Abstract
The endothelial protein C receptor (EPCR) facilitates protein C activation and plays a protective role in the response toEscherichia coli–mediated sepsis in primates. Previously, a soluble form of EPCR (sEPCR) in human plasma was characterized, and several studies indicated that generation of sEPCR is regulated by inflammatory mediators, including thrombin-mediated up-regulation of surface metalloproteolytic activity in vitro. This study addressed the question of whether plasma sEPCR levels reflect changes in thrombin generation in patients undergoing anticoagulant treatment. The sEPCR levels in patients treated with coumarin-type oral anticoagulants were significantly lower than those in healthy asymptomatic adult volunteers (105.3 ± 70.8 ng/mL [n = 55] versus 165.8 ± 115.8 ng/mL [n = 200]; P < .0001). A similar decline in plasma sEPCR levels was found in patients treated with unfractionated heparin. In healthy volunteers, sEPCR levels declined to about 100 ng/mL within 3 days after initiation of an 8-day period of warfarin administration and increased within 2 days after its cessation. Plasma sEPCR levels returned to pretreatment values within 1 week, and the changes in plasma sEPCR levels mirrored changes in values for international normalized ratios. A similar decline in sEPCR levels with time was observed in 7 patients beginning treatment with warfarin for a thrombotic disorder. Prothrombin fragment 1 + 2 levels also decreased in volunteers and patients given warfarin. These results show that plasma sEPCR levels decline in response to treatment with anticoagulants whose mechanism of action is known to decrease in vivo thrombin production.
Introduction
Perturbations of hemostasis are central to the pathogenesis of a hypercoagulable state and may be triggered by multiple, overlapping influences. These include but are not limited to environmental effects (surgery, diet, smoking, childbirth, and trauma), manifestations of a primary disease (cardiovascular or cerebrovascular disease, diabetes, sepsis, hypertension, autoimmune disease, and malignant disease), or inheritable defects in hemostatic factors (protein C, protein S, factor V Leiden, prothrombin, and antithrombin).1-3
Currently, the therapeutic approach for patients with thrombotic disease typically starts with an intravenous course of unfractionated heparin or low-molecular-weight heparin, followed by a course of oral anticoagulation. In North America and Europe, warfarin (4-hydroxycoumarin) and other coumarin derivatives are the most widely used oral anticoagulants for preventing and treating venous or arterial thrombosis and embolism. The basic premise of anticoagulant therapy is that it will ultimately reduce formation of thrombin by interfering with the vitamin K–dependent coagulation factors required for activation of prothrombin.4 5 Thrombin is the final enzyme product of the coagulation cascade and is needed to form fibrin and to activate platelets, both of which are essential components of a clot.
Thrombin is also needed for the protein C anticoagulant regulatory system to function properly. The protein C system is a primary regulatory pathway of coagulation and thrombin formation.6The activated protein C generated by this pathway inactivates critical coagulation factors required for thrombin formation. An endothelial protein C receptor (EPCR) has been identified7,8 and shown to facilitate the protein C activation by the thrombin/thrombomodulin system.9 Immunohistochemistry studies found that membrane-bound EPCR is expressed on endothelial cells, with increasing expression corresponding to increasing vessel diameter.10,11 A soluble form of EPCR (sEPCR) exists in plasma, and retains its ability to bind both protein C zymogen and activated protein C, although it cannot augment protein C activation.12 Recently it was shown that recombinant sEPCR binds to activated neutrophils partly by means of proteinase 3, a neutrophil granule serine proteinase, and may be involved in modulating autoantibody-mediated neutrophil inflammatory events.13
Previous studies found that sEPCR is generated in vitro from its endothelial-bound parent by metalloproteinase activity, and this activity is inducible by thrombin and inflammatory mediators.14 In an in vivo rodent model of sepsis, up-regulation of EPCR messenger RNA (mRNA) and sEPCR generation by thrombin infusion was inhibited by hirudin.15 Furthermore, the murine EPCR gene contains a thrombin response element, and expression of a transgene driven by the murine EPCR promoter was found to be responsive to thrombin.16 Although the physiologic importance of sEPCR in vivo is unknown, elevated sEPCR levels are found in patients with sepsis and systemic lupus erythematosus, conditions associated with considerable thrombin production and coagulopathy.17 In this study, we addressed the question of whether plasma sEPCR levels are related to thrombin production in vivo by evaluating the effect of anticoagulant administration on circulating sEPCR levels in patients and healthy volunteers.
Patients and methods
Patients and volunteers
Plasma samples were obtained from an apparently healthy population of 200 adult volunteers (100 men and 100 women) recruited at a French health-care center (IPC, Paris, France). Blood was collected by venipuncture into Vacutainer tubes containing 0.129 M trisodium citrate (1:10). Plasma was stored at −70°C until testing. Plasma samples were obtained from patients undergoing vitamin K–antagonist oral anticoagulant therapy with acenocoumarol, fluindione, or warfarin and recruited at a French general hospital (Assistance Hôpitaux Publique de Paris, France). All samples were obtained with informed consent.
Six healthy Italian volunteers (3 men and 3 women; mean age ± SD; 38 ± 11 years) agreed to undergo an 8-day course of warfarin administration. All received an initial dose of 10 mg/day, which was then adjusted on the basis of international normalized ratio (INR) results, aiming for a target of 2.5. Blood samples were obtained at 11:00 am from an antecubital vein 2 days before the first dose of warfarin and 24 hours after the intake of warfarin for the first 5 days. Blood samples were also collected on days 8 through 12 and on day 15. To monitor changes in thrombin production, levels of prothrombin fragment 1 + 2 (F1 + 2) were measured with a commercial enzyme-linked immunosorbent assay (ELISA; Enzygnost F1 + 2; Dade-Behring, Milan, Italy). Plasma protein C antigen was measured by Laurell rocket immunoelectrophoresis using purified goat antihuman protein C IgG.18
Blood samples were collected from 10 Italian patients (5 men and 5 women; mean age, 70 ± 5 years) treated for at least 2 days with therapeutic dosages of intravenous unfractionated sodium heparin (mean activated partial thromboplastin time [APTT] ratio, 2.65 ± 0.89) because of unstable angina, myocardial infarction (n = 5), or venous thromboembolism (n = 5). Samples were also collected from 7 additional patients (3 men and 4 women; mean age, 71 ± 13 years) before and at different times after initiation of warfarin treatment (without previous heparin therapy) for atrial fibrillation (n = 4), dilative cardiomyopathy, mitral valve disease, or deep vein thrombosis. All blood samples (4.5 mL) were collected in Vacutainer tubes containing 0.5 mL 0.129 M trisodium citrate (Becton Dickinson, Plymouth, United Kingdom). Platelet-poor plasma was obtained within 2 hours by centrifugation at room temperature for 10 minutes at 1500g. INR (Hemoliance Recombiplastin) and APTT (STA PTTA; Diagnostica Stago, Gennevilliers, France) determinations were done in samples of fresh plasma. The remaining plasma was divided into aliquots in Eppendorf tubes (0.4 mL), snap-frozen with methanol and dry ice, and stored at −70°C until assayed.
Assay of plasma sEPCR levels
The sEPCR assay was modified from an ELISA described previously.17 Briefly, microtiter plates were coated with anti-EPCR 1494 monoclonal antibody (mAb) F(ab′)2 fragments prepared by pepsin digestion using standard procedures. The wells were washed, blocked, and incubated for 1 hour at room temperature with plasma samples diluted (1:50) in Tris buffer containing EDTA. The 1494 mAb binds to the ligand binding site on EPCR, and assay in the presence of EDTA dissociates any sEPCR–protein C complexes to permit measurement of sEPCR only. Bound antigen was detected with horseradish peroxidase–labeled anti-EPCR 1495 mAb (1 hour). This was followed by washing and 3 minutes of incubation with a substrate of o-phenylenediamine and urea peroxide. The reaction was stopped with a one-fifth volume of 3 M sulfuric acid, and the optical density (490 nm) was read with a Vmax microplate reader (Molecular Devices, Sunnyvale, CA) after an additional 30 minutes of incubation. The assay was calibrated with recombinant human sEPCR19 (provided by Dr Charles Esmon, Oklahoma Medical Research Foundation) and had a measuring range of 50 to 1000 ng/mL with an intra-assay and interassay variation of less than 8%. In preliminary studies, blood collected into either 0.109-M or 0.129-M citrate had no effect on assay results (data not shown).
Results
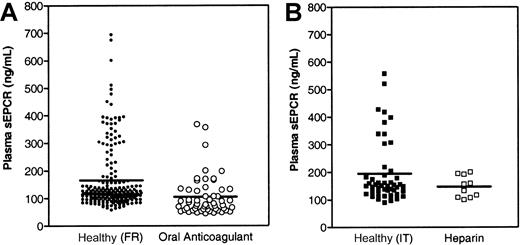
It was found previously that sEPCR is generated from its endothelial-bound parent in vitro by metalloproteinase activity induced by thrombin and some inflammatory mediators,14 thus raising the question of whether sEPCR levels in plasma may reflect in vivo thrombin generation. To address this question, we determined sEPCR levels in patients receiving vitamin K–antagonist oral anticoagulant therapy and compared those with sEPCR levels in an apparently healthy adult population. Because the effect, if any, of diet or other environmental factors on plasma sEPCR levels is unknown, data from patient samples were compared with data from healthy adult samples obtained from the same geographic region. A French population of patients receiving oral anticoagulant therapy with acenocoumarol, fluindione, or warfarin had significantly lower sEPCR levels (105.3 ± 70.8 ng/mL; n = 55) than a healthy French population (165.8 ± 115.8 ng/mL [range, 57.8-693.5 ng/mL]; n = 200;P < .0001; Figure 1A). In the healthy population, sEPCR levels tended to be higher in the men than in the women (184.51 ± 129.2 ng/mL [n = 100] versus 147.1 ± 97.8 ng/mL [n = 100]; P < .01). Additional analysis of data from the healthy population revealed a bimodal distribution in which the majority of volunteers had plasma sEPCR levels of approximately 75 to 175 ng/mL, but about 20% had much higher levels (up to nearly 700 ng/mL).
Plasma levels of sEPCR decrease in patients undergoing anticoagulant therapy.
Plasma sEPCR levels in (A) a healthy French adult population and in patients undergoing oral anticoagulant therapy and in (B) a healthy Italian adult population and in patients undergoing intravenous anticoagulant therapy with unfractionated heparin. The mean value for each population is indicated.
Plasma levels of sEPCR decrease in patients undergoing anticoagulant therapy.
Plasma sEPCR levels in (A) a healthy French adult population and in patients undergoing oral anticoagulant therapy and in (B) a healthy Italian adult population and in patients undergoing intravenous anticoagulant therapy with unfractionated heparin. The mean value for each population is indicated.
To evaluate the relation between plasma sEPCR levels and anticoagulant therapy further, samples were obtained from Italian patients undergoing intravenous unfractionated heparin therapy. Samples from an apparently healthy Italian adult population had a mean sEPCR level of 195.1 ± 114.0 ng/mL (n = 49; Figure 1B), a value that did not differ significantly from that in the healthy French population. In addition, a similar bimodal distribution was observed. Patients receiving intravenous heparin therapy had lower sEPCR levels (147.1 ± 39.3 ng/mL [n = 10]; P < .09) than the healthy volunteers (Figure 1B). Although the number of samples from patients receiving heparin therapy was small, the reduction in plasma sEPCR levels followed the trend observed in patients undergoing oral anticoagulant therapy with vitamin K antagonists.
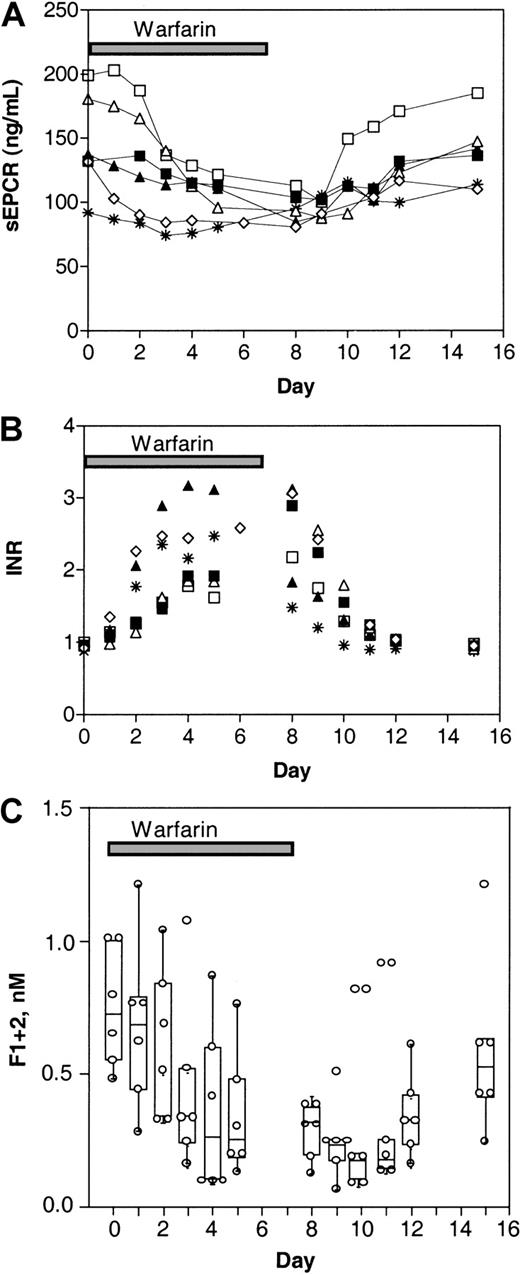
The effect of warfarin treatment on plasma sEPCR levels in healthy adults was studied in 6 volunteers (Figure2A). The volunteers received warfarin (oral anticoagulation) for 8 days, and follow-up was extended to day 15 after the beginning of warfarin administration. Blood for evaluation of INR levels was obtained before the warfarin regimen began (day 0) and on each day of the 15 days of the study. The warfarin dosage was adjusted as needed for each subject based on the subject's daily INR value. As shown in Figure 2A, plasma sEPCR levels declined during warfarin administration. There was a lag time of 24 to 48 hours before sEPCR levels decreased, and the extent of the decline varied among the volunteers. Approximately 24 to 48 hours after cessation of warfarin administration, sEPCR levels began to increase in all volunteers, with most reaching pretreatment values by day 15. The INR values in the samples from these volunteers were essentially a mirror image of the sEPCR levels (Figure 2B).
Levels of sEPCR respond to warfarin in healthy adult volunteers.
Warfarin was administered to 6 adult volunteers for 8 days. The sEPCR levels (A), INR values (B), and F1 + 2 levels (C) were determined in blood drawn just before warfarin administration (day 0), throughout the administration period, and during a 1-week follow-up period. Data from each individual donor are indicated. The F1 + 2 levels are shown with the median, interquartile ranges, and 95% confidence intervals for the means.
Levels of sEPCR respond to warfarin in healthy adult volunteers.
Warfarin was administered to 6 adult volunteers for 8 days. The sEPCR levels (A), INR values (B), and F1 + 2 levels (C) were determined in blood drawn just before warfarin administration (day 0), throughout the administration period, and during a 1-week follow-up period. Data from each individual donor are indicated. The F1 + 2 levels are shown with the median, interquartile ranges, and 95% confidence intervals for the means.
To confirm that administration of warfarin is accompanied by a decrease in thrombin production in healthy individuals, F1 + 2 levels were monitored (Figure 2C). Levels of F1 + 2 decreased as a result of the warfarin administration, with a significant decrease observed on day 5 (to 39% of initial values; P < .05). The F1 + 2 levels appeared to return to pretreatment levels more slowly than did the sEPCR levels.
Table 1 shows the changes in sEPCR and protein C antigen values in the 6 healthy volunteers given warfarin. The data are expressed as percent variations relative to baseline values (day 0), with the corresponding INR values. A significant decrease in sEPCR levels was first observed on day 3 (to 77% of initial values; P < .05), and this persisted until about 48 hours after the last warfarin administration. Protein C antigen levels dropped rapidly, to 46% of baseline values by day 2, and returned to normal levels 96 hours after the last warfarin administration. In a previous study of volunteers given an 8-day warfarin regimen, factor II levels reached a nadir more slowly (between days 2 and 5) than other vitamin K–dependent coagulation factors, after which F1 + 2 levels declined.20 Thus, in healthy volunteers, prevention of prothrombin activation by warfarin was not observed until after a significant drop in factor II levels, coinciding with the reductions in sEPCR and F1 + 2 levels observed in the current study. Together, these data suggest that the decrease in sEPCR is not dependent on reduced circulating levels of protein C but rather mirrors the inhibition of in vivo thrombin generation.
Percent changes over baseline of sEPCR, protein C antigen, and INR mean values in 6 healthy subjects starting warfarin treatment
| Day of Study . | 0 . | 1 . | 2 . | 3 . | 4 . | 5 . | 8 . | 9 . | 10 . | 11 . | 12 . | 15 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose of Warfarin, mg/d | 10 | 10 | 7.3 (4.1-10.4) | 6.3 (2.6-9.9) | 5.8 (2.5-9.1) | 6.7 (2.7-10.6) | 6.7 (2.7-10.6) | — | — | — | — | — |
| sEPCR | 100 | 93 (82-105) | 89 (77-102) | 77 (67-89)* | 74 (63-87)* | 71 (57-88)* | 67 (51-87)* | 69 (50-94)* | 80 (53-120) | 80 (66-98) | 90 (76-106) | 97 (82-114) |
| PC:Ag | 100 | 65 (43-99) | 42 (25-70)* | 31 (18-53)* | 32 (23-44)† | 28 (19-44)† | 33 (27-41)‡ | 42 (35-50)‡ | 49 (38-63)* | 77 (55-106) | 86 (57-128) | 98 (75-126) |
| INR | 0.95 | 1.13 | 1.57 | 1.99 | 2.17 | 2.20 | 2.34 | 1.90 | 1.39 | 1.13 | 1.01 | 0.94 |
| (0.90-0.99) | (1.01-1.27) | (1.16-2.13) | (1.47-2.69)* | (1.73-2.73)‡ | (1.70-2.85)† | (1.69-3.23)† | (1.41-2.57)† | (1.10-1.75)* | (0.99-1.28) | (0.96-1.07) | (0.90-0.98) |
| Day of Study . | 0 . | 1 . | 2 . | 3 . | 4 . | 5 . | 8 . | 9 . | 10 . | 11 . | 12 . | 15 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose of Warfarin, mg/d | 10 | 10 | 7.3 (4.1-10.4) | 6.3 (2.6-9.9) | 5.8 (2.5-9.1) | 6.7 (2.7-10.6) | 6.7 (2.7-10.6) | — | — | — | — | — |
| sEPCR | 100 | 93 (82-105) | 89 (77-102) | 77 (67-89)* | 74 (63-87)* | 71 (57-88)* | 67 (51-87)* | 69 (50-94)* | 80 (53-120) | 80 (66-98) | 90 (76-106) | 97 (82-114) |
| PC:Ag | 100 | 65 (43-99) | 42 (25-70)* | 31 (18-53)* | 32 (23-44)† | 28 (19-44)† | 33 (27-41)‡ | 42 (35-50)‡ | 49 (38-63)* | 77 (55-106) | 86 (57-128) | 98 (75-126) |
| INR | 0.95 | 1.13 | 1.57 | 1.99 | 2.17 | 2.20 | 2.34 | 1.90 | 1.39 | 1.13 | 1.01 | 0.94 |
| (0.90-0.99) | (1.01-1.27) | (1.16-2.13) | (1.47-2.69)* | (1.73-2.73)‡ | (1.70-2.85)† | (1.69-3.23)† | (1.41-2.57)† | (1.10-1.75)* | (0.99-1.28) | (0.96-1.07) | (0.90-0.98) |
Values are means (95% confidence intervals). All comparisons were adjusted according to the Bonferroni method.
P < .05 compared with baseline values.
P ≤ .01 compared with baseline values.
P < .001 compared with baseline values.
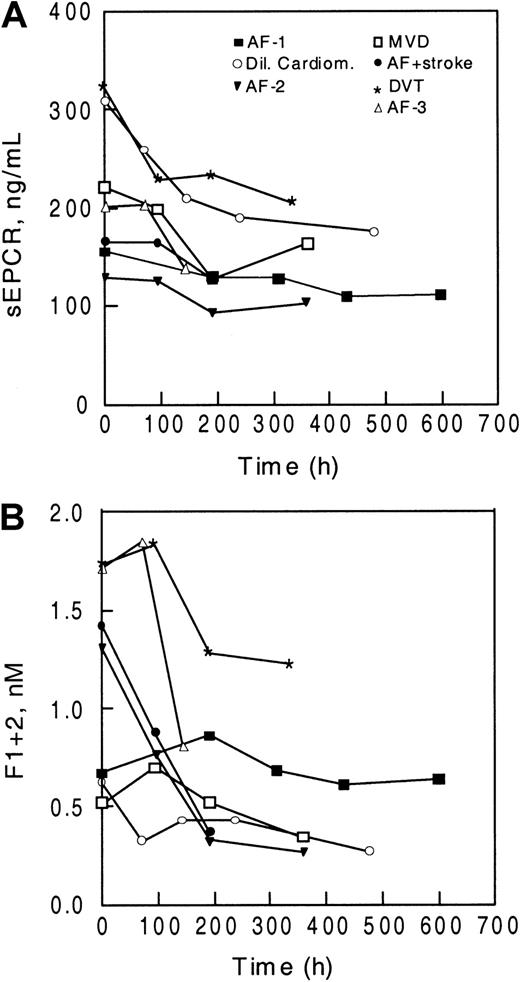
A similar reduction in sEPCR values with time was observed in patients beginning therapy with oral anticoagulants. Blood was obtained before the start of anticoagulation, and sEPCR levels (Figure3A) and F1 + 2 levels (Figure 3B) were determined during the course of therapy. In all patients studied, sEPCR levels declined with time after the start of oral anticoagulant therapy, and as was also observed in healthy volunteers, the extent of the decrease varied. As expected, F1 + 2 levels declined in most of the patients after initiation of warfarin therapy, particularly in those with higher prothrombin fragment levels (Figure 3C).
Levels of sEPCR decline in patients beginning warfarin treatment.
Levels of sEPCR (A) and F1 + 2 (B) were determined in 7 patients before the start of oral anticoagulant therapy (time 0) and during treatment. The diagnoses for the patients were atrial fibrillation (AF), dilative cardiomyopathy (Dil Cardiom), mitral valve disease (MVD), and deep vein thrombosis (DVT).
Levels of sEPCR decline in patients beginning warfarin treatment.
Levels of sEPCR (A) and F1 + 2 (B) were determined in 7 patients before the start of oral anticoagulant therapy (time 0) and during treatment. The diagnoses for the patients were atrial fibrillation (AF), dilative cardiomyopathy (Dil Cardiom), mitral valve disease (MVD), and deep vein thrombosis (DVT).
Discussion
The current results indicate that anticoagulant therapy not only reduces thrombin generation but also decreases plasma sEPCR levels. Patients undergoing anticoagulant therapy with oral vitamin K antagonists (acenocoumarol, fluindione, or warfarin) or intravenous unfractionated heparin had lower sEPCR plasma levels than populations of healthy volunteers. In both the patients and the healthy volunteers given warfarin, the time frame for the changes in sEPCR levels also coincided with the known changes in vitamin K–dependent factors induced by warfarin.5,18,20 21 On initiation of warfarin administration, there was a delay of 2 to 3 days before sEPCR levels declined. Subsequently, when warfarin administration to the healthy volunteers ended after 8 days, plasma sEPCR levels began to rise by day 10 to 11 and, in most cases, reached pretreatment levels within a week after warfarin administration ended. Rather than paralleling the changes in protein C antigen levels, the changes in sEPCR mirrored the changes in INR values.
Plasma F1 + 2 levels also declined with warfarin administration in both the volunteers and the patients, a finding that supports the idea that thrombin production decreased. However, the kinetics of the changes in F1 + 2 levels and sEPCR levels with warfarin administration did not coincide entirely in the healthy volunteers. We observed that sEPCR levels responded more rapidly to the start and cessation of warfarin administration. The in vivo pharmacokinetics of sEPCR has not been studied, but the vast difference between the modes of production and/or plasma clearance of the 2 markers may contribute to this observation.
This responsiveness of plasma sEPCR levels to anticoagulant therapy is consistent with the current model for generation of sEPCR from endothelial cells. Studies in cultured cells or in a rat sepsis model suggested that EPCR mRNA and ectodomain shedding into the circulation are responsive to thrombin activity, probably as an indirect result of thrombin-induced metalloproteinase activity.14-16 The current study does present the mechanism of sEPCR formation in humans, but it did have the novel finding that changes in plasma sEPCR levels in both patients and healthy volunteers can be induced by anticoagulant treatment.
The effect of anticoagulant treatment was not absolute, however, in that sEPCR levels never approached zero but tended to stabilize at about 100 ng/mL in healthy volunteers and at somewhat higher levels in patients (Figures 2 and 3). Thus, there may be an alternative pathway of sEPCR production that is independent of thrombin. We also cannot rule out the possibility that a downstream product of thrombin activity (eg, plasmin) contributes to changes in sEPCR levels. However, the observation that sEPCR levels declined with administration of either warfarin or unfractionated heparin, which have distinctly different mechanisms for inhibiting thrombin formation, suggests that the reduction in plasma sEPCR levels was directly related to a reduction in thrombin generation.
Earlier studies showed that plasma sEPCR is functional, at least with respect to its ability to bind protein C and activated protein C.12 Thus, it differs from soluble thrombomodulin, which is proteolyzed by neutrophil elastase and cathepsin G at the endothelial surface to release a variety of degraded forms into the circulation.22 Because the mechanisms for release of these 2 endothelial-derived proteins differ considerably, the plasma levels of these soluble receptors may be reporting different facets of endothelial status during a pathologic process. Soluble thrombomodulin is an accepted marker for endothelial damage, having been shown to be elevated in a variety of disease states with histological evidence of endothelial damage.23-25 It is possible that soluble thrombomodulin levels reflect endothelial damage, whereas soluble EPCR levels monitor an endothelial response to injury. This idea is consistent with the observation that plasma sEPCR levels, but not soluble thrombomodulin levels, are related to disease activity in patients with Wegener granulomatosis, an autoimmune vasculitis characterized by activation of both immune and coagulation systems (manuscript submitted, October 2001).
There are several ways to evaluate thrombin generation, including assessment of enzymatic activity,26 or the more common measurements of plasma thrombin-antithrombin complexes, F1 + 2, and D-dimer used clinically.1 Clinical evaluation of a patient's prothrombotic status and the efficacy of an anticoagulant therapy regimen takes into account the levels of these markers as well as the integrity of coagulation-pathway members and inhibitors by means of plasma thromboplastin (PT) and APTT clotting assays. Each of these approaches involves technical difficulties, including thrombin generation during phlebotomy and standardization of test reagents (INR).27 The PT clotting assay is the most useful screening method, yet it is more sensitive to agents or events that result in prolongation of the clotting times. There is a good correlation between prolonged PT times and bleeding episodes in patients undergoing anticoagulant therapy. However, it can be difficult to detect underdosing of anticoagulant therapy by assessment of PT and INR values. Long-term inadequate treatment maintains a hypercoagulable state, so patients unknowingly remain at risk for thrombotic events.28 The current observation that sEPCR levels decrease in response to anticoagulant therapy suggests that sEPCR may have potential as a marker of thrombin generation. Given the probable mode of generation of sEPCR and its assay method, measurement of plasma sEPCR levels would most likely not be affected by the technical difficulties associated with current tests of thrombin generation.
Related to this notion is the observation that there appear to be 2 subpopulations within the apparently healthy population in terms of sEPCR levels. In both the French and the Italian volunteers we studied, most had sEPCR levels in the range of 75 to 175 ng/mL (Figure 1). The rest had levels scattered in the range of 200 to 700 ng/mL. Although we cannot rule out an effect of environmental (smoking and diet) or genetic (polymorphisms and sex) factors, the observation that sEPCR levels fell in healthy volunteers during warfarin administration suggests that those with exceedingly high sEPCR levels may have had higher levels of endogenous thrombin production. This observation suggests that sEPCR levels may be a marker for a hypercoagulable state. However, more studies in a variety of populations are necessary to support this speculation.
In summary, our findings demonstrate a relation between plasma sEPCR levels and thrombin generation in healthy volunteers and in patients undergoing anticoagulant therapy. We anticipate that additional studies, particularly those examining different thrombin inhibitors, will provide insight into the potential usefulness of plasma sEPCR levels in evaluating a hypercoagulable state.
Supported by an Initial Investigator Award (D.J.S.-K.) and a Scientist Development Grant (S.K.) from the American Heart Association; grants from the National Institutes of Health (HL64787 and AI47575 to S.K.) and Telethon-Italy (A.D.); and research funding from Diagnostica Stago (N.C., M.G., and B.W.)
N.C., M.G., and B.W. are employed by Diagnostica Stago whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shinichiro Kurosawa, Free Radical Biology & Aging Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: kurosawas@omrf.ouhsc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal