Abstract
Emerging data suggest an involvement of angiogenesis in the pathophysiology of acute myeloid leukemia (AML). Thus, antiangiogenic therapy could constitute a novel strategy for the treatment of AML. To test this hypothesis, a phase I/II dose-escalating trial was performed to study the safety and efficacy of thalidomide, a putative inhibitor of angiogenesis, in 20 patients with AML. Thirteen patients were assessable for both toxicity and response, tolerating a maximum dose of 200 to 400 mg daily for at least 1 month. Seven patients had to be prematurely withdrawn from drug administration owing to progressive disease and death (3 patients), personal decision (2 patients), or inability to tolerate thalidomide (2 patients). Overall, adverse events were fatigue, constipation, rash, and neuropathy (grade 1 to 2 in most patients). In 4 patients, a partial response, defined as reduction of at least 50% in the blast cell infiltration of the bone marrow accompanied by increases in platelet counts and hemoglobin values, was observed. One additional patient showed a hematologic improvement without fulfilling the criteria of a partial response. The responses lasted a median of 3 months (range, 1-8 months). In parallel, microvessel densities significantly decreased in these 5 patients during treatment with thalidomide (P < .05). This decrease was accompanied by declining plasma levels of basic fibroblast growth factor, one of the most potent angiogenic growth factors. In conclusion, single-agent thalidomide has antiangiogenic and antileukemic activity in AML, although a causal relationship between both effects has still to be proven.
Introduction
Angiogenesis, a complex process of pericellular proteolysis, endothelial migration, and proliferation, is an absolute requirement for the viability and growth of solid tumors.1Recently, we reported increased angiogenesis in the bone marrow of patients with acute myeloid leukemia (AML) and normalization of bone marrow microvessel density when patients achieved a complete remission (CR).2 This suggests an involvement of angiogenesis in the pathophysiology of AML as well. Thus, antiangiogenic therapy could constitute a novel strategy for the treatment of AML.
Thalidomide, a derivative of glutamic acid, was introduced in Europe in 1954 as a sedative/hypnotic agent,3 but was removed from the market when its teratogenic effects were discovered. Recently, it was demonstrated that thalidomide has potent antiangiogenic activity in animal models,4,5 possibly owing to its direct inhibition of endothelial cell proliferation.6 This prompted the initiation of a number of clinical trials of thalidomide for the treatment of advanced malignancies. In contrast to its limited activity reported in advanced solid tumors so far,7-9 its efficacy in acquired immunodeficiency syndrome (AIDS)–related Kaposi sarcoma and refractory multiple myeloma is striking.10,11 However, since thalidomide has a variety of different modes of action,12a causal relationship between its antiangiogenic effect and clinical activity against neoplastic diseases has still to be demonstrated.
We conducted a phase I/II dose-escalating study in AML patients not qualifying for intensive cytotoxic chemotherapy in order to assess the efficacy and safety of thalidomide in AML.
Patients and methods
Study design
This prospective phase I/II dose-escalating clinical trial was conducted at the University of Muenster (Germany) in accordance with the Helsinki protocol and with the approval of the local institutional review board. Thalidomide was supplied by Gruenenthal (Aachen, Germany). Eligible patients had to be 18 years or older and have had AML according to the criteria of the French-American-British Cooperative Group.13 The study included only patients who were poor candidates for intensive cytotoxic chemotherapy (eg, congestive heart failure according to New York Heart Association classification III/IV, severe chronic obstructive lung disease, serious active infections, age older than 80 years, duration of first CR not exceeding 6 months) or who had proved to be refractory toward at least 2 standard induction chemotherapies and were not eligible for allogeneic stem cell transplantation because of advanced age (older than 60 years) and/or serious comorbidity. Negative serum pregnancy testing was required within 48 hours of the first dose and monthly thereafter for all women with childbearing potential. Patients had to have an Eastern Cooperative Oncology Group performance status not exceeding 2 and a liver and renal function as follows: bilirubin not more than 34.2 μM (2 mg/dL); aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels no greater than 10.0 times the upper limit of normal; and serum creatinine no more than 305 μM (4.0 mg/dL). Pre-existing peripheral neuropathy of grade 2 or higher was an exclusion criterion. All patients had to give written informed consent to the study.
Patients were treated with 200 mg/d thalidomide, escalating by 200 mg/d every 2 weeks until a total dose of 800 mg/d was achieved. Safety was assessed at least weekly through physical examinations, recording of vital signs, toxicity assessments, and laboratory tests (hematology, coagulation, clinical chemistry). Grade 2 toxicities were considered to be dose limiting, requiring withdrawal from further dose escalation. Additionally, neurotoxicity of grade 2 required a dose reduction of 200 mg. Persistent grade 2 neurotoxicity lasting more than 6 days after dose reduction resulted in discontinuation of the study drug. Patients were taken off the study if they had grade 3 neurotoxicity, other grade 3 toxicity persisting more than 6 days after dose reduction, or any grade 4 toxicity. Thalidomide was given in the evening to avoid sedative side effects during daytime. Patients were asked to take lactulose (Bifiteral, Solvay Arzneimittel, Hannover, Germany) to prevent constipation.
Evaluation and criteria of response
Complete blood cell counts were examined weekly, and bone marrow biopsies were performed before and 1 month after initiation of treatment with thalidomide. CR was defined according to a consensus definition.14 Partial response (PR) was defined as a decrease in the blast cell infiltration of the bone marrow of at least 50% of the pretreatment value. An increase in hemoglobin values greater than 100 g/L (10 g/dL) and platelet counts exceeding 30 × 109 L (30 000/μL) during treatment with thalidomide resulting in the patient's becoming independent of previously required transfusions was considered to be hematologic improvement (HI). The determination of the degree of bone marrow angiogenesis was performed by immunohistochemical identification of microvascular endothelial cells with antihuman thrombomodulin antibodies (clone 1009) (DAKO, Carpinteria, CA) by means of the alkaline phosphatase/antialkaline phosphatase double-bridge technique (DAKO-APAAP kit) (DAKO, Glostrup, Denmark) as previously described.2 The degree of marrow vascularization was expressed as microvessel counts per × 500 field. The median and interquartile range for the microvessel density of 22 control patients were 13.2 and 11.4 to 14.8 × 500 field, respectively.2
Measurement of angiogenic growth factors
Blood samples for measurements of the circulating angiogenic factors, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) were obtained after 1 month of treatment with thalidomide and quantitatively determined in citrate anticoagulated plasma by enzyme-linked immunosorbent assay (ELISA) (Quantikine human VEGF, Quantikine HS human FGF basic) (R&D Systems, Minneapolis, MN). Owing to the known release of VEGF from platelets,15 venous blood sampling for VEGF measurement was performed within glass collection tubes in the presence of citrate, theophylline, adenosine, dipyridamole as platelet stabilizer (Becton Dickinson Vacutainer Systems, Plymouth, United Kingdom). Tubes were centrifuged at 3000g for 10 minutes at 20°C to generate platelet-poor plasma.16 Aliquots were stored at −70°C until analysis and assayed for each patient on the same ELISA plate. The normal range (mean ± 1 SD), determined in 25 healthy volunteers, was 22.5 ± 20.8 pg/mL for VEGF and 9.4 ± 6.4 pg/mL for bFGF.
Statistical analyses
Data are presented as individual data plots or medians with interquartile ranges including low quartile and high quartile (LQ-HQ). Differences in peripheral blood cell counts, percentage of leukemic blasts, and microvessel density in the bone marrow before and after treatment with thalidomide were analyzed by the Wilcoxon matched-pair signed rank test. The Mann-Whitney rank sum test for independent groups was used to compare bone marrow microvessel counts in thalidomide-responding, nonresponding, and control patients. Comparisons of VEGF/bFGF plasma level changes and dose of thalidomide between responders and nonresponders were evaluated by the Fisher exact test. For all tests, P < .05 was considered significant. The 95% confidence intervals were calculated by employing exact binomial confidence limits.
Results
A total of 20 patients aged 58 to 85 years (median, 69 years) were accrued to the study. Patient and disease characteristics are summarized in Table 1. Ten patients had secondary AML defined as a history of myelodysplasia, other antecedent hematologic disorder, or previous exposure to cytostatic drugs or radiotherapy. Cytogenetic analyses were available in 13 patients; these showed prognostically unfavorable chromosomal aberrations in 5 patients, standard-risk cytogenetic abnormalities in 3 patients, and normal karyotypes in 5 patients. Eleven patients had AML relapse. Fourteen patients had received prior chemotherapy regimens (4 patients had received 1 regimen, and 10 patients, 2 or more regimens). Before starting thalidomide administration, patients had been off previous therapy for a median of 31 weeks (range, 10-48 weeks). Duration of treatment with thalidomide ranged from 1 up to 40 weeks, with a median of 7 weeks (Table 2). In 7 patients, thalidomide administration had to be stopped within the first month because of early disease progression and death (3 patients), personal decision (2 patients), or drug intolerance (2 patients) on the lowest dose level of 200 mg/d. Of the 3 patients who had progressive disease and died, 2 showed a white blood cell count of 21.4 × 109/L (21 400/μL) and 27 × 109/L (27 000/μL), respectively, before the start of treatment. The remaining 13 patients were treated for more than 4 weeks with thalidomide after achieving the maximal tolerable dose of 200 to 400 mg/d.
Patient and disease characteristics
| Patient No. . | Age, y . | Sex . | AML FAB subtype/karyotype* . | Treatment history . | Duration of first CR, mo . | No. reinduction attempts . |
|---|---|---|---|---|---|---|
| 1 | 64 | Male | M1/del(7)(q31), t(1;7)(q11;q22) | 2nd relapse | 17 | 1 |
| 2 | 73 | Female | M1/tetrasomy 22 | 1st relapse | 5 | N/A |
| 3 | 85 | Female | Secondary/normal | Untreated | N/A | N/A |
| 4 | 67 | Male | Secondary, M5/del(7)(p22) | 1st relapse | 6 | N/A |
| 5 | 69 | Female | M1/normal | 1st relapse | 30 | N/A |
| 6 | 62 | Female | M2/normal | 2nd relapse | 31 | 2 |
| 7 | 78 | Female | Secondary, M1/normal | Untreated | N/A | N/A |
| 8 | 76 | Male | Secondary/† | 1st relapse | 19 | N/A |
| 9 | 68 | Male | Secondary, M1/del(5q) | Refractory | N/A | N/A |
| 10 | 62 | Male | Secondary/† | Untreated | N/A | N/A |
| 11 | 72 | Male | Secondary, M4/† | Refractory | N/A | N/A |
| 12 | 65 | Male | M7/del(7)(q22) | 2nd relapse | 58 | 2 |
| 13 | 64 | Female | M4/del(9p) | 2nd relapse | 15 | 2 |
| 14 | 82 | Female | M1/† | Untreated | N/A | N/A |
| 15 | 82 | Female | M6/† | Untreated | N/A | N/A |
| 16 | 69 | Male | Secondary, M4/† | Refractory | N/A | N/A |
| 17 | 58 | Male | Secondary, M6/complex | 1st relapse | 5 | N/A |
| 18 | 68 | Male | Secondary, M5/† | Untreated | N/A | N/A |
| 19 | 63 | Female | M2/inv(3q), del(7)(q31) | 1st relapse | 8 | N/A |
| 20 | 79 | Female | M5/normal | 1st relapse | 2 | N/A |
| Patient No. . | Age, y . | Sex . | AML FAB subtype/karyotype* . | Treatment history . | Duration of first CR, mo . | No. reinduction attempts . |
|---|---|---|---|---|---|---|
| 1 | 64 | Male | M1/del(7)(q31), t(1;7)(q11;q22) | 2nd relapse | 17 | 1 |
| 2 | 73 | Female | M1/tetrasomy 22 | 1st relapse | 5 | N/A |
| 3 | 85 | Female | Secondary/normal | Untreated | N/A | N/A |
| 4 | 67 | Male | Secondary, M5/del(7)(p22) | 1st relapse | 6 | N/A |
| 5 | 69 | Female | M1/normal | 1st relapse | 30 | N/A |
| 6 | 62 | Female | M2/normal | 2nd relapse | 31 | 2 |
| 7 | 78 | Female | Secondary, M1/normal | Untreated | N/A | N/A |
| 8 | 76 | Male | Secondary/† | 1st relapse | 19 | N/A |
| 9 | 68 | Male | Secondary, M1/del(5q) | Refractory | N/A | N/A |
| 10 | 62 | Male | Secondary/† | Untreated | N/A | N/A |
| 11 | 72 | Male | Secondary, M4/† | Refractory | N/A | N/A |
| 12 | 65 | Male | M7/del(7)(q22) | 2nd relapse | 58 | 2 |
| 13 | 64 | Female | M4/del(9p) | 2nd relapse | 15 | 2 |
| 14 | 82 | Female | M1/† | Untreated | N/A | N/A |
| 15 | 82 | Female | M6/† | Untreated | N/A | N/A |
| 16 | 69 | Male | Secondary, M4/† | Refractory | N/A | N/A |
| 17 | 58 | Male | Secondary, M6/complex | 1st relapse | 5 | N/A |
| 18 | 68 | Male | Secondary, M5/† | Untreated | N/A | N/A |
| 19 | 63 | Female | M2/inv(3q), del(7)(q31) | 1st relapse | 8 | N/A |
| 20 | 79 | Female | M5/normal | 1st relapse | 2 | N/A |
AML indicates acute myeloid leukemia; FAB, French-American-British classification, N/A, not applicable.
See Bennett et al.13
Not available.
Thalidomide doses and response
| Patient no. . | Treatment duration, wk . | Final dose, mg/d . | Response to thalidomide . | Toxicity (WHO grade) . |
|---|---|---|---|---|
| 1 | 40 | 400 | PR | Constipation (2) |
| 2 | 15 | 400 | No response | Constipation (1) |
| 3 | 19 | 200 | PR | Constipation (2) |
| 4 | 3 | 400 | Withdrawal (early progression) | N/A |
| 5 | 14 | 200 | HI | Constipation (3) |
| 6 | 2 | 200 | Withdrawal (drug intolerance) | Neuropathy (2) |
| 7 | 1 | 200 | Withdrawal (personal decision) | Fatigue (1) |
| 8 | 4 | 400 | Withdrawal (personal decision) | Fatigue (2), rash (2) |
| 9 | 4 | 200 | Withdrawal (early progression) | Fatigue (2) |
| 10 | 7 | 400 | No response | Fatigue (1), constipation (2) |
| 11 | 5 | 200 | PR | Fatigue (1), rash (2), neuropathy (2) |
| 12 | 9 | 400 | No response | Constipation (2) |
| 13 | 8 | 200 | Progression | Fatigue (2), neuropathy (1) |
| 14 | 2 | 200 | Withdrawal (early progression) | Rash (2) |
| 15 | 5 | 200 | Progression | Fatigue (1), constipation (2), rash (1) |
| 16 | 11 | 200 | No response | Fatigue (2) |
| 17 | 7 | 200 | Progression | Fatigue (1), constipation (2) |
| 18 | 3 | 200 | Withdrawal (drug intolerance) | Fatigue (1), neuropathy (2) |
| 19 | 9 | 200 | No response | Fatigue (2) |
| 20 | 8 | 200 | PR | Fatigue (1), constipation (2), rash (1) |
| Patient no. . | Treatment duration, wk . | Final dose, mg/d . | Response to thalidomide . | Toxicity (WHO grade) . |
|---|---|---|---|---|
| 1 | 40 | 400 | PR | Constipation (2) |
| 2 | 15 | 400 | No response | Constipation (1) |
| 3 | 19 | 200 | PR | Constipation (2) |
| 4 | 3 | 400 | Withdrawal (early progression) | N/A |
| 5 | 14 | 200 | HI | Constipation (3) |
| 6 | 2 | 200 | Withdrawal (drug intolerance) | Neuropathy (2) |
| 7 | 1 | 200 | Withdrawal (personal decision) | Fatigue (1) |
| 8 | 4 | 400 | Withdrawal (personal decision) | Fatigue (2), rash (2) |
| 9 | 4 | 200 | Withdrawal (early progression) | Fatigue (2) |
| 10 | 7 | 400 | No response | Fatigue (1), constipation (2) |
| 11 | 5 | 200 | PR | Fatigue (1), rash (2), neuropathy (2) |
| 12 | 9 | 400 | No response | Constipation (2) |
| 13 | 8 | 200 | Progression | Fatigue (2), neuropathy (1) |
| 14 | 2 | 200 | Withdrawal (early progression) | Rash (2) |
| 15 | 5 | 200 | Progression | Fatigue (1), constipation (2), rash (1) |
| 16 | 11 | 200 | No response | Fatigue (2) |
| 17 | 7 | 200 | Progression | Fatigue (1), constipation (2) |
| 18 | 3 | 200 | Withdrawal (drug intolerance) | Fatigue (1), neuropathy (2) |
| 19 | 9 | 200 | No response | Fatigue (2) |
| 20 | 8 | 200 | PR | Fatigue (1), constipation (2), rash (1) |
WHO indicates World Health Organization; PR, partial response; NA, not applicable; and HI, hematologic improvement.
No patient tolerated a thalidomide dose above 400 mg daily without adverse events of grade 2 or more according to the classification system of the World Health Organization (Table 2). Especially, fatigue up to grade 2 (12 patients) and constipation up to grade 3 (9 patients) despite the use of laxatives required dose reduction. Five patients developed rashes after the second week of thalidomide administration. As additional side effect, peripheral neuropathy of grade 2 with tremor as the main symptom, occurred in 4 patients and resulted in withdrawal from the study in 2 of the patients on the lowest dose level. After dose reduction or discontinuation, symptoms of neuropathy completely resolved.
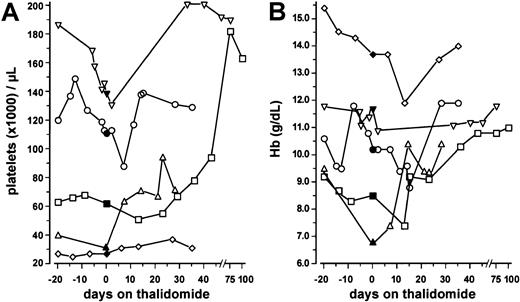
Of the 13 patients receiving thalidomide for longer than 4 weeks, 4 patients achieved PR and 1 patient achieved HI (Table 2). Three of these 5 patients had de novo AML; 2, secondary AML. Cytogenetic analyses, available in 4 of the responding patients, revealed an unfavorable karyotype in 1 patient, del(7)(q31), t(1;7)(q11;q22), and normal karyotypes in the 3 other patients. Four of the responding patients had received prior chemotherapy regimens (1 patient had had 1 regimen; 3 patients, 2 or more regimens). Two patients were in first, 1 patient in second AML relapse; 1 patient was refractory to previous chemotherapy; 1 patient was untreated. The other 8 patients had progressive disease after 4 weeks of thalidomide and were taken off the study. In all 5 responding patients (4 PRs, 1 HI), a significant increase in the platelet counts was observed when median values of the period before (day −20 until day 0) and during (day +1 until day +45) thalidomide administration were compared (P = .042, Wilcoxon test; Figure 1A).
Platelet counts and hemoglobin values before and during therapy with thalidomide in 5 responding patients with AML.
Data represent individual values within a period of 20 days before and during treatment with thalidomide, the latter period independent of transfusion effects (■, patient no. 1; ▵, patient no. 3; ▿, patient no. 5; ⋄, patient no. 11; ○, patient no. 20). Closed symbols denote the values obtained on the day when thalidomide was started (day 0). (A) Median platelet counts were significantly higher during thalidomide treatment (day +1 to +45) compared with the pretreatment period (day −20 until day 0) (P = .042, Wilcoxon matched-pair signed rank test). (B) Hemoglobin values were not significantly different between the two time periods (P = .893).
Platelet counts and hemoglobin values before and during therapy with thalidomide in 5 responding patients with AML.
Data represent individual values within a period of 20 days before and during treatment with thalidomide, the latter period independent of transfusion effects (■, patient no. 1; ▵, patient no. 3; ▿, patient no. 5; ⋄, patient no. 11; ○, patient no. 20). Closed symbols denote the values obtained on the day when thalidomide was started (day 0). (A) Median platelet counts were significantly higher during thalidomide treatment (day +1 to +45) compared with the pretreatment period (day −20 until day 0) (P = .042, Wilcoxon matched-pair signed rank test). (B) Hemoglobin values were not significantly different between the two time periods (P = .893).
In the 2 previously anemic patients, the hemoglobin concentrations increased independently from red blood cell (RBC) transfusions or erythropoietin administration (Figure 1B). In patient no. 1, this increase was sustained until day +282, reaching a maximum hemoglobin value of 144 g/L (14.4 g/dL) on day +205 of thalidomide treatment. However, median hemoglobin values of the entire group obtained in the period from 20 days before thalidomide treatment until treatment were not significantly different from those obtained during (day +1 to +45) thalidomide treatment (P = .893, Wilcoxon test; Figure1B). The absolute neutrophil counts remained unchanged (data not shown). The median treatment duration was 7 weeks (range, 1-40 weeks), and the duration of the response lasted a median of 3 months (range, 1-8 months). There was no relationship between thalidomide dose and response (P = .49 for the comparison of thalidomide dose between responders and nonresponders; Fisher exact test).
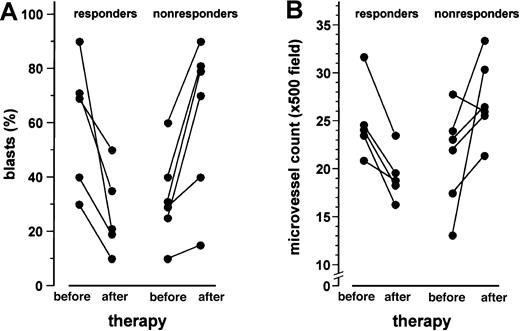
Parallel to the decrease of the leukemic blast cell infiltration in the bone marrow of responding patients, a significant drop of the microvessel counts was observed after 1 month of thalidomide treatment (P < .05, Wilcoxon test; Figure2). Figure3 illustrates the significant decrease in microvessel density after 1 month of treatment with thalidomide in a patient who achieved a PR (patient no. 11). In contrast, the progression of the leukemic blast cell infiltration in the bone marrow of nonresponding patients was associated with a significant increase in the bone marrow microvessel density (P < .05, Wilcoxon test; Figure 2). Apart from intraindividual differences, the responding patients revealed significantly lower microvessel densities after thalidomide administration than nonresponders (median [LQ-HQ] 18.5 [18.3-19.3] versus 26.1 [25.9-29.4]/ × 500 field;P = .014, Mann-Whitney rank sum test for independent groups), whereas there was no difference in marrow vascularity before treatment (P = .24). Nevertheless, microvessel counts in the responding patients did not reach the normal range of the previously described control group of 22 patients (median [LQ-HQ] 13.2 [11.4-14.8]/ × 500 field;P = .001).2
Leukemic blast cell infiltration and microvessel density of the bone marrow before and after 1 month of treatment with thalidomide.
Data are presented as individual values. A significant reduction of the blast cell infiltration in the 5 patients responding to thalidomide (panel A) with a concomitant decrease of the microvessel counts (panel B) was observed (P < .05, Wilcoxon matched-pair signed rank test). In contrast, in the 6 nonresponding patients, the percentage of blast cell infiltration (panel A) and microvessel density (panel B) increased (P < .05).
Leukemic blast cell infiltration and microvessel density of the bone marrow before and after 1 month of treatment with thalidomide.
Data are presented as individual values. A significant reduction of the blast cell infiltration in the 5 patients responding to thalidomide (panel A) with a concomitant decrease of the microvessel counts (panel B) was observed (P < .05, Wilcoxon matched-pair signed rank test). In contrast, in the 6 nonresponding patients, the percentage of blast cell infiltration (panel A) and microvessel density (panel B) increased (P < .05).
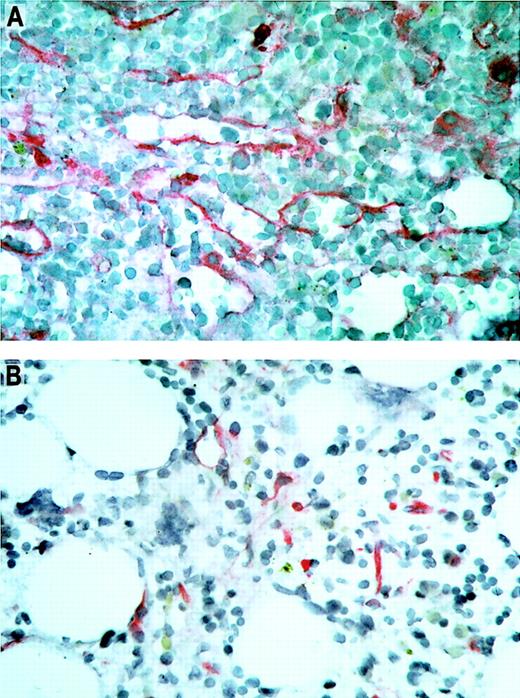
Immunohistochemical staining of bone marrow sections.
Microvascular endothelial cells were highlighted by antibodies against thrombomodulin. The bone marrow biopsies were taken from patient no. 11 with AML before (panel A) and 1 month after (panel B) treatment with thalidomide. Note the visible reduction in microvessel density of the bone marrow during thalidomide treatment in this patient with a partial response. Original magnification × 500.
Immunohistochemical staining of bone marrow sections.
Microvascular endothelial cells were highlighted by antibodies against thrombomodulin. The bone marrow biopsies were taken from patient no. 11 with AML before (panel A) and 1 month after (panel B) treatment with thalidomide. Note the visible reduction in microvessel density of the bone marrow during thalidomide treatment in this patient with a partial response. Original magnification × 500.
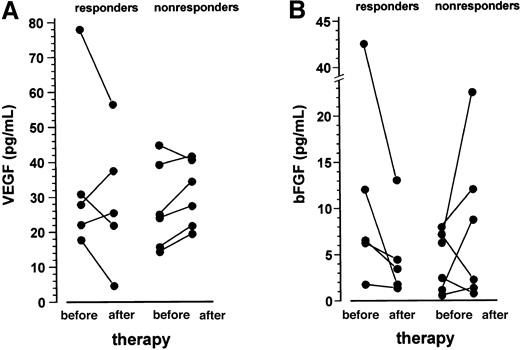
VEGF and bFGF plasma levels of the entire patient population were within the normal range of the controls. After 1 month of treatment with thalidomide, VEGF plasma levels decreased in 3 of 5 responding patients, whereas an increase was observed in 5 of 6 nonresponders (P = .197 for the comparison of change in VEGF between responders and nonresponders, Fisher exact test; Figure4A). A similar pattern was observed for the bFGF plasma levels, with a drop in all responding patients and an increase in 4 of 6 nonresponders (P = .045 for the comparison of change in bFGF between responders and nonresponders, Fisher exact test; Figure 4B).
VEGF and bFGF plasma levels before and after 1 month of treatment with thalidomide.
Data are presented as individual values. VEGF plasma levels (panel A) decreased in 3 of 5 responding patients, whereas an increase was observed in 5 of 6 nonresponders (P = .197 for the comparison of change in VEGF between responders and nonresponders, Fisher exact test). The bFGF levels (panel B) declined in all responding patients and increased in 4 of 6 nonresponders (P = .045 for the comparison of change in bFGF between responders and nonresponders; Fisher exact test).
VEGF and bFGF plasma levels before and after 1 month of treatment with thalidomide.
Data are presented as individual values. VEGF plasma levels (panel A) decreased in 3 of 5 responding patients, whereas an increase was observed in 5 of 6 nonresponders (P = .197 for the comparison of change in VEGF between responders and nonresponders, Fisher exact test). The bFGF levels (panel B) declined in all responding patients and increased in 4 of 6 nonresponders (P = .045 for the comparison of change in bFGF between responders and nonresponders; Fisher exact test).
Discussion
In the search for novel therapeutic targets for AML, angiogenesis seems to be a promising candidate, given the observation of increased microvessel density in AML2,17,18 and the growing biologic data that demonstrate overexpression of inducers of angiogenesis such as VEGF and bFGF.17-22 This phase I/II dose-escalating study of thalidomide is the first prospective trial of an antiangiogenic agent in a small group of patients with AML, most of whom had proved to be refractory toward intensive cytotoxic chemotherapy. The results demonstrate that thalidomide has antileukemic activity in this patient population, with 4 patients achieving PR and 1 patient achieving HI (response rate, 25%; 95% confidence intervals, 8.6%-49.1%). In the responding patients, platelet counts significantly increased, demonstrating a biologically relevant effect of thalidomide. In contrast, although the hemoglobin concentration increased in the 2 previously anemic patients, this did not reach statistical significance for the entire group. This observation is most likely due to the fact that the change in hemoglobin concentrations is underestimated because anemic patients had received RBC transfusions in the period before thalidomide administration. No complete or partial remissions according to consensus definitions were observed.14 23 However, this was not unexpected given the fact that most of the patients had relapsed/refractory AML.
The exact mechanism of the antileukemic activity of thalidomide remains to be elucidated. The significant decrease in the microvessel density in responding patients in contrast to the nonresponders is consistent with the hypothesis that the observed antileukemic activity might have been mediated through an antiangiogenesis mechanism. This would be in line with recent reports of the antiangiogenic activity of thalidomide in animal models4,5 and its direct inhibitory effect on endothelial proliferation.6 However, it is also possible that the apparent antileukemic effects of thalidomide may not be solely attributable to antiangiogenesis, but rather were mediated directly through other mechanisms, such as the demonstrated ability of thalidomide to induce oxidative damage to DNA mediated by free radicals.24 Thus, the observed decrease in microvessel density in responding patients may be in part indirectly mediated by a loss of survival signals from leukemic blasts undergoing apoptosis, since a decrease in microvessel density has also been noted after administration of cytotoxic chemotherapy.2 Furthermore, the ability of thalidomide to modulate the immune system25-31 or to alter the profile of adhesion molecules32 may contribute to its antileukemic effects. Of course, antiangiogenic and antileukemic activities of thalidomide could enhance each other, since paracrine growth signal exchange between AML blasts and endothelial cells has been proposed.19 22
To gain further insight into the mechanism of thalidomide activity, we studied circulating markers of angiogenesis. There was a significant relationship between the decrease in bFGF plasma levels and response to thalidomide treatment. Whether bFGF is directly down-regulated by thalidomide, or whether the decrease merely reflects an antileukemic effect of treatment, remains to be elucidated. In contrast, we did not observe a consistent effect on VEGF levels in responding patients. This might be due to interindividual variation and/or the small number of patients studied.
The profile of side effects observed in this trial is less favorable than that observed in previous studies in solid tumors and AIDS-related Kaposi sarcoma.7,9 10 According to the strict criteria of the study protocol, no patient tolerated thalidomide doses above 400 mg daily without adverse events of grade 2 or higher. Factors contributing to the higher observed incidence of toxicities, especially fatigue, constipation, and neuropathy, may be the advanced age, the anemia, and the pretreatment with intensive cytotoxic chemotherapy of our patient population. However, it has to be emphasized that 13 of 20 patients did stay on treatment for a median of 9 weeks (range, 5-40 weeks). Therefore, thalidomide should be explored earlier in the course of the disease, and other antiangiogenic drugs or analogs of thalidomide with fewer side effects are needed.
We did not observe any relationship between dose of thalidomide and response. This might be due to the low variation in the final dose of thalidomide because we considered grade 2 toxicities to be dose limiting in order not to further compromise quality of life with this investigational therapy. Thus, we cannot exclude the possibility that higher doses of thalidomide have greater efficacy. On the other hand, the nature of the dose-response curve for thalidomide is not well characterized in either AML or other hematologic11 or nonhematologic malignancies.7-9 Therefore, dose escalation to the maximum tolerable dose may or may not be the most appropriate dose-finding strategy. This has to be addressed in future studies.
Since thalidomide has only modest activity as a single agent, its future role in the treatment of AML has to be determined. There are growing data demonstrating synergism between antiangiogenic agents and traditional cytotoxic therapies.33-35 Therefore, it might be promising to include thalidomide in standard induction chemotherapy regimens. A recent abstract report on a study randomizing patients with newly diagnosed AML or myelodysplastic syndrome with excessive blasts and abnormal karyotypes, except inv(16), t(8;21), and t(15;17) to liposomal daunorubicin plus ara-C with or without thalidomide showed no difference in early CR rates in the 2 arms.36 However, early CR rate may not be the appropriate clinical end point for an antiangiogenic agent, because antiangiogenic treatment strategies might have higher efficacy in a state of minimal residual disease. Therefore, evaluation of thalidomide or other more selective antiangiogenic agents as maintenance therapy may be more promising.
In summary, thalidomide has antiangiogenic and antileukemic activity in AML. Thus, thalidomide and more specific antiangiogenic principles should be further evaluated in clinical trials for the treatment of AML.
The authors are indebted to Achim Heinecke, PhD, of the Department of Biostatistics, University of Muenster, Germany, for his biostatistical advice and assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rolf M. Mesters or Wolfgang E. Berdel, Dept of Medicine/Hematology and Oncology, University of Muenster, Albert-Schweitzer-Strasse 33, D-48129 Muenster, Germany; e-mail:mesters@uni-muenster.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal