To determine whether production of type 1 and type 2 cytokines defines discrete stages of natural killer (NK) cell differentiation, cytokine expression was analyzed in human NK cells generated in vitro in the presence of interleukin-15 (IL-15) and/or IL-2 from umbilical cord blood hematopoietic progenitors. Like peripheral NK cells, the CD161+/CD56+ NK cells from these cultures contained a tumor necrosis factor alpha (TNF-α)+/granulocyte macrophage–colony-stimulating factor (GM-CSF)+ subset, an interferon gamma (IFN-γ)+ subset, mostly included within the former, and very few IFN-γ−/IL-13+ cells. Instead, most immature CD161+/CD56− NK cells, detectable only in the cultures with IL-2, produced IL-13, TNF-α, and GM-CSF, but not IFN-γ, and contained an IL-5+ subset. In short-term cultures with IL-12 and feeder cells, a proportion of the immature cells acquired the ability to produce IFN-γ. Part of these produced both IFN-γ and IL-13, irrespective of induced CD56 expression. These in vitro data indicate that ability to produce the type 2 cytokines IL-13 and IL-5 defines CD161+ NK cells at intermediate stages of differentiation, and is lost upon terminal functional differentiation, concomitant with acquired ability to produce IFN-γ.
Introduction
Natural killer (NK) cells mediate the early, nonadaptive, responses against virus-, intracellular bacteria-, and parasite-infected cells,1 and modulate the activity of other effector cells of the adaptive and innate systems of defense. In the mouse, interleukin-12 (IL-12)–induced interferon gamma (IFN-γ) production by mature NK cells2,3 directs the development of Ag-specific cell-mediated responses to intracellular pathogens, controlling Th1 cell differentiation4 (reviewed in Trinchieri and Scott5 and Coffman et al6). NK cells participate in the regulation of myeloid hematopoiesis,7 and activation of myeloid8 and monocytic cells (reviewed in Trinchieri et al9) via production of granulocyte macrophage–colony-stimulating factor (GM-CSF), IL-3, IFN-γ and tumor necrosis factor alpha (TNF-α).10-14 They have also been proposed to participate in the regulation of humoral immune responses,15,16 and to play a significant role in the asthma-associated eosinophilia17,18 via IL-5 production, and possibly other factors. A minor subset or subsets of IL-1319 and of IL-5 producing NK cells exists in adult peripheral and umbilical cord blood.20 21 Whether combinations of different cytokines are produced by NK cells at distinct stages of differentiation or by distinct mature NK cell subsets remains to be established.
NK cell differentiation is controlled by cytokines produced in an intact bone marrow microenvironment22,23 (reviewed in Sivakumar et al24). In the murine system, these include Flt-3 ligand1 (Flt3-L),25 c-kitligand (stem cell factor, SCF),25,26 and IL-1525,27 that act, alone or together, on NK cells at different stages of differentiation.25 Flt3-L and IL-15 sustain differentiation of human CD34+ bone marrow cells to cells functionally and phenotypically similar to mature peripheral blood NK cells.28-30 Produced by stromal and monocytic/myeloid cells,31 they likely also act in vivo in physiologic conditions (reviewed in Carson and Calgiuri32). Possible differential effects of these cytokines on NK cell differentiation have been analyzed only at very early developmental stages,30,33 34 when NK lineage-specific markers are not yet identifiable.
In rodents (mouse35,36 and rat37) and humans38-42 IL-2 efficiently substitutes for IL-15 in vitro to support NK cell differentiation from CD34+ or lineage negative (Lin−) hematopoietic progenitor cells. We have reported an in vitro model of human NK cell differentiation involving coculture of umbilical cord blood Lin− hematopoietic progenitor cells with IL-2 and a murine stromal cell line expressing the membrane-bound form of SCF (mSCF).21 Using this system, we established that expression of CD161 in the absence of other mature NK cell markers defines NK cells at a relatively immature stage of differentiation. CD161+/CD56− NK cells mediate TRAIL-L–mediated, but not FasL-mediated or granule exocytosis–mediated cytotoxicity,43 and do not express IFN-γ upon stimulation.21 However, in culture conditions including IL-12 and feeder cells, a proportion of these cells is induced to differentiate to mature cells expressing CD56 and, constitutively, IFN-γ mRNA.21 It is currently not known whether NK cells at this stage of differentiation produce IFN-γ or other cytokines produced by mature NK cell subsets or if they can be induced to produce them when cultured with the cytokines we have previously shown able to induce expression of IFN-γ mRNA.
Here, we have analyzed cytokine production in human NK cells at distinct stages of differentiation. Our data demonstrate that most CD161+/CD56− immature NK cells produce IL-13, TNF-α, and GM-CSF and contain a minor fraction, undetectable in the mature CD161+/CD56+ cells, that produces IL-5. As previously suggested based on mRNA expression, the ability to produce IFN-γ is acquired late, approximately at the same time as acquisition of CD56 expression. Interestingly, late differentiation is concurrent with decreased ability to produce IL-13 and/or IL-5 only, and with the appearance of IFN-γ/IL-13 (or IL-5) double-positive, CD56−/dim cells. The data support the conclusion that production of type 2 (IL-13 and IL-5) cytokines is transient and defines an intermediate stage of NK cell differentiation in this in vitro system. This is unlike production of TNF-α and GM-CSF, which are produced throughout differentiation.
Materials and methods
Monoclonal and polyclonal antibodies
Monoclonal antobodies (mAbs) to CD2 (B67.1, B67.6), CD4 (B66.6), CD5 (B36.1), CD8 (B116.1), CD11b (B43.4), CD14 (B52.1), CD15 (B40.9),44 CD16 (3G8),45 CD56 (B159.5),44 CD161/NKR-P1A (B199.2),21 TNF-α (B154.1, B154.9, nonneutralizing; B154.2, B154.7, neutralizing),10 and IFN-γ (B133.1, B133.3, neutralizing)46 were previously characterized in our laboratory. mAbs to CD3 (OKT3), CD21 (THB5), CD32 (IV.3), CD34 (My10), CD64 (32.2), and the irrelevant P3 × 63-Ag8.653 Ig were produced from cells obtained from the American Type Culture Collection (ATCC, Rockville, MD). mAb to CD94/NKG2 (HP-3B1)47 48 was kindly provided by Dr M. Lopez-Botet (Pompeu Fabra University, Barcelona, Spain). When indicated, mAbs were labeled with biotin or fluorescein isothiocyanate (FITC) according to standard procedures, after purification on protein G-Sepharose (Pharmacia Fine Chemicals, Uppsala, Sweden). Phycoerythrin (PE)–anti-CD56 (N901) and electron coupled dye (ECD)–anti-CD3 (UCHT1) were from Coulter (Beckman Immunotec, Marseille, France). FITC–anti–IFN-γ (B27), FITC–anti–GM-CSF (BVD2-21C11), FITC–anti-CD3 (S4.1), FITC-control mouse IgG1, and PE–anti–TNF-α (MP9-20A4) were from Caltag Laboratories (Burlingame, CA); PE–anti–IL-5 (TRFK5 or JES1-39D10), PE–anti–IL-13 (JES10-5A2) (the only available reagents to these cytokines), PE-rat IgG2a (R35-95) and PE-rat IgG1 (R3-34) were from Pharmingen (San Diego, CA). FITC–goat F(ab′)2-anti–mouse F(ab′)2 (GaMIg) (Cappel Laboratories, Durham NC) was used for indirect immunofluorescence, and the GaMIgs (produced in our laboratory) used for panning were adsorbed on human Ig-purified and affinity-purified mouse Ig-Sepharose before use. The rabbit IgG anti–sheep erythrocytes (E) used to prepare immune complex monolayers was from Organon-Teknika (Cappel).
Cell isolation
Lymphocytes were isolated from umbilical cord blood samples (provided by Dr R. Wapner, Department of Obstetrics and Gynecology, Thomas Jefferson University Hospital, Philadelphia, PA) collected at delivery from full-term pregnancies, and anticoagulated with heparin. Lin− cells were purified according to our previously published protocol21 after sequential depletion of (1) aminoethylisothiouronium bromide (AET)-E rosetting cells (AET, Sigma Chemical); (2) FcγR+ cells on rabbit IgG immune complex monolayers; and (3) most other mature leukocytes following panning (30 minutes, 4°C) on GaMIg-treated dishes after sensitization of the cells with the panel of mAbs to differentiation antigens on mature hematopoietic cells listed above. The Lin− cells were more than 99% CD3−/CD161−/CD16−/CD56−in immunofluorescence analysis; they were not cytotoxic, and did not express CD16 mRNA, as determined using reverse transcription polymerase chain reaction (RT-PCR)21 (and data not shown). When indicated, CD34+ cells were positively selected from cells at step (3) after sensitization with anti-CD34 (My10) mAb and panning as above.
Homogeneous immature CD161+/CD56− NK cell populations used in secondary cultures (see below) were purified from 30-day cultures of Lin− cells with IL-2 depleting (panning or fluorescence-activated cell sorting) CD3+/CD5+ T cells (if needed), CD32+/CD64+ myeloid cells, and CD94+/CD56+ mature NK cells after sensitizing the cells with a panel of mAbs to the indicated surface antigens. These cell populations were more than 99% CD3−/CD161+/CD56− in direct immunofluorescence. When indicated, total cell populations from primary cultures, and homogeneous NK cell populations from 10-day cultures of umbilical cord blood lymphocytes with 50 Gy irradiated RPMI-8866 cells as described,44 were used as a source of NK cells. The latter are referred to as 10-day NK cells.
Progenitor cell cultures
For primary cultures, Lin− or CD34+cells, as indicated, were incubated (37°C, humidified 8% CO2 atmosphere) in 24-well tissue culture plates (2 × 105 cells per well/mL RPMI-1640 medium [Biowhittaker, Walkersville, MD] supplemented with 10% heat-inactivated fetal bovine serum [FBS; Sigma Chemical]). When indicated, the murine bone marrow stromal cell line Sl/Sl4hSCF,220 expressing human mSCF49 (provided by Dr D. Williams, University of Indiana School of Medicine, Indianapolis, IN) was used as feeder, after irradiation (30 Gy). Alternatively, Flt-3/Flk-2 ligand (5 ng/mL; specific activity 3 × 106 U/mg; R&D Systems, Minneapolis, MN) was used in a feeder cell-free system. rIL-2 (50 U/mL; Hoffman-LaRoche, Nutley, NJ, obtained through the Biological Response Modifiers Program, National Cancer Institute, Bethesda, MD) and rIL-15 (10 ng/mL; specific activity 2.95 × 108 U/mg protein; provided by Immunex, Seattle, WA) and/or rIL-12 (2 ng/mL; specific activity 4.5 × 106 U/mg protein in an IFN-γ induction assay; provided by Dr S. Wolf, Genetics Institute, Andover, MA) were added at the beginning of the cultures and every 3 to 4 days during a 20- to 30-day culture period. The culture medium was partially replaced once a week, and nonadherent cells were subcultured when confluent.
For secondary cultures, 106 unseparated or CD3−/CD161+/CD56− NK cells from 30-day primary cultures of Lin− or CD34+ cells with IL-2 were cultured for 8 to 10 days in 24-well culture plates (2 mL medium/well). rIL-2, rIL-15, rIL-12 (concentrations indicated above), or rIL-4 (10 ng/mL, specific activity ≥ 107U/mg protein in a proliferation assay with CTLLhuIL-4R1.d cells; Genzyme, Cambridge, MA) and their combinations were added at the beginning of the culture and every 3 to 4 days. When indicated, 50 Gy–irradiated Daudi cells (5:1 lymphocyte-to-feeder cell ratio), anti–TNF-α and/or anti–IFN-γ mAb (ascites, 1:500 final dilution) were added throughout primary or secondary culture.
Intracellular cytokine detection
Cells were incubated (5 × 106/mL, 6 hours, 37°C) in medium with or without phorbol myristate acetate (PMA) (10−9 M) and Ca++ ionophore (A23187, 0.1 μg/mL) (all reagents from Sigma Chemical). Brefeldin A (10 μg/mL) was added during the last 3 hours. A Fix/Perm cell permeabilization kit (Caltag Laboratories, Burlingame, CA), or formaldehyde (3.7% in phosphate buffered saline [PBS], 10 minutes, room temperature) and 18-hour incubation in PBS containing 0.5% saponin, 0.2% FBS, 0.005% Tween 20, 0.01% NaN3, were used to fix and permeabilize the cells for intracellular cytokine detection combined with surface phenotyping as described in detail.50 Single-color and multiple-color (up to 4) immunofluorescence analyses (flow cytometry) were performed with the indicated FITC-, PE-, ECD-, or biotin-labeled mAbs, as described.21 FITC-labeled (Vector Laboratories, Burlingame, CA), PE-labeled (Becton Dickinson), R670-labeled (Gibco BRL, Gaithersburg, MD), or CyChrome-labeled (CyC, Pharmingen) streptavidin was used to detect biotin-labeled mAbs. Samples were analyzed on an EPICS Elite, a Profile II, or an XL-MCL automated flow cytofluorimeter (Beckman Coulter, Miami, FL). Listmode data were analyzed with WinMDI Flow Cytometry Application (J. Trotter, the Scripps Research Institute, La Jolla, CA,http://www.facs.scripps.edu/). When 4-color analysis was performed, the percentage of CD56− cells within the total CD161+ NK cells (including CD161+/CD56− and CD161+/CD56+ cells) was calculated, within the gated CD3− cells, as follows: (% CD161+cells − % CD56+ cells)/CD161+ cells. The proportion of cytokine-positive cells within these was calculated, taking into account the fraction of cytokine-positive CD56+cells within the total cytokine-positive CD161+ NK cells, using the following formula: X = [A − (B × C)]/(100 − C) × 100, where X indicates cytokine-positive cells within CD3−/CD161+/CD56− cells; A and B indicate percentage of cytokine-positive cells within gated CD3−/CD161+ and CD3−/CD56+ cells, respectively, and C indicates percentage of CD56+ cells within gated CD3−/CD161+ cells.
RT-PCR analyses
These were performed as previously described,21using total cellular RNA from cells (5 × 105/sample) incubated (5 × 106/mL, 2 hours, 37°C) in medium with or without combinations of IL-2, IL-12, and IL-15 (50 U/mL, 2 ng/mL, and 10 ng/mL, respectively) for stimulation. The conditions and primers used to detect β actin, IFN-γ, and TNF-α mRNA (Clontech Laboratories, Palo Alto, CA) have been reported.21 The IL-5 and GM-CSF primer sequences used were those defined in a previous report.51
Statistical analysis
Data were analyzed using the 2-tailed, paired Studentt test (Minitab statistical analysis software, State College, PA). Values of P < .05 were considered significant.
Results
Kinetics of CD56+ NK cell generation from Lin− cell cultures
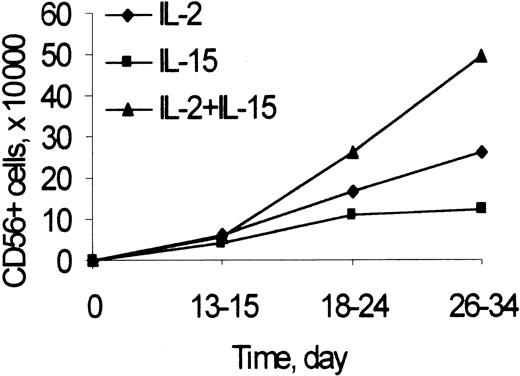
The number of CD3−/CD56+ NK cells was recorded at different time points during primary cultures of umbilical cord blood Lin− cells (including CD34+ and CD34− cells) with feeder cells and IL-2 or IL-15, alone or combined (Figure 1). Starting on the third week of culture and up to 34 days, at the last time point analyzed, the numbers of CD56+ NK cells generated from cultures with IL-2 and IL-15, IL-2, or IL-15 alone at the doses used were highest, intermediate, and lowest, respectively. The kinetics of generation of NK cells in cultures of CD34+ cells with Flt3-L and IL-2 were similar to those reported in Figure 1 (not shown).
CD56 expression to determine the kinetics of generation of NK cells from Lin− cells in cultures with IL-2 and IL-15.
Lin− cells (2 × 105/well) were cultured with Sl/Sl4hSCF220 cells and rIL-2 (50 U/mL), rIL-15 (10 ng/mL), or their combination, as described in “Materials and methods.” At the indicated times, the number of NK cells was calculated as the product of the number of viable cells recovered by the percentage of CD3−/CD56+ cells in 2-color immunofluorescence. Points are mean values from 3 to 20 experiments performed for each time and condition. In 26- to 34-day culture: IL-2 and IL-15 versus IL-2 or IL-15 alone, P < .05.
CD56 expression to determine the kinetics of generation of NK cells from Lin− cells in cultures with IL-2 and IL-15.
Lin− cells (2 × 105/well) were cultured with Sl/Sl4hSCF220 cells and rIL-2 (50 U/mL), rIL-15 (10 ng/mL), or their combination, as described in “Materials and methods.” At the indicated times, the number of NK cells was calculated as the product of the number of viable cells recovered by the percentage of CD3−/CD56+ cells in 2-color immunofluorescence. Points are mean values from 3 to 20 experiments performed for each time and condition. In 26- to 34-day culture: IL-2 and IL-15 versus IL-2 or IL-15 alone, P < .05.
In agreement with our previous data52CD3−/CD161+/CD56+ cells were not detected in cultures with IL-12 only, and NK cell generation in cultures with IL-2 or IL-15 was inhibited by IL-12 regardless of the presence of IFN-γ and/or TNF-α neutralizing mAbs (not shown).
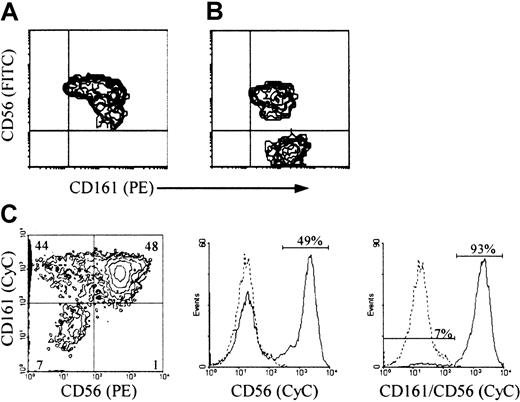
CD56 and CD161 expression in NK cells generated from progenitor cell cultures with IL-2 and IL-15
Most CD3−/CD161+ cells in primary cultures of Lin− cells with feeder cells and IL-15 were CD56+ (Figure 2A). Like the corresponding population in cultures with IL-2 and feeder cells,21 or Flt3-L and IL-2, variable proportions of these cells (lower than those in mature NK cells from the corresponding cord blood samples) expressed all other mature NK cell markers (CD2, CD8, CD16, CD94, and killer Ig-like receptors, not shown). All CD3−/CD56+ cells (herein referred to as CD56+ NK cells) from cultures with IL-2 and Flt3-L, like those from cultures with IL-2 and feeder cells (Figure 2B and Bennett et al21), were included in the CD161+population (Figure 2C), as confirmed independently in multiple-color and single-color immunofluorescence with anti-CD56 mAb alone or combined with anti-CD161 mAb. However, unlike the cultures with IL-15 and feeder cells, both cultures with IL-2 contained a significant proportion (up to 50%) of CD3−/CD161+/CD56− cells (herein referred to as CD56− NK cells). Most of these immature NK cells21 expressed CD7 (not shown) and CD161 at an average density higher than that on their CD56+ counterpart (Figure 2B-C).
CD161 and CD56 expression on CD3− NK cells from cultures of progenitor cells with feeder cells and IL-2 or IL-15, or Flt3-L and IL-2, to determine the conditions that support accumulation of immature CD56− NK cells.
(A, B) 2-color and (C) 3-color immunofluorescence was performed, with the indicated FITC- or PE-, and biotin-labeled mAb detected with streptavidin-PE or CyC, on cells generated from Lin− (A, B) or CD34+ cells (C) after 30-day culture with IL-15 (A) or IL-2 (B) and the Sl/Sl4hSCF220 feeder cells, or with Flt3-L and IL-2 (C), as described in “Materials and methods.” Correlate measurements of red and green fluorescence (x and y axis, respectively, log10 scale) are displayed as 2-dimensional contour plots. In C, analysis was performed on gated CD3− cells (CD3-FITC). The contours were divided into quadrants in which less than 0.5% control cells (treated with irrelevant isotype-matched mAbs) were included: top left, cells with green fluorescence (binding FITC-labeled Ab only); top right, double positive cells; bottom right, cells with red fluorescence (binding PE-labeled Ab only); bottom left, double-negative cells. Histograms in (C) are from samples treated with biotin-labeled anti-CD56 +/− anti-CD161 mAb detected with streptavidin-CyC on the same CD3− cells (dotted line, negative control; solid line, mAb+ cells; x axis, fluorescence intensity, y axis, relative cell number). The experiment in A and B is representative of 10, and that in C is representative of 3 performed with similar results.
CD161 and CD56 expression on CD3− NK cells from cultures of progenitor cells with feeder cells and IL-2 or IL-15, or Flt3-L and IL-2, to determine the conditions that support accumulation of immature CD56− NK cells.
(A, B) 2-color and (C) 3-color immunofluorescence was performed, with the indicated FITC- or PE-, and biotin-labeled mAb detected with streptavidin-PE or CyC, on cells generated from Lin− (A, B) or CD34+ cells (C) after 30-day culture with IL-15 (A) or IL-2 (B) and the Sl/Sl4hSCF220 feeder cells, or with Flt3-L and IL-2 (C), as described in “Materials and methods.” Correlate measurements of red and green fluorescence (x and y axis, respectively, log10 scale) are displayed as 2-dimensional contour plots. In C, analysis was performed on gated CD3− cells (CD3-FITC). The contours were divided into quadrants in which less than 0.5% control cells (treated with irrelevant isotype-matched mAbs) were included: top left, cells with green fluorescence (binding FITC-labeled Ab only); top right, double positive cells; bottom right, cells with red fluorescence (binding PE-labeled Ab only); bottom left, double-negative cells. Histograms in (C) are from samples treated with biotin-labeled anti-CD56 +/− anti-CD161 mAb detected with streptavidin-CyC on the same CD3− cells (dotted line, negative control; solid line, mAb+ cells; x axis, fluorescence intensity, y axis, relative cell number). The experiment in A and B is representative of 10, and that in C is representative of 3 performed with similar results.
Cytokine production by NK cells derived from progenitor cells
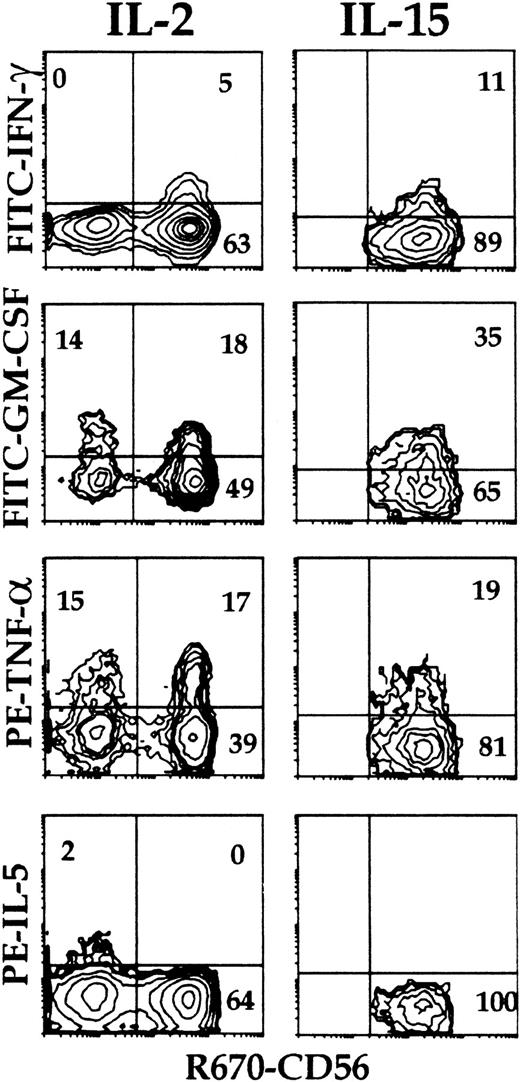
Cytokine expression and surface phenotype were analyzed simultaneously at the single-cell level (multiple-color immunofluorescence, flow cytometry) (Figure3) in cells from primary cultures of Lin− cells with feeder cells and IL-2, and from parallel cultures of cord blood lymphocytes with B-lymphoblastoid cell lines (10-day NK cells). Intracellular cytokines were not detectable in control, nonstimulated cells (not shown). Coexpression of IFN-γ, TNF-α, and GM-CSF was detected in approximately 75% of the umbilical cord blood CD3−/CD56+ mature NK cells from 10-day cultures (Figure 3, top) within 6 hours of stimulation. IL-5, when present, was detected in a minor (<1.0%) NK cell subset that did not produce IFN-γ but contained cells expressing GM-CSF. About 35% of the CD3−/CD161+ (including both CD56+ and CD56−) NK cells from primary cultures of Lin− cells with IL-2 and feeder cells expressed intracellular cytokines upon stimulation (Figure 3, bottom). Most if not all IFN-γ– and GM-CSF–expressing cells were included within those expressing TNF-α. A minor (<5%) cell subset expressed IL-5; this was significantly greater than that detectable in mature lymphocytes, was distinct from that producing IFN-γ, and overlapped only minimally with that producing GM-CSF.
Intracellular cytokine accumulation in CD161+ NK cells from cultures of Lin− cells with IL-2 to determine the proportion of cells capable of cytokine production, and cytokine production by distinct subsets.
Umbilical cord blood 10-day NK cells (top), and CD3−/CD161+ cells from 30-day primary cultures of Lin− cells with IL-2 and Sl/Sl4hSCF220 feeder cells (bottom) were stimulated (6 hours, 37°C) with PMA and Ca++ ionophore (see “Materials and methods”). Surface phenotype and expression of the indicated cytokines were detected simultaneously (3-color immunofluorescence) on gated CD161+ cells as described in “Materials and methods,” and analyzed as in Figure 2. Percent positive cells is indicated in each quadrant. Experiment representative of at least 4 performed with similar results with each Ab combination.
Intracellular cytokine accumulation in CD161+ NK cells from cultures of Lin− cells with IL-2 to determine the proportion of cells capable of cytokine production, and cytokine production by distinct subsets.
Umbilical cord blood 10-day NK cells (top), and CD3−/CD161+ cells from 30-day primary cultures of Lin− cells with IL-2 and Sl/Sl4hSCF220 feeder cells (bottom) were stimulated (6 hours, 37°C) with PMA and Ca++ ionophore (see “Materials and methods”). Surface phenotype and expression of the indicated cytokines were detected simultaneously (3-color immunofluorescence) on gated CD161+ cells as described in “Materials and methods,” and analyzed as in Figure 2. Percent positive cells is indicated in each quadrant. Experiment representative of at least 4 performed with similar results with each Ab combination.
Cytokine production by CD56− and CD56+NK cells
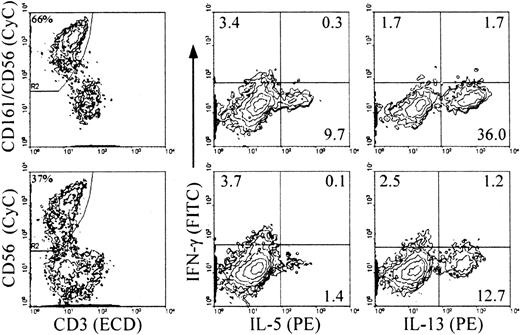
RT-PCR analysis on cells from cultures with IL-2 and feeder cells revealed inducible expression of IFN-γ mRNA only in the CD56+ cells, and constitutive expression of GM-CSF, TNF-α, and IL-5 in both mature CD56+ and immature CD56− cells21 (and data not shown). Analysis of intracellular cytokine expression upon stimulation indicated that about 30% of the NK cells from primary cultures with IL-2 and feeder cells (both CD56+ and CD56−) expressed TNF-α and GM-CSF (Figure 4). The levels of GM-CSF on a per cell basis were lower in the CD56+ cells than in the CD56− cells, as indicated by the mean fluorescence intensity (MFI) values in the 2 populations. IL-5 was detected in a small proportion of the CD56− cells. In NK cells from all primary culture conditions, IFN-γ+ cells were detected mostly, if not exclusively, in the CD56+subset (Table 1 and Figure 4, IL-2 and IL-15 with feeder cells; Table 2 and Figure 5, IL-2 and Flt3-L). Similar to the CD3−/CD161+ NK cells from cultures with feeder cells and IL-2, approximately 10% of those from cultures with Flt3-L and IL-2 expressed IL-5, and up to approximately 40% of them expressed IL-13 (Figure 5). The majority of these, like those of the IL-5+ cells, were included in the CD56− cells, and only a minor proportion of the CD56+ cells expressed IL-13 (Table 2). The percentage of cytokine+ NK cells detected with anti–IL-5 mAb added to the anti–IL-13 mAb was identical to that of the cells positive with the anti–IL-13 mAb alone, indicating that the IL-5–producing NK cells overlapped completely with those producing IL-13 (not shown). Although IL-5 and IFN-γ were produced independently, a minor proportion of IFN-γ+/IL-13+ cells was reproducibly detected in both the CD56+ and the CD56− cells (Figure 5).
Intracellular accumulation of IFN-γ, IL-5, TNF-α, and GM-CSF in CD56+ and CD56− NK cells from cultures of Lin− cells to determine the sequence with which the ability to produce type 1 and type 2 cytokines is acquired during differentiation.
Cells from primary cultures of Lin− cells with Sl/Sl4hSCF220 feeder cells and IL-2 (left panels) or IL-15 (right panels) were stimulated as in Figure 3. Intracellular cytokines and surface phenotype were analyzed simultaneously (3-color immunofluorescence) on gated CD161+cells within purified CD3− cells using FITC-, PE-, or biotin-labeled mAbs to the indicated molecules and streptavidin-R670. Quadrants were set to distinguish CD56+ and CD56− cells. Percent positive cells is indicated in each quadrant. Experiment representative of 4 performed with similar results.
Intracellular accumulation of IFN-γ, IL-5, TNF-α, and GM-CSF in CD56+ and CD56− NK cells from cultures of Lin− cells to determine the sequence with which the ability to produce type 1 and type 2 cytokines is acquired during differentiation.
Cells from primary cultures of Lin− cells with Sl/Sl4hSCF220 feeder cells and IL-2 (left panels) or IL-15 (right panels) were stimulated as in Figure 3. Intracellular cytokines and surface phenotype were analyzed simultaneously (3-color immunofluorescence) on gated CD161+cells within purified CD3− cells using FITC-, PE-, or biotin-labeled mAbs to the indicated molecules and streptavidin-R670. Quadrants were set to distinguish CD56+ and CD56− cells. Percent positive cells is indicated in each quadrant. Experiment representative of 4 performed with similar results.
Cytokine expression in CD56+ and CD56−, CD161+ natural killer cells generated in primary cultures of Lin− cells
| Culture conditions* . | CD161+cells† . | IFN-γ %‡ . | GM-CSF . | TNF-α . | IL-5 % . | ||
|---|---|---|---|---|---|---|---|
| % . | MFI1-153 . | % . | MFI . | ||||
| rIL-2 | CD56− | < 0.5* (6)1-155 | 31.3 ± 19.5 (4) | 3.7 ± 1.7 (4) | 25.7 ± 6.9 (4) | 5.1 ± 1.2 (4) | 5.5 ± 4.71-154 (4) |
| CD56+ | 6.9 ± 2.7 (6) | 11.6 ± 7.7 (4) | 2.8 ± 1.2 (4) | 16.8 ± 7.9 (4) | 4.7 ± 1.3 (4) | < 0.5 (4) | |
| rIL-15 | CD56+ | 8.7 ± 3.9 (3) | 18.6 ± 13.2 (3) | 1.4 ± 0.3 (3) | 19.0 ± 4.6 (3) | 3.4 ± 0.1 (3) | < 0.5 (3) |
| Culture conditions* . | CD161+cells† . | IFN-γ %‡ . | GM-CSF . | TNF-α . | IL-5 % . | ||
|---|---|---|---|---|---|---|---|
| % . | MFI1-153 . | % . | MFI . | ||||
| rIL-2 | CD56− | < 0.5* (6)1-155 | 31.3 ± 19.5 (4) | 3.7 ± 1.7 (4) | 25.7 ± 6.9 (4) | 5.1 ± 1.2 (4) | 5.5 ± 4.71-154 (4) |
| CD56+ | 6.9 ± 2.7 (6) | 11.6 ± 7.7 (4) | 2.8 ± 1.2 (4) | 16.8 ± 7.9 (4) | 4.7 ± 1.3 (4) | < 0.5 (4) | |
| rIL-15 | CD56+ | 8.7 ± 3.9 (3) | 18.6 ± 13.2 (3) | 1.4 ± 0.3 (3) | 19.0 ± 4.6 (3) | 3.4 ± 0.1 (3) | < 0.5 (3) |
IFN-γ indicates interferon gamma; GM-CSF, granulocyte macrophage–colony-stimulating factor; TNF-α, tumor necrosis factor alpha; MFI, mean fluorescence intensity.
The indicated cytokines were added to primary cultures of Lin− cells with Sl/Sl4hSCF220feeder cells, as described in “Materials and methods.”
Expression of the cytokines listed on top was analyzed (3-color immunofluorescence) in cells stimulated with PMA and Ca2+ ionophore. Analysis was performed on cells gated on the basis of their reactivity with anti-CD161 or anti-CD56 mAb, as described in “Materials and methods,” and Figure 3.
Percent positive cells (mean ± SD).
Fluorescence intensity (mean channel of fluorescence, mean ± SD).
Number of experiments.
Percentage positive cells in the CD56−population significantly different (P < .05) from that in the CD56+ population.
Expression of IL-13 and IL-5 in the CD56+ and CD56− natural killer cell subsets
| Culture conditions* . | (CD161/CD56)+cells† . | IFN-γ . | IL-5 . | IL-13 . |
|---|---|---|---|---|
| Flt3-L + IL-2 | CD56− | 2 ± 3‡ | 21 ± 92-153 | 74 ± 212-153 |
| CD56+ | 11 ± 9 | 1 ± 1 | 8 ± 6 |
| Culture conditions* . | (CD161/CD56)+cells† . | IFN-γ . | IL-5 . | IL-13 . |
|---|---|---|---|---|
| Flt3-L + IL-2 | CD56− | 2 ± 3‡ | 21 ± 92-153 | 74 ± 212-153 |
| CD56+ | 11 ± 9 | 1 ± 1 | 8 ± 6 |
IFN-γ indicates interferon gamma.
Flt3-L and IL-2 were added to primary cultures of CD34+ cells, as described in “Materials and methods.”
Expression of the cytokines listed on top was analyzed (4-color immunofluorescence) in cells stimulated as described in “Materials and methods.” Analysis was performed on gated CD3−/(CD161/CD56)+ and CD3−/CD56+ cells. The proportion of CD56+ cells within the CD161+ natural killer cells were 40% ± 25% (mean ± SD, n = 3). The proportion of cells producing cytokines within the CD161+/CD56− cells was calculated as described in “Materials and methods.”
Numbers are percent positive cells (mean ± SD, n = 3).
Percentage positive cells in the CD56−population significantly different (P < .05) from that in the CD56+ population.
Analysis of intracellular accumulation of IL-5 and IL-13 in CD161+ and CD56+ NK cells during differentiation to determine the stage(s) at which IL-13 is produced and whether the same or distinct NK cell subsets produce the 2 cytokines.
Cells from primary cultures of CD34+ cells with Flt3-L and IL-2 were stimulated as in Figure 3. Intracellular cytokines and surface phenotype were analyzed simultaneously in 4-color immunofluorescence with ECD–anti-CD3, biotin-labeled anti-CD56 alone (bottom) or combined with anti-CD161 (top) detected with streptavidin-CyC, FITC–anti–IFN-γ, and PE–anti–IL-5 or PE–IL-13 mAbs, as indicated. Analysis was performed, as indicated, on gated CD3−/CD56+ or CD3−/(CD161/CD56)+ cells (referred to as CD161+ NK cells in the text). Percent positive cells is indicated in each quadrant. Percentages in the left-hand plots are percent gated cells in the total population. Experiment representative of 3 performed with similar results.
Analysis of intracellular accumulation of IL-5 and IL-13 in CD161+ and CD56+ NK cells during differentiation to determine the stage(s) at which IL-13 is produced and whether the same or distinct NK cell subsets produce the 2 cytokines.
Cells from primary cultures of CD34+ cells with Flt3-L and IL-2 were stimulated as in Figure 3. Intracellular cytokines and surface phenotype were analyzed simultaneously in 4-color immunofluorescence with ECD–anti-CD3, biotin-labeled anti-CD56 alone (bottom) or combined with anti-CD161 (top) detected with streptavidin-CyC, FITC–anti–IFN-γ, and PE–anti–IL-5 or PE–IL-13 mAbs, as indicated. Analysis was performed, as indicated, on gated CD3−/CD56+ or CD3−/(CD161/CD56)+ cells (referred to as CD161+ NK cells in the text). Percent positive cells is indicated in each quadrant. Percentages in the left-hand plots are percent gated cells in the total population. Experiment representative of 3 performed with similar results.
Cytokine-induced differentiation of immature CD161+/CD56− NK cells
Cells from primary cultures of CD34+ cells with Flt3-L and IL-2 (either unseparated or depleted of mature CD56+ NK cells) were cultured with IL-2, IL-12, and Daudi feeder cells, conditions we previously demonstrated capable of supporting functional NK cell maturation, as indicated by the acquisition of IFN-γ mRNA expression.21 Intracellular cytokines and surface phenotype were analyzed simultaneously after stimulation (3- or 4-color immunofluorescence) in gated CD3−/CD161+(total) and CD3−/CD56+ (mostly mature) NK cells. The initial cell number was maintained throughout the culture. Within NK cells from nonseparated cultures (Table3, donors 1 and 2) there was a variable (range 2- to 20-fold) but consistent increase in the percentages of IFN-γ+ cells. These were detected both among the CD56+ and the CD56− subsets. Instead, the percentage of NK cells capable of producing IL-13 or IL-5 did not change, but a significant percentage of IFN-γ/IL-13 double-positive cells was detected in both CD56+ and CD56−cells. These results suggested that cells originally capable of producing only IL-13 had become capable of also producing IFN-γ, and that differentiation to mature IFN-γ+ cells involves an intermediate double-positive stage. To further analyze this, CD3−/CD161+/CD56− NK cells (purified to homogeneity from primary cultures and containing only approximately 0.1% and 52% of cells producing, respectively, IFN-γ and IL-13 exclusive of each other), were cultured with IL-2 and IL-12 with or without irradiated Daudi cells as feeder (Table 3, donor 3). After secondary culture, approximately 50% of the cells expressed CD56 and the proportion of IFN-γ+ cells increased to 7.6%. Whereas approximately 85% of the IFN-γ+ cells in the population that were still CD56− also produced IL-13, only about 50% of the IFN-γ+ CD56+ cells were capable of doing so. As in the cultures using total cells, the majority of the IL-13+ cells did not produce IFN-γ. In cultures with IL-2 and IL-12, alone or in combination, without feeder cells CD56 and IFN-γ expression was induced only in a minor proportion of the cells. Similarly, secondary cultures of the CD56− NK cells with IL-15 alone or with added IL-2, IL-4, and/or IL-12 induced minimal to no CD56 and IFN-γ expression (not shown). The data support the conclusion that IL-12, but none of the other cytokines tested, is needed, together with other cellular or soluble factors, to induce differentiation of CD161+/CD56− cells to cells capable of producing IFN-γ, and that this occurs gradually, concurrent with decreased ability to produce IL-13 and expression of CD56.
IL-5, IL-13, and IFN-γ accumulation in CD56+and CD56− natural killer cells from secondary cultures
| Donor3-150 . | Cells . | Day 0 . | IL-2 + IL-12 + Daudi, day 8 . | ||||
|---|---|---|---|---|---|---|---|
| IFN-γ+ . | IL-13+ . | IL-5+3-151 . | IFN-γ+(IL-13+, IL-5+) . | IL-13+ (IL-5+, IFN-γ+) . | IL-5+(IFN-γ+) . | ||
| 1. CD3− | Total | 23-152 | 42 | 16 | 40 (37, 8)3-153 | 54 (33, 28) | 18 (17) |
| CD56+3-155 | 4 | 9 | 2 | 39 (38, 5) | 56 (29, 27) | 16 (12) | |
| CD56− | 0 | 85 | 33 | 42 (38, 10) | 52 (40, 31) | 21 (19) | |
| 2. CD3− | Total | 18 | 7 | 2 | 42 (31, 2) | 27 (15, 48) | 4 (25) |
| CD56+ | 20 | 2 | 1 | 46 (30, 2) | 27 (15, 52) | 4 (25) | |
| CD56− | 1 | 50 | 12 | 12 (42, 8) | 22 (32, 23) | 7 (14) | |
| Donor3-150 . | Cells . | Day 0 . | IL-2 + IL-12 + Daudi, day 8 . | ||||
|---|---|---|---|---|---|---|---|
| IFN-γ+ . | IL-13+ . | IL-5+3-151 . | IFN-γ+(IL-13+, IL-5+) . | IL-13+ (IL-5+, IFN-γ+) . | IL-5+(IFN-γ+) . | ||
| 1. CD3− | Total | 23-152 | 42 | 16 | 40 (37, 8)3-153 | 54 (33, 28) | 18 (17) |
| CD56+3-155 | 4 | 9 | 2 | 39 (38, 5) | 56 (29, 27) | 16 (12) | |
| CD56− | 0 | 85 | 33 | 42 (38, 10) | 52 (40, 31) | 21 (19) | |
| 2. CD3− | Total | 18 | 7 | 2 | 42 (31, 2) | 27 (15, 48) | 4 (25) |
| CD56+ | 20 | 2 | 1 | 46 (30, 2) | 27 (15, 52) | 4 (25) | |
| CD56− | 1 | 50 | 12 | 12 (42, 8) | 22 (32, 23) | 7 (14) | |
| . | Cells . | Day 0 . | IL-2 + IL-12 + Daudi, day 9 . | IL-2 + IL-12, day 9 . | ||||
|---|---|---|---|---|---|---|---|---|
| IFN-γ+ . | IL-13+ . | IL-5+ . | IFN-γ+(IL-13+) . | IL-13+(IFN-γ+) . | IFN-γ+(IL-13+) . | IL-13+ (IFN-γ+) . | ||
| 3. CD3−/CD56− | Total | 0.1 | 51.7 | nd3-154 | 3.8 (79) | 55.4 (5.4) | 0.6 (50.0) | 57.7 (0.5) |
| CD56+ | —3-159 | — | — | 1.7 (53) | 37.2 (2.4) | 0.4 (0.0) | 3.1 (0.0) | |
| CD56− | 0.1 | 51.7 | nd | 5.8 (86) | 72.8 (6.9) | 0.6 (50.0) | 63.7 (0.5) | |
| . | Cells . | Day 0 . | IL-2 + IL-12 + Daudi, day 9 . | IL-2 + IL-12, day 9 . | ||||
|---|---|---|---|---|---|---|---|---|
| IFN-γ+ . | IL-13+ . | IL-5+ . | IFN-γ+(IL-13+) . | IL-13+(IFN-γ+) . | IFN-γ+(IL-13+) . | IL-13+ (IFN-γ+) . | ||
| 3. CD3−/CD56− | Total | 0.1 | 51.7 | nd3-154 | 3.8 (79) | 55.4 (5.4) | 0.6 (50.0) | 57.7 (0.5) |
| CD56+ | —3-159 | — | — | 1.7 (53) | 37.2 (2.4) | 0.4 (0.0) | 3.1 (0.0) | |
| CD56− | 0.1 | 51.7 | nd | 5.8 (86) | 72.8 (6.9) | 0.6 (50.0) | 63.7 (0.5) | |
For abbreviations, see Table 1.
Cells from primary cultures of CD34+ cells with Flt3-L and IL-2 from 2 independent donors (1 and 2) were cultured for 8 days in secondary culture with IL-12 (2 ng/mL), IL-2 (50 U/mL) and 50-Gy-irradiated Daudi feeder cells. CD161+/CD56− cells from similar cultures from a third donor were prepared by sorting, after sensitization with anti-CD3, anti-CD5, and anti-CD56 mAbs. Upon reanalysis, the proportion of CD161+ cells in this donor was more than 99%, with no detectable CD56+ cells. Intracellular cytokines and surface phenotype (CD56 or CD56 and CD161) were analyzed simultaneously before (day 0, 3-color immunofluorescence with anti-CD3, anti-CD56 alone, or with anti-CD161, and anti-IFN-γ, anti-IL-5, or IL-13 mAbs) and at the end of the culture (day 8 or 9, 4-color immunofluorescence with anti-CD3, anti-CD56 alone, or with anti-CD161, anti-IFN-γ, and anti-IL-5 or IL-13 mAbs) on cells stimulated as in Figure 4. Analysis was performed on gated CD3− cells.
All IL-5+ cells were always included within the IL-13+ population.
Figures are percent cells positive for the cytokine indicated above them.
Numbers in parentheses are the percentages of cells within the indicated cytokine+ population producing also the cytokines indicated in the parentheses above them.
The percentages of CD3−/CD56+cells within total CD161+ NK cells in donor 1 were 56 on day 0 and 53 on day 8; in donor 2 they were 90 on day 0 and 87 on day 8. On day 0, before secondary culture, more than 99% of the cells from donor 3 were CD3−/CD161+/CD56−; these contained no detectable CD56+ or IFN-γ+/IL-13+ cells. After a 9-day culture, the proportion of CD161+/CD56+ cells increased to 49% with IL-2 and IL-12 and Daudi and to 10% with IL-2 and IL-12 only.
Not done.
Not applicable (0% CD56+ cells).
Discussion
Using 3 in vitro models of hematopoietic cell differentiation of Lin− or CD34+ cells to analyze cytokine production at distinct stages of human NK cell differentiation and its regulation by IL-15 and IL-12, we present evidence that (1) like in cord blood, most CD56+ NK cells generated in these cultures produce TNF-α and GM-CSF, contain a subset of cells producing exclusively IFN-γ, a small proportion of cells producing IL-13 only, and none producing IL-5 at detectable levels; (2) IL-13 and IL-5 production, evident in immature CD161+/CD56− cells unable to produce IFN-γ, is lost gradually upon differentiation to phenotypically mature IFN-γ+ cells, indicating that IFN-γ and IL-13 and/or IL-5 production, characterize, respectively, final and intermediate stages of functional NK cell differentiation; (3) IL-12, likely together with other factors, supports terminal functional maturation of the IFN-γ− CD56− NK cells, as indicated by the appearance of a significant proportion of both IFN-γ+/IL-13+/IL-5+ cells and cells producing only IFN-γ in cultures of the immature NK cells with this cytokine and feeder cells; and (4) IL-2, IL-4, and IL-15 alone support survival/proliferation, but not differentiation, of the CD56− NK cells, as indicated by the lack of significant changes in cytokine production in secondary cultures with these cytokines without (this report) or with feeder cells.21
NK cell differentiation in vivo is mediated, in part, by IL-15, produced by stromal/myeloid cells,53 and NK cells are not generated in mice lacking IRF-1,22,23 essential for induced expression of IL-15. In agreement with previous reports using different culture systems and bone marrow–derived or umbilical cord blood–derived progenitor cells,27,28,30 IL-15 supports the generation of phenotypically mature NK cells in all 3 systems used, and our data show that the relative proportion and phenotype of the cytokine-producing NK cell subsets in either condition are similar to those identified in peripheral blood. However, immature CD161+/CD56− NK cells are not generated in cultures with IL-15. This may depend on the inability of IL-15 to induce their proliferation, and consequent detectable accumulation, whereas IL-2 may be directly or indirectly mitogenic for the same cells. Supporting this possibility, IL-15 maintains the CD161+/CD56− cells without inducing their proliferation in secondary cultures. However, we cannot exclude that IL-15 has a more pronounced differentiation-inducing effect than IL-2, resulting in faster differentiation and consequent difficulty in experimentally identifying cells at intermediate stages of differentiation. Alternatively, progenitor cells at different stages of maturation may be differentially susceptible to the 2 cytokines, like in the murine system,25 54 where distinct growth factors act on cells at different stages of maturation. Undefined cytokines contributing to differentiation of earlier NK cell progenitors may be produced endogenously in response to IL-2 by other (eg, myeloid) cells and be depleted at later times during culture, and/or receptors for IL-15 may not be expressed on these cells. Finally, the accumulation of immature NK cells in the cultures with IL-2 may be only apparent and due to susceptibility of the mature cells to death induced by membrane-bound or soluble factors expressed by the NK or other cells in the cultures in response to IL-2 but not, or at lower and ineffective levels, to IL-15.
Whatever the reason for our observation, the results of the studies on the CD161+/CD56− NK cells generated necessarily from cultures with IL-2 indicate that in vitro systems containing this cytokine are appropriate for studying the origin and significance of NK cell subsets in the peripheral blood. Likely because of the sharing of the β and γc signal transduction subunits of its receptor,53,55 IL-2 substitutes for IL-15 in vitro to study NK cell differentiation in the murine system.54 A CD161+/CD56− NK cell subset and IL-13+ NK cells have been detected, although in minor proportions, in peripheral adult and neonatal blood19,21; and NK cells producing high levels of IL-13 and IL-5, with correspondingly lower IFN-γ levels, can be expanded/induced from peripheral NK cells.56 Finally, although IL-2 is unlikely to be a growth factor for NK cells in physiologic conditions, it may influence differentiation of NK cells from their progenitors in the bone marrow under pathologic conditions in which IL-2–producing activated T cells are present, or in the peripheral blood during infection, once specific activated T cells have been generated.
As previously reported,52 IL-12 alone does not support NK cell differentiation although, in concert with other growth/differentiation factors, it supports differentiation of hematopoietic progenitors (preferentially myeloid) both in vitro57-60 and in vivo,61 and affects later stages of differentiation of the CD56− NK cells generated in cultures with IL-2.21,52 The inability of IL-12 to support IL-2 and IL-15 in allowing NK cell differentiation in vitro, and the observation that NK cells are generated, although functionally impaired, in IL-12 p40−/− mice,62adds to the contention that this cytokine does not participate in early steps of NK cell differentiation. The inhibitory effect of IL-12 indicates a negative regulatory effect of this cytokine. This may depend on preferential induction of differentiation or proliferation of immature myeloid cells, and may be mediated, in part, via induced TNF-α production and/or TNF-R1 expression.63-65 However, neutralization of TNF-α or IFN-γ, and addition of either cytokine to IL-2 or IL-15 (not shown) had no significant effects on NK cell differentiation, and direct or indirect inhibitory effects of IL-12 (eg, induction of Fas/FasL and consequent apoptosis) on progenitors or differentiating NK cells cannot be excluded.
Our data indicate that most immature CD161+/CD56− cells produce IL-13, and a discrete subset of them coexpresses IL-5, in the absence of IFN-γ. Interestingly, it has been recently reported that an asialoGM-1+, DX5+, non-T, non-NK/T, nonmature NK cell type found in association with immature B cells in the bone marrow66 is responsible for protecting these cells from antigen-induced, apoptosis-mediated deletion. Given the phenotype reminiscent of the human immature IL-13+ NK cells we report here, and the antiapoptotic effects of IL-13 on B cells,67it will be important to determine whether immature bone marrow–resident NK cells play a role to control B-cell selection. Additionally, from our data, the suggestion can be made that resting IL-5+ or IL-13+ peripheral blood NK cells may represent NK cells that exited the bone marrow at relatively late, although not final, stages of differentiation.
A major proportion of cells with phenotype (CD56 and other differentiation antigens) and functions (IFN-γ, but not IL-13 production) of mature NK cells and, importantly, CD56−/dim/IL-13+/IFN-γ+ NK cells with intermediate phenotype, are generated from the CD161+/CD56− cells derived from both in vitro culture models containing IL-2 after switch to culture conditions including IL-12 and B lymphoblastoid cells as feeder. This occurs both using total NK or purified CD161+/CD56− NK cells. Also, similar numbers of cells were recovered from the cultures without feeder cells (not shown), and cytokines alone did not support significant changes in cytokine production. Thus, it is unlikely that the cells with mature or intermediate phenotype derive from expansion of minor contaminants in the original population. We favor the interpretation that type 2 and type 1 cytokine production is a functional characteristic of NK cells at distinct stages, rather than of distinct subsets of cells at an identical stage of differentiation. Unlike IL-221 (and IL-15, reported here), IL-12 in the presence of feeder cells promotes terminal functional differentiation of the immature CD161+/CD56− NK cells, as previously proposed based on induced expression of IFN-γ mRNA and CD56.21 Unlike IFN-γ and type 2 cytokine production, that of GM-CSF and TNF-α does not define distinct stages of differentiation, although the lower levels of GM-CSF detected on a per cell basis in the CD56+ cells suggest its decreased production during differentiation.
Only a fraction of the CD56+ NK cells from the primary cultures were induced to express IFN-γ, similar to what is observed, with interdonor variability, in peripheral blood NK cells. The observation that only a proportion of the CD161+/CD56− cells differentiate under the appropriate conditions most likely depends on lack of additional factors. NK cell functions are impaired in vivo (decreased cytotoxicity and lower IFN-γ production by either T or NK cells) in mice with a disrupted IL-12R β1 chain gene68 and in those lacking IL-18.69 Further functional impairment is observed in the NK1.1 (NKRP-1A, CD161)+ NK cells from IL-12 p40−/−/IL-18−/− mice,69paralleling our in vitro data. This suggests that together with IL-12, IL-18 (and possibly other factors) may be involved in the functional final maturation of NK cells to IFN-γ production. It will be important to determine whether peripheral NK cells in the IL-12 p40−/−/IL-18−/− mice are arrested at a stage of differentiation equivalent to that of the CD161+/CD56− NK cells reported here.
IL-12 with IL-2 or IL-15 alone (the latter 2 likely needed to support survival/proliferation) induce CD56 expression at low density and IFN-γ in a minor proportion of the cells, without concomitant decrease in the proportion of IL-5+ and IL-13+cells. The observation that most IL-13+ cells under these conditions are still CD56− supports the hypothesis that cytokines alone are incompletely effective to support differentiation of the CD161+ NK cells. No significant changes were detected, at the times analyzed, in the proportion of IL-13+ and IL-5+ cells in cultures with IL-4 (not shown). In keeping with the data of Warren et al in mature NK cells,20 IL-4 may support survival and/or proliferation, rather than differentiation, of the relatively immature CD161+/CD56− NK cells.
Our data support the conclusion that the ability to produce IFN-γ is acquired by NK cells at intermediate to late stages of differentiation, concomitant with the shut-off of the type 2 cytokines IL-5 and IL-13 specifically and irreversibly induced by IL-12 and possibly other cytokines. We have recently demonstrated that, like in the 2 culture systems that recapitulate and mimic the bone marrow environment (including culture of progenitor cells with IL-2), the CD161+/CD56− NK cells detectable in peripheral blood produce IL-13, but not IFN-γ. These cells undergo IL-12–induced differentiation to IFN-γ+/IL-13−/CD56+ NK cells, transiting through an IL-13+/IFN-γ+ stage at which the cells start expressing CD56.70 Thus, the 2 in vitro systems dissected here will be invaluable in future studies of the cytokine-mediated and molecular regulation of the differentiation and functions of NK cells at stages preceding or following the intermediate CD161+/IL-13+ stage.
We thank Dr R. Wapner and the staff in the Obstetrics and Gynecology Department of Thomas Jefferson University Hospital for providing the umbilical cord blood samples, Mr B. Abebe for technical assistance, and Mr D. Dicker and P. Hallberg for assistance with flow cytometry.
Supported, in part, by USPHS grants CA45284 (B.P.) and T32-CA09683 (M.J.L.); and by a grant from CNR (Italy), posizione 12115645, and the MURST Funds 1997, Project “Meccanismi immunologici di resistenza alle neoplasie” (E.R.).
M.J.L. and L.Z. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bice Perussia, Jefferson Medical College, Kimmel Cancer Center, BLSB Room 750, 233 S 10th St, Philadelphia, PA 19107; e-mail: bice.perussia@mail.tju.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal