The prophylaxis of the hemolytic disease of the newborn requires significant amounts of plasma-derived polyclonal human anti-D. Because of procurement problems, there is a growing interest in replacing plasma-derived anti-D by in vitro–produced human monoclonal anti-D. Hundreds of monoclonal anti-D have been prepared, but the selection of the most potent for in vivo use is difficult because it cannot be predicted by in vitro characterization. This study evaluated the possibility of using nonobese diabetic/severe combined immunodeficient (NOD-scid) mice for the in vivo evaluation of human monoclonal anti-D. Human red blood cells (RBCs) were found to circulate normally in the blood of NOD-scid mice previously injected with a physiologic amount of human immunoglobulin G (10 mg). The addition of a small amount of anti-D (1 to 5 μg) resulted in the clearance of Rh D+RBCs within 4 hours. The comparative testing of 8 monoclonal anti-Ds showed a wide range of potency (15% to 87%) relative to plasma-derived polyclonal anti-D. There was no strong correlation between the in vivo potency index and the immunoglobulin G isotype, affinity, or fine specificity of the antibodies. These results show the usefulness of NOD-scid mice for the initial in vivo screening of human monoclonal anti-D before testing the most active antibodies in clinical trials done in human volunteers.
Introduction
Human monoclonal antibodies of various specificities can now be prepared by using cellular or molecular technologies. Several of these antibodies could have interesting therapeutic applications, such as human monoclonal anti-D that could be used to replace plasma-derived polyclonal antibodies for the prevention of the hemolytic disease of the newborn.1 Contrary to plasma-derived anti-D preparations that rely on the availability of immunized donors, monoclonal anti-D prepared by culture of immortalized cell lines give access to an unlimited supply of standardized preparations. However, as is the case for other antibody specificities, the selection of the most appropriate monoclonal anti-D for therapeutic use represents a significant challenge.
The mechanism by which anti-Ds suppress Rh D immunization is still unclear. Some of the proposed mechanisms rely on the interaction between the Fc portion of anti-D and different Fcγ receptors (FcγR) and could lead either to the inhibition of antigen-specific B cells by the binding of antigen-antibody complexes or to the rapid clearance and destruction of the Rh D+ red blood cells (RBCs) coated with anti-D.2 These 2 mechanisms are not mutually exclusive, and both require the efficient binding of the antibodies to the Rh D+ RBCs in vivo. However, work with FcγR-deficient mice suggested that immune suppression could occur by antigenic epitope masking and thus be independent of the Fc portion of immunoglobulin G (IgG).3 Although the mechanism of inhibition of immune response is still unresolved, it is likely that the anti-D preparations suitable for the prevention of the hemolytic disease of the newborn will be among those exhibiting the most efficient antigen binding in vivo.
Because of obvious ethical problems in studying Rh D+ RBC destruction in Rh D− individuals, the biologic activity of monoclonal anti-D has been evaluated mainly by using in vitro tests intended to reproduce the processes involved in antibody-dependent in vivo RBC destruction. Clearance of RBCs coated with antibodies may depend to a large extent on the interaction of antibodies with the FcγRs present on macrophages in the spleen.4 However, because splenic macrophages are difficult to obtain, only indirect assessment of antibody-FcγR interactions can be done in vitro by using natural killer cells to measure FcγRIII-mediated interactions5,6 and monocytes to measure FcγRI/II-mediated interactions.7,8 One of the main problems associated with in vitro tests is that the results of these assays will vary, depending on a number of parameters that are likely to differ from the in vivo situation (eg, RBC-phagocyte ratio, degree of RBC sensitization, etc). Thus, the suitability of these in vitro assays to predict the ability of monoclonal anti-D to prevent Rh D alloimmunization has not been demonstrated.9 10
In the past 10 years, the isolation of immunodeficient mouse strains has opened new possibilities for the early in vivo testing of human proteins and cells. The nonobese diabetic/severe combined immunodeficient (NOD-scid) mice exhibit a combination of immunologic defects, including a lack of functional lymphoid cells, no serum immunoglobulin, low natural killer cell activity, and lack of hemolytic complement activity. These defects make them particularly good recipients for xenogenic cells and tissues.11 For example, NOD-scid mice have been used to study the human hematopoietic progenitor development,12-14 HIV pathogenesis,15-17 and human leukemic cell biology and treatment.18,19 We selected these mice for this study because it has been shown that human RBCs could circulate for several days in the peripheral blood of NOD-scid mice but not in other immunodeficient strains of mice.20
In this work, we have used NOD-scid mice to compare the ability of several human monoclonal anti-Ds to mediate Rh D+ RBC clearance in vivo. The use of NOD-scid mice to study the in vivo clearance of human RBCs depends on 3 parameters. The first parameter is the ability to detect human RBCs in the peripheral blood of NOD-scid mice for a sufficient period of time, and the second parameter is the ability of the mouse cells to phagocytose RBCs coated with human antibodies. Finally, the antibody content of the mouse serum should mimic as closely as possible the situation in humans. Indeed, it has been observed many years ago that the rate of IgG catabolism in humans is proportional to the serum IgG concentration.21,22 Our previous study with immunodeficient mice also showed the strong correlation between antibody concentration and catabolism rate.23 Because NOD-scid mice do not have immunoglobulins in their serum, all animals used in this study were injected with human IgGs to reconstitute a serum immunoglobulin level comparable to normal. The results obtained showed the suitability of NOD-scid mice for comparing the ability of anti-D to mediate RBC clearance and indicated that the relative in vivo potency of the various monoclonal anti-Ds is not directly related to the antibody IgG subclass, agglutination titer, and fine specificity.
Materials and methods
Mice
Male or female 4- to 6-week-old NOD.CB17-Prkdcscid/J (formerly known as NOD/LtSz-scid) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The animals were kept in a pathogen-free environment and fed with sterilized food pellets and water. All manipulations involving the animals were performed by trained technicians at the Service des Animaux de Laboratoire, Laval University (Quebec, Canada). The absence of mouse immunoglobulins was evaluated by enzyme-linked immunosorbent assay (ELISA) as described,23 and mice having murine immunoglobulin levels higher than 10 μg/mL were excluded from the study.
Antibodies and F(ab′)2 fragments
The human monoclonal anti-Ds were prepared from culture supernatants of heterohybridoma cells cultured in Iscoves modified Dulbecco media (Gibco BRL, Life Technologies, Ontario, Canada) supplemented with 5% Low IgG fetal bovine serum (Hyclone Laboratories, Logan, UT) at 37°C in a humid atmosphere containing 10% CO2. The characteristics of the anti-Ds used in this study were described elsewhere23,24 and are summarized in Table1. The anti-Ds were purified from the culture supernatants by chromatography on Protein G-agarose (Life Technologies), and the IgG concentrations were determined with the Bradford protein assay (Bio-Rad, Ontario, Canada) or by ELISA as previously described.23 F(ab′)2 fragments of anti-D were prepared by digestion with immobilized pepsin (300 U pepsin/mg anti-D; Pierce, Rockford, IL) at pH 4.5 for 4 hours at 37°C. The preparation was passed over a protein A-agarose (Life Technologies) column to remove the undigested material. The F(ab′)2 fraction was tested for the presence of contaminating undigested IgG by indirect Rh D+RBC agglutination by using an antihuman IgG (Fc-specific) monoclonal antibody (C5-1) developed in our laboratory.25 The removal of the Fc fragment was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis of the F(ab′)2 fragments. However, the F(ab′)2 fragments were functional because they could agglutinate enzyme-treated Rh D+ RBCs (titer of 64 compared with 512 for undigested anti-D, when tested at 2 μg/mL). All antibody solutions used were dialyzed against phosphate-buffered saline, sterile filtered, and stored frozen in aliquots until used.
In vitro and in vivo characteristics of monoclonal and polyclonal anti-D
| Anti-D . | IgG subclass . | Agglutination titer* . | Epitope specificity . | Dose tested in vivo, μg . | Relative in vivo clearance potency, % (± SD) . |
|---|---|---|---|---|---|
| Polyclonal | IgG1/IgG3 | 512 | ND | 5 | 100 |
| 13E10 | IgG1 | 32 | 1† | 5 | 50 ± 23 |
| 25F5 | IgG1 | 256 | 6/7 | 5 | 86 ± 8 |
| 21H9 | IgG1 | 256 | 6/7† | 5 | 61 ± 15 |
| 46E7 | IgG1 | 32 | 2 | 5 | 87 ± 9 |
| 19A5 | IgG1 | 32 | 6/7† | 5 | 15 ± 19 |
| 10 | 50 ± 28 | ||||
| 20 | 47 ± 8 | ||||
| 13D2 | IgG3 | 256 | 1† | 5 | 42 ± 16 |
| 24A5 | IgG3 | 64 | 2† | 5 | 72 ± 20 |
| 26C1 | IgG3 | 256 | 6/7 | 1 | 62 ± 21 |
| 5 | 82 ± 11 | ||||
| 10 | 96 ± 14 | ||||
| Blend‡ | 5 | 82 ± 4 | |||
| 10 | 92 ± 3 |
| Anti-D . | IgG subclass . | Agglutination titer* . | Epitope specificity . | Dose tested in vivo, μg . | Relative in vivo clearance potency, % (± SD) . |
|---|---|---|---|---|---|
| Polyclonal | IgG1/IgG3 | 512 | ND | 5 | 100 |
| 13E10 | IgG1 | 32 | 1† | 5 | 50 ± 23 |
| 25F5 | IgG1 | 256 | 6/7 | 5 | 86 ± 8 |
| 21H9 | IgG1 | 256 | 6/7† | 5 | 61 ± 15 |
| 46E7 | IgG1 | 32 | 2 | 5 | 87 ± 9 |
| 19A5 | IgG1 | 32 | 6/7† | 5 | 15 ± 19 |
| 10 | 50 ± 28 | ||||
| 20 | 47 ± 8 | ||||
| 13D2 | IgG3 | 256 | 1† | 5 | 42 ± 16 |
| 24A5 | IgG3 | 64 | 2† | 5 | 72 ± 20 |
| 26C1 | IgG3 | 256 | 6/7 | 1 | 62 ± 21 |
| 5 | 82 ± 11 | ||||
| 10 | 96 ± 14 | ||||
| Blend‡ | 5 | 82 ± 4 | |||
| 10 | 92 ± 3 |
IgG indicates immunoglobulin G; ND, not done.
Antibody concentration adjusted to 1 μg/mL.
Indicates probable epitope specificity.
Blend of 26C1, 46E7, and 25F5 (1:1:1).
Serologic tests
The agglutination titer of the anti-Ds used in this study was determined by using the indirect agglutination assay.26Briefly, the anti-D was diluted serially in saline containing 1% bovine serum albumin (100 μL) and was added to 50 μL of a 3% Rh D+ RBC suspension. After incubation (45 minutes at 37°C), the RBCs were washed with saline, and the antihuman globulin reagent (100 μL; Dominion Biological Limited, Nova Scotia, Canada) was added. The tubes were centrifuged for 15 seconds at 700 RPM (Automatic Centrifuge, Dade, Miami, FL). The agglutination titer was defined as the reciprocal of the last dilution giving macroscopic agglutinates (1+ reaction). A commercial preparation of polyclonal anti-D was used as a reference.
RBC labeling and conservation
RBCs were obtained from volunteer donors in acid citrate dextrose Vacutainer tubes (Becton Dickinson, Ontario, Canada). Because we did not observe differences in RBC survival in NOD-scid mice by using RBCs of different ABO blood groups, the donors were not selected according to their ABO blood group. The RBCs were labeled with chromium-51 (51Cr) by using standard techniques.27 Briefly, RBCs were washed in citrate-dextrose solution (110 mM sodium citrate, 10 mM dextrose, 1 mM sodium phosphate, pH 6.8), and 150 μL packed RBCs were mixed with 11.1 MBq (300 μCi) 51Cr (Amersham Pharmacia Biotech, Quebec, Canada) and incubated at room temperature for 1 hour. The RBCs were then washed twice with saline to remove unincorporated51Cr. Finally, RBCs were suspended in saline at a final 40% concentration for injection into the animals.
Anti-D–mediated RBC clearance in vivo
To determine the antibody-mediated RBC clearance, groups of 3 animals were first injected intraperitoneally with a volume of 250 μL containing 10 mg human IgG (IVIg 5%; Bayer, Ontario, Canada) and 5 μg of the antibodies to be tested, unless otherwise indicated. After 18 to 24 hours, the animals received 100 μL of the radiolabeled RBC suspension by injection into the tail vein. Blood was collected by retroorbital bleeding, starting 15 minutes after the intravenous injection and at a maximum of 3 different time points thereafter. A volume of 150 μL was collected in microtubes already containing a small amount of saponin to lyse RBCs, and aliquots of 100 μL were counted in a Gamma Counter to determine the amount of radiolabeled RBCs in the peripheral blood of the animals.
Results
Human RBC survival in NOD-scid mice
To reconstitute a serum immunoglobulin level comparable to normal animals, all NOD-scid mice used in this study were injected with 10 mg human IgGs (about 4 mg/mL IgG with a blood volume of 2 to 3 mL).28 Human intravenous IgGs was prepared from pools of plasma obtained from thousands of donors and, thus, contained immunoglobulins with a very wide range of specificities. We first determined whether these human IgGs could by themselves promote clearance of human RBCs in NOD-scid mice, because this would interfere with the subsequent evaluation of the biologic activity of anti-D. The survival of human RBCs in NOD-scid mice in the presence or absence of 10 mg human IgGs was evaluated in groups of animals injected (intraperitoneally) or not with 10 mg human IgGs. It is known that the IgG distribution from the peritoneal cavity to the vascular compartment reaches equilibrium 8 hours after intraperitoneal injection.23 However, for logistical reasons, RBC survival was determined 18 to 24 hours after human IgG injection. RBCs were injected into the tail vein of the animals. In initial experiments, a volume of 100 μL containing 5 μL packed 51Cr-labeled Rh D+ RBCs was injected into each animal. This volume of injected RBCs was similar to the volume used in clinical trials of anti-D in humans,29 taking into account the difference in blood volume between the 2 species. However, we found that the proportion of injected 51Cr-labeled RBCs that was present in the NOD-scid mouse circulation was low (data not shown), suggesting a nonspecific clearance mechanism. To overcome this problem, the labeled Rh D+ RBCs (5 μL) were mixed with a larger volume (35 μL) of unlabeled Rh D− RBCs before injection. This approach resulted in an increase in the proportion of injected51Cr-labeled RBCs found in the animal circulation (80% on average) and in more consistent results between animals from the same experimental group (data not shown). After RBC injection, blood samples were collected at various intervals, starting at 15 minutes. This sample represented the starting point (100% survival). Samples were also taken 90 and 240 minutes after the initial bleeding. The results obtained are shown in Figure 1. A rapid decrease in circulating 51Cr-labeled RBCs was observed during the first hour after injection. The rate of clearance decreased slowly thereafter, and about 50% of the injected RBCs were still in the circulation 4 hours after the injection. The small reduction in RBC survival between 1 and 4 hours after injection (about 15%) could be due in part to the volume of collected blood (150 μL) relative to the total blood volume of the mice (about 3 mL). This could result in a reduction of the hematocrit at the late time points and an apparent decrease in RBC survival. The results in Figure 1 also showed that the amount of 51Cr-labeled RBCs in NOD-scid mouse circulation was similar over a 4-hour period in animals injected or not with human IgGs, indicating that human IgGs do not interfere with human RBC survival in NOD-scid mice.
Human IgGs do not affect human RBC survival in NOD-scid mice.
Groups of 3 NOD-scid mice were injected with (○) or without (●) 10 mg human IgGs 18 hours before injection of a volume of 100 μL containing 5 μL 51Cr-labeled Rh D+ RBCs and 35 μL unlabeled Rh D− RBCs in saline. At the indicated times, the amount of circulating 51Cr-labeled RBCs was determined as described in “Materials and methods.”
Human IgGs do not affect human RBC survival in NOD-scid mice.
Groups of 3 NOD-scid mice were injected with (○) or without (●) 10 mg human IgGs 18 hours before injection of a volume of 100 μL containing 5 μL 51Cr-labeled Rh D+ RBCs and 35 μL unlabeled Rh D− RBCs in saline. At the indicated times, the amount of circulating 51Cr-labeled RBCs was determined as described in “Materials and methods.”
Antibody-mediated Rh D+ RBC clearance in NOD-scid mice
The effect of polyclonal and monoclonal anti-Ds on RBC clearance was tested in the above system. A dose of 5 μg of a plasma-derived polyclonal anti-D preparation was used, which was estimated to be almost in excess considering the low volume of 51Cr-labeled Rh D+ RBCs injected (5 μL). Groups of 3 NOD-scid mice were thus injected with 10 mg of human IgGs containing or not 5 μg of polyclonal anti-D, 16 to 18 hours before the intravenous injection of RBCs. The results obtained are shown in Figure2. In animals injected with human IgGs only, about 40% to 50% of the injected RBCs were still in the peripheral blood of NOD-scid mice 4 hours after injection, whereas less than 5% of the injected Rh D+ RBCs were detected at that time point in animals treated with human IgGs and polyclonal anti-D. These results indicated that antibody-mediated human RBC clearance occurred in NOD-scid mice. Anti-D–mediated RBC clearance was highly reproducible in different experiments. Indeed, in a series of 10 experiments done over a 6-month period, the mean RBC survivals at 0.5, 2.0, and 4.0 hours were, respectively, 35% ± 9%, 11% ± 3%, and 5% ± 2%.
Anti-D–mediated RBC clearance is easily detected in NOD-scid mice.
Groups of 3 NOD-scid mice were injected with 10 mg human IgGs only (○), or in the presence of 5 μg intact anti-D (■) or 5 μg F(ab′)2 fragments of polyclonal anti-D (▪). RBCs were prepared and injected as in Figure 1. RBC survival was measured as described in “Materials and methods.”
Anti-D–mediated RBC clearance is easily detected in NOD-scid mice.
Groups of 3 NOD-scid mice were injected with 10 mg human IgGs only (○), or in the presence of 5 μg intact anti-D (■) or 5 μg F(ab′)2 fragments of polyclonal anti-D (▪). RBCs were prepared and injected as in Figure 1. RBC survival was measured as described in “Materials and methods.”
In the same experiment, we also determined if the antibody-mediated RBC clearance was Fc-dependent. This determination was done by using F(ab′)2 fragments of polyclonal anti-D prepared by pepsin digestion. The results obtained (Figure 2) showed that RBC survival was similar in animals injected with human IgGs only or with human IgGs containing F(ab′)2 fragments of polyclonal anti-D, indicating that only whole IgG molecules were able to mediate RBC clearance. To rule out the possibility that this result could be due to a shorter half-life of F(ab′)2 fragments in NOD-scid mice, we first titrated the anti-D present in the mouse serum 18 hours after injection, using enzyme-treated RBCs to allow direct agglutination by F(ab′)2 fragments and whole anti-D. Results obtained showed that anti-D activity could be detected in the serum of mice injected with F(ab′)2 fragments (titer of 32) and with whole anti-D (titer of 512). This difference in titer was similar to the one measured in the 2 antibody preparations used to inject the animals. The involvement of Fc regions was also assessed by injecting NOD-scid mice with normal Rh D+ RBCs previously sensitized in vitro with whole anti-D or F(ab′)2 fragments. The results obtained (data not shown) were similar to the ones shown in Figure 2(injection of anti-D 18 hours before RBCs). Altogether, these observations indicated that the anti-D–mediated RBC clearance was an Fc-dependent process in NOD-scid mice.
Monoclonal anti-D–mediated RBC clearance
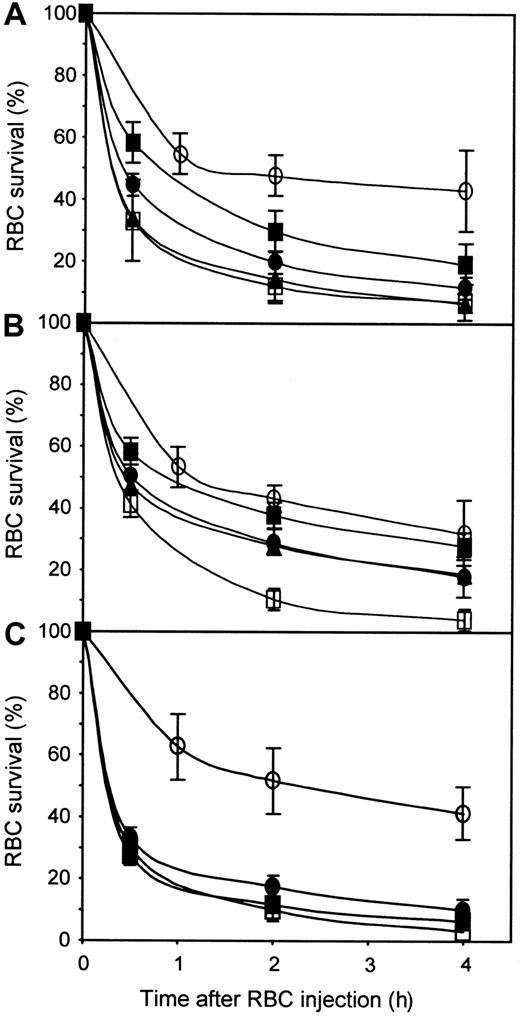
Eight different monoclonal anti-Ds (5 IgG1 and 3 IgG3) were tested in NOD-scid mice for their comparative ability to mediate Rh D+ RBC clearance. These anti-Ds have different epitope specificities and exhibit very different potency as tested by RBC agglutination assays done with similar IgG concentrations (Table 1). These monoclonal anti-Ds were tested individually for RBC clearance in groups of 3 NOD-scid mice at a dose of 5 μg/mouse, as described above for the polyclonal anti-D preparation. The results showed that these anti-Ds have a variable capacity to mediate RBC clearance in vivo (Figure 3) and that no monoclonal was as potent as the polyclonal anti-D preparation. We calculated the relative potency of each monoclonal anti-D by using the data obtained at 4 hours and the clearance observed with the polyclonal anti-D preparation as the 100% reference (Table 1). The relative RBC clearance ranged from 87% (46E7) to 15% (19A5). Three of the monoclonal anti-D tested gave relative RBC clearance higher than 80% (46E7, 26C1, and 25F5). The 26C1 (IgG3) and 25F5 (IgG1) anti-Ds gave agglutination titers of 256, which are in the same range as the one obtained with the polyclonal anti-D preparation (512; see Table 1). However, 46E7 has a significantly lower agglutination titer (32; Table 1), which indicates that there is no direct relationship between the ability to agglutinate RBCs in vitro and the ability to mediate RBC clearance in vivo. Indeed, 46E7 has an agglutination titer similar to that of 19A5, the latter being the less-efficient anti-D for mediating RBC clearance in vivo. The absence of relationship between the agglutination titer and the ability to mediate RBC clearance is also illustrated by the 13E10 and 13D2 anti-Ds, which are both comparable in vivo (50% versus 42%) and having significantly different agglutination titers (32 versus 256). Furthermore, comparison of the relative clearance potencies and the recognized D epitopes did not permit us to derive a significant correlation between these 2 characteristics
Monoclonal anti-Ds exhibit variable efficiencies in mediating RBC clearance.
Groups of 3 NOD-scid mice were injected with 10 mg human IgGs and 5 μg of each individual anti-D. RBCs were prepared and injected as in Figure 1. RBC survival was measured as described in “Materials and methods.” The SD at the 4-hour time points were between 0.4% and 13% (mean of 5%) for all the preparations tested.
Monoclonal anti-Ds exhibit variable efficiencies in mediating RBC clearance.
Groups of 3 NOD-scid mice were injected with 10 mg human IgGs and 5 μg of each individual anti-D. RBCs were prepared and injected as in Figure 1. RBC survival was measured as described in “Materials and methods.” The SD at the 4-hour time points were between 0.4% and 13% (mean of 5%) for all the preparations tested.
To determine whether the antibody concentration used was limiting the extent of RBC clearance, we performed dose-response analyses by using 26C1 and 19A5. The results obtained with 26C1 (Figure4A and Table 1) showed that the RBC clearance increased with the dose of 26C1 used. Indeed a dose of 10 μg 26C1 resulted in a RBC clearance comparable to that obtained with 5 μg of polyclonal anti-D (relative RBC clearance of 96%). In the case of 19A5 (Figure 4B and Table 1), the relative RBC clearance was very low at a dose of 5 μg (15%). The use of larger amounts of 19A5 permitted to some extent to increase the RBC clearance to about 50% but not to the level observed with the polyclonal and some other monoclonal anti-D. Furthermore, the relative RBC clearance observed with 10 μg 19A5 did not increase with 20 μg, indicating that factors other than anti-D concentration are involved in RBC clearance in vivo.
Dose-response of anti-D used individually or in a blend.
Groups of 3 NOD-scid mice were injected with human IgGs only (○), or in the presence of 5μg polyclonal anti-D (■), or the following amounts of the indicated monoclonal anti-D: (A) 1 μg 26C1 (▪), 5 μg 26C1 (●), and 10 μg 26C1 (▴); (B) 5μg 19A5 (▪), 10 μg 19A5 (●), and 20 μg 19A5 (▴); and (C) 5 μg of blend (●) and 10 μg blend (▪). The blend was made of equal proportions of 26C1, 46E7, and 25F5. RBCs were prepared and injected as in Figure1.
Dose-response of anti-D used individually or in a blend.
Groups of 3 NOD-scid mice were injected with human IgGs only (○), or in the presence of 5μg polyclonal anti-D (■), or the following amounts of the indicated monoclonal anti-D: (A) 1 μg 26C1 (▪), 5 μg 26C1 (●), and 10 μg 26C1 (▴); (B) 5μg 19A5 (▪), 10 μg 19A5 (●), and 20 μg 19A5 (▴); and (C) 5 μg of blend (●) and 10 μg blend (▪). The blend was made of equal proportions of 26C1, 46E7, and 25F5. RBCs were prepared and injected as in Figure1.
Synergistic functional effects have been reported for anti-D in in vitro tests such as antibody-dependent cellular cytotoxicity (ADCC) and monocyte chemiluminescence in which blends containing both IgG1 and IgG3 anti-Ds appeared to be the most active, as did blends containing antibodies recognizing 2 distinct D epitopes.10 We thus tested in NOD-scid mice a blend of monoclonal anti-Ds containing 2 IgG1 (25F5 and 46E7) and one IgG3 (26C1). These anti-Ds recognized 2 different epitopes on the Rh D antigen (epitope 6/7 for 25F5 and 26C1 and epitope 2 for 46E7; Table1). The blend containing an equal proportion of each monoclonal anti-D was tested at doses of 5 and 10 μg and was compared with a dose of 5 μg polyclonal anti-D. The results obtained (Figure 4C) indicated that the blend did not show synergistic effects at 5 μg and 10 μg and remained less potent than the polyclonal anti-D preparation.
Discussion
Hundreds of different human monoclonal anti-Ds are now available, but very few of them exhibit a biologic activity comparable to polyclonal anti-D preparations in clinical trials.2 In this work, we have tested the hypothesis of using NOD-scid mice for the in vivo testing of human anti-D. The results obtained showed that NOD-scid mice can be used to evaluate the relative biologic activity of different human anti-Ds in an in vivo environment. This conclusion is derived from experiments in which we observed that the reduction in the amount of human RBCs circulating in the blood of NOD-scid mice was related to the nature and amount of the injected anti-D, thus allowing comparison of the potency of each monoclonal with the one of the plasma-derived polyclonal preparations currently used in humans for the prophylaxis of the hemolytic disease of the newborn.
This mouse assay has several characteristics that make it suitable for extensive in vivo testing of human monoclonal anti-D. To shorten the time necessary for the in vivo assay, we used a 4-hour post-RBC injection to determine RBC clearance. Very small volumes of human RBCs are injected in NOD-scid mice, and we initially observed nonspecific clearance of human RBCs. This problem was resolved by diluting the labeled Rh D+ RBCs in a large excess of unlabeled Rh D− RBCs. This observation suggests that human RBCs are not intrinsically unable to circulate in NOD-scid mice and that the number of sites responsible for nonspecific RBC destruction is limited because they can be saturated with unlabeled Rh D− RBCs. However, it appears that the human RBC clearance in NOD-scid mice is much faster than in the only other animal model described, based on the use of chimpanzees. Indeed the half-life of human RBCs in chimpanzees was about 48 hours compared with about 4 hours in NOD-scid mice. The difference may well be related to the smaller mean corpuscular volume of mouse RBCs (41 to 51 μm3) compared with human RBCs (80 to 100 μm3), resulting in the clearance of the less deformable human RBCs injected. It is known that NOD-scid mice have a mild macrocytic anemia that could explain why human RBCs can circulate in these animals and not in CB.17-scid mice, albeit for limited times.20 Other differences between NOD-scid and other immunodeficient mice, such as mutated FcγRI, decreased natural killer cell, and hemolytic activities, could also account for the ability of human RBCs to circulate in NOD-scid mice. Thus, apart from the difference in RBC sizes, it is unclear what mechanism is responsible for the antibody-independent human RBC clearance in NOD-scid mice.
RBC clearance assays using a polyclonal anti-D preparation in NOD-scid mice showed that a dose of 5 μg resulted in the almost complete clearance of 5μL of Rh D+ RBCs from NOD-scid mouse circulation within 4 hours (1 μg was less efficient; data not shown). When considering that 20 to 25 μg is the amount of polyclonal anti-D required to prevent immunization against 1 mL Rh D+ RBCs in humans,2,9 it appears that the amount of anti-D required to clear human RBCs is much higher in NOD-scid mice. The FcγRs present in NOD mice have now been more fully characterized. The mutated NOD FcγRI has been shown to have a broader IgG subclass specificity than the BALB/c FcγRI.30 Also the NOD mice express a strongly reduced FcγRII on macrophages and a normal level of FcγRIII.31 Altogether, these studies indicate that the NOD mice have the ability to mediate Fc-dependent clearance of RBCs. The ability of human IgG of various subclasses to interact with mouse Fc receptors has already been documented.32 However, it is possible that the interaction of human IgG with mouse Fc receptors in vivo is less efficient than the interaction of human IgG with human Fc receptors, which would explain the difference in the amount of anti-D required to clear Rh D+ RBCs. Another explanation could be that the short circulation times (4 hours) used to emphasize the differences between anti-D were responsible for the necessity to use higher doses. Testing of extended circulation times (eg, 8 to 16 hours) may permit us to more precisely determine the relationship between clearance potency and injected doses of anti-D.
The in vivo analysis of 8 monoclonal anti-Ds tested revealed a wide range of RBC clearance potency when used at similar doses in NOD-scid mice (15% to 87% of polyclonal control). Thus, the NOD-scid mouse assay permits a good discrimination between weak and more active monoclonal anti-Ds. Also we could observe a dosage effect with 26C1 and 19A5, suggesting that the anti-D could also be classified according to their specific activity if additional dose-response experiments were performed. No single monoclonal anti-D could equal the clearance activity of polyclonal anti-D. This result was not totally unexpected, as none of the tested monoclonal anti-D was as potent as the polyclonal anti-D in standard agglutination assays. Two of the 3 most active monoclonal anti-D (26C1, 46E7, and 25F5) were among the group of 4 monoclonal anti-Ds that were the best agglutinators, suggesting a relationship between these 2 parameters. However, the link was not absolute because 46E7 was highly active in vivo and was a poor agglutinator in vitro, whereas the opposite results were obtained with 21H9. Also, no clear relationship could be established between the in vivo results and the IgG subclass or the recognized Rh D epitopes. Altogether these results further emphasize the limitations of in vitro tests for the prediction of in vivo efficacy of anti-D as previously suggested in other studies.9,10 We could not detect a synergy between 26C1, 25F5, and 46E7 when injected as a blend. However, the result may be caused by the experimental design and the fact that the 3 individual anti-Ds were at least 80% as active as the polyclonal preparation. The putative synergy would certainly be more easily detected by using a lower dose of each monoclonal anti-D selected in a range in which the clearance rate would be expected to be proportional to the injected amount (eg, 0.5 μg) as suggested by the dose-response effect observed with 26C1. Concerning the effect of blending, it should be pointed out that our results are in agreement with 2 previous studies done in humans33 and in chimpanzees34 in which no synergy between 2 anti-D was observed.
The mechanism by which anti-Ds suppress Rh D immunization is still unclear and may be explained either by efficient RBC clearance or inhibition of B-lymphocyte functions.2 Nevertheless, the selection of monoclonal anti-Ds able to efficiently prevent Rh D immunization should be based on 2 parameters that are the ability of the antibody to bind significantly to Rh D+ RBCs in vivo and the ability of the bound monoclonal to lead to efficient recognition of the opsonized RBCs by the immune cells. The NOD-scid mouse assay described in this work permits us to assess these 2 criteria in the context of phagocytosis, but this assay will obviously not replace the need for clinical trials. However, the use of an in vivo environment to evaluate the ability of anti-D to mediate RBC clearance may reflect more accurately the intrinsic biologic activity of these antibodies when compared with results of in vitro tests because the in vivo environment provided by an animal model is clearly more physiologically relevant than the simple in vitro interaction of antibodies with their antigens. Thus, although not perfect, the NOD-scid mouse assay described here could be very useful to select the best combination and dose of monoclonal anti-D to reproduce the effect of plasma-derived polyclonal preparations before conducting clinical trials in humans.
We thank Ms Huguette Collin for performing the agglutination assays and Dr Patricia Tippett for the determination of the D epitopes recognized by the monoclonal anti-Ds.
Supported by research funding from the Bayer/Canadian Blood Services/ Héma-Québec Partnership Fund (R.B. and R.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Renée Bazin, Héma-Québec, R&D Department, 2535 Boul Laurier, Sainte-Foy (Que), Canada, G1V 4M3; e-mail: rbazin@hema-quebec.qc.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal