recipient mismatched for maternal antigens is associated with less GVHD than transplantation from a sibling donor mismatched for paternal antigens and with less GVHD and TRM than transplantation from the mother or the father. This study may indicate that exposure to NIMA has a strong lifelong modulating impact on the immune response, resulting in a down-regulation of this response to later antigen challenge. No statistically significant differences in graft failure rates were found. This is not surprising considering the drastic immune ablation of pretransplantation conditioning regimens and the limited statistical power of the study. Maternal grafts tended to involve less graft failure and significantly less early chronic GVHD than grafts from the father, but they were not associated with less acute GVHD, less TRM, or better survival than paternal grafts. This is contrary to expectation, if one assumes that the mother develops, during pregnancy, partial tolerance to the haplotypes of the father. Such an assumption is, however, an oversimplification. Evidence for a tolerising effect through pregnancy exists, but a large percentage of pregnant women form antibodies against paternal HLA antigens.19 This tolerising effect is certainly not always present. Priming of T cells against paternal antigens has also been documented. This does not exclude the possibility that partial tolerance to paternal HLA antigens might exist in some women. The situation is further complicated by the fact that such partial tolerance to the paternal HLA antigens might be counteracted by priming of T (and B?) cells to paternal minor histocompatibility antigens.
Our findings show striking similarity to the results of a recent study on the outcome of parental and haploidentical sibling kidney allografts4 and to those of a small (n = 15) study of haploidentical sibling cord blood transplantations.20 In the first study, grafts from haploidentical siblings mismatched for NIMA had significantly better survival than those from parents or from siblings mismatched for paternal antigens. In contrast to that study, in which there was no difference between maternal and paternal transplantations, the current study found less chronic GVHD with maternal than with paternal grafts.
Comparing the outcome of a transplant donated by a haploidentical sibling with one donated by a parent is further complicated by the fact that the shared haplotypes differ (Figure 1). If the mother is the donor, her T cells might be primed against the minor histocompatibility antigens of the father through restriction by the shared maternal haplotype. If a sibling is the donor, the shared haplotype originates from the parent other than the one contributing the mismatched haplotype. This might reduce T-cell reactivity to the minor histocompatibility antigens. The mother and the child have been exposed to the other's histocompatibility antigens. However, during this exposure, the immune system of the child is more naive than that of the mother. It is possible that this, too, plays a role in the different outcome for maternal grafts than for sibling grafts mismatched for maternal antigens. It should be noted that the parental donors in this study tended to have more HLA disparity with the recipient than the sibling donors. In addition, transplants from older donors are more often associated with GVHD. Although we attempted to adjust for this effect in multivariate analysis, it is possible that it contributed to the observed differences in outcome between parental and sibling transplantations. Another limitation of the study is that no information on (mis)matches of HLA-C, -DQ, or -DP was available, nor was information available on HLA typing at the allele level. These shortcomings will be addressed during the forthcoming 13th International Histocompatibility Workshop.
It may be of interest that transplants mismatched for HLA-A with the recipient, in which the HLA-A–mismatched antigens were for the donor NIMA, involved the least GVHD (P = .10) (data not shown). Although our data are not statistically significant, similar and significant observations have been reported in kidney transplantation.11
Whether our findings can be used to improve the results of hematopoietic stem cell transplantation from unrelated donors remains to be investigated. Taken together, our observations could be interpreted as evidence for a lifelong(!) immune-modulating effect of pregnancy. Unraveling the cellular and molecular mechanism underlying this effect might provide information regarding allograft recognition and its regulation. The mechanism whereby exposure to maternal cells or soluble HLA antigens minimizes the risk for GVHD and, consequently, TRM remains unclear. Several interactions are possible and have been discussed elsewhere, but none of them have been formally tested and shown to be correct.4 21-23
The recent observation that the stimulation of umbilical cord blood T cells with peripheral blood lymphocytes of the mother causes a significant increase in immune-modulating CD8dim T cells—in contrast to an increase of cytotoxic CD8bright T cells after stimulation with the paternal cells—might provide a new avenue to elucidate the mechanisms underlying the NIMA effect.24 Understanding these mechanisms might be of crucial importance in our quest to obtain tolerance to allografts in humans. In this context it might be profitable to explore whether the NIMA effect and the immune-modulating effect of pretransplantation blood transfusions from donors who share an HLA haplotype (HST) with the recipient have a common basis.22 In both instances, haploidentical allogeneic cells have a tolerising effect on haploidentical grafts. HSTs with initial, but temporary, immunosuppression have led to true tolerance for renal allografts from 2 to more than 18 years in monkeys.25,26 It is well recognized that an encounter with allo-antigens before organ or stem cell transplantation can influence transplantation outcome. It makes a major difference whether this allogeneic stimulation3-5,8 9 is MHC restricted. If it is MHC restricted, partial tolerance can ensue; if it is not, rejection is more likely. Thus, our findings might be relevant not only when selecting 1 or 2 HLA antigen-mismatched haploidentical donors. They might be relevant for our understanding of the cellular basis of autoimmunity or resistance to infection. It is surprising that the impact of the first major encounter with allo-antigens, those of the mother, has so far received little attention.
Supported by Public Health Service grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; grants from Amcell; Amgen; Anonymous; Aventis Pharmaceuticals; Baxter Oncology; Berlex Laboratories; Blue Cross and Blue Shield Association; Lynde and Harry Bradley Foundation; Ceros; Chimeric Therapies; Chiron Therapeutics; Eleanor Naylor Dana Charitable Trust; Empire Blue Cross Blue Shield; Fujisawa Healthcare; Gambro BCT; Center for Advanced Studies in Leukemia; Genentech; GlaxoSmithKline; Human Genome Sciences; ICN Pharmaceuticals; IDEC Pharmaceuticals; Immunex Corporation; IntraBiotics Pharmaceuticals; Kettering Family Foundation; Kirin Brewery; Robert J. Kleberg Jr and Helen C. Kleberg Foundation; LifeTrac/Allianz; Nada and Herbert P. Mahler Charities; Market Certitude; Mayer Ventures; MedImmune; Merck; Milliman & Robertson; Milstein Family Foundation; Greater Milwaukee Foundation/Elsa Schoeneich Research Fund; NeoRx; Novartis Pharmaceuticals; Orphan Medical; Ortho Biotech; John Oster Family Foundation; Pfizer; Pharmacia; Principal Life Insurance; Response Oncology; RGK Foundation; Roche Laboratories; SangStat; Schering AG; Schering Oncology/Biotech; Stackner Family Foundation; The Starr Foundation; SuperGen; TheraTechnologies; Unicare Life and Health Insurance; Wyeth/Genetics Institute; and Macropa Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mary M. Horowitz, IBMTR/ABMTR Statistical Center, Medical College of Wisconsin, PO Box 26509, Milwaukee, WI 53226; e-mail: marymh@mcw.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal