Angiogenesis, an essential process for tumor growth, is regulated by endothelial proliferation factors and their inhibitors such as endostatin. Endostatin, a carboxyl-terminal fragment of type XVIII collagen, inhibits endothelial proliferation, angiogenesis, and tumor growth. Ornithine decarboxylase (ODC), a molecule that is overexpressed in various cancers, is associated with promoting tumor growth and angiogenesis. We found that ODC-overexpressing human cancer cells and breast cancer specimens showed suppressed expression of type XVIII collagen and endostatin. We hypothesized that ODC overexpression may facilitate angiogenesis in tumors by suppressing endostatin expression. ODC-overexpressing COS cells, which showed suppressed type XVIII collagen and endostatin expression, were established. Conditioned media derived from these cells, containing decreased levels of endostatin, induced significant endothelial proliferation. ODC-overexpressing cells, when transplanted into nude mice, suppressed type XVIII collagen expression and promoted neovascularization in vivo. Thus, overexpression of ODC facilitates endothelial proliferation by suppressing endostatin expression.
Introduction
Endostatin, a 20-kd carboxyl-terminal fragment of type XVIII collagen, inhibits endothelial proliferation, angiogenesis, and tumor growth.1-3 Endostatin induces endothelial cell apoptosis.4 Type XVIII collagen consists of an amino-terminal region, collagenlike domains, and a 35-kd carboxyl-terminal noncollagenous (NC1) domain. The NC1 domain,5 consisting of 3 major segments, a 5-kd N-terminal association domain, a central proteinase-sensitive hinge region, and the compact 20-kd C-terminal endostatin domain, inhibits vascular endothelial growth factor (VEGF)–induced endothelial cell migration.6 The transcripts of type XVIII collagen are reported to be 4.5 kb and 5.5 kb in multiple organs by Northern blot analysis with probe for NC1 domain containing endostatin.7Therefore, it is considered that NC1 domain and endostatin are processed from type XVIII collagen by proteolytic cleavage. Several proteinases that cleave type XVIII collagen are also reported.8-10
Ornithine decarboxylase (ODC) catalyzes the biosynthesis of polyamines, and ODC activities are associated with cell transformation, tumor invasion, and angiogenesis.11-14 An ODC inhibitor, DL-α-difluoromethylornithine (DFMO), inhibits B16 melanoma angiogenesis.15 16
We hypothesized that ODC overexpression may facilitate angiogenesis by suppressing endostatin expression.
Study design
Sample collection
Thirty-three paired specimens of cancerous and noncancerous tissues adjacent to the cancer tissue and 38 cancerous tissues of breast were obtained (Cancer Institute Hospital, Tokyo). Written informed consents were obtained from all patients before surgery.
Plasmid construction
Full-length human ODC cDNA (1.8 kb) was cloned into pTargeT expression vector (Promega, Tokyo, Japan).
Cells and stable transfection
COS-1 and calf pulmonary artery endothelial (CPAE) cells (Health Science Research Resource Bank, Osaka, Japan) were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. COS cells transfected with the plasmid carrying ODC cDNA were selected in the presence of G418 for 2 weeks. The surviving cells were pooled and established as ODC-overexpressing cells (ODC transfectants). We also established mock transfectants.
ODC enzyme activity assay
ODC activity was determined as described previously.12
Semiquantitative reverse transcriptase–polymerase chain reaction
Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed by using the following primers: glyceraldehyde phophate dehydrogenase (GAPDH) (5′-ACGCATTTGGTCGTATTGGG-3′ and 5′-TGATTTTGGAGGGATCTC-GC-3′), ODC (5′-GTATGCTGCTAATGGAG-3′ and 5′-TTACGCCGGTGATCTCTT-CA-3′), VEGF (5′-CATCCCTGTGGGCCTTGCTC-3′ and 5′-GCTCACCGCCTCGG-CTTGTC-3′), and type XVIII collagen (5′-ACGCATCTTCTCCTTTGACG-3′ and 5′-TGGCTACTTGGAGGCAGTCA-3′). Band intensities for the ODC, VEGF, and type XVIII collagen PCR products were quantitated by using National Institutes of Health image software and normalized to the intensity of the GAPDH signal.
Western blot analysis
Western blotting was performed by using cell and tissue lysates. Basic fibroblast growth factor was observed by using a specific antibody (Transduction Lab, Lexington, KY). Endostatin was detected by using an antibody generated against the sequence IFSDGKDVLRHPTWPQKSV from human endostatin. Bands were visualized by using the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Tokyo, Japan) or an Amplified AP immune kit (Bio-Rad, Tokyo, Japan).
Endostatin enzyme immunoassay
Concentrations of endostatin were determined by using an Accucyte human endostatin enzyme immunoassay kit (Cytoimmune Sciences, College Park, MD), using 100 μL conditioned media.
Endothelial proliferation assay
CPAE cells plated on 96-well plates were incubated for 24 hours at 37°C in Dulbecco modified Eagle medium. After the addition of 50 μL conditioned media, the cells were further incubated for 18 hours. Cell number was then counted by using a cell counting kit-8 (Wako, Osaka, Japan), according to the manufacturer's instruction.
In vivo transplantation
Male Balb/c slc/nu mice (4- to 5-weeks old) were obtained from SLC (Hamamatsu, Japan). Cells (ODC transfectants and mock transfectants; 1 × 107), suspended in 100 μL phosphate-buffered saline after mixing with 100 μL matrigel (Becton Dickinson, Franklin Lakes, NJ) were inoculated subcutaneously on the backs of mice. Transplanted cells formed a tumor. The tumors were removed after 25 days, fixed with formalin, and embedded in paraffin. The tumor weight was approximately 200 mg. Sections were stained by using the hematoxylin-eosin method.
Immunohistochemistry
For enumerating endothelial cells, tumor sections derived from ODC transfectants and mock transfectants were stained with anti–von Willebrand factor (VWF) antibody. The tumor sections were primed with goat polyclonal anti-VWF antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at 25°C. Then, the sections were washed 3 times with phosphate-buffered saline and incubated with horseradish peroxidase–conjugated antigoat immunoglobulin G for 1 hour at 25°C. After washing 3 times with phosphate-buffered saline, they were stained with diaminobenzidine. As a negative control, only the second antibody was used and stained with diaminobenzidine. No specific immunoreactivity was detected in negative-control sections. The number of stained endothelial cells (vessels) in 10 fields was counted and quantitated.
Statistical analysis
Results were expressed as mean ± SD from 3 independent experiments. The significance of the differences was determined by using analysis of variance Fisher PSLD test or the Studentt test.
Results and discussion
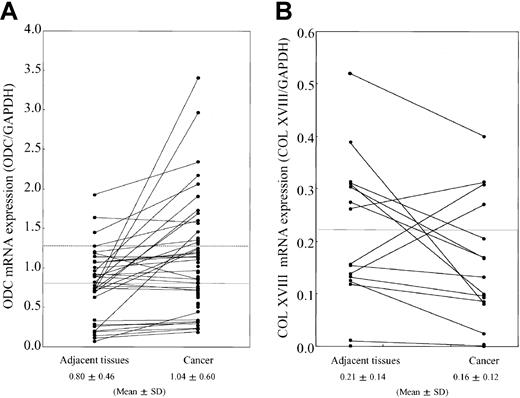
We analyzed the relationship between ODC expression levels and type XVIII collagen production in various tumor cells. In the ODC-overexpressing cell lines, A549 (human lung cancer cell) and MDA-MB-231(mammary cancer cell), the expression of type XVIII collagen, NC1, and endostatin were remarkably suppressed in comparison to normal cells, as determined by Western blotting (data not shown). We next analyzed the expression levels of ODC and type XVIII collagen at the messenger RNA (mRNA) level in human breast cancer (n = 71) and the adjacent normal tissue (n = 33) by using RT-PCR. Fifteen (21.1%) of 71 cancer tissues overexpressed ODC mRNA (> normal mean + SD levels), in comparison to the adjacent tissue (Figure1A). Eleven (73.3%) of 15 ODC-overexpressing human breast cancers showed suppressed type XVIII collagen mRNA expression when compared with the adjacent normal tissue (Figure 1B).
mRNA expression levels of ODC and type XVIII collagen.
Total RNA was extracted from breast cancer tissues and the noncancerous adjacent tissue. (A) RT-PCR was performed by using primers specific for ODC. Band intensities were quantitated by using National Institutes of Health image software and normalized to the intensity of the GAPDH signal. Straight and broken lines indicate normal mean and mean + SD levels, respectively. (B) ODC overexpressing breast cancer tissue (15 pairs) was analyzed for type XVIII collagen mRNA expression. Band intensities were quantitated by using National Institutes of Health image software and normalized to the intensity of the GAPDH signal. The line indicates the normal mean level.
mRNA expression levels of ODC and type XVIII collagen.
Total RNA was extracted from breast cancer tissues and the noncancerous adjacent tissue. (A) RT-PCR was performed by using primers specific for ODC. Band intensities were quantitated by using National Institutes of Health image software and normalized to the intensity of the GAPDH signal. Straight and broken lines indicate normal mean and mean + SD levels, respectively. (B) ODC overexpressing breast cancer tissue (15 pairs) was analyzed for type XVIII collagen mRNA expression. Band intensities were quantitated by using National Institutes of Health image software and normalized to the intensity of the GAPDH signal. The line indicates the normal mean level.
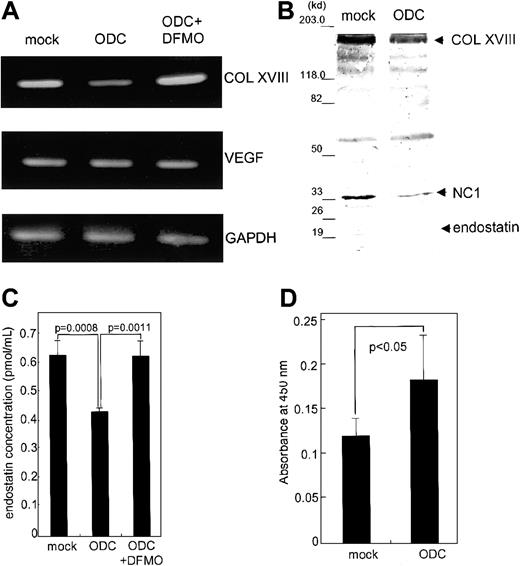
We next examined the effect of ODC overexpression on endostatin expression and endothelial cell proliferation. We established transfectants overexpressing ODC and mock transfectants containing vector alone. ODC transfectants exhibited 6.1- ± 0.3-fold higher ODC mRNA expression, as compared with mock transfectants. The expression of type XVIII collagen in ODC transfectants at both the protein and mRNA levels was significantly suppressed, 40.2% ± 6.8% (P < .005; data not shown) and 47.9% ± 5.3% (P < .005; Figure 2A), respectively, compared with mock transfectants. DFMO (1 mM), an ODC inhibitor, rescued the suppression of type XVIII collagen mRNA to return to the expression levels to that of mock transfectants (Figure2A), demonstrating the specificity of the suppression. VEGF mRNA expression levels remained unchanged (Figure 2A). Basic fibroblast growth factor was undetectable in ODC and mock transfectants by using Western blotting (data not shown).
Effect of ODC overexpression on type XVIII collagen and endostatin expression, as well as endothelial proliferation.
Both total RNA and whole cell lysates were prepared from ODC transfectants and mock transfectants, as well as DFMO-treated ODC transfectants. (A) Type XVIII collagen (COL XVIII), VEGF, and GAPDH mRNA expression was analyzed by RT-PCR. (B) Concentrated conditioned media from both ODC transfectants and mock transfectants were used for endostatin analysis by Western blotting. (C) The concentration of endostatin was quantitated by using an endostatin enzyme immunoassay kit. (D) Conditioned media derived from both ODC transfectants and mock transfectants were assayed for CPAE cell proliferation.
Effect of ODC overexpression on type XVIII collagen and endostatin expression, as well as endothelial proliferation.
Both total RNA and whole cell lysates were prepared from ODC transfectants and mock transfectants, as well as DFMO-treated ODC transfectants. (A) Type XVIII collagen (COL XVIII), VEGF, and GAPDH mRNA expression was analyzed by RT-PCR. (B) Concentrated conditioned media from both ODC transfectants and mock transfectants were used for endostatin analysis by Western blotting. (C) The concentration of endostatin was quantitated by using an endostatin enzyme immunoassay kit. (D) Conditioned media derived from both ODC transfectants and mock transfectants were assayed for CPAE cell proliferation.
We next analyzed endostatin secretion by immunoblot analysis of concentrated conditioned media by using a specific antiendostatin antibody. The levels of type XVIII collagen (180 kd), NC1 (35 kd), and endostatin (20 kd) in ODC transfectants were significantly suppressed 46.5% ± 11.3% (P < .02), 62.5% ± 10.0% (P < .01), and 73.9% ± 12.2% (P < .01), respectively, compared with mock transfectants (Figure 2B). The concentration of immunoreactive endostatin analyzed by using endostatin enzyme immunoassay was significantly suppressed in ODC transfectants, in comparison to mock transfectants (P < .0008; Figure 2C). An 18-hour treatment with 1 mM DFMO restored endostatin concentrations to the levels seen in mock transfectants, indicating the specificity of ODC-mediated endostatin suppression (P < .0011). Conditioned media derived from ODC transfectants induced 1.6- ± 0.3-fold more CPAE cell proliferation than media derived from the mock transfectants (P < .05; Figure 2D). These data indicate that ODC overexpression facilitates endothelial cell proliferation by suppressing endostatin secretion.
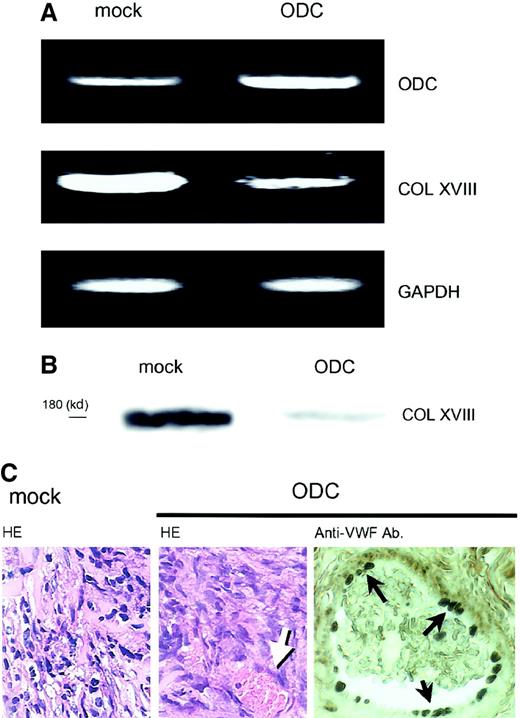
We further examined whether ODC-overexpressing cells possessed similar properties in vivo. As ODC transfectants formed very small transplants in nude mice, and matrigel is reported to facilitate tumor growth,17 ODC transfectants were injected into nude mice with matrigel, according to the method as described.17 ODC transfectants (1 × 107 cells) and mock transfectants (1 × 107 cells) within a matrigel were injected subcutaneously into nude mice. Both ODC transfectant and mock transfectant cells formed solid tumors. However, the ODC-overexpressing tumor in nude mice showed 6.5- ± 0.8-fold greater ODC mRNA expression than the mock transplants (Figure3A), and suppressed type XVIII collagen expression 50.8% ± 4.2% and 48.3% ± 6.4% at the mRNA and protein levels, respectively, compared with mock transplants (Figure3A,B). Histologic examination revealed that ODC-overexpressing transplantations exhibited neovascularization in the tumor tissue (Figure 3C). Neovascularization was confirmed by staining endothelial cells with the use of anti-VWF antibody (Figure 3C). The number of stained vessels was counted in 10 fields microscopically. ODC transplants showed significant increase in tumor neovascularization compared with mock transplants (2.1 ± 1.2 versus 0.8 ± 0.6/field,P = .0277). Because matrigel was used for in vivo experiment, mock transplants formed tumor unexpectedly. However, the data clearly have shown that not mock transfectants but ODC transfectants in vivo possessed similar properties to those observed in vitro and induced neovascularization. The data further support our idea. The data are not inconsistent with previous reports in which antitumor activity of DFMO is due to its inhibition of tumor-induced angiogenesis by inhibition of proliferation of endothelial cells, although endostatin was not analyzed.16
In vivo effects of ODC overexpression on type XVIII collagen expression and neovascularization.
ODC-overexpressing transfectants and mock transfectants within a matrigel were injected subcutaneously into nude mice. Both transfectants formed tumors. (A) Total RNA was extracted from the transplanted cells; mRNA expression was then analyzed by RT-PCR. (B) Transplanted cells were homogenized and protein was extracted. Type XVIII collagen (COL XVIII) expression was analyzed by Western blotting by using a specific antiendostatin antibody. (C) Tumors were removed, fixed in formalin, and embedded in paraffin. Sections were then stained by using the hematoxylin-eosin and immunohistochemistry methods. Left and center panels show mock transplants and ODC transplants stained with the hematoxylin-eosin method, respectively. Right panel shows the endothelial cells in ODC transplants stained with anti-VWF antibody. Arrows indicate vessel in the tumor tissue. Original magnification for both HE, × 200; anti-VWF, × 400.
In vivo effects of ODC overexpression on type XVIII collagen expression and neovascularization.
ODC-overexpressing transfectants and mock transfectants within a matrigel were injected subcutaneously into nude mice. Both transfectants formed tumors. (A) Total RNA was extracted from the transplanted cells; mRNA expression was then analyzed by RT-PCR. (B) Transplanted cells were homogenized and protein was extracted. Type XVIII collagen (COL XVIII) expression was analyzed by Western blotting by using a specific antiendostatin antibody. (C) Tumors were removed, fixed in formalin, and embedded in paraffin. Sections were then stained by using the hematoxylin-eosin and immunohistochemistry methods. Left and center panels show mock transplants and ODC transplants stained with the hematoxylin-eosin method, respectively. Right panel shows the endothelial cells in ODC transplants stained with anti-VWF antibody. Arrows indicate vessel in the tumor tissue. Original magnification for both HE, × 200; anti-VWF, × 400.
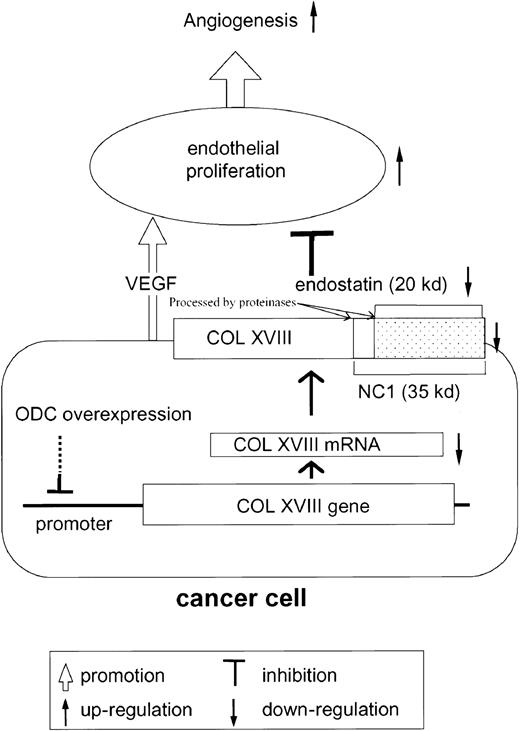
In summary, these results support our novel hypothesis that ODC overexpression facilitates endothelial cell proliferation (angiogenesis) by suppressing type XVIII collagen and endostatin expression (Figure 4).
Scheme of hypothesis.
Cancer cells overexpressing ODC suppress type XVIII collagen (COL XVIII) expression. As endostatin is generated from type XVIII collagen by proteolytic cleavage, ODC-overexpressing cancer cells secrete reduced quantities of endostatin. As endostatin inhibits VEGF-mediated endothelial proliferation, VEGF secreted from cancer cells further facilitates endothelial proliferation, leading to angiogenesis.
Scheme of hypothesis.
Cancer cells overexpressing ODC suppress type XVIII collagen (COL XVIII) expression. As endostatin is generated from type XVIII collagen by proteolytic cleavage, ODC-overexpressing cancer cells secrete reduced quantities of endostatin. As endostatin inhibits VEGF-mediated endothelial proliferation, VEGF secreted from cancer cells further facilitates endothelial proliferation, leading to angiogenesis.
Supported by a grant under the Ministry of Education, Science, Sports, and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shunichiro Kubota, Department of Physiological Chemistry and Metabolism, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; e-mail:kubota@bio.m.u-tokyo.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal