Abstract
The GIMEMA ALL 0288 trial was designed to evaluate the impact of a 7-day prednisone (PDN) pretreatment on complete remission (CR) achievement and length, the influence of the addition of cyclophosphamide (random I) to a conventional 4-drug induction on CR rate and duration, and whether an early post-CR intensification (random II) by an 8-drug consolidation could improve CR duration. Median follow-up of this study was 7.3 years. From January 1988 to April 1994, among 794 adult (> 12 but < 60 years) patients registered, 778 were eligible. Their median age was 27.5 years; 73% had B-lineage acute lymphoblastic leukemia (ALL) and 22% had T-lineage disease; 18% showed associated myeloid markers; 47 of 216 analyzed patients (22%) had Philadelphia chromosome–positive ALL. Response to PDN pretreatment was observed in 65% of cases. CR was achieved in 627 patients (82%). Resistant patients and induction death rates were 11% and 7%, respectively. Random II was applied to 388 patients with CR; 201 had maintenance alone and 187 had consolidation followed by maintenance. The relapse rate was 60%; isolated central nervous system relapses were 8% of all CRs and 13% of all relapses. Median survival (overall survival [OS]), continuous complete remission (CCR), and disease-free survival (DFS) were 2.2, 2.4, and 2 years, respectively. PDN pretreatment response resulted the main independent factor influencing CR achievement, OS, CCR, and DFS; the addition of cyclophosphamide in induction significantly influenced CR achievement in a multivariate analysis. Neither induction intensification nor early consolidation appeared to influence CCR and DFS duration. For the first time PDN pretreatment response proved to be a powerful factor predicting disease outcome in adult ALL patients.
Introduction
In adult acute lymphoblastic leukemia (ALL), unlike in childhood ALL, the percentage of long-term remitters and survivors has not improved significantly during recent decades, although several trials have attempted to intensify the induction and postremission strategy, including early bone marrow transplantation (BMT).1-12 In the previous GIMEMA trial ALL 0183, we demonstrated that a mild 4-drug induction led to an excellent complete remission (CR) rate (79.3%), and that a short intensive post-CR therapy did not prevent recurrence of leukemia, leaving only 25% of remitter patients as more than 10-year long-term survivors.13
Some recent studies have focused on the hypothesis that an intense initial cytoreduction followed by multidrug intensification could increase the likelihood of long-term disease-free survival (DFS) in adults with ALL.6 14
The GIMEMA ALL 0288 trial was started in 1988 with the aim of testing whether (1) a 7-day prednisone (PDN) pretreatment response could have a prognostic significance on CR achievement and duration, as demonstrated in childhood ALL; (2) the randomized addition of cyclophosphamide (Cy) in induction could improve CR rate and length; and (3) an early post-CR intensification by an 8-drug consolidation phase could influence the percentage of long-term remitters and survivors.
After a median potential follow-up of 7.3 years (range, 3.9-10.4 years; all surviving patients were followed until June 1999), we are now able to determine the real proportion of adult patients with ALL who become long-term disease-free survivors and investigate the prognostic factors that significantly influenced the disease outcome.
Patients, materials, and methods
Treatment program
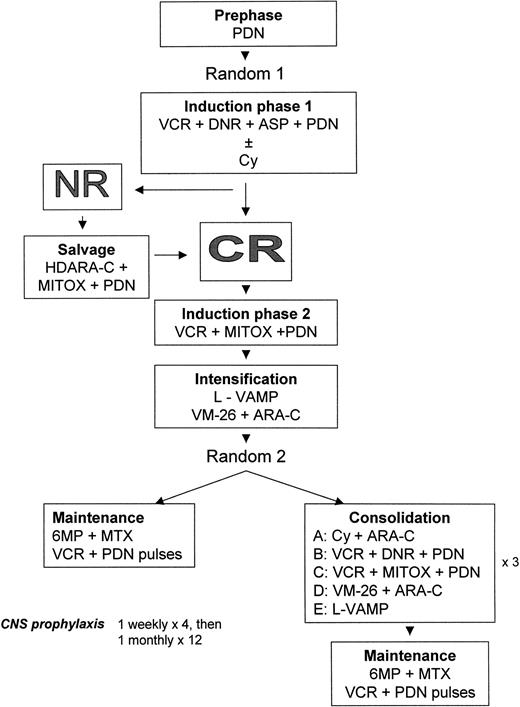
GIMEMA ALL 0288 treatment plan.
The trial includes 2 randomizations. Total post-CR treatment duration is 30 months.
GIMEMA ALL 0288 treatment plan.
The trial includes 2 randomizations. Total post-CR treatment duration is 30 months.
Chemotherapy courses and drug doses in the GIMEMA ALL 0288 randomized trial
| Course . | Route . | Dosage . | Days . |
|---|---|---|---|
| Prephase | |||
| Prednisone | PO | 20 to 60 mg/m2 | Day −7 to −1 |
| Induction phase I | |||
| Cyclophosphamide | IV | 800 mg/m2 | Day 1, 2 |
| Daunorubicin | IV | 40 mg/m2 | Day 1, 8, 15, 22 |
| Vincristine | IV | 2 mg/m2 | Day 1, 8, 15, 22 |
| Prednisone | PO | 60 mg/m2 | Day 1-14 |
| 40 mg/m2 | Day 15-31 | ||
| L-asparaginase | SC | 6000 U/m2 | Day 22-31 |
| Salvage | |||
| Cytosine arabinoside | CI | 1000 mg/m2 | Day 33-36 |
| Mitoxantrone | IV | 6 mg/m2 | Day 33-36 |
| Prednisone | PO | 40 mg/m2 | Day 32-39 |
| Induction phase II | |||
| Vincristine | IV | 2 mg/m2 | Day 32 |
| Mitoxantrone | IV | 10 mg/m2 | Day 32-34 |
| Prednisone | PO | 40 mg/m2 | Day 32-39 |
| Intensification | |||
| L-VAMP × 3 courses | |||
| Vincristine | IV | 1.5 mg/m2 | Day 1 |
| Methotrexate | CI | 1000 mg/m2 | Day 1 |
| Cytosine arabinoside | IV | 100 mg/m2 | Day 1 |
| Cytosine arabinoside | CI | 400 mg/m2 | Day 1 |
| Dexamethasone | IV | 10 mg/m2 | Day 1-5 |
| VM-26 + CA × 4 doses | |||
| VM-26 | IV | 165 mg/m2 | Day 1, 5, 9, 13 |
| Cytosine arabinoside | IV | 300 mg/m2 | Day 1, 5, 9, 13 |
| Consolidation: every course × 3 | |||
| A | |||
| Cyclophosphamide | IV | 800 mg/m2 | Day 1 |
| Cytosine arabinoside | SC | 75 mg/m2 | Day 3-10 |
| B | |||
| Vincristine | IV | 1.5 mg/m2 | Day 1 |
| Daunorubicin | IV | 40 mg/m2 | Day 1 |
| Prednisone | PO | 40 mg/m2 | Day 1-7 |
| C | |||
| Vincristine | IV | 1.5 mg/m2 | Day 8 |
| Mitoxantrone | IV | 10 mg/m2 | Day 8-10 |
| Prednisone | PO | 40 mg/m2 | Day 8-15 |
| D | |||
| VM-26 | IV | 165 mg/m2 | Day 1, 5 |
| Cytosine arabinoside | IV | 300 mg/m2 | Day 1, 5 |
| E | |||
| L-VAMP | |||
| Maintenance 5-wk courses × 2 y | |||
| Methotrexate | IM | 30 mg/m2 | Day 1, 8, 15 |
| 6-Mercaptopurine | PO | 70 mg/m2 | Day 1-21 |
| Vincristine | IV | 1.5 mg/m2 | Day 22, 29 |
| Prednisone | PO | 40 mg/m2 | Day 22-36 |
| CNS prophylaxis × 16 doses | |||
| Methotrexate | IT | 12 mg | |
| Methylprednisolone | IT | 40 mg |
| Course . | Route . | Dosage . | Days . |
|---|---|---|---|
| Prephase | |||
| Prednisone | PO | 20 to 60 mg/m2 | Day −7 to −1 |
| Induction phase I | |||
| Cyclophosphamide | IV | 800 mg/m2 | Day 1, 2 |
| Daunorubicin | IV | 40 mg/m2 | Day 1, 8, 15, 22 |
| Vincristine | IV | 2 mg/m2 | Day 1, 8, 15, 22 |
| Prednisone | PO | 60 mg/m2 | Day 1-14 |
| 40 mg/m2 | Day 15-31 | ||
| L-asparaginase | SC | 6000 U/m2 | Day 22-31 |
| Salvage | |||
| Cytosine arabinoside | CI | 1000 mg/m2 | Day 33-36 |
| Mitoxantrone | IV | 6 mg/m2 | Day 33-36 |
| Prednisone | PO | 40 mg/m2 | Day 32-39 |
| Induction phase II | |||
| Vincristine | IV | 2 mg/m2 | Day 32 |
| Mitoxantrone | IV | 10 mg/m2 | Day 32-34 |
| Prednisone | PO | 40 mg/m2 | Day 32-39 |
| Intensification | |||
| L-VAMP × 3 courses | |||
| Vincristine | IV | 1.5 mg/m2 | Day 1 |
| Methotrexate | CI | 1000 mg/m2 | Day 1 |
| Cytosine arabinoside | IV | 100 mg/m2 | Day 1 |
| Cytosine arabinoside | CI | 400 mg/m2 | Day 1 |
| Dexamethasone | IV | 10 mg/m2 | Day 1-5 |
| VM-26 + CA × 4 doses | |||
| VM-26 | IV | 165 mg/m2 | Day 1, 5, 9, 13 |
| Cytosine arabinoside | IV | 300 mg/m2 | Day 1, 5, 9, 13 |
| Consolidation: every course × 3 | |||
| A | |||
| Cyclophosphamide | IV | 800 mg/m2 | Day 1 |
| Cytosine arabinoside | SC | 75 mg/m2 | Day 3-10 |
| B | |||
| Vincristine | IV | 1.5 mg/m2 | Day 1 |
| Daunorubicin | IV | 40 mg/m2 | Day 1 |
| Prednisone | PO | 40 mg/m2 | Day 1-7 |
| C | |||
| Vincristine | IV | 1.5 mg/m2 | Day 8 |
| Mitoxantrone | IV | 10 mg/m2 | Day 8-10 |
| Prednisone | PO | 40 mg/m2 | Day 8-15 |
| D | |||
| VM-26 | IV | 165 mg/m2 | Day 1, 5 |
| Cytosine arabinoside | IV | 300 mg/m2 | Day 1, 5 |
| E | |||
| L-VAMP | |||
| Maintenance 5-wk courses × 2 y | |||
| Methotrexate | IM | 30 mg/m2 | Day 1, 8, 15 |
| 6-Mercaptopurine | PO | 70 mg/m2 | Day 1-21 |
| Vincristine | IV | 1.5 mg/m2 | Day 22, 29 |
| Prednisone | PO | 40 mg/m2 | Day 22-36 |
| CNS prophylaxis × 16 doses | |||
| Methotrexate | IT | 12 mg | |
| Methylprednisolone | IT | 40 mg |
PO indicates orally; IV, intravenously, SC, subcutaneously; CI, continuous infusion; IM, intramuscularly; IT, intrathecally. A, B, C, D, and E are abbreviations of each course.
Pretreatment with PDN was given over 7 days (day −7 to day −1) in escalating doses: 20 mg/m2 day −7, 30 mg/m2day −6, 40 mg/m2 day −5, and 60 mg/m2 from day −4 to day −1. Response to PDN, as defined by peripheral blast cell count of at least 1000/μL, was evaluated at day 0.
Induction phase I (random I) was given over 4 weeks either as a 5-drug regimen (arm A) with Cy, vincristine (VCR), daunorubicin (DNR), asparaginase (ASP), and prednisone (PDN), or as a 4-drug regimen (arm B) with VCR, DNR, ASP, and PDN.
CR was evaluated on day +32. Patients not in CR received a salvage regimen that included high-dose cytosine-arabinoside (HDARA-C) in continuous infusion and mitoxantrone (MITOX). Patients in CR after induction phase I or after salvage chemotherapy were given induction phase II (VCR, MITOX, and PDN) followed by intensification, which consisted of 3 courses of L-VAMP followed by 4 doses of teniposide (VM-26) plus ARA-C. L-VAMP is a combination of VCR, methotrexate (MTX), ARA-C, and dexamethasone (Dexa). A rescue with folinic acid was provided thereafter. L-VAMP courses were given at 15-day intervals.
Postintensification therapy (random II) consisted of consolidation plus maintenance (C+M) or maintenance alone (M). Consolidation therapy included 8 drugs in 5 courses (A, B, C, D, E) that were repeated 3 times each (Figure 1). The expected consolidation time was 6 months.
Maintenance chemotherapy was based on daily oral 6 mercaptopurine (6-MP) plus intramuscular MTX weekly for 3 weeks, followed by VCR and PDN pulses. Total duration of postintensification therapy (C+M or M alone) was 24 months.
Central nervous system (CNS) prophylaxis was based on intrathecal MTX plus PDN weekly, times 4 during induction (day 0, 8, 15, 22), monthly during intensification and postintensification for a total of 16 doses, associated with the systemic high-dose MTX given during the 3 courses of L-VAMP.
All patients were given low-dose trimethoprim (80 mg/d), sulfamethoxazole (400 mg/d) for 3 days a week as prophylaxis against Pneumocystis carinii pneumonia. The use of prophylactic oral antibiotic therapy and the management of febrile episodes were carried out according to GIMEMA infection program guidelines.15
Criteria for response
After induction phase I, patients were considered to be in CR if they had normal peripheral blood count (polymorphonuclear cells [PMNs] > 1.5 × 109/L; hemoglobin > 10 g/dL; platelets > 100 × 109/L; no circulating blast cells) and fewer than 5% blast cells with normal cellularity at bone marrow examination. Patients who exhibited more than 5% blasts in bone marrow were given salvage chemotherapy; if they did not obtain CR after the salvage course, they were considered resistant.
Patients
Patients aged 12 to 60 years, with previously untreated ALL of French-American-British (FAB) classification subtypes L1 and L2 or undifferentiated leukemia (AUL) were considered eligible for this study. Patients with B S(Ig)+ ALL were excluded.
All patients were registered by telephone at the GIMEMA Data Center before treatment; at registration cytology, cytochemistry, and immunophenotype, performed at the local institution, were required. Diagnosis of ALL was confirmed by the Central Committee's review of blood smears and bone marrow specimens for cytologic and cytochemical characteristics, according to FAB criteria.16 Central review of immunophenotype data were also required and done.17
Pretreatment cytogenetics was recommended to be performed at the local center level; however, it was not mandatory, and a centralized karyotype review was not planned. At the beginning of this study, pretreatment molecular analysis for bcr/ablrearrangement was not included; then, from May 1990, when in the context of this trial a pilot study for patients positive for Philadelphia chromosome (Ph+) or bcr/ablrearrangements (or both) was planned,18 the GIMEMA group activated the first framework for centralized molecular analysis. Thebcr/abl fusion gene was analyzed in 3 centers, Rome (F. Lo Coco), Turin (G. Saglio), and Naples (F. Pane).
No therapeutical lumbar puncture for cerebrospinal fluid examination was mandatory at diagnosis for all patients; 3 patients with CNS involvement (> 3 definable blast cells in cytospin sample) were considered not eligible for the randomized study and excluded from this analysis.
Statistical methods
The primary goals of the trial were to conduct a multicenter, randomized controlled clinical trial to evaluate the effect of addition of Cy during induction in increasing the CR rate and the efficacy of intensified postremission therapy in reducing relapse risk.
Allocation to study groups
Randomization was performed using randomly permuted blocks. For the first random, the only stratifying variable was white blood cell (WBC) count at entry; for the second random, the type of allocated induction group was considered. A central repository of randomization schedules was kept by the Trial Secretariat at the GIMEMA Data Center. After obtaining written informed consent, the hospital investigator had to call the Data Center to obtain the allocation for that patient. Allocation was granted only after the data on eligibility had been verbally transferred, recorded, and checked.
Sample size
We assumed a percentage of CR of 80% in the conventional chemotherapy group (no Cy) with an increase in this frequency to 90% with Cy addition, and a reduction of 33% of relapse risk in patients treated with C+M versus M only. To have an 80% chance of detecting these differences required a sample size of 390 patients in the first randomization and 152 events after the second randomization (α = 0.05, 2-sided test). The follow-up period was established at 5 years.
Analysis
All primary analyses were conducted using the intention-to-treat rule; that is, the outcome for each patient was counted against the trial group to which he/she was originally allocated, regardless of whether he/she later deviated from the protocol.
We compared the prevalence of risk factors and the incidence of end points in randomized groups using χ2.
Survival was defined as the time from randomization to death or date of last follow-up. Continuous complete remission (CCR) was calculated from the time of achieving CR to relapse or date of last follow-up; DFS was defined as the time from achieving CR to relapse, death, or date of last follow-up.
The probability of survival, CCR, and DFS were estimated using the Kaplan-Meier method.19
The log-rank test20 was used to compare treatment effect and risk factor categories. The 95% confidence intervals (CIs) for these probabilities and the median survival times were obtained using the Simon and Lee method.21
All tests were 2-sided, accepting P < .05 as indicating a statistically significant difference.
Median follow-up time was estimated by reversing the codes for the censoring indicator in a Kaplan-Meier analysis.
Logistic regression and Cox proportional hazards regression models22,23 were performed to examine and check for treatment results and the risk factors affecting CR rate and time to event. These were performed using the SAS procedures LOGISTIC and PHREG, respectively.24
Analysis of the full trial population was followed by analysis of relevant subgroups.
Results
Patient accrual
From January 1988 to April 1996, 794 adult patients with ALL were registered in the GIMEMA ALL 0288 study from 41 centers throughout Italy. At central reviewing of the initial data, 16 patients were considered ineligible because of misdiagnosis (n = 7), age over 60 years (n = 2), CNS involvement (n = 3), B S(Ig)+ ALL (n = 2), and severe organ failure (n = 2).
Patient characteristics
Of the 778 eligible patients, 459 were men; median age was 27.5 years (range, 12-59.9 years); 231 (30%) were aged 20 years or younger, 188 (24%) were 21 to 30 years old, 121 (16%) were aged 31 to 40 years, 113 (14%) were aged 41 to 50 years, and 125 (16%) were older than 50 years.
Initial median WBC count was 13.6 × 109/L (range, 0.5-527 × 109/L); 575 (74%) had an initial WBC count of 50 × 109/L or less, 12% had WBC counts of 50 to 100 × 109/L; and 14% had WBC counts more than 100 × 109/L.
At diagnosis, performance status (PS) according to World Health Organization (WHO) criteria was available for 740 patients. Only 58 patients (8%) were classified as having WHO PS more than 3. Fever was present in 90 cases (11.6%); infection was documented in 34 patients.
Splenomegaly more than 3 cm and adenomegaly more than 3 cm were documented (by physical examination) in 304 patients (38%) and in 121 patients (16%), respectively, among 778 eligible patients. A mediastinal mass was detected in 74 of 764 patients (10%).
A centralized review of immunophenotype data was undertaken in 706 of 778 cases; the remaining 72 could not be reviewed. Of the reviewed cases, 513 (73%) were classified as having B-lineage ALL, 154 (22%) as T-lineage ALL, and 39 (5%) were considered not classifiable. In 122 cases (102 B lineage, 20 T lineage) myeloid (My) markers (CD13 or CD33 or both) were found, and they were classified as My+ ALL.
Response to induction therapy
PDN pretreatment.
In vivo response to PDN pretreatment (defined as circulating blasts at day 0 ≤ 1000/μL) was evaluable in 657 of the 778 eligible patients, whereas in the remaining 121 cases response to PDN pretreatment could not be assessed because the patients were already taking PDN before enrollment in the study. At diagnosis, of 657 evaluable patients 154 had at least 1000 blasts and 503 had more than 1000 blasts; 429 (65%) were considered responders (143 and 286 among patients with initial peripheral blasts ≤ 1000 and > 1000, respectively) and 228 were nonresponders. Response to PDN was not related to age, whereas it was associated with initial WBC count (Table2). A significantly higher percentage of PDN responder patients was recorded in B-lineage ALL cases (71%) as compared to T-lineage ALL (56%) and My+ ALL (56%) (P = .002). In T-lineage ALL the lower PDN response rate did not show a relationship to higher (> 100 × 109/L) WBC count, because even among patients with lower (≤ 100 × 109/L) WBC count, fewer were PDN responders (62%) as compared to those with B-lineage ALL (74%) (P = .02).
Response to PDN pretreatment according to WBC count at diagnosis
| Initial WBC count . | % patients responding to PDN pretreatment* . |
|---|---|
| 10 × 109/L | 84 |
| > 10 and 20 × 109/L | 60 |
| > 20 and 30 × 109/L | 61 |
| > 30 and 50 × 109/L | 52 |
| > 50 and 100 × 109/L | 44 |
| > 100 × 109/L | 39 |
| Initial WBC count . | % patients responding to PDN pretreatment* . |
|---|---|
| 10 × 109/L | 84 |
| > 10 and 20 × 109/L | 60 |
| > 20 and 30 × 109/L | 61 |
| > 30 and 50 × 109/L | 52 |
| > 50 and 100 × 109/L | 44 |
| > 100 × 109/L | 39 |
P = .0001.
Response to induction and toxicity.
Of the 778 eligible patients, 391 were randomized in arm A (Cy+) and 387 in arm B (Cy−). Five patients died before starting treatment and 4 responses could not be evaluated; thus, 769 patients, 387 in arm A and 382 in arm B, were evaluable for response. Among those, 627 (82%) patients achieved a CR, 87 (11%) were resistant, and 55 (7%) died during induction. No difference between the 2 randomized induction arms was found concerning CR rate (81% in arm A, 83% in arm B), resistant patients, and induction deaths (Table 3).
Induction results by treatment arm
| . | Total . | Percent . | Cy+ . | Percent . | Cy− . | Percent . |
|---|---|---|---|---|---|---|
| Patients evaluable | 769 | 387 | 382 | |||
| CR | 627 | 82 | 312 | 81 | 315 | 83 |
| Resistant | 87 | 11 | 44 | 11 | 43 | 11 |
| Induction death | 55 | 7 | 31 | 8 | 24 | 6 |
| . | Total . | Percent . | Cy+ . | Percent . | Cy− . | Percent . |
|---|---|---|---|---|---|---|
| Patients evaluable | 769 | 387 | 382 | |||
| CR | 627 | 82 | 312 | 81 | 315 | 83 |
| Resistant | 87 | 11 | 44 | 11 | 43 | 11 |
| Induction death | 55 | 7 | 31 | 8 | 24 | 6 |
Of 87 resistant patients, 62 were given the salvage regimen; 33 (53%) obtained a CR, 22 remained resistant, and 7 died during treatment. Thus, after the salvage regimen overall CR rate increased to 86%.
Hematologic recovery and toxicity after induction are summarized in Table 4. There was a significantly faster neutrophil recovery in arm B, with fewer severe infections and less cardiac toxicity. All patients were hospitalized during induction phase I. The main cause of induction death was infection (27 patients); 12 patients died or went off the study because of hemorrhage or a thromboembolic event mainly due to Escherichia coli ASP.
Postinduction hematologic recovery and grade III to IV toxicity (WHO)
| . | Arm A (Cy+) n = 371 . | Arm B (Cy−) n = 366 . | P . |
|---|---|---|---|
| Days to PMN count greater than or equal to 1 000/μL | 20.7 (0-50) | 18.1 (0-61) | .002 |
| Days to platelet count greater than or equal to 100 000/μL | 16 (0-58) | 14.2 (0-58) | .04 |
| Patients with severe infection | 47 | 35 | NS |
| Patients with liver toxicity | 64 | 52 | NS |
| Patients with neurologic toxicity | 40 | 38 | NS |
| Patients with cardiac toxicity | 9 | 1 | .01 |
| . | Arm A (Cy+) n = 371 . | Arm B (Cy−) n = 366 . | P . |
|---|---|---|---|
| Days to PMN count greater than or equal to 1 000/μL | 20.7 (0-50) | 18.1 (0-61) | .002 |
| Days to platelet count greater than or equal to 100 000/μL | 16 (0-58) | 14.2 (0-58) | .04 |
| Patients with severe infection | 47 | 35 | NS |
| Patients with liver toxicity | 64 | 52 | NS |
| Patients with neurologic toxicity | 40 | 38 | NS |
| Patients with cardiac toxicity | 9 | 1 | .01 |
NS indicates not significant.
The induction response rate was related to age (P = .001) and WBC count (P = .004), even if in the subgroup of patients with hyperleukocytosis (> 100 × 109/L) the CR rate was 83%. The CR rate according to revised immunophenotype was 83% in B-lineage ALL, 85% in T-ALL, and 71% in My+ ALL (P = .001; Table 5).
Univariate analysis of prognostic factors on CR rate, OS, and DFS
| Variable . | . | No. patients . | % CR . | P . | % OS at 8 years . | P . | % DFS at 8 years . | P . |
|---|---|---|---|---|---|---|---|---|
| Age (y) | 20 | 288 | 87 | 34 | 38 | |||
| > 20 and 30 | 187 | 83 | 36 | 32 | ||||
| > 30 and 40 | 120 | 86 | .001 | 20 | .0001 | 15 | .0164 | |
| > 40 and 50 | 113 | 75 | 21 | 29 | ||||
| > 50 and 60 | 121 | 68 | 15 | 19 | ||||
| WBC (× 109/L) | 50 | 568 | 83 | 32 | 33 | |||
| > 50 and 100 | 93 | 78 | .004 | 15 | .0001 | 19 | .0001 | |
| > 100 | 108 | 83 | 18 | 19 | ||||
| Immunophenotype | B lineage | 411 | 83 | 30 | 30 | |||
| T lineage | 134 | 85 | .001 | 27 | NS | 24 | .0204 | |
| My+ | 122 | 71 | 26 | 32 | ||||
| PDN response | Yes | 429 | 87 | 33 | 32 | |||
| No | 228 | 70 | .001 | 17 | .0001 | 22 | .0003 |
| Variable . | . | No. patients . | % CR . | P . | % OS at 8 years . | P . | % DFS at 8 years . | P . |
|---|---|---|---|---|---|---|---|---|
| Age (y) | 20 | 288 | 87 | 34 | 38 | |||
| > 20 and 30 | 187 | 83 | 36 | 32 | ||||
| > 30 and 40 | 120 | 86 | .001 | 20 | .0001 | 15 | .0164 | |
| > 40 and 50 | 113 | 75 | 21 | 29 | ||||
| > 50 and 60 | 121 | 68 | 15 | 19 | ||||
| WBC (× 109/L) | 50 | 568 | 83 | 32 | 33 | |||
| > 50 and 100 | 93 | 78 | .004 | 15 | .0001 | 19 | .0001 | |
| > 100 | 108 | 83 | 18 | 19 | ||||
| Immunophenotype | B lineage | 411 | 83 | 30 | 30 | |||
| T lineage | 134 | 85 | .001 | 27 | NS | 24 | .0204 | |
| My+ | 122 | 71 | 26 | 32 | ||||
| PDN response | Yes | 429 | 87 | 33 | 32 | |||
| No | 228 | 70 | .001 | 17 | .0001 | 22 | .0003 |
In vivo response to PDN pretreatment appeared to influence significantly CR achievement; 374 of 429 PDN responders (87%) obtained CR as compared to 159 of 228 PDN nonresponders (70%) (P = .001; Table 5).
No association was found between CR rate and sex, WHO PS status, the presence or absence of fever or infection, or organomegaly.
In multivariate analysis, the CR rate proved to be strongly influenced by age (P = .0001), PDN pretreatment response (P = .0001), and by the addition of Cy (P = .0441).
Postremission treatment and outcome
Of 627 patients showing a CR, 239 did not undergo the second randomization because of treatment toxicity (n = 37), death in CR (n = 24), early relapse (n = 83), early transplantation (n = 44), and protocol violation or treatment refusal or lost to follow-up (n = 51). Thus, 388 patients (62%) were randomized for postintensification therapy; 187 received C+M and 201 M alone.
Of the 187 patients allocated to receive C+M, 79 completed the assigned treatment regularly, whereas 108 (58%) went off the study because of early relapse (n = 76), chemotherapy-related toxicity (n = 11), treatment refusal (n = 17), or BMT (3 allogeneic, 1 autologous). Only 28 patients (35%) completed consolidation in the expected 6 months; the median time to complete consolidation was 10 months. Hematologic toxicity, mainly during or after courses A and D (which did not include prednisone), was the main cause of treatment delay or interruption. The median duration of neutropenia (PMN count ≤ 1000/μL) and thrombocytopenia (platelets ≤ 100 000/μL) during these 2 courses was 21 days (range, 0-98 days) and 10.2 days (range, 0-67 days), respectively.
Of 201 patients receiving M only, 113 completed the assigned treatment regularly, whereas 88 (44%) went off the study because of early relapse (n = 68), chemotherapy-related toxicity (n = 6), treatment refusal (n = 4), or BMT (5 allogeneic, 5 autologous).
Of the 627 CR patients, 375 (60%) had a relapse in a median time of 10 months (range, 1 month to 6.6 years); late relapse (> 24 months from CR) occurred in 101 patients (27%). Relapses were mainly hematologic; there were 50 isolated CNS relapses, representing 8% of all CRs and 13% of all relapses; this type of relapse occurred in a median time of 14 months (range, 1 month to 6.5 years) from CR achievement. In 17 cases, isolated CNS relapse occurred after more than 2 years (median CCR time was 2.3 years) when patients were still on treatment (n = 9) or off therapy (n = 8).
Prognostic factors for overall survival, CCR, and DFS
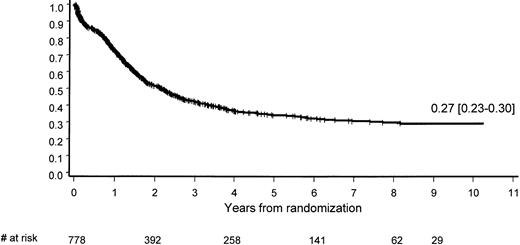
The median overall survival (OS) of the 778 eligible patients was 2.2 years; at 9 years, 27% (95% CI, 23%-30%) are projected to be long-term survivors (Figure 2). OS appeared to be significantly influenced by age (P = .0001) and WBC count (P = .0001; Table 5).
The PDN pretreatment response proved to have a significant impact on survival. At 8 years 33% (95% CI, 28%-38%) of PDN responder patients are survivors compared to 17% (95% CI, 12%-23%) of those classified as nonresponders (P = .0001).
The type of induction did not influence survival. At 8 years 28% of patients treated with Cy were survivors compared to 27% of those treated without Cy (P = .9261).
Median survival from the second randomization in the 388 patients involved was 4 years; at 8 years, 38% (95% CI, 32%-44%) of these are projected to survive. The type of post-CR treatment (C+M or M only) did not reveal any significant influence on survival from randomization. At 8 years, 38% (95% CI, 31%-46%) of patients in arm C+M were survivors versus 37% (95% CI, 28%-46%) of patients in arm M only (P = .1710).
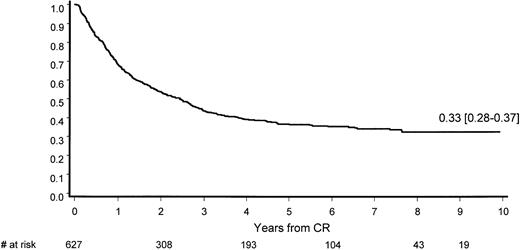
After a median potential follow-up of 7.3 years (range, 3.9-10.4 years), median CR duration was 2.4 years; at 9 years, 33% (95% CI, 28%-37%) of all responders are in CCR (Figure3).
CCR duration.
Patients who achieved CR after salvage therapy are excluded.
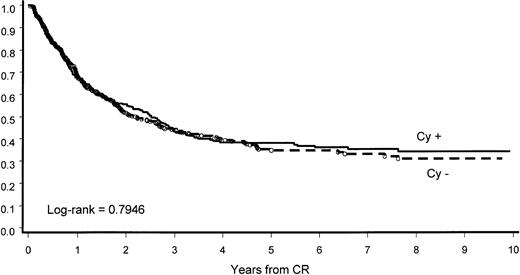
The intensification of induction by the addition of Cy did not influence percentage and length of CCR. At 8 years, 34% of patients in arm A and 31% in arm B had CCR; median CCR length in the 2 groups was 2.5 and 2.2 years, respectively (P = .7946; Figure4).
CCR by induction arm.
The addition of Cy in induction did not influence CCR.
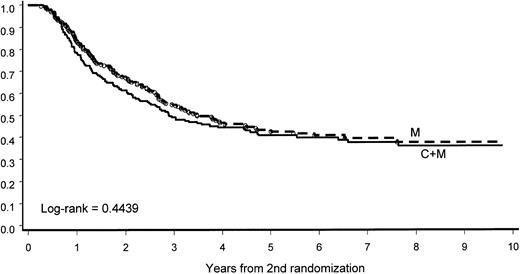
Intensification of post-CR treatment (C+M) did not show any influence on CCR. At 8 years, 36% (95% CI, 28%-44%) of patients in the C+M group and 37% (95% CI, 29%-45%) in the M group showed CCR. The median CCR was 2.9 and 3.5 years in the 2 groups, respectively (P = .4439; Figure 5).
CCR according to the second randomization.
The addition of intensive consolidation (C) to maintenance (M) did not improve CCR duration.
CCR according to the second randomization.
The addition of intensive consolidation (C) to maintenance (M) did not improve CCR duration.
Patient age did not significantly associate with CCR. The probability of CCR at 8 years is 39% for young (≤ 20 years) patients, 33% for those 21 to 30, 18% for those 31 to 40, 33% for those 41 to 50, and 25% for those 51 to 60 years old (P = .4540).
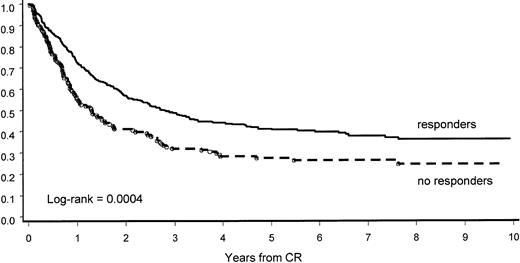
The PDN pretreatment response was confirmed to have a significant impact on CCR. At 8 years, 36% (95% CI, 30%-42%) of PDN responders had CCR versus 24% (95% CI, 16%-32%) of those considered nonresponders (P = .0004; Figure6).
CCR by response to PDN pretreatment.
PDN pretreatment response predicted longer CR duration.
CCR by response to PDN pretreatment.
PDN pretreatment response predicted longer CR duration.
B-lineage ALL appeared to have a significantly longer CCR compared to T-lineage ALL. At 8 years, 34% (95% CI, 28%-40%) of B-lineage ALL cases showed CCR compared to 27% (95% CI, 17%-37%) of the T-ALL cases (P = .0059).
Initial WBC count also appeared to have a significant influence on CCR probability at 8 years: 36%, 22%, and 21% for patients with no more than 50, more than 50 or less than 100, and more than 100 × 109/L, respectively (P = .0001).
Median DFS was 2 years; at 9 years, 29% (95% CI, 24%-33%) of patients are disease-free survivors. The intensification of induction by the addition of Cy did not influence DFS probability and length. At 8 years, 31% of patients are disease-free survivors in arm A and 28% in arm B; median DFS in the 2 groups was 2.3 and 1.9 years, respectively (P = .8055).
Failure to achieve CR after the first induction course strongly influenced DFS. In the group of 33 patients who achieved CR after salvage therapy, median DFS was 7 months; at 3 years, only 12% of these were disease-free survivors.
More intensified post-CR treatment (C+M) did not prove to influence DFS. At 8 years, 34% of patients are disease-free survivors in the C+M group and 35% in the M group; median DFS was 2.4 and 2.9 years, respectively (P = .3811).
Disease-free survival was not influenced by the entire more intensified treatment, that is, Cy+ in induction and C+M in post-CR. At 8 years, 37% of patients, who were given the more intensified approach, were disease-free survivors compared to 34% of those who received the less intense treatment (Cy− and M alone); median DFS was 2.2 and 2.9 years, respectively (P = .7337).
Disease-free survival appeared significantly influenced by age, initial WBC count, immunophenotype, and response to PDN pretreatment (Table 5).
The results of multivariate analysis are shown in Table6. PDN pretreatment was a powerful independent prognostic factor influencing OS, DFS, and CCR. Age at diagnosis influenced significantly OS and DFS, whereas initial WBC count appeared to influence DFS and CCR only.
Significant factors for predicting OS, DFS, and CCR obtained by multivariate analysis
| . | Risk ratio . | 95% CI . | P . |
|---|---|---|---|
| Survival | |||
| Age | 1.018 | 1.011 -1.026 | .0001 |
| PDN response (yes, no) | 1.632 | 1.305 -2.040 | .0001 |
| CCR | |||
| WBC count | 1.000 | 1.000 -1.000 | .0007 |
| PDN response (yes, no) | 1.495 | 1.133 -1.972 | .0044 |
| DFS | |||
| Age | 1.009 | 1.001 -1.018 | .0343 |
| WBC count | 1.000 | 1.000 -1.000 | .0029 |
| PDN response (yes, no) | 1.491 | 1.148 -1.938 | .0028 |
| . | Risk ratio . | 95% CI . | P . |
|---|---|---|---|
| Survival | |||
| Age | 1.018 | 1.011 -1.026 | .0001 |
| PDN response (yes, no) | 1.632 | 1.305 -2.040 | .0001 |
| CCR | |||
| WBC count | 1.000 | 1.000 -1.000 | .0007 |
| PDN response (yes, no) | 1.495 | 1.133 -1.972 | .0044 |
| DFS | |||
| Age | 1.009 | 1.001 -1.018 | .0343 |
| WBC count | 1.000 | 1.000 -1.000 | .0029 |
| PDN response (yes, no) | 1.491 | 1.148 -1.938 | .0028 |
Both randomized additions of Cy (during induction as well as in consolidation) failed to show any influence on OS, DFS, and CCR.
Transplantation
In this study, 61 patients underwent a transplantation procedure, 41 allogeneic and 20 autologous. Of these, 14 were Ph+ ALL and will be discussed later. Of the 47 remaining patients, 34 underwent allotransplantation and 13 autotransplantation; 37 (79%) were younger than 40 years and 10 (21%) had an initial WBC count more than 50 × 109/L. Posttransplantation median CR duration was 50 and 29 months in patients undergoing autotransplantation and allotransplantation, respectively; 18 patients had relapse (7 autotransplantation and 11 allotransplantation) and 8 deaths related to the allotransplantation were recorded.
Ph+ or bcr/abl rearranged patients
Cytogenetic analysis at diagnosis was performed in 216 patients; in 158 there was a sufficient number of evaluable metaphases. The Ph chromosome was identified in 30 patients (19%). Molecular analysis forBCR gene rearrangement from blood or marrow was performed in 200 cases; 38 (19%) were rearranged. When the results of cytogenetic and molecular tests were combined, 47 patients were positive for one or both techniques (Table 7).
Results of cytogenetic and molecular analysis for t(9;22)
| . | bcr rearranged . | bcr not rearranged . | bcr unknown . | Total . |
|---|---|---|---|---|
| Ph+ | 21 | 0 | 9 | 30 |
| Ph− | 4 | 1177-150 | 7 | 128 |
| Ph unknown | 13 | 45 | — | 58 |
| Total | 38 | 162 | 16 | 216 |
| . | bcr rearranged . | bcr not rearranged . | bcr unknown . | Total . |
|---|---|---|---|---|
| Ph+ | 21 | 0 | 9 | 30 |
| Ph− | 4 | 1177-150 | 7 | 128 |
| Ph unknown | 13 | 45 | — | 58 |
| Total | 38 | 162 | 16 | 216 |
Including 6 cases with t(4;11)(q21;q23) and 37 with other abnormalities.
In this subset, the median age was 35.7 years (range, 13.4-58.3 years) and initial median WBC count was 20.9 × 109/L (range, 1.4-190 × 109/L); 38 were classified as having B-lineage ALL, 2 as T-lineage ALL, 5 with My+ ALL, and 2 were not classified.
Thirty-six of these 47 patients were evaluable for PDN pretreatment response; 26 (72%) were responders and 10 (28%) nonresponders. Thirty-nine patients (83%) achieved complete hematologic remission (CHR) and 8 (17%) were resistant; no patient died during induction.
For post-CR therapy, 14 of 39 patients with CR (36%) underwent BMT, 7 allogeneic and 7 autologous transplantations. The remaining 25 did not undergo early BMT because of early relapse (n = 15), chemotherapy-related toxicity (n = 5), or because when they were diagnosed the program of early transplantation for Ph+ ALL had not been activated yet (n = 5).
Overall CHR duration was 9 months in the whole group of patients with Ph+ ALL and 18 months in patients undergoing BMT.
Discussion
This study represents one of the largest adult ALL series reported in literature. Patients were treated with an intensified therapy, which compares well with the intensive approaches designed and applied during the past 10 years.1-13
In childhood leukemia, PDN pretreatment (plus intrathecal MTX) response was recognized as a new, independent, powerful prognostic factor. Poor response to PDN identifies 10% of all pediatric ALL with a dismal prognosis, whose estimated event-free survival is less than 50%.25 In this study the influence of PDN pretreatment alone was for the first time tested in adult ALL and revealed to be an independent prognostic factor for CR achievement as well as for OS, CCR, and DFS. Response to PDN pretreatment correlated directly with initial WBC count and immunophenotype, but not with age and the presence of cytogenetic or molecular abnormalities.
One of the main aims of this study was to test whether the intensification of a conventional 4-drug induction by adding Cy might improve CR rate. Overall CR rate was 82%, which is equal to or higher than the one reported in previous, contemporary, and successive monocentric and multi-institutional studies of adult ALL.1-10 The addition of Cy did not improve the percentage of CR, which was 81% in arm A (Cy+) and 83% in arm B (Cy−). Thus, at variance with the Cancer and Leukemia Group B experience,6 reporting an 85% CR rate and a 46% CCR at 3 years with a similar induction including Cy (although not identical to the present one), we demonstrate that the addition of Cy does not induce a significant increase of cytoreduction translating into CR rate and length improvement.
Induction failure rates, that is, resistant patients and induction deaths, were 11% and 7%, respectively. However, the percentage of true resistant cases was lower, considering that salvage therapy rescued 53% of the cases, thus increasing overall CR rate to 86%. The main cause of induction death was infection, whereas hemorrhage and thromboembolic events, due to E coli ASP, disappeared when the drug was substituted by Erwinia ASP.26
Achievement of CR was influenced by age, initial WBC count, and response to PDN pretreatment, but not by immunophenotype or cytogenetic or molecular abnormality. The CR rate in T-lineage ALL was slightly higher (85%); even in this subset, the impact of Cy during induction did not translate into a significantly better disease outcome. At 8 years, DFS was the same, 20% in arm A (Cy+) and 22% in arm B (Cy−). The presence of myeloid markers appeared to have an impact on the possibility of achieving CR; in this subset, CR rate (71%) was significantly lower than the one observed in B- and T-lineage ALL without myeloid markers.
In this study, 47 (22%) patients of 216 tested were identified as Ph+ or with bcr/abl rearrangement (or both); 72% of them were responders to PDN pretreatment and 83% achieved CR. This CR rate is higher than the one reported in other adult Ph+ ALL multi-institutional studies using a similar induction regimen.1-3,6,10 This suggests that for these patients a more intensive induction may not be necessary. Because in this series of adult Ph+ patients the response rate to PDN pretreatment was the same (72%) as observed in childhood Ph+ cases,27 it can be argued that, as in children, even in adult patients, response to PDN pretreatment could be useful in identifying those with a relatively better prognosis. In fact, among our Ph+ cases, 7 patients with low initial WBC count (median 12.8 × 109/L), who were PDN responders and did not undergo a transplantation procedure, had a prolonged (> 24 months) first CHR (one of them is still in first CHR, off therapy for 12 years). Thus, as in children,27-29 low initial WBC count and PDN pretreatment response could be taken into account in designing therapeutic approaches for Ph+ adult patients ineligible for a transplantation procedure. In view of the use of new agents, such as tyrosine kinase inhibitors, this information may acquire importance because chemotherapy combined with tyrosine kinase inhibitors could be an innovative approach for selected patient categories.30 31
Of 6 patients recognized to have t(4;11)(q21;q23), 5 achieved CR and 4 of them were PDN pretreatment responders; 2 of the patients with CHR achieved molecular remission during post-CR chemotherapy and became long-term remitters and survivors.32
Because in our previous GIMEMA ALL 0183 study we ascribed the high percentage (64%) of early leukemia recurrence to the short duration of post-CR therapy,13 in the present study post-CR therapy was intensified and prolonged for 24 months. Nevertheless, in the present group of patients the median CCR duration was 2.4 years, only marginally influenced by a more intensive post-CR treatment.
The randomized addition of intensive consolidation to a conventional maintenance failed to show a significant impact on CCR. At 8 years both long-term DFS fraction and median DFS duration were similar in the C+M and M arms: 34% and 35% and 2.4 and 2.9 years, respectively. In a high percentage of patients consolidation was not fulfilled in the expected 6 months, requiring nearly 1 year; we cannot exclude that this delay could have hampered the intensification, bringing about early relapses, which occurred in 41% of cases randomized in this arm. The present trial did not include the use of growth factors to overcome myelosuppression during the consolidation phase, because at the time of activation of the study these recombinant molecules were not readily available in this country. Only a few patients received growth factors during consolidation, thus no definitive conclusion about their efficacy can be drawn. However, in childhood high-risk ALL, growth factors during intensive consolidation demonstrated only a moderate increase of chemotherapy dose intensity, which did not prove to have a significant impact on DFS.33
Conventional maintenance—daily 6-MP and weekly MTX—is considered essential to control and to possibly eradicate the minimal residual disease.34-36 Some recent approaches with intensive post-CR chemotherapy not followed by maintenance failed to demonstrate that this strategy could play a positive role in maintaining durable remission both in adult and in childhood ALL.7 37 In the present study, half the patients with CR, assigned to the second randomization, received 24-month conventional maintenance only, 35% of them were disease-free survivors at 8 years. As compared to our previous ALL 0183 trial, in patients who received a similar maintenance treatment, DFS percentage (27% versus 35%) and length (18 versus 35 months) were improved.
However, even in this study the relapse rate (60%) remains intolerably high; relapses were mainly hematologic (52% of patients) and the majority occurred early, with a median time from CR to relapse of 10 months. Isolated CNS relapse occurred in 8% of all CRs and 13% of all relapses. In this trial CNS prophylaxis was intensified by systemic intermediate doses of MTX and Dexa; nevertheless, isolated CNS relapse rate was the same as recorded in our previous GIMEMA ALL 0183 study.13 One may thus infer that intermediate-dose MTX, combined with a standard dose of Dexa, given for 3 courses only, is not sufficient to eradicate CNS leukemia. Because Dexa penetrates into CNS better than prednisolone, thus showing a higher antileukemic activity,38 a larger use of Dexa at high dose might improve CNS prophylaxis in adult patients as well as in children. In the successive GIMEMA ALL 0394 study, in which systemic high-dose (20 mg/m2) Dexa was used during induction and subsequently during systemic CNS prophylaxis, the incidence of isolated CNS relapse dropped to 2% (unpublished data, May 2001), similar to that reported both in M. D. Anderson Center (Houston, TX) adult series39and in a pediatric one.40 The role of cranial irradiation in adult ALL still remains a debated issue; in some monocentric experiences8 it proved to be a successful approach, whereas in larger studies radiotherapy did not completely prevent CNS leukemia recurrence.6 The question is whether in adult patients, as in children, CNS cranial radiotherapy should be reserved for patients with CNS-3 status (5 or more leucocytes/μL with definable blast cells or the presence of cranial nerve palsies)39 only and whether a cyclic intensification with systemic high dose of MTX plus Dexa could help in eradicating CNS leukemia.
In multivariate analysis, so-called conventional factors such as age and initial WBC count confirmed their significant role in influencing DFS (Table 6). Response to PDN pretreatment arose as an important independent factor predicting disease outcome; in PDN responder patients, a high CR rate (87%) translated into a significantly higher percentage of long-term CCRs (Figure 6). Initial rapid cytoreduction is considered a basic step in the treatment of adult ALL; it directly correlates with prolonged remission and survival.1,2 This goal can be achieved by intensifying the induction regimen or by applying a preinduction chemotherapy not including PDN.6 9However, unlike the PDN pretreatment, neither of these approaches can be used to allocate patients into 2 different risk categories.
In this study, as in our previous ALL 0183 trial,13 we were not able to demonstrate an independent prognostic role for the immunophenotype. B-lineage ALL was confirmed to show a marginally better outcome. Compared to B-lineage, T-lineage ALL was less responsive to PDN pretreatment without reaching statistical significance; nevertheless CR rate was slightly higher (83% versus 85%). However, this excellent induction result did not translate into a higher percentage of long-term CCR and DFS. The addition of Cy during induction as well as during the consolidation phase did not significantly influence the outcome of T-ALL. Other multi-institutional studies suggested that Cy plus intermediate-dose ARA-C may be the most appropriate consolidation approach for this patient subset.1,6 41 In the present study, both drugs were used at standard dose after a double randomization; as a consequence, 48 of our T-ALL cases received these drugs in induction or consolidation or both and 35% of them resulted in DFS at 3 years, compared to 39% of the remaining 23 T-ALL patients who received neither Cy+induction nor consolidation. However, due to the small number of patients in each subgroup, we do not think that these results are sufficient to argue against the data obtained from larger series of homogeneously treated T-ALL patients.
Nevertheless, we cannot definitively explain the prognosis of T ALL in our series; it may be speculated both that T ALL is a heterogeneous disease as well as that in our population more aggressive forms were predominant.
Bone marrow transplantation in the first CR is retained as the best approach for high-risk ALL patients, especially for those with genetic abnormalities10-12,36,42-44; for the remaining patients it remains a controversial issue, because long-term follow-up analysis did not show a significant impact of bone marrow transplantation on leukemia-free survival with respect to chemotherapy.45 In our study, in which early BMT was planned from 1990 onward only for Ph+ patients,18 fewer than 10% of those with CR underwent early BMT, half of them coming from high-risk categories. The median CR duration in the whole transplantation series was 30 months and 50% were CCR at 5 years. Considering the low number of patients undergoing transplantation, we cannot draw any factual conclusion about the role of this procedure.
In conclusion, the present long-term follow-up analysis shows, compared to our previous GIMEMA study, that the percentage of long-term survivors and those with DFS improved with this protocol. However, the relapse rate remained disappointingly high, even using a more intensive schedule in induction and in consolidation. The reasons for the persistently high relapse rate may include the following: (1) no specific strategy was applied for high-risk patient subsets and (2) treatment delay, mainly in the consolidation arm, may have hampered an optimal dose intensity of the drugs used. It may also be that long-term follow-up analysis shows the true outcome of adult ALL, for which, regardless of different intensive approaches, leukemia recurrence represents a constant phenomenon. The observation that response to PDN pretreatment is one of the main prognostic factors even in adults indicates that the evaluation of this early therapeutic phase should not be omitted in the future trials for adult ALL and may be useful to stratify patients for different postremission approaches.
Participating centers are listed in alphabetical order by city, with the responsible individuals given and the number of cases provided in parentheses.
Ancona: Nuovo Ospedale Torrette, P. Leoni, M. Montillo, M. Offidani (21); Avellino: Ospedale Civile, E. Volpe, N. Cantore (17); Aviano: Centro di Referimento Oncologico, S. Monfardini, G. Cartei, V. Zagonel (9); Bari: Policlinico, V. Liso, G. Specchia (70); Bergamo: Ospedali Riuniti, T. Barbui, M. Buelli (8); Bologna: Policlinico S. Orsola, S. Tura, M. Baccarani, G. Visani (35); Cagliari: Ospedale Oncologico A. Businco, G. Broccia, †W. Deplano (32); Catania: Ospedale Ferrarotto, †E. Cacciola, R. Giustolisi, F. Di Raimondo (25); Catanzaro: Ospedale Regionale A. Pugliese, A. Alberti, A. Peta, F. Iuliano (33); Cremona: Istituti Ospitalieri, A. Porcellini, P. Bodini, S. Morandi (2); Firenze: Policlinico di Careggi, P. Rossi Ferrini, S. Ciolli (33); Foggia: Ospedali Riuniti, M. Monaco, E. Capussela (2); Genova: Università degli Studi, M. Gobbi, M. Clavio (3); Latina: Ospedale S. M. Goretti, L. De Riu, A. De Blasio, A. Chierichini (12); Milano: Ospedale Niguarda, De Cataldo, E. Morra, G. Muti (15); Napoli: Ospedale A. Cardarelli, R. Cimino, F. Ferrara (31); Napoli: Ospedale Nuovo Pellegrini R. De Biasi, E. Miraglia, D. De Blasi (10); Napoli: Università Federico II, B. Rotoli, A. Camera (43); Napoli: Ospedale A. Cardarelli L. De Rosa, V. Mettivier (6); Nuoro: Ospedale S. Francesco, A. Gabbas. F. Latte (2); Orbassano: Ospedale S. Luigi Gonzaga, G. Saglio, F. Vischia (1); Palermo: Policlinico, †A. Cajozzo, G. Mariani, M. Musso (13); Palermo: Ospedale V. Cervello, F. Caronia, S. Mirto, F. Fabbiano (31); Palermo: Policlinico, P. Citarrella, S. Miceli (6); Pavia: Policlinico S. Matteo, E. Ascari, R. Invernizzi (21); Perugia: Policlinico Monteluce, M. Martelli, A. Tabilio (22); Perugia: Policlinico Monteluce, F. Grignani, A.M. Liberati (1); Pesaro: Ospedale S. Salvatore, G. Lucarelli, G. Sparaventi (11); Pescara: Ospedale Civile, G. Fioritoni, A. Recchia (34); Potenza: Ospedale S. Carlo, F. Ricciuti, M. Pizzuti (11); Reggio Calabria: Azienda Ospedaliera, F. Nobile, P. Iacopino (15); Roma: UniversitàCattolica del Sacro Cuore B. Bizzi, G. Leone, S. Sica (30); Roma: Università degli Studi “La Sapienza,” F. Mandelli, L. Annino, F. Giona (86); Roma: Ospedale S. Camillo A. De Laurenzi, I. Majolino, L. Pacilli (7); Roma: Ospedale S. Eugenio, †G. Papa, S. Amadori, G. Del Poeta (23); S.G. Rotondo: Ospedale Casa Sollievo della Sofferenza, †M. Carotenuto, S. Ladogana, N. Cascavilla (23); Sassari: Università degli Studi, M. Longinotti, F. Dore (1); Udine: Ospedale S. Maria della Misericordia, M. Baccarani, R. Fanin (15); Vicenza: Ospedale Civile S. Bortolo E. Dini, F. Rodeghiero, R. Battista (16); Taranto: Ospedale S.S. Annunziata, P. Mazza, B. Amurri (1); Torino: Ospedale Maggiore S. Giovanni Battista, L. Resegotti, E. Gallo, M. Falda (1).
Supported by grants progetto finalizzato CNR no. 92.02240.PF39, no. 93.02217.PF39, no. 94.01182.PF39.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Franco Mandelli, Department of Cellular Biotechnology and Hematology, Via Benevento, 6-00161, Rome, Italy; e-mail: mandelli@bce.med.uniroma1.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal