Abstract
V(D)J recombination in lymphocytes is mediated by 2 recombination-activating genes, RAG1 and RAG2,which are expressed during lymphocyte development in bone marrow and thymus. Prompted by studies reporting re-expression of the RAGs in germinal center B cells, the expression of RAGs and terminal deoxynucleotidyl transferase (TdT) in human lymphoid tissues was examined using in situ hybridization and immunohistochemistry, respectively. Here it is shown that RAGs and TdT are not reinduced in germinal center reactions. However, RAG+/TdT+ cells are frequently present in extrafollicular areas of tonsils mainly at the boundary between lymphoid tissue and fibrous scaffold. Phenotypic analyses suggest that these cells are B cells. Finally, it is shown that RAG+/TdT+ cells are found more frequently in tonsils than in other peripheral lymphoid tissues. This may reflect an increased influx of RAG+/TdT+ cells as a result of higher antigenic stimulation at this site. Alternatively, this observation may indicate that the tonsils are an additional site of lymphocyte ontogeny.
Introduction
V(D)J rearrangement of immunoglobulin (Ig) and T-cell receptor (TCR) genes is mediated by the products of 2 recombination- activating genes (RAG1 and RAG2) which recognize specific recombination signal sequences (RSS) flanking the V, D, and J gene segments.1,2 The RAGs are transiently and coordinately expressed during B-cell and T-cell ontogeny in bone marrow and thymus, respectively.3-5 Upon completion of V(D)J rearrangement, RAG expression is shut down.3IgM-expressing immature bone marrow B cells recognizing self antigens can re-express the RAGs, leading to further rearrangement of the light chain locus.6 This process, termed receptor editing, is thought to play a role in the elimination of autoreactive B cells.6 Mature lymphocytes were generally believed to be incapable of expressing the RAG, thus maintaining allelic exclusion. A number of recent studies, however, have raised the possibility that secondary V(D)J recombination may occur in peripheral lymphoid tissue B cells in the context of germinal center reactions. Thus, RAG transcripts and proteins have been detected in germinal center B cells from lymph nodes, spleens, and Peyer patches in mice.7,8Using polymerase chain reaction (PCR), RSS DNA strand breaks characteristic of V(D)J recombination were detected in interleukin 4 (IL-4)–stimulated mature mouse B cells and in germinal center B cells from immunized mice.9,10 Han et al have suggested that reinduction of RAG expression may occur in germinal center B cells as a result of diminished or abolished antigen binding, thus rescuing cells which would otherwise have been lost to apoptotic cell death.10 Although initial studies have used mouse models, subsequent reports have provided evidence that similar mechanisms may operate in humans. RAG1 and RAG2 transcripts have been detected in human germinal center B lymphocytes by reverse transcriptase (RT)–PCR.11 In human tonsils, RSS DNA breaks were detectable in germinal center centrocytes but not in follicle mantle cells.12 Moreover, RAG, terminal deoxynucleotidyl transferase (TdT), VpreB, and λ-like transcripts were detected in centrocytes by PCR analysis of fluorescence activated cell sorting (FACS) sorted cell populations.12,13 Nevertheless, the exact nature and localization of RAG-expressing cells in peripheral lymphoid tissues is still controversial and immunohistochemical studies have not clarified this issue. Expression of RAG genes has been variably reported in the light zones of germinal centers,8in germinal center apoptotic bodies,14 and in scattered cells of germinal center, mantle zone, and extrafollicular area.13 To resolve this issue, we have examined the expression of RAGs and TdT in human lymphoreticular tissues using in situ hybridization and immunohistochemistry, respectively.
Materials and methods
Tissues
Formalin-fixed and paraffin-embedded tonsillectomy specimens from 25 patients aged 3 to 49 years were studied. All cases showed prominent follicular hyperplasia but no evidence of neoplastic disease. In addition, paraffin blocks from splenectomy specimens from 5 patients aged 19 to 79 years were studied. Reasons for splenectomy were traumatic rupture in 4 cases and pancreatitis in one case. Paraffin blocks from 5 biopsy and 3 surgical specimens from the terminal ileum were also available. The age of these patients ranged from 1 to 71 years. Hyperplastic lymph nodes from 8 patients aged 12 to 56 years showing nonspecific hyperplastic changes were also studied. Three of these patients were clinically diagnosed with systemic lupus erythematosus. For control purposes, paraffin sections from a human thymus and from a bone marrow trephine from a 7-month-old child were studied. All biopsy specimens were submitted to the Department of Pathology, Friedrich-Alexander-University, for diagnostic purposes.
Plasmids and probes
The plasmid H36CD containing the human RAG1 cDNA was kindly provided by Dr David G. Schatz (New Haven, CT). From this plasmid, a 700-bp fragment was amplified using the primers TCGAGAATTCAGGAATTAACTCACAAACTGC and TCGAGAATTCGCCATGAAGAGCAGTGAATTA, and cloned into the pCRII-TOPO vector (Invitrogen, Leek, The Netherlands) between an SP6 and a T7 promoter site. For the generation of a RAG2-specific probe, a 607-bp RAG2-specific fragment was amplified from NALM6 cDNA using the primers TCGAGAATTCACTATTTGCTTCTGCACTGA and TCGAGAATTCGTGTGCTACATCATACATTC. This PCR product was cloned into the pGEM-T vector (Promega, Mannheim, Germany) between a T7 and an SP6 promoter site. Both inserts were sequenced and compared with published RAG1 and RAG2 cDNA sequences (accession nos. M29474 and M94633, respectively). From all plasmids, radiolabeled antisense and sense RNA probes were generated by in vitro transcription using 35S-labeled uridine triphosphate (UTP) as described.15
In situ hybridization
In situ hybridization was carried out as previously described.15 16 In brief, NALM6 cytospin preparations were air-dried, fixed in 4% paraformaldehyde, dehydrated through graded ethanols, air-dried, and stored at −80°C until use. Paraffin sections were dewaxed and rehydrated through graded ethanols, treated with 0.2N HCl for 20 minutes, digested with 0.5 mg/mL pronase (Boehringer, Mannheim, Germany) for 10 minutes, fixed in cold 4% paraformaldehyde for 20 minutes, and acetylated using a freshly prepared 1-to-400 solution of acetic anhydride in 0.1 M triethanolamin, pH 8.0, for 10 minutes. Sections were dehydrated through graded ethanols and air-dried. Cytospin preparations were thawed and treated as above, except that a reduced pronase concentration (0.125 mg/mL) was used. Approximately 25 μL of hybridization mixture [50% deionized formamide/10% dextran sulfate/2x standard saline citrate (SSC)/0.2 mg/mL yeast tRNA/10 mM dithiothreitol (DTT)] containing between 4 × 104 cpm and 2 × 105 cpm of labeled probe was added to each slide. After hybridization overnight at 50°C, sections were washed in 50% formamide/1x SSC/10 mM DTT at 52°C for 4 hours. To remove nonspecifically bound probe, sections were subjected to RNase A digestion (Sigma, Steinheim, Germany, 20 μg/mL, 30 minutes, 37°C). Sections were then rinsed in 2x SSC, 0.1x SSC, dehydrated through graded ethanols, and dipped into Ilford G5 emulsion (Ilford, Mobberley, Cheshire, United Kingdom) and exposed at 4°C for up to 6 weeks.
Immunohistochemistry
For immunohistochemical staining, paraffin sections were dewaxed, rehydrated, and transferred into Tris buffered saline (0.05 M Tris, 0.15 M NaCl, pH 7.6). Sections were then subjected to microwave irradiation in 0.1 M citrate buffer, pH 6.0, at 800 W and 600 W for 10 minutes each. Subsequently, sections were incubated with appropriately diluted rabbit anti-TdT antiserum (Dako, Glostrup, Denmark; or Zymed, San Francisco, CA). Bound primary antibody was detected using biotinylated goat antirabbit antiserum (Dako) and a streptavidin-biotinylated alkaline phosphatase complex. Fast Red (Sigma) was used as a chromogen and sections were counterstained using hematoxylin. Alternatively, peroxidase-labeled streptavidin (Zymed) was employed with diaminobenzidine as a chromogen. For double-labeling immunohistochemistry, TdT staining was carried out as described but using Fast Blue (Sigma) as chromogen. Subsequently, sections were incubated in Double Staining Enhancer (Zymed) for 30 minutes at room temperature. Sections were then incubated with mouse monoclonal CD10, CD20, CD34, CD79a, or polyclonal CD3 antibodies (all from Dako). Bound CD3 reagent was detected as described for TdT using Fast Red as chromogen. Immobilized CD10, CD20, CD34, or CD79a antibodies were visualized using biotinylated rabbit antimouse antiserum and a streptavidin-biotinylated alkaline phosphatase complex as described above. Fast Red was used as a chromogen. Statistical analysis was done using the chi-square test.
Results
Validation of methods
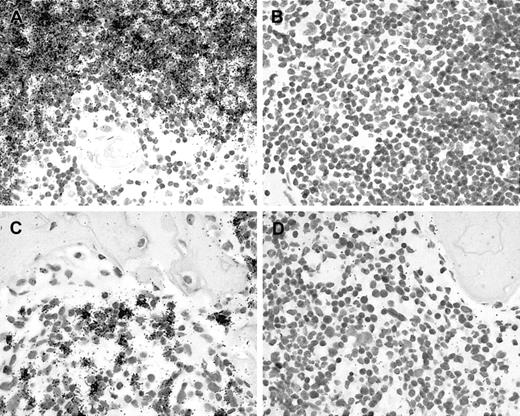
In preliminary experiments, we have tested the suitability of commercially available RAG1- and RAG2-specific antibodies for immunohistochemical staining. However, these reagents did not produce the expected results in frozen or paraffin sections from human thymus nor in cytospin preparations from NALM-6 cells. Therefore, we decided to generate RAG1- and RAG2-specific probes for use in in situ hybridization experiments.16 Using appropriate transcription vectors, single-stranded 35S-labeled RNA probes were obtained.16 To validate this approach, several control studies were carried out. In situ hybridization with antisense probes resulted in a strong specific labeling of cortical thymocytes (Figure 1A) and of NALM-6 cells (not shown). Furthermore, there were numerous RAG+ cells detected in a bone marrow trephine from a 7-month-old child showing a reactive increase of immature B cells (Figure 1C). As negative controls, in situ hybridization with sense probes derived from the same plasmids yielded negative results (Figure1B,D). Furthermore, we have previously reported the detection of RAG-specific transcripts in lymphoblastic non-Hodgkin lymphomas using this method.16 Thus, the validation studies demonstrate that this assay yields the expected results in our hands. However, in situ hybridization with RAG1- and RAG2-specific probes required prolonged exposure (up to 6 weeks) to yield clearly detectable results. In the control experiments, antisense probes derived from both plasmids gave identical results, as expected, since both genes are expressed coordinately.5 We therefore decided to apply RAG1- and RAG2-specific antisense probes as a mixture in all subsequent experiments. This approach increases the available target and, therefore, affords a higher sensitivity.
Validation of in situ methods.
(A) In situ hybridization with 35S-labeled antisense probes results in an accumulation of black silver grains, indicating RAG expression in cortical but not in medullary thymocytes of a human thymus. (B) Hybridization with sense control probes shows no specific labeling. (C) In situ hybridization reveals scattered RAG-positive cells in bone marrow, whereas (D) hybridization with sense probes shows no specific labeling.
Validation of in situ methods.
(A) In situ hybridization with 35S-labeled antisense probes results in an accumulation of black silver grains, indicating RAG expression in cortical but not in medullary thymocytes of a human thymus. (B) Hybridization with sense control probes shows no specific labeling. (C) In situ hybridization reveals scattered RAG-positive cells in bone marrow, whereas (D) hybridization with sense probes shows no specific labeling.
For the detection of TdT expression, we employed immunohistochemistry with commercially available polyclonal antisera from 2 different suppliers. Application of this method to paraffin sections from human thymus resulted in strong nuclear staining of cortical but not medullary thymocytes (Figure3A). Immature lymphocytes in bone marrow also showed a clear nuclear labeling (Figure 3B). Immunostaining with TdT-specific antisera from both suppliers gave identical results. Thus, control experiments with TdT-specific reagents yielded the expected results, confirming the specificity of this assay.
RAG and TdT expression in peripheral lymphoid tissues
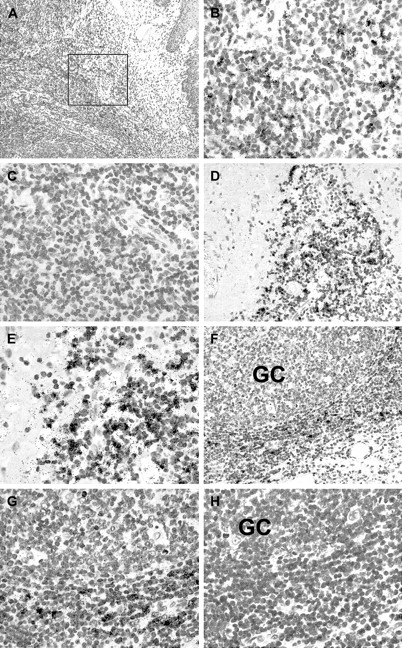
Paraffin sections from 25 hyperplastic tonsils showing numerous prominent germinal centers were subjected to in situ hybridization with RAG-specific probes. RAG+ cells were identified in 12 cases. The remaining cases did not show specific labeling above background. In the positive cases, variable numbers of RAG+cells were identified mainly at the interface between the lymphoreticular tissue and the fibrous connective tissue of the tonsillar scaffolding (Figure 2A-B,D-E). In addition, RAG+cells were sometimes located just outside germinal centers near the dark zone (Figure 2F-G; compare also Figure4E-F). Hybridization with sense probes derived from the same plasmids yielded negative results (Figure 2C,H). Attempts to characterize the RAG+ cells by double-labeling in situ hybridization and immunohistochemistry were unsuccessful for technical reasons.
In situ expression of recombination-activating genes in human tonsil.
In situ hybridization reveals focal clusters of RAG-positive cells in human tonsil sections as indicated by the presence of black silver grains. (A) Low power magnification shows tonsillar epithelium at the right margin of this figure. The boxed area is shown at high power in B and C. (B) Using 35S-labeled RAG-specific antisense probes, several labeled cells are detected in this area, whereas in (C) no specific labeling is seen in the same area using the corresponding sense probes. (D) In a different tonsil, numerous RAG-expressing cells are seen at the boundary between lymphoid tissue and fibrous connective tissue. (E) A higher power view of the same areas as in D. (F) In another tonsil, RAG-positive cells are observed in the vicinity of a germinal center (GC). (G) A high power view of the same area as in F. (H) No specific labeling is seen in the same case using the corresponding sense probes.
In situ expression of recombination-activating genes in human tonsil.
In situ hybridization reveals focal clusters of RAG-positive cells in human tonsil sections as indicated by the presence of black silver grains. (A) Low power magnification shows tonsillar epithelium at the right margin of this figure. The boxed area is shown at high power in B and C. (B) Using 35S-labeled RAG-specific antisense probes, several labeled cells are detected in this area, whereas in (C) no specific labeling is seen in the same area using the corresponding sense probes. (D) In a different tonsil, numerous RAG-expressing cells are seen at the boundary between lymphoid tissue and fibrous connective tissue. (E) A higher power view of the same areas as in D. (F) In another tonsil, RAG-positive cells are observed in the vicinity of a germinal center (GC). (G) A high power view of the same area as in F. (H) No specific labeling is seen in the same case using the corresponding sense probes.
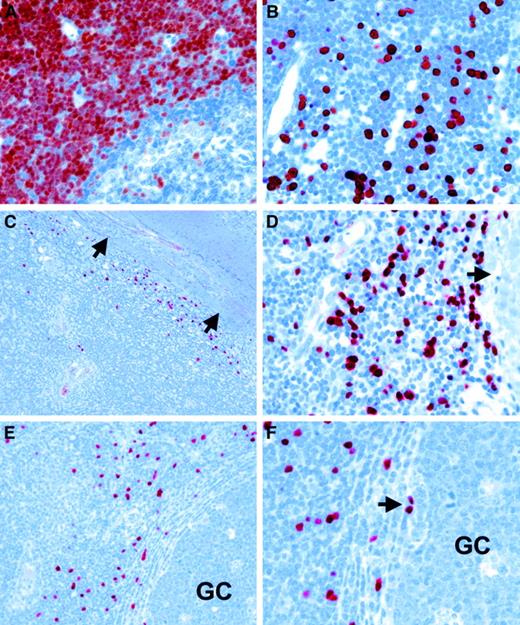
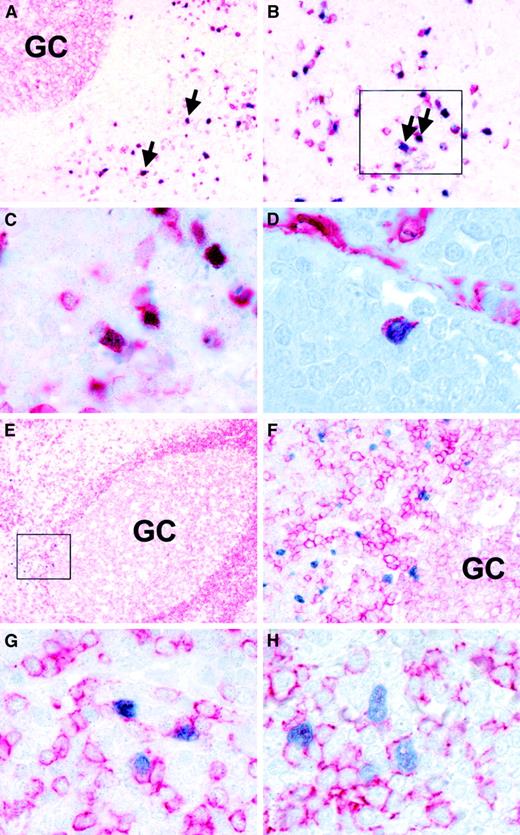
Immunohistochemistry with TdT-specific antisera identified variable numbers of labeled cells in all tonsils. TdT-expressing cells showed a distribution identical to the RAG+ cells, that is, they were localized near the fibrous stroma of the tonsils (Figure 3C-D) and in the immediate vicinity of germinal centers (Figure 3E-F; Figure 4E-F). Occasional TdT+ cells appeared to be localized just within germinal centers (Figure 3F, Figure 4F). TdT staining clearly showed the location of these cells at the base of the germinal centers near the dark zone and opposite to the mantle zone (Figure 4E-F). The identical distribution of RAG+ and TdT+ cells suggests that both gene products are expressed in the same cells, as has been reported for proB and preB cells.3 The distribution of RAG+/TdT+ cells in correlation to tonsillar architecture is shown schematically in Figure5. Double-staining experiments were subsequently carried out on 6 tonsils with larger numbers of TdT+ cells in order to characterize the nature of these cells. This revealed that the vast majority of TdT+ cells coexpressed CD10, which is expressed in early lymphoid progenitor cells and in germinal center cells (Figure 4A-C).17 Moreover, isolated TdT+ cells showed coexpression of CD34 (Figure4D).18 Double-labeling with CD3-, CD20-, and CD79a-specific reagents showed that the majority of TdT+cells lacked lineage-specific markers (Figure 4E-F). However, a small proportion of TdT+ cells showed unequivocal coexpression of the B-cell antigens CD79a and CD20 (Figure 4G-H).
In situ expression of TdT in human tonsil.
(A) Immunohistochemistry with a TdT-specific antiserum (note red-brown staining) shows strong nuclear labeling of cortical thymocytes in a human thymus. (B) Scattered TdT-positive cells are also detected in bone marrow. (C, D) Immunohistology with a TdT-specific reagent (note red nuclear labeling) reveals numerous TdT-positive cells at the boundary between lymphoid tissue and fibrous scaffold at (C) low and (D) high power (arrows indicate fibrous tissue in C and D). (E, F) In a different case, TdT-positive cells are present in the vicinity of a germinal center (GC) at (E) low power and (F) high power. Note the presence of isolate-labeled cells at the edge of a germinal center in F (arrow).
In situ expression of TdT in human tonsil.
(A) Immunohistochemistry with a TdT-specific antiserum (note red-brown staining) shows strong nuclear labeling of cortical thymocytes in a human thymus. (B) Scattered TdT-positive cells are also detected in bone marrow. (C, D) Immunohistology with a TdT-specific reagent (note red nuclear labeling) reveals numerous TdT-positive cells at the boundary between lymphoid tissue and fibrous scaffold at (C) low and (D) high power (arrows indicate fibrous tissue in C and D). (E, F) In a different case, TdT-positive cells are present in the vicinity of a germinal center (GC) at (E) low power and (F) high power. Note the presence of isolate-labeled cells at the edge of a germinal center in F (arrow).
Phenotypic characterization of TdT-positive cells.
Double-staining immunohistochemistry with a TdT-specific antiserum (blue nuclear labeling) and cell surface markers (red staining) shows (A, B, C) coexpression of TdT and CD10 (arrows). C represents the boxed area in B. (D) Double-labeling reveals coexpression of TdT and CD34 in a single cells. Note reactivity of endothelial cells with the CD34 reagent in the top right hand corner. (E, F) Double-staining with TdT- and CD79a-specific antibodies reveals localization of TdT-positive cells outside a germinal center near the dark zone. F represents the boxed area in E at high power. Note that TdT-positive cells are negative for CD79a in this image. (G) This panel illustrates the presence of a few TdT-positive cells coexpressing CD79a. (H) Double-staining with TdT- and CD20-specific antibodies also reveals isolated TdT-positive cells coexpressing CD20.
Phenotypic characterization of TdT-positive cells.
Double-staining immunohistochemistry with a TdT-specific antiserum (blue nuclear labeling) and cell surface markers (red staining) shows (A, B, C) coexpression of TdT and CD10 (arrows). C represents the boxed area in B. (D) Double-labeling reveals coexpression of TdT and CD34 in a single cells. Note reactivity of endothelial cells with the CD34 reagent in the top right hand corner. (E, F) Double-staining with TdT- and CD79a-specific antibodies reveals localization of TdT-positive cells outside a germinal center near the dark zone. F represents the boxed area in E at high power. Note that TdT-positive cells are negative for CD79a in this image. (G) This panel illustrates the presence of a few TdT-positive cells coexpressing CD79a. (H) Double-staining with TdT- and CD20-specific antibodies also reveals isolated TdT-positive cells coexpressing CD20.
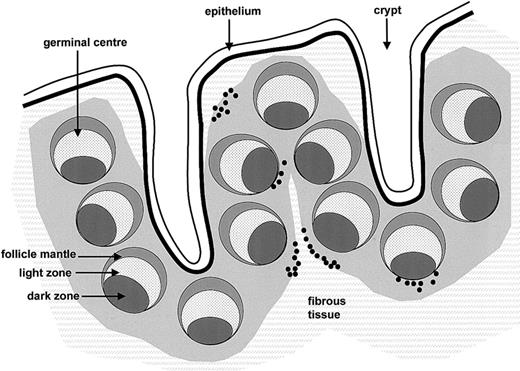
Distribution of RAG+/TdT+ cells in human tonsil.
RAG+/TdT+ cells (black dots) are shown in relation to tonsillar architecture.
Distribution of RAG+/TdT+ cells in human tonsil.
RAG+/TdT+ cells (black dots) are shown in relation to tonsillar architecture.
TdT+ cells, as well as RAG+ cells, were not distributed homogeneously throughout the sections. In order to quantify their number, TdT+ cells were counted in 10 high power fields (hpf; x400) in areas showing most TdT+ cells as assessed by low power examination. This confirmed that there were variable numbers of TdT+ cells, ranging from 1 to 512 positive cells per 10 hpf (median 149). The same approach was also used to determine the frequency of RAG+ cells. This revealed between 24 and 287 labeled cells per 10 hpf (median 60) in the 12 cases with detectable RAG+ cells. Comparison of results revealed that RAG+ cells were preferentially detected in those tonsils showing numerous TdT+ cells, suggesting that the apparent absence of RAG+ cells from some tonsils had technical reasons, for example, related to the lower stability of RAG mRNA detected by in situ hybridization as compared with TdT protein identified by immunohistochemistry. There was no statistical correlation of the numbers of TdT+ cells with age (P = .732).
In addition to tonsils, we examined the expression of RAGs and TdT in paraffin sections from 5 spleens, 10 ileal Peyer patches, and 8 lymph nodes. In the spleens, between 0 and 14 TdT+ cells per 10 hpf were detected. In the ileal samples, there were between 0 and 67 TdT+ cells per 10 hpf, with 6 cases containing no labeled cells. Finally, in the lymph nodes, between 0 and 254 (median 3) TdT+ cells were detected per 10 hpf. These included 3 lymph nodes from patients with systemic lupus erythematosus, which harbored 3, 16, and 254 TdT+ cells per 10 hpf, respectively. The distribution of these cells was similar to that seen in tonsils. Specifically, in all of these samples, there were no TdT+ cells detected in germinal centers. Using RAG in situ hybridization, no specific signal above background was detected in any of these tissues.
Discussion
We have used 2 independent methods to detect expression of 2 gene products associated with V(D)J recombination, RAGs and TdT, in situ in human peripheral lymphoid tissues. The first conclusion from this study is that RAG+/TdT+ cells are frequently detectable in human tonsils. Double-labeling experiments suggest that these cells are likely to be B cells. This conclusion is supported by a previous study reporting the presence of TdT+ immature B cells but not T cells in fetal lymph nodes and spleens.19However, since the majority of RAG+/TdT+ cells did not express lineage-specific markers and since RAG expression in peripheral T-cells has been reported,20 21 this question requires further examination.
The second important observation regards the distribution of RAG+/TdT+ cells (Figure 5). Several recent studies have suggested that expression of the RAGs and other components of the V(D)J recombination machinery may be reinduced in peripheral mature B cells in the context of germinal center reactions, and it has been proposed that this mechanism may rescue B cells producing low avidity antibodies.7-9,11-13,22 However, the precise nature and localization of these cells has remained uncertain. Immunohistochemical studies of mouse and human tissues have variably detected RAG expression in the light zones of germinal centers,8 in germinal center apoptotic bodies,14 and in scattered cells of germinal center, mantle zone, and extrafollicular area,13 respectively. In contrast, we clearly demonstrate that detectable RAG expression is absent from germinal center cells. This conclusion appears to be justified since we were able to detect RAG expression in situ in all situations where this was expected, notably in immature B and T cells of bone marrow and thymus, respectively. Moreover, we identified RAG-positive cells localized outside germinal centers in approximately 50% of the tonsils investigated. Lastly, virtually identical results were obtained by TdT immunostaining. Thus, we conclude that RAG and TdT expression are not reinduced in germinal center cells. This conclusion is in agreement with recent studies of RAG2-GFP knock-in mice, which have identified RAG expression in immature splenic B cells but not in germinal center B cells.23-25 Moreover, it has been shown that the number of peripheral RAG-expressing cells may increase in response to infection or immunization.25 This may well be the mechanism underlying the variable numbers of RAG/TdT-expressing cells observed in our study. The majority of RAG+/TdT+ cells were detected in a poorly defined compartment at the interface between lymphoid tissue and the fibrous scaffold of the tonsils (Figure 5). It is currently unclear if these cells represent mature lymphocytes which have reacquired features of immature cells or genuinely immature lymphocytes. The expression of CD10 in most of these cells and of CD34 in at least a small proportion of these cells would seem to suggest the latter. However, further studies are required to clarify this issue. It has been shown that secondary V(D)J recombination may occur in B cells following somatic hypermutation.26-28 Our results suggest that this is not due to re-expression of the RAGs in germinal center reactions. It has been proposed that V(D)J recombination may occur in germinal centers in a RAG-independent fashion.28 Alternatively, it is conceivable that somatic mutation is not restricted to germinal center reactions.
Finally, our studies demonstrate that RAG+/TdT+cells are more frequently detectable in tonsils than in other peripheral lymphoid tissues. This may be due to a higher exposure of the tonsils to antigenic stimuli, leading to an increased influx of immature lymphoid cells.25 Alternatively, it may be that in humans the tonsils are an additional site of lymphocyte ontogeny.
We are indebted to Renate Lisner and Christa Winkelmann for excellent technical assistance. We are also grateful to Prof Antony Basten for advice and for critical reading of the manuscript.
Supported by the Deutsche Forschungsgemeinschaft (SFB466).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gerald Niedobitek, Pathologisches Institut, Friedrich-Alexander-Universität, Krankenhausstr 8-10, 91054 Erlangen, Germany; e-mail:gerald.niedobitek@patho.imed.uni-erlangen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal