Treatment of severe, chronic idiopathic thrombocytopenic purpura (ITP) refractory to most usual therapies is a difficult challenge. Little information exists on the clinical use of cyclosporin A (CyA) in the treatment of ITP. This report describes long-term treatment with CyA (median, 40 months) and follow-up (median, 36.8 months) in 12 adult patients with resistant ITP. CyA used in relatively low doses (2.5-3 mg/kg of body weight per day) led to a clinical improvement in 10 patients (83.3%). Five had a complete response (41.1%), 4 a complete response to maintenance therapy (33.3%), and one a partial response (8.3%). Two patients had no response. Most patients with a response (60%) had a long-term remission (mean, 28.6 months) after discontinuation of CyA. One patient had a relapse of ITP 4 years after CyA therapy was stopped. Side effects were moderate and transient, even in patients dependent on continued CyA treatment. CyA seems to represent reasonable salvage treatment in severe, potentially life-threatening, refractory ITP.
Introduction
Idiopathic thrombocytopenic purpura (ITP) is characterized by the appearance of circulating platelet autoantibodies that opsonize the platelet membrane, leading to platelet destruction by the reticuloendothelial system. The only clinical manifestations of the disorder are hemorrhages of various degrees. In children, ITP is usually acute and self-limiting. In contrast, the chronic form of the disease prevalent in adults persists for years and, in those with marked thrombocytopenia (platelet count < 30 × 109/L) and bleeding manifestations, requires treatment.
Although a prompt remission after steroid treatment is achieved in about two thirds of cases of ITP, many patients have a relapse or become dependent on high-dose therapy. Splenectomy is the treatment of choice in patients with no response to steroids and has been reported to induce a complete and sustained response in two thirds of cases. Nevertheless, in many patients, resistance to steroids and splenectomy develops, requiring additional treatments, such as immunosuppression by high-dose methylprednisolone; chemotherapeutic drugs (one third to two thirds of patients have a complete response); intravenous high-dose immunoglobulins (two thirds have a complete but transient improvement in platelet levels); and investigative therapies such as protein A immunoabsorption, interferon α, heparin, tamoxifen, combination chemotherapy, monoclonal antibodies, autologous stem cell transplantation, and cyclosporin A (CyA).1-10 The efficacy of the investigative therapeutic approaches in obtaining long-term remissions is not known. Notably, older patients with a higher risk of bleeding may have much lower response rates.11,12 Reports on the use of CyA in the treatment of ITP are anecdotal and describe therapy with a variety of drug dosages.13-17
Patients who have severe ITP that becomes progressively resistant to the usual treatments and who, as a result, are expected to have the worst outcomes, often represent a dramatic therapeutic dilemma. To address this challenge, we investigated the use of long-term salvage CyA therapy in 12 patients with refractory ITP.
Study design
The study was conducted in accordance with the guidelines of the Helsinki Declaration and was approved by the provincial ethics committee, and informed consent to participate was obtained from all patients. Twelve adult patients with severe, refractory chronic ITP were treated with CyA (Table 1). In all patients, the disease had become progressively resistant to other treatments, including high-dose prednisone, splenectomy (8 patients), cyclophosphamide, azathioprine, danazol, high-dose γ-globulins, antilymphocyte globulin, or a combination of these therapies. Four patients (7, 9, 11, and 12) in whom splenectomy was not feasible or who chose not to undergo the procedure received only high-dose prednisone, high-dose γ-globulins, and cyclophosphamide. Initially, all patients had a transient response to the therapies listed above, and all subsequently received maintenance prednisone therapy (0.4 mg/kg of body weight per day) to keep platelet counts at about 25 × 109/L until CyA therapy was begun. Patients 1 to 4 were described previously15; the information on these patients given here represents a follow-up update.
Patients' characteristics
| Patient no. . | Age, y/sex . | Months from diagnosis . | Platelets (× 109/L) . | Bone marrow . | Bleeding* . | Previous treatment† . |
|---|---|---|---|---|---|---|
| 1 | 66/F | 110 | 15 | Normal | Major skin, mucosal | P, G, A, Sple |
| 2 | 84/F | 78 | 29 | Normal | Minor skin, retinal | P, G, ALG, Sple |
| 3 | 69/F | 108 | 18 | Normal | Minor skin, mucosal | P, G, ALG, Sple |
| 4 | 70/F | 50 | 20 | Normal | Major skin, intestinal | P, G, A, Sple |
| 5 | 56/F | 504 | 10 | Normal | Major skin, mucosal | P, G, C, Sple |
| 6 | 75/M | 168 | 27 | Normal | Minor skin, mucosal | P, C, ALG, Sple |
| 7 | 78/F | 146 | 28 | Normal | Minor skin, intestinal | P, G, C |
| 8 | 64/F | 132 | 3 | Normal | Major skin, mucosal | P, G, A, D, Sple |
| 9 | 42/M | 52 | 27 | Normal | Minor skin, retinal | P, G, C |
| 10 | 68/F | 86 | 12 | Normal | Major skin, menorrhagia | P, G, Sple |
| 11 | 85/F | 46 | 19 | Normal | Minor skin, mucosal | P, G, C |
| 12 | 62/M | 41 | 25 | Normal | Minor skin, mucosal | P, G, C |
| Patient no. . | Age, y/sex . | Months from diagnosis . | Platelets (× 109/L) . | Bone marrow . | Bleeding* . | Previous treatment† . |
|---|---|---|---|---|---|---|
| 1 | 66/F | 110 | 15 | Normal | Major skin, mucosal | P, G, A, Sple |
| 2 | 84/F | 78 | 29 | Normal | Minor skin, retinal | P, G, ALG, Sple |
| 3 | 69/F | 108 | 18 | Normal | Minor skin, mucosal | P, G, ALG, Sple |
| 4 | 70/F | 50 | 20 | Normal | Major skin, intestinal | P, G, A, Sple |
| 5 | 56/F | 504 | 10 | Normal | Major skin, mucosal | P, G, C, Sple |
| 6 | 75/M | 168 | 27 | Normal | Minor skin, mucosal | P, C, ALG, Sple |
| 7 | 78/F | 146 | 28 | Normal | Minor skin, intestinal | P, G, C |
| 8 | 64/F | 132 | 3 | Normal | Major skin, mucosal | P, G, A, D, Sple |
| 9 | 42/M | 52 | 27 | Normal | Minor skin, retinal | P, G, C |
| 10 | 68/F | 86 | 12 | Normal | Major skin, menorrhagia | P, G, Sple |
| 11 | 85/F | 46 | 19 | Normal | Minor skin, mucosal | P, G, C |
| 12 | 62/M | 41 | 25 | Normal | Minor skin, mucosal | P, G, C |
P indicates prednisone; G, γ-globulins; A, azathioprine; Sple, splenectomy; ALG, antilymphocyte globulin; C, cyclophosphamide; and D, danazol.
Major skin indicates diffuse ecchymosis; mucosal, intrabuccal hemorrhagic vesicles or prolonged epistaxis; and intestinal and menorrhagia, gastrointestinal and genitourinary bleeding, respectively.
All patients took prednisone before starting cyclosporin A therapy.
The patients were 9 women and 3 men, with a mean age of 66.6 years (range, 42-85 years). The mean duration of ITP before CyA treatment was 89.9 months (range, 11-467 months). Only patients with platelet levels below 30 × 109/L were enrolled in the study.
ITP was diagnosed in accordance with standard criteria and after other causes of thrombocytopenia were excluded. All patients had a normal or increased number of megakaryocytes in the bone marrow, and none had signs of fibrosis or dysplasia. In the 8 patients who underwent splenectomy, the pathological findings in spleen specimens were consistent with ITP. All patients had a history of major or minor bleeding (Table 1); these episodes were often transient but recurrent. Major hemorrhagic events included gastrointestinal or genitourinary bleeding, intrabuccal hemorrhagic vesicles, diffuse ecchymosis, prolonged epistaxis, and retinal hemorrhages. Minor bleeding events included mild purpura, mild epistaxis, gingival bleeding, and easy bruising.
CyA treatment was started at a dose of 5 mg/kg per day administered orally twice daily for 6 days. The dose was then reduced to 2.5-3 mg/kg per day and continued at that level, with only slight changes, to maintain a therapeutic serum level between 200 and 400 ng/mL. Monitoring included monthly blood cells counts and examination of renal and hepatic functions every week for 1 month and then every 1 or 2 months. When the serum creatinine level exceeded 150% of the baseline value, the CyA dose was reduced by 25% for 6 to 10 days. In all patients, renal function, evaluated before CyA therapy began, was normal. In patients who had a response to CyA, administration of the agent was tentatively discontinued for 2 weeks every 10 to 12 months to evaluate the persistence of remission. Some patients (1-5) with clinical bleeding manifestations or older age (> 60 years) were given low-dose prednisone (0.3 mg/kg per day) during the first 3 or 4 weeks of CyA treatment. In the other patients, prednisone was stopped at initiation of CyA therapy.
A complete response (CR) was defined as a platelet count in the normal range for at least 3 months after CyA treatment was discontinued. A partial response (PR) was defined as a platelet count between 80 and 120 × 109/L for at least 3 months while the patient was not receiving CyA. A response to maintenance therapy (MTR) was defined as a platelet count in the normal range during continuous administration of CyA. No response (NR) was defined as a platelet count that did not rise above 40 × 109/L.
Results and discussion
All patients who entered the CyA protocol had ITP that did not respond to common multiple-treatment approaches (Table 1). In 4 patients, splenectomy was not done because of advanced age (patients 7 and 11) or because the patient chose not to undergo the procedure (patients 9 and 12). The mean platelet count at initiation of therapy was 19.4 × 109/L (range, 3-29 × 109/L). No patient had a disease known to be associated with ITP (ie, connective tissue disorder, viral infection, lymphoproliferative disorder, or liver or thyroid disease). The total mean follow-up time for the patients was 118.4 months (range, 41-504 months), and the mean follow-up time from the beginning of CyA treatment was 36.8 months (range, 3-86 months). For patients 1 to 4, the median follow-up from the beginning of treatment was 51.2 months (range, 20-86 months).
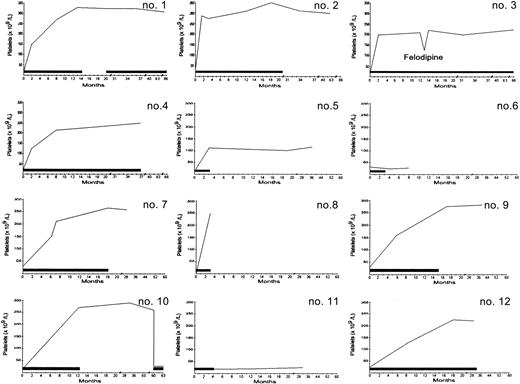
The results of CyA treatment are shown in Table2 and Figure1. Platelet counts usually began to increase in the third or fourth week of treatment. Two patients (6 and 11) had NR to CyA. Ten patients (83.3%) had a response: 5 patients had a CR, one patient had a PR, and 4 patients had an MTR (platelet counts in the normal range were achieved and maintained with continued administration of CyA). Patient 8 died during a 3-month course of CyA therapy, before an attempt to discontinue the drug, although her platelet counts were in the normal range. Patients 1, 2, 7, 9, and 10 had a CR after a mean of 13.8 months of therapy (range, 3-21 months), and their platelet counts remained in the normal range for a mean of 27.3 months (range, 6-48 months) without any treatment. However, one of these patients (10) had a relapse of ITP 4 years after CyA was stopped, with the disease becoming resistant to the drug. Ultimately, 9 patients had a sustained remission for the duration of the observation period.
Patients' characteristics after cyclosporin A (CyA) treatment
| Patient no. . | CyA therapy (mo) . | Platelets (× 109/L) . | CyA stopped . | Response duration (mo) . | Outcome . | Bleeding . | |||
|---|---|---|---|---|---|---|---|---|---|
| Before therapy . | After therapy . | Total . | Off CyA . | Before therapy . | After therapy . | ||||
| 1 | 14 | 15 | 325 | Yes | 20 | 6 | CR | ++ | − |
| 2 | 21 | 29 | 300 | Yes | 62 | 41 | CR | ++ | − |
| 3 | 86 | 18 | 225 | No | 86 | — | MTR | + | − |
| 4 | 37 | 20 | 260 | No | 37 | — | MTR | ++ | − |
| 5 | 3 | 10 | 118 | Yes | 37 | 34 | PR | ++ | − |
| 6 | 3 | 27 | 28 | Yes | — | — | NR | + | + |
| 7 | 18 | 28 | 255 | Yes | 28 | 18 | CR | ++ | − |
| 8 | 3 | 3 | 250 | No | 3 | — | MTR | ++ | − |
| 9 | 15 | 27 | 290 | Yes | 40 | 25 | CR | ++ | − |
| 10 | 12 | 12 | 280 | Yes | 60 | 48 | CR | ++ | − |
| 11 | 3 | 19 | 25 | Yes | — | — | NR | + | + |
| 12 | 30 | 25 | 215 | No | 30 | — | MTR | + | − |
| Patient no. . | CyA therapy (mo) . | Platelets (× 109/L) . | CyA stopped . | Response duration (mo) . | Outcome . | Bleeding . | |||
|---|---|---|---|---|---|---|---|---|---|
| Before therapy . | After therapy . | Total . | Off CyA . | Before therapy . | After therapy . | ||||
| 1 | 14 | 15 | 325 | Yes | 20 | 6 | CR | ++ | − |
| 2 | 21 | 29 | 300 | Yes | 62 | 41 | CR | ++ | − |
| 3 | 86 | 18 | 225 | No | 86 | — | MTR | + | − |
| 4 | 37 | 20 | 260 | No | 37 | — | MTR | ++ | − |
| 5 | 3 | 10 | 118 | Yes | 37 | 34 | PR | ++ | − |
| 6 | 3 | 27 | 28 | Yes | — | — | NR | + | + |
| 7 | 18 | 28 | 255 | Yes | 28 | 18 | CR | ++ | − |
| 8 | 3 | 3 | 250 | No | 3 | — | MTR | ++ | − |
| 9 | 15 | 27 | 290 | Yes | 40 | 25 | CR | ++ | − |
| 10 | 12 | 12 | 280 | Yes | 60 | 48 | CR | ++ | − |
| 11 | 3 | 19 | 25 | Yes | — | — | NR | + | + |
| 12 | 30 | 25 | 215 | No | 30 | — | MTR | + | − |
Patients 1 to 4 were described previously15; the information here represents a follow-up update. Patient 8 died of a myocardial infarction during CyA treatment.
CR indicates complete response; MTR, response to maintenance therapy; PR, partial response; NR, no response; ++, major bleeding; +, minor bleeding; and −, no bleeding.
Platelet counts over time.
Each small number represents a value from one patient identified by number in Table 1. The bars indicate the time of CyA administration. The first 4 numbers represent a follow-up update for the 4 patients described previously.15 In patient 1, CyA treatment was resumed at 20 months because of development of an autoimmune hemolytic anemia. Patient 10 had a relapse at 4 years, with her condition becoming resistant to CyA. The decrease in platelet values in patient 3 occurred concomitantly with administration of felodipine. Patient 8 died of a myocardial infarction during a 3-month course of CyA therapy.
Platelet counts over time.
Each small number represents a value from one patient identified by number in Table 1. The bars indicate the time of CyA administration. The first 4 numbers represent a follow-up update for the 4 patients described previously.15 In patient 1, CyA treatment was resumed at 20 months because of development of an autoimmune hemolytic anemia. Patient 10 had a relapse at 4 years, with her condition becoming resistant to CyA. The decrease in platelet values in patient 3 occurred concomitantly with administration of felodipine. Patient 8 died of a myocardial infarction during a 3-month course of CyA therapy.
In patient 1, an autoimmune hemolytic anemia (AHA) developed 6 months after CyA was stopped. At that time, administration of CyA was resumed to control the AHA. The disease was controlled by the drug for 47 months, with platelet counts remaining in the normal range. Patient 3, who had a 4-year history of hypertension, underwent hypotensive treatment before and during CyA therapy. She died of a myocardial infarction after 86 months of CyA treatment, even though her hypertension was well controlled. Patient 8 also died of a myocardial infarction, without having any history of hypertension or heart disease either before or during CyA treatment.
Side effects and other complications of CyA treatment are shown in Table 3. Transient and reversible intolerance was observed in several patients. There was a slight increase in creatinine level in 3, moderate hypertension in 3, fatigue in 2, paraesthesias in 2, gingival hyperplasia in 3, myalgia in 2, dyspepsia in 2, hypertrichosis in one, and tremor in one. These conditions usually resolved spontaneously or responded to a reduction in dose of CyA or discontinuation of the agent for a few days. In patient 7, a case of oropharynx candidiasis resolved when CyA was discontinued and fluconazole therapy begun. In patient 3, an adverse effect of felodipine was observed: use of the agent led to a dramatic decrease in CyA serum level. This resolved when the hypotensive drug was discontinued and a β-blocker given instead.
Complications during and after CyA treatment
| Patient no. . | Side effects . | Other complications . |
|---|---|---|
| 1 | Fatigue, hypertension | — |
| 2 | Gingival hyperplasia | — |
| 3 | Hypertrichosis, increased creatinine | Interaction of felodipine and CyA; death after 86 mo of CyA |
| 4 | Hypertension, fatigue | — |
| 5 | Gingival hyperplasia, tremor | — |
| 6 | Paraesthesias, dyspepsia, increased creatinine | Death 6 mo after CyA stopped |
| 7 | Hypertension | Candidiasis |
| 8 | Myalgia | Death during 3-mo course of CyA |
| 9 | Gingival hyperplasia | — |
| 10 | Myalgia | Relapse 48 mo after CyA stopped |
| 11 | Dyspepsia, increased creatinine | — |
| 12 | Paraesthesias | — |
| Patient no. . | Side effects . | Other complications . |
|---|---|---|
| 1 | Fatigue, hypertension | — |
| 2 | Gingival hyperplasia | — |
| 3 | Hypertrichosis, increased creatinine | Interaction of felodipine and CyA; death after 86 mo of CyA |
| 4 | Hypertension, fatigue | — |
| 5 | Gingival hyperplasia, tremor | — |
| 6 | Paraesthesias, dyspepsia, increased creatinine | Death 6 mo after CyA stopped |
| 7 | Hypertension | Candidiasis |
| 8 | Myalgia | Death during 3-mo course of CyA |
| 9 | Gingival hyperplasia | — |
| 10 | Myalgia | Relapse 48 mo after CyA stopped |
| 11 | Dyspepsia, increased creatinine | — |
| 12 | Paraesthesias | — |
All side effects were transient. Patient 3 received hypotensive drugs for 4 years.
Nonanecdotal, comprehensive information on the clinical use of CyA in the treatment of ITP is lacking. We studied a series of 12 selected patients in whom ITP had become progressively resistant to commonly used multiple-treatment modalities. These patients either underwent splenectomy or received at least 3 different immunosuppressive drugs. Spontaneous remission of chronic ITP months or years after failure of therapy has been reported in some patients.18Nevertheless, clinical management of resistant ITP, which naturally evolves toward a severe and uncontrolled thrombocytopenia, is problematic. The mortality rate for this disorder is relatively low (4%-5%), but because bleeding and infection contribute equally to the deaths that occur,12 19 patients with resistant ITP must be considered to have a potentially life-threatening condition.
Our study suggests that CyA treatment is safe and effective in patients with resistant ITP. Clinical improvements were sustained in at least half of the patients, even after they stopped taking the drug. On the other hand, patients who continued to be dependent on CyA (one third) remained in long-term remission with continuous low-dose treatment and without major side effects.
A study by Kappers-Klunne and van't Veer20 reported on the use of CyA in combination with prednisone in patients with ITP refractory to corticosteroids alone or to corticosteroids and splenectomy. The authors stated that CyA can be useful in such patients but highlighted the strong toxicity of the treatment (30% of patients stopped taking CyA because of side effects). The toxicity observed in their study appears to be related to the high dose of CyA used (an average of 5 to 7 mg/kg per day, about twice as high as that in our series). Moreover, the action of CyA is likely to be potentiated by corticosteroid administration.21
In our patients, intolerance of CyA was minor, even during long-term treatment. Although hypertension and an increase in creatinine levels were observed in some patients, no persistent nephrotoxicity occurred and hypertension was always controlled by adjustments in dose. Since CyA began to be used clinically, long-term maintenance of therapeutic CyA levels has been made problematic by fears of chronic nephrotoxicity. However, reports on a broad series of patients with renal transplants who were treated with CyA showed that chronic nephrotoxicity is exceedingly rare.23 23 In our series, other minor side effects were transient and always resolved with tapering the dose of CyA or discontinuing use of the drug for a short time. When a fungal infection occurred in one of our patients, the response to standard antifungal therapy was good and discontinuation of CyA was required for only a short period.
Treatment with CyA seems to be a reasonable approach in ITP in particularly serious clinical situations, such as cases that do not respond to standard treatments. To avoid mutagenic effects or myelotoxicity, this therapeutic option could, in selected cases, be considered even before immunosuppressive chemotherapy.
Supported by AIRC, Milan, Italy (M.L.); and the AIL Section, Modena, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giovanni Emilia, Department of Medical Sciences, Section of Internal Medicine, Policlinico, via del Pozzo 71, 41100 Modena, Italy; e-mail: emilia.giovanni@unimo.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal