Abstract
Acute promyelocytic leukemia (APL) is characterized by the specific chromosome translocation t(15;17) with promyelocytic leukemia-retinoic acid receptor-α (PML-RARA) fusion gene and the ability to undergo terminal differentiation as an effect of all-trans retinoic acid (ATRA). Recently, arsenic trioxide (As2O3) has been identified as an alternative therapy in patients with both ATRA-sensitive and ATRA-resistant APL. At the cellular level, As2O3 triggers apoptosis and a partial differentiation of APL cells in a dose-dependent manner; both effects are observed in vivo among patients with APL and APL animal models. To further explore the mechanism of As2O3-induced differentiation, the combined effects of arsenic and a number of other differentiation inducers on APL cell lines (NB4 and NB4-R1) and some fresh APL cells were examined. The data show that a strong synergy exists between a low concentration of As2O3 (0.25 μM) and the cyclic adenosine monophosphate (cAMP) analogue, 8-CPT-cAMP, in fully inducing differentiation of NB4, NB4-R1, and fresh APL cells. Furthermore, cAMP facilitated the degradation of As2O3-mediated fusion protein PML-RARα, a process considered to play a key role in overcoming the differentiation arrest of APL cells. On the other hand, cAMP could significantly inhibit cell growth by modulating several major players in G1/S transition regulation. Interestingly, H89, an antagonist of protein kinase A, could block the differentiation-inducing effect of As2O3potentiated by cAMP. These results thus support the existence of a novel signaling cross-talk for APL maturation, which may deepen understanding of As2O3-induced differentiation in vivo, and thus furnish insights for new therapeutic strategies.
Introduction
Acute promyelocytic leukemia (APL), characterized by differentiation arrest of granulopoiesis at the promyelocytic stage, is the first human malignancy that can be efficiently treated with a cell differentiation inducer, all-trans retinoic acid (ATRA).1 Despite the great success of ATRA and chemotherapy in both remission induction and maintenance therapy in APL, a fair percentage (30%-40%) of patients still have a relapse after initial remission and develop resistance to ATRA treatment.2 Recently, arsenic trioxide (As2O3), an ancient traditional Chinese medicine, has proven to be an effective drug in the treatment of patients with APL, not only in primary cases, but also in patients with relapses or refractory disease after ATRA or chemotherapy or both.3 4
It is well known that APL blasts harbor a specific translocation t(15;17),5 fusing promyelocytic leukocyte (PML) and retinoic acid receptor-α (RARA) genes and leading to a PML-RARα oncoprotein,6-9 which exerts dominant-negative effects over PML and RARα,10 acquires an abnormally tight binding ability to the nuclear receptor corepressor complex including N-CoR and silencing mediator for retinoid and thyroid hormone receptor (SMRT), and thus disturbs myeloid differentiation through transcriptional repression.11-13 It is well established that the therapeutic effect of ATRA relies on the binding of the drug to the ligand-binding domain on the RARα portion of PML-RARα resulting in dissociation of the corepressor complex to relieve the transcriptional repression, whereas arsenic targets the PML-RARα fusion protein through the PML moiety and triggers a prompt degradation of the chimeric protein.12-14 In vitro experiments showed that, at the cellular level, arsenic could exert a dual effect on APL cells: triggering apoptosis at relatively high doses (0.5-2 μM) with the collapse of mitochondrial transmembrane potentials in a thiol-dependent manner and inducing partial differentiation at low concentration (0.1-0.25 μM).15,16In vivo, both differentiation and apoptosis were observed among patients with APL or in APL animal models.15,17 However, the mechanism of arsenic-mediated differentiation is far from clear. Recently, efforts from several groups have been focused on the effects of combination of As2O3 and other drugs, such as ATRA, granulocyte colony-stimulating factor (G-CSF),17-20 and inhibitors of histone deacetylase (HDAC) including trichostatin A (TSA), on differentiation in APL cells (Z.C. et al, in preparation). Although some results obtained in vitro need further investigation, these studies strongly support the notion that differentiation of APL cells exposed to arsenic was the result of the synergism of distinct signaling pathways.
Cyclic adenosine monophosphate (cAMP), one of the most common second messengers, plays an important role in the response to hormonal signals for cell proliferation, differentiation, and apoptosis, including that in hemopoietic development.21 It was shown that cAMP was able to induce monocytic differentiation of M1 mouse myeloid leukemia cells22 and of HL60 cells.23 cAMP could also potentiate granulocytic differentiation of retinoid- or rexinoid-induced maturation of human APL cell.24-26 In the present work, in an attempt to investigate the possible interaction between As2O3 and several differentiation inducers, we found that the 8-CPT (4-chlorophenylthio)–cAMP, a cAMP analogue, synergizes with arsenic and significantly induces the differentiation of APL cell lines NB4 and NB4-R1, which are retinoid-induced maturation sensitive and resistant, respectively.24 27 This synergy was also observed in fresh APL cells. We provide here mechanistic evidence for synergic effects of As2O3 and cAMP on APL cell maturation, involving As2O3-induced degradation of oncoprotein PML-RARα, and cAMP-mediated inhibition of cell cycling.
Materials and methods
Cell culture and reagents
The NB4 and NB4-R1 cells27 were cultured in RPMI-1640, supplemented with 10% fetal calf serum (FCS; Gibco BRL, Gaithersburg, MD), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. COS-7 cells were cultured in Dulbecco modified Eagle medium supplemented with 10% bovine serum, 2 mMl-glutamine. Fresh bone marrow cells were obtained with informed consent from 2 patients with de novo APL, who were diagnosed as M3 by the French-American-British criteria and showed an initial percentage of circulating blasts over 90%. Confirmation of the presence of t(15;17) and PML-RARA transcripts was performed by karyotype and reverse transcription-polymerase chain reaction (RT-PCR) analysis. Leukemia cells were isolated and enriched on Ficoll solution. All cell cultures were incubated at 37°C in humidified air with 5% CO2. As2O3 (Sigma, St Louis, MO) was dissolved in 1 N NaOH and further diluted to 5 mM in phosphate-buffered saline (PBS) as stock solution. 8-CPT-cAMP, ATRA, and TSA (Sigma) were dissolved in PBS, ethanol, and dimethyl sulfoxide as stock solution at 10 mM, 1 mM, and 50 μg/mL, respectively. H-89 (N-(2-[p-bromocinnamylamino]ethyl)-5-isoquinolinesulfonamide) purchased from Calbiochem (San Diego, CA) was dissolved in 50% ethanol as stock solution at 10 mM.
Characterization of cell differentiation
The NB4 and NB4-R1 cells were grown in the presence of As2O3 (0.25 μM), ATRA (1 nM to 1 μM), TSA (10-50 ng/mL), 8-CPT-cAMP (200 μM), or their combination for 4 to 6 days. Cell maturation was evaluated on the basis of cellular morphology, changes in cell surface antigen, and nitroblue-tetrazolium (NBT; Sigma) assay. Briefly, cytospin slides stained with May-Grunwald-Giemsa stain were analyzed for cell morphology by means of light microscopy. Analysis of cell surface myeloid-specific antigen CD11b (Coulter, Marseilles, France) was performed by flow cytometry (EPICS XL, Coulter, Hialeah, FL). For NBT assay, 1 × 106cells were centrifuged, suspended in 500 μL PBS, and incubated at 37°C for 30 minutes with 1 mg/mL NBT and 30 ng/mL 12-O-tetradecanoylphorbol-13-acetate (Sigma). The percentage of NBT+ cells with formazan deposits in the cytoplasm was determined by microscopically counting at least 300 cells per experimental condition.
Analysis of cell cycle
Briefly, cells were collected, washed, and fixed overnight in 70% cold ethanol. After washing with PBS, cells were treated by Tris-HCl buffer (pH 7.4) supplemented with 1% RNase. Cell cycle distribution was then analyzed by staining the cells with 50 μg/mL propidium iodine (PI; Sigma) and evaluated in a flow cytometer. All data were collected, stored, and analyzed by Multicycle software (Coulter, Miami, FL).
Confocal immunofluorescence staining
For immunofluorescence analysis, the cells were smeared onto histologic slides by using cytospin centrifugation (Shandon, Astmoor, Runcorn, Cheshire, United Kingdom). After drying, the cells were fixed in acetone at 4°C for 10 minutes and allowed to air dry for 20 minutes. The slides were preincubated for 15 minutes with PBS. PML-RARα and PML were detected with the monoclonal antiserum directed against PML (Santa Cruz Biotechnology, Santa Cruz, CA). Fluorescein isothiocyanate (FITC)–coupled antimouse antibody (Biosys, Compiègne, France) was used as a second antibody. All incubations were carried out at room temperature and followed by 3 washes in PBS; slides were finally mounted with 5 μL fluorescent mounting medium (Dako, Kyoto, Japan). Preparations were examined by confocal laser scanning microscopy (MRC-600 Confocal Imaging System; Bio-Rad Microscience, Hertfordshire, United Kingdom), mounted on an Optiphot II Nikon microscope. Images were collected using an oil immersion lens (× 60, NA I.4 plan Apochromat) and using excitation wavelength of 488 nm for FITC.
Western blotting analysis
Whole cell protein extracts were prepared from 2 × 106 cells. Briefly, cultured cells were washed in PBS, then the pellets were immediately lysed by 100 μL of a boiling Laemmli solution containing β-mercaptoethanol and disrupted with a pestle. Samples were then boiled for 5 minutes and insoluble material was removed by centrifugation at 13 000 rpm for 5 minutes. The quality and the quantity of all samples were detected with sodium dodecyl sulfate (SDS)–polyacrylamide gels. The protein loading volume was adjusted according to Coomassie blue staining. For Western analysis, 10 μg protein extracts were loaded on SDS-polyacrylamide gels, subjected to electrophoresis, and blotted onto polyvinylidene difluoride membranes (Amersham, Buckinghamshire, United Kingdom). To ensure that the protein loading was as equal as possible, we generally performed 2 gels in parallel, one for transfer, another for staining with Coomassie blue as a loading control. After transfer, membranes were blocked with 5% nonfat milk in PBS pH 7.6, 0.1% Tween-20 (PBS-T), then incubated with a specific antiserum raised against the indicated protein in PBS-T/3% milk for 18 hours at 4°C. Subsequently, membranes were incubated with horseradish peroxidase (HRP)–conjugated antirabbit or antimouse secondary antibody (Jackson Laboratories, Bar Harbor, ME) for 30 minutes at 25°C. Each step was followed by three 10-minute washes in PBS-T. Detection was performed by using an enhanced chemoluminescence kit (Amersham) according to the manufacturer's instruction. All antibodies used in the present work (CDK4, cyclin D1, E2F, p21, p27, p53) were purchased from Santa Cruz Biotechnology.
Cell transient transfection
The PML-RARα expression plasmid was subcloned in pSG5 vector as described.28 RARE-TK-luciferase reporter plasmid contains the DR5-RARE retinoic acid response element of the RARβ upstream of thymidine kinase promoter.29 For luciferase assay, COS-7 cells were cotransfected with 1.0 μg PML-RARα expression plasmid and 0.1 μg RARE-TK-luciferase reporter plasmid by using LipofectAmine reagent (Gibco BRL) according to the manufacturer's procedure. The total amount of plasmid was adjusted to 1.1 μg using empty vector to maintain constant in all transfection assays. Twenty-four hours after transfection, cells were respectively treated without or with ATRA (10 nM), As2O3(0.25 μM), or 8-CPT-cAMP (200 μM) alone or their combination for 24 hours followed by transcriptional activity assay using the luciferase assay system (Promega, Madison, WI).
Results
Synergism of As2O3 and several differentiation inducers
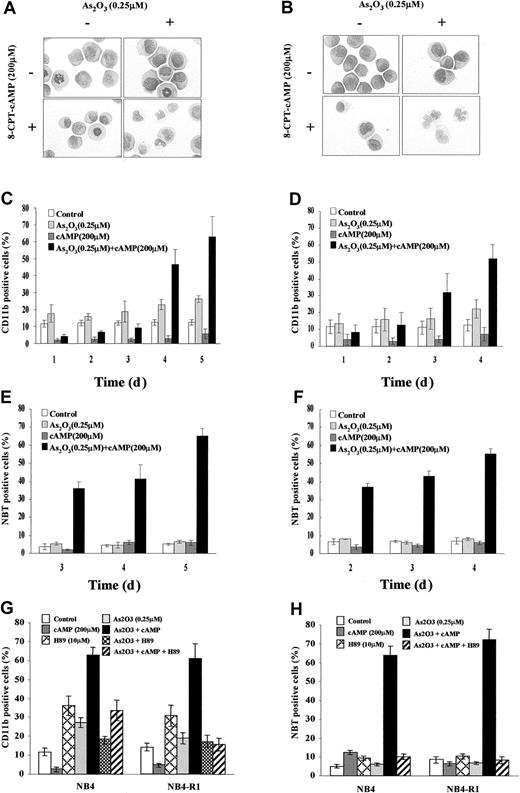
Previous work showed that As2O3 at low concentration (0.1-0.25 μM) could induce a certain degree of maturation of NB4 cells. However, this differentiation is far from terminal because most cells could not mature beyond the myelocyte-metamyelocyte stage and the NBT reduction test remained negative.15 Moreover, this partial differentiation could be observed only after long (> 10 days) exposure of the cells to the drug. As shown in the Figure 1A of this work, assessed by morphologic criteria, no obvious maturation was observed in NB4 cells after treatment with arsenic alone for 4 days, except an increased number of cytoplasmic granules and vacuoles (Figure1A). The expression of CD11b was only slightly increased (Figure 1C). Further mechanism studies showed that 0.25 μM arsenic exerted no direct effect on modulating the interaction between SMRT and the wild-type RARα/retinoid X receptor (RXR) or PML-RARα (data not shown), although it caused increased acetylation of histones H3 and H4 in favor of opening chromatin structure.30 It is thus unlikely that the low-dose effect of As2O3 on APL cells is mediated directly through RARα pathway, although the integrity of the latter seems to be important for granulocytic differentiation at promyelocyte stage.16 To test possible synergistic effects between As2O3 and other differentiation pathways, we added to the culture system different concentrations of other inducers such as ATRA, TSA, and cAMP. Consistent with a recent report,20 the As2O3-induced CD11b elevation in NB4 cells could be slightly enhanced by a physiologic concentration (10−9 M) of ATRA and low-dose (10 ng/mL) TSA (Z.C. et al, in preparation). However, the 2 above combinations showed a limited effect in triggering morphologic maturation of NB4 cells and they could not significantly induce differentiation in ATRA-induced maturation-resistant NB4 sublines such as NB4-R1 and MR2. To our surprise, arsenic could induce an almost terminal maturation of NB4 cells in the presence of 8-CPT-cAMP, which on its own exhibit little differentiating effect.
Evidence for cooperation between As2O3 and cAMP signaling pathways.
Morphologic features of NB4 (A) and NB4-R1 (B) cells after a 96-hour exposure to the drugs indicated. Original magnification × 1000. Expression of CD11b integrin and NBT-reducing activity of NB4 (C,E) and NB4-R1 cells (D,F) following the indicated treatments. Inhibitory effect of H89 on CD11b expression (G) and NBT activity (H) in NB4 and NB4-R1 cells treated by the indicated drugs for 5 and 4 days, respectively, is shown. Each value represented the mean ± SD of 3 independent measurements.
Evidence for cooperation between As2O3 and cAMP signaling pathways.
Morphologic features of NB4 (A) and NB4-R1 (B) cells after a 96-hour exposure to the drugs indicated. Original magnification × 1000. Expression of CD11b integrin and NBT-reducing activity of NB4 (C,E) and NB4-R1 cells (D,F) following the indicated treatments. Inhibitory effect of H89 on CD11b expression (G) and NBT activity (H) in NB4 and NB4-R1 cells treated by the indicated drugs for 5 and 4 days, respectively, is shown. Each value represented the mean ± SD of 3 independent measurements.
Synergism of As2O3 and cAMP in inducing differentiation of NB4 and NB4-R1 cells
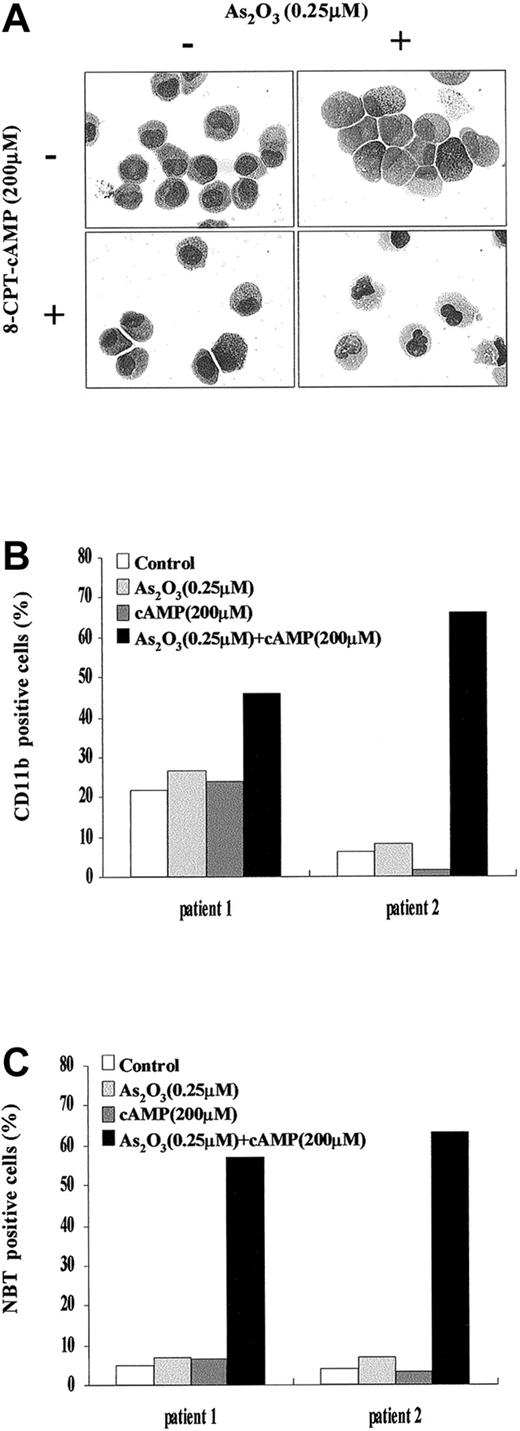
During a 4-day dual treatment of NB4 cells with 0.25 μM As2O3 and 200 μM 8-CPT-cAMP, an increasing proportion of cells presented lobed or multiple nuclei, a reduced nucleus-cytoplasm ratio, more neutrophilic granules and vacuoles, as well as a smaller cell size and less basophilic cytoplasm (Figure 1A). Similar morphologic changes also were observed in NB4-R1 cells (Figure1B), which have no maturation in the presence of ATRA. This situation is reminiscent of the previous report that addition of cAMP-elevating agents could trigger retinoid-primed NB4-R1 cells to undergo terminal differentiation.24 31 Our morphologic data on the cooperation between arsenic and cAMP were further supported by the results of NBT test and CD11b expression detection (Figure 1C-F). A gradual increasing of CD11b expression and of NBT+ cells was observed in both NB4 and NB4-R1 cells during the treatment by 0.25 μM As2O3 and 200 μM 8-CPT-cAMP in combination, whereas there was no prominent change in the presence of each of the inducers alone. Notably, cell maturation seemed to proceed faster in NB4-R1 cells than in NB4 cells. In line with these observations, similar synergic effects of As2O3and cAMP were also seen in fresh cells from 2 de novo APL patients (Figure 2A-C).
Effects of As2O3 or cAMP or both on primary leukemia cells from 2 patients with de novo APL.
(A) Morphologic features of primary leukemia cells from patient 1 after 9 days of exposure to the drugs as indicated. Similar morphologic changes were also observed in patient 2. Original magnification × 1000. (B) Expression of CD11b integrin of primary leukemia cells from patient 1 and patient 2 following the indicated treatments. (C) NBT-reducing activity of primary leukemia cells from patient 1 and patient 2 following the indicated treatments.
Effects of As2O3 or cAMP or both on primary leukemia cells from 2 patients with de novo APL.
(A) Morphologic features of primary leukemia cells from patient 1 after 9 days of exposure to the drugs as indicated. Similar morphologic changes were also observed in patient 2. Original magnification × 1000. (B) Expression of CD11b integrin of primary leukemia cells from patient 1 and patient 2 following the indicated treatments. (C) NBT-reducing activity of primary leukemia cells from patient 1 and patient 2 following the indicated treatments.
Because protein kinase A (PKA) represents a major regulator in cAMP signal transduction pathway, H89, a specific PKA inhibitor, was further used to examine the possible role of PKA pathway in the differentiation of NB4 and NB4-R1 cells induced by As2O3 or cAMP or both. Indeed, H89 was able to decrease the As2O3-triggered CD11b expression of NB4 cells in a weak but significant way (Figure 1G). Moreover, this PKA inhibitor could dramatically inhibit the differentiation of NB4 and NB4-R1 cells induced by the combination of As2O3 and cAMP judging from CD11b expression (Figure 1G), NBT reduction activity (Figure 1H), and morphologic change (data not shown). These observations suggested that the synergism effect of As2O3 and cAMP in inducing differentiation of APL cells might reside at the PKA level.
Inhibition of cell growth induced by As2O3or cAMP in NB4 and NB4-R1 cells
Next, the effects of As2O3, 8-CPT-cAMP or their combination on the growth of NB4 and NB4-R1 cells were examined. Only a slight decrease in cell growth was observed in NB4 and NB4-R1 cells treated with arsenic alone (Figures3A and 4A). In contrast, the growth of both cells could be dramatically inhibited by 8-CPT-cAMP alone or As2O3 combined with cAMP, particularly in NB4-R1 cells. The proliferation of NB4-R1 cells was almost completely inhibited within 2 days (Figure 4A). In agreement with this, analysis of the cell cycle distribution showed that NB4 and NB4-R1 cells significantly accumulated at the G0/G1 phase under 8-CPT-cAMP treatment alone or when As2O3and 8-CPT-cAMP treatments were combined, but not in the presence of As2O3 alone (Figures 3B and 4B). These data suggest that the cAMP pathway should play an important role in the inhibition of cell cycle.
Effects of As2O3 or cAMP or both on NB4 cell proliferation.
(A) Cell viability was assessed by trypan blue dye exclusion method following the indicated treatments. Each value represented the mean ± SD of 3 independent measurements. (B) Flow cytometric analysis of cell cycle distribution by PI staining. One representative experiment among 3 independent assays was shown. (C) Western blot analysis of some cell cycle-involved proteins (E2F, p21, p27, cyclin D1, CDK4, and p53) expression during the treatments.
Effects of As2O3 or cAMP or both on NB4 cell proliferation.
(A) Cell viability was assessed by trypan blue dye exclusion method following the indicated treatments. Each value represented the mean ± SD of 3 independent measurements. (B) Flow cytometric analysis of cell cycle distribution by PI staining. One representative experiment among 3 independent assays was shown. (C) Western blot analysis of some cell cycle-involved proteins (E2F, p21, p27, cyclin D1, CDK4, and p53) expression during the treatments.
Effects of As2O3 or cAMP or both on NB4-R1 cell proliferation.
(A) Cell viability was assessed by trypan blue dye exclusion method following the indicated treatments. Each value represented the mean ± SD of 3 independent measurements. (B) Flow cytometric analysis of cell cycle distribution by PI staining. One representative experiment among 3 independent assays is shown. (C) Western blot analysis of some cell cycle-involved proteins (E2F, p21, p27, cyclin D1, CDK4, and p53) expression level during the treatments.
Effects of As2O3 or cAMP or both on NB4-R1 cell proliferation.
(A) Cell viability was assessed by trypan blue dye exclusion method following the indicated treatments. Each value represented the mean ± SD of 3 independent measurements. (B) Flow cytometric analysis of cell cycle distribution by PI staining. One representative experiment among 3 independent assays is shown. (C) Western blot analysis of some cell cycle-involved proteins (E2F, p21, p27, cyclin D1, CDK4, and p53) expression level during the treatments.
To further understand the G1 phase arrest of NB4 and NB4-R1 cells in the presence of 8-CPT-cAMP alone or in combination with As2O3, we examined the expression level of several G1/S transition-related proteins. Although some positive regulatory proteins for the cell cycle, such as CDK4 and cyclin D1, seemed unchangeable during the treatment, E2F, a protein necessary for the progression of the cell into S phase, was greatly down-regulated in both cells treated by 8-CPT-cAMP. It has been well known that p21 and p27, 2 important cyclin kinase inhibitors, negatively regulate the progression from G1 into S phase.32-34 Here we showed a dramatic increase of p21 during 8-CPT-cAMP treatment of NB4 and NB4-R1 cells, whereas p27 showed little change (Figures 3C and 4C). Furthermore, we demonstrated that this up-regulation of p21 was p53 independent despite the existence of transcriptional targets of p53 in the promoter of the p21gene.35-37 Noteworthy, all the above proteins showed no variations when the cells were treated with arsenic alone. These observations supported the notion that cAMP may play a major role in inducing G1 arrest and cell cycle exit of NB4 and NB4-R1 cells.
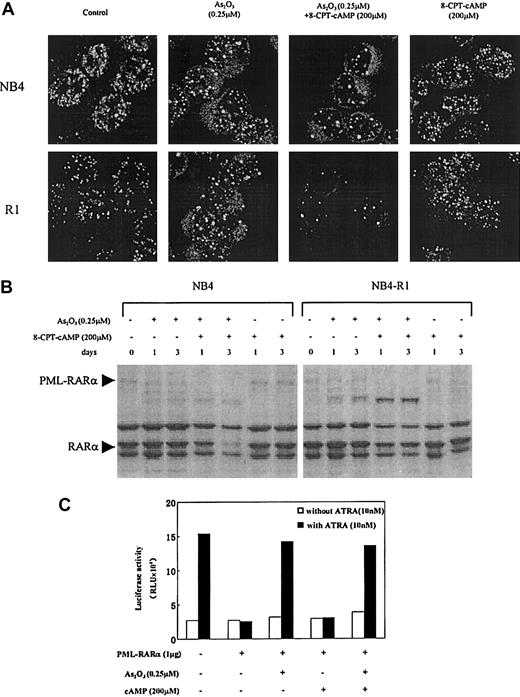
As2O3-associated PML-RARα degradation and PML-nuclear body reorganization in NB4 and NB4-R1 cells
It was reported that in APL cells, 0.1 to 1 μM arsenic targets PML and PML-RARα onto nuclear bodies (NBs) and induces their degradation.14,15 19 In the present work, confocal microscopy was used to analyze intracellular distribution of PML and PML-RARα in NB4 and NB4-R1 cells in the context of arsenic and cAMP synergism. As shown in Figure 5A, the abnormally organized fine and numerous labeled micro-PML-NBs in these 2 cell lines were modified by 0.25 μM As2O3, with the number of NBs gradually decreased, and the size of these structures increased. Moreover, this process was accelerated by the addition of 8-CPT-cAMP which, on its own, failed to cause NB reorganization. In accordance with this, Western blot analysis with anti-RARα antibodies showed that in the presence of 8-CPT-cAMP, As2O3 caused a near total degradation of PML-RARα (Figure 5B) as well as the wild-type PMLs (data not shown), whereas 8-CPT-cAMP alone did not cleave the chimeric protein (Figure5B). These observations suggested that the PML-RARα differentiation block in NB4 and NB4-R1 cells can be, to some degrees, partially released by As2O3-induced degradation, diminishing the dominant effect of PML-RARα. This conclusion was further supported by data from in vitro transient transactivation on a DR5 reporter (Figure 5C). We showed that PML-RARα inhibited the endogenous transcriptional activity of RARα and that this dominant-negative effect was abrogated by As2O3alone or combined with 8-CPT-cAMP, but not by 8-CPT-cAMP alone.
Effects of As2O3 or cAMP or both on PML-NB reorganization and PML-RARα degradation.
(A) Confocal microscopic analysis of PML-NBs in NB4 and NB4-R1 cells after a 72-hour exposure to the compounds indicated. Original magnification × 600. (B) Western blot analysis of PML-RARα during NB4 and NB4-R1 cell treatment with As2O3 in the presence and absence of 8-CPT-cAMP as indicated. (C) Transcriptional inhibition of PML-RARα protein on a DR5-RARE reporter with and without treatment of As2O3 or cAMP or both. COS-7 cells were cotransfected with 1 μg PML-RARα expression plasmid and 0.1 μg RARE-TK-luciferase reporter plasmid (see “Materials and methods”). The amount of plasmid was kept constant using empty vector. Twenty-four hours after transfection, COS-7 cells were treated with or without 10 nM ATRA, 0.25 μM As2O3, and 200 μM cAMP, as indicated. Luciferase activity was measured 48 hours after the transfection. The value indicated is a representative experiment among 3 independent assays. RLU indicates relative light units.
Effects of As2O3 or cAMP or both on PML-NB reorganization and PML-RARα degradation.
(A) Confocal microscopic analysis of PML-NBs in NB4 and NB4-R1 cells after a 72-hour exposure to the compounds indicated. Original magnification × 600. (B) Western blot analysis of PML-RARα during NB4 and NB4-R1 cell treatment with As2O3 in the presence and absence of 8-CPT-cAMP as indicated. (C) Transcriptional inhibition of PML-RARα protein on a DR5-RARE reporter with and without treatment of As2O3 or cAMP or both. COS-7 cells were cotransfected with 1 μg PML-RARα expression plasmid and 0.1 μg RARE-TK-luciferase reporter plasmid (see “Materials and methods”). The amount of plasmid was kept constant using empty vector. Twenty-four hours after transfection, COS-7 cells were treated with or without 10 nM ATRA, 0.25 μM As2O3, and 200 μM cAMP, as indicated. Luciferase activity was measured 48 hours after the transfection. The value indicated is a representative experiment among 3 independent assays. RLU indicates relative light units.
Discussion
Currently, ATRA and arsenic are specific drugs available for the treatment of patients with APL. It has been known that ATRA, under both in vitro and in vivo conditions, induces terminal differentiation (followed by natural apoptosis) of malignant cells. The underlying molecular mechanisms include the modulation of PML-RARα homodimer and activation of the wild-type RAR/RXR pathway, both depending on specific ligand binding to the appropriate domain of the receptors and resulting in the relief of transcriptional repression.12,13 On the contrary, the mechanisms of action of As2O3 seem to be much more complex, because the compound is pleiotropic and the mode of action shows dose dependency. Several recent studies suggested that the in vivo therapeutic effect of As2O3 could be primarily ascribed to the induction of differentiation.15,17Clinically, it was reported that after 2 to 3 weeks of either standard-dose (0.16 mg/kg daily) or low-dose (0.08 mg/kg daily) As2O3 treatment, not only the differentiated granulocytes at the stage of myelocytes and metamyelocytes increased significantly in the bone marrow, but also hyperleukocytosis developed in the majority of patients.4,38 The apoptotic cells increased in marrow usually after this “wave” of differentiation, sometimes even giving rise to a clinical setting mimicking the “retinoic acid syndrome,” most likely representing an event secondary to myeloid maturation. Pharmacokinetic analysis showed that, during the standard treatment course, the plasma concentrations of arsenic fluctuated over most time between 0.1 and 0.5 μM, except a short peak level superior to 1 μM caused by intravenous drip. Interestingly, the arsenic concentration was around 0.2 μM in the plasma 20 hours after intravenous administration in a group of patients treated with low-dose arsenic.38 It can thus be speculated that in vivo, APL cells are primarily exposed to a drug concentration in favor of differentiation rather than apoptosis. Moreover, it becomes now clear that the apoptotic effect of arsenic can be observed in many cell types, whereas the differentiating one is relatively selective to APL.
However, a number of issues should be clarified and further addressed. First, the differentiation of APL cells induced by arsenic is by no means a complete one, in that most cells are blocked at the metamyelocyte stage in both in vivo and in vitro settings. Second, the mechanisms underlying this incomplete or partial differentiation are still obscure. Third, the differentiation phenomenon observed in vitro may not really reflect that in vivo, because many naturally existing differentiation regulators, including the physiologic concentrations of retinoids, could be cooperating with the therapeutic effect of arsenic in patients. To this end, it is noteworthy that in a mice APL model, the induced leukemia could be cured by the association of ATRA with arsenic through enhanced differentiation and apoptosis, whereas the use of one of the drugs on its own only prolonged survival but did not produce complete cure.17 In this study, we found that not only ATRA and the histone deacetylase inhibitor TSA, but particularly cAMP, synergized with arsenic and triggered maturation of APL cells. It has been well known that, as a ubiquitous second messenger, cAMP plays a pivotal role in the proliferation and differentiation of human myeloid, lymphoid, and erythroid progenitor cells. Strikingly, cAMP can substantially enhance the differentiating effect of As2O3 not only on ATRA-sensitive NB4 parental cells, but also on NB4-R1 cells characterized by resistance to the ATRA-induced maturation, as shown by morphologic criteria (Figure1A,B), expression of CD11b integrin (Figure 1C,D), and the NBT test (Figure 1E,F). Moreover, this ability of cAMP was also observed in fresh APL cells (Figure 2A-C). Thus, investigation of the mechanisms underlying the synergy between cAMP and As2O3may shed new light on the differentiation therapy.
It has long been recognized that signals that stimulate cell cycle progression usually produce a block in differentiation, whereas interventions that produce a cell cycle arrest sensitize cells to the induction of differentiation.39,40 In fact, many pharmacologic differentiating agents, including phorbol esters, phenylbutyrate, and retinoids, share a common biologic activity to inhibit cell cycling.41-43 Our data showed that cAMP could cause a marked inhibition of NB4 and NB4-R1 cell proliferation by preventing these cells from entering into S phase (Figures 3B and 4B). Furthermore, we found that in the presence of cAMP, the mitotic inhibitor p21 was dramatically up-regulated in both NB4 and NB4-R1 cells, whereas the cellular transcription factor E2F greatly down-regulated (Figures 3C and 4C). p21 has been considered to best inhibit cyclin/cdk (cyclin-dependent kinase) complexes involving cdk2 and cdk4 at the G1, G1/S, and S phases and transitions in the cell cycle,32,33 and E2F has been shown to be able to regulate a number of genes that encode proteins with putative functions in the G1 to S phases of the cell cycle.44 These results strongly indicated that cAMP could exert an antiproliferative effect on myeloid cells. In contrast, no expression modulation of these cell cycle regulatory proteins was observed in the presence of arsenic alone. Nevertheless, the fact that cAMP alone was unable to induce differentiation in both NB4 and NB4-R1 cells suggested that full induction of terminal differentiation after induction of cell cycle arrest required additional lineage-specific signals.
In the case of APL, the fusion protein PML-RARα due to a specific chromosome translocation t(15;17) has been considered as a dominant-negative receptor that blocks myeloid differentiation most likely through the impairment of retinoid response.10Thus, one requisite for APL cell maturation is to restore retinoid signaling pathways. Two strategies may be taken into account: (1) a targeted degradation or inactivation of PML-RARα and (2) or an activation of alternative signaling pathways bypassing PML-RARα, such as recently demonstrated by Benoit et al.26Indeed, a RAR-independent RXR signaling, which requires cooperation with cAMP signaling, can trigger APL cells maturation.26 45 In this study, we showed that arsenic could induce PML-RARα degradation in both NB4 and NB4-R1 cells (Figure 5A,B) and circumvent the dominant-negative effect of PML-RARα on RAR-dependent gene expression (Figure 5C). Moreover, we observed that cAMP could cooperate with arsenic to accelerate the degradation of PML-RARα/PML. We also found that PKA inhibitor could significantly abrogate the granulocytic differentiation induced by As2O3 and cAMP (Figure 1G,H). It is possible that the actions of As2O3 and cAMP converge at the level of PKA pathway. Further study on arsenic- and cAMP-modulated gene expression profiles may be conducive to reveal the cross-talk point between these 2 agents.
Based on the above data, we may propose the following scenario. Although neither arsenic nor cAMP is sufficient alone in promoting terminal differentiation, these 2 signals cooperated to induce the maturation of APL cells. The cooperation might result from the concomitant degradation of fusion protein PML-RARα induced by As2O3 and the cell growth inhibition by cAMP. These findings are in line with the capacity of pharmacologic levels of ATRA to induce NB4 cell maturation. In this case also the relief of the differentiation block could be the result of both PML-RARα modulation/degradation induced by ATRA associated with a quick increase in intracellular cAMP level following ATRA stimulation.46In addition, the fact that an antagonist of cAMP can block ATRA-induced NB4 cell maturation supports that retinoid-induced maturation of NB4 cells needs also cooperation with cAMP signaling.24,25This idea could be further substantiated by the evidence that cAMP solely suffices to induce the differentiation of HL60, another myeloid leukemia cell line that lacks t(15;17) translocation.23 47
In summary, our study demonstrated the existence of synergism between arsenic and cAMP that triggers maturation pathway for APL cells. It is suggested that differentiation of arsenic-induced APL cells in vivo may probably result from cooperative effects of arsenic with other factors present in the cellular microenvironment of patients with APL. In addition, the demonstration that the combined treatment of arsenic with cAMP induces the maturation not only in NB4 but also in NB4-R1 cells might to some extent account for the effective improvement in the arsenic treatment of patients with relapsed APL who are resistant to ATRA and conventional chemotherapy. Finally, consideration of the facts that chronic toxicity and carcinogenicity of As2O3 has been reported and that the toxic and cumulative effect of As2O3 is dose dependent, our work may encourage the trials of new treatment protocols for the differentiation therapy of cancer.
The authors are grateful to Dr E. Ségal-Bendirdjian for help in reviewing the manuscript. The authors acknowledge Prof Zhen-yi Wang and Prof Ting-dong Zhang for their continuous support and all members of SIH for their encouragement and constructive discussion.
Supported in part by the National Key Program for Basic Research (973), the National Natural Science Foundation of China, the Shanghai Commission for Education, the Shanghai Commission for Science and Technology, the Shanghai “New Star” Research Program, L'Association Franco-Chinoise pour la Recherche Scientifique et Technique (PRA), L'Association pour la Recherche contre le Cancer (ARC), the Samuel Waxman Cancer Research Foundation, and the Clyde Wu Foundation of Shanghai Institute of Hematology (SIH).
Q.Z. and J-W.Z. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dr Jian-Hua Tong and Zhu Chen, Shanghai Institute of Hematology, Rui Jin Hospital, Shanghai Second Medical University, 197 Rui Jin Road II, Shanghai, 200025, P. R. China; e-mail:jhtong@yahoo.com.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal