Nitric oxide (NO), essential for maintaining vascular tone, is produced from arginine by nitric oxide synthase. Plasma arginine levels are low in sickle cell anemia, and it is reported here that low plasma arginine is also found in our sickle transgenic mouse model that expresses human α, human βS, and human βS-Antilles and is homozygous for the mouse βmajor deletion (S+S-Antilles). S+S-Antilles mice were supplemented with a 4-fold increase in arginine that was maintained for several months. Mean corpuscular hemoglobin concentration (MCHC) decreased and the percent high-density red cells was reduced. Deoxy K+ efflux is characteristic of red cells in sickle cell disease and contributes to the disease process by increasing the MCHC and rendering the cells more susceptible to polymer formation. This flux versus the room air flux was reduced in S+S-Antilles red cells from an average value of 1.6 ± 0.3 mmol per liter of red cells × minute (FU) in nonsupplemented mice to 0.9 ± 0.3 FU (n = 4, P < .02, paired t test) in supplemented mice. In room air, Vmax of the Ca++-activated K+ channel (Gardos) was reduced from 4.1 ± 0.6 FU (off diet) to 2.6 ± 0.4 FU (n = 7 and 8,P < .04, t test) in arginine-supplemented mice versus clotrimazole. In conclusion, the major mechanism by which arginine supplementation reduces red cell density (MCHC) in S+S-Antilles mice is by inhibiting the Ca++-activated K+ channel.
Introduction
Nitric oxide (NO) plays a major role in maintaining vascular tone. Production of NO results from the action of nitric oxide synthase (NOS) on arginine and molecular oxygen to form NO and citrulline. The role of NO is of particular importance in sickle cell disease, where the vasodilatory effects of NO may be critical1 and vasoconstriction can play a role in obstruction of the microcirculation by sickle cells.1 2
Increased levels of inducible and constitutive NOS have been reported in cremaster muscle of transgenic mice that express human α, human βS, and human βS-Antilles and are homozygous for the βmajor deletion (S+S-Antilles mice1) and in kidney, liver, and brain of transgenic mice that express human α and human βS and are homozygous for the βmajor deletion (NY1DD 3 4).
Arginine depletion has been reported in sickle cell patients5-7 and may be related to increased NOS activity. Arginine is a nonessential amino acid that can be synthesized from citrulline and other sources; however, it can be depleted either globally or locally. Global depletion has been demonstrated in both humans with sickle cell disease and is reported here for transgenic sickle mice; local depletion could result from the high tissue-specific activity of NOS that has been demonstrated in sickle transgenic mice.
Whether red cells themselves can produce NO is controversial. Both endothelial NOS (eNOS) and inducible NOS (iNOS) can be detected in red cells by immunohistochemistry and Western blot. Some investigators8 have reported inhibitable NOS activity in red cells, but it has been suggested by other investigators9 that the red cell enzyme is inactive and that the reaction product detected was ornithine (produced by arginase) rather than citrulline produced by NOS.10 Red cell preparation is a potential pitfall in all of these studies because removal of contaminating macrophages and platelets, which are known to contain active NOS, from the red cell preparation is crucial and has not been well documented in many studies.
An increase in nitrotyrosine has been reported in kidneys of NY1DD mice.11 Nitrotyrosine is formed when peroxynitrite interacts with tyrosine and may interfere with the activity of enzymes and proteins involved in regulatory cascades or ion transport. One mechanism of peroxynitrite formation occurs when NOS is active at low levels of arginine. Under these conditions, NOS forms both superoxide and NO, and peroxinitrite is then formed by a nonenzymatic reaction between them.12 13
We hypothesized that arginine supplementation might have beneficial effects by increasing vasodilatation and decreasing peroxynitrite levels in mice expressing high levels of sickle transgenes. We have examined the effects of arginine supplementation on our arginine-depleted, sickle transgenic mice (S+S-Antilles) and control mice (C57BL) by a 4-fold augmentation of the arginine level of standard mouse chow. We found that in arginine-supplemented S+S-Antilles mice, red cell density was decreased, the percent of high-density red blood cells (RBCs) was reduced, and theVmax of the Ca++-activated K+ channel was reduced.
Materials and methods
Transgenic mice
We produced transgenic mice expressing human α, human βS, and human βS-Antilles by crossing mice expressing our previously described sickle constructs (cointegrated human miniLCRα2 and miniLCRβS generated by F. Costantini, Dept of Genetics and Development, Columbia University, New York, NY) or NY114 with a line generated by Rubin et al expressing human cointegrated HSIIα2 and HSIIβS-Antilles.15 We hypothesized that mice expressing both hemoglobins would have a more severe phenotype because the reduced oxygen affinity and solubility of the βS-Antilles might enhance the rate and extent of polymer formation and the high human α expression of the NY1 line would compensate for the low human α expression of the S-Antilles line. We obtained mice that expressed both βS and βS-Antilles and were homozyous for the mouse βmajor deletion (Hbbth-1//Hbbth-1)16—hereafter called S+S-Antilles. These mice have been backcrossed an average of 6 generations to C57BL/6J mice; therefore, C57BL/6J are appropriate control mice. We also used C57BL/6J mice that are homozyous for the mouse βmajor deletion, which we will call THAL mice, and NY1 mice that express the NY1 transgene (human α and βS) and are homozyous for the mouse βmajordeletion, which we will call NY1DD mice (previously αHβS[βMDD]). Characteristics of the mice studied are presented in Table 1.
Genetic and hematologic properties of mice used
| Name . | Description . | βX* . | βX%† . | αH %‡ . | MCH, pg/cell . | Reticulocyte %, Sysmex . | Hematocrit . | Urine concentration, mOsm1-153 . |
|---|---|---|---|---|---|---|---|---|
| Control | C57BL/6J | — | — | — | 14.5 ± 1.0 | 2.2 ± 0.5 | 48 ± 1 | 3147 ± 653 |
| THAL | C57BL/6J | — | — | — | 12.7 ± 0.5 | 24.6 ± 3.3 | 32.3 ± 1.6 | 2820 ± 405 |
| Hbbth-1//Hbbth-1 | ||||||||
| NY1DD | miniLCRα2 miniLCRβS | βS | 75 | 56 | 14.1 ± 0.5 | 3.2 ± 0.6 | 47.0 ± 2.4 | 2821 ± 484 |
| Hbbth-1//Hbbth-1 | ||||||||
| S+S-Antilles | miniLCRα2 miniLCRβS | βS | 42 | 58 | 14.9 ± 0.5 | 11.1 ± 4.7 | 44.5 ± 4.0 | 1302 ± 477 |
| HSIIα2 HSIIβS-Antilles | βS-Ant | 38 | ||||||
| Hbbth-1//Hbbth-1 |
| Name . | Description . | βX* . | βX%† . | αH %‡ . | MCH, pg/cell . | Reticulocyte %, Sysmex . | Hematocrit . | Urine concentration, mOsm1-153 . |
|---|---|---|---|---|---|---|---|---|
| Control | C57BL/6J | — | — | — | 14.5 ± 1.0 | 2.2 ± 0.5 | 48 ± 1 | 3147 ± 653 |
| THAL | C57BL/6J | — | — | — | 12.7 ± 0.5 | 24.6 ± 3.3 | 32.3 ± 1.6 | 2820 ± 405 |
| Hbbth-1//Hbbth-1 | ||||||||
| NY1DD | miniLCRα2 miniLCRβS | βS | 75 | 56 | 14.1 ± 0.5 | 3.2 ± 0.6 | 47.0 ± 2.4 | 2821 ± 484 |
| Hbbth-1//Hbbth-1 | ||||||||
| S+S-Antilles | miniLCRα2 miniLCRβS | βS | 42 | 58 | 14.9 ± 0.5 | 11.1 ± 4.7 | 44.5 ± 4.0 | 1302 ± 477 |
| HSIIα2 HSIIβS-Antilles | βS-Ant | 38 | ||||||
| Hbbth-1//Hbbth-1 |
Human β-globin as indicated.
Percent human β-globin as indicated.
Percent human α-globin.
Water withheld for a 24 hour period.
Arginine supplementation
Arginine was increased from 1.3% by weight (normal mouse chow) to 5% by weight. The diet was formulated from Purina mouse chow and arginine by Harlan-Teklad (Madison, WI). Arginine supplementation was maintained for a minimum of 6 weeks to a maximum of 11 months. Except where noted, the data presented are based on mice supplemented for a minimum of 6 weeks and a maximum of 6 months.
Amino acid analysis
Blood was collected in heparinized capillary tubes, and plasma was removed after centrifugation and stored frozen. Samples were shipped on dry ice to the Molecular Structure Facility at the University of California at Davis. On receipt, the samples were centrifuged for 15 minutes at 4°C and deproteinized with 10% sulfasalicylic acid. The sample was then diluted 1:1 with a buffer containing the internal standard S-aminocysteine. Fifty microliters of the sample was then loaded onto a Beckman 6300 Amino Acid Analyzer (Beckman Coulter, Fullerton, CA) using lithium-citrate–based ion exchange chromatography followed by postcolumn ninhydrin derivatization for separation and quantitation of amino-containing components.
Reticulocytes and red cell indices
Mice were bled from the tail using protocols approved by the animal studies committee. Blood samples were analyzed for reticulocytes and red cell indices using the Sysmex SE 9000 system (Toa, Japan). Manual counts after staining with new methylene blue were used to validate the Sysmex reticulocyte counts in a limited number of cases, and good agreement was found.
Red cell density
Measurement of volume-stimulated Cl−-dependent K+ efflux
K-Cl cotransport activity in mouse RBCs was measured as described previously by us.19 Briefly, we determined the volume-stimulated and Cl−-dependent K+component of this flux by estimating K+ efflux into isotonic (330 mOsm) and hypotonic (250 mOsm) media. Net K+efflux was calculated by addition of RBCs into prewarmed flux media and duplicate sampling at 0, 10, 25, 40, and 60 minutes. K+efflux was then estimated from the nonlinear regression analysis of K+ concentration versus time.
Measurement of deoxygenation-stimulated K+efflux
Measurements under deoxygenated conditions were performed as previously described.20 Briefly, K+ efflux under deoxygenated conditions was determined by incubating mouse RBCs in Na+ media containing 150 mM NaCl, 10 mM Tris-Mops (pH 7.4 at 37°C), 1 mM ouabain (to inhibit the Na+ pump), 10 mM glucose, 10 mM sucrose, 0.15 mM MgCl2, 0.01 mM bumetanide (to inhibit the Na-K-Cl cotransporter), and 1 mM CaCl2. Measurements under deoxygenated conditions were performed as previously described. A sealed 14 mL flask containing Na+ media was deoxygenated for 20 minutes at 37°C with bubbling nitrogen; at the end of 20 minutes 100 μL of RBCs, hematocrit 0.5 (50%), were injected into the sealed flask and gently mixed by hand. Upon addition of the cells, nitrogen bubbling was terminated, but a positive nitrogen pressure was maintained. An aliquot of the flux medium was centrifuged, and the hemoglobin content of the supernatant was measured to estimate the contribution from red cell lysis, which was found to be less than 5% of the total flux. Initial rates of K+ loss were used to calculate K+efflux by sampling the efflux media with a syringe in duplicate at 2, 5, 7, and 10 minutes. To measure the K+ efflux under oxygenated conditions, RBCs were treated in a similar manner except that the media were oxygenated by exposure to room air. Potassium in the flux media was measured by atomic absorption spectrophotometry. To determine the contribution of the Gardos channel to the deoxy K+ efflux, the K+ efflux from mouse RBCs was also studied in the absence or presence of 10 μM clotrimazole. Deoxy K+ efflux is calculated from the difference between the flux under deoxygenated conditions minus the flux under oxygenated (room air) conditions.
Measurement of calcium-activated K+ channel activity, Vmax, and EC50
Ca++-activated K+ channel activity in room air was measured as K+ efflux from calcium-loaded mouse RBCs, as previously described.20 RBCs were incubated in Na+ media containing 150 mM NaCl, 10 mM Tris-Mops (pH 7.4 at 37°C), 1 mM ouabain, 10 mM glucose, 10 mM sucrose, 0.15 mM MgCl2, 0.1 mM bumetanide, 1 mM ethyleneglycotetraacetic acid or 1 mM Tris-citrate, total CaCl2 either 0 or 50 μM, and A23187 (2.5 μM) at 0.01 (1%) hematocrit. Cytosolic ionized Ca++ was buffered at 5 μM Ca++ with 1 mM citrate to maximally stimulate the Ca++-activated K+ efflux. Initial rates of K+ loss were used to calculate K+efflux in RBCs by sampling the efflux media in duplicates at 1, 2, 3.5, and 5.0 minutes in the presence or absence of 10 μM clotrimazole. The maximal rate of channel activity was determined as previously described20 by measuring the Gardos channel activity as a function of extracellular calcium concentration. From this we obtained the Vmax and the median effective concentration (EC50) for the channel activity in RBCs from mice that were supplemented with 5% arginine and nonsupplemented (regular chow).
Results
Plasma arginine is decreased in S+S-Antilles mice
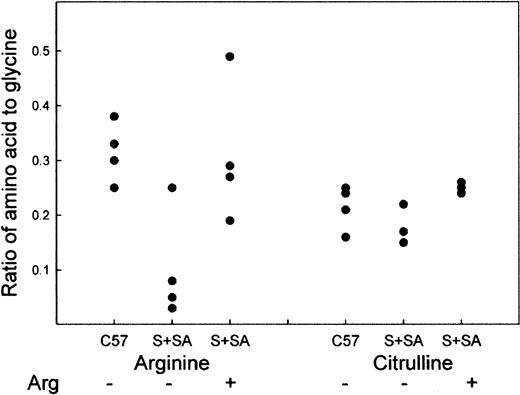
Plasma arginine and citrulline were measured at the Molecular Structure Facility at the University of California at Davis on groups of 4 mice. If the ratio of any given pair of plasma amino acids is compared, there is interindividual variation; however, there is greater variation in the absolute values for individual mice. Therefore, the results are expressed as the ratio of arginine or citrulline to glycine (Figure 1). We found that C57BL mice on Purina mouse chow had an average Arg/Gly ratio of 0.31 ± 0.05, S+S-Antilles mice on Purina mouse chow had an average ratio of 0.10 ± 0.09 (P < .005, C57 vs S+S-Antilles), and S+S-Antilles mice receiving 5% arginine supplementation had an average ratio of 0.31 ± 0.11 (P < .02, S+S-Antilles–off diet vs S+S-Antilles–on diet). The absolute values for unsupplemented C57BL mice versus S+S-Antilles were 100.3 ± 23.2 μmol per liter and 35.5 ± 49.5 μmol per liter, respectively,P < .004. For comparison, humans homozygous for hemoglobin A have 78.2 ± 6.4 μmol per liter versus 48.6 ± 3.8 μmol per liter for patients with homozygous hemoglobin S, which makes the decrease in mice larger than that seen in humans with sickle cell disease.6 The 5% arginine diet restored the plasma arginine level to that found in C57BL mice. The ratio of citrulline to glycine was also measured: C57BL, regular chow 0.21 ± 0.03; S+S-Antilles, regular chow 0.17 ± 0.03; and S+S-Antilles, 5% arginine 0.25 ± 0.01 (P < .002, S+S-Antilles–off diet vs S+S-Antilles–on diet).
Plasma arginine and citrulline levels in C57BL and S+S-Antilles mice.
Amino acid analysis of plasma arginine and citrulline levels expressed as the ratio of the amino acid to the glycine level in the same animal. S+S-Antilles mice were either fed regular Purina mouse chow (−) or were supplemented with mouse chow containing 5% arginine (+) in their diet for a 10-week period.
Plasma arginine and citrulline levels in C57BL and S+S-Antilles mice.
Amino acid analysis of plasma arginine and citrulline levels expressed as the ratio of the amino acid to the glycine level in the same animal. S+S-Antilles mice were either fed regular Purina mouse chow (−) or were supplemented with mouse chow containing 5% arginine (+) in their diet for a 10-week period.
Red cell density is decreased only in sickle transgenic mice that have been supplemented with arginine
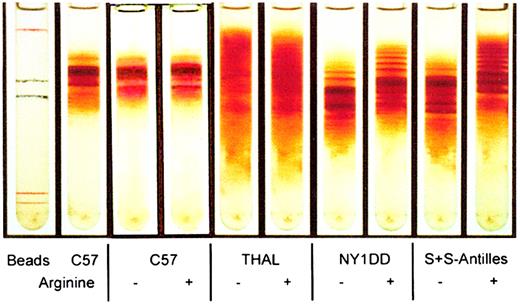
Red cell density distribution was examined on Percoll-Larex continuous density gradients for mice that had been fed a 5% arginine diet for 6 or 10 weeks or 11 months. In all cases, we found that red cells from NY1DD and S+S-Antilles mice supplemented with 5% arginine had decreased red cell density and a reduction in the percent of high-density red cells. The red cell density of C57BL/6J and THAL mice was not affected by the arginine diet. The results for mice fed 5% arginine for 10 weeks are illustrated in Figure2. There was no significant effect on reticulocyte levels in S+S-Antilles mice that were supplemented with arginine or nonsupplemented (11% ± 4.7% vs 11% ± 3.6%).
Arginine supplementation causes a reduction of mean corpuscular hemoglobin concentration and dense cells in transgenic mice.
Percoll-Larex continuous density gradient of mouse red cells obtained from mice either not supplemented with arginine (−) or mice supplemented with 5% arginine (+) for a 10-week period.
Arginine supplementation causes a reduction of mean corpuscular hemoglobin concentration and dense cells in transgenic mice.
Percoll-Larex continuous density gradient of mouse red cells obtained from mice either not supplemented with arginine (−) or mice supplemented with 5% arginine (+) for a 10-week period.
Incubation of mouse red cells with arginine increases red cell density
To determine if incubation of red cells with arginine is capable of reducing red cell density, we incubated red cells from S+S-Antilles mice with 10 mM arginine for 30 minutes. This resulted in a substantial increase in red cell density (data not shown). A high concentration of arginine was chosen to obtain a measurable result without a long incubation, which could result in complex metabolic changes. This demonstrates that high intracellular arginine per se does not result in reduced red cell density.
Volume-stimulated Cl-dependent K+ efflux
K-Cl or volume-stimulated Cl-dependent K+ efflux was measured for S+S-Antilles mice both off diet (2.9 ± 1.1 mmol per liter of red cells × hour) and on diet (3.3 ± 0.9 mmol per liter of red cells × hour) on 2 separate occasions using the pooled blood of 3 mice on each occasion (6 on and 6 off). No statistically significant difference was found.
Deoxygenation-stimulated K+ efflux is decreased in red cells from mice that have been supplemented with 5% dietary arginine
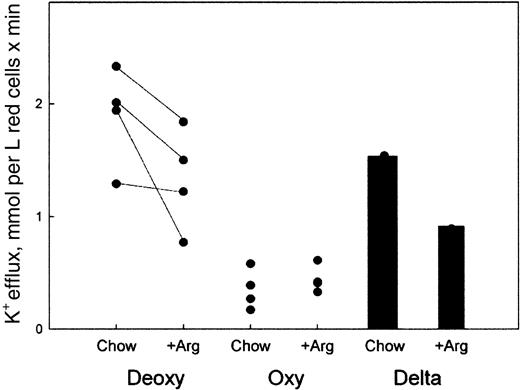
Low oxygen tension stimulates a K+ efflux that is characteristic of RBCs containing hemoglobin S and has been shown to play a role in red cell volume regulation. To determine if deoxygenation-stimulated K+ efflux was altered by an arginine diet supplementation, we measured deoxy-stimulated K+ efflux from RBCs of mice on normal chow or supplemented with 5% arginine for 10 weeks. Deoxy-stimulated K+ efflux from RBCs was estimated as flux into media that was bubbled with nitrogen (deoxygenation) versus flux into media exposed to room air. Our results show that in S+S-Antilles RBCs, deoxy-stimulated K+ efflux was significantly higher in nonsupplemented mice (1.6 ± 0.3 mmol per liter of red cells × minute [FU]) than in arginine-supplemented mice (0.9 ± 0.3 FU [n = 4,P < .02, paired t test]), as seen in Figure3. All arginine-supplemented mice tested showed a decrease in deoxy-stimulated K+ efflux. In addition, the effect of the arginine diet was only observed when the fluxes were measured under deoxygenated conditions. When potassium fluxes were measured in oxygenated samples, the effect of the arginine diet was not statistically significant, suggesting an effect of arginine on deoxy-stimulated K+ fluxes only.
Deoxygenation-stimulated K+ efflux is reduced in red cells from S+S-Antilles mice that have been supplemented with 5% arginine.
K+ efflux was measured in RBCs that were incubated in media that were either exposed to room air (Oxy) or bubbled with nitrogen to deoxygenate them (Deoxy), from mice that were on regular chow (Chow) and after they were supplemented with the 5% arginine diet (+Arg) for 10 weeks. Each point represents the average of one experiment performed in triplicate on one mouse as described in “Materials and methods.” The lines connect the same mouse. The Delta bar graphs represent the average of the deoxy-oxy flux in RBCs from mice on normal chow or arginine-supplemented chow.
Deoxygenation-stimulated K+ efflux is reduced in red cells from S+S-Antilles mice that have been supplemented with 5% arginine.
K+ efflux was measured in RBCs that were incubated in media that were either exposed to room air (Oxy) or bubbled with nitrogen to deoxygenate them (Deoxy), from mice that were on regular chow (Chow) and after they were supplemented with the 5% arginine diet (+Arg) for 10 weeks. Each point represents the average of one experiment performed in triplicate on one mouse as described in “Materials and methods.” The lines connect the same mouse. The Delta bar graphs represent the average of the deoxy-oxy flux in RBCs from mice on normal chow or arginine-supplemented chow.
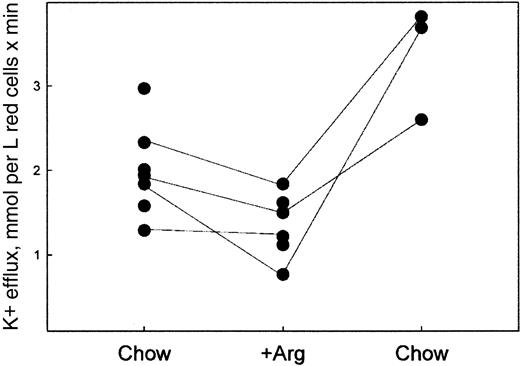
The effects of 5% arginine in the diet were reversible. When mice were then taken off the arginine diet and placed on normal chow and retested 15 weeks later, we observed a return to a high deoxy-stimulated K+ efflux, as shown in Figure4. The postdiet values are higher than those observed at baseline.
Removal from the 5% arginine diet causes a restoration of deoxy-stimulated K+ efflux to high values.
The fluxes were measured as described in “Materials and methods” on RBCs from mice on normal chow (Chow) after 10 weeks on the 5% arginine diet (Arg) and after 15 weeks off arginine and on regular chow (Chow). Each point represents the average of one experiment performed in triplicate on one mouse as described in “Materials and methods.” The lines connect the same mouse.
Removal from the 5% arginine diet causes a restoration of deoxy-stimulated K+ efflux to high values.
The fluxes were measured as described in “Materials and methods” on RBCs from mice on normal chow (Chow) after 10 weeks on the 5% arginine diet (Arg) and after 15 weeks off arginine and on regular chow (Chow). Each point represents the average of one experiment performed in triplicate on one mouse as described in “Materials and methods.” The lines connect the same mouse.
When the magnitude of the deoxy K+ efflux was analyzed with respect to the time that the mice had received arginine supplementation, there was no statistically significant time dependence of the deoxy K+ efflux for mice receiving chow for periods ranging between 6 weeks and 6 months.
Clotrimazole-sensitive deoxy-stimulated K +efflux is decreased in red cells from mice that have been supplemented with 5% dietary arginine
Deoxy K+ efflux from hemoglobin S containing red cells is partially inhibited by clotrimazole and charybdotoxin. These compounds have been shown to be specific inhibitors of the erythrocyte calcium-activated K+ channel also called the Gardos channel. It is believed that the increased permeability to calcium, induced by deoxygenation, causes activation of the Gardos channel. Deoxy-stimulated K+ efflux from S+S-Antilles RBCs showed sensitivity to these inhibitors. The clotrimazole-inhibitable fraction of the deoxy K+ efflux was reduced in S+S-Antilles RBCs from an average value of 1.3 ± 0.3 FU in nonsupplemented mice to 0.7 ± 0.2 FU (n = 4, P < .04, paired ttest) in arginine-supplemented mice when measured under deoxy conditions in the presence or absence of 10 μM clotrimazole. This strongly suggests that the effect of an arginine diet on RBC volume regulation is mediated via the inhibition of the Ca++-activated K+ channel also known as the Gardos channel. In unsupplemented S+S-Antilles mice 81% of the deoxy-stimulated K+ efflux can be inhibited by clotrimazole, and after supplementation 77% of the deoxy-stimulated K+ efflux can be inhibited by clotrimazole. One interpretation of this result is that the number of channels may be reduced in supplemented mice.
The Vmaxof the Ca++-activated K+channel (Gardos channel) is reduced in red cells from mice on the 5% arginine diet
To determine the effect of arginine supplementation on the Gardos channel itself, we studied the kinetic properties of the channel in supplemented mice. We measured the Gardos channel activity as a function of extracellular calcium concentration under room air conditions. The Vmax of the clotrimazole-sensitive Ca++-activated K+channel was reduced from 4.1 ± 0.6 FU (off diet) to 2.6 ± 0.4 FU (on diet) (n = 7 and 8, P < .04,t test), as shown in Figure 5. The EC50 for Ca++ was measured in duplicate in 3 (normal chow) and 3 (5% arginine diet) separate experiments on pooled blood that came from 3 different mice in each experiment (9 on and 9 off). No significant difference in EC50 was observed between these 2 groups.
Arginine diet inhibits the
Vmax of the Gardos channel activity in S+S-Antilles mouse red cells but does not change the EC50. Gardos channel activity under oxygenated conditions was measured as described in “Materials and methods” on RBCs from mice on normal chow (Chow) and after 10 weeks on the 5% arginine diet (5% Arg). The clotrimazole-sensitive, calcium-activated K+ efflux was estimated from the difference between fluxes measured in the presence or absence of 10 μM clotrimazole. (A) TheVmax data shown represent the mean ± SE of assays performed in duplicate on 7 (normal chow) and 8 (5% arginine diet) different experiments on either individual mice or pooled blood from at least 2 mice (P < .04, t test). (B) The EC50 data shown represent the mean ± SE of experiments performed in duplicate on 3 (normal chow) and 3 (5% arginine diet) different experiments on pooled blood from 3 different mice.
Arginine diet inhibits the
Vmax of the Gardos channel activity in S+S-Antilles mouse red cells but does not change the EC50. Gardos channel activity under oxygenated conditions was measured as described in “Materials and methods” on RBCs from mice on normal chow (Chow) and after 10 weeks on the 5% arginine diet (5% Arg). The clotrimazole-sensitive, calcium-activated K+ efflux was estimated from the difference between fluxes measured in the presence or absence of 10 μM clotrimazole. (A) TheVmax data shown represent the mean ± SE of assays performed in duplicate on 7 (normal chow) and 8 (5% arginine diet) different experiments on either individual mice or pooled blood from at least 2 mice (P < .04, t test). (B) The EC50 data shown represent the mean ± SE of experiments performed in duplicate on 3 (normal chow) and 3 (5% arginine diet) different experiments on pooled blood from 3 different mice.
Discussion
NO is produced from arginine and oxygen by NOS. There are several NOSs, each of which has a distinct physiologic function. Endothelial NOS (eNOS, NOS3, NOSIII) is the physiologic regulator of vascular tone. Inducible NOS (iNOS, NOS2, NOSII), which is found in macrophages and many other tissues, produces the bulk of the NO in the body and would be expected to be a major source of NO under conditions of arginine supplementation. The expression of both eNOS and iNOS are up-regulated in sickle transgenic mice in kidney and liver.3 4 In addition to these sources, there is neuronal NOS (nNOS, NOS1, NOSI) found in brain and also in kidney. In arginine-supplemented mice we anticipate that NO production from all isozymes of NOS will be stimulated.
We found that plasma arginine was significantly depleted in S+S-Antilles mice to about one third of the value found in C57BL mice. This is even greater than the reduction seen in sickle cell anemia patients. Supplementation with 5% arginine restored the level of plasma arginine to that of C57BL mice. Although citrulline levels were decreased in S+S-Antilles mice, this effect was not statistically significant; however, in supplemented mice the increase in plasma citrulline was statistically significant.
We found that both NY1DD and S+S-Antilles mice have reduced red cell density and a smaller percent of high-density red cells after the mice are fed a diet supplemented with 5% arginine for 6 weeks or more. In contrast, supplementing C57BL or THAL mice with 5% arginine had no effect on red cell density. Incubating mouse red cells with arginine resulted in increased red cell density rather than the decrease observed in mice receiving an arginine dietary supplement, suggesting that the effect on red cell volume is not mediated via a direct effect of arginine but rather by some other mechanism.
The deoxy-stimulated potassium efflux was found to be significantly diminished in mice supplemented with arginine. The most likely mechanism for reduction of red cell density in NY1DD mice and S+S-Antilles mice while the red cell density of C57BL and THAL mice remained unaffected is an effect on the deoxy-stimulated potassium efflux that is unique to red cells expressing hemoglobin S or other forms of mutant hemoglobin capable of forming polymer on deoxygenation. When mice that had been on the arginine diet were removed from the diet for 6 weeks or more, the deoxy-stimulated K+ efflux returned to baseline.
The primary mechanisms for deoxy-stimulated potassium efflux in red cells with a polymerizable hemoglobin are via K/Cl cotransport and the Ca++-activated K+ channel or Gardos channel.21-23 We measured K/Cl cotransport activity for S+S-Antilles mice supplemented with 5% arginine. No significant differences in K/Cl cotransport activity were observed in these cells. However, because the activity of the K/Cl cotransport is small in these cells, it is possible that we were unable to detect differences due to lack of sensitivity in our assay. We then measured the deoxy-simulated K+ efflux in the presence or absence of clotrimazole to determine the contribution of the Gardos channel to this deoxy flux. We observed that the clotrimazole-sensitive portion of the deoxy K+ efflux was reduced in arginine-supplemented mice. This suggests that the effect of arginine is mediated by inhibition of the Gardos channel. Therefore, we investigated the effect of arginine supplementation on the kinetics of the Gardos channel and found that the Vmax for the channel activity was reduced in S+S-Antilles mice receiving 5% arginine supplementation with no effect on the EC50. This last experiment definitively demonstrates that Gardos channel activity is reduced in arginine-supplemented mice.
The decrease in red cell density observed in our experiments is consistent with the effect of inhibition of the Gardos channel in the SAD mouse by administration of oral clotrimazole observed by de Franceschi et al.24
There are a number of possible explanations for the reduction in Gardos channel activity in animals receiving arginine-supplemented chow. First, it is well documented in humans25,26 and rats27 but not in mice that arginine reduces endothelin levels. The results reported by Rivera et al28 indicate that endothelin stimulation of the Gardos channel in the mouse red cell changes both the EC50 for Ca++ and theVmax. We report here that arginine supplementation reduces Vmax but leaves EC50 for Ca++ unchanged. However, the details of the dose response for changes in endothelin levels and its effect on the EC50 for Ca++ and theVmax of the Gardos channel in the mouse have not yet been worked out, and this remains a possible explanation.
Secondly, arginine could alter the density distribution of red cells by some mechanism unrelated to Gardos channel activity, and the population with high Gardos channel activity could be decreased. However, we observed that the percent of the densest red cells was reduced, and Rivera (personal communication, August 30, 2001) has demonstrated that in humans the densest cells have the least Gardos channel activity. Reduction in transport activity in the densest cells has been reported for several other transporters in human red cells. Therefore, decreasing the number of cells with highest density could actually increase Gardos channel activity. It also is possible that Gardos channel activity is unequally distributed among red cells and that the population of cells with high channel activity is selectively reduced by arginine supplementation; however, this seems unlikely.
Finally, as proposed in the introduction, peroxynitrite may play a role in inactivating either the channel itself or a regulatory protein; however, we have not yet definitively tested this hypothesis, and it too remains a possible explanation.
We conclude that a 5% arginine supplementation of sickle S+S-Antilles transgenic mice significantly reduces deoxy K+efflux and results in long-term normalization of RBC density primarily via its effect on the Gardos channel. Nevertheless, before considering clinical applications of arginine supplementation, possible pathogenic effects in sickle cell patients with potentially elevated NOS must be excluded.
Supported by National Institutes of Health grants P01HL55435, 1M01RR12248, P60HL38655, and DK02817.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mary E. Fabry, Dept of Medicine/Ullmann Rm 921, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461; e-mail: fabry@aecom.yu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal