Abstract
Angiogenesis and enhanced microvascular permeability are hallmarks of a large number of inflammatory diseases. Although up-regulation of proangiogenic factors such as vascular endothelial growth factor and interleukin-8 have been previously reported in inflamed tissue, the biologic role of endogenous inhibitors of angiogenesis in inflammation has remained unclear. To investigate the biologic role of the potent angiogenesis inhibitor thrombospondin-2 (TSP-2) in the control of cutaneous inflammation, delayed-type hypersensitivity reactions were elicited in the ear skin of wild-type and TSP-2–deficient mice by topical sensitization and challenge with oxazolone. Cutaneous TSP-2 expression was up-regulated in the inflamed skin of wild-type mice, predominantly in dermal fibroblasts and microvessels. Lack of TSP-2 resulted in a significantly enhanced inflammatory response with increased angiogenesis, edema formation, and inflammatory infiltration. Ear swelling and inflammation persisted for more than 2 weeks in TSP-2–deficient mice, as compared with 1 week in wild-type mice. Although baseline vascular permeability was unchanged, significantly enhanced microvascular leakage was found in the inflamed skin of TSP-2–deficient mice. Moreover, the fraction of rolling leukocytes was significantly increased in the untreated skin of TSP-2–deficient mice. These results reveal an important role of TSP-2 in limiting the extent and the duration of edema formation, angiogenesis, and inflammatory cell infiltration during acute and chronic inflammation.
Introduction
In addition to its important role in malignant tumor growth and tissue repair,1 angiogenesis is also a hallmark of a large variety of inflammatory diseases, including psoriasis2 and rheumatoid arthritis.3 There is considerable evidence to suggest that angiogenesis and chronic inflammation are closely linked.4 Angiogenic blood vessels at the site of inflammation are enlarged and hyperpermeable to maintain the blood flow and to meet the increased metabolic demands of the tissue.4 Several proangiogenic factors, including vascular endothelial growth factor (VEGF)5-7 and members of the CXC-chemokine family,8 9 have been found to be up-regulated during inflammation. However, the biologic role of endogenous inhibitors of angiogenesis in the control of the inflammatory process has remained unknown.
Several naturally occurring angiogenesis inhibitors have been identified, including thrombospondin-1 (TSP-1),10angiostatin,11 endostatin,12 and vasostatin.13 We have identified thrombospondin-2 (TSP-2) as a potent endogenous inhibitor of tumor growth and angiogenesis.14 TSP-2 is a 420-kd homotrimeric matricellular glycoprotein15 that inhibits the angiogenic activity of fibroblast growth factor16 and the formation of focal adhesions in bovine endothelial cells in vitro.17 During embryonic development, TSP-2 is predominantly expressed in areas of chondrogenesis, osteogenesis, and in early connective tissues.18 In adult mouse skin, low-level TSP-2 expression is predominantly associated with dermal fibroblasts.18 TSP-2 is also expressed in normal human skin and most likely contributes to the antiangiogenic barrier that separates the avascular epidermis from the richly vascularized dermis.5,19 Targeted disruption of the TSP-2 gene in mice resulted, among other abnormalities, in an increased number of small and medium-sized blood vessels in several tissues including the skin,20 confirming the biologic role of TSP-2 as an endogenous inhibitor of angiogenesis.
To characterize directly the biologic role of TSP-2 in inflammation, we evaluated cutaneous delayed-type hypersensitivity (DTH) reactions, an established experimental model for skin inflammation,21 in wild-type and in TSP-2–deficient mice. Here, we report that TSP-2 expression is up-regulated in acute inflammation and that TSP-2 deficiency results in a dramatically enhanced and prolonged inflammatory response, associated with increased angiogenesis, edema formation, and inflammatory cell infiltration. Lack of TSP-2 also leads to increased plasma extravasation and leukocyte recruitment. These findings reveal an important role of an endogenous antiangiogenic factor in the control of inflammation and suggest inhibition of angiogenesis as a potential new approach for anti-inflammatory therapy.
Materials and methods
Induction of DTH reactions
As a first step, DTH reactions were induced in the skin of 10-week-old male wild-type 129/SvJ mice (n = 6) and of TSP-2–deficient mice (n = 6) as previously described.22The construction of the targeting vector and the generation of TSP-2–deficient mice on a homogenous 129/SvJ background have been previously described.20 The mice were sensitized by topical application of a 2% oxazolone (4-ethoxymethylene-2-phenyl-2-oxazoline-5-one; Sigma, St Louis, MO) solution in acetone/olive oil (4:1 vol/vol) to the shaved abdomen (50 μL) and to each paw (5 μL). Five days after sensitization, the right ears were challenged by topical application of 10 μL of a 1% oxazolone solution, whereas the left ears were treated with vehicle alone. The extent of inflammation was measured daily, using the mouse ear-swelling test.23 All studies were performed at least twice with comparable results. The unpaired Studentt test was used for statistical analyses.
Intravital microscopy of cutaneous blood vessels
The animal preparation as well as the technical and experimental aspects of the customized intravital microscopy apparatus have been previously described.24 In brief, 10- to 14- week-old male 129/SvJ wild-type (n = 6) and TSP-2–deficient mice (n = 6) were anesthetized by intraperitoneal injection of physiologic saline (10 mL/kg body weight) containing ketamine HCl (5 mg/mL) and xylazine (1 mg/mL). The right jugular vein was catheterized with PE-10 polyethylene tubing. The fluorescent dye rhodamine 6G (20 mg/kg in phosphate-buffered saline [PBS]; Sigma) was administered intravenously to visualize circulating leukocytes by video-triggered stroboscopic epi-illumination. At least 3 superficially located postcapillary and small collecting venules in the ear skin were randomly chosen and recorded during 1- to 3-minute intervals to assess the baseline rolling interactions of leukocytes. The rolling fraction was calculated as the number of rolling leukocytes × 100/total leukocyte flux as described.24 Blood was withdrawn from the tail vein of wild-type and TSP-2–deficient mice, and leukocytes were counted by using a hematocytometer after ammonium chloride lysis.
Histology and immunohistochemistry
Mice were killed 24 hours, 48 hours, and 28 days after oxazolone challenge. Two hours before death, mice received intraperitoneal injections of 40 mM 5-bromodeoxyuridine (Sigma). One half of each ear was fixed for 1 hour in 10% formalin and was processed and embedded in paraffin. The other half was embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA) and snap frozen in liquid nitrogen. Immunohistochemical stains were performed on 6-μm paraffin sections or cryostat sections as previously described,25 using monoclonal rat antibodies against mouse CD31, CD3, CD4, CD8, CD11b, 5-bromodeoxyuridine, E-selectin, P-selectin (all Pharmingen, San Diego, CA), or a polyclonal rabbit antimouse TSP-2 antibody.26Naphtol-AS-d-chloroacetate-esterase stains were performed on paraffin sections as described.27
Computer-assisted morphometric analysis of blood vessels
Frozen sections (6 μm) of ear tissue were obtained from wild-type and TSP-2–deficient mice 24 hours (n = 2), 48 hours (n = 2), and 28 days (n = 2) after challenge and were stained with a monoclonal rat antibody against the endothelial cell junction molecule CD31. The sections were examined with the use of a Nikon E-600 microscope, and images were captured with a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI). Morphometric analyses of digital images were performed by using the IP-LAB software (Scanalytics, Fairfax, VA). Eight individual fields per section were examined at × 60 magnification, and the number of vessels per squared millimeter and the average vessel size were determined as described.14 The two-sided unpaired Student ttest was used for statistical analyses.
Vascular perfusions
DTH reactions were induced in the right ears of 10-week-old male wild-type (n = 3) and TSP-deficient (n = 3) mice. Twenty-four hours after challenge, mice were anesthetized by using a mixture of ketamine (800 μg/10 g body weight; Ketaset; Fort Dodge Laboratories, Fort Dodge, IA) and Avertin (0.5 μg/10 g body weight of 2,2,2-tribromoethanol in 2.5% t-amyl alcohol; Sigma). The retroorbital venous sinus of each animal was injected intravenously with 150 μL biotinylated Lycopersicon esculentum lectin (0.5 mg/mL; Vector, Burlingame, CA), which binds toN-acetyl-d-glucosamine residues on the luminal surface of vascular endothelial cells.28 Subsequently, mice were perfused with a fixative (1% paraformaldehyde, 0.5% glutaraldehyde in PBS) via the left ventricle at a constant pressure of 120 mmHg.29 The ears were removed and processed as previously described.30 The vascular architecture was analyzed in whole mounts of mouse ears, using a Nikon E-600 microscope. Images of blood vessels and adherent leukocytes were captured, using a Spot digital camera, and the length and the diameter of cutaneous blood vessels were measured by using the IP-LAB software. The luminal surface was estimated, using a formula to calculate the surface area of a cylinder (area = π × diameter × length). Adherent leukocytes, expressed as the number per luminal surface area, were counted in at least 30 blood vessels of wild-type and TSP-2–deficient mice (n = 3). The unpaired Student t test was used for statistical analysis.
Measurement of plasma leakage
The inflammatory agent mustard oil has been shown to cause plasma leakage in mouse skin.29,31 Mustard oil (allyl-isoyhiocyanate; Sigma) was diluted to 5% in mineral oil. The solution (10 μL) was applied to the dorsal and ventral surfaces of the right ears of wild-type (n = 3) and TSP-2–deficient mice (n = 4) 1 minute after intravenous injection of 100 μL Evans blue (30 mg/kg; Sigma) and was reapplied after 15 minutes.32The left ears were treated with mineral oil alone. After 30 minutes, the vasculature was perfusion-fixed (1% paraformaldehyde in 50 mM citrate buffer, pH 3.5) for 1 minute at a constant pressure of 120 mmHg. The animals were killed, and the ears were removed, dried for 6 hours at 55°C, and weighed. Evans blue was extracted from the tissues by incubation in 1 mL formamide for 5 days. The Evans blue content was measured by using a spectrophotometer at λ = 610 nm, and vascular leakage was calculated as nanogram of Evans blue per milligram of tissue.32 Statistical analyses were performed by using the unpaired Student t test.
Fluorescence-activated cell sorter analysis of leukocytes and cutaneous endothelial cells
For fluorescence-activated cell sorter analysis (FACS), spleens and peripheral lymph nodes of wild-type (n = 2) and TSP-2–deficient mice (n = 2) were passed through cell strainers (100 μm to 35 μm) in Dulbecco modified Eagle medium containing 1% fetal calf serum, 20 mM HEPES pH 7.4, and 5 mM EDTA. Cells were harvested from spleens and peripheral lymph nodes, and blood samples were taken from each animal and were depleted of erythrocytes by ammonium chloride lysis. The cells were stained with monoclonal antibodies directed against CD3, B220, or CD11b/Mac1 for 20 minutes at 4°C. Thereafter, cells were washed and analyzed by flow cytometry (FACScan; Becton Dickinson), using the CELLQuest software (Becton Dickinson) after gating for viable leukocytes by forward- and side-scatter characteristics. In an additional experiment, skin samples were obtained from the dorsal skin of TSP-2–deficient (n = 3) and wild-type mice (n = 3), cut into small pieces, and incubated for 45 minutes in 0.1% collagenase A (Boehringer, Indianapolis, IN) in Hanks balanced salt solution at 37°C, followed by mechanical release of cells by scraping as described.33 The cell suspension was passed through cell strainers, washed in PBS, and incubated for 15 minutes with fluorescence-labeled antibodies directed against CD31 and E-selectin or P-selectin (Pharmingen). The cells were washed and fixed for 10 minutes in 4% paraformaldehyde. Subsequently, the ratio of CD31+ endothelial cells expressing E- or P-selectin per total number of CD31-expressing cells and the mean fluorescence intensity were determined by flow cytometry. All studies were performed at least twice with comparable results. Statistical analysis was performed by using the two-sided unpaired Student ttest.
Results
Up-regulation of TSP-2 expression in cutaneous inflammation
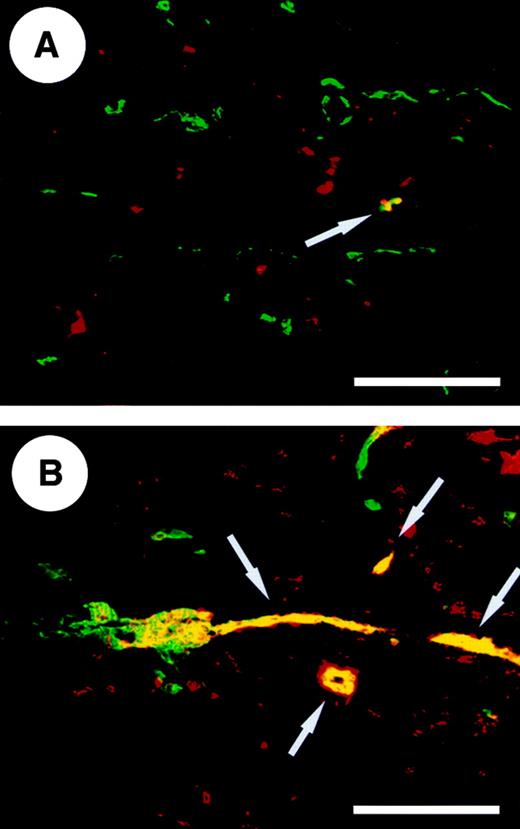
To investigate the expression of TSP-2 in cutaneous inflammation, we performed combined immunofluorescent stains with antibodies directed against mouse TSP-2 and against the endothelial cell junction molecule CD31. In normal mouse skin, TSP-2 expression was predominantly found in nonendothelial cells within the dermis and was only occasionally detected in association with blood vessels (Figure1A). In contrast, TSP-2 expression was highly up-regulated in inflamed skin 24 hours after oxazolone challenge and was frequently observed in angiogenic blood vessels and in dermal cells (Figure 1B).
Up-regulation of TSP-2 expression in cutaneous inflammation.
Differential immunofluorescent staining for CD31 (green) and TSP-2 (red) in normal wild-type mouse skin (A) revealed several TSP-2–expressing nonendothelial cells within the dermis but only a few TSP-2–positive blood vessels (yellow, arrow). (B) Twenty-four hours after oxazolone challenge the TSP-2 expression is up-regulated in fibroblasts, and an increased number of TSP-2–positive blood vessels (yellow, arrows) were observed. Scale bar = 100 μm.
Up-regulation of TSP-2 expression in cutaneous inflammation.
Differential immunofluorescent staining for CD31 (green) and TSP-2 (red) in normal wild-type mouse skin (A) revealed several TSP-2–expressing nonendothelial cells within the dermis but only a few TSP-2–positive blood vessels (yellow, arrow). (B) Twenty-four hours after oxazolone challenge the TSP-2 expression is up-regulated in fibroblasts, and an increased number of TSP-2–positive blood vessels (yellow, arrows) were observed. Scale bar = 100 μm.
Increased and prolonged DTH reactions in TSP-2–deficient mice
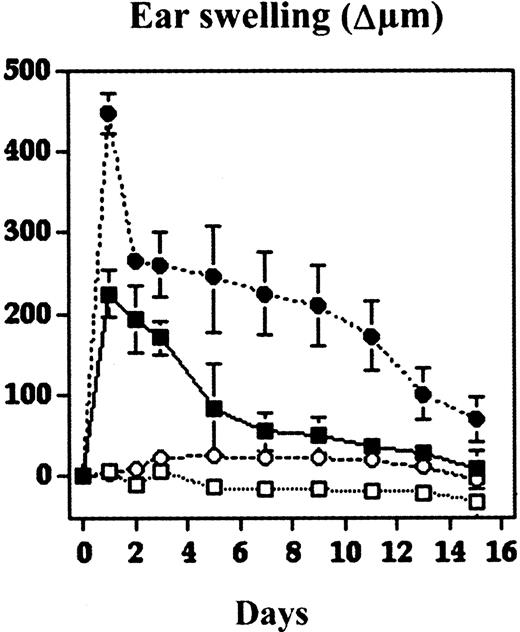
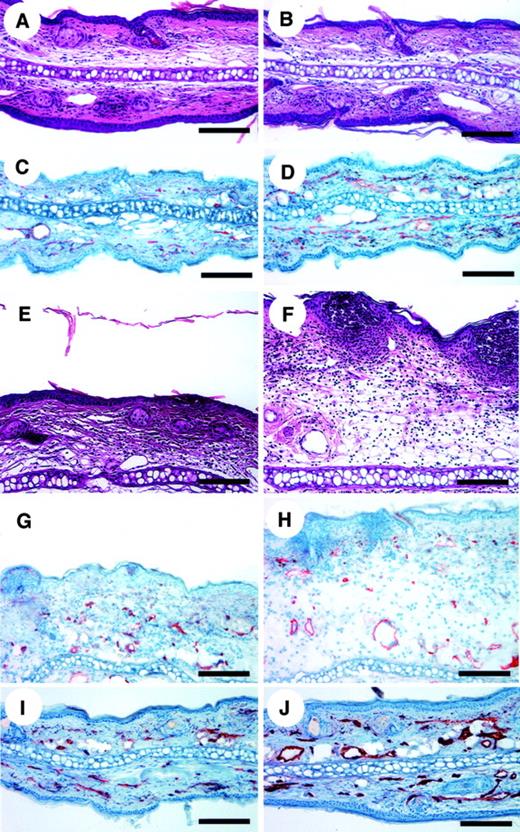
To study directly the biologic role of TSP-2 in cutaneous inflammation, DTH reactions were elicited in wild-type and in TSP-2–deficient mice by using oxazolone as a sensitizing agent. 24 hours after challenge. TSP-2–deficient mice showed a significantly (P < .01) increased ear swelling (+99.6%), as compared with wild-type mice (Figure 2). The ear swelling persisted for more than 2 weeks in TSP-2–deficient mice, whereas ear thickness returned to normal levels after approximately 7 days in wild-type mice (Figure 2). We found no apparent differences in the morphology of untreated skin of wild-type and TSP-2–deficient mice as revealed by routine histology stains (Figure3A,B). In contrast, histologic analysis revealed increased edema formation and inflammatory cell infiltration 24 hours and 48 hours after challenge in the inflamed skin of TSP-2–deficient mice (Figure 3E,F). Both wild-type and TSP-2–deficient mice showed accumulation of neutrophils in the upper dermis and epidermis (Figure 3E,F). However, these changes were more pronounced in TSP-deficient mice (Figure 3F). The inflammatory infiltrate in both types of mice consisted mainly of neutrophils and macrophages, as visualized by naphtol-AS-d-chloroacetate esterase stains and by immunostains for CD11b. No apparent differences were found in the percentage of CD3+, CD4+, or CD8+ cells in wild-type versus TSP-2–deficient mice (data not shown). The rapidly and highly increased tissue volume at sites of cutaneous edema formation precluded a meaningful analysis of vessel densities, defined as the number of vessels per tissue area.
Increased and prolonged ear swelling in DTH reactions elicited in TSP-2–deficient mice.
DTH reactions were induced in the ear skin of TSP-2–deficient and wild-type mice using oxazolone. Ear swelling is expressed as the increase (Δ) over the original ear thickness in micrometers. TSP-2–deficient mice ● showed a significantly increased ear swelling (99.6%; P < .01) 24 hours after challenge, as compared with wild-type mice ▪. Moreover, the ear swelling in TSP-2–deficient mice persisted for more than 2 weeks, whereas ear thickness returned to normal levels after about 1 week in wild-type mice. Unchallenged mice: ○, TSP2−/−; ■, wild type. Data are expressed as mean ± SD. *P < .05; **P < .01.
Increased and prolonged ear swelling in DTH reactions elicited in TSP-2–deficient mice.
DTH reactions were induced in the ear skin of TSP-2–deficient and wild-type mice using oxazolone. Ear swelling is expressed as the increase (Δ) over the original ear thickness in micrometers. TSP-2–deficient mice ● showed a significantly increased ear swelling (99.6%; P < .01) 24 hours after challenge, as compared with wild-type mice ▪. Moreover, the ear swelling in TSP-2–deficient mice persisted for more than 2 weeks, whereas ear thickness returned to normal levels after about 1 week in wild-type mice. Unchallenged mice: ○, TSP2−/−; ■, wild type. Data are expressed as mean ± SD. *P < .05; **P < .01.
Increased angiogenesis and inflammation in cutaneous DTH reactions elicited in TSP-2–deficient mice.
No apparent morphologic differences were found between the untreated ear skin of wild-type (A) and TSP-2–deficient mice (B). However, CD31 stains revealed increased skin vascularization in TSP-2–deficient mice (D), as compared with wild-type mice (C). Highly increased edema formation and inflammatory infiltration in the skin of TSP-2–deficient mice (F) at 24 hours after antigen challenge, as compared with wild-type mice (E). CD31 stains demonstrated enhanced angiogenesis 24 hours after challenge in TSP-2–deficient mice (H), as compared with wild-type mice (G). Twenty-eight days after challenge, the ear thickness of wild-type mice (I) and TSP-2–deficient mice (J) returned to normal levels, whereas blood vessels in the skin of TSP-2–deficient mice remained enlarged (J). Hematoxylin/eosin stains were used to produce panels A, B, E, and F, and CD31 stains for panels C, D, and G-J. Scale bars = 125 μm.
Increased angiogenesis and inflammation in cutaneous DTH reactions elicited in TSP-2–deficient mice.
No apparent morphologic differences were found between the untreated ear skin of wild-type (A) and TSP-2–deficient mice (B). However, CD31 stains revealed increased skin vascularization in TSP-2–deficient mice (D), as compared with wild-type mice (C). Highly increased edema formation and inflammatory infiltration in the skin of TSP-2–deficient mice (F) at 24 hours after antigen challenge, as compared with wild-type mice (E). CD31 stains demonstrated enhanced angiogenesis 24 hours after challenge in TSP-2–deficient mice (H), as compared with wild-type mice (G). Twenty-eight days after challenge, the ear thickness of wild-type mice (I) and TSP-2–deficient mice (J) returned to normal levels, whereas blood vessels in the skin of TSP-2–deficient mice remained enlarged (J). Hematoxylin/eosin stains were used to produce panels A, B, E, and F, and CD31 stains for panels C, D, and G-J. Scale bars = 125 μm.
Increased angiogenesis in the inflamed skin of TSP-2–deficient mice
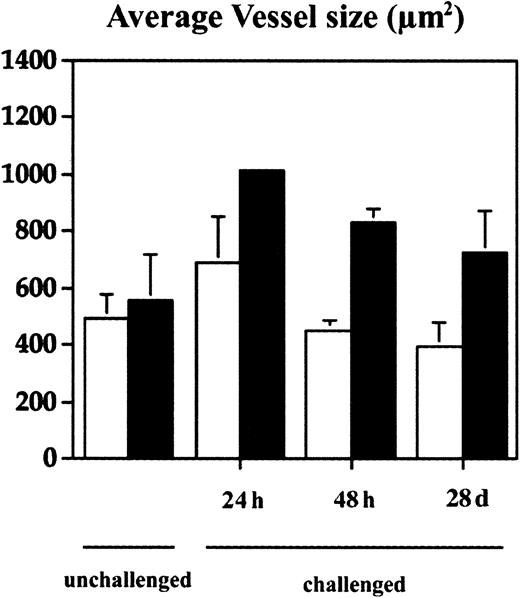
With the use of computer-assisted morphometric analysis of CD31-stained sections, we found an increased density of blood vessels (50.8%) and a slightly increased average vessel size (11.5%) in the normal skin of TSP-2–deficient mice (Figure 3C,D). In inflamed skin of wild-type mice (Figure 3G), the average vessel size was increased by 28.4% in the 24 hours after challenge and returned to normal levels after 48 hours (Figure 4). In contrast, TSP-2–deficient mice showed a pronounced, more than 45%, increase of vessel size 24 hours after challenge (Figures 3H and 4) and a more than 44% increase after 48 hours (Figure 4). Importantly, blood vessels still remained enlarged 28 days after challenge in the skin of TSP-2–deficient mice (33%) (Figures 3J and 4), whereas wild-type mice showed regular vessel sizes (Figures 3I and 4).
Increased vascular remodeling in the inflamed skin of TSP-2–deficient mice.
Computer-assisted morphometric analysis of CD31-stained tissue sections revealed a moderately increased average vessel size at 24 hours after oxazolone challenge with normalization after 48 hours in the ear skin of wild-type mice (■). In contrast, cutaneous vessels in the inflamed skin of TSP-2–deficient mice (▪) were significantly larger after 24 hours, and the increase in vessel size persisted over 28 days. Data are expressed as mean ± SD. *P < .05; **P < .01.
Increased vascular remodeling in the inflamed skin of TSP-2–deficient mice.
Computer-assisted morphometric analysis of CD31-stained tissue sections revealed a moderately increased average vessel size at 24 hours after oxazolone challenge with normalization after 48 hours in the ear skin of wild-type mice (■). In contrast, cutaneous vessels in the inflamed skin of TSP-2–deficient mice (▪) were significantly larger after 24 hours, and the increase in vessel size persisted over 28 days. Data are expressed as mean ± SD. *P < .05; **P < .01.
Increased plasma leakage in the ear skin of TSP-2–deficient mice
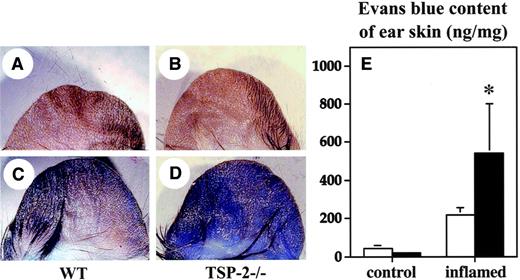
We next investigated whether increased baseline vascular permeability might have contributed to the increased edema formation in TSP-2–deficient mice. No major differences in the extravasation of Evans blue were detected in the normal skin of wild-type (Figure5A,E) and TSP-2–deficient mice (Figure5B,E). However, topical application of the proinflammatory agent mustard oil resulted in a significant (P < .05) 2.4-fold enhanced vascular leakage in TSP-2–deficient mice (Figure 5D,E), as compared with wild-type mice (Figure 5C,E).
Enhanced vascular leakage in the inflamed skin of TSP-2–deficient mice.
No major differences were found in the extent of plasma extravasation in untreated skin between wild-type mice (A) and TSP-2–deficient mice (B), as evaluated after intravenous injection of Evans blue. In contrast, topical application of mustard oil to the ear skin resulted in a more pronounced vascular leakage of Evans blue in TSP-2–deficient mice (D), as compared with wild-type mice (C). To quantify the plasma extravasation, Evans blue was extracted from the skin. No major differences were found in the amount of Evans blue extravasation in untreated skin of TSP-2–deficient (▪) and wild-type (■) mice (E), whereas leakage was increased more than 2.4-fold in the inflamed skin of TSP-2–deficient mice, as compared with wild-type mice. Data are expressed as mean ± SD. *P < .05.
Enhanced vascular leakage in the inflamed skin of TSP-2–deficient mice.
No major differences were found in the extent of plasma extravasation in untreated skin between wild-type mice (A) and TSP-2–deficient mice (B), as evaluated after intravenous injection of Evans blue. In contrast, topical application of mustard oil to the ear skin resulted in a more pronounced vascular leakage of Evans blue in TSP-2–deficient mice (D), as compared with wild-type mice (C). To quantify the plasma extravasation, Evans blue was extracted from the skin. No major differences were found in the amount of Evans blue extravasation in untreated skin of TSP-2–deficient (▪) and wild-type (■) mice (E), whereas leakage was increased more than 2.4-fold in the inflamed skin of TSP-2–deficient mice, as compared with wild-type mice. Data are expressed as mean ± SD. *P < .05.
Increased leukocyte-endothelial cell interactions in the skin of TSP-2–deficient mice
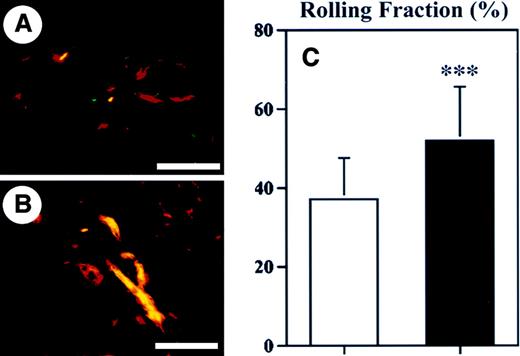
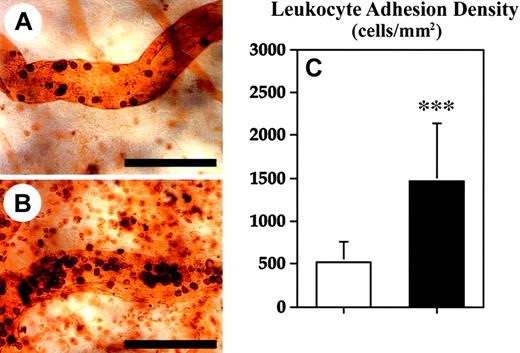
As a next step, we investigated whether enhanced baseline leukocyte–endothelial cell interactions might have contributed to the increased inflammatory response observed in TSP-2–deficient mice. Therefore, we performed intravital microscopy of blood vessels in the normal skin of wild-type and TSP-2–deficient mice and determined the ratio of rolling leukocytes to the total leukocyte flux in postcapillary venules. A significant increase in the rolling fraction (39.6%; P < .001) of leukocytes was detected in cutaneous blood vessels of TSP-2–deficient mice (51.8% ± 10.1%), as compared with wild-type mice (37.1% ± 13.7%) (Figure6C). The rolling fraction of leukocytes in the cutaneous blood vessels of wild-type mice was comparable with previous results.34 Only very few (< 1%) adherent leukocytes were observed in cutaneous blood vessels of normal skin, and no significant differences were found between wild-type and TSP-2–deficient mice (data not shown). Because reliable measurements of leukocyte rolling and adhesion were not feasible in inflamed ear skin, because of the marked edema formation and hence reduced resolution, we next studied leukocyte adhesion by in vivo perfusions with the lectin L esculentum. We found a significant (P < .001) 2.8-fold increase of the fraction of adhering leukocytes in postcapillary venules of inflamed skin in TSP-2–deficient mice (Figure 7B,C), as compared with wild-type mice (Figure 7A,C).
Enhanced rolling of leukocytes in cutaneous blood vessels of TSP-2–deficient mice.
Immunofluorescent stains for CD31 (red) and P-selectin (green) demonstrated increased P-selectin expression in cutaneous blood vessels (yellow) of TSP-2–deficient mice (B), as compared with wild-type mice (A). Scale bars = 100 μm. (C) Intravital microscopy revealed that the rolling fraction of leukocytes in cutaneous blood vessels of TSP-2–deficient mice (▪) was increased by 39.6% over wild-type mice (■). Data are expressed as mean ± SD. ***P < .001.
Enhanced rolling of leukocytes in cutaneous blood vessels of TSP-2–deficient mice.
Immunofluorescent stains for CD31 (red) and P-selectin (green) demonstrated increased P-selectin expression in cutaneous blood vessels (yellow) of TSP-2–deficient mice (B), as compared with wild-type mice (A). Scale bars = 100 μm. (C) Intravital microscopy revealed that the rolling fraction of leukocytes in cutaneous blood vessels of TSP-2–deficient mice (▪) was increased by 39.6% over wild-type mice (■). Data are expressed as mean ± SD. ***P < .001.
Enhanced leukocyte adhesion in blood vessels of TSP-2–deficient mice during skin inflammation.
Twenty-four hours after oxazolone challenge, mice were perfused with the lectin L esculentum to visualize leukocytes adherent to the luminal surface of blood vessels. Although only sparse leukocytes were found to be adherent to blood vessels of wild-type mice (A), an increased density of adherent leukocytes was detected in TSP-2–deficient mice (B). Scale bars = 100 μm. (C) Quantitative analyses of lectin-perfused skin revealed a 2.8-fold increase in leukocyte adhesion density in TSP-2–deficient mice (▪), as compared with wild-type mice (■). Data are expressed as mean ± SD. ***P < .001.
Enhanced leukocyte adhesion in blood vessels of TSP-2–deficient mice during skin inflammation.
Twenty-four hours after oxazolone challenge, mice were perfused with the lectin L esculentum to visualize leukocytes adherent to the luminal surface of blood vessels. Although only sparse leukocytes were found to be adherent to blood vessels of wild-type mice (A), an increased density of adherent leukocytes was detected in TSP-2–deficient mice (B). Scale bars = 100 μm. (C) Quantitative analyses of lectin-perfused skin revealed a 2.8-fold increase in leukocyte adhesion density in TSP-2–deficient mice (▪), as compared with wild-type mice (■). Data are expressed as mean ± SD. ***P < .001.
Because leukocyte rolling on endothelium is mediated predominantly by cell adhesion molecules of the selectin family, we next investigated whether the enhanced leukocyte rolling observed in the normal skin of TSP-2–deficient mice might have been caused by increased levels of endothelial E-selectin or P-selectin expression. Combined immunofluorescent stains for P- or E-selectin and CD31 revealed a slightly increased number of P-selectin–positive blood vessels in normal skin of TSP-2–deficient mice (Figure 6B) compared with wild-type mice (Figure 6A), whereas no major differences of E-selectin expression were detected (data not shown). To obtain a quantitative analysis of endothelial selectin expression, we isolated endothelial cells from the skin of wild-type and TSP-2–deficient mice and compared their expression of E- and P-selectin by FACS analysis. The percentage of P-selectin–expressing endothelial cells was increased by more than 40% in TSP-2–deficient mice (1.03% of all endothelial cells), as compared with wild-type mice (0.73% of all endothelial cells). Moreover, the mean fluorescence intensity of P-selectin on TSP-2–deficient endothelial cells was significantly increased by 63% (P < .05) when compared with endothelial cells derived from wild-type mice. The percentage of E-selectin–expressing endothelial cells was increased by 13.5% in TSP-2–deficient endothelial cells (8.79% of all CD31+ endothelial cells), as compared with wild-type mice (7.74% of all CD31+endothelial cells). However, no differences in the mean fluorescence levels of E-selectin on endothelial cells were observed.
To investigate whether the increased cutaneous inflammatory reaction in TSP-2–deficient mice might have been mediated by increased numbers of circulating leukocytes, we performed total leukocyte counts and determined the percentage of neutrophils and monocytes in the peripheral blood of mice of both genotypes. No significant differences were found in the total leukocyte count or in the percentage of the different leukocyte subpopulations between TSP-2–deficient and wild-type mice (n = 5 in each group; data not shown). Moreover, we found a similar ratio of B and T lymphocytes, harvested from peripheral lymph nodes and spleens, in wild-type and TSP-2–deficient mice (data not shown).
Discussion
Angiogenesis is of critical importance for tumor progression and tissue repair.35 Evidence also suggests a codependence of angiogenesis and inflammation,36 and several proangiogenic mediators such as VEGF21 and interleukin 8 have been shown to be increased in inflamed tissue.4 We hypothesized that up-regulation of endogenous inhibitors of angiogenesis might occur during inflammation to protect the tissue from excessive inflammatory angiogenesis. With the use of an established experimental model for skin inflammation, we induced DTH reactions in mouse ear skin by sensitization and challenge with the contact sensitizer oxazolone.37 Our results reveal that the expression of the endogenous angiogenesis inhibitor TSP-2 is indeed up-regulated during skin inflammation, suggesting a potential counterbalancing effect of TSP-2 in the control of inflammation. The observed mesenchymal up-regulation of TSP-2 is in agreement with the previously reported increased TSP-2 expression in cutaneous wounds26 and foreign body reactions,38 as well as with our recent findings of increased stromal TSP-2 expression during skin carcinogenesis.39
To test directly the role of TSP-2 during inflammation, we next induced cutaneous DTH reactions in mice lacking TSP-2. TSP-2–deficient mice showed significantly increased ear swelling after challenge with oxazolone, associated with pronounced enlargement of blood vessels and enhanced inflammatory infiltrates. Moreover, the duration of the inflammatory response was prolonged in TSP-2–deficient mice, as compared with wild-type mice. These findings indicated that TSP-2 plays an important role in the control of cutaneous inflammation and edema formation through inhibition of vascular remodeling.
To quantify the effects of TSP-2 on inflammatory angiogenesis, we performed computer-assisted morphometric analyses of tissue sections stained for the endothelial junction molecule CD31. These analyses revealed a significantly increased average vessel size in the inflamed skin of TSP-2–deficient mice, as compared with wild-type mice. These findings are in agreement with the previously reported vascular abnormalities in inflammatory skin diseases. In particular, psoriatic skin lesions are characterized by elongation and enlargement of cutaneous microvessels without the formation of new vessel sprouts.40 Although angiogenesis has traditionally been defined as the growth of new capillaries from preexisting vessels,41 it has been recently proposed that a second type of angiogenesis involves enlargement and elongation of preexisting vessels.42 Our findings suggest that inflammatory angiogenesis represents predominantly the vascular enlargement type, similar to the angiogenesis that occurs physiologically during the growth phase of the hair cycle.43 The determination of vessel densities, ie, the number of vessels per tissue area unit, has been frequently used to characterize tumor and wound angiogenesis.39 However, the marked increase of tissue area in inflamed skin, caused by increased vascular leakage and resulting edema formation, prevented a meaningful analysis of vessel densities in our study.
The increase in average vessel size observed in TSP-2–deficient mice indicates an important role of TSP-2 in the regulation of vascular remodeling in cutaneous inflammation. Vessel enlargement is a characteristic feature of angiogenic blood vessels, as compared with quiescent blood vessels. We have previously shown that inhibition of wound angiogenesis by the related molecule TSP-1 potently inhibited vascular enlargement in TSP-1–overexpressing transgenic mice, whereas no major differences in vessel size were found in the normal skin of these mice, as compared with wild-type mice.44 Although the exact mechanisms for this preferential effect of angiogenesis inhibition on the size of angiogenic vessels remain unknown, increasing evidence suggests that the increased vessel size of angiogenic vessels appears to be a more sensitive target for angiogenesis inhibition than vessel density or sprouting. Indeed, treatment of human tumor xenografts with a neutralizing anti-VEGF antibody resulted predominantly in a dramatic reduction in tumor vessel diameters.45 These findings indicate that absence of the angiogenesis inhibitor TSP-2 in the inflamed skin of TSP-2–deficient mice resulted in a less potent inhibition of the size increase of angiogenic vessels and, therefore, in increased average vessel size, as compared with wild-type mice. These results are supported by our recent findings that the average vessel size of perifollicular blood vessels is also significantly increased during the angiogenesis associated with the anagen hair growth phase in TSP-2–deficient mice (Yano K et al, unpublished results, 2001).
To investigate whether the increased edema formation in the inflamed skin of TSP-2–deficient mice might have been due to already increased baseline vascular permeability, we evaluated the cutaneous vascular leakage in normal skin after intravenous application of Evans blue. Our results demonstrate that the baseline vascular leakage was unaltered by the lack of TSP-2 in the skin. However, topical application of the proinflammatory agent mustard oil31,32 46 to the skin of TSP-2–deficient mice resulted in significantly enhanced vascular leakage, as compared with wild-type mice. These results indicate that TSP-2 contributes to the control of vascular permeability induced by inflammation and are in agreement with our findings that TSP-2 expression was up-regulated in blood vessels of inflamed skin. They also suggest that TSP-2 deficiency promoted the inflammatory edema formation, at least in part, independently from any direct effects on the oxazolone-induced immune response.
Recruitment of leukocytes from the blood into tissues requires a cascade of molecular events that mediate leukocyte rolling, adhesion, and extravasation in postcapillary venules.47,48 To examine whether enhanced baseline leukocyte interactions in the normal skin of TSP-2–deficient mice might have contributed to the increased extent of cutaneous inflammation, we next evaluated leukocyte rolling in postcapillary venules by intravital microscopy. We found that the fraction of rolling leukocytes was significantly increased by 39.6% in postcapillary venules of TSP-2–deficient mice as compared to wild-type mice. The molecular pathways that mediate constitutive rolling of leukocytes in cutaneous blood vessels have been recently described.34 E-selectin is expressed at low levels in dermal vessels and enables leukocytes to roll at low velocities, whereas P-selectin expression regulates the rolling fraction.34 To determine whether the enhanced rolling fraction in TSP-2–deficient mice was due to increased P-selectin expression on postcapillary venule endothelial cells, we next performed immunostains for P-selectin that revealed an increased number of P-selectin–positive blood vessels in the normal skin of TSP-2–deficient mice, as compared with wild-type mice. Because the significance of immunostains for P-selectin is limited, because of the predominant storage of P-selectin in Weibel-Palade bodies,49 we next investigated the cell surface expression of P-selectin in cutaneous endothelial cells after isolation from the skin of wild-type and of TSP-2–deficient mice. FACS analyses revealed a 40% increase in the number of CD31+ endothelial cells that expressed P-selectin in mice lacking TSP-2. Endothelial cells were isolated from the skin according to their expression of CD31, an endothelial junction molecule. Immunostains confirmed that CD31 was almost exclusively expressed by vascular endothelial cells in the skin of both wild-type and TSP-2–deficient mice, excluding, with a high degree of confidence, the possibility of contamination of the analyzed endothelial cells with other cell lineages. The mean fluorescence intensity of P-selectin on TSP-2–deficient endothelial cells was increased by 63% compared with endothelial cells derived from wild-type mice. These findings indicate that TSP-2 may affect baseline expression levels of distinct endothelial adhesion molecules involved in leukocyte recruitment, thereby modulating endothelial-leukocyte interactions. Similarly, we have previously demonstrated that transgenic mice overexpressing the angiogenesis factor VEGF in the skin are also characterized by enhanced baseline leukocyte rolling.50 It remains to be established whether TSP-2 can directly down-regulate P-selectin expression in endothelial cells or whether TSP-2 acts indirectly by modulation of other cytokines.
Because the increased thickness of inflamed ear skin resulted in low resolution of blood vessel images by intravital microscopy, we examined the leukocyte adhesion in inflamed skin by lectin perfusions. L esculentum lectin binds to the luminal surface of blood vessels and adherent leukocytes and permits quantification of the adhesion density of leukocytes in the inflamed tissue.51 These studies revealed a significantly increased number of adherent leukocytes in cutaneous blood vessels of TSP-2–deficient mice, further confirming a potential role of TSP-2 in modulating leukocyte recruitment to the skin. Importantly, we did not detect any significant differences in the number of circulating leukocytes between wild-type and TSP-2–deficient mice, indicating that the increased adhesion of leukocytes to postcapillary venules of TSP-2–deficient mice was the direct result of enhanced leukocyte-endothelial cell interactions, leading to an increased number of extravasating leukocytes.
It remains to be established whether effects of TSP-2 on the clearance of inflammatory cells from the inflamed tissue might also have contributed to the observed differences in the extent of inflammation between wild-type and TSP-2–deficient mice. It has been shown that the CD36-receptor, expressed on the surface of macrophages, is involved in the phagocytosis of apoptotic neutrophils at the site of inflammation,52 and the related molecule, TSP-1, has been demonstrated to act as a “molecular bridge” between macrophages and apoptotic neutrophils in this clearance process. TSP-2 has a 65% sequence identity with TSP-1 and, like TSP-1, contains 2 CSVTCG domains within the properdinlike type I repeats that have been shown to interact with the CD36 receptor.16 53 Therefore, it is conceivable that lack of TSP-2 may result in decreased activation of macrophage phagocytosis of apoptotic neutrophils, further contributing to the prolongation of the inflammatory response.
Taken together, our findings reveal a novel biologic function of the endogenous angiogenesis inhibitor, TSP-2, in the control of cutaneous inflammation, vascular leakage, and edema formation. It remains to be established whether other endogenous antiangiogenic factors also play a role in the control of the inflammatory process and whether therapeutic application of angiogenesis inhibitors might represent a novel approach for the treatment of inflammatory diseases.
Supported by National Institutes of Health grants CA69184 and CA86410 (M.D.), HL54936, HL62524 and AR42689 (U.H.v.A.), HL18645 and AR45418 (P.B.), American Cancer Society Research Project Grant 99-23901 (M.D.), Deutsche Forschungsgemeinschaft (B.L.-A.), Max Kade Foundation (W.W.), and by the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Agreement (M.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Detmar, Cutaneous Biology Research Center, Department of Dermatology, Massachusetts General Hospital, Bldg 149, 13th St, Charlestown, MA 02129; e-mail:michael.detmar@cbrc2.mgh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal