Phosphatidlyserine (PS) exposure on the erythrocyte surface endows the cell with the propensity of adhering to vascular endothelium. Because individuals with sickle cell disease (SCD) manifest loss of erythrocyte membrane asymmetry with PS exposure, we have assessed the contribution of this marker to the process of sickle erythrocyte–microendothelial adhesion. Assays for plasma-induced adhesion were conducted on unactivated endothelium, in the absence of immobilized ligands, such that PS was compared to the erythrocyte adhesion receptor CD36. Blocking studies with erythrocytes pretreated with annexin V (to cloak PS) or anti-CD36 or both revealed an inhibitory effect on adhesion of 36% ± 10% and 23% ± 8% with blocking of both sites suggestive of an additive effect. We next evaluated 87 blood samples from patients with SCD and grouped them into 4 categories based on adhesion marker (CD36 and PS) levels. Results revealed a striking correlation between erythrocyte PS positivity and adhesion. Analyses of the individual patient data demonstrated a positive correlation between PS and adhesion (R = 0.52,P < .000 001), whereas none was noted between adhesion and CD36 (R = 0.2, P > .07). The effect of PS on adhesion appears to be related to the quantitative differences in erythrocyte markers in SCD, with PS the predominant marker when compared to CD36 both in the total erythrocyte population, and when the adherence-prone erythrocyte, the CD71+ stress reticulocyte, was evaluated. Our study signals the entrance of an important new contributor to the field of sickle erythrocyte–endothelial adhesion. The implications of erythrocyte PS exposure in relation to the vascular pathology of SCD need to be assessed.
Introduction
Since the initial observation by Hebbel and coworkers that sickle red cell–endothelial adherence is correlated with clinical disease severity,1 the last 2 decades have seen a large body of work related to the surface adhesion molecules and markers present on the erythrocyte and endothelium that play a role in the adhesion process.2,3 Receptors on the red blood cell (RBC) include the integrin α4β1 also known as the very late activation antigen 4 (VLA-4),4-6 and the thrombospondin (TSP) receptor CD365,7,8; nonreceptor markers include aggregated membrane band-39 and sulfated glycolipids.10 Endothelial receptors include the vitronectin receptor αVβ3integrin7,11,12 and the vascular cell adhesion molecule 1 (VCAM-1),4,6,13 whereas CD36 and glycoprotein (GP) Ib may also be of potential significance.2 Ligands in plasma and endothelial matrix proteins that promote adhesion include TSP,14,15 von Willebrand factor (VWF),12,16and laminin,17 and fibrinogen and fibronectin may also be involved.2
Although the asymmetrical distribution of phospholipids appears to be well conserved throughout the lifespan of the cell, studies using annexin V (a calcium-dependent phospholipid-binding protein) have demonstrated that in patients with sickle cell disease (SCD), the anionic phospholipid phosphatidylserine (PS) is present on the RBC surface.18-20 Potential consequences of such pathologic PS exposure include an exacerbation of the anemia due to enhanced reticuloendothelial clearance and activation of coagulation.21 Recent reports have demonstrated that PS exposing human erythrocytes (produced by the action of ionophore on control RBCs) adhere to vascular endothelium and the endothelial matrix protein TSP.22 23 In the present study, we have sought to extend these in vitro observations (on treated control erythrocytes) to the clinical arena by performing studies in patients with SCD and assessing the relative roles of some of the important erythrocyte adhesion markers described to date. We demonstrate by in vitro blocking experiments and in the patient-related studies that erythrocyte PS exposure appears to be a critical determinant in the adhesion process.
Materials and methods
Materials
For flow cytometric analyses, phycoerythrin (PE)–and fluorescein isothiocyanate (FITC)–labeled mouse monoclonal antibodies against human antigens and the isotypic negative control antibodies were obtained from Immunotec (Beckman-Coulter, Miami, FL). These antibodies included anti–glycophorin A (CD235a)–PE (clone 11E4B7.6 [KC16]), anti-TSP receptor (CD36)–pure, anti-CD36–FITC (clone FA6.152), anti–integrin α4 (CD49d)–FITC (α chain of VLA-4, clone HP2/1), antitransferrin receptor (CD71)–FITC, anti-CD71–PE (clone YDJ1.2.2), and the isotypic control antibody (clone 679.1Mc7). TRI-COLOR (TC)–labeled goat anti–mouse IgG and annexin V–pure (product A9460) were obtained from Caltag Laboratories (Burlingame, CA) and Sigma Immunochemicals (St Louis, MO), respectively. Annexin V–FITC was purchased from R & D Systems (Minneapolis, MN). In flow cytometric studies, CD235a was used as a marker for erythrocytes; CD49d, CD71, and annexin V were used as markers for VLA-4, stress reticulocytes, and PS positivity, respectively. 51Cr-sodium chromate (400-1200 mCi/mg; 14.8-44.4 TBq/g) was purchased from New England Nuclear (Boston, MA). Tissue culture supplies were obtained from Gibco Laboratories (Grand Island, NY).
Collection of blood
Venous blood was obtained from 68 children with SCD in steady state (40 with SS, 24 with SC, and 4 with Sβthal+genotypes; ages 3 months to 22 years), and 10 age-matched hemoglobin AA (HbAA) controls. Patients were considered to be in steady state if they were afebrile, had not been hospitalized or received a transfusion within 8 weeks, and had had no vaso-occlusive episode for at least 2 weeks before or after blood sampling. Blood was drawn by a well-trained phlebotomist using a 2-syringe technique. Eighty-seven blood samples were obtained from 68 patients such that 2 blood samples were obtained at least 1 year apart on 12 infants with SS and 7 with SC genotypes. Because fetal hemoglobin (HbF) levels decrease during early childhood, and because an inverse correlation exists between HbF and adhesion marker–positive erythrocytes,24 results from these blood samples were treated as individual data points for analyses of adhesion results. This study was reviewed and approved by the Institutional Review Committee for the protection of human subjects at St Christopher's Hospital for Children and Thomas Jefferson University. Blood was obtained from controls and patients after informed consent was given. For minors, the patient's assent, where appropriate, was obtained in addition to parental permission. For both flow cytometry analyses of RBC markers and adhesion assays, blood (1 mL) was collected using sodium heparin as the anticoagulant and assessed within 2 to 20 hours after collection. For adhesion blocking studies described below, 3 mL blood was obtained from children 5 years or older and adolescents.
RBC adhesion assay
Adhesion of sickle RBCs to endothelial cell monolayers was evaluated in a static adhesion assay1 using51Cr-labeled sickle RBCs and human retinal capillary microvascular endothelial cell (HRCMEC) monolayers. The51Cr-labeled sickle RBCs were prepared as previously described.25 HRCMECs were isolated, identified, and cultured in minimal essential medium supplemented with 10% fetal calf serum.26 Cells from passages 8 to 16 were used in the adhesion experiments, each passage representing 2-cell doublings. For adhesion assay, endothelial cells were plated at a density of 200 000 cells per well into wells of 12-well plates, grown to confluence, and then coincubated with 51Cr-labeled sickle RBCs. Adhesion assays were conducted in the presence of 10% autologous plasma at 37°C for 45 minutes, and the nonadherent RBCs were removed. The monolayers were then washed and adherent RBCs were determined by51Cr release following cell lysis. 51Cr-labeled control HbAA RBCs were concomitantly evaluated in adhesion assays in the presence and the absence of 10% autologous plasma. The adhesion potential of the test RBC was expressed as an adhesion ratio, which was determined by dividing the number of adherent erythrocytes by control HbAA RBCs assayed in the absence of plasma.
Flow cytometric analysis of adhesive receptors and other surface markers on erythrocytes
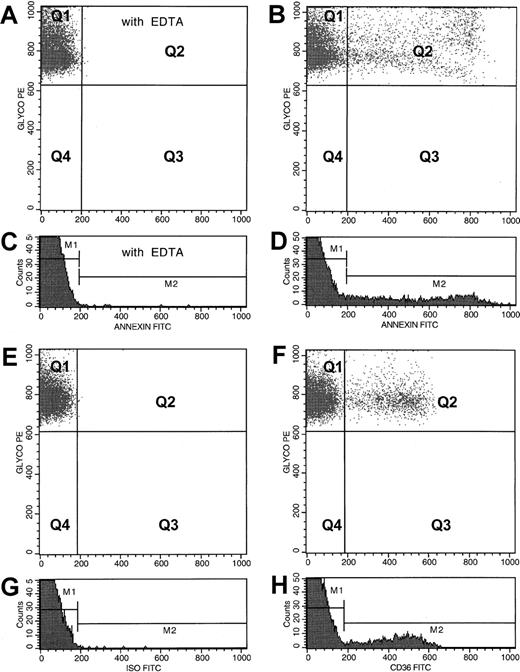
One million washed RBCs (suspended in a final volume of 100 μL Hanks balanced salt solution [HBSS]–HEPES buffer) were incubated for 30 minutes at room temperature with 20 μL PE-labeled anti-CD235a and 10 μL FITC-labeled annexin V in the presence of either 2.5 mM CaCl2 or 2.5 mM EDTA.19,20 27 Incubation mixtures were then diluted with 1 mL HBSS-HEPES buffer containing either 2.5 mM CaCl2 or 2.5 mM EDTA and analyzed in a Becton Dickinson Flow Cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) formatted for 2-color analysis. Fluorescence compensation settings were established using cells stained with anti-CD235a–PE alone, annexin V–FITC alone, PE-labeled isotopic negative control antibody, and annexin V–FITC in the presence of EDTA. Data from 50 000 events were collected for analysis. Using size scatter properties, RBC-associated events were separated from non-RBC–associated events (which did not exceed 0.5%) and were gated out from the analysis. As shown in Figure1B, PS+ RBCs (events in quadrant Q2), defined as anti-CD235a+ events simultaneously stained for annexin V were determined by setting quadrants on the FL1 (annexin V) and FL2 (CD235a) dot plot. Nonspecific membrane immunofluorescence was determined using cells stained with anti-CD235a–PE and annexin V–FITC in the presence of EDTA, and these values (events in quadrant Q2, Figure 1A) were subtracted from the respective sample fluorescence. As shown in panels C and D of Figure 1, analysis of the data using the respective histograms of the annexin V–FITC fluorescence provided identical results. Data were expressed as percent PS+ RBCs.
Flow cytometric analyses of PS+ and CD36+ erythrocytes from a representative patient with SCD.
For the analysis of PS+ RBCs, dot plots of anti-CD235a–PE fluorescence and annexin V–FITC fluorescence from an RBC preparation stained in the presence of EDTA and calcium are presented in panels A and B, respectively. PS− (quadrant Q1) and PS+(quadrant Q2) dot plot regions were set using the erythrocyte sample stained in the presence of EDTA (A). Corresponding histogram profiles of annexin V–FITC fluorescence are presented in panels C and D, respectively. PS− (gate M1) and PS+ (gate M2) histogram regions were set using the RBC sample stained in the presence of EDTA (C). Values obtained for PS+ RBCs from both dot plot and histogram analyses were identical. For the analysis of CD36+ erythrocytes, dot plots of anti-CD235a–PE fluorescence and either FITC fluorescence from an isotype-matched negative control antibody or anti-CD36–FITC fluorescence from an RBC preparation are presented in panels E and F, respectively. Corresponding histogram profiles of FITC fluorescence from RBCs stained with the negative control antibody and anti-CD36 are presented in panels G and H, respectively. Events in quadrant Q1 (F) and gate M1 (H) represent CD36− RBCs, and those in quadrant Q2 (F) and gate M2 (H) are from the CD36+ erythrocytes. Analyses procedures similar to those described for PS+ RBCs were also used to assess data related to CD36 presented in panels E through H.
Flow cytometric analyses of PS+ and CD36+ erythrocytes from a representative patient with SCD.
For the analysis of PS+ RBCs, dot plots of anti-CD235a–PE fluorescence and annexin V–FITC fluorescence from an RBC preparation stained in the presence of EDTA and calcium are presented in panels A and B, respectively. PS− (quadrant Q1) and PS+(quadrant Q2) dot plot regions were set using the erythrocyte sample stained in the presence of EDTA (A). Corresponding histogram profiles of annexin V–FITC fluorescence are presented in panels C and D, respectively. PS− (gate M1) and PS+ (gate M2) histogram regions were set using the RBC sample stained in the presence of EDTA (C). Values obtained for PS+ RBCs from both dot plot and histogram analyses were identical. For the analysis of CD36+ erythrocytes, dot plots of anti-CD235a–PE fluorescence and either FITC fluorescence from an isotype-matched negative control antibody or anti-CD36–FITC fluorescence from an RBC preparation are presented in panels E and F, respectively. Corresponding histogram profiles of FITC fluorescence from RBCs stained with the negative control antibody and anti-CD36 are presented in panels G and H, respectively. Events in quadrant Q1 (F) and gate M1 (H) represent CD36− RBCs, and those in quadrant Q2 (F) and gate M2 (H) are from the CD36+ erythrocytes. Analyses procedures similar to those described for PS+ RBCs were also used to assess data related to CD36 presented in panels E through H.
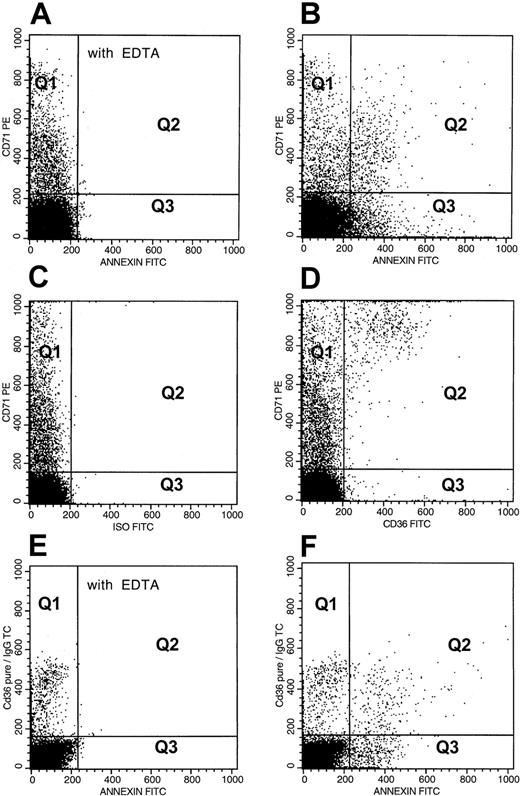
The RBC adhesion receptors including CD36 and VLA-4, and CD71 were analyzed by 2-color flow cytometry using erythrocytes stained with anti-CD235a–PE and one of the FITC-labeled antibodies: anti-CD36, anti-CD49d, anti-CD71, or an isotypic negative control antibody as previously described.24 Adhesion receptor–positive and CD71+ erythrocytes were analyzed using the steps outlined as above for PS+ RBCs. A representative analysis for CD36+ RBCs is depicted in panels E to H of Figure 1. Marker-positive stress reticulocytes were analyzed by flow cytometry using 2-color analysis as detailed above using erythrocytes stained for the transferrin receptor (anti-CD71–PE) and one of the FITC-labeled reagents: anti-CD36 or an isotypic negative control IgG, or annexin V in the presence of 2.5 mM CaCl2 or 2.5 mM EDTA. PS+ and CD36+ stress reticulocytes were analyzed using the dot plots of anti-CD71–PE fluorescence and either annexin V–FITC fluorescence (Figure2A,B) or anti-CD36–FITC fluorescence (Figure 2C,D), respectively. For analyses of RBCs positive for both PS and CD36, anti-CD36–pure plus anti–mouse IgG–TC–labeled washed sickle RBCs were stained with annexin V–FITC in the presence of CaCl2 or EDTA and analyzed as shown in panels E and F of Figure 2.
Flow cytometric analyses of adhesion marker–positive stress reticulocytes and double adhesion marker–positive erythrocytes from a representative patient with SCD.
For analysis of PS+ stress reticulocytes, dot plots of anti-CD71–PE fluorescence and annexin V–FITC fluorescence from an RBC preparation stained in the presence of EDTA and calcium are presented in panels A and B. PS− (Q1) and PS+ (Q2) dot plot regions were set using erythrocytes stained in the presence of EDTA (A). The events in quadrants Q1 and Q2 (B) represent PS− and PS+ stress reticulocytes, whereas Q3 and Q4 represent PS+ and PS− nonreticulocytes. The following analyses were made: (1) Percent PS+ stress reticulocytes in the total stress reticulocyte fraction = (Q2 × 100)/(Q1 + Q2); (2) Percent PS+ stress reticulocytes in the total PS+ RBC fraction = (Q2 × 100)/(Q2 + Q3); (3) Percent PS+ stress reticulocytes in the total RBC fraction = (Q2 × 100)/(Q1 + Q2 +Q3 +Q4). Analyses of CD36+ stress reticulocytes (C,D) were performed in a manner similar to that described above for PS relationships. CD36− (Q1) and CD36+ (Q2) dot plot regions were set using erythrocytes stained with anti-CD71–PE and FITC-labeled negative isotype (C). For analysis of erythrocytes positive for both PS and CD36, dot plots of mouse anti-CD36–pure plus goat anti–mouse IgG–TC fluorescence and annexin V–FITC fluorescence from an RBC preparation stained in the presence of EDTA and calcium are presented in panels E and F. The quadrants in panel F represent PS− and CD36+ RBCs (Q1), erythrocytes positive for both PS and CD36 (Q2), CD36− and PS+ RBCs (Q3), and erythrocytes negative for both PS and CD36 (Q4). Data were analyzed in a manner similar to that presented above for PS+ stress erythrocytes.
Flow cytometric analyses of adhesion marker–positive stress reticulocytes and double adhesion marker–positive erythrocytes from a representative patient with SCD.
For analysis of PS+ stress reticulocytes, dot plots of anti-CD71–PE fluorescence and annexin V–FITC fluorescence from an RBC preparation stained in the presence of EDTA and calcium are presented in panels A and B. PS− (Q1) and PS+ (Q2) dot plot regions were set using erythrocytes stained in the presence of EDTA (A). The events in quadrants Q1 and Q2 (B) represent PS− and PS+ stress reticulocytes, whereas Q3 and Q4 represent PS+ and PS− nonreticulocytes. The following analyses were made: (1) Percent PS+ stress reticulocytes in the total stress reticulocyte fraction = (Q2 × 100)/(Q1 + Q2); (2) Percent PS+ stress reticulocytes in the total PS+ RBC fraction = (Q2 × 100)/(Q2 + Q3); (3) Percent PS+ stress reticulocytes in the total RBC fraction = (Q2 × 100)/(Q1 + Q2 +Q3 +Q4). Analyses of CD36+ stress reticulocytes (C,D) were performed in a manner similar to that described above for PS relationships. CD36− (Q1) and CD36+ (Q2) dot plot regions were set using erythrocytes stained with anti-CD71–PE and FITC-labeled negative isotype (C). For analysis of erythrocytes positive for both PS and CD36, dot plots of mouse anti-CD36–pure plus goat anti–mouse IgG–TC fluorescence and annexin V–FITC fluorescence from an RBC preparation stained in the presence of EDTA and calcium are presented in panels E and F. The quadrants in panel F represent PS− and CD36+ RBCs (Q1), erythrocytes positive for both PS and CD36 (Q2), CD36− and PS+ RBCs (Q3), and erythrocytes negative for both PS and CD36 (Q4). Data were analyzed in a manner similar to that presented above for PS+ stress erythrocytes.
Treatment of sickle erythrocytes with anti-CD36 and annexin V
The 51Cr-labeled and washed sickle red cells (2.5% hematocrit) from 6 donors were pretreated for 45 minutes at 37°C with 40 μg/mL anti-CD36 (to cloak cell surface CD36) or 40 μg/mL annexin V (to cloak cell surface PS) or both and then simultaneously evaluated for their residual adhesion potential and surface adhesion markers. Concentrations of anti-CD36 and annexin V for the blocking studies described above were selected from preliminary dose-response experiments (n = 3) performed using 1 to 100 μg/mL blocking agent. Both anti-CD36 and annexin V at a concentration of 40 μg/mL blocked 70% to 90% of RBC surface CD36 and PS in these preliminary experiments. To assess whether blocking one marker affected surface expression of the other, in additional preliminary experiments, RBCs were pretreated with either anti-CD36 (40 μg/mL) or annexin V (40 μg/mL), and then analyzed for both CD36 and PS by flow cytometry. Anti-CD36 treatment (n = 6) had no significant effect on PS exposure (4.9% ± 2.6% on untreated versus 4.4% ± 2.5% PS positivity on anti-CD36–treated RBCs, P > .25, paired ttest). Similarly, annexin V treatment (n = 7) had no effect on CD36 exposure (2.63% ± 2.32% on untreated versus 2.53% ± 2.16% CD36 positivity on annexin V–treated RBCs, P > .45).
Statistical analysis
Statistical evaluation was performed using the Sigmastat Statistical Package (Jandel Scientific, San Rafael, CA). All results are presented as the mean ± SD. Because analyses of the adhesion marker data showed a nonparametric distribution, significant differences between control and the patient groups were analyzed using the Kruskal-Wallis test. If the P value for this overall comparison was significant at P < .05, group-wise comparisons were performed using the Mann-Whitney test. Statistical significance between 2 paired variables was analyzed using the paired Student t test. Both Pearson and Spearman correlation tests were used to determine the relationship between 2 variables. Both tests yielded similar results for the same pair of variables analyzed. R andP values presented for adhesion markers (except for VLA-4 related) were obtained by Pearson tests on log-transformed data. Because no VLA-4+ RBCs or reticulocytes were detected in many donors including patients with SCD, Pearson tests on VLA-4–related data were performed using nontransformed data.
Results
Relationship between erythrocyte PS, CD36, VLA-4, and adhesion
To assess the relative roles of adhesion markers in sickle erythrocyte–endothelial adhesion, we grouped patients into 4 arbitrary categories based on their adhesion marker values as depicted in Table 1. The surface levels of the main adhesion-related markers (PS, CD36, and VLA-4) and the adhesive properties of each patient group as compared to HbAA controls are shown in Table 2. Minimal levels of adhesion marker–positive RBCs were measured in controls. Both PS+and CD36+ erythrocytes were elevated in all 4 SCD patient groups when compared to controls (P < .002 to <.000 001). Although the mean levels of VLA-4+ RBCs in SCD groups 1 and 4 were identical to control values, increased VLA-4 positivity was seen in SCD groups 2 and 3, with significant changes noted in group 2 (P < .05). The elevations in erythrocyte adhesion markers noted in the SCD patient groups 1 through 4 when compared to controls was accompanied by an increase in the adhesion of these RBCs to microvessel endothelium.
SCD patient groups evaluated
| Group 1 (n = 19) | Low levels of both PS+ and CD36+ erythrocytes |
| Group 2 (n = 45) | Elevated levels of both PS+ and CD36+erythrocytes |
| Group 3 (n = 10) | Low levels of erythrocyte PS in combination with elevated CD36 levels |
| Group 4 (n = 13) | Elevated levels of erythrocyte PS in combination with low CD36 levels |
| Group 1 (n = 19) | Low levels of both PS+ and CD36+ erythrocytes |
| Group 2 (n = 45) | Elevated levels of both PS+ and CD36+erythrocytes |
| Group 3 (n = 10) | Low levels of erythrocyte PS in combination with elevated CD36 levels |
| Group 4 (n = 13) | Elevated levels of erythrocyte PS in combination with low CD36 levels |
Levels of erythrocyte PS and CD36 were considered elevated if the values were more than 3 SD above that seen in the control population, that is, 2.5% and 0.4% for PS and CD36, respectively.
Adhesion characteristics of RBCs from patients with SCD
| Marker/parameter assayed . | HbAA controls (n = 10) . | SCD-1 low PS/low CD36 (n = 19) . | SCD-2 high PS/high CD36 (n = 45) . | SCD-3 low PS/high CD36 (n = 10) . | SCD-4 high PS/low CD36 (n = 13) . |
|---|---|---|---|---|---|
| PS+erythrocytes | 0.78% ± 0.60% | 1.44% ± 0.42% | 5.59% ± 2.30%* | 1.76% ± 0.35%† | 4.38% ± 1.56%* |
| (0.59%, 0.31%, 1.12%) | (1.49%, 1.03%, 1.81%) | (5.05%, 3.71%, 7.60%) | (1.93%, 1.38%, 2.06%) | (3.96%, 3.41%, 4.97%) | |
| CD36+erythrocytes | 0.06% ± 0.07% | 0.23% ± 0.16% | 2.22% ± 1.69%* | 2.12% ± 1.73%* | 0.30% ± 0.15%† |
| (0.02%, 0.00%, 0.11%) | (0.24%, 0.08%, 0.30%) | (1.33%, 0.94%, 3.39%) | (1.53%, 1.01%, 2.82%) | (0.30%, 0.15%, 0.38%) | |
| VLA-4+erythrocytes | 0.05% ± 0.05% | 0.05% ± 0.08% | 0.23% ± 0.30%* | 0.33% ± 0.64%* | 0.05% ± 0.11%† |
| (0.04%, 0.00%, 0.11%) | (0.03%, 0.00%, 0.07%) | (0.14%, 0.05%, 0.30%) | (0.09%, 0.04%, 0.22%) | (0.00%, 0.00%, 0.04%) | |
| Erythrocytes positive | ND | 0.10% ± 0.09% | 1.18% ± 0.83%* | 0.96% ± 0.54%* | 0.10% ± 0.06%† |
| for both PS and CD36 | (0.07%, 0.03%, 0.14%) | (0.93%, 0.64%, 1.18%) | (0.79%, 0.60%, 1.36%) | (0.09%, 0.06%, 0.15%) | |
| Plasma-induced | 1.18 ± 0.46 | 2.20 ± 1.36 | 6.49 ± 4.10* | 2.08 ± 1.47† | 6.39 ± 1.8* |
| adhesion | (1.07, 0.91, 1.40) | (1.90, 1.20, 2.73) | (5.92, 3.03, 9.88) | (1.38, 1.22, 2.35) | (6.80, 5.30, 7.90) |
| Marker/parameter assayed . | HbAA controls (n = 10) . | SCD-1 low PS/low CD36 (n = 19) . | SCD-2 high PS/high CD36 (n = 45) . | SCD-3 low PS/high CD36 (n = 10) . | SCD-4 high PS/low CD36 (n = 13) . |
|---|---|---|---|---|---|
| PS+erythrocytes | 0.78% ± 0.60% | 1.44% ± 0.42% | 5.59% ± 2.30%* | 1.76% ± 0.35%† | 4.38% ± 1.56%* |
| (0.59%, 0.31%, 1.12%) | (1.49%, 1.03%, 1.81%) | (5.05%, 3.71%, 7.60%) | (1.93%, 1.38%, 2.06%) | (3.96%, 3.41%, 4.97%) | |
| CD36+erythrocytes | 0.06% ± 0.07% | 0.23% ± 0.16% | 2.22% ± 1.69%* | 2.12% ± 1.73%* | 0.30% ± 0.15%† |
| (0.02%, 0.00%, 0.11%) | (0.24%, 0.08%, 0.30%) | (1.33%, 0.94%, 3.39%) | (1.53%, 1.01%, 2.82%) | (0.30%, 0.15%, 0.38%) | |
| VLA-4+erythrocytes | 0.05% ± 0.05% | 0.05% ± 0.08% | 0.23% ± 0.30%* | 0.33% ± 0.64%* | 0.05% ± 0.11%† |
| (0.04%, 0.00%, 0.11%) | (0.03%, 0.00%, 0.07%) | (0.14%, 0.05%, 0.30%) | (0.09%, 0.04%, 0.22%) | (0.00%, 0.00%, 0.04%) | |
| Erythrocytes positive | ND | 0.10% ± 0.09% | 1.18% ± 0.83%* | 0.96% ± 0.54%* | 0.10% ± 0.06%† |
| for both PS and CD36 | (0.07%, 0.03%, 0.14%) | (0.93%, 0.64%, 1.18%) | (0.79%, 0.60%, 1.36%) | (0.09%, 0.06%, 0.15%) | |
| Plasma-induced | 1.18 ± 0.46 | 2.20 ± 1.36 | 6.49 ± 4.10* | 2.08 ± 1.47† | 6.39 ± 1.8* |
| adhesion | (1.07, 0.91, 1.40) | (1.90, 1.20, 2.73) | (5.92, 3.03, 9.88) | (1.38, 1.22, 2.35) | (6.80, 5.30, 7.90) |
Values presented are the mean ± SD from 10 to 45 donors as indicated. Corresponding median, 25th and 75th percentile values are shown in the parentheses. Marker levels from either the SCD-1 (low PS/low CD36) or SCD-2 (high PS/high CD36) group were compared to the other 3 patient groups. For comparison, marker levels from HbAA control donors are also shown. Adhesion is the ratio of test erythrocyte adhesion in the presence of plasma to HbAA control RBC adhesion performed in the absence of plasma to retinal microendothelium. F cells (erythrocytes containing HbF) in SCD-2 and SCD-4 with the highest adhesion ratios were not significantly different from values in SCD-1. Number of control HbAA RBCs (measured as 51Cr radioactivity) adhered to retinal capillary microendothelium in the absence and the presence of plasma ranged from 30 to 90 cpm/well, equivalent to 50 to 150 RBCs/mm2 (calculated from a specific radioactivity of 1700 cpm/1 × 106 RBCs).
ND indicates measurements not done.
Indicates values significantly different from SCD-1 atP < .05 to .0001.
Indicates values significantly different from SCD-2 atP < .0001.
The relative role of erythrocyte PS versus CD36 in adhesion is demonstrated in the results presented in Table 2. Comparison of SCD groups 1 and 2 revealed that the mean levels of both PS+and CD36+ erythrocytes in group 2 were significantly increased when compared to group 1 (5.6% versus 1.4% for PS and 2.2% versus 0.2% for CD36; P < .0001), with a concomitant increase in RBC–endothelial adhesion (6.5 versus 2.2;P < .0001). To further assess whether the increased adhesion noted in group 2 patients was due to the elevation in erythrocyte PS, RBC CD36, or a combination of both these adhesive markers, 2 further SCD patient groups were evaluated. Although group 3 demonstrated a significant elevation in erythrocyte CD36 levels when compared to group 1 (2.1% versus 0.2%, P < .0001) with the mean CD36 level in this patient group approximating that previously noted in group 2, mean adhesion values in group 3 were similar to those noted in group 1, and were markedly decreased when compared to group 2 (2.1% in group 3 versus 6.5% in group 2, P < .0001). This observation that the enhanced adhesion in group 2 individuals did not appear to be due to elevations in the levels of erythrocyte CD36 was further confirmed by the data we have obtained in group 4. Although levels of CD36 in this patient group were no different from those in group 1 (0.3% versus 0.23%, P > .2), adhesion was significantly increased (6.4% in group 4 versus 2.2% in group 1,P < .0001) and appeared to be related to the elevated levels of RBC PS in this group (4.4% versus 1.4% in group 1) with values for both erythrocyte PS positivity and adhesion not significantly different from those observed in group 2. When the group data from VLA-4+ erythrocytes was evaluated for a potential association with adhesion, no relationship was noted. Groups 1 and 4, in which the mean values of VLA-4+ erythrocytes were no different from each other or from the control population (0.05%), demonstrated significant differences in adhesion (2.2 versus 6.4,P < .0001), which thus could not be attributed to VLA-4 positivity.
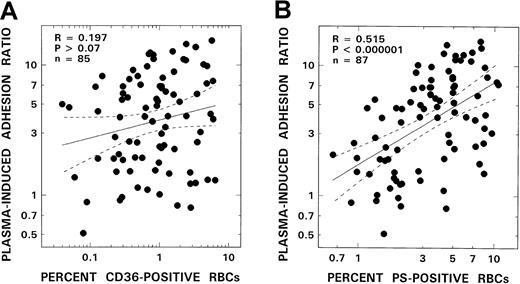
In additional analyses of the clinically related data, correlations between adhesion and the individual levels for all adhesion-related markers in the patient groups were sought. Although a striking positive correlation was noted between erythrocyte PS positivity and adhesion (R = 0.52, P < .000 001, Figure3B), no correlation was noted either with CD36+ RBCs (R = 0.2, P > .07, Figure 3A) or VLA-4 positivity (R = 0.09, P > .35).
Relationship between RBC adhesion and adhesion markers from patients with SCD.
Adhesion correlation with number of CD36+ RBCs is shown in panel A, and that with PS+ erythrocytes in panel B. The solid line represents the linear-regression fit to the data; the dotted lines represent the 95% confidence interval curves.
Relationship between RBC adhesion and adhesion markers from patients with SCD.
Adhesion correlation with number of CD36+ RBCs is shown in panel A, and that with PS+ erythrocytes in panel B. The solid line represents the linear-regression fit to the data; the dotted lines represent the 95% confidence interval curves.
Effects of blocking erythrocyte CD36 and PS on adhesion
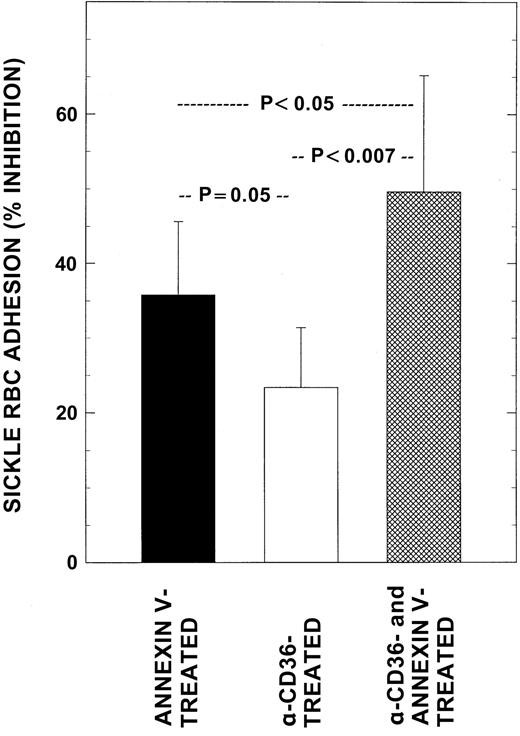
To further test the hypothesis that erythrocyte PS is a crucial mediator of RBC–endothelial adhesion, sickle RBCs were pretreated with anti-CD36 or annexin V or both and assessed for both cell surface markers and adhesion potential. For these studies we used sickle erythrocytes with the highest levels of both CD36 and PS (2.5% ± 1.2% and 9.5% ± 1.9%, respectively; n = 6). Following anti-CD36 and annexin V treatment, CD36 and PS sites on the RBC surface were blocked by 75% ± 5% and 87% ± 2%, respectively, with similar cloaking results noted in both single and double cloaking experiments. In concomitant functional adhesion assays, although anti-CD36–treated erythrocytes decreased adhesion by 23% ± 8%, annexin V–treated (PS cloaked) cells demonstrated a significantly greater inhibitory effect on adhesion of 36% ± 10% (P < .05). The inhibitory response observed with RBCs that were pretreated with both annexin V and anti-CD36 (50% ± 16%) was significantly different when compared to those noted with RBCs treated with either blocking agent alone (Figure4, P < .05 and <.007 respectively), and was suggestive of an additive effect. One of the explanations for the observed partial inhibition of RBC adhesion (23%-35%) by anti-CD36 or annexin V treatment, despite blocking surface CD36 or PS by 75% to 85%, is that the same erythrocyte might be carrying multiple adhesion markers on its surface, which could therefore participate in adhesion reactions with endothelium even when one of the other adhesion markers/receptors is blocked. We have therefore analyzed the marker data for cells positive for both CD36 and PS. As shown in Table 2, approximately 50% of CD36+ RBCs in all groups were also positive for PS, whereas the levels of concomitant PS positivity for these dual positive erythrocytes varied between the groups, from a low of 2% to high of 54%.
Effects of blocking RBC adhesion markers on adhesion.
Washed RBCs from patients with SCD were pretreated with either anti-CD36 alone, annexin V alone, or in combination to cloak cell surface CD36 and PS and then assessed simultaneously for their adhesion potential and surface adhesion marker levels as described. Mean adhesion ratio for untreated control sickle RBCs in these experiments was 7.03 ± 1.28. Values presented are the mean ± SD from 6 patients.
Effects of blocking RBC adhesion markers on adhesion.
Washed RBCs from patients with SCD were pretreated with either anti-CD36 alone, annexin V alone, or in combination to cloak cell surface CD36 and PS and then assessed simultaneously for their adhesion potential and surface adhesion marker levels as described. Mean adhesion ratio for untreated control sickle RBCs in these experiments was 7.03 ± 1.28. Values presented are the mean ± SD from 6 patients.
Relationship between circulating levels of CD71+ stress reticulocytes and adhesion
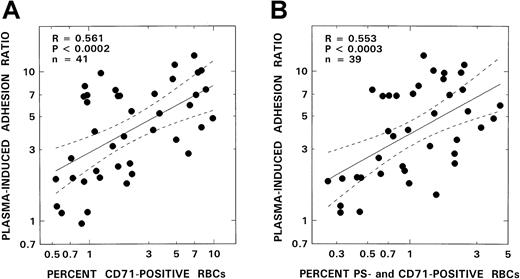
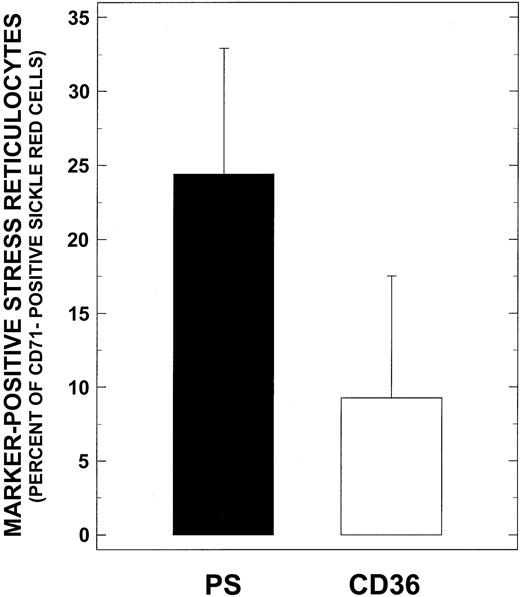
Because stress reticulocytes are known to express the highest levels of adhesive markers and have marked adhesion properties, we analyzed our adhesion data for potential correlates with CD71 positivity. As depicted in Figure 5A, significant positive correlations were noted between the levels of stress reticulocytes and adhesion (R = 0.561,P < .0002, n = 41). Additionally, when these stress reticulocytes were assessed for adhesion marker positivity (Figure6), PS+ stress reticulocytes were found to be the most abundant cells present in this RBC fraction (24.4% ± 8.5% and 9.3% ± 8.2%, for PS and CD36 positivity, respectively). Levels of PS+ stress reticulocytes reported here are comparable to those reported in the literature.28A positive correlation was also observed between PS+ stress reticulocytes and adhesion (Figure 5B, R = 0.553,P < .0003, n = 39) with correlation parameters almost identical to those obtained with stress reticulocytes (Figure 5A). No correlations were noted with CD36+ stress reticulocytes and adhesion (R = 0.166, P > .34, n = 39).
RBC adhesion and stress reticulocytes.
The relationship between RBC adhesion and number of stress reticulocytes (A) or PS+ stress reticulocytes (B) from patients with SCD is shown. The solid line represents the linear-regression fit to the data; the dotted lines represent the 95% confidence interval curves.
RBC adhesion and stress reticulocytes.
The relationship between RBC adhesion and number of stress reticulocytes (A) or PS+ stress reticulocytes (B) from patients with SCD is shown. The solid line represents the linear-regression fit to the data; the dotted lines represent the 95% confidence interval curves.
Relative distribution of CD36+ and PS+ stress reticulocytes from patients with SCD.
Washed sickle RBCs were incubated with anti-CD71–PE and one of the following reagents in the FITC form: anti-CD36, isotypic negative control IgG, annexin V in the presence of CaCl2, or EDTA; and analyzed by flow cytometry as described in “Materials and methods.” Values presented are the mean ± SD from 39 patients.
Relative distribution of CD36+ and PS+ stress reticulocytes from patients with SCD.
Washed sickle RBCs were incubated with anti-CD71–PE and one of the following reagents in the FITC form: anti-CD36, isotypic negative control IgG, annexin V in the presence of CaCl2, or EDTA; and analyzed by flow cytometry as described in “Materials and methods.” Values presented are the mean ± SD from 39 patients.
Discussion
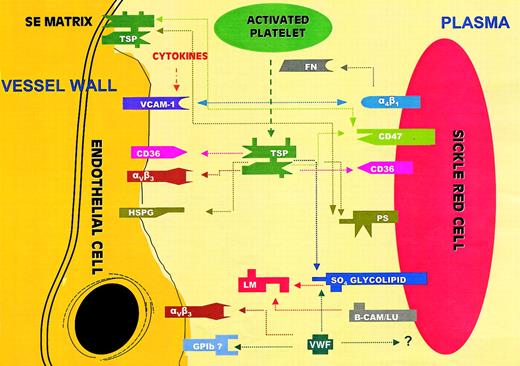
The microvascular occlusive event of SCD has a multifactorial etiology dependent primarily on whether the rate of sickle hemoglobin polymer formation is within the range of the capillary transit time.29 Events that therefore slow the transit of sickle RBCs in the microcirculation, such as factors enhancing RBC–endothelial adhesion, can play a critical role in this process. Vaso-occlusion appears to be initiated by the adhesion of the least dense and highly adhesinogenic sickle reticulocyte to the endothelium of the postcapillary venule, followed by the secondary accumulation of poorly deformable dense cells leading to vascular occlusion.2 To date, the adhesive relationships delineated between the sickle RBC and the endothelium have been, for the most part, receptor-mediated events, as depicted in Figure7. One of the main mechanisms described is the bridging role of the plasma ligand TSP14,15 between the RBC receptor CD36 on the one hand and several constitutively present endothelial receptors on the other. Because TSP is comprised of a number of heterogeneously distinct domains, vascular adhesion to TSP depends on several endothelial sites, including the vitronectin receptor (αvβ3), the transmembrane glycoprotein CD36, and endothelial cell surface heparan sulfate proteoglycans (HSPGs). A second well-characterized coupling includes the binding of the erythrocyte integrin α4β1 (VLA-4) to endothelial VCAM-1.4-6,13 Although VCAM-1 is not constitutively expressed on the endothelial cell, expression occurs following exposure to several agonists including cytokines and hypoxia. A second mechanism of endothelial adhesion via erythrocyte α4β1 is thought to occur through the ligand fibronectin, although the endothelial receptor involved in this process has not been clarified.2 Another important ligand, VWF,12,16 also serves as a bridging molecule between the endothelial vitronectin receptor αvβ3 or the potential endothelial adhesion receptor complex GPIb-IX-V on the one hand and the erythrocyte on the other. The receptor on the RBC surface mediating this latter adhesive process has not been identified. Sickle erythrocyte interactions with the vessel wall may also involve adhesion to subendothelial immobilized matrix components such as laminin, and the previously mentioned ligands TSP, VWF, and fibronectin.3 These latter molecules, besides being present in plasma in a soluble form, are also matrix components that can be exposed following vascular injury or endothelial retraction. The interaction of the sickle RBC with laminin via the erythrocyte basal cell adhesion molecule-Lutheran protein receptor (B-CAM/LU) is one such well-characterized high-affinity interaction.30 A recent study has demonstrated that sickle RBCs can also bind to immobilized TSP via the integrin-associated protein (IAP or CD47) present on the RBCs.31 Additionally, these investigators have identified the SS erythrocytes as an active signal transducing cell, which via IAP and shear stress activated G proteins and tyrosine kinases, resulting in its enhanced adhesion to immobilized TSP.32Nonreceptor-mediated adhesive mechanisms include a role for RBC sulfated glycolipids and PS. A sulfated glycolipid from the sickle erythrocyte has been shown to bind to laminin and to TSP,10 whereas PS-exposing human erythrocytes have been demonstrated to adhere to vascular endothelium in both static and flow adhesion systems.22,23 One of the described mechanisms involved in erythrocyte PS–endothelial binding appears to be the interaction between PS and immobilized matrix TSP, because adherence was reduced by antibodies to TSP and to the endothelial receptor αvβ3, a molecule that binds to several adhesion proteins including TSP.23
Adhesive interactions between sickle RBCs and endothelium or plasma proteins.
PS indicates phosphatidylserine; GP1b, glycoprotein 1b; α4β1, integrin receptor VLA-4; VCAM-1, vascular cell adhesion molecule-1; αVβ3, integrin vitronectin receptor; B-CAM/LU, basal cell adhesion molecule/Lutheran protein; HSPG, heparan sulfate proteoglycan; SO4 glycolipid, sulfated glycolipid; TSP, thrombospondin; FN, fibronectin; VWF, von Willebrand factor; LM, laminin; and SE matrix, subendothelial matrix. CD47 is also known as the integrin-associated protein or IAP.
Adhesive interactions between sickle RBCs and endothelium or plasma proteins.
PS indicates phosphatidylserine; GP1b, glycoprotein 1b; α4β1, integrin receptor VLA-4; VCAM-1, vascular cell adhesion molecule-1; αVβ3, integrin vitronectin receptor; B-CAM/LU, basal cell adhesion molecule/Lutheran protein; HSPG, heparan sulfate proteoglycan; SO4 glycolipid, sulfated glycolipid; TSP, thrombospondin; FN, fibronectin; VWF, von Willebrand factor; LM, laminin; and SE matrix, subendothelial matrix. CD47 is also known as the integrin-associated protein or IAP.
Because adhesion as described above involves interactions of some complexity, in this study we have only attempted a delineation of the relative roles of the adhesion markers erythrocyte PS when compared to CD36. Our in vitro system, which used unactivated endothelium for the plasma-induced adhesion assays we performed, effectively eliminated VLA-4/VCAM-1–mediated adhesive mechanisms because VCAM-1 is not constitutively expressed on unactivated endothelial cells (a finding that was confirmed by flow cytometric analyses of our microvascular retinal capillary endothelial cells; data not shown). The blocking experiments using anti-CD36 and annexin V unequivocally demonstrated that cloaking of the PS sites led to a significantly greater degree of inhibition of adhesion as compared to blocking of the erythrocyte CD36 receptor. In addition, when both these RBC adhesion markers were unavailable for interaction with plasma-soluble ligands and endothelial adhesive receptors, the inhibitory effect appeared to be additive, suggesting that the interactions with TSP or endothelial receptors occurred via distinct adhesive sites or domains.
Our patient related data (Table 2 and Figure 3) was unexpected. It suggests that under the conditions of our study, that is, in unactivated endothelium, PS appears more critical to the adhesive process than mechanisms involving erythrocyte CD36. Because the endothelial receptor sites involved in RBC CD36 binding via TSP are constitutively expressed, the study undertaken appears to be a valid assessment of the comparative role of RBC PS versus CD36 in the adhesive process. Previous investigations and our present study do not address the quality or affinity of the adhesive interaction of PS versus CD36 with soluble ligands, subendothelial matrix proteins, or endothelial receptors. However, evaluation of the number of PS+ and CD36+ erythrocytes (Table 2) suggests that part of the dominant PS effect on adhesion appears to be due to the quantitative differences noted which favor PS positivity 2 to 3 times over CD36+ RBCs in SCD. In keeping with this finding is our additional observation that stress reticulocytes (which hitherto have had their strong adherent properties ascribed to their CD36 positivity8) are significantly more positive for PS than for the adhesion marker CD36 (Figure 6), and that the adhesion potential of these cells correlates exclusively with their PS positivity (Figure 5). Additionally, the coexpression marker data also favors PS in that 50% of the CD36+ cells also expressed PS. An alternative explanation for the results we obtained in our patient-related studies, as has been suggested by other investigators, is that erythrocyte CD36-TSP binding may not play such a central role in the sickle erythrocyte adhesion process as previously believed. A recent patient-related study demonstrating that the presence or absence of CD36 on sickle reticulocytes and RBCs had no effect on the clinical severity of sickle cell anemia also supports this latter conclusion.33 TSP binding to PS or other RBC markers not addressed in our study, such as erythrocyte sulfated glycolipids, band-3 peptide, or the integrin-associated protein, may be of more relevance.10,15,23,31,32,34 35
We used 10% autologous plasma for our adhesion studies. Although the individual patient with SCD exhibits quantitative differences in plasma adhesion-related ligands, these values are usually relatively high in all patients due to the SCD-related chronic proinflammatory and procoagulant phenotype.36 Thus, although the differences in adhesion we have noted in the patient groups appear from our composite data to be PS related, we cannot rule out some minimal effect on adhesion ratios in all patient groups evaluated due to the fact that we used diluted plasma rather than an undiluted preparation for these experiments. However, a previous study has demonstrated that the maximum plasma effect on sickle RBC–endothelial adhesion occurs in vitro at a 10% plasma dilution.37 A static adhesion assay was performed for the erythrocyte–microendothelial adhesion experiments and relationships described. Although it has been suggested that RBC adhesion mechanisms identified under conditions of flow are of more pathophysiologic relevance, it has been also noted that this view does not give consideration to the fact that microvessel blood flow can be intermittent,38 and that stasis may be enhanced by slow-moving and relatively large granulocytes or cellular aggregates,39 thus simulating more closely adhesion assays where flow is absent. On the other hand, in vivo data demonstrate that sickle erythrocyte adherence occurring in capillaries during periods of incipient flow stasis can be dislodged on removal of the pressure cuff and restoration of blood flow.40,41 However, because the initial correlation described between sickle erythrocyte adhesion to endothelium and microvascular complications in patients with SCD was observed using the static adhesion assay, Hebbel has suggested that low-affinity adhesion mechanisms (as reflected by static assays) are of major relevance to SCD pathophysiology.1 42
Several adhesive surface receptors on macrophages have been identified to mediate the recognition and removal of PS+ cells in vitro during apoptosis or programmed cell death. These include CD68, CD36, CD14, and the class B scavenger proteins SR-BI that do not discriminate between PS and other anionic phospholipids.43Most recently, a receptor on macrophages and fibroblasts for PS-specific clearance of apoptotic cells has also been identified.44 In addition, plasma proteins such as β2-glycoprotein 1 may facilitate adhesion by linking PS on the apoptotic cell surface to receptors on the macrophage.45 Although specific PS recognition sites on the endothelium have not been identified to date, the observation of Manodori et al23 that PS+ ionophore-treated control erythrocytes demonstrate a marked propensity to adhere to endothelium via an interaction with the subendothelial matrix protein TSP is the first documented mechanism for endothelial binding of this anionic phospholipid. Our studies demonstrating that PS is a major determinant of RBC–endothelial adhesion provides an ex vivo counterpoint to these recently reported in vitro PS-adhesion related results, and suggests that disruption of normal erythrocyte phospholipid asymmetry with PS surface exposure may be of importance in the phenomenon of SCD vaso-occlusion via its effect on the adhesion process. In fact, preliminary evidence in a small cohort of infants and toddlers with SCD, whom we are prospectively following, has demonstrated a clinical relationship between vaso-occlusion and erythrocyte PS positivity.46 Subpopulations of PS+ erythrocytes have also been reported to correlate with the risk of stroke in patients with SCD.47 Such findings suggest that further investigations are necessary into the mechanisms by which PS+ erythrocytes interact with the vessel wall and the implications of these interactions in relation to the microvessel and macrovessel pathophysiology of SCD.
Supported by grants HL51497 and 1P60HL62148 from the National Heart, Lung and Blood Institute of the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
B. N. Yamaja Setty, Department of Pediatrics, Thomas Jefferson University, 1025 Walnut St, Suite 727, Philadelphia, PA 19107; e-mail: yamaja.setty@mail.tju.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal