Interferon-α (IFN-α) has significantly prolonged survival in chronic myeloid leukemia (CML), but some patients do not respond and many responses are not durable. To improve the results, IFN-α has been combined with other treatments, but so far only the association with low-dose arabinosyl cytosine (LDAC) has been shown to increase the response rate and to prolong survival. Here are reported the results of a study of 538 Philadelphia chromosome–positive CML patients who were assigned at random to treatment with IFN-α2a alone or in combination with LDAC. The scheduled dose of IFN-α2a was 56IU/m2/d. The scheduled dose of AC was 40 mg/d for the first 10 days of each month of treatment. The efficacy endpoints were a complete hematologic response rate at 6 months (62% in the IFN-α–plus–LDAC arm versus 55% in the IFN-α arm; P = .11), major cytogenetic response (MCgR) rate at 24 months (28% versus 18%; P = .003), and overall survival (5-year survival, 68% versus 65%; P = .77). Treatment did not affect overall survival within different prognostic risk groups: low, intermediate, or high. Also the duration of MCgR was identical. The results of this study confirm the results of a similar French study only for the response rate, not for survival, suggesting that the relationship between cytogenetic response and survival may be extremely variable and that a meta-analysis of these and other studies of IFN-α versus IFN-α plus LDAC is required to settle the issue of the role of LDAC in the treatment of CML.
Introduction
Interferon-α (IFN-α) became the treatment of choice for chronic myeloid leukemia (CML) when it was shown that IFN-α was able to induce hematologic and cytogenetic remissions,1 that some cytogenetic remissions were stable,2 and that survival was prolonged by comparison with conventional chemotherapy.3-8 However, not all patients respond to IFN-α, and not all patients have their survival prolonged. In particular, only 70% of the patients achieve a complete hematologic response (HR), and fewer then 50% of them achieve the major and stable cytogenetic response that is associated with long survival and, sometimes, with a clinical cure.3-11Therefore, although the introduction of IFN-α has marked a significant advance, only a minority of the patients receive substantial benefit, and there is still much need for improvement, especially in the rate, quality, and duration of cytogenetic response.12 For patients who do not achieve a complete HR with IFN-α, treatment with a conventional cytotoxic drug like hydroxyurea (HU) is usually employed, although it is not clear if the combination is more advantageous than conventional chemotherapy alone.3,5,10,13-15 To improve the results, IFN-α has been systematically combined with arabinosyl cytosine (AC),16-18 intensive chemotherapy,19,21 or homoharringtonine22 and has been associated with autologous bone marrow transplantation,20,21,23,24 with some promising results but without firm evidence of benefit, except for AC. The association with AC has been pursued because it was shown that at a low concentration, AC was more toxic against leukemic cells than against normal cells24 and that treatment with low-dose AC (LDAC) was able to obtain hematologic and cytogenetic responses, also in patients who had failed IFN-α alone or who were in advanced phases of the disease.26-29 A prospective randomized study of IFN-α alone versus IFN-α and LDAC was performed in France and was reported to show the combination to be superior in terms of hematologic and cytogenetic response and survival.16 That study was begun in 1991. Three years later, when the results of the French study had not not yet been discovered, the Italian Cooperative Study Group on CML undertook a similar study, with the aim of providing independent data on the important issue that was being tested in the French study.
Patients and methods
This phase 3, prospective, open-labeled, randomized study was designed to evaluate whether the addition of LDAC to IFN-α increased and improved the hematologic and the cytogenetic response rates, prolonged the duration of the cytogenetic response, and increased overall survival. Patients were eligible for the study if they had Philadelphia chromosome–positive (Ph+) CML in chronic phase (CP) for fewer than 7 months; were younger than 66 years old; had not been treated, or had been minimally pretreated, with HU (less than 50 g, total dose) or busulfan (less than 100 mg, total dose); and were free of any concurrent disorder that could interfere with treatment or become life threatening. Written informed consent was required to participate in the study, which was planned and managed according to the Helsinki declaration.
Treatment protocol
Pretreatment with HU, 40 mg/kg daily for 15 days, was allowed for all patients and was recommended in cases of a white blood cell (WBC) count higher than 100 × 109/L or a splenomegaly more than 10 cm below the costal margin. Subsequent treatment with HU was also allowed at any time during the study if it was required to control counts or symptoms, with the need for treatment as well as the dose and schedule at the investigator's discretion. Study treatment was randomly assigned and consisted of human recombinant IFN-α2a (Roferon-A) (Roche, Basel, Switzerland), alone or in combination with LDAC. Roferon-A was given subcutaneously or intramuscularly at a dose of 36 IU daily for the first 2 weeks, at 66 IU daily for the next 2 weeks, and at 56 IU/m2 daily thereafter (maximum dose). AC was given at a dose of 40 mg daily for the first 10 days of each month of treatment. The guidelines for treatment and dose adjustment were specified in the protocol, for the various types and degrees of toxicity. The adjustment was decided every month and was valid for 1 month, after which it was reviewed. In cases of hematologic toxicity or in cases of nonhematologic toxicity of uncertain attribution, LDAC was discontinued while Roferon-A was continued, since the conservation of the dose of Roferon-A had priority over the conservation of the dose of AC. The assigned treatment was continued, if tolerated and accepted, until treatment failure. Failure was defined by (1) lack of an at least partial HR after 6 months, (2) lack of any cytogenetic response (no Ph− metaphases) after 12 months, (3) lack of an at least minor cytogenetic response (fewer than 33% Ph−metaphases) after 24 months, (4) loss of a complete HR, (5) loss of a major cytogenetic response, or (6) progression to accelerated or blast phase (ABP). In cases of treatment failure, the patients who had been assigned to IFN-α alone were transferred to the IFN-α–plus–LDAC arm, while the patients who had been assigned to IFN-α plus LDAC went off study treatment and were managed at the investigators' discretion. In cases of progression to ABP, study treatment was always terminated irrespective of the assigned arm. After 3 years of treatment, the patients who were in cytogenetic response (at least minor) had to be randomly assigned to continue IFN-α at maximum tolerated dose or at a low dose (3 MIU 3 times a week). However, no information on the results of this second randomization will be disclosed in this report, since the observation time is still too short. The option of allogeneic bone marrow transplantation (alloBMT) was always open, irrespective of the response to the treatment, at the patient's and investigator's discretion.
Definitions
The accelerated and blast phases were not separated; the ABP was defined, as previously described,3 by at least 2 of the following 5 criteria: a peripheral blood sample containing more than 10% blast cells or more than 30% blast cells and promyelocytes; a bone marrow aspirate containing more than 15% blast cells or more than 50% blast cells and promyelocytes; a spleen that could be palpated more than 10 cm below the left costal margin and a WBC count lower than 25 × 109/L; the involvement of the central nervous system, bone, lymph nodes, or other extrahematologic sites; and cytogenetic evaluation revealing trisomy Ph, trisomy 8, or isochromosome 17. The HR was defined as complete (CHR) if all the following criteria were met: WBC count below 10 × 109/L; platelet count below 450 × 109/L; a differential with no promyelocytes or myeloblasts and fewer than 5% myelocytes or metamyelocytes; and a nonpalpable spleen. The HR was defined as partial (PHR) if any of the following criteria were met: WBC count between 10 and 20 × 109/L; platelet count between 450 and 1000 × 109/L; a differential with more than 5% metamyelocytes and myelocytes, or with promyelocytes or myeloblasts; and a spleen palpable less than 6 cm below costal margin. It should be appreciated that PHR was defined only to decide if the treatment had to be continued for more than 6 months. The degree of HR was assessed and checked monthly during the first year, every 2 months during the second year, and every 3 months thereafter. The cytogenetic response (CgR) was defined on the basis of the percentage of Ph− metaphases: complete CgR (CCgR) in cases of 100%; partial (PCgR) in cases of 66% to 99%; minor in cases of 33% to 65%; minimal in cases of 1% to 32%; and none if no Ph− metaphase was detected. CCgR and PCgR were pooled and named major (MCgR). The CgR was evaluated after 6, 9, and 12 months, and every 6 months thereafter. Cytogenetic evaluations were made on marrow cells, were performed in different laboratories, and were not centrally reviewed. The minimum required number of metaphases was 20, but data based on fewer metaphases, down to a minimum of 10, were accepted if they were congruent with prior or subsequent evaluations. The risk was calculated by means of Sokal formulation,30 as it was originally stated in the protocol. Subsequently, risk was also calculated by means of the more recent Euro formulation, derived from an international study of patients treated with IFN-α–based regimens.31The variables contributing to the calculation of the risk are age, spleen size, platelet count, and the percentage of blast cells in peripheral blood. In addition, the Euro formulation also takes into account the percentage of eosinophils and basophils in peripheral blood.
Statistical design and procedures
Randomization was required and obtained by fax at registration, prior to any study treatment, in a ratio of 1:1 on the basis of lists that were computer generated for each participating center. The lists were blind to the investigators and to the secretariat. The primary efficacy endpoint of the study was overall survival. It was established that the first assessment of survival would be made at 5 years and that a difference of 25% or more between the test arm (IFN-α plus LDAC) and the control arm would be clinically relevant. From a prior study,3 the 5-year survival probability in the control study was settled at 60%. The number of cases that were required to detect a change of 25% of the predicted 5-year survival was 450, with a type I error (α, 2 sided) of 0.05 and a power (1-β) of 80%, allowing a 20% loss for alloBMT. The number of patients who were actually randomized was in excess of 450, simply because it was decided to continue in the same randomized treatment policy even after the required case number had been reached. Secondary efficacy endpoints were the rate of CHR after 6 months and the rate of MCgR after 12 and 24 months. It was established that any calculations and any comparisons would be based on all randomized cases according to the intention-to-treat principle. Calculations for censored data were made by the Kaplan-Meier method,32 with the use of the randomization date as the starting time and the date of the relevant event (first CHR, first MCgR, death, or progression) as the end time. The patients who received alloBMT in first CP were censored at the date of BMT (the results, however, did not change if the patients were not censored). Patients who received alloBMT after progression to ABP and patients who received autografts in any disease phase were not censored. Comparisons were performed by the Student t test, the χ2 test, and the log rank test for trend and for heterogeneity. All P values were 2 sided.
Results
Between February 1994 and March 1997, 688 patients were registered in 64 university and general hospitals located throughout the country, but only 538 were enrolled and randomized, because 20 patients were already in ABP, 18 patients refused to be enrolled, and the remaining 112 patients were not eligible for various reasons, such as prior treatment, being more than 6 months from diagnosis, other diseases, or logistical problems. The 538 enrolled patients were assigned to receive IFN-α and LDAC (275 patients) or IFN-α alone (263 patients). The main demographic, clinical, and laboratory characteristics of these patients are reported in Table1, showing that there was no difference of case distribution in the 2 treatment arms. The median age was 45 years. Spleen was palpable in 58% of cases. The karyotype was mixed Ph+ and Ph− in 15% of cases. The transcript was B3A2 in 59% of cases. Low-risk patients were 50% by both Sokal and Euro formulation. High-risk patients were 20% by Sokal formulation and 8% by Euro.
Patients' distribution by treatment arm and major demographic, clinical, and laboratory characteristics
| . | IFN-α + LDAC . | IFN-α . |
|---|---|---|
| No. cases | 275 | 263 |
| Prior treatment, no. (%) | 75 (27) | 68 (26) |
| Gender, male, no. (%) | 162 (59) | 162 (61) |
| Age, y, mean ± SD | 45 ± 13 | 45 ± 13 |
| Symptoms, no. (%) | 95 (34) | 100 (38) |
| Spleen palpable, no. cases (%) | 162 (59) | 169 (64) |
| Spleen, cm below costal margin, mean ± SD | 5 ± 5 | 5 ± 5 |
| Hb, g/L, mean ± SD | 119 ± 21 | 121 ± 21 |
| WBC count, × 109/L, mean ± SD | 126 ± 101 | 128 ± 95 |
| Platelet count × 109/L, mean ± SD | 431 ± 282 | 446 ± 356 |
| Myeloblasts, % in PB, mean ± SD | 1.5 ± 2.3 | 1.6 ± 2.3 |
| Eosinophils, % in PB, mean ± SD | 2.0 ± 2.4 | 2.0 ± 2.0 |
| Basophils, % in PB, mean ± SD | 2.9 ± 2.7 | 3.2 ± 3.7 |
| Sokal RR, no. (%) | ||
| less than 0.8 (low) | 139 (50) | 124 (47) |
| 0.8-1.2 (intermediate) | 82 (30) | 87 (33) |
| greater than 1.2 (high) | 54 (20) | 52 (20) |
| Euro RR, no. (%) | ||
| at most 780 (low) | 157 (57) | 125 (47) |
| 781-1479 (intermediate) | 92 (33) | 116 (44) |
| at least 1480 (high) | 26 (9) | 22 (8) |
| Ph+/Ph− cytogenetic mosaicism, no. (%) | 47 (18) | 34 (14) |
| Transcript type, no. (%) | ||
| B2A2 | 76 (33) | 84 (37) |
| B3A2 | 145 (64) | 139 (62) |
| Other | 6 (3) | 2 (1) |
| . | IFN-α + LDAC . | IFN-α . |
|---|---|---|
| No. cases | 275 | 263 |
| Prior treatment, no. (%) | 75 (27) | 68 (26) |
| Gender, male, no. (%) | 162 (59) | 162 (61) |
| Age, y, mean ± SD | 45 ± 13 | 45 ± 13 |
| Symptoms, no. (%) | 95 (34) | 100 (38) |
| Spleen palpable, no. cases (%) | 162 (59) | 169 (64) |
| Spleen, cm below costal margin, mean ± SD | 5 ± 5 | 5 ± 5 |
| Hb, g/L, mean ± SD | 119 ± 21 | 121 ± 21 |
| WBC count, × 109/L, mean ± SD | 126 ± 101 | 128 ± 95 |
| Platelet count × 109/L, mean ± SD | 431 ± 282 | 446 ± 356 |
| Myeloblasts, % in PB, mean ± SD | 1.5 ± 2.3 | 1.6 ± 2.3 |
| Eosinophils, % in PB, mean ± SD | 2.0 ± 2.4 | 2.0 ± 2.0 |
| Basophils, % in PB, mean ± SD | 2.9 ± 2.7 | 3.2 ± 3.7 |
| Sokal RR, no. (%) | ||
| less than 0.8 (low) | 139 (50) | 124 (47) |
| 0.8-1.2 (intermediate) | 82 (30) | 87 (33) |
| greater than 1.2 (high) | 54 (20) | 52 (20) |
| Euro RR, no. (%) | ||
| at most 780 (low) | 157 (57) | 125 (47) |
| 781-1479 (intermediate) | 92 (33) | 116 (44) |
| at least 1480 (high) | 26 (9) | 22 (8) |
| Ph+/Ph− cytogenetic mosaicism, no. (%) | 47 (18) | 34 (14) |
| Transcript type, no. (%) | ||
| B2A2 | 76 (33) | 84 (37) |
| B3A2 | 145 (64) | 139 (62) |
| Other | 6 (3) | 2 (1) |
There are no differences between the 2 arms. Some data were missing for cytogenetics (17 items in the IFN-α + LDAC arm and 21 in the IFN-α arm) and for molecular biology (48 items in the IFN-α + LDAC arm and 38 in the IFN-α arm).
IFN indicates interferon; LDAC, low-dose arabinosyl cytosine; Hb, hemoglobin; WBC, white blood cell; PB, peripheral blood; RR, relative risk; Ph, Philadelphia chromosome.
Hematologic response
At 6 months after randomization, 81% of cases in the IFN-α–plus–LDAC arm and 74% of cases in the IFN-α arm had achieved and maintained a complete or partial HR and thus were eligible to continue in the assigned treatment. At the same time point (sixth month), the CHR rate, one of the secondary efficacy endpoints of the study, was 62% in the IFN-α–plus–LDAC arm versus 55% in the IFN-α arm (P = .11, χ2 test). The time that was required to achieve a CHR ranged between 1 and 7 months in the IFN-α–plus–LDAC arm (median, 3.5 months) and between 1 and 12 months in the IFN-α arm (median, 4 months).
Cytogenetic response
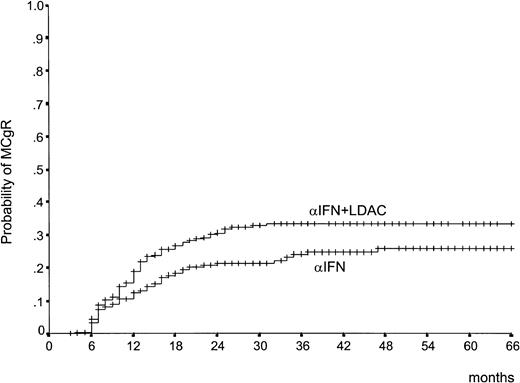
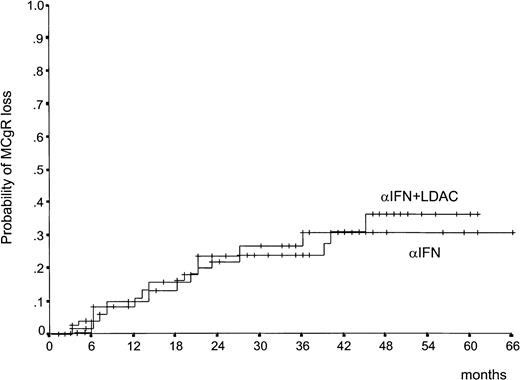
The CgR rate is reported in Table 2. There was a difference in the rate of the MCgR (complete plus partial, 66% through 100% Ph−) in favor of IFN-α plus LDAC both at 1 year (21% versus 13%) and at 2 years (28% versus 18%). In Table 2, the rates are calculated on the basis of all randomized cases, according to the intention-to-treat principle. Several cases were not cytogenetically evaluable for reasons other than treatment failure, including inadequate cytogenetic examinations, treatment refusal, and treatment discontinuation for side effects or for alloBMT. If these cases were removed, the difference would remain the same. Figure1 shows that the patients who were assigned to IFN-α plus LDAC not only achieved more responses, but responded more rapidly than the patients who were assigned to IFN-α alone. The duration of the MCgR is shown in Figure2. About two thirds of the major responders have maintained the response, with no detectable difference between the 2 treatment arms.
Comparison of the cytogenetic response at 1 and 2 years
| Cytogenetic response . | After 1 y, no. (%) . | After 2 y, no. (%) . | ||
|---|---|---|---|---|
| IFN-α + LDAC . | IFN-α . | IFN-α + LDAC . | IFN-α . | |
| Nonevaluable | 81 (29) | 91 (35) | 76 (28) | 89 (34) |
| None, 0 Ph− | 51 (18) | 56 (21) | 50 (18) | 50 (19) |
| Minimal, 1%-32%, Ph− | 40 (14) | 45 (17) | 31 (11) | 42 (16) |
| Minor, 33%-65% Ph− | 44 (16) | 36 (14) | 40 (14) | 35 (13) |
| Partial, 66%-99% Ph− | 38 (14) | 28 (11) | 39 (14) | 27 (10) |
| Complete, 100% Ph− | 21 (8)* | 7 (3)* | 39 (14)† | 20 (8)† |
| Total | 275 | 263 | 275 | 263 |
| Major, 66%-100% Ph− | 59 (21)‡ | 35 (13)‡ | 78 (28)2-153 | 47 (18)2-153 |
| Cytogenetic response . | After 1 y, no. (%) . | After 2 y, no. (%) . | ||
|---|---|---|---|---|
| IFN-α + LDAC . | IFN-α . | IFN-α + LDAC . | IFN-α . | |
| Nonevaluable | 81 (29) | 91 (35) | 76 (28) | 89 (34) |
| None, 0 Ph− | 51 (18) | 56 (21) | 50 (18) | 50 (19) |
| Minimal, 1%-32%, Ph− | 40 (14) | 45 (17) | 31 (11) | 42 (16) |
| Minor, 33%-65% Ph− | 44 (16) | 36 (14) | 40 (14) | 35 (13) |
| Partial, 66%-99% Ph− | 38 (14) | 28 (11) | 39 (14) | 27 (10) |
| Complete, 100% Ph− | 21 (8)* | 7 (3)* | 39 (14)† | 20 (8)† |
| Total | 275 | 263 | 275 | 263 |
| Major, 66%-100% Ph− | 59 (21)‡ | 35 (13)‡ | 78 (28)2-153 | 47 (18)2-153 |
Data are presented as number and percentage. The response rate is calculated on all randomized cases, according to the intention-to-treat principle. The overall response rate was slightly better in the IFN-α-plus-LDAC arm than in the IFN-α arm (P = .07 at 1 year and P = .05 at 2 years, χ2test). Moreover, in the IFN-α-plus-LDAC arm, there were more complete responses (P = .009 and P = .012) and more major responses (P = .014 and P= .003).
Abbreviations are explained in Table 1.
P = .009.
P = .012.
P = .014.
P = .003.
Time to MCgR.
Time from randomization to the achievement of the first MCgR (complete or partial) (66% to 100% Ph−). The difference is significant (P = .038, log rank test).
Time to MCgR.
Time from randomization to the achievement of the first MCgR (complete or partial) (66% to 100% Ph−). The difference is significant (P = .038, log rank test).
Duration of MCgR.
Time from the first MCgR (complete or partial) (66% to 100% Ph−) to loss of MCgR (lower than 66% Ph−) (P = .97, log rank test). About two thirds of cases are projected to maintain the response for more than 4 years.
Duration of MCgR.
Time from the first MCgR (complete or partial) (66% to 100% Ph−) to loss of MCgR (lower than 66% Ph−) (P = .97, log rank test). About two thirds of cases are projected to maintain the response for more than 4 years.
Survival and response duration
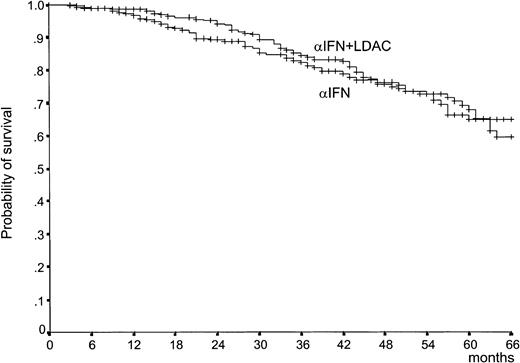
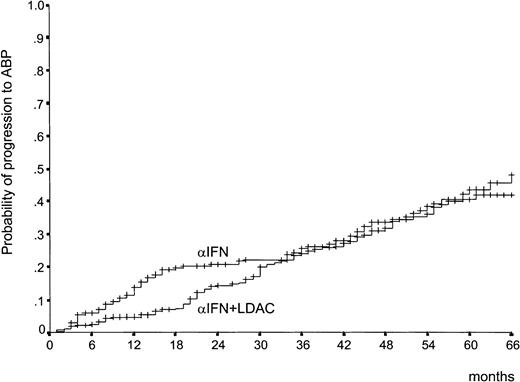
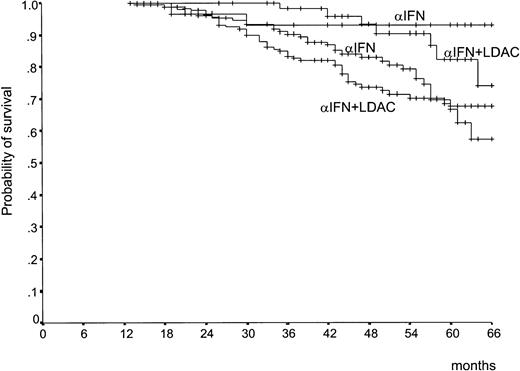
Survival by treatment arm was the primary efficacy endpoint of the study. It is shown in Figure 3. There was a trend in favor of the IFN-α–plus–LDAC arm during the first 3 years, but the 2 curves became almost identical (P = .77), with a 5-year survival rate of 68% (95% confidence interval [CI], 60%-75%) in the IFN-α–plus–LDAC arm versus 65% (95% CI, 57%-73%) in the IFN-α arm. The early trend in favor of IFN-α plus LDAC is accounted for by the observation that during the first 2 years the rate of the progression from CP to ABP was less rapid in the IFN-α–plus–LDAC arm than in the IFN-α arm (Figure4). However, after 3 years, the progression rates became identical. The relationship between overall survival, treatment, and prognostic risk score is illustrated in Table3. With both formulations, Sokal and Euro, survival duration was clearly and significantly related with the risk score, but within each risk group there was no survival difference between IFN-α and IFN-α plus LDAC.
Survival.
Survival from randomization (P = .77, log rank test). The patients who received alloBMT in first CP were censored at the date of BMT. The number of cases at risk at 12, 24, 36, 48, and 60 months is 246, 217, 181, 111, and 53, respectively, in the IFN-α–plus–LDAC arm, and 225, 178, 151, 100, and 49, respectively, in the IFN-α arm.
Survival.
Survival from randomization (P = .77, log rank test). The patients who received alloBMT in first CP were censored at the date of BMT. The number of cases at risk at 12, 24, 36, 48, and 60 months is 246, 217, 181, 111, and 53, respectively, in the IFN-α–plus–LDAC arm, and 225, 178, 151, 100, and 49, respectively, in the IFN-α arm.
Time to progression to ABP.
This shows the time lapsed from randomization to progression to ABP. The early divergence disappeared after 30 months, and the 2 curves are not different (P = .35, log rank test).
Time to progression to ABP.
This shows the time lapsed from randomization to progression to ABP. The early divergence disappeared after 30 months, and the 2 curves are not different (P = .35, log rank test).
Survival by treatment and by prognostic risk score
| Risk groups . | 5-year survival, % (95% CI) . | ||
|---|---|---|---|
| IFN-α + LDAC . | IFN-α . | P . | |
| Sokal RR | |||
| Low | 79 (70-88) | 72 (61-83) | .35 |
| Intermediate | 62 (48-76) | 64 (49-79) | .56 |
| High | 55 (40-70) | 51 (33-69) | .83 |
| P, trend | .0004 | .0134 | |
| Euro RR | |||
| Low | 82 (75-90) | 75 (66-84) | .30 |
| Intermediate | 63 (51-75) | 67 (55-79) | .18 |
| High | 51 (29-72) | 57 (36-78) | .69 |
| P, trend | .0144 | .0412 | |
| Risk groups . | 5-year survival, % (95% CI) . | ||
|---|---|---|---|
| IFN-α + LDAC . | IFN-α . | P . | |
| Sokal RR | |||
| Low | 79 (70-88) | 72 (61-83) | .35 |
| Intermediate | 62 (48-76) | 64 (49-79) | .56 |
| High | 55 (40-70) | 51 (33-69) | .83 |
| P, trend | .0004 | .0134 | |
| Euro RR | |||
| Low | 82 (75-90) | 75 (66-84) | .30 |
| Intermediate | 63 (51-75) | 67 (55-79) | .18 |
| High | 51 (29-72) | 57 (36-78) | .69 |
| P, trend | .0144 | .0412 | |
The risk was calculated according both available formulations: the old Sokal30 formulation, derived from conventionally treated cases, and the new Euro31 formulation, derived from IFN-α-treated cases. A significant risk stratification was achieved with either risk system, but within each risk group there was no difference between IFN-α plus LDAC and IFN-α alone.
CI indicates confidence interval. Other abbreviations are explained in Table 1.
Overall survival was also related to the achievement of a CgR, but the survival of patients who had achieved the same degree of CgR was not affected by the assigned treatment. This is shown in Figure5, which is a landmark analysis of the patients who were alive and in CP after 12 months; the analysis was done according to the extent of the best CgR that had been obtained during that period and according to treatment arm. We also examined other variables that were not included in either prognostic formulations and that might interact with treatment; these included prior treatment, gender, symptoms, hemoglobin concentration, WBC count, and transcript type. These variables did not influence the relationship of treatment to survival. We also examined whether there was any center-related effect in terms of the number of cases per center and the compliance with treatment, but no effect could be detected.
Landmark analysis of survival according to CgR and treatment arm.
The calculation includes only patients who were alive and in CP after 1 year of treatment. Patients are divided into (1) those who during the first year of treatment had achieved a MCgR (complete or partial) (66% to 100% Ph−) (upper curves, no difference between IFN-α plus LDAC and IFN-α) (P = .33, log rank test) and (2) those who during the same period had not achieved a MCgR (66% or lower Ph−, or Ph− not available) (lower curves, no difference between IFN-α plus LDAC and IFN-α) (P = .15, log rank test).
Landmark analysis of survival according to CgR and treatment arm.
The calculation includes only patients who were alive and in CP after 1 year of treatment. Patients are divided into (1) those who during the first year of treatment had achieved a MCgR (complete or partial) (66% to 100% Ph−) (upper curves, no difference between IFN-α plus LDAC and IFN-α) (P = .33, log rank test) and (2) those who during the same period had not achieved a MCgR (66% or lower Ph−, or Ph− not available) (lower curves, no difference between IFN-α plus LDAC and IFN-α) (P = .15, log rank test).
Treatment discontinuation
The study protocol required the discontinuation of the assigned treatment in cases of treatment failure, study failure, or alloBMT. Treatment failure was a priori defined as failure of the patient to achieve an HR, either complete or partial, after 6 months; failure to achieve a CgR, at least minimal after 12 months and at least minor after 24 months; loss of a CHR at any time; loss of an MCgR at any time; and progression to ABP. Study failure included the patients who died in CP and patients for whom study treatment was discontinued for causes related to compliance with treatment, adverse events or toxic or side effects, or refusal of the patient or the doctor to continue in the assigned treatment. Table4 shows the treatment discontinuation by treatment arm, according to cause of treatment failure and study failure. In the IFN-α–plus–LDAC arm, slightly more patients went off treatment because they had failed to achieve a response or had lost the response, while in the IFN-α arm more patients discontinued the treatment for progression to ABP. However, when one considers total treatment failures, the percentages were similar: 39% in the IFN-α–plus–LDAC arm versus 35% in the IFN-α arm. Also, the rate of treatment discontinuation for study failure, for alloBMT, and for other causes was the same (Table 4).
The rate of treatment discontinuation for treatment failure, study failure, and other causes
| Cause of discontinuation of study treatment . | IFN-α + LDAC, no. (%) . | IFN-α, no. (%) . |
|---|---|---|
| Treatment failure | ||
| No HR (less than partial) at 6 mo | 23 (8.4) | 15 (5.7) |
| Loss of complete HR | 23 (8.4) | 7 (2.7) |
| No CgR at 12 mo or less than minor CgR at 24 mo | 24 (8.7) | 18 (6.8) |
| Loss of MCgR | 16 (5.8) | 8 (3.0) |
| Progression to ABP | 22 (8.0) | 41 (15.6) |
| Death in CP | 1 (0.4) | 4 (1.5) |
| Treatment failure total | 109 (39.6) | 93 (35.4) |
| Study failure | ||
| Adverse events, toxicity | 32 (11.6) | 42 (16.0) |
| Refusal to continue | 25 (9.1) | 20 (7.6) |
| Study failure, total | 57 (20.7) | 62 (23.6) |
| Other causes | ||
| AlloBMT in CP | 49 (17.8) | 56 (21.3) |
| Other cancers | 2 (0.7) | 3 (1.1) |
| Other, or undefined | 20 (7.3) | 24 (9.1) |
| Total other causes | 71 (25.8) | 83 (31.5) |
| Total causes | 237 (86.2) | 238 (90.5) |
| Cause of discontinuation of study treatment . | IFN-α + LDAC, no. (%) . | IFN-α, no. (%) . |
|---|---|---|
| Treatment failure | ||
| No HR (less than partial) at 6 mo | 23 (8.4) | 15 (5.7) |
| Loss of complete HR | 23 (8.4) | 7 (2.7) |
| No CgR at 12 mo or less than minor CgR at 24 mo | 24 (8.7) | 18 (6.8) |
| Loss of MCgR | 16 (5.8) | 8 (3.0) |
| Progression to ABP | 22 (8.0) | 41 (15.6) |
| Death in CP | 1 (0.4) | 4 (1.5) |
| Treatment failure total | 109 (39.6) | 93 (35.4) |
| Study failure | ||
| Adverse events, toxicity | 32 (11.6) | 42 (16.0) |
| Refusal to continue | 25 (9.1) | 20 (7.6) |
| Study failure, total | 57 (20.7) | 62 (23.6) |
| Other causes | ||
| AlloBMT in CP | 49 (17.8) | 56 (21.3) |
| Other cancers | 2 (0.7) | 3 (1.1) |
| Other, or undefined | 20 (7.3) | 24 (9.1) |
| Total other causes | 71 (25.8) | 83 (31.5) |
| Total causes | 237 (86.2) | 238 (90.5) |
The rate of treatment discontinuation for treatment failure, study failure, and other causes was the same in the 2 treatment arms. However, while all the patients who failed IFN-α plus LDAC went off treatment, 29 patients who failed IFN-α were crossed to IFN-α plus LDAC.
HR indicates hematologic response; CgR, cytogenetic response; MCgR, major cytogenetic response; ABP, accelerated or blast phase; CP, chronic phase; alloBMT, allogeneic bone marrow transplantation. Other abbreviations are explained in Table 1.
Treatment compliance, adverse events
The type and frequency of adverse events are reported in Table5. There were 140 patients in the IFN-α–plus–LDAC arm and 110 patients in the IFN-α arm who reported one or more adverse events of grade 2 or higher (51% versus 42%) (P = .0034, χ2 test). The total number of adverse events or toxic side effects grade 2 or higher was 214 in the IFN-α–plus–LDAC arm versus 135 in the IFN-α arm, for a frequency of 0.78 (95% CI, 0.73-0.83) and 0.51 (95% CI, 0.45-0.57) per patient, respectively. This difference is significant (P = .008, χ2 test). In the IFN-α–plus–LDAC arm, there were more cases of psychiatric disorders, mainly depression (21 versus 11); hematologic toxicity (61 versus 21, but with no case of severe marrow aplasia recorded); oral mucositis (12 versus 2); skin toxicity (13 versus 1); and vomiting (9 versus 2). Table 5 also lists the events that led to permanent discontinuation of the assigned treatment. While adverse events were more frequent in the IFN-α–plus–LDAC arm, the rate of treatment discontinuation for adverse events was slightly higher in the IFN-α arm than in the IFN-α–plus–LDAC arm (16% versus 12%) (P = .145, χ2 test). These data confirm that the addition of LDAC to the basic regimen of IFN-α resulted in an increased toxicity but suggest that the policy of treatment adjustment that was devised and applied in this study, and which was based on the priority of the conservation of IFN-α over the conservation of AC, prevented an increased loss rate from the IFN-α–plus–LDAC arm. In fact, the events that motivated treatment discontinuation were similar in the 2 arms, with a prevalence of autoimmune complications (5 cases of autoimmune hemolytic anemia and 2 cases of thyroiditis) and psychiatric or neurologic disorders (16 and 8 cases, respectively). Table 5 also shows the adverse events that were fatal and were the cause of a death in CP. There was one such case in the IFN-α–plus–LDAC arm, consisting in a fatal cerebral ischemic stroke. There were 4 such cases in the IFN-α arm: namely, a case of congestive heart failure, a case of myocardial infarction, a case of infection with a septic shock, and a case of fatal traumatic bleeding. Five cases of other cancers were detected during the study: 2 in the IFN-α–plus–LDAC arm and 3 in the IFN-α arm.
Adverse events, grade 2 or higher, recorded during the study
| Adverse events . | Patients with adverse events grade 2 or higher, no. . | Adverse events grade 2 or higher, no. . | Patients with permanent treatment discontinuation, no. . | |||
|---|---|---|---|---|---|---|
| IFN-α + LDAC . | IFN-α . | IFN-α + LDAC . | IFN-α . | IFN-α + LDAC . | IFN-α . | |
| Anorexia, weight loss | 14 | 10 | 18 | 16 | 0 | 0 |
| Autoimmunity | 3 | 14 | 3 | 4 | 3 | 4 |
| Diarrhea | 2 | 1 | 3 | 1 | 0 | 0 |
| Flulike syndrome | 30 | 36 | 52 | 51 | 6 | 12 |
| Heart, failure | 1 | 25-150 | 1 | 25-150 | 1 | 2 |
| Heart, infarction | 0 | 15-150 | 0 | 15-150 | 0 | 1 |
| Hematologic | 31 | 20 | 61 | 21 | 4 | 3 |
| Hemorrhagy | 0 | 15-150 | 0 | 15-150 | 0 | 1 |
| Infection | 1 | 15-150 | 1 | 15-150 | 1 | 1 |
| Kidney | 0 | 1 | 0 | 1 | 0 | 1 |
| Liver | 8 | 9 | 10 | 11 | 1 | 2 |
| Lung | 0 | 1 | 0 | 1 | 0 | 1 |
| Mucositis, oral | 10 | 2 | 12 | 2 | 0 | 0 |
| Neurologic, encephalitis | 1 | 0 | 1 | 0 | 1 | 0 |
| Neurologic, motor | 2 | 4 | 3 | 4 | 0 | 3 |
| Neurologic, sensory | 4 | 4 | 5 | 4 | 2 | 2 |
| Psychiatric disorder | 15 | 10 | 21 | 11 | 7 | 9 |
| Skin | 9 | 1 | 13 | 1 | 3 | 0 |
| Vascular, thrombosis | 15-150 | 0 | 15-150 | 0 | 1 | 0 |
| Vomiting | 8 | 2 | 9 | 2 | 2 | 0 |
| Total | 140 | 110 | 214 | 135 | 32 | 42 |
| Adverse events . | Patients with adverse events grade 2 or higher, no. . | Adverse events grade 2 or higher, no. . | Patients with permanent treatment discontinuation, no. . | |||
|---|---|---|---|---|---|---|
| IFN-α + LDAC . | IFN-α . | IFN-α + LDAC . | IFN-α . | IFN-α + LDAC . | IFN-α . | |
| Anorexia, weight loss | 14 | 10 | 18 | 16 | 0 | 0 |
| Autoimmunity | 3 | 14 | 3 | 4 | 3 | 4 |
| Diarrhea | 2 | 1 | 3 | 1 | 0 | 0 |
| Flulike syndrome | 30 | 36 | 52 | 51 | 6 | 12 |
| Heart, failure | 1 | 25-150 | 1 | 25-150 | 1 | 2 |
| Heart, infarction | 0 | 15-150 | 0 | 15-150 | 0 | 1 |
| Hematologic | 31 | 20 | 61 | 21 | 4 | 3 |
| Hemorrhagy | 0 | 15-150 | 0 | 15-150 | 0 | 1 |
| Infection | 1 | 15-150 | 1 | 15-150 | 1 | 1 |
| Kidney | 0 | 1 | 0 | 1 | 0 | 1 |
| Liver | 8 | 9 | 10 | 11 | 1 | 2 |
| Lung | 0 | 1 | 0 | 1 | 0 | 1 |
| Mucositis, oral | 10 | 2 | 12 | 2 | 0 | 0 |
| Neurologic, encephalitis | 1 | 0 | 1 | 0 | 1 | 0 |
| Neurologic, motor | 2 | 4 | 3 | 4 | 0 | 3 |
| Neurologic, sensory | 4 | 4 | 5 | 4 | 2 | 2 |
| Psychiatric disorder | 15 | 10 | 21 | 11 | 7 | 9 |
| Skin | 9 | 1 | 13 | 1 | 3 | 0 |
| Vascular, thrombosis | 15-150 | 0 | 15-150 | 0 | 1 | 0 |
| Vomiting | 8 | 2 | 9 | 2 | 2 | 0 |
| Total | 140 | 110 | 214 | 135 | 32 | 42 |
This Table shows the adverse events grade 2 or higher both by the number of patients who reported them and by the number of adverse events. The frequency of the events was 0.78 in the IFN-α-plus-LDAC arm versus 0.51 in the IFN-α arm (P= .008, χ2 test). The last 2 columns report the number of patients who permanently discontinued the assigned treatment by specific cause. The frequency of treatment discontinuation for adverse events did not differ by treatment arm (0.12 in the IFN-α-plus-LDAC arm versus 0.16 in the IFN-α arm; P = .145, χ2 test).
Abbreviations are explained in Table 1.
Fatal adverse events.
Treatment dose
The mean daily dose of IFN-α was the same in either treatment arm during the first quarter (3.76 IU/m2 in the IFN-α–plus–LDAC arm versus 3.6 in the IFN-α arm) and in any subsequent period (3.9 and 4.1 in the second quarter, 3.6 and 3.7 during the second half year, and 3.4 and 3.3 during the second year). Therefore, the ratio of the administered to the scheduled dose of of IFN-α ranged from 0.72:1 and 0.68:1 during the first year and from 0.66:1 to 0.68:1 during the second year (Table6). This ratio was lower for AC, ranging from 0.82:1 in the first quarter down to 0.26:1 in the second year, mainly because an increasing number of patients skipped AC, from 5% in the first quarter up to 40% in the second year. Note that maintaining IFN-α and skipping AC in cases of toxicity was dictated by the protocol. The study protocol required that the patients who failed IFN-α alone were crossed over to the IFN-α–plus–LDAC arm. The crossing over should have been done in 48 cases, but in 19 of these cases it was not done because of toxicity, refusal, alloBMT, or other reasons. In the remaining 29 cases, crossing over to the combined treatment resulted in the gain of 4 CHRs out of 14 cases and of 1 MCgR out of 15 cases.
Ratio of the administered to the scheduled dose of IFN-α and arabinosyl cytosine in the first 2 years of study
| . | IFN-α . | IFN-α + LDAC . | |
|---|---|---|---|
| IFN-α . | LDAC . | ||
| First quarter | 0.72:1 | 0.74:1 | 0.82:1 |
| Second quarter | 0.82:1 | 0.78:1 | 0.63:1 |
| Second half-year | 0.74:1 | 0.72:1 | 0.49:1 |
| Second year | 0.66:1 | 0.68:1 | 0.26:1 |
| . | IFN-α . | IFN-α + LDAC . | |
|---|---|---|---|
| IFN-α . | LDAC . | ||
| First quarter | 0.72:1 | 0.74:1 | 0.82:1 |
| Second quarter | 0.82:1 | 0.78:1 | 0.63:1 |
| Second half-year | 0.74:1 | 0.72:1 | 0.49:1 |
| Second year | 0.66:1 | 0.68:1 | 0.26:1 |
The ratios were done on the basis of the patients who remained in study during each period. For IFN-α, the ratio basically reflects the average reduction of the dose, because almost all the patients continued to receive IFN-α. For arabinosyl cytosine, the ratio reflects more the number of the patients who skipped LDAC completely (the frequency of these cases was 5% during the first quarter, 21% during the second quarter, 31% during the second half-year, and 40% in the second year). In the patients who received arabinosyl cytosine, the administered dose ranged from 0.75 to 0.82 of the scheduled dose.
Abbreviations are explained in Table 1.
Bone marrow transplantation
There were 115 patients who went off treatment protocol to receive alloBMT. Another 25 patients received alloBMT after their treatment had been discontinued for other causes. Therefore, a total of 130 patients received allografts in CP: 63 in the IFN-α–plus–LDAC arm and 67 in the IFN-α arm. The time from randomization to alloBMT ranged between 3 and 44 months, with a median of 12 months in each arm. The 5-year survival of these patients was 68%, but in all the calculations that were reported in this paper they were censored at the date of alloBMT. Another 34 patients (16 in the IFN-α–plus–LDAC arm and 18 in the IFN-α arm) received alloBMT after progression to ABP, and another 19 patients (9 in the IFN-α–plus–LDAC arm and 10 in the IFN-α arm) received autografts in advanced CP or in ABP. The survival of these patients was not censored at the date of the transplant.
Discussion
In this study, we have evaluated the overall survival at 5 years, the CHR rate at 6 months, and the MCgR rate at 1 and 2 years in a cohort of 538 patients with early chronic phase Ph+ CML who were enrolled over a 3-year period and were assigned at random to treatment with IFN-α alone or in combination with LDAC. The composition of the 2 treatment arms was identical. The CHR at 6 months was slightly, but not significantly, higher in the IFN-α–plus–LDAC arm. The MCgR rate at 1 and 2 years was significantly higher in the IFN-α–plus–LDAC arm. The overall survival and the time to progression to ABP were identical, with a slight and not significant superiority of IFN-α–plus–LDAC arm during the first 2 years. Overall survival was also identical when the comparison was made within the same risk group by means of both the old Sokal formulation30 and the new Euro prognostic score31 (Table 3). The duration of the MCgR was also identical (Figure 2). The frequency of the adverse events was significantly greater in the IFN-α–plus–LDAC arm than in the IFN-α arm, but overall the compliance with the assigned treatment did not differ (Table 5). However, since treatment protocol was designed with the intention of assigning priority to the continuation of IFN-α over the continuation of LDAC, in cases of toxicity, almost all patients received the same amount of IFN-α in the 2 arms, corresponding to about 0.75 of the scheduled dose, while the amount of AC that was actually given decreased from 0.82 of the scheduled dose in the first quarter to 0.26 after 2 years (Table 6).
The results of this study provide only a partial support to a general extension of the combination of IFN-α and LDAC to the treatment of CML. It is useful to remember that the therapeutic application of AC to the treatment of CML was based on a study25 published in 1987 reporting a preferential inhibition by AC of granulomonocytic colony-forming units (CFU-GMs) from patients with CML, by comparison with CFU-GMs from normal controls and showing that the inhibition of Ph+ CFU-GM could be achieved at a low AC concentration (4.0 ± 0.9 ng/mL), in a range that is achievable in vivo with a continuous infusion of a low dose of the drug.33Subsequent reports suggested that LDAC was effective for treatment of CML26,29 and that the combination of LDAC and IFN-α could achieve stable hematologic and cytogenetic responses, either in CP27 or in more advanced phases.28 A national French study, which was begun in 1991, enrolled 721 patients who were assigned at random to receive IFN-α alone at a daily dose of 5 MIU/m2, or the same dose of IFN-α plus AC at a daily dose of 20 mg/m2 for 10 days every month.16 The current Italian study was begun in 1991, when the results of the French study were not yet known, and enrolled 538 patients who were randomly assigned to the same treatment. The main results of the 2 studies are summarized in Table 7. The CHR rate at 6 months was identical in the IFN-α arms of the 2 studies (55%) while in the IFN-α–plus–LDAC arms it was slightly higher in the French study (66%) than in the Italian one (62%). Therefore, the difference between the 2 arms was significant in the French study (P = .003) but not in the Italian study (P = .11). The MCgR rate was higher in the French than in the Italian study, in each arm, but the difference in favor of the IFN-α–plus–LDAC arm was significant in both studies. The 5-year survival in the IFN-α arm was slightly higher in the Italian study (65% versus 62%), while in the IFN-α–plus–LDAC arm it was slightly higher in the French study (70% versus 68%). In spite of the smallness of these differences, the difference between the 2 arms, which was measured with the same log rank method, was significant in the French study (P = .02) but not in the Italian one (P = .77).
Comparison of the main results of this study and the French study
| . | French study16 (n = 721) . | This study (n = 538) . | ||||
|---|---|---|---|---|---|---|
| IFN-α + LDAC, % . | P . | IFN-α, % . | IFN-α + LDAC, % . | P . | IFN-α, % . | |
| CHR at 6 months | 66 | .003 | 55 | 62 | .11 | 55 |
| MCgR at 12 months | 35 | .001 | 21 | 21 | .012 | 13 |
| MCgR at 24 months | N/A | N/A | N/A | 28 | .003 | 18 |
| 5-year survival | 70 | .02 | 62 | 68 | .77 | 65 |
| . | French study16 (n = 721) . | This study (n = 538) . | ||||
|---|---|---|---|---|---|---|
| IFN-α + LDAC, % . | P . | IFN-α, % . | IFN-α + LDAC, % . | P . | IFN-α, % . | |
| CHR at 6 months | 66 | .003 | 55 | 62 | .11 | 55 |
| MCgR at 12 months | 35 | .001 | 21 | 21 | .012 | 13 |
| MCgR at 24 months | N/A | N/A | N/A | 28 | .003 | 18 |
| 5-year survival | 70 | .02 | 62 | 68 | .77 | 65 |
A difference in favor of IFN-α plus LDAC was confirmed for MCgR, was supported for CHR and was not confirmed for overall survival.
Since it is generally believed that with IFN-α any substantial survival benefit is related to cytogenetic response,3,7,9-12,16,34 the main question is why the advantage in terms of MCgR, which was significant in both studies, was associated with a survival advantage only in the French study. A second question, which may, however, be relevant to the first, is why the MCgR rate was higher in the French than in the Italian study for both treatment arms. Answers to these questions may be helped by a scrutiny of the data in Table 8, which lists the results of the main studies of IFN-α alone or with LDAC. These studies are ordered according to the MCgR rate, from the lowest value of 6.0%, which was reported in the German multicenter prospective study,4 up to the highest value of 45.1%, which was recorded in a series of patients who were treated with IFN-α and LDAC at a single center in Houston.17 Table 8 also lists the 5-year survival rates, which range between 50% and 70%; this shows that although there is a trend toward a relationship between the MCgR rate and survival, there is great variability, and the relationship between the MCgR rate and survival is neither linear nor constant. Therefore, a reasonable conclusion is that the differences between the French and the Italian study are not surprising and are within the order of magnitude that would be expected to occur when many studies are compared. Moreover, it should be noted that although the 2 protocols were very similar for several important issues, such as dose and schedule of both drugs, priority to IFN-α over AC in cases of toxicity, and the criteria for the definition of HR and CgR, there were also differences. To cite a few, the upper age was 69 in the French study and 65 in this study, so the median (and mean) ages were 50 years and 45 years, respectively. In the French study, the patients who were eligible for alloBMT were neither registered nor enrolled, while in this study enrollment and randomization were not dependent on the decision to perform alloBMT, and the option to receive alloBMT was open at any time. Moreover, the criteria for crossover or discontinuation of treatment differed, and the Italian protocol did not provide for any increase of AC dose. A joint study of the Italian and the German Group has already shown that different results can be explained by a careful comparative analysis of the protocols and the individual data.15 The same methodology should be applied to the French study and the present one in a meta-analysis that could also include the data of another, smaller study that is being performed in Austria.37 This is important, because a noncontrolled study of IFN-α plus LDAC has shown a possible benefit in terms of CgR and survival,17 while a second smaller study has failed to show any advantage.18 New prospective studies of IFN-α plus LDAC will not be designed, while it is urgent to settle the relationship between the CgR rate and survival and to establish which treatment should be used as a control to evaluate the efficacy of other treatments, specifically by protein tyrosine kinase inhibitors.38-40
Percentage of partial cytogenetic responses, complete cytogenetic responses, and partial plus complete, or major, cytogenetic responses, and 5-year survival in the main chronic myeloid leukemia studies of interferon-α alone or in combination with low-dose arabinosyl cytosine
| Study . | Year . | Treatment . | Cases, no. . | PCgR, % . | CCgR, % . | MCgR (PCgr + CCgR), % . | 5-year survival, % . |
|---|---|---|---|---|---|---|---|
| Hehlmann et al8-1504 | 1994 | IFN-α | 133 | 1.5 | 4.5 | 6.0 | 58 |
| Allan et al8-1505 | 1995 | IFN-α | 293 | 5.8 | 4.4 | 10.2 | 50 |
| Lindauer et al8-15118 | 1999 | IFN-α + LDAC | 65 | 6.1 | 4.6 | 10.7 | 56 |
| Ohnishi et al8-1506 | 1995 | IFN-α | 85 | 8.2 | 7.0 | 15.2 | 63 |
| Benelux CML Study Group8-15014 | 1988 | IFN-α | 100 | 7.0 | 9.0 | 16.0 | 54 |
| This study8-152 | 2002 | IFN-α | 263 | 10.3 | 7.6 | 17.98-155 | 65 |
| Italian Cooperative Study Group on CML8-1503 | 1994 | IFN-α | 218 | 11.0 | 7.8 | 18.8 | 60 |
| Guilhot et al8-15216 | 1997 | IFN-α | 361 | 13.0 | 7.8 | 20.88-154 | 62 |
| Ozer et al8-15335 | 1993 | IFN-α | 112 | 15.2 | 12.5 | 27.7 | 54 |
| This study8-152 | 2002 | IFN-α + LDAC | 275 | 14.2 | 14.2 | 28.48-155 | 68 |
| Kloke et al8-15136 | 2000 | IFN-α | 71 | 16.8 | 12.7 | 29.6 | 60 |
| Guilhot et al8-15216 | 1997 | IFN-α + LDAC | 360 | 22.2 | 12.7 | 34.98-154 | 70 |
| Kantarjian et al8-1517 | 1995 | IFN-α | 274 | 11.7 | 26.3 | 38.0 | 62 |
| Kantarjian et al8-15117 | 1999 | IFN-α + LDAC | 186 | 17.7 | 27.4 | 45.1 | 688-159 |
| Study . | Year . | Treatment . | Cases, no. . | PCgR, % . | CCgR, % . | MCgR (PCgr + CCgR), % . | 5-year survival, % . |
|---|---|---|---|---|---|---|---|
| Hehlmann et al8-1504 | 1994 | IFN-α | 133 | 1.5 | 4.5 | 6.0 | 58 |
| Allan et al8-1505 | 1995 | IFN-α | 293 | 5.8 | 4.4 | 10.2 | 50 |
| Lindauer et al8-15118 | 1999 | IFN-α + LDAC | 65 | 6.1 | 4.6 | 10.7 | 56 |
| Ohnishi et al8-1506 | 1995 | IFN-α | 85 | 8.2 | 7.0 | 15.2 | 63 |
| Benelux CML Study Group8-15014 | 1988 | IFN-α | 100 | 7.0 | 9.0 | 16.0 | 54 |
| This study8-152 | 2002 | IFN-α | 263 | 10.3 | 7.6 | 17.98-155 | 65 |
| Italian Cooperative Study Group on CML8-1503 | 1994 | IFN-α | 218 | 11.0 | 7.8 | 18.8 | 60 |
| Guilhot et al8-15216 | 1997 | IFN-α | 361 | 13.0 | 7.8 | 20.88-154 | 62 |
| Ozer et al8-15335 | 1993 | IFN-α | 112 | 15.2 | 12.5 | 27.7 | 54 |
| This study8-152 | 2002 | IFN-α + LDAC | 275 | 14.2 | 14.2 | 28.48-155 | 68 |
| Kloke et al8-15136 | 2000 | IFN-α | 71 | 16.8 | 12.7 | 29.6 | 60 |
| Guilhot et al8-15216 | 1997 | IFN-α + LDAC | 360 | 22.2 | 12.7 | 34.98-154 | 70 |
| Kantarjian et al8-1517 | 1995 | IFN-α | 274 | 11.7 | 26.3 | 38.0 | 62 |
| Kantarjian et al8-15117 | 1999 | IFN-α + LDAC | 186 | 17.7 | 27.4 | 45.1 | 688-159 |
Studies are ordered according to the percentage of MCgR, from the lowest to the highest. Because 2 of the studies, this study and the French study,16 studied 2 treatment arms, each of these studies appears twice as row headings. Note also that the percentage of CgR may be lower than reported in each original paper because, wherever possible, CgR was calculated again on the basis of all enrolled cases, according to the intention-to-treat principle. The data show that the relationship between the MCgR rate and 5-year survival is extremely variable.
PCgR indicates partial cytogenetic response; CCgR, complete cytogenetic response; CML, chronic myeloid leukemia. Other abbreviations are explained in Tables 1 and 4.
Multicenter prospective study of IFN-α versus chemotherapy.
Single-center study.
Multicenter prospective study of IFN-α versus IFN-α + LDAC.
Multicenter prospective study of IFN-α alone.
Calculated at 2 years.
Calculated at 1 year.
Calculated for 140 patients who received LDAC daily.
Among the many persons who have contributed to this study, special mention is made of the skilled cooperation of Dr Eliana Zuffa and Katia Vecchi.
This list is ordered by the number of cases that have contributed to the study. Active study members: S. Bassi, E. Trabacchi, Bologna; F. Mandelli, G. Alimena, Roma; R. Giustolisi, P. Guglielmo, Catania; E. Gallo, M. Bertini, Torino; P. Leoni, S. Rupoli, Ancona; V. Liso, G. Specchia, Bari; G. Broccia, A. Di Tucci, Cagliari; M. Carotenuto, C. A. Bodenizza, San Giovanni Rotondo; A. Rambaldi, P. Viero, Bergamo; M. Martelli, A. Tabilio, Perugia; R. Fanin, M. Tiribelli, Udine; B. Rotoli, L. Luciano, Napoli; E. Volpe, F. Palmieri, Avellino; T. Izzi, A. Capucci, Brescia; F. Nobile, B. Martino, Reggio Calabria; A. Peta, F. Iuliano, Catanzaro; F. Ferrara, E. M. Schiavone, Napoli; O. A. Spada, Napoli; E. Pogliani, I. Miccolis, Monza; F. Ricciuti, M. Pizzuti, Potenza; S. Amadori, M. Cantonetti, Roma; G. Castoldi, G. L. Scapoli, Ferrara; G. Pizzolo, A. Ambrosetti, Verona; E. Miraglia, A. Gagliardi, Napoli; F. Leoni, S. Ciolli, Firenze; C. Delfini, G. Nicolini, Pesaro; G. Mariani, D. Turri, Palermo; M. Lazzarino, S. Merante, Pavia; G. Leone, S. Sica, Roma; M. Boccadoro, D. Ferrero, Torino; A. Zaccaria, E. Zuffa, Ravenna; A. Venco, G. Pinotti, Varese; G. Fioritoni, R. Di Lorenzo, Pescara; F. Lauria, M. Bocchia, Siena; G. Rege Cambrin, Orbassano; M. Petrini, F. Papineschi, Pisa; F. Grignani, A. M. Liberati, Perugia; F. Rodeghiero, A. D'Emilio, Vicenza; P. Mazza, M. Cervellera, Taranto; A. T. Maiolo, F. Radaelli, Milano; M. Gobbi, M. Clavio, Genova; P. Bodini, C. Bergonzi, Cremona; S. Nardelli, F. Ciccone, Latina; L. Gugliotta, P. Avanzini, Reggio Emilia; R. Quaini, Bolzano; A. Capaldi, Torino; M. Spina, Aviano; D. Noli, Nuoro; M. Pini, Alessandria; A. M. Gatti, Genova; R. Battista, Chioggia; G. Polimeno, Acquaviva delle Fonti; M. Badone, Biella; A. Gallamini, Cuneo; M. Risso, Genova; L. Gargantini, Milano; S. Iaccarino, Napoli; S. Pardini, Sassari; M. Candela, Ancona; E. Capussela, Foggia; A. Di Francesco, Teramo; C. Musolino, Messina; D. Dini, Modena; E. Aitini, Mantova.
Supported by the National Research Council (CNR) of Italy, by The Italian Association for Cancer Research (AIRC), Milan, and by MURST, COFIN 1999 (Regulation of Ph-positive leukemia).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michele Baccarani, Institute of Hematology and Medical Oncology, L and A Seràgnoli, S Orsola Hospital, Via Massarenti 9, 40138 Bologna, Italy; e-mail:baccarani@med.unibo.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal