Abstract
The present study investigated the potential role of stromal cell–derived factor 1 (SDF-1) in human intrathymic T-cell differentiation. Results show that SDF-1 is produced by human thymic epithelial cells from the subcapsular and medullary areas, and its receptor, CXCR4, is up-regulated on CD34+ precursor cells committed to the T-cell lineage. Chimeric human-mouse fetal thymus organ culture (FTOC) seeded with purified CD34+thymic progenitors and treated with neutralizing antibodies against SDF-1 or CXCR4 showed a significant reduction of the number of human thymocytes and an arrested thymocyte differentiation in the transition between CD34+ precursor cells and CD4+ immature thymocytes. SDF-1–treated FTOC showed an increase of human thymocyte numbers, mainly affecting the most immature subpopulations. Moreover, these results suggest that CXCR4/SDF-1 signaling is not critical for the CD34+ cell precursor recruitment to the thymus. On the other hand, SDF-1 significantly increased the viability of CD34+ T-cell precursors modulating the expression ofBCL-2 and BAX genes, and stimulated the proliferation of CD34+ thymic precursor cells, particularly in synergy with interleukin 7 (IL-7), but not with other cytokines, such as stem cell factor or flt3-ligand. Accordingly, only IL-7 was able to up-regulate CXCR4 expression on CD34+ thymic progenitors. In addition, deprivation of SDF-1 partially inhibited human thymocyte expansion induced by IL-7 in human-mouse FTOC. This study indicates that SDF-1/CXCR4 signaling is required for the survival, expansion, and subsequent differentiation of human early thymocytes and identifies a new mechanism by which IL-7 mediates its effects on human thymopoiesis.
Introduction
When T cells derived from fetal liver or bone marrow hematopoietic precursors enter the thymus, they pass through a series of discrete stages defined by the differential expression of a variety of phenotypic markers. The most primitive hematopoietic progenitors in the human thymus express high levels of CD34 and CD45RA and low levels of CD38; they lack CD2, CD5, CD1, CD4, CD8, or CD3.1,2 On further differentiation, these cells acquire CD2, CD5, and CD1. The CD34+CD1+ cells gradually become CD34− and up-regulate first CD4 followed by CD8α before acquisition of CD8β.3,4 Following entrance into the CD4+CD8+ double-positive (DP) stage, thymocytes express low levels of CD3/T-cell receptor-αβ complex and are the main targets for positive selection. Positively selected cells up-regulate CD3 and CD69, followed by CD27. Finally, thymocytes lose CD4 or CD8, followed by loss of CD1, and switch their phenotype from CD45RO to CD45RA, with these cells then ready to leave the thymus.5 In this process, the relevance of developing thymocyte-thymic stromal cell interactions, through cell-to-cell contacts or via soluble cell mediators, has been repeatedly recognized.6 7
Stromal cell–derived factor 1 (SDF-1), a member of the chemokine CXC subfamily, was first identified as a pre–B-cell growth factor8 and was later demonstrated to be involved in migration and also survival, proliferation, and activation of different cell types.9-14 CXCR4, the receptor for SDF-1, is a G protein– coupled 7-transmembrane receptor that also functions as a coreceptor for the entry of T-tropic strains of human immunodeficiency virus 1 into CD4+ cells.15-17 The widespread distribution of SDF-1, its constitutive expression pattern, and its highly conserved nucleotide and amino acid sequences18,19suggest that it could play a key biologic role. In correlation with this biologic relevance, targeted gene knockout mice of either SDF-1 or its receptor CXCR4 die in utero and show severe abnormalities in cardiogenesis, as well as vascular, cerebellar, and hematopoietic development.20-23 Mice deficient in CXCR4 and SDF-1 have fewer myeloid progenitors in fetal liver and bone marrow than normal mice and display severe defects in the generation of B cells but not in T cells.20,22-24 Although these gene-disruption experiments suggest that the SDF-1/CXCR4 complex is not required for the generation of normal cell populations in the mouse thymus, other results indicate, however, a role for this chemokine also in T-cell differentiation: (1) SDF-1 messenger RNA (mRNA) is abundantly expressed in fetal and adult thymus25-28; (2) CXCR4 is expressed on different subpopulations of human and mouse thymocytes25,28,29; (3) SDF-1 induces calcium flux primarily in the most immature human thymocytes28; (4) SDF-1 is a chemoattractant for thymocytes30,31; (5) transplantation of fetal liver–derived hematopoietic cells from CXCR4 knockout mice into lethally irradiated syngenic mice results in reduced numbers of donor-derived T cells in the thymus32; and (6) mice reconstituted with bone marrow–hematopoietic progenitor cells transduced with SDF-1–intrakine gene display reduced numbers of total thymocytes and an arrest in thymocyte maturation.33
To demonstrate the possible role of SDF-1/CXCR4 signaling in human thymocyte development, we report the production of SDF-1 protein by thymic epithelial cells, as well as the expression of CXCR4 in the more mature subsets of CD34+ thymocytes. Studies using a hybrid human/severe combined immunodeficiency (SCID) mouse fetal thymus organ culture (FTOC) system demonstrate a key role for SDF-1 in the proliferation, survival, and subsequent differentiation of human thymocytes. Furthermore, we demonstrate that SDF-1 partially mediates the effects of interleukin 7 (IL-7) on human thymopoiesis.
Materials and methods
Animals
The C.B17 SCID mice were purchased from Harlan (Harlan Iberica, Barcelona, Spain) and maintained in our own specific pathogen-free breeding facilities. Fetal mice were obtained by timed mating; the day of finding a vaginal plug was designated day 0 of pregnancy.
Histology and immunofluorescence
Thymic cryosections (7 μm thick) were air-dried for 2 hours at room temperature and fixed in acetone for 10 minutes. Nonspecific binding of antibodies was blocked by incubation with diluted donkey serum (Santa Cruz Biotechnology, Santa Cruz, CA) and avidin-biotin (Vector Laboratories, Burlingame, CA). Sections were then sequentially incubated with antihuman SDF-1α antibodies (R & D Systems, Minneapolis, MN), biotinylated donkey anti–goat IgG (Jackson Immunoresearch Laboratories, West Grove, PA), Texas red–conjugated streptavidin (Amersham Pharmacia Biotec, Buckinghamshire, United Kingdom), and anti–type I collagen (COL-1) (Sigma España), anticortical epithelial cells (TE-3), or antimedullary/subcapsullary epithelial cells (TE-4) (both kindly provided by Dr B. F. Haynes, Duke University Medical Center, Durham, NC), followed by fluorescein isothiocyanate (FITC)–conjugated human adsorbed F(ab′)2fragments of rabbit anti–mouse IgG (Jackson Immunoresearch Laboratories). Slides were mounted in Vectashield (Vector Laboratories) and then examined using a Zeiss Axioplan-2 microscope.
Isolation of human thymocyte subsets
Human thymus samples were obtained from children, aged 1 month to 3 years, undergoing corrective cardiovascular surgery. Thymus tissues were obtained and used following the guidelines of the Medical Ethics Commission of the University Hospital Gregorio Marañón (Madrid, Spain). Informed consent was provided according to the Declaration of Helsinki. Thymuses were dissected free of surrounding connective tissue and then gently disrupted with a Potter homogenizer until completely disaggregated. Cell suspensions were enriched in immature thymocytes by using the sheep red blood cell rosetting technique as described previously.34 Recovered cells were then depleted of mature T, natural killer (NK), B, myeloid, and dendritic cells by treatment with saturating concentrations of anti-CD3 (SK7) (Becton Dickinson, San Jose, CA), anti-CD56 (B159), anti-CD19 (B43), anti-CD14 (M5E2), and anti-CD11c (B-ly6) (all from Pharmingen, San Diego, CA) bound to sheep anti–mouse Ig–coated magnetic beads (Dynal, Oslo, Norway). CD34+ cells or CD4+ immature thymocytes were then purified by magnetic sorting using VarioMACS (Miltenyi Biotec, Bergisch Gladbach, Germany) in conjunction with either CD34 or CD4 Multisort kit (Miltenyi Biotec) following the manufacturer's instructions. CD4+CD8+ thymocytes were purified from the whole population using CD4 Multisort Kit and CD8-Microbeads (Miltenyi Biotec). The purity of the enriched CD34+, CD4+, or CD4+CD8+ cells was always more than 98%.
Antibodies and flow cytometry
Directly labeled monoclonal antibodies (mAbs) against the following antigens were used: CD4 (SK3-FITC, -phycoerythrin [PE], or –peridinin chlorophyll protein [PerCP]); CD8 (SK1-FITC, -PE, or -PerCP); CD45 (HI30-FITC); HLA-DR (G46-6-FITC); CD117(c-kit 4B5.B8-PE); CD45RA (HI100–FITC); CD7 (M-T701-FITC); CD38 (HIT2-FITC); CXCR4 (12G5-FITC or -PE); CD33 (WM53-PE); CD5 (UCHT2–FITC); CD1a (HI149-FITC); CD34 (581-FITC, -PE, or -Cychrome); CD3 (SK7-FITC, -PE, or -PerCP) from Becton Dickinson; CD127 (R34.34-PE) from Coulter Immunotech (Beckman Coulter, Marseille, France). Three-color immunofluorescence staining was performed by incubating the cells in phosphate-buffered saline (PBS) containing 1% fetal calf serum (FCS) and 0.1% NaN3 in the presence of saturating amounts of FITC-, PE-, PerCP-, and Cychrome-conjugated mAbs for 30 minutes at 4°C. All specific mAbs against human antigens were checked for negative staining on SCID thymocytes. Isotype-matched irrelevant mAbs were used as negative controls to define background fluorescence. For intracellular detection of Bcl-2 and bax antigens, cells were treated with a FACS permeabilizing solution according to the manufacturer's instructions (Becton Dickinson), and stained with antihuman bcl-2–PE mAb (Pharmingen) or antihuman bax antibodies (Pharmingen) followed by FITC-conjugated donkey anti–rabbit IgG (Jackson Immunoresearch Laboratories) for 30 minutes at room temperature. The percentage of stained cells was evaluated by comparison with the isotype control. Analysis was conducted in a FACScan flow cytometer (Becton Dickinson) from the Servicio Común de Investigación, Faculty of Biology, Complutense University of Madrid. Debris, and dead cells were excluded from the analysis by forward (FSC) and side light scatter (SSC), and analysis was performed on at least 10 000 events. The data were analyzed using PC-lysis research software (Becton Dickinson).
Hybrid human-mouse fetal thymic organ culture
Thymic lobes removed from 15-day-old embryos of SCID mice were cocultured for 2 days in hanging drop in Terasaki wells with purified human CD34+ cells, CD4+ immature thymocytes, or CD4+CD8+ thymocytes (1-2 × 104cells/lobe), transferred to 0.8-μm polycarbonate filters (Millipore Iberica, Madrid, Spain) that rested on stainless steel screen pieces attached to the central well of organ tissue culture dishes (Becton Dickinson), and cultured for the indicated number of days. Culture medium consisted of RPMI-1640 supplemented with 7% human AB serum and 3% FCS. Where indicated, cultures were supplemented with recombinant human SDF-1α (rhSDF-1α) at a concentration of 10 ng/mL; neutralizing mouse anti–human CXCR4 antibodies at a concentration of 10 μg/mL; neutralizing goat anti–human SDF-1α antibodies at a concentration of 10 μg/mL; neutralizing mouse anti–human/mouse IL-7 antibodies at a concentration of 20 μg/mL (all from R & D Systems); recombinant human IL-7 (rhIL-7) at a concentration of 1000 U/mL (ampule code 90/530; National Institute for Biological Standards and Control, Hertfordshire, United Kingdom) throughout the culture period, or only during part of the experimental procedure. The doses of rhSDF-1α, rhIL-7, and the blocking antibodies were shown to be optimal on the basis of previous titrations in FTOC (data not shown). Purified mouse IgG2a (Pharmingen) was used as control. Medium was replaced every week. To analyze differentiation of human cells, mouse thymuses were dispersed into single-cell suspensions and stained with mAbs specific for human cell surface antigens. Flow cytometric analysis was then performed on electronically gated CD45+ human cells.
Culture of thymic CD34+ precursors
Purified thymic CD34+ precursors (3-5 × 104) were cultured in 96-well flat-bottom culture plates in 0.2 mL AIMV serum-free lymphocyte medium (Life Technologies, Eragny, France) in the presence of rhSDF-1α (10 ng/mL), rhIL-7 (1000 U/mL), recombinant human stem cell factor (rhSCF; 5 ng/mL; Peprotech, London, United Kingdom) or rhflt3-ligand (10 ng/mL; PeproTechec). After 2 days in culture at 37°C in a 5% CO2- in air incubator, cells were harvested and processed for proliferative assays, viability analysis, and bcl-2 and bax stainings.
Proliferation assay
Cultures were pulsed for the last 12 hours with 10 μM 5-bromo-2′-deoxyuridine (BrdU). A specific kit from Boehringer Mannheim (Mannheim, Germany; BrdU Labeling and Detection kit III) was used to measure BrdU incorporation into newly synthesized DNA. Briefly, the labeling medium was removed, and cells were dried (2 hours at 60°C), fixed in ethanol in HCl (0.5 M) for 30 minutes at −20°C, treated with nucleases (30 minutes at 37°C), and then incubated with peroxidase-conjugated Fab fragments of mouse anti-BrdU antibodies (30 minutes at 37°C). The peroxidase reaction was developed with ABTS substrate (Boehringer Mannheim), and the sample absorbance was measured using an enzyme-linked immunoabsorbent assay reader at 405 nm with a reference wavelength at 492 nm.
Apoptosis assay
Cells were washed twice with PBS containing 1% FCS and then stained with annexin V–FITC (Boehringer Mannheim) according to the supplier's instructions. Cells were analyzed on a FACScan and gated according to FSC, SSC, and their ability to exclude propidium iodide. Apoptotic cells were considered as those positive for annexin V and negative for propidium iodide.
Results
Expression of SDF-1 on human thymus
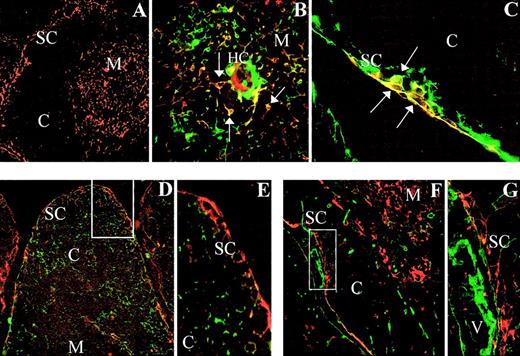
The expression of SDF-1 was assayed by immunofluorescence on frozen sections of human thymus (Figure1A). SDF-1α protein was detected in epithelial cells as demonstrated by double staining with TE-4 mAbs, which recognize subcapsular and medullary epithelial cells (Figure1B,C). SDF-1α+ epithelial cells appeared as small cell clusters in the subcapsular region (Figure 1B), and scattered SDF 1α-expressing epithelial cells were also found in the medullary compartment (Figure 1C). No reactivity for SDF-1α was found in cortical epithelial cells as evidenced by double staining with TE-3 mAbs (Figure 1D,E). Furthermore, no coexpression of SDF-1 protein and fibroblast-associated type I collagen was found in the human thymus (Figure 1F,G).
Expression of SDF-1α in the human thymus.
Frozen sections of human thymus were double stained with anti–SDF-1α alone (red fluorescence; A) or in combination with anti–TE-4 (green fluorescence; B,C), anti–TE-3 (green fluorescence; D,E), or anti–type I collagen (green fluorescence; F,G). SDF-1α expression is detected in TE-4+ epithelial cells (yellow cells, arrows) appearing in the thymic subcapsular (C) and medullary areas (B). Note the absence of SDF-1α expression in TE-3+ cortical epithelial cells (D,E), and type I collagen-positive fibroblasts (F,G). SC indicates subcapsullary area; C, cortex; M, medulla; HC, Hassal corpuscle; V, blood vessel. Original magnifications: panel A, × 50; panels B, E, and G, × 250; panel C, × 150; and panels D and F, × 75.
Expression of SDF-1α in the human thymus.
Frozen sections of human thymus were double stained with anti–SDF-1α alone (red fluorescence; A) or in combination with anti–TE-4 (green fluorescence; B,C), anti–TE-3 (green fluorescence; D,E), or anti–type I collagen (green fluorescence; F,G). SDF-1α expression is detected in TE-4+ epithelial cells (yellow cells, arrows) appearing in the thymic subcapsular (C) and medullary areas (B). Note the absence of SDF-1α expression in TE-3+ cortical epithelial cells (D,E), and type I collagen-positive fibroblasts (F,G). SC indicates subcapsullary area; C, cortex; M, medulla; HC, Hassal corpuscle; V, blood vessel. Original magnifications: panel A, × 50; panels B, E, and G, × 250; panel C, × 150; and panels D and F, × 75.
Phenotypic characterization of CD34+CXCR4−and CD34+CXCR4+ thymocytes
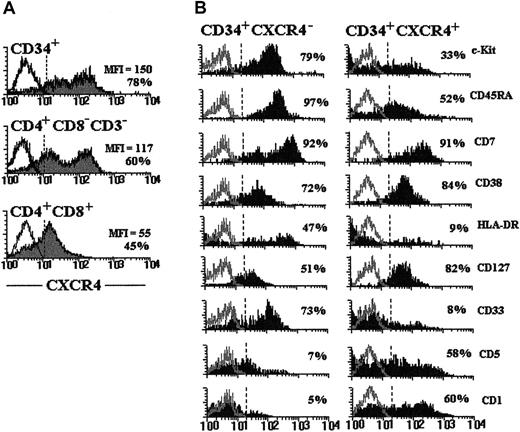
To elucidate whether SDF-1 could play a role in early human T-cell development, we first defined the phenotype of the thymic progenitor cells that have been previously reported to express CXCR4.28 As shown in Figure2A, on average about 80% of thymic CD34+ cells were positive for CXCR4. CD34+thymic progenitor cells were found to express the highest density of the receptor for the chemokine SDF-1 compared with more mature thymocyte subsets. Likewise, the proportion of cells staining positive gradually decreased in the CD3−4+8− intermediate cell population and more in CD4+CD8+ DP thymocytes (Figure 2A). The analysis of the expression of differentiation-specific antigens indicated that the vast majority of CD34+CXCR4− cells expressed CD45RA, c-kit, CD33, CD7, and CD38; about 40% to 50% of this subpopulation was positive also for HLA-DR and CD127 antigens, but was mostly negative for CD5 and CD1 antigens (Figure 2B). Progenitor cells exhibiting this surface phenotype represent primitive tripotential T/dendritic cell/NK precursors in the postnatal human thymus.35 In contrast, a reduced percentage of CD34+CXCR4+ cells positively reacted with c-kit, CD45RA, CD33, and HLA-DR antigens, and about 60% of this subpopulation expressed the T- lineage markers, CD5 and CD1 (Figure2B). These results indicate that the CD34+CXCR4− subpopulation contains more primitive cells than the CD34+CXCR4+ cell subset. Supporting this, most CD34bright cells, which contain the earliest T-cell precursors in the human thymus,4 36 were found to be in the CXCR4−fraction (data not shown).
CXCR4 expression in CD34+ thymic progenitor cells.
(A) Expression of CXCR4 on immature thymocyte subsets. CD34+ and CD4+ immature thymocytes purified as described in “Materials and methods” were stained with anti–CXCR4-PE. CXCR4 expression on DP cells was assayed by staining total thymocytes with anti–CD4-FITC, anti–CD8-PerCP, and anti–CXCR4-PE. Gray histograms show the expression of CXCR4 in each thymic cell subpopulation indicated in the figure. The percentages of positive cells and their mean fluorescence intensity (MFI) are indicated in each histogram. (B) Purified CD34+ cells were double stained with anti–CXCR4-FITC or -PE and a range of mAbs against differentiation-specific antigens. Solid histograms represent the expression of the indicated cell surface antigens on CD34+CXCR4− and CD34+CXCR4+ thymocytes. The percentages of positive cells are showed in each histogram. Open histograms represent background fluorescence using isotype-matched irrelevant mAbs. Similar staining patterns were obtained in 4 different experiments.
CXCR4 expression in CD34+ thymic progenitor cells.
(A) Expression of CXCR4 on immature thymocyte subsets. CD34+ and CD4+ immature thymocytes purified as described in “Materials and methods” were stained with anti–CXCR4-PE. CXCR4 expression on DP cells was assayed by staining total thymocytes with anti–CD4-FITC, anti–CD8-PerCP, and anti–CXCR4-PE. Gray histograms show the expression of CXCR4 in each thymic cell subpopulation indicated in the figure. The percentages of positive cells and their mean fluorescence intensity (MFI) are indicated in each histogram. (B) Purified CD34+ cells were double stained with anti–CXCR4-FITC or -PE and a range of mAbs against differentiation-specific antigens. Solid histograms represent the expression of the indicated cell surface antigens on CD34+CXCR4− and CD34+CXCR4+ thymocytes. The percentages of positive cells are showed in each histogram. Open histograms represent background fluorescence using isotype-matched irrelevant mAbs. Similar staining patterns were obtained in 4 different experiments.
SDF-1 deprivation drastically reduces the yield of human thymocytes and arrests their differentiation in chimeric human-mouse FTOC
To establish the role of SDF/CXCR4 signaling in human T-cell development, we investigated the effects of the addition of neutralizing antibodies against human CXCR4 or SDF-1 proteins to chimeric human-mouse FTOC. Addition of anti-CXCR4 (10 μg/mL) or anti–SDF-1 (10 μg/mL) antibodies resulted in a dramatic decrease in the cell recovery from the thymus seeded with CD34+thymocyte precursors. By 10 to 18 days of FTOC, a 60% to 80% reduction in human cellularity was observed. The reduction in cell number was always more pronounced in the anti–CXCR4-treated cultures (Table 1).
Effects of SDF-1α, anti–SDF-1α, or anti-CXCR4 treatment on human thymocyte development in chimeric human-mouse FTOC
| Treatment . | Length of culture . | |
|---|---|---|
| 10 d . | 18 d . | |
| Control | 26 677 ± 3 496* | 29 348 ± 4 002 |
| IgG2a | Not determined | 27 830 ± 4 453 |
| SDF-1α | 100 600 ± 13 294 | 95 312 ± 7 539 |
| Antihuman CXCR4 | 4 291 ± 750 | 5 932 ± 899 |
| Antihuman SDF-1α | 9 576 ± 1 524 | 7 997 ± 1 039 |
| Treatment . | Length of culture . | |
|---|---|---|
| 10 d . | 18 d . | |
| Control | 26 677 ± 3 496* | 29 348 ± 4 002 |
| IgG2a | Not determined | 27 830 ± 4 453 |
| SDF-1α | 100 600 ± 13 294 | 95 312 ± 7 539 |
| Antihuman CXCR4 | 4 291 ± 750 | 5 932 ± 899 |
| Antihuman SDF-1α | 9 576 ± 1 524 | 7 997 ± 1 039 |
Number of human cells was calculated by multiplying the total number of cells by the percentage of human CD45+ cells determined by flow cytometry analysis. The data represent the mean ±SD from 3 to 8 independent experiments wherein a pool of at least 5 thymic lobes per test were analyzed. Differences between cell numbers in control and rhSDF-1α–, antihuman SDF-1α–, or antihuman CXCR4–treated FTOC were statistically significant, with P≤ .05 for 10 and 18 days of culture.
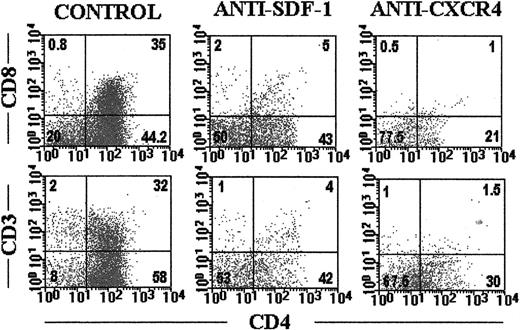
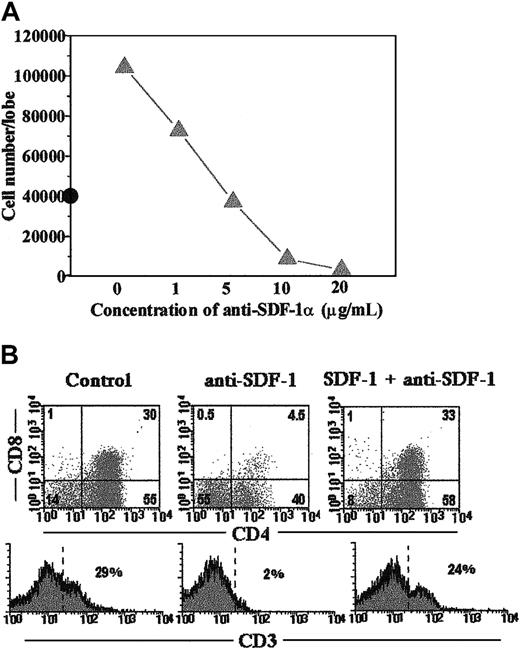
We next examined the thymocyte subsets affected by the treatments. After 18 days of culture, the frequency of CD34+ thymic precursors was increased 2 to 3 times (control, 5.4% ± 3%; anti-CXCR4, 11.5% ± 4.8%; anti–SDF-1α, 16% ± 5.2%), whereas the proportion of immature CD4+ thymocytes was reduced in the anti–SDF-1α- and anti–CXCR4-treated FTOC with respect to control values (Figure 3). At that time point of the culture, about 30% of thymocytes in control cultures expressed both CD4 and CD8 antigens, whereas in the presence of either blocking anti-CXCR4 or anti–SDF-1 antibodies the production of CD4+CD8+ thymocytes was inhibited (Figure3). Moreover, in the antibody-treated cultures the expression of CD3 was virtually absent in the human thymocytes (Figure 3). The numbers of all thymocyte subpopulations were decreased, but the reduction was more pronounced in the immature CD4+ and CD4+CD8+ cell subsets. Similar differences between control and antibody-treated cultures were obtained after 28 days of culture (data not shown).
Blockade of SDF-1/CXCR4 signaling arrests human thymocyte development.
SCID fetal thymic lobes seeded with highly purified CD34+human thymic precursors were organ cultured for 18 days in the absence (control) or presence of neutralizing anti–SDF-1α antibodies or blocking anti-CXCR4 mAbs. Cells were triple labeled as described in “Materials and methods” with antihuman CD45-FITC, anti–CD4-PerCp, anti–CD3-PE, or anti–CD8-PE. Dot plots show CD4 versus CD8 or CD3 expression on gated human CD45-FITC+ cells. Data are representative of 4 independent experiments.
Blockade of SDF-1/CXCR4 signaling arrests human thymocyte development.
SCID fetal thymic lobes seeded with highly purified CD34+human thymic precursors were organ cultured for 18 days in the absence (control) or presence of neutralizing anti–SDF-1α antibodies or blocking anti-CXCR4 mAbs. Cells were triple labeled as described in “Materials and methods” with antihuman CD45-FITC, anti–CD4-PerCp, anti–CD3-PE, or anti–CD8-PE. Dot plots show CD4 versus CD8 or CD3 expression on gated human CD45-FITC+ cells. Data are representative of 4 independent experiments.
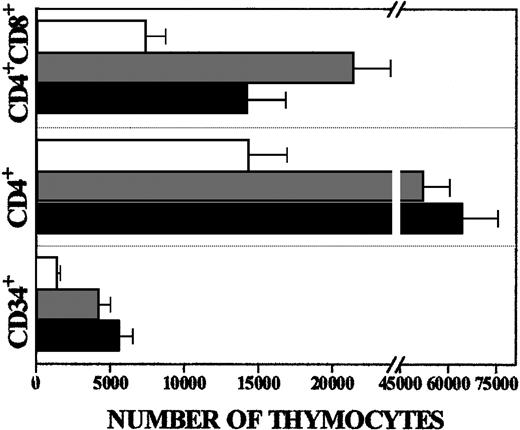
Given that both anti-CXCR4 and anti–SDF-1 antibodies arrested human thymocyte development, we tested the effects of rhSDF-1α protein on T-cell differentiation. When rhSDF-1α (10 ng/mL) was added to the cultures there was a 3- to 4-fold increase in the number of human thymocytes recovered after 10 or 18 days in FTOC (Table 1). The absolute numbers of all thymocyte subsets were higher in the SDF-1α–treated FTOC than in control cultures, but the increase was more pronounced in the CD34+ and immature CD4+cell subsets (3.5 and 4.5 times, respectively) than in the CD4+CD8+ cells (2 times) (Figure4).
Preferential expansion of immature thymocytes after rhSDF-1α treatment.
Absolute numbers of different thymocyte subsets from FTOC cultured in absence (white bars) or presence of rhSDF-1α (gray bars, 5 ng/mL; black bars, 10 ng/mL). The absolute numbers of each thymocyte subset were calculated by multiplying the percentage of the cell subsets by the total number of human cells. The bars represent the mean ± SD from 4 independent experiments.
Preferential expansion of immature thymocytes after rhSDF-1α treatment.
Absolute numbers of different thymocyte subsets from FTOC cultured in absence (white bars) or presence of rhSDF-1α (gray bars, 5 ng/mL; black bars, 10 ng/mL). The absolute numbers of each thymocyte subset were calculated by multiplying the percentage of the cell subsets by the total number of human cells. The bars represent the mean ± SD from 4 independent experiments.
Because blocking anti–SDF-1 antibodies and rhSDF-1α protein had opposite effects on human thymocyte development (Table 1), it seemed unlikely that the arrest in differentiation caused by anti–SDF-1 was the result of nonspecific toxicity. To confirm whether SDF-1α was able to counteract the inhibition mediated by anti–SDF-1 antibodies, we simultaneously added rhSDF-1α and anti–SDF-1 antibodies to the FTOC. As shown in Figure5, panels A and B, the addition of rhSDF-1α (10 ng/mL) neutralized the inhibitory effect of anti–SDF-1 (5 μg/mL) on human T-cell maturation. The ability of the antibody to overcome the action of 10 ng/mL SDF-1 was dose dependent (Figure 5A).
The influence of rhSDF-1α on growth and development of human thymocytes can be neutralized by anti–SDF-1 antibodies.
(A) FTOC carried out as described in “Material and methods” were cultured in the presence of rhSDF-1α (10 ng/mL) and different doses of neutralizing anti–SDF-1α antibodies. The numbers of human cells recovered per lobe after 18 days of treatment are shown (solid line). Cell number in control FTOC is also indicated (•). (B) FTOC were cultured with anti–SDF-1α (5 μg/mL) in the absence or presence of rhSDF-1α (10 ng/mL) for 18 days. Cells expressing human CD45 antigen were gated and the expression of CD4, CD8, and CD3 antigens is shown for this gated population. Results are representative of 2 different experiments.
The influence of rhSDF-1α on growth and development of human thymocytes can be neutralized by anti–SDF-1 antibodies.
(A) FTOC carried out as described in “Material and methods” were cultured in the presence of rhSDF-1α (10 ng/mL) and different doses of neutralizing anti–SDF-1α antibodies. The numbers of human cells recovered per lobe after 18 days of treatment are shown (solid line). Cell number in control FTOC is also indicated (•). (B) FTOC were cultured with anti–SDF-1α (5 μg/mL) in the absence or presence of rhSDF-1α (10 ng/mL) for 18 days. Cells expressing human CD45 antigen were gated and the expression of CD4, CD8, and CD3 antigens is shown for this gated population. Results are representative of 2 different experiments.
These results indicated a role for the SDF-1 signal in the early steps of human T-cell differentiation. To further characterize the thymocyte subpopulations that are the main targets for this chemokine, we next established chimeric human-mouse FTOC seeded with either CD34+ or immature CD4+ thymocytes. As shown in Figure 6, although both types of cultures were affected by the treatment with rhSDF-1α protein or anti-CXCR4 antibodies after 10 or 18 days of culture, those repopulated with CD34+ cell precursors were more drastically affected (Figure 6). No effect was seen when FTOC were seeded with CD4+CD8+ thymocytes (data not shown). These results indicate that the main cell targets for SDF-1 are CD34+ thymocyte precursors and, to a lesser extent, immature CD4+ cells.
Comparative analysis of the changes in human cell yield provoked by the addition of rhSDF-1α protein or anti-CXCR4 mAbs to FTOC established with either CD34+ cells or immature CD4+ thymocytes.
SCID thymic lobes were seeded with either highly purified CD34+ (white bars) or CD4+ immature thymocytes (black bars) and organ cultured for 10 or 18 days in the presence of either rhSDF-1α protein or anti-CXCR4 mAbs. At each time point, cells were harvested and counted. Numbers of human cells were calculated by multiplying the total number of cells per lobe by the percentage of human CD45-FITC+ cells determined by flow cytometric analysis. The bars represent the mean of 3 independent experiments. SDs represented less than 18% of the mean values. Results are expressed as the percentage of values from the control FTOC. Control values are represented as 0.
Comparative analysis of the changes in human cell yield provoked by the addition of rhSDF-1α protein or anti-CXCR4 mAbs to FTOC established with either CD34+ cells or immature CD4+ thymocytes.
SCID thymic lobes were seeded with either highly purified CD34+ (white bars) or CD4+ immature thymocytes (black bars) and organ cultured for 10 or 18 days in the presence of either rhSDF-1α protein or anti-CXCR4 mAbs. At each time point, cells were harvested and counted. Numbers of human cells were calculated by multiplying the total number of cells per lobe by the percentage of human CD45-FITC+ cells determined by flow cytometric analysis. The bars represent the mean of 3 independent experiments. SDs represented less than 18% of the mean values. Results are expressed as the percentage of values from the control FTOC. Control values are represented as 0.
Therefore, the current data show that SDF-1 plays a pivotal role in the expansion and differentiation of early human T-cell precursors at a stage between the transition of CD34+cells toward CD4+ cells.
SDF-1 does not play a functional role in thymic colonization
Previous reports have described a role for SDF-1 in the migration of CD34+ progenitor cells.12 To determine whether SDF-1 has a role in thymus colonization, we examined the effects of anti-CXCR4 antibodies or rhSDF-1α on the ability of human thymic CD34+ precursors to repopulate alymphoid fetal thymus lobes. As shown in Table 2, exposure of CD34+ T-cell precursors to inhibiting anti-CXCR4 antibodies or rhSDF-1α only in the hanging drop did not significantly modify the recolonization of recipient thymus lobes by T-cell precursors. The addition of anti-CXCR4 antibodies after hanging drop resulted in a drastic reduction of human cell number, comparable to the inhibition that was found after continuous treatment during the hanging drop period and the FTOC afterward (Table 2). Similar increases in the human thymocyte yields were observed when rhSDF-1α was present during either the FTOC period or the whole culture period.
SDF-1 chemokine is not required for migration of T-cell precursors into the thymus
| Treatment* . | Presence of rhSDF-1α or α-CXCR4 . | ||
|---|---|---|---|
| Hanging drop . | FTOC . | No. of human cells per lobe after 18 d of FTOC† . | |
| Control | No | No | 26 830 |
| Control IgG2a | No | Yes | 25 690 |
| Control IgG2a | Yes | No | 30 520 |
| rhSDF-1α | No | Yes | 98 315 |
| rhSDF-1α | Yes | No | 26 963 |
| rhSDF-1α | Yes | Yes | 115 312 |
| Antihuman CXCR4 | No | Yes | 6 213 |
| Antihuman CXCR4 | Yes | No | 22 210 |
| Antihuman CXCR4 | Yes | Yes | 6 004 |
| Treatment* . | Presence of rhSDF-1α or α-CXCR4 . | ||
|---|---|---|---|
| Hanging drop . | FTOC . | No. of human cells per lobe after 18 d of FTOC† . | |
| Control | No | No | 26 830 |
| Control IgG2a | No | Yes | 25 690 |
| Control IgG2a | Yes | No | 30 520 |
| rhSDF-1α | No | Yes | 98 315 |
| rhSDF-1α | Yes | No | 26 963 |
| rhSDF-1α | Yes | Yes | 115 312 |
| Antihuman CXCR4 | No | Yes | 6 213 |
| Antihuman CXCR4 | Yes | No | 22 210 |
| Antihuman CXCR4 | Yes | Yes | 6 004 |
Comparative analysis of the influence of rhSDF-1 or antihuman CXCR4 treatment on the development of human cells in chimeric human-mouse FTOC as function of time of addition of the recombinant human protein or of the blocking mAb.
Data represent the mean human cell number per lobe from 2 to 4 independent experiments.
These results indicate, therefore, that SDF-1/CXCR4 signaling is not involved in the migration of human T-cell precursors into the thymus.
SDF-1 enhances the proliferation and the survival of human thymocyte precursors
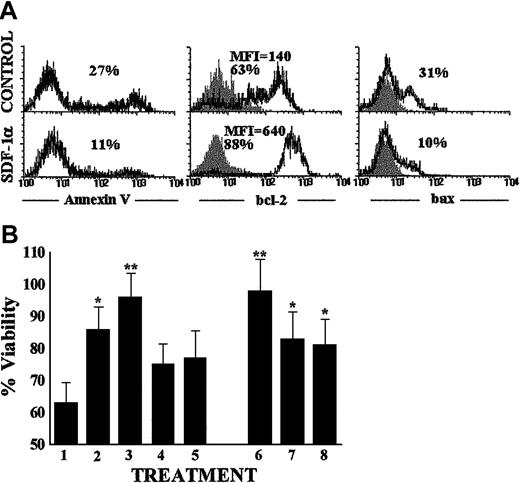
To determine whether the results obtained with chimeric human-mouse FTOC could be reflecting a role of SDF-1 in promoting the expansion or survival of human T-cell precursors, experiments were performed in which CD34+ thymocytes were cultured for 48 hours in serum-free medium with rhSDF-1α alone or in combination with various cytokines, and cellular proliferation and viability were evaluated. The addition of rhSDF-1α to thymocyte precursor cultures always increased the viability of CD34+ cells. The percentage of annexin-positive propidium iodide–negative apoptotic cells in the absence of the chemokine was 29% ± 6% (n = 4), decreasing to 12% ± 4% (n = 4) in cultures supplemented with rhSDF-1α (Figure 7A). To elucidate possible mechanisms of the inhibitory effects of SDF-1 on serum depletion–induced apoptosis in T-cell progenitors, we examined the expression of bcl-2 and bax proteins on CD34+ thymocyte suspensions cultured in the presence or absence of rhSDF-1α. As previously described, the ratio of bcl-2/bax can determine survival or death on triggering of apoptosis.37 As shown in Figure 7A, rhSDF-1α significantly induced an increase of the bcl-2/bax ratio in CD34+ thymocytes, due to both an up-regulation of bcl-2 expression (4-5 times in mean fluorescence intensity and 1.5-2 times in percentage of positive cells) and down-regulation of bax expression (2.5-3 times in percentage of positive cells). On the other hand, the ability of SDF-1 alone to promote CD34+ thymic precursor survival did not increase in the presence of SCF or flt3-ligand, and the viability of IL-7–treated cultures was not improved by the addition of rhSDF-1α (Figure 7B).
SDF-1 enhances the survival of human thymocyte precursors.
(A) Purified CD34+ thymic precursor cells were cultured in triplicate for 48 hours in serum-free medium alone (control) or containing rhSDF-1α protein. Annexin V, bcl-2, and bax expression (open histograms) measured by flow cytometry in the thymocytes harvested from control and rhSDF-1α–treated cellular suspensions is shown. Background staining is shown by isotype-matched control antibody (solid histograms). Data are representative results from 3 independent experiments. MFI is shown for bcl-2 staining. (B) Determination of CD34+ thymic cell viability after culture for 48 hours in serum-free medium alone (indicated by 1) or supplemented with either rhSDF-1α (2), IL-7 (3), SCF (4), flt3-ligand (5), or a combination of rhSDF-1α plus IL-7 (6), SCF (7) or flt3-ligand (8). Percentage of viable cells was determined by staining with annexin V and propidium iodide. Viable cells were defined as annexin V negative and propidium iodide negative. Data represent the means ± SD of 4 independent experiments, including 3 cultures per point. Asterisks refer to the statistical significance differences between control and the different treatments. *P ≤ .05; **P ≤ .01.
SDF-1 enhances the survival of human thymocyte precursors.
(A) Purified CD34+ thymic precursor cells were cultured in triplicate for 48 hours in serum-free medium alone (control) or containing rhSDF-1α protein. Annexin V, bcl-2, and bax expression (open histograms) measured by flow cytometry in the thymocytes harvested from control and rhSDF-1α–treated cellular suspensions is shown. Background staining is shown by isotype-matched control antibody (solid histograms). Data are representative results from 3 independent experiments. MFI is shown for bcl-2 staining. (B) Determination of CD34+ thymic cell viability after culture for 48 hours in serum-free medium alone (indicated by 1) or supplemented with either rhSDF-1α (2), IL-7 (3), SCF (4), flt3-ligand (5), or a combination of rhSDF-1α plus IL-7 (6), SCF (7) or flt3-ligand (8). Percentage of viable cells was determined by staining with annexin V and propidium iodide. Viable cells were defined as annexin V negative and propidium iodide negative. Data represent the means ± SD of 4 independent experiments, including 3 cultures per point. Asterisks refer to the statistical significance differences between control and the different treatments. *P ≤ .05; **P ≤ .01.
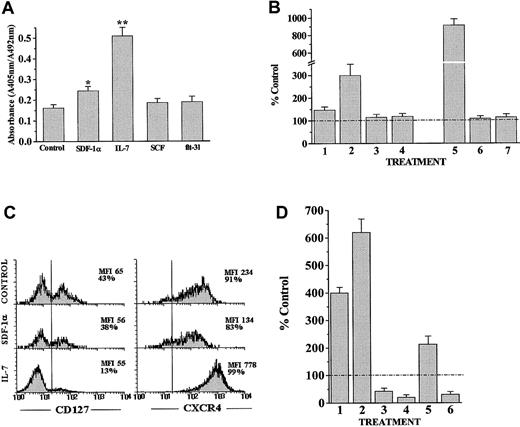
We next determined the direct proliferative response of CD34+ thymocyte precursors to SDF-1. Figure8A shows that rhSDF-1α alone stimulated the proliferation of T-cell precursors (1.5-2 times), but to a lesser extent than IL-7 (3-4 times). No proliferative effect was observed after culturing CD34+ cells with either SCF or flt3-ligand (Figure 8A).
SDF-1α synergizes with IL-7 to stimulate the proliferation of human thymocyte precursors.
(A) Determination of CD34+ thymic cell proliferation after culture for 48 hours in serum-free medium alone or supplemented with rhSDF-1α, IL-7, SCF, or flt3-ligand. Cells were pulsed for 12 hours with BrdU. A specific kit was used to measure BrdU incorporation into newly synthesized DNA. Full details are given in “Materials and methods.” Results are the means of 4 independent experiments, each with 3 cultures per point. Asterisks refer to the statistical significance differences between control and the different treatments. *P ≤ .05; **P ≤ .01. (B) Determination of CD34+ thymic cell proliferation after culture for 48 hours in serum-free medium alone or supplemented with rhSDF-1α (1), IL-7 (2), SCF (3), flt3-ligand (4), or a combination of rhSDF-1α plus IL-7 (5), SCF (6), or flt3-ligand (7). Results are expressed as the percentage of values from untreated cultures (number of cells × 100/number of cells in control cultures). The dashed line represents the control values. The index of synergy between SDF-1α and IL-7 was 1.97, with SCF 0.50 and with flt3-ligand 0.54. This index was calculated as BrdU incorporation in the presence of IL-7, SCF or flt3-ligand with rhSDF-1α divided by the BrdU incorporation by rhSDF-1α alone plus IL-7, SCF, or flt3-ligand alone. (C) Expression of CD127 and CXCR4 was analyzed in CD34+ thymic cell precursors after culture for 48 hours in serum-free medium alone or supplemented with rhSDF-1α or IL-7. The percentages of positive cells and their MFI are indicated in each histogram. Results are representative of 4 independent experiments. (D) SCID fetal thymic lobes were seeded with CD34+ human thymic precursors and organ-cultured for 10 days in the presence of rhSDF-1α (1), rhIL-7 (2), neutralizing anti–SDF-1α antibodies (3), neutralizing anti–IL-7 antibodies (4), rhIL-7 plus anti–SDF-1α antibodies (5), or rhSDF-1α plus anti–IL-7 antibodies (6). The numbers of human cells were calculated by multiplying the total number of cells per lobe by the percentage of human CD45-FITC+ cells determined by flow cytometry analysis. Results are expressed as the percentage of values from the control FTOC (number of cells × 100/number of cells in control culture). The dashed line represents the control values.
SDF-1α synergizes with IL-7 to stimulate the proliferation of human thymocyte precursors.
(A) Determination of CD34+ thymic cell proliferation after culture for 48 hours in serum-free medium alone or supplemented with rhSDF-1α, IL-7, SCF, or flt3-ligand. Cells were pulsed for 12 hours with BrdU. A specific kit was used to measure BrdU incorporation into newly synthesized DNA. Full details are given in “Materials and methods.” Results are the means of 4 independent experiments, each with 3 cultures per point. Asterisks refer to the statistical significance differences between control and the different treatments. *P ≤ .05; **P ≤ .01. (B) Determination of CD34+ thymic cell proliferation after culture for 48 hours in serum-free medium alone or supplemented with rhSDF-1α (1), IL-7 (2), SCF (3), flt3-ligand (4), or a combination of rhSDF-1α plus IL-7 (5), SCF (6), or flt3-ligand (7). Results are expressed as the percentage of values from untreated cultures (number of cells × 100/number of cells in control cultures). The dashed line represents the control values. The index of synergy between SDF-1α and IL-7 was 1.97, with SCF 0.50 and with flt3-ligand 0.54. This index was calculated as BrdU incorporation in the presence of IL-7, SCF or flt3-ligand with rhSDF-1α divided by the BrdU incorporation by rhSDF-1α alone plus IL-7, SCF, or flt3-ligand alone. (C) Expression of CD127 and CXCR4 was analyzed in CD34+ thymic cell precursors after culture for 48 hours in serum-free medium alone or supplemented with rhSDF-1α or IL-7. The percentages of positive cells and their MFI are indicated in each histogram. Results are representative of 4 independent experiments. (D) SCID fetal thymic lobes were seeded with CD34+ human thymic precursors and organ-cultured for 10 days in the presence of rhSDF-1α (1), rhIL-7 (2), neutralizing anti–SDF-1α antibodies (3), neutralizing anti–IL-7 antibodies (4), rhIL-7 plus anti–SDF-1α antibodies (5), or rhSDF-1α plus anti–IL-7 antibodies (6). The numbers of human cells were calculated by multiplying the total number of cells per lobe by the percentage of human CD45-FITC+ cells determined by flow cytometry analysis. Results are expressed as the percentage of values from the control FTOC (number of cells × 100/number of cells in control culture). The dashed line represents the control values.
It has been previously shown that SDF-1 stimulates the expansion of peripheral blood CD34+ cells in synergy with thrombopoietin (TPO), SCF, and flt3-ligand.11 However, our results show that neither SCF nor flt3-ligand alone or in combination with rhSDF-1α could induce proliferation of CD34+thymocyte precursors (Figure 8B). On the contrary, a high proliferative response was observed when CD34+ precursor cells were cultured with rhSDF-1α in combination with IL-7. In those cultures, the proliferative response of CD34+ thymocyte precursors was 6 times higher than for rhSDF-1α–treated cells and 3 times higher than for IL-7–treated cell cultures (Figure 8B). To establish whether the synergistic effect of IL-7 and SDF-1 could be a consequence of the modulation of their receptors by the other growth factor, we analyzed the expression of CD127 and CXCR4 in these cultures. As expected, CXCR4 expression was down-regulated in rhSDF-1α–treated cell suspensions (Figure 8C). rhSDF-1α hardly changed CD127 expression, whereas IL-7 induced a 3- to 4-fold increase in the surface expression of CXCR4 on CD34+ thymic precursors (Figure 8C), explaining, at least in part, the synergistic proliferative effect of SDF-1 and IL-7 on human CD34+ cell precursors.
These data indicate that the SDF-1–mediated expansion of early human thymic precursors involve both survival and proliferative signals. Previous reports have shown that IL-7 is also an essential growth and survival factor in early human T-cell development,38suggesting that SDF-1 and IL-7 could be functionally redundant. To test this hypothesis, we used chimeric human/mouse FTOC and analyzed the effects of the addition of rhSDF-1α, rhIL-7, or blocking antibodies against these growth factors. Treatment with anti–IL-7 resulted in a more pronounced reduction (70%-85%) of human cellularity as compared with anti–SDF-1 treatment (50%-60%; Figure 8D). Interestingly, IL-7–mediated expansion was partially blocked by anti–SDF-1 antibodies, whereas the addition of rhSDF-1α was not able to counteract the reduction in human cellularity mediated by anti–IL-7 antibodies (Figure 8D).
Discussion
Stromal cell–derived factor 1 has been described as an important factor in the maturation and proliferation of different cell types of the hematopoietic and immune systems. We show here that in addition to its apparent role in myelopoiesis, B differentiation, or the peripheral immune system, SDF/CXCR4 signaling is critical for early human T-cell development.
In the present report, we show SDF-1 expression on human thymic subcapsular and medullary epithelial cells. In agreement, other authors have described SDF-1 mRNA expression in mouse thymic epithelium positive for major histocompatibility complex class II,26 especially in the subcapsular compartment.25 However, Zaitseva et al28reported SDF-1 mRNA expression on collagen III+fibroblastlike cells derived from human thymic stromal cell monolayers.
The preferential expression of SDF-1 in the thymic subcapsular area suggests a role for this chemokine in the early steps of T-cell differentiation, because primitive progenitors in the human thymus have been described to be located in that thymic compartment.4We describe the expression of CXCR4 on thymic CD34+progenitors, confirming the observations of Zaitseva et al28 and extending them by further specifying the expression of SDF-1 receptor on those thymic CD34+progenitors committed to the T-cell lineage. In agreement, Suzuki et al25 described that in adult mice the population of CD44+CD25−CD4−CD8−CD3−cells, comprising the earliest thymic precursor cells that are not committed to the T-cell lineage, does not express CXCR4, whereas their descendant CD44+CD25+ cells do. In addition, the highest density of CXCR4 is found on adult human bone marrow CD34+ cell subsets enriched for progenitor cells committed to the lymphoid lineage.29 Likewise, Ishii et al39 found that multipotential progenitors located in the bone marrow are negative for CXCR4 expression and that the acquired expression of CXCR4 on CD34+ human bone marrow cells during differentiation results in the loss of myeloid differentiation potential, whereas the ability to develop into lymphoid progenitors is retained, suggesting that CXCR4 on CD34+ human bone marrow cells is one of the earliest markers for lymphoid commitment.
To test whether SDF/CXCR4 signaling might be important for human thymocyte development, we added anti–SDF-1 antibodies, anti-CXCR4 antibodies, or rhSDF-1α protein to chimeric human-mouse FTOC, and determined their impact after 10 to 18 days. Treatment of FTOC with either anti–SDF-1 or anti-CXCR4 antibodies causes similar changes in T-cell development, suggesting that in thymus, as in other systems, SDF-1 acts through a single receptor, CXCR4. The current data also show that the addition of rhSDF-1α induces a 3- to 4-fold increase in the human cell yield, and as a consequence, the absolute numbers of all thymic cell subsets increase, being the CD34+ and CD4+ cell subsets the most affected by the cell expansion. The comparative analysis of FTOC seeded with either CD34+or immature CD4+ cells confirms that the earliest stages of differentiation are the most dependent on SDF-1 chemokine. On the contrary, the disruption of SDF-1/CXCR4 interactions provokes a dramatic reduction in the human cell yield and a partial blockade in the maturation of CD34+ thymocyte precursors. An impairment in T lymphopoiesis has been described in mice reconstituted with bone marrow-hematopoietic progenitor cells expressing SDF-1-intrakine. Intrakine-transduced mice, in which the SDF-1 chemokine remains inside the cells preventing the transport of the CXCR4 receptor to cell surface, show a reduction of the number of thymocytes and a partial arrest of thymocyte maturation at the step from double-negative to double-positive cells.33 Likewise, mice reconstituted with CXCR4-deficient fetal liver cells show a severe reduction in thymocyte number.32 Mice with disrupted CXCR4 orSDF1 genes display, however, normal T lymphopoiesis,20,22-24 suggesting that the SDF-1/CXCR4 interaction is not required for the generation of a normal cell population in the mouse thymus. The explanation for these contradictory results is currently unknown, but in our case could be related to species-specific differences. Also, SDF-1 may serve redundant functions during thymocyte development, possibly acting together with other chemokines or cytokines, such as IL-7, as we discuss later. In addition, it is also possible that the requirement of a functional expression of CXCR4 for T-cell precursor development could be different during fetal, neonatal, and adult life. In this sense, because CXCR4−/− and SDF-1−/− mice die in utero, there are no available data on thymic cellularity or T-cell development in these knockout mice after birth. In humans, CD34+ cells from fetal liver show important changes in CXCR4 expression in different developmental stages,29 and CD34+CXCR4+ cells from bone marrow or cord blood exhibit a different myeloid potential,39 suggesting that SDF-1/CXCR4 signaling could change during development.
Because various studies have involved SDF-1 in the homing and mobilization of CD34+ progenitor cells,12,29,39 the severe reduction of thymocyte number obtained in the absence of SDF-1/CXCR4 signaling could be reflecting an altered migration of T-cell progenitors into the thymus. Our data suggest that SDF-1/CXCR4 signaling is not critical for CD34+ precursor cell recruitment to the thymus, because the treatment with either rhSDF-1α protein or anti-CXCR4 antibodies only during the hanging drop procedure does not alter human cell recovery or T-cell development as compared to the control cultures. Furthermore, a comparable effect is observed when the chemokine or the blocking antibody is added to the cultures only during the FTOC period or during both the hanging drop and the FTOC afterward. Also in support, we describe that the earliest T-cell precursors found in the thymus are CXCR4−, which correlates with the fact that most peripheral blood CD34+ progenitor cells do not express CXCR4 (our unpublished observations, January 2001; and Lataillade et al11; Deichmann et al40; and Mohle et al41). Recently, it has been reported that fetal blood prothymocytes respond to SDF-1 in chemotaxis assays.27 However, Wilkinson et al26previously described that the level of expression of SDF-1 in thymic epithelial cells does not exceed that in fetal rudiments that lack the ability to attract precursors, suggesting that this chemokine is unlikely to be primarily involved in thymus colonization.
The reduction in thymocyte numbers and the blockade of T-cell differentiation observed in both anti–CXCR4- and anti–SDF-1–treated FTOC may be also related to an alteration in the intrathymic trafficking of developing thymocytes toward the appropriate stromal cell niches where they would receive growth- and maturation-promoting signals. In this respect, a similar migratory behavior of different thymocyte subsets to SDF-1 has been described, unlike other chemokines such as thymus-expressed chemokine (TECK), secondary lymphoid tissue chemokine (SLC), or macrophage inflammatory protein-3β, which only attract specific thymic cell populations.30,31Therefore, a potential role for SDF-1 in migratory events during T-cell development does not seem to be apparent. Alternatively, proliferation or differentiation signals could be directly affected by the SDF-1/CXCR4 signaling disruption. Our results favor this hypothesis, implying a new role for SDF-1 and its receptor in thymus different from the migratory events. Remarkably, we show that SDF-1 protects T-cell precursors from serum depletion-induced apoptosis, considerably increasing their viability. This protective effect involves both the down-regulation of the proapoptotic bax protein and the up-regulation of the antiapoptotic bcl-2 protein. Burger et al13 reported that blood-derived nurselike cells protect leukemia B cells from undergoing spontaneous apoptosis through an SDF-1–dependent mechanism. In agreement with our results, these authors found a transient activation of p44/42 MAPK, known to promote cell survival by inactivating proapoptotic protein and activating the transcription factor CREB, which is important for the up-regulation of theBCL2 gene.42
Stromal cell–derived factor 1 was first described as a growth factor for murine pre-B cells.8 More recently, Lataillade et al11 have extended this proliferative effect of SDF-1 also to adult peripheral blood CD34+ cells. We demonstrate that SDF-1 alone stimulates the proliferation of CD34+thymic precursors. In the presence of IL-7, but not other cytokines, such as SCF or flt3-ligand, this effect is significantly enhanced, indicating that SDF-1 and IL-7 synergistically act in the human thymus to promote the proliferation of early T-cell precursors. Interestingly, we also show that IL-7, but not SCF or ftl3-ligand, up-regulates CXCR4 expression on CD34+ thymic precursor cells. In addition, SDF-1 deprivation with anti–SDF-1 antibodies partially inhibits the IL-7–mediated thymocyte expansion, whereas the profound reduction in thymic cellularity induced by anti–IL-7 antibodies cannot be counteracted by the addition of rhSDF-1α. These findings, together with the above described role for SDF-1 in promoting proliferation and survival of T-cell precursors, indicate that the effects described for IL-7 as an essential growth and maturation factor for early steps in human thymocyte development38 are partially mediated through SDF-1.
In summary, our findings provide evidence that SDF-1/CXCR4 signaling plays a key role in human thymocyte development, promoting survival and expansion of human early T-cell progenitors. Therefore, SDF-1 may be a good additional candidate to include in the list of trophic cytokines that could be used to therapeutically promote reconstitution of T-cell immunity by enhancing thymopoiesis in some of those pathologic situations associated with loss or damage to the peripheral T-cell pool.
We thank Dr Barton F. Haynes for the generous gift of mAbs, and the Pediatric Cardiosurgery Unit from the Hospital Gregorio Marañón (Madrid) for the thymus samples. The flow cytometry analysis was performed on a FACScan flow cytometer from the Servicio Común de Investigación, Facultad de Biologı́a, Universidad Complutense de Madrid.
Supported by grant 98/0041 from the Fondo de Investigaciones Sanitarias; grants 08.3/0014/1997, 08.3/0027/1998, 08.3/0041./2000 from the Comunidad Autónoma de Madrid; and grants PB97-0032 and PM99-0060 from the Ministerio de Educación y Cultura.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Angeles Vicente, Departamento de Biologı́a Celular, Facultad de Biologı́a, Universidad Complutense, 28040 Madrid, Spain; e-mail: avicente@bio.ucm.es.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal