Abstract
Addition of a delayed-intensification (DI) phase after standard induction/consolidation therapy was previously shown to improve outcome for patients younger than 10 years of age with intermediate-risk acute lymphoblastic leukemia (ALL). The current trial randomized 1204 patients to regimens containing a single DI phase (405 patients), 2 DI phases (DDI) (402 patients), or a single DI phase in conjunction with increased vincristine and prednisone pulses during maintenance (DIVPI) (397 patients). Estimates of event-free survival (EFS) and survival at 6 years are 79% ± 1% and 89% ± 1%, respectively. EFS was improved on DDI compared with either DI (log-rankP = .04; Kaplan-Meier [KM] P = .04; relative risk [RR] = 1.38) or DIVPI (log-rankP = .04; KM P = .01; RR = 1.39).There was no difference in EFS for the DI and DIVPI regimens (log-rankP = .96; KM P = .75). Survival estimates at 6 years were 87% (SD = 2%) for DI; 91% (SD = 2%) for DDI; and 90% (SD = 2%) for DIVPI (P = .17). Significant univariate risk factors for the overall cohort included poor day-7 marrow response, black race, and age of at least 5 years. These data demonstrate that DDI improves EFS of patients younger than 10 years of age with intermediate-risk ALL.
Introduction
Current National Cancer Institute (NCI) criteria1 for risk assignment in acute lymphoblastic leukemia (ALL) define standard-risk disease as that occurring in children older than 1 year of age and younger than 10 years of age who have white blood cell (WBC) counts lower than 50 × 109L (50 000/μL). Previous Children's Cancer Group (CCG) studies stratified such children with ALL into low- and intermediate-risk groups based on presenting age, WBC count, sex, and platelet count. These studies have shown that 60% to 70% of such children can be cured with induction therapy consisting of vincristine, prednisone, L-asparaginase, central nervous system (CNS) prophylaxis consisting of intrathecal methotrexate without cranial radiation therapy, and 2 to 3 years of maintenance therapy based on oral 6-mercaptopurine and methotrexate.2 The CCG-161 trial for lower-risk patients found that addition of monthly vincristine and prednisone pulses during maintenance increased the event-free survival (EFS) of lower-risk patients from 64% to 77%.3 In relapsed patients, most of whom had already received vincristine and prednisone pulses on frontline therapy, addition of vincristine to sequential methotrexate and asparaginase doubled the duration of second remissions compared with standard therapy.4 This result raised the possibility that more frequent vincristine and prednisone pulses might be more effective than the monthly pulses.
Subsequently, the CCG-105 study for intermediate-risk ALL found that the addition of a delayed-intensification phase (DI) based on that used in the Berlin-Frankfurt-Muenster (BFM) 76/79 study5prior to maintenance with 6-mercaptopurine, methotrexate, and vincristine/prednisone pulses was advantageous for the subset of patients younger than 10 years of age who had WBC counts higher than 10 × 109L (10 000/μL) and lower than 50 × 109L (50 000/μL).6 The 5-year EFS in this study was 61% for patients who did not receive DI and 77% for patients who received DI (P = .001). Adverse risk factors for intermediate-risk patients treated on CCG-105 included WBC count of at least 20 × 109L (20 000/μL), male sex, and CD24 negativity. In addition, as has now been shown for all risk groups, day-14 marrow response was a highly significant predictor of EFS. An M2 marrow status (5% to 25% blasts) at day 14 conferred a 1.3-fold excess risk of an event, and M3 (more than 25% blasts), a 3.4-fold excess risk of an event.7
On the basis of these findings, the successor study for intermediate-risk ALL, CCG-1891, was initiated with the primary objective of further improving outcome by modifying the single DI-based regimen employed on CCG-105 with either a second DI phase (DDI) or with an increased number of vincristine and prednisone pulses (DIVPI) given during the maintenance phase. In this report, we show that DDI improves outcome, particularly for subsets of patients with intermediate-risk ALL who show delayed early responses to induction therapy.
Patients and methods
Patients
CCG-1891 opened in January 1990 and closed in July 1993. Eligible patients included those aged 1 through 1.99 years with WBC count lower than 50 × 109L (50 000/μL); those aged 2 years through 9.99 years with WBC count from 10 × 109L (10 000/μL) to lower than 50 × 109L (50 000/μL); and males aged 2 years through 9.99 years with WBC count lower than 10 × 109L (10 000/μL) and platelet counts lower than 100 × 109L (100 000/μL). Patients with lymphoma syndrome or L3 lymphoblasts were excluded. Diagnosis was based on morphological, biochemical, and immunological features of leukemic cells, including lymphoblast morphology on Wright-Giemsa–stained bone marrow smears, negative staining for myeloperoxidase, and reactivity with monoclonal antibodies to B-lineage–associated or T-lineage–associated lymphoid differentiation antigens, or the myeloid antigens, CD13 and CD33, as described previously.8 9 Remission was defined as fewer than 5% blasts with recovery of trilineage hematopoiesis.
Treatment protocol
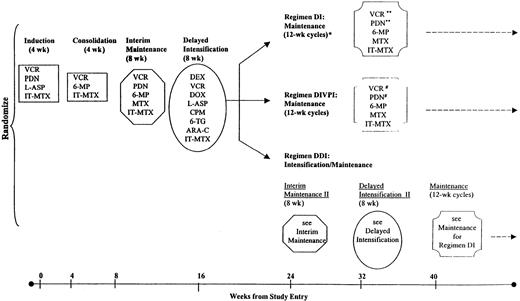
Patients were randomly assigned at diagnosis to 1 of 3 postconsolidation intensification regimens (DI, DDI, DIVPI). Therapy was identical for all patients from the start of treatment until the end of the first DI; thereafter, therapy was as shown in Figure1. Patients were required to have M1 or M2 marrow status by the end of induction and M1 marrow status by the end of consolidation therapy to continue on the study. The protocol was approved by the NCI and CCG Institutional Review Boards. Written, informed consent was obtained from parents or guardians.
Therapy on CCG-1891.
Eligible enrolled patients were randomized at study entry to receive standard therapy with 1 delayed intensification phase (DI); standard therapy with 2 delayed intensification phases (DDI); or standard therapy with 1 delayed intensification phase and intensified vincristine and prednisone pulses during maintenance (DIVPI). Cumulative doses of anthracyclines were 75 mg/m2 in DI and DIVPI and 150 mg/m2 in DDI, and cumulative doses of cyclophosphamide were 1000 mg/m2 in DI and DIVPI and 2000 mg/m2 in DDI. There were 15 doses of L-asparaginase in DI and DIVPI and 21 in DDI. There were 8 doses of cytarabine at 75 mg/m2 in DI and DIVPI and 16 in DDI. DI prescribed 31 doses of 1.5 mg/m2 of vincristine for girls and 43 for boys; DIVPI, 41 doses for girls and 57 for boys; DDI, 34 doses for girls and 47 for boys. In DI, excluding tapers, there were 138 days of corticosteroid for girls and 198 for boys; in DIVPI, 188 days for girls and 268 for boys; and in DDI, 159 days for girls and 219 for boys. VCR indicates vincristine; PDN, prednisone; L-ASP, L-asparaginase; IT-MTX, intrathecal methotrexate; MP, mercaptopurine; MTX, methotrexate; DEX, dexamethasone; DOX, doxorubicin; CPM, cyclophosphamide; TG, thioguanine; ARA-C, cytosine arabinoside; *, total duration of therapy was 2 years for girls and 3 years for boys on all regimens; **, pulses given every 4 weeks; and #, pulses given every 3 weeks.
Therapy on CCG-1891.
Eligible enrolled patients were randomized at study entry to receive standard therapy with 1 delayed intensification phase (DI); standard therapy with 2 delayed intensification phases (DDI); or standard therapy with 1 delayed intensification phase and intensified vincristine and prednisone pulses during maintenance (DIVPI). Cumulative doses of anthracyclines were 75 mg/m2 in DI and DIVPI and 150 mg/m2 in DDI, and cumulative doses of cyclophosphamide were 1000 mg/m2 in DI and DIVPI and 2000 mg/m2 in DDI. There were 15 doses of L-asparaginase in DI and DIVPI and 21 in DDI. There were 8 doses of cytarabine at 75 mg/m2 in DI and DIVPI and 16 in DDI. DI prescribed 31 doses of 1.5 mg/m2 of vincristine for girls and 43 for boys; DIVPI, 41 doses for girls and 57 for boys; DDI, 34 doses for girls and 47 for boys. In DI, excluding tapers, there were 138 days of corticosteroid for girls and 198 for boys; in DIVPI, 188 days for girls and 268 for boys; and in DDI, 159 days for girls and 219 for boys. VCR indicates vincristine; PDN, prednisone; L-ASP, L-asparaginase; IT-MTX, intrathecal methotrexate; MP, mercaptopurine; MTX, methotrexate; DEX, dexamethasone; DOX, doxorubicin; CPM, cyclophosphamide; TG, thioguanine; ARA-C, cytosine arabinoside; *, total duration of therapy was 2 years for girls and 3 years for boys on all regimens; **, pulses given every 4 weeks; and #, pulses given every 3 weeks.
Common therapy
Induction chemotherapy consisted of prednisone (40 mg/m2 per day for 28 days with a 7- to 10-day taper); vincristine (1.5 mg/m2 per week × 4); L-asparaginase (6000 IU/m2 intramuscularly [IM] × 9); and age-adjusted intrathecal methotrexate (age 1 through 1.99 years, 8 mg; age 2 through 2.99 years, 10 mg; age at least 3 years, 12 mg on days 0 and 14). Patients with CNS disease at diagnosis also received intrathecal methotrexate on days 7 and 21. Consolidation consisted of daily oral 6-mercaptopurine (75 mg/m2 per day); vincristine (1.5 mg/m2 on day 0); and age-adjusted intrathecal methotrexate (doses as given above) weekly × 4. Patients with CNS leukemia at diagnosis, ie, at least 0.005 × 109L WBCs (5 WBCs/μL) with blasts on cytospin,10 received 2400 cGy cranial radiation and 600 cGy spinal radiation. Patients with testicular leukemia received 2400 cGy testicular irradiation. Interim maintenance therapy consisted of continued daily oral 6-mercaptopurine (75 mg/m2 per day); weekly oral methotrexate (20 mg/m2 per week); intravenous vincristine (1.5 mg/m2 on days 0 and 28); oral prednisone (40 mg/m2 on days 0 through 4 and 28 through 32); and intrathecal methotrexate (age-adjusted doses, as above) on day 0. The DI phase consisted of intravenous vincristine (1.5 mg/m2 on days 0, 7, and 14); dexamethasone (10 mg/m2 per day for 21 days with 7-day taper); doxorubicin (25 mg/m2 intravenously [IV] bolus on days 0, 7, and 14); L-asparaginase (6000 IU/m2 IM 3 times a week for 6 doses); cyclophosphamide (1 g/m2 on day 28); cytosine arabinoside (75 mg/m2IV or subcutaneously on days 29 through 32 and 36 through 39); oral 6-thioguanine (60 mg/m2 on days 28 through 41); and age-adjusted intrathecal methotrexate (doses as above on days 28 and 35).
Regimen-specific intensification/maintenance
Treatment on the 3 regimens diverged following DI. The DI and DIVPI regimens proceeded to standard or intensified maintenance therapy, respectively (see below). The DDI regimen included a second interim maintenance phase and a second DI phase, each of which was identical to the interim maintenance and DI phases described above, before proceeding to standard maintenance. The standard 12-week maintenance courses for the DI regimen consisted of intravenous vincristine (1.5 mg/m2 on days 0, 28, and 56); oral prednisone (40 mg/m2 on days 0 through 4, 28 through 32, and 56 through 60); daily oral 6-mercaptopurine (75 mg/m2per day); weekly oral methotrexate (20 mg/m2 per week); and age-adjusted intrathecal methotrexate (doses given as above on day 0 of each course). The DIVPI maintenance regimen was identical to standard maintenance except that vincristine (1.5 mg/m2) was given every 3 weeks (days 0, 21, 42, and 63) and oral prednisone (40 mg/m2) was given every 3 weeks (on days 0 through 4, 21 through 25, 42 through 46, and 63 through 67). The legend to Figure 1lists the cumulative doses and numbers of doses of agents in the 3 regimens. On all regimens, therapy continued for 2 years for girls and 3 years for boys, timed from the beginning of interim maintenance.
Statistical methods
In the initial planning of this study, sample size and power calculations were based on a proportional hazards assumption for the treatment regimens, with few treatment failures assumed to occur after 5 years of follow-up. The planned accrual of 400 patients per randomized regimen yielded in excess of 80% power for a 2-sided log-rank test with a multiple comparison adjustment to detect a change in outcome from a baseline EFS of 80% to 88%. This change in EFS represents a reduction in the hazard rate by 43% (ie, relative hazard rate of 0.57 for the improved treatment). EFS is defined in this study as the time to the first occurrence of any one of the following events: induction death, nonresponse to induction therapy, relapse after initial remission at any site, death in remission, or second malignant neoplasm. The study protocol also emphasized the importance of directly comparing EFS estimates at later periods of follow-up, when the EFS estimates would be close to their plateau values.
Randomization occurred at the time of study entry. Comparison of treatment outcome used the intent-to-treat philosophy: all events that occurred after randomization were attributed to the regimen initially assigned. Since the treatment regimens did not diverge until the end of the initial DI phase (which was planned to be 24 weeks from study entry), there was some diminution of treatment effect estimates and reduction of significance levels for the regimen comparisons. EFS outcome was the primary end point used for life table comparisons of treatment regimen outcomes and prognostic factor effects. Comparison of overall survival was a secondary end point. Life table estimates used the Kaplan-Meier (KM) method,11 and KM estimates were provided at 6 years of follow-up, which represent a stable estimate of long-term outcome, since very few events occurred subsequent to this time point and almost all event-free patients had follow-up beyond 6 years. The SD of the KM estimate was calculated by means of Peto variance formula.12 Relative hazard rates were estimated by the log-rank ratio of observed-to-expected method. Chi-square tests for homogeneity of distributions were used in some comparisons (similarity of patient characteristics, patterns of outcome events, etc). Multivariate analysis of prognostic factors used the Cox proportional hazards model.13 For reporting purposes, conventional significance for statistical comparisons was defined asP ≤ .05, and borderline significance as .05 < P ≤ .15. Reported P values were unadjusted. The Bonferroni procedure, which would require multiplication of the P value by 3, could be used to adjust the pairwise treatment comparisons. Comparisons of duration of hospitalization among the 3 regimens used the nonparametric Wilcoxon rank test.14
Results
Patient characteristics
A total of 1219 patients were entered in the study; of these patients, 1204 were deemed eligible. Nine patients were ineligible owing to inadequate Institutional Review Board approval; of the remaining 6 patients, 3 had incorrect diagnoses, 2 had low-risk ALL, and 1 had received systemic steroid therapy prior to study entry. In the study, 405 patients were randomized to DI; 397 patients were randomized to DIVPI; and 402 patients were randomized to DDI. Presenting characteristics of patients randomized to each of the 3 regimens are shown in Table1. As expected, nearly all characteristics were distributed homogeneously among the 3 regimens, although hepatomegaly and splenomegaly were less frequent in patients who received the DDI regimen. Overall, the clinical criteria used to assign patients to the intermediate-risk classification resulted in twice as many boys as girls in the current cohort.
Presenting features of children with intermediate-risk acute lymphoblastic leukemia
| Variable and category . | DI (N = 405) . | DIVPI (N = 397) . | DDI (N = 402) . | P* . | |||
|---|---|---|---|---|---|---|---|
| N . | (%) . | N . | (%) . | N . | (%) . | ||
| Age, y | |||||||
| 1-1.99 | 52 | (13) | 62 | (16) | 73 | (18) | .22 |
| 2-4.99 | 231 | (57) | 220 | (55) | 228 | (57) | |
| 5 or older | 122 | (30) | 115 | (29) | 101 | (25) | |
| WBC count, × 109/L | |||||||
| Less than 20 | 286 | (71) | 276 | (70) | 277 | (69) | .87 |
| 20-49 | 119 | (29) | 121 | (30) | 125 | (31) | |
| Sex | |||||||
| Male | 279 | (69) | 275 | (69) | 264 | (66) | .49 |
| Female | 126 | (31) | 122 | (31) | 138 | (34) | |
| Race | |||||||
| White | 310 | (77) | 300 | (76) | 304 | (76) | .96 |
| Black | 13 | (3) | 15 | (4) | 19 | (5) | |
| Hispanic | 56 | (14) | 57 | (14) | 53 | (13) | |
| Other | 26 | (6) | 25 | (6) | 26 | (6) | |
| Down syndrome | |||||||
| Yes | 11 | (3) | 12 | (3) | 10 | (2) | .90 |
| No | 394 | (97) | 385 | (97) | 391 | (98) | |
| Liver† | |||||||
| Normal | 158 | (39) | 162 | (41) | 188 | (47) | .03 |
| Moderately enlarged | 231 | (57) | 217 | (55) | 207 | (52) | |
| Markedly enlarged | 16 | (4) | 18 | (5) | 6 | (2) | |
| Spleen† | |||||||
| Normal | 169 | (42) | 156 | (39) | 199 | (50) | .03 |
| Moderately enlarged | 218 | (54) | 226 | (57) | 193 | (48) | |
| Markedly enlarged | 18 | (4) | 15 | (4) | 10 | (2) | |
| Lymph nodes‡ | |||||||
| Normal | 206 | (51) | 210 | (53) | 204 | (51) | .83 |
| Moderately enlarged | 191 | (47) | 176 | (44) | 186 | (46) | |
| Markedly enlarged | 8 | (2) | 11 | (3) | 12 | (3) | |
| Mediastinal mass1-153 | |||||||
| Absent | 393 | (97) | 384 | (97) | 397 | (99) | .24 |
| Small | 12 | (3) | 12 | (3) | 5 | (1) | |
| Large | 0 | (0) | 1 | (0) | 0 | (0) | |
| Hemoglobin (g/dL) | |||||||
| 1-7.9 | 285 | (70) | 269 | (68) | 260 | (65) | .53 |
| 8.0-10.9 | 97 | (24) | 106 | (27) | 117 | (29) | |
| 11.0 or more | 23 | (6) | 22 | (6) | 25 | (6) | |
| Platelets (× 109/L) | |||||||
| 1-49 | 248 | (61) | 243 | (61) | 242 | (60) | .82 |
| 50-149 | 122 | (30) | 115 | (29) | 115 | (29) | |
| 150 or more | 35 | (9) | 39 | (10) | 45 | (11) | |
| CNS disease at diagnosis | |||||||
| Yes | 4 | (1) | 8 | (2) | 3 | (1) | .38 |
| No | 399 | (99) | 389 | (98) | 397 | (99) | |
| Testicular disease at diagnosis | |||||||
| Yes | 6 | (2) | 8 | (3) | 5 | (2) | .96 |
| No | 273 | (98) | 267 | (97) | 256 | (98) | |
| Immunophenotype1-155 | |||||||
| B-lineage | 271 | (97) | 268 | (94) | 270 | (97) | .24 |
| T-lineage | 9 | (3) | 16 | (6) | 9 | (3) | |
| Myeloid lineage | |||||||
| Yes | 36 | (20) | 48 | (24) | 27 | (14) | .04 |
| No | 147 | (80) | 148 | (76) | 161 | (86) | |
| Ploidy group | |||||||
| Normal | 44 | (28) | 40 | (30) | 52 | (34) | .21 |
| Pseudodiploid | 6 | (4) | 4 | (3) | 14 | (9) | |
| Hypodiploid | 30 | (19) | 32 | (24) | 23 | (15) | |
| Hyperdiploid (47-50) | 18 | (12) | 13 | (10) | 14 | (9) | |
| Hyperdiploid (more than 50) | 58 | (37) | 43 | (33) | 51 | (33) | |
| N | (%) | N | (%) | N | (%) | ||
| Translocation | |||||||
| t(4;11)(q21;q23) | |||||||
| Yes | 2 | (1) | 1 | (1) | 0 | (0) | .39 |
| No | 154 | (99) | 131 | (99) | 154 | (100) | |
| t(9;22)(q34;q11) | |||||||
| Yes | 1 | (1) | 2 | (2) | 1 | (1) | .68 |
| No | 155 | (99) | 130 | (98) | 153 | (99) | |
| t(1;19)(q23;p13) | |||||||
| Yes | 7 | (4) | 4 | (3) | 4 | (3) | .63 |
| No | 149 | (96) | 128 | (97) | 150 | (97) | |
| Variable and category . | DI (N = 405) . | DIVPI (N = 397) . | DDI (N = 402) . | P* . | |||
|---|---|---|---|---|---|---|---|
| N . | (%) . | N . | (%) . | N . | (%) . | ||
| Age, y | |||||||
| 1-1.99 | 52 | (13) | 62 | (16) | 73 | (18) | .22 |
| 2-4.99 | 231 | (57) | 220 | (55) | 228 | (57) | |
| 5 or older | 122 | (30) | 115 | (29) | 101 | (25) | |
| WBC count, × 109/L | |||||||
| Less than 20 | 286 | (71) | 276 | (70) | 277 | (69) | .87 |
| 20-49 | 119 | (29) | 121 | (30) | 125 | (31) | |
| Sex | |||||||
| Male | 279 | (69) | 275 | (69) | 264 | (66) | .49 |
| Female | 126 | (31) | 122 | (31) | 138 | (34) | |
| Race | |||||||
| White | 310 | (77) | 300 | (76) | 304 | (76) | .96 |
| Black | 13 | (3) | 15 | (4) | 19 | (5) | |
| Hispanic | 56 | (14) | 57 | (14) | 53 | (13) | |
| Other | 26 | (6) | 25 | (6) | 26 | (6) | |
| Down syndrome | |||||||
| Yes | 11 | (3) | 12 | (3) | 10 | (2) | .90 |
| No | 394 | (97) | 385 | (97) | 391 | (98) | |
| Liver† | |||||||
| Normal | 158 | (39) | 162 | (41) | 188 | (47) | .03 |
| Moderately enlarged | 231 | (57) | 217 | (55) | 207 | (52) | |
| Markedly enlarged | 16 | (4) | 18 | (5) | 6 | (2) | |
| Spleen† | |||||||
| Normal | 169 | (42) | 156 | (39) | 199 | (50) | .03 |
| Moderately enlarged | 218 | (54) | 226 | (57) | 193 | (48) | |
| Markedly enlarged | 18 | (4) | 15 | (4) | 10 | (2) | |
| Lymph nodes‡ | |||||||
| Normal | 206 | (51) | 210 | (53) | 204 | (51) | .83 |
| Moderately enlarged | 191 | (47) | 176 | (44) | 186 | (46) | |
| Markedly enlarged | 8 | (2) | 11 | (3) | 12 | (3) | |
| Mediastinal mass1-153 | |||||||
| Absent | 393 | (97) | 384 | (97) | 397 | (99) | .24 |
| Small | 12 | (3) | 12 | (3) | 5 | (1) | |
| Large | 0 | (0) | 1 | (0) | 0 | (0) | |
| Hemoglobin (g/dL) | |||||||
| 1-7.9 | 285 | (70) | 269 | (68) | 260 | (65) | .53 |
| 8.0-10.9 | 97 | (24) | 106 | (27) | 117 | (29) | |
| 11.0 or more | 23 | (6) | 22 | (6) | 25 | (6) | |
| Platelets (× 109/L) | |||||||
| 1-49 | 248 | (61) | 243 | (61) | 242 | (60) | .82 |
| 50-149 | 122 | (30) | 115 | (29) | 115 | (29) | |
| 150 or more | 35 | (9) | 39 | (10) | 45 | (11) | |
| CNS disease at diagnosis | |||||||
| Yes | 4 | (1) | 8 | (2) | 3 | (1) | .38 |
| No | 399 | (99) | 389 | (98) | 397 | (99) | |
| Testicular disease at diagnosis | |||||||
| Yes | 6 | (2) | 8 | (3) | 5 | (2) | .96 |
| No | 273 | (98) | 267 | (97) | 256 | (98) | |
| Immunophenotype1-155 | |||||||
| B-lineage | 271 | (97) | 268 | (94) | 270 | (97) | .24 |
| T-lineage | 9 | (3) | 16 | (6) | 9 | (3) | |
| Myeloid lineage | |||||||
| Yes | 36 | (20) | 48 | (24) | 27 | (14) | .04 |
| No | 147 | (80) | 148 | (76) | 161 | (86) | |
| Ploidy group | |||||||
| Normal | 44 | (28) | 40 | (30) | 52 | (34) | .21 |
| Pseudodiploid | 6 | (4) | 4 | (3) | 14 | (9) | |
| Hypodiploid | 30 | (19) | 32 | (24) | 23 | (15) | |
| Hyperdiploid (47-50) | 18 | (12) | 13 | (10) | 14 | (9) | |
| Hyperdiploid (more than 50) | 58 | (37) | 43 | (33) | 51 | (33) | |
| N | (%) | N | (%) | N | (%) | ||
| Translocation | |||||||
| t(4;11)(q21;q23) | |||||||
| Yes | 2 | (1) | 1 | (1) | 0 | (0) | .39 |
| No | 154 | (99) | 131 | (99) | 154 | (100) | |
| t(9;22)(q34;q11) | |||||||
| Yes | 1 | (1) | 2 | (2) | 1 | (1) | .68 |
| No | 155 | (99) | 130 | (98) | 153 | (99) | |
| t(1;19)(q23;p13) | |||||||
| Yes | 7 | (4) | 4 | (3) | 4 | (3) | .63 |
| No | 149 | (96) | 128 | (97) | 150 | (97) | |
DI indicates delayed-intensification phase; DIVPI, a single DI phase in conjunction with increased vincristine and prednisone pulses during maintenance; DDI, 2 DI phases; WBC, white blood cell; and CNS, central nervous system.
Chi-square test.
Markedly enlarged is defined as organ below the umbilicus; data from one DDI patient missing.
Normal is defined as normal or shotty nodes; markedly enlarged is defined as visible nodes.
Large is defined as mass below the umbilicus.
Among 843 patients with data on B-lineage and T-lineage antigen expression and 576 patients with data on myeloid antigen expression.
Treatment outcome
On day 28, 1190 patients were eligible to proceed to phase 2: 12 had M2 marrows, and 38 lacked the blast percentage. Day-7 marrow response was analyzed among the overall cohort of patients who achieved M1 or M2 marrow status at the end of induction therapy. Among this cohort, 51% of all patients were M1, 24% were M2, and 24% were M3 at day 7 of induction. At the end of induction, 98% of all patients were in remission (M1 or M2 marrow status). Nine patients (2 on DI; 3 on DIVPI; 4 on DDI) were M3 at the end of induction and were taken off protocol. Five patients (1 on DI; 2 on DIVPI; 2 on DDI) died during induction. Similar day-7 marrow and end-of-induction results were observed for patients on each of the randomized regimens.
The 6-year EFS and survival estimates for the overall cohort of patients on CCG-1891 were 79% (SD = 1%) and 89% (SD = 1%), respectively. EFS estimates at 6 years were 76% (SD = 2%) for DI; 83% (SD = 2%) for DDI; and 77% (SD = 2%) for DIVPI (P = .08; Figure 2). EFS was improved on DDI compared with either DI (log-rank P = .04; KM P = .04; relative risk [RR] = 1.38) or DIVPI (log-rank P = .04; KM P = .01; RR = 1.39). In addition, a comparison of outcome for patients on DDI versus patients who did not receive DDI (DI and DIVPI regimens combined) showed a significant difference favoring DDI (log-rankP = .02; KM P = .02). These data indicate an approximate 28% reduction in events for DDI compared with the other 2 regimens. There was no difference in EFS for the DI and DIVPI regimens (log-rank P = .96; KM P = .75). Survival estimates at 6 years were 87% (SD = 2%) for DI; 91% (SD = 2%) for DDI; and 90% (SD = 2%) for DIVPI (P = .17). There was no significant difference in EFS estimates or P value if the 12 patients with M2 marrows or the 38 without a recorded blast percentage are excluded from the analysis. Because Figure 2 shows a few more early events in the DIVIP arm prior to week 24, the results were examined from week 24. Overall outcomes and P value were unchanged.
EFS by randomized regimen for patients with intermediate risk ALL.
The probability of surviving event-free is higher for patients randomized to DDI, compared with those randomized to DIVPI or DI. The numbers of patients remaining in follow-up at 6 and 9 years, respectively, were 282 and 38 on DI; 268 and 33 on DIVPI; and 299 and 45 on DDI.
EFS by randomized regimen for patients with intermediate risk ALL.
The probability of surviving event-free is higher for patients randomized to DDI, compared with those randomized to DIVPI or DI. The numbers of patients remaining in follow-up at 6 and 9 years, respectively, were 282 and 38 on DI; 268 and 33 on DIVPI; and 299 and 45 on DDI.
The number and type of events on each of the randomized regimens are shown in Table 2. Both isolated bone marrow and isolated CNS relapses were lower on the DDI regimen compared with the DI and DIVPI regimens. Remission deaths, however, were more frequent on the DIVPI and DDI regimens compared with the DI regimen. There were 4 second malignancies: 2 on DDI and 2 on DIVPI.
Number and type of first event according to randomized regimen
| Event . | Randomized regimen . | ||
|---|---|---|---|
| DI . | DIVPI . | DDI . | |
| Induction failure* | 2 | 3 | 4 |
| Induction death | 1 | 2 | 2 |
| Relapse | |||
| Marrow | 40 | 35 | 23 |
| Marrow + CNS | 4 | 3 | 7 |
| Marrow + testicular | 3 | 3 | 0 |
| Marrow + SMN | 0 | 0 | 1 |
| CNS | 32 | 27 | 19 |
| Testicular | 7 | 8 | 5 |
| Other | 2 | 3 | 0 |
| SMN | 0 | 2 | 2 |
| Remission death | 4 | 8 | 9 |
| Total | 95 | 94 | 72 |
| Event . | Randomized regimen . | ||
|---|---|---|---|
| DI . | DIVPI . | DDI . | |
| Induction failure* | 2 | 3 | 4 |
| Induction death | 1 | 2 | 2 |
| Relapse | |||
| Marrow | 40 | 35 | 23 |
| Marrow + CNS | 4 | 3 | 7 |
| Marrow + testicular | 3 | 3 | 0 |
| Marrow + SMN | 0 | 0 | 1 |
| CNS | 32 | 27 | 19 |
| Testicular | 7 | 8 | 5 |
| Other | 2 | 3 | 0 |
| SMN | 0 | 2 | 2 |
| Remission death | 4 | 8 | 9 |
| Total | 95 | 94 | 72 |
SMN indicates second malignant neoplasm. See Table 1 footnote for other abbreviations.
M3 (> 25% blasts) marrow status at day 28 of induction.
Prognostic factors
Univariate analysis of the overall cohort indicated that age of at least 5 years (P = .001), nonwhite race (P < .0001), marked splenomegaly (P = .002), and hemoglobin of at least 11 g/dL (P = .0003) were significant adverse risk factors and that the presence of a t(4;11) or t(9;22) translocation (occurring in 3 and 4 patients, respectively) was marginally significant (P = .09). In addition, normal chromosomes or high hyperdiploidy (greater than 50 chromosomes) conferred decreased risk of treatment failure (P = .001). With respect to race, white patients had the best outcome (6-year EFS = 82%, SD = 1%); Hispanic or “other” patients had intermediate outcome (6-year EFS = 71%, SD = 4%; and 72%, SD = 6%, respectively); and black patients had the worst outcome (6-year EFS = 54%, SD = 9%) (P < .0001) (Figure 3). Male children had worse outcome than females, although the log-rank P value did not reach conventional significance (P = .07): EFS probabilities were similar for males and females during the first 3 years from study entry and subsequently diverged owing to more posttherapy events among males (data not shown). Patients whose leukemic cells expressed markers for B-lineage ALL had the same outcomes as those for T-lineage ALL (P = .90), and patients whose cells coexpressed lymphoid and myeloid markers had similar outcomes to those with only lymphoid markers (P > .99).
EFS for patients with intermediate-risk ALL according to race.
The probability of surviving event free is highest for those classified as white, lowest for those classified as black, and intermediate for the other groups. The numbers of patients remaining in follow-up at 6 years and 9 years were, respectively, 690 and 95 for whites; 95 and 10 for Hispanics; 18 and 2 for blacks; and 46 and 9 for others.
EFS for patients with intermediate-risk ALL according to race.
The probability of surviving event free is highest for those classified as white, lowest for those classified as black, and intermediate for the other groups. The numbers of patients remaining in follow-up at 6 years and 9 years were, respectively, 690 and 95 for whites; 95 and 10 for Hispanics; 18 and 2 for blacks; and 46 and 9 for others.
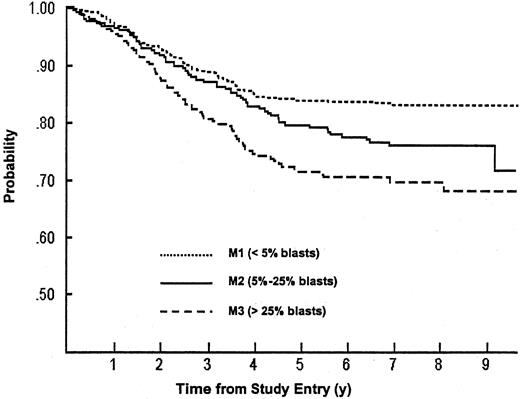
Day-7 response was also a significant prognostic factor, with 6-year EFS of 84% (SD = 2%) for patients with M1 marrow status, as compared with 78% (SD = 3%) with M2 or 71% (SD = 3%) with M3 (P < .0001) (Figure 4). Within the DI regimen, day-7 marrow status was also a significant predictor of outcome (P = .0008), but within the DIVPI and DDI regimens, day-7 marrow status reached only borderline prognostic significance (P = .07 for both groups) (Table3). Although there was no clear EFS advantage for patients on the DDI regimen within the subset of patients with a day-7 M1 marrow (RR, DI versus DDI = 1.16), DDI appeared superior to DI for patients who were M2 (RR, DI versus DDI = 1.61) or M3 (RR, DI versus DDI = 1.64) at day 7 of induction (Table 3).
EFS for patients with intermediate-risk ALL according to early bone marrow status.
The probability of surviving event free is highest for patients with M1 (fewer than 5% blasts); intermediate for those with M2 (5% to 25% blasts); and lowest for those with M3 (more than 25% blasts) bone marrow status at day 7 of induction therapy. The numbers of patients remaining in follow-up at 6 years and 9 years were, respectively, 398 and 58 for M1 patients; 186 and 23 for M2 patients; and 146 and 18 for M3 patients.
EFS for patients with intermediate-risk ALL according to early bone marrow status.
The probability of surviving event free is highest for patients with M1 (fewer than 5% blasts); intermediate for those with M2 (5% to 25% blasts); and lowest for those with M3 (more than 25% blasts) bone marrow status at day 7 of induction therapy. The numbers of patients remaining in follow-up at 6 years and 9 years were, respectively, 398 and 58 for M1 patients; 186 and 23 for M2 patients; and 146 and 18 for M3 patients.
Relative risk for events according to day-7 bone marrow status and intensification regimen
| Day-7 bone marrow status . | 5-year EFS by randomized regimen % (SD) . | RR (DI vs DDI) . | ||
|---|---|---|---|---|
| DI (N = 405) . | DIVPI (N = 397) . | DDI (N = 402) . | ||
| M1 (less than 5% blasts) | 84 (3) | 82 (3) | 86 (3) | 1.16 |
| M2 (5%-25% blasts) | 77 (4) | 74 (5) | 89 (4) | 1.61 |
| M3 (more than 25% blasts) | 63 (6) | 77 (5) | 74 (5) | 1.64 |
| RR | ||||
| M2 vs M1 | 1.43 | 1.75 | 1.00 | |
| M3 vs M1 | 2.61 | 1.57 | 1.81 | |
| Log-rankP | .0008 | .07 | .07 | |
| Day-7 bone marrow status . | 5-year EFS by randomized regimen % (SD) . | RR (DI vs DDI) . | ||
|---|---|---|---|---|
| DI (N = 405) . | DIVPI (N = 397) . | DDI (N = 402) . | ||
| M1 (less than 5% blasts) | 84 (3) | 82 (3) | 86 (3) | 1.16 |
| M2 (5%-25% blasts) | 77 (4) | 74 (5) | 89 (4) | 1.61 |
| M3 (more than 25% blasts) | 63 (6) | 77 (5) | 74 (5) | 1.64 |
| RR | ||||
| M2 vs M1 | 1.43 | 1.75 | 1.00 | |
| M3 vs M1 | 2.61 | 1.57 | 1.81 | |
| Log-rankP | .0008 | .07 | .07 | |
EFS indicates event-free survival; RR, relative risk. See Table 1footnote for other abbreviations.
An analysis of the multivariate effect of significant univariate prognostic factors on the treatment effect of the DDI regimen was conducted with a model that included day-7 marrow status (M1, M2, M3; restricted to those who achieved remission at the end of induction); race (white, Hispanic, black, other); hemoglobin (lower than 8 g/dL, 8 through 10.99 g/dL, at least 11 g/dL); splenomegaly (normal; moderately enlarged; significantly enlarged); platelet count (fewer than 50 × 109L [50 000/μL], 50 × 109L through 149 × 109L [50 000 through 149 000/μL], and at least 150 × 109L [150 000/μL]); sex; and age (1 through 4.99 years, 5 through 9.99 years). The significant difference in EFS observed for DI compared with DDI in the univariate analysis was similar in the multivariate analysis (P = .07; RR = 1.37, confidence interval, 0.979-1.92).
Toxicities and resource use
The first 4 phases of therapy were identical on the 3 treatment regimens, and incidence of grade 3 and 4 nonhematological toxicity was similar on each of the 3 regimens during this period.3 6Morbidity and supportive-care interventions were tabulated from the point at which the 3 regimens diverged (Figure 1; Table4). Episodes of pancreatic and other gastrointestinal dysfunction, coagulation abnormalities, and infection were more common in patients receiving DDI than in patients on DI or DIVPI. There was a 4-fold increase in the number of patients receiving red cell transfusions and a 6-fold increase in the number of patients receiving platelet transfusions on the DDI regimen compared with the DI regimen. Mean (± SD) number of days of hospitalization was significantly higher (Wilcoxon P = .0001) for the DDI regimen (mean, 15 ± 15 days; median, 11 days; 75th percentile, 21 days) compared with DI (mean, 8 ± 11 days; median, 5 days; 75th percentile, 11 days) or DIVPI (mean, 10 ± 13 days; median, 6 days; 75th percentile, 14 days). Thus, DDI involved a median of 6 more hospital days compared with DI. Four patients developed second malignant neoplasms: AML in marrow (1); chloroma (1); byphenotypic leukemia (1); and B-cell lymphoproliferative disease in a patient with t(9;22) ALL who discontinued protocol therapy for a marrow transplant (1).
Episodes of grade 3 and 4 toxicities on CCG-1891
| . | DI . | DIVPI . | DDI . | |||
|---|---|---|---|---|---|---|
| No. episodes . | Episodes per 100 patient days4-150 . | No. episodes . | Episodes per 100 patient days4-150 . | No. episodes . | Episodes per 100 patient days4-150 . | |
| Hepatic | ||||||
| SGOT | 69 | 0.023 | 71 | 0.025 | 59 | 0.020 |
| SGPT | 205 | 0.068 | 213 | 0.074 | 240 | 0.083 |
| Alkaline phosphatase | 9 | 0.003 | 16 | 0.006 | 6 | 0.002 |
| Bilirubin | 33 | 0.011 | 35 | 0.012 | 27 | 0.009 |
| Pancreatic | 0 | 0.0 | 2 | 0.001 | 8 | 0.003 |
| Renal | 3 | 0.001 | 0 | 0.0 | 4 | 0.001 |
| Gastrointestinal | 11 | 0.004 | 10 | 0.004 | 24 | 0.008 |
| Pulmonary | 6 | 0.002 | 9 | 0.003 | 10 | 0.003 |
| Cardiac | 0 | 0.0 | 0 | 0.0 | 3 | 0.001 |
| Nervous system | ||||||
| Peripheral | 4 | 0.001 | 8 | 0.003 | 7 | 0.002 |
| Central | 1 | 0.0 | 2 | 0.001 | 2 | 0.001 |
| Dermatological | 8 | 0.003 | 12 | 0.004 | 12 | 0.004 |
| Blood coagulation | 1 | 0.0 | 4 | 0.001 | 16 | 0.006 |
| Infection | 21 | 0.007 | 20 | 0.007 | 46 | 0.016 |
| . | DI . | DIVPI . | DDI . | |||
|---|---|---|---|---|---|---|
| No. episodes . | Episodes per 100 patient days4-150 . | No. episodes . | Episodes per 100 patient days4-150 . | No. episodes . | Episodes per 100 patient days4-150 . | |
| Hepatic | ||||||
| SGOT | 69 | 0.023 | 71 | 0.025 | 59 | 0.020 |
| SGPT | 205 | 0.068 | 213 | 0.074 | 240 | 0.083 |
| Alkaline phosphatase | 9 | 0.003 | 16 | 0.006 | 6 | 0.002 |
| Bilirubin | 33 | 0.011 | 35 | 0.012 | 27 | 0.009 |
| Pancreatic | 0 | 0.0 | 2 | 0.001 | 8 | 0.003 |
| Renal | 3 | 0.001 | 0 | 0.0 | 4 | 0.001 |
| Gastrointestinal | 11 | 0.004 | 10 | 0.004 | 24 | 0.008 |
| Pulmonary | 6 | 0.002 | 9 | 0.003 | 10 | 0.003 |
| Cardiac | 0 | 0.0 | 0 | 0.0 | 3 | 0.001 |
| Nervous system | ||||||
| Peripheral | 4 | 0.001 | 8 | 0.003 | 7 | 0.002 |
| Central | 1 | 0.0 | 2 | 0.001 | 2 | 0.001 |
| Dermatological | 8 | 0.003 | 12 | 0.004 | 12 | 0.004 |
| Blood coagulation | 1 | 0.0 | 4 | 0.001 | 16 | 0.006 |
| Infection | 21 | 0.007 | 20 | 0.007 | 46 | 0.016 |
Data shown are frequencies of grade 3 or 4 nonhematological toxicities, enumerated from the beginning of the second interim maintenance (DDI) or maintenance (DI and DIVPI).
SGOT indicates serum glutamic-oxaloacetic transaminase; SGPT, serum glutamic-pyruvic transaminase. See Table 1 footnote for other abbreviations.
Patient days were defined as the total number of days spent on therapy for all patients entering interim maintenance 2 (DDI) or maintenance (DI and DIVPI). Episodes per 100 patient days were calculated as number of episodes per total patient days divided by 100.
Discussion
Children with intermediate-risk ALL represent approximately two thirds of NCI-defined standard-risk patients. The CCG-1891 study for this subset of patients has suggested that the addition of a second DI phase given 16 weeks after the first DI phase results in improved EFS. Treatment-related toxicities, attributable primarily to the myelosuppressive effects of daunorubicin, cytosine arabinoside, and cyclophosphamide, or effects of L-asparaginase on coagulation were more frequent for patients on DDI than on DI. These complications did not result in significantly increased treatment-related mortality or major late effects that might compromise the life or functioning of these patients. For example, among patients treated with DDI, there was no occurrence of aseptic necrosis, a debilitating complication that occurred at 3-year cumulative rates of 14% (SD = 4%) and 26% (SD = 5%)15 among patients 10 to 20 years of age who received 1 or 2 DI phases, respectively, as part of an overall augmented treatment strategy on the CCG-1882 higher-risk ALL study.16 It is possible that older age and use of higher steroid doses during induction, as well as the second DI phase itself, mediated aseptic necrosis on the higher-risk study. Also, although 2 patients on DDI developed a second malignant neoplasm, one neoplasm occurred following unrelated donor stem cell transplantation in a patient with t(9;22) who was removed from protocol therapy. The total doses of cyclophosphamide (2 g/m2 on DDI and 1 g/m2 on DI) used on this study are not likely to be associated with a further increase in second malignant neoplasms as the study matures.17 Few cardiac abnormalities were observed on this study; this is consistent with the relatively low cumulative dose of anthracycline (150 mg/m2 on DDI; 75 mg/m2 on DI or DIVPI) used. The number of days of hospitalization was significantly higher in the DDI regimen than in either DI or DIVPI, indicating that resource use is increased by the more intensive regimen. The CCG has calculated a relapse-adjusted marginal cost-effectiveness, based on duration of hospitalization on frontline and relapsed ALL studies. Each relapse that is prevented by DDI saves the cost of 52 additional hospital days per 100 patients given DDI or about one-half day of hospitalization per patient.18 This suggests that the increase in EFS afforded by DDI offsets its cost in hospital days.
The difference in outcome according to race is striking. Previous studies have reported poorer outcome for black children with ALL compared with white children, with an approximate 10% to 15% difference in EFS at late periods of follow-up.19-21 An analysis of more recent studies by the St Jude Children's Research Hospital (SJCRH) (Memphis, TN) indicated that differences in outcome between race groups, like those between males and females, may have been canceled out by more intensive therapy.22 In contrast, a recent CCG analysis of the entire cohort of patients treated between 1989 and 1995 revealed highly significant differences in EFS among all ethnic groups (P < .0001), with worse outcome for both Hispanic and African-American children.23This analysis suggested that differences in outcome among race groups have persisted even in the face of intensive contemporary treatment strategies. The underlying causes for these differences may be multifactorial and include issues related to socioeconomic status, access to health-care systems, compliance with or adherence to therapy, or genetic differences in the ability to metabolize different chemotherapeutic agents.23
In contrast to earlier studies,24-26 male sex, age between 1 and 2 years, coexpression of myeloid and lymphoid antigens, or T-lineage immunophenotype were not unfavorable characteristics for the current group of intermediate-risk ALL patients. Similar findings on the prognostic significance of immunophenotype9 27 have been observed for concurrently enrolled patients with low-risk, higher-risk, infant, or lymphomatous syndrome ALL.
As has been reported for other CCG-defined risk groups, day-7 marrow status was an important predictor of outcome for the overall cohort of intermediate-risk patients.7 Although the subset of patients with fewer than 5% marrow blasts on day 7 had relatively similar outcome regardless of regimen, patients with at least 5% blasts treated with DDI had an approximate 40% reduction in relative risk compared with their counterparts on the DI regimen. This finding suggests that patients with a modest residual tumor burden are most likely to benefit from DDI. However, these results must be interpreted with caution since they are based on subset analyses that were not part of the overall design of the study. Since half of all patients achieved a favorable day-7 response, these data also suggest that improved methods are needed to identify the subset of patients with a favorable day-7 M1 response who will nevertheless experience events with current intensive therapies.
Timing of DI therapy may be a critical determinant of outcome. On CCG-105, the predecessor CCG intermediate-risk ALL study, randomized comparisons of both induction/consolidation intensification (weeks 1 through 8) and DI (weeks 16 through 24) were employed. EFS was similar for patients treated with standard or intensive induction/consolidation, but was significantly improved for patients treated with DI compared with patients who did not receive DI.6 Interestingly, the effect of DI was reduced when it was given in the context of intensive induction/consolidation.
The 6-year EFS of 83% achieved by patients treated with DDI is comparable to that of the other major cooperative groups or single institutions using different strategies and different definitions of standard-, moderate-, and intermediate-risk patients.28-31For example, among the lowest-risk patients, 6-year EFS was 87% on BFM-86, and 2-year EFS was 86% on BFM-90. Strategy on the BFM-86 and BFM-90 protocols was based on stratification according to response to 7 days of prednisone. Therapy involved intensive 8-drug induction, consolidation with 6-mercaptopurine and high-dose MTX; reinduction (with substitution of dexamethasone for prednisone and oral 6-thioguanine for oral 6-mercaptopurine); late intensification (prednisone, vindesine, teniposide, ifosfamide, and high dose-cytarabine) for all but the lowest-risk patients; and maintenance.32
Antimetabolite-based therapy favored by the Pediatric Oncology Group30 and SJCRH has resulted in 5-year EFS rates of 78% and 81%, respectively, for lower- or standard-risk patients, and the intensified L-asparaginase and anthracycline therapy employed by Dana-Farber Cancer Institute investigators has yielded an EFS of 89% at 4 years for the standard-risk subset.28,29 Pui et al33 reported better outcome for patients with B-lineage leukemia who received individualized dosing of methotrexate, cytarabine, and teniposide. In a CCG study following CCG-1891, Bostrom et al34 observed a 3-year EFS of 91% for standard-risk patients treated on a regimen that included a single DI phase and employed dexamethasone as the only steroid.
The different strategies have different serious toxicities, but it is not clear that one strategy is better than another. Advantages of the CCG-1891 study and the BFM-derived studies are that they are relatively simple in structure, do not demand patient-specific protocols, and are highly reproducible and adaptable around the world. On the currently open CCG-1991 trial for standard-risk ALL, the CCG is comparing DI and DDI in the context of a dexamethasone-based regimen. Results from this study will determine whether DDI provides a clinically and statistically important benefit for the full group of patients with NCI-defined standard-risk ALL. Further improvements in outcome among children with standard-risk ALL are likely to require the continued manipulation of dose, route, and schedule of conventional chemotherapeutic agents; patient-specific adjustments of therapy based on pharmacogenetic profiling; and the addition of novel biologically based methods.
We are indebted to Diane Arthur, MD, for her extensive contribution to the cytogenetics database, Ms Lucia Noll for expert editing, and Ms Christine Curran for typing and revising.
The following are contributing Children's Cancer Group institutions, investigators, and grant numbers: Group Operations Center, Arcadia, California, (W. Archie Bleyer, MD, Anita Khayat, PhD, Harland Sather, PhD, Mark Krailo, PhD, Jonathan Buckley, MBBS, PhD, Daniel Stram, PhD, Richard Sposto, PhD) CA 13539; University of Michigan Medical Center, Ann Arbor, Michigan, (Raymond Hutchinson, MD) CA 02971; University of California Medical Center, San Francisco, California, (Katherine Matthay, MD) CA 17829; University of Wisconsin Hospital, Madison, Wisconsin, (Diane Puccetti, MD) CA 05436; Children's Hospital and Medical Center, Seattle, Washington (J. Russell Geyer, MD) CA 10382; Rainbow Babies and Children's Hospital, Cleveland, Ohio (Susan Shurin, MD) CA 20320; Children's National Medical Center, Washington, D.C. (Gregory Reaman, MD) CA 03888; Children's Hospital of Los Angeles, California (Paul Gaynon, MD) CA 02649; Children's Hospital of Columbus, Ohio (Frederick Ruymann, MD) CA 03750; Columbia Presbyterian College of Physicians and Surgeons, New York, New York (Leonard Wexler, MD) CA 03526; Children's Hospital of Pittsburgh, Pennsylvania (A. Kim Ritchey, MD) CA 36015; Vanderbilt University School of Medicine, Nashville, Tennessee (John Lukens, MD) CA 26270; Doernbecher Memorial Hospital for Children, Portland, Oregon (H. Stacy Nicholson, MD) CA 26044; University of Minnesota Health Sciences Center, Minneapolis, Minnesota (Joseph Neglia, MD) CA 07306; Children's Hospital of Philadelphia, Pennsylvania (Beverly Lange, MD) CA 11796; Memorial Sloan-Kettering Cancer Center, New York, New York (Peter Steinherz, MD) CA 42764; James Whitcomb Riley Hospital for Children, Indianapolis, Indiana (Philip Breitfeld, MD) CA 13809; University of Utah Medical Center, Salt Lake City (William L. Carroll, MD) CA 10198; University of British Columbia, Vancouver, Canada (Christopher Fryer, MD) CA 29013; Children's Hospital Medical Center, Cincinnati, Ohio (Robert Wells, MD) CA 26126; Harbor/UCLA and Miller Children's Medical Center, Torrance/Long Beach, California (Jerry Finklestein, MD) CA 14560; University of California Medical Center (UCLA), Los Angeles, California (Stephen Feig, MD) CA 27678; University of Iowa Hospitals and Clinics, Iowa City, (Raymond Tannous, MD) CA 29314; Children's Hospital of Denver, Colorado (Lorrie Odom, MD) CA 28851; Mayo Clinic and Foundation, Rochester, Minnesota (Gerald Gilchrist, MD) CA 28882; Izaak Walton Killam Hospital for Children, Halifax, NS, Canada (Dorothy Barnard, MD); University of North Carolina, Chapel Hill (Stuart Gold, MD); University of Medicine and Dentistry of New Jersey, Camden; (Richard Drachtman, MD); Children's Mercy Hospital, Kansas City, Missouri (Maxine Hetherington, MD); University of Nebraska Medical Center, Omaha (Peter Coccia, MD); Wyler Children's Hospital, Chicago, Illinois (James Nachman, MD); M.D. Anderson Cancer Center, Houston, Texas (Beverly Raney, MD); Princess Margaret Hospital, Perth, Western Australia (David Baker, MD); New York University Medical Center, New York (Aaron Rausen, MD); and Children's Hospital of Orange County, California (Violet Shen, MD).
Supported in part by the Children's Cancer Group Chairman's grant CA-13539 from the National Cancer Institute, National Institutes of Health. The contribution of B.J.L. is supported in part by the Yetta Deitch Novotny Chair in Pediatric Oncology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Beverly J. Lange, Children's Cancer Group, Attn: Lucia Noll, PO Box 60012, Arcadia, CA 91066-6012; e-mail:lange@emailchop.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal