Vascular smooth muscle cells (SMCs) generate carbon monoxide (CO) via the catabolism of heme by the enzyme heme oxygenase (HO). In the present study, we found that serum stimulated a time- and concentration-dependent increase in the levels of HO-1 messenger RNA (mRNA) and protein in vascular SMCs. The induction of HO-1 expression by serum was inhibited by actinomycin D or cycloheximide. In addition, serum stimulated HO activity, as reflected by an increase in the concentration of bilirubin in the culture media. Treatment of vascular SMCs with serum stimulated DNA synthesis and this was potentiated by the HO inhibitors, zinc and tin protoporphyrin-IX as well as by the CO scavenger, hemoglobin. The iron chelator desferrioxamine had no effect on DNA synthesis. However, exposure of vascular SMCs to exogenous CO inhibited serum-stimulated SMC proliferation and the phosphorylation of retinoblastoma protein. In addition, CO arrested SMCs at the G1/S transition phase of the cell cycle and selectively blocked the serum-stimulated expression of cyclin A mRNA and protein without affecting the expression of cyclin D1 and E. CO also inhibited the serum-stimulated activation of cyclin A–associated kinase activity and cyclin-dependent kinase 2 activity. These results demonstrate that serum stimulates HO-1 gene expression and CO synthesis. Furthermore, they show that CO acts in a negative feedback fashion to inhibit vascular SMC growth by regulating specific components of the cell cycle machinery. The capacity of vascular mitogens to induce CO synthesis may provide a novel mechanism by which these agents modulate cell growth.
Introduction
Heme oxygenase (HO) catalyzes the initial and rate-limiting step in heme metabolism. It oxidatively degrades heme into equimolar amounts of carbon monoxide (CO), free iron, and biliverdin.1 Biliverdin is subsequently metabolized to the potent antioxidant bilirubin by biliverdin reductase.1Three distinct isoforms of HO have been identified and cloned.2-4 These isozymes are products of different genes and differ markedly in their tissue distribution. Both HO-2 and HO-3 are constitutively expressed isoforms that are present in high concentration in selected mammalian tissues.3,4 In contrast, the HO-1 isoform is ubiquitously distributed and is strongly induced by a variety of physiologic and pathophysiologic stimuli, including heme, heavy metals, inflammatory cytokines, endotoxin, nitric oxide, and hemodynamic forces.3,5-7 All 3 isoforms of HO are inhibited by various metalloprotoporphyrins, such as zinc (Zn) and tin (Sn) protoporphryin-IX (ZnPP, SnPP)8. In addition, the HO-1 metabolites iron and CO can be inactivated by the iron chelator desferrioxamine and by the CO scavenger hemoglobin (Hb), respectively.9 10
Recent studies indicate that HO-1 plays an important role in the circulation. The induction of HO-1 by endotoxin contributes to the severe hypotension associated with septic shock.6 In addition, the expression of HO-1 in the blood vessel impairs contractile function and causes a marked decrease in blood pressure in spontaneously hypertensive rats.11,12 In contrast, HO inhibitors increase blood pressure and peripheral resistance, suggesting a critical vasoregulatory role for HO-1.13 HO-1 also modulates platelet–vessel wall interactions. Using a platelet-vascular smooth muscle cell (SMC) coincubation system, we found that the induction of HO-1 in SMCs inhibits platelet aggregation, indicating a potentially important antithrombotic role for this enzyme.7 These HO-1–mediated effects on cell function are mediated via the release of CO.7,12-14 Finally, HO-1 also confers significant cytoprotective effects on vascular cells. The induction of HO-1 in vascular cells leads to an increased resistance to oxidative stress, whereas HO-1 deficiency results in enhanced cell injury.15-17 The cytoprotective effect of HO-1 is dependent on the generation of bilirubin.15 16
Interestingly, we and others have demonstrated that growth factors are potent inducers of HO-1.10,18 19 These findings raise the possibility that HO-1 may also affect cell growth. Accordingly, the present study examined whether HO-1 affects the proliferative response of vascular SMCs. We now report that serum stimulates HO-1 gene expression and that the HO-1–catalyzed release of CO functions in an autocrine manner to limit vascular SMC proliferation. The ability of growth factors to induce CO synthesis may represent a novel mechanism by which vascular mitogens regulate SMC growth.
Materials and methods
Materials
Collagenase, sodium dodecyl sulfate (SDS), β-mercaptoethanol, cycloheximide, actinomycin, EDTA, Tris, Tes, HEPES, Tween 20, urea, bilirubin, biliverdin, benzene, barium chloride, Hb, acrylamide, trichloroacetic acid, aprotinin, leupeptin, phenylmethylsulfonyl fluoride (PMSF), NaF, dithiothreitol (DTT), guanidine isothiocyanate, CsCl, desferrioxamine, and minimum essential medium were purchased from Sigma Chemical (St Louis, MO). Penicillin, streptomycin, neomycin, elastase, trypsin, and calf serum were from Gibco BRL (Rockville, MD); RNase and propidium iodide were from Boehringer Mannheim (Indianapolis, IN); and GAPDH complementary DNA (cDNA) and molecular markers were from Ambion (Austin, TX). A polyclonal HO-1 antibody was from Stressgen (Victoria, BC, Canada); a monoclonal α-tubulin antibody was from Oncogene Research Products (Boston, MA); a monoclonal antibody that recognizes both the hypophosphorylated and hyperphosphorylated forms of retinoblastoma protein (pRb) was from Pharmingen (San Diego, CA); a polyclonal phosphorylation (Ser87/811)–specific antibody against pRb was from Cell Signaling Technology (Beverly, MA); monoclonal antibodies against cyclin D1, cyclin E, and cyclin A, and a polyclonal antibody against cyclin-dependent kinase-2 (cdk2) were from Santa Cruz Biotechnology (Santa Cruz, CA); and a neutralizing antibody against platelet-derived growth factor (PDGF) was from Upstate Biotechnology (Lake Placid, NY). A neutralizing antibody against transforming growth factor-β1 (TGF-β1) was from R & D Systems (Minneapolis, MN); [32P]UTP (400 Ci/mmol [1480 × 1010Bq]) was from Amersham (Arlington Heights, IL); [3H]thymidine (90 Ci/mmol [333 × 1010Bq]) and γ-[32P] adenosine triphosphate (ATP; 3000 Ci/mmol [11 100 × 1010 Bq]) were from NEN-Dupont (Boston, MA).
Cell culture
Vascular SMCs were isolated by elastase and collagenase digestion of rat thoracic aorta and characterized by morphologic and immunologic criteria.20 Cells were serially cultured in minimum essential medium containing 10% calf serum, Earle balanced salts, 5.6 mM glucose, 2 mM l-glutamine, 5 mM Tes-HEPES, and 100 U/mL penicillin, streptomycin, and neomycin. Cells were passaged twice a week by harvesting with trypsin/EDTA and seeded onto 6-well plates. When cells reached approximately 50% confluence, the culture media were replaced with serum-free minimum essential media containing bovine serum albumin (BSA; 0.1%, wt/vol) for 48 hours and then exposed to serum.
Messenger RNA analysis
HO-1 messenger RNA (mRNA) levels were determined by ribonuclease protection analysis using a commercially available kit (Ambion). Total RNA (10 μg) was hybridized with approximately 1 × 106cpm of [32P]UTP-labeled antisense HO-1 (284–base pair [bp]) and GAPDH (316-bp) riboprobes. The HO-1 antisense RNA probe was prepared as described earlier.5 Protected RNA was analyzed by electrophoresis using 6% acrylamide/8 mM urea gel. Gels were exposed overnight to x-ray film at −70°C in the presence of intensifying screens. The size of the predicted nucleotide-protected fragments was confirmed by using a 32P-labeled RNA ladder.
Cyclin A mRNA levels were determined by Northern blotting. Total RNA (30 μg) was loaded on 1.2% agarose gels and fractionated by electrophoresis. RNA was blot transferred to Gene Screen Plus membranes and prehybridized for 4 hours at 68°C in hybridization buffer (rapid-hyb buffer, Amersham). Membranes were hybridized overnight at 68°C in hybridization buffer containing [32P]DNA probes (1 × 108 cpm) for cyclin A and 18 S ribosomal RNA. DNA probes were labeled with α-[32P]dCTP using a random priming kit (Amersham). After hybridization, membranes were washed sequentially with 2 times standard saline citrate (SSC; 1 times SSC is 0.15 mM NaCl plus 0.015 mM sodium citrate)/0.1% sodium dodecyl sulfate (SDS) at 68°C for 20 minutes, and twice with 0.1 times SSC/0.1% SDS at 68°C for 20 minutes. Membranes were then exposed to x-ray film at −70°C in the presence of intensifying screens.
Protein analysis
The SMCs were lysed in electrophoresis buffer (125 mM Tris, pH 6.8, 12.5% glycerol, 2% SDS, and trace bromophenol blue) and boiled for 10 minutes. SDS–polyacrylamide gel electrophoresis (PAGE) was performed on gels with 20 μg protein using the buffer system of Laemmli.21 The separated blots were electrophoretically transferred to nitrocellulose membranes and blocked for one hour in phosphate-buffered saline (PBS) containing 0.1% Tween-20 and 3% nonfat milk. Blots were incubated with antibodies directed against HO-1 (1:500 dilution), pRb (1:750 dilution), cyclin D1 (1 μg/mL), cyclin E (2.5 μg/mL), cyclin A (0.7 μg/mL), cdk2 (1 μg/mL), or the housekeeping protein α-tubulin (5 μg/mL) in PBS containing Tween-20 (0.1%) for 1 hour. Membranes were then washed in PBS and incubated for 1 hour with horseradish peroxidase–conjugated goat antirabbit or goat antimouse antibody. After further washing with PBS, blots were incubated in commercial chemoluminescence reagents (Amersham) and exposed to photographic film.
Cyclin A–associated kinase and cdk2 assay
The SMCs were collected in lysis buffer (150 mM Tris, pH 7.5, 200 mM NaCl, 2.0 mM EDTA, 2.0 mM EGTA, 10% glycerol, 0.1% Tween-20, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 mM PMSF, 1 mM Na3VO4, 50 mM NaF, and 1 mM DTT), and sonicated twice on ice at 30% for 10 seconds. Protein (300-500 μg) was immunoprecipitated using protein A agarose beads (40 μL; Santa Cruz Biotechnology), cyclin A (2 μg), or cdk2 (1 μg) antibody, and lysis buffer (400 μL) overnight at 4°C. Agarose beads were washed 3 times with lysis buffer, 2 times with kinase buffer (50 mM Tris, pH 7.5, 10 mM MgCl2, 1 mM DTT, 1 mM Na3VO4,and 1 mM DTT), and all wash buffer removed. For kinase assays, kinase buffer (40 μL), histone H1 (2.0 μg; Roche, Indianapolis, IN), ATP (50 μM), and γ-[32P]ATP (6 μCi [.222 MBq]) were added to the agarose beads for 20 minutes at 30°C. Reactions were stopped by adding Laemmli buffer and samples boiled for 5 minutes. Proteins were separated by SDS-PAGE, fixed, and exposed to x-ray film.
HO activity
The activity of HO was measured spectrophotometrically by quantifying the release of bilirubin into the culture media.22 Culture media (500 μL) were collected and combined with barium chloride (250 mg) and benzene (750 μL). After vortexing, the benzene phase containing the extracted bilirubin was separated from the aqueous phase by centrifugation at 13 000g for 30 minutes. Bilirubin was determined spectrophotometrically as a difference in absorbance between 450 and 600 nm using an excitation coefficient of 27.3 mM−1cm−1.
SMC proliferation
The SMCs were seeded at a density of 5 × 104cells/well in 12-well plates in serum (10%)–containing media. After 24 hours, culture media were exchanged for serum-free media, and SMCs were incubated for an additional 48 hours. SMCs were then treated with serum in the presence or absence of CO. Media with appropriate additions were replenished every second day. Cell number determinations were performed after 4 days of treatment by dissociating cells with trypsin (0.025%)/EDTA (1 mM) and counting cells in a calibrated Coulter Counter (model ZF, Coulter Electronics, Hialeah, FL).
DNA synthesis
Quiescent SMCs were treated with serum in the presence or absence of CO for 20 hours. Then [3H]thymidine (1 μCi/mL [0.037 MBq]) was added and cells incubated for an additional 4 hours. For [3H]thymidine incorporation, SMCs were washed 3 times with ice-cold PBS, fixed with 10% trichloroacetic acid for 30 minutes at 4°C, and DNA extracted with 0.2% SDS/0.2 N NaOH. Radioactivity was determined by scintillation spectrophotometry (Tricarb liquid scintillation analyzer, model 1900, Packard, Meriden, CT). In some experiments the uptake of [3H]thymidine by SMC was monitored. For these experiments, SMCs were incubated with [3H]thymidine (1 μCi/mL for 4 hours [0.037 MBq]), lysed in 0.2% SDS/0.2 N NaOH, and radioactivity measured by scintillation counting.
Cell cycle analysis
Quiescent SMCs were treated with serum in the presence and absence of CO for 24 hours. Cells were then dispersed with trypsin (0.025%)/EDTA (1 mM), suspended in PBS, and fixed in 70% ethanol for 30 minutes. The ethanol was removed and SMCs were incubated in PBS containing RNase (0.5 mg/mL) for 30 minutes at 37°C, and then stained with 0.005% propidium iodide. DNA fluorescence was measured on a Dickinson FACScan flow cytometer (Franklin Lakes, NJ). The area versus the width of the fluorescent signal was analyzed to gate out cellular multiplets. Histograms of DNA content were analyzed using Modfit Lt V1.01 (Verity Software House, Topsham, ME) to determine fractions of the population in each phase of the cell cycle.
CO exposure
The SMCs were exposed to CO via a previously described environmental chamber.23 24 CO at a concentration of 1% in air was combined with air containing 5% CO2 in a stainless steel mixing cylinder prior to delivery into the humidified 37°C environmental chamber. Flow into the chamber was at 1 L/min and CO levels in the chamber were continuously monitored by electrochemical detection using a CO analyzer (Interscan, Chatsworth, CA).
Statistics
Results are expressed as the means ± SEM. Statistical analysis was performed with the use of a Student 2-tailed ttest, and an analysis of variance when more than 2 treatment regimens were compared. P < .05 was considered statistically significant.
Results
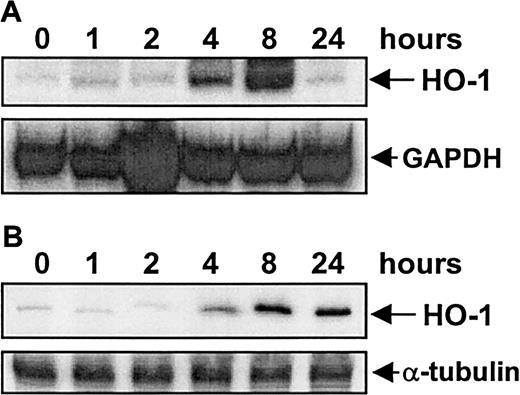
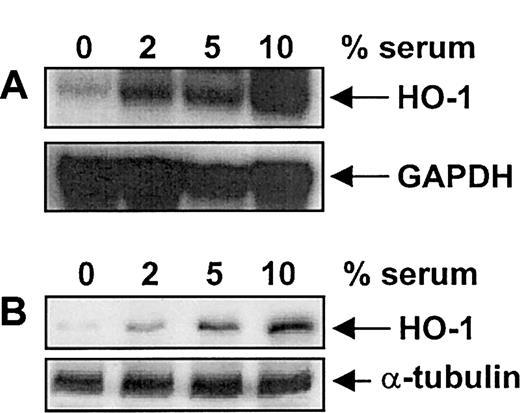
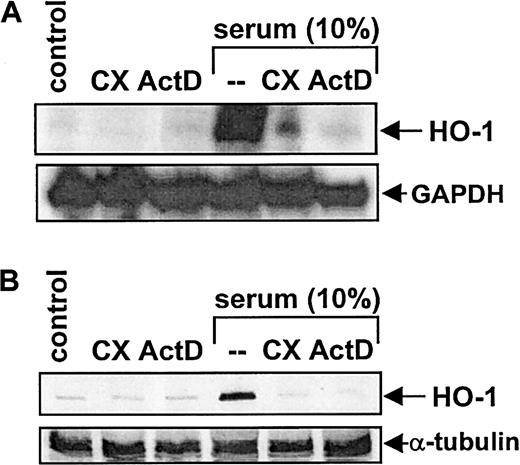
Treatment of vascular SMCs with serum (10%) resulted in a time-dependent increase in HO-1 mRNA and protein. An increase in HO-1 message was first detected after 4 hours of exposure, reached a maximum at 8 hours, and then declined to basal levels by 24 hours (Figure1A). Serum (10%) also induced a marked increase in HO-1 protein that was first detected after 4 hours of exposure; however, this increase was sustained for 24 hours (Figure1B). Serum-mediated increases in HO-1 mRNA and protein were concentration-dependent (Figure 2) and were specific for the HO-1 isoform (data not shown). Incubation of SMCs with the protein synthesis inhibitor cycloheximide (5 μg/mL) or with the transcriptional inhibitor actinomycin D (2 μg/mL) blocked the serum-mediated increase in HO-1 mRNA and protein (Figure3). In addition, serum had no effect on the stability of HO-1 mRNA. Incubation of SMCs with actinomycin D (2 μg/mL) resulted in the decay of HO-1 message with a half-life of about 2 hours, and this remained unchanged in the presence of serum (data not shown). Finally, because PDGF and TGF-β1are established inducers of HO-1,10 19 we examined whether they contribute to the induction of HO-1 by serum. However, incubation of serum with neutralizing antibodies directed against either of these growth factors had no effect on serum-stimulated HO-1 expression (data not shown).
Time-dependent increases in HO-1 mRNA and protein.
The time course of HO-1 mRNA (A) and protein (B) expression by serum in vascular SMCs is shown. Cells were treated with serum (10%) and then analyzed for HO-1 mRNA and protein at the indicated time. Similar findings were observed in 3 separate experiments.
Time-dependent increases in HO-1 mRNA and protein.
The time course of HO-1 mRNA (A) and protein (B) expression by serum in vascular SMCs is shown. Cells were treated with serum (10%) and then analyzed for HO-1 mRNA and protein at the indicated time. Similar findings were observed in 3 separate experiments.
Serum-mediated increases in HO-1 mRNA and protein.
Concentration-dependent increases in HO-1 mRNA (A) and protein (B) by serum in vascular SMCs are shown. Cells were treated with serum (0%-10%) for 8 hours and then analyzed for HO-1 mRNA and protein. Similar findings were made in 4 separate experiments.
Serum-mediated increases in HO-1 mRNA and protein.
Concentration-dependent increases in HO-1 mRNA (A) and protein (B) by serum in vascular SMCs are shown. Cells were treated with serum (0%-10%) for 8 hours and then analyzed for HO-1 mRNA and protein. Similar findings were made in 4 separate experiments.
Incubation of SMCs with cycloheximide (CX) and actinomycin D.
The effects of CX and ActD on HO-1 mRNA (A) and protein (B) expression by serum in vascular SMCs are shown. Cells were treated with serum (10%) in the presence or absence of CX (5 μg/mL) or actinomycin D (ActD) (2 μg/mL) for 8 hours and then analyzed for HO-1 mRNA and protein. Similar findings were observed in 3 separate experiments.
Incubation of SMCs with cycloheximide (CX) and actinomycin D.
The effects of CX and ActD on HO-1 mRNA (A) and protein (B) expression by serum in vascular SMCs are shown. Cells were treated with serum (10%) in the presence or absence of CX (5 μg/mL) or actinomycin D (ActD) (2 μg/mL) for 8 hours and then analyzed for HO-1 mRNA and protein. Similar findings were observed in 3 separate experiments.
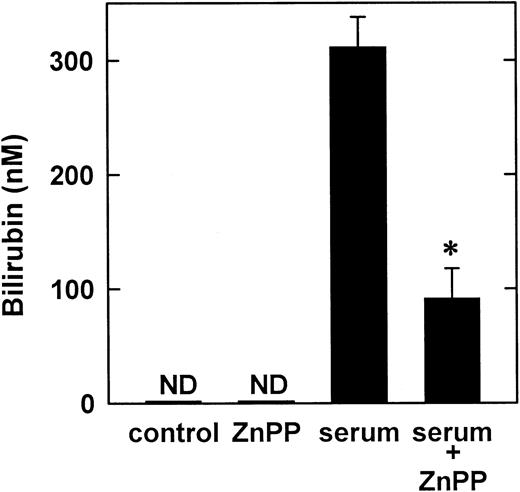
In subsequent experiments, HO-1 activity was measured by monitoring the release of bilirubin by vascular SMCs. In the absence of serum, bilirubin was not detected in culture media (Figure4). However, serum (10%) induced a marked rise in bilirubin concentration that was dependent on HO activity because it was blocked by ZnPP (Figure 4).
Effect of serum on the synthesis of bilirubin by vascular SMCs.
Bilirubin was measured in the culture media after cells were treated with serum (10%) for 8 hours. Results are means ± SEM of 3 separate experiments. *Statistically significant effect of ZnPP. ND indicates not detected.
Effect of serum on the synthesis of bilirubin by vascular SMCs.
Bilirubin was measured in the culture media after cells were treated with serum (10%) for 8 hours. Results are means ± SEM of 3 separate experiments. *Statistically significant effect of ZnPP. ND indicates not detected.
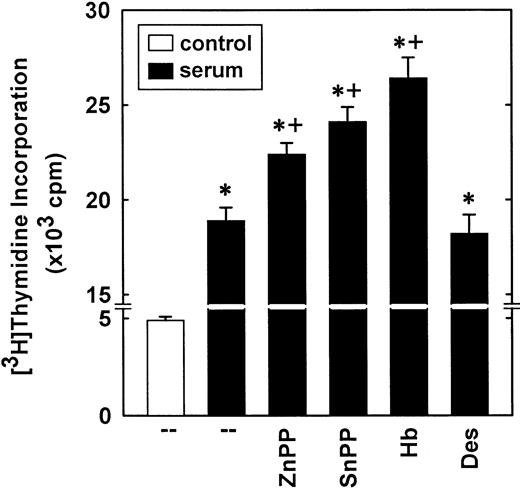
Treatment of vascular SMCs with serum stimulated over a 3-fold increase in DNA synthesis that was potentiated by the HO inhibitors, ZnPP (20 μM) and SnPP (20 μM; Figure 5). In addition, the CO scavenger Hb (50 μM) further increased serum-stimulated DNA synthesis (Figure 5). In contrast, the iron chelator desferrioxamine (100 μM) had no effect on DNA synthesis (Figure 5). In the absence of serum, the HO inhibitors or Hb minimally affected DNA synthesis (data not shown).
Effect of serum on DNA synthesis in vascular SMCs.
Cells were treated with serum (10%) for 24 hours in the presence or absence of ZnPP (20 μM), SnPP (20 μM), Hb (50 μM), or desferrioxamine (100 μM). The heme oxygenase inhibitors and desferrioxamine were added simultaneously with serum, whereas Hb was given 12 hours after serum addition. Results are means ± SEM of 4 separate experiments. *Statistically significant effect of serum.+Statistically significant effect of HO inhibitors and Hb.
Effect of serum on DNA synthesis in vascular SMCs.
Cells were treated with serum (10%) for 24 hours in the presence or absence of ZnPP (20 μM), SnPP (20 μM), Hb (50 μM), or desferrioxamine (100 μM). The heme oxygenase inhibitors and desferrioxamine were added simultaneously with serum, whereas Hb was given 12 hours after serum addition. Results are means ± SEM of 4 separate experiments. *Statistically significant effect of serum.+Statistically significant effect of HO inhibitors and Hb.
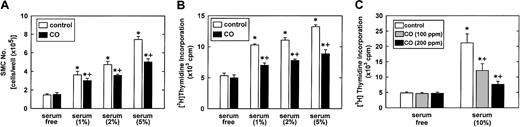
To confirm that CO exerts an antiproliferative effect, vascular SMCs were treated with SnPP (20 μM), to prevent the endogenous formation of CO, placed in an environmental chamber, and exposed to CO.23 24 Exposure of vascular SMCs to CO (100 ppm) significantly attenuated serum-stimulated SMC proliferation and thymidine incorporation (Figure 6A,B). In addition, the inhibition of thymidine incorporation by CO (0-200 ppm) was concentration dependent (Figure 6C). Control experiments indicated that CO had no effect on the uptake of thymidine by SMCs (data not shown).
Effect of CO on vascular SMC growth.
(A) SMC proliferation was monitored in cells treated with serum (0%-5%) and SnPP (20 μM) for 4 days in the presence or absence of CO (100 ppm). (B) SMC DNA synthesis was monitored in cells treated with serum (0%-5%) and SnPP (20 μM) for 24 hours in the presence and absence of CO (100 ppm). (C) Concentration-dependent inhibitory effect of CO (0-200 ppm) on DNA synthesis in SMCs treated with serum (10%) and SnPP (20 μM) for 24 hours. Results are means ± SEM of 4 separate experiments. *Statistically significant effect of serum.+Statistically significant effect of CO.
Effect of CO on vascular SMC growth.
(A) SMC proliferation was monitored in cells treated with serum (0%-5%) and SnPP (20 μM) for 4 days in the presence or absence of CO (100 ppm). (B) SMC DNA synthesis was monitored in cells treated with serum (0%-5%) and SnPP (20 μM) for 24 hours in the presence and absence of CO (100 ppm). (C) Concentration-dependent inhibitory effect of CO (0-200 ppm) on DNA synthesis in SMCs treated with serum (10%) and SnPP (20 μM) for 24 hours. Results are means ± SEM of 4 separate experiments. *Statistically significant effect of serum.+Statistically significant effect of CO.
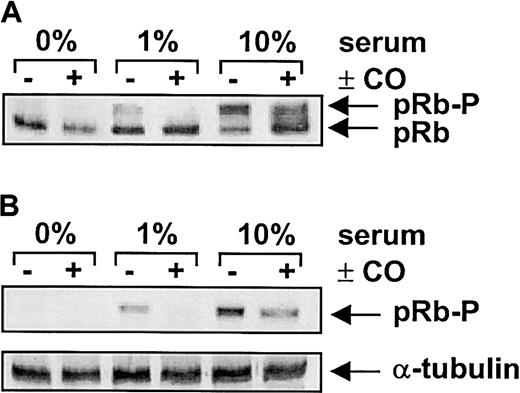
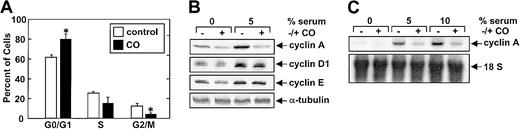
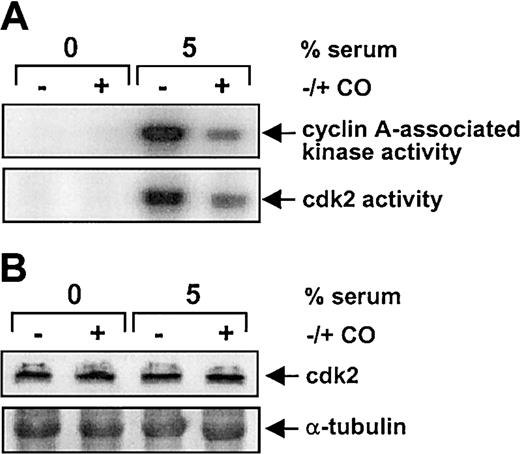
The CO-mediated inhibition of SMC growth was associated with a marked reduction in serum-mediated phosphorylation of pRb (Figure7). In addition, flow cytometric data indicated that CO arrested SMCs in the G1/S transition phase of the cell cycle (Figure 8A). Treatment of vascular SMC with serum also stimulated the expression of cyclin A, cyclin D1, and cyclin E protein (Figure 8B). However, CO exposure selectively attenuated the serum-stimulated expression of cyclin A protein without affecting the expression of cyclin D1 or E (Figure 8B). CO also blocked the induction of cyclin A mRNA expression by serum (Figure 8C). Because cyclin A is an activating subunit of cdk2, we also examined the effect of CO on cdk activity.25CO potently inhibited the serum-mediated increase in both cyclin A-associated kinase and cdk2 activity (Figure9A). However, CO had no effect on the expression of cdk2 protein (Figure 9B). Finally, the administration of CO did not induce cell necrosis or apoptosis (data not shown).
Effect of CO on retinoblastoma phosphorylation in vascular SMCs.
Cells were treated with serum (0%-10%) and SnPP (20 μM) for 24 hours in the presence and absence of CO (100 ppm) and retinoblastoma expression determined using an antibody that recognizes both the hypophosphorylated (pRb) and hyperphosphorylated (pRb-P) forms of pRb (A) or a phosphorylation-specific pRb antibody (B). Similar findings were observed in 4 separate experiments.
Effect of CO on retinoblastoma phosphorylation in vascular SMCs.
Cells were treated with serum (0%-10%) and SnPP (20 μM) for 24 hours in the presence and absence of CO (100 ppm) and retinoblastoma expression determined using an antibody that recognizes both the hypophosphorylated (pRb) and hyperphosphorylated (pRb-P) forms of pRb (A) or a phosphorylation-specific pRb antibody (B). Similar findings were observed in 4 separate experiments.
Effect of CO on cell cycle progression and cyclin expression in vascular SMCs.
(A) Effect of CO on the distribution of SMCs in the cell cycle. Cells were treated with serum (5%) and SnPP (20 μM) for 24 hours in the presence and absence of CO (200 ppm). Results are means ± SEM of 4 separate experiments. *Statistically significant effect of CO. (B) Effect of CO on the expression of cyclins A, E, and D1 protein. Cells were treated with serum (5%) and SnPP (20 μM) for 24 hours in the presence and absence of CO (200 ppm). Similar findings were observed in 3 separate experiments. (C) Effect of CO on the expression of cyclin A mRNA. Cells were treated with serum (0%-10%) and SnPP (20 μM) for 24 hours in the presence and absence of CO (200 ppm). Similar findings were observed in 3 separate experiments.
Effect of CO on cell cycle progression and cyclin expression in vascular SMCs.
(A) Effect of CO on the distribution of SMCs in the cell cycle. Cells were treated with serum (5%) and SnPP (20 μM) for 24 hours in the presence and absence of CO (200 ppm). Results are means ± SEM of 4 separate experiments. *Statistically significant effect of CO. (B) Effect of CO on the expression of cyclins A, E, and D1 protein. Cells were treated with serum (5%) and SnPP (20 μM) for 24 hours in the presence and absence of CO (200 ppm). Similar findings were observed in 3 separate experiments. (C) Effect of CO on the expression of cyclin A mRNA. Cells were treated with serum (0%-10%) and SnPP (20 μM) for 24 hours in the presence and absence of CO (200 ppm). Similar findings were observed in 3 separate experiments.
Effect of CO on cdk activity and cdk2 protein in vascular SMCs.
Cells were treated with serum (5%) and SnPP (20 μM) for 24 hours in the presence and absence of CO (200 ppm) and cyclin A–associated kinase and cdk2 activity (A) determined as described in “Materials and methods.” The cdk2 protein levels (B) were monitored by Western blotting. Similar findings were observed in 3 separate experiments.
Effect of CO on cdk activity and cdk2 protein in vascular SMCs.
Cells were treated with serum (5%) and SnPP (20 μM) for 24 hours in the presence and absence of CO (200 ppm) and cyclin A–associated kinase and cdk2 activity (A) determined as described in “Materials and methods.” The cdk2 protein levels (B) were monitored by Western blotting. Similar findings were observed in 3 separate experiments.
Discussion
The present study demonstrates that serum induces the expression of the HO-1 gene and that HO-1 acts in a negative feedback manner to limit vascular SMC proliferation. Serum stimulates HO-1 mRNA and protein in a time- and concentration-dependent manner. The serum-mediated up-regulation of HO-1 gene expression is dependent on de novo RNA and protein synthesis and likely involves the transcriptional activation of the gene because serum does not alter the stability of HO-1 mRNA. In addition, the induction of HO-1 by serum is associated with a marked increase in HO activity.
The ability of serum to stimulate HO-1 gene expression is also observed after the administration of PDGF, TGF-β1, or angiotensin II and may represent a mechanism by which mitogens regulate SMC growth.10,18,19 In support of this proposal, we found that serum-stimulated DNA synthesis is augmented in the presence of HO inhibitors, indicating a growth inhibitory role for HO-1. Consistent with this, a recent study26 observed that vascular SMCs from HO-1 null mice exhibit enhanced SMC proliferation and DNA synthesis compared to SMCs from wild-type animals. The antiproliferative effect of HO-1 is likely mediated via the release of CO because the CO scavenger Hb potentiates serum-stimulated DNA synthesis. Moreover, the exogenous administration of CO at concentrations of 100 to 200 ppm can substitute for HO-1 in blocking SMC proliferation. These concentrations of exogenous CO have been demonstrated to be comparable to that produced by HO-1 activity in cultured cells and represent a physiologically relevant concentration.23 24 These results clearly demonstrate that the HO-1–catalyzed release of CO inhibits the mitogenic activity of vascular SMCs.
We also found that CO interacts with various components of the cell cycle machinery. CO inhibits the phosphorylation of pRb, a critical event required for S-phase entry and DNA synthesis.27 The inhibition of pRb phosphorylation was demonstrated by a mobility shift assay where CO blocked the serum-mediated formation of the slower-migrating hyperphosphorylated form of pRb. Moreover, Western blotting using a phosphorylation-specific antibody against pRb directly confirmed the inhibitory effect of CO on pRb phosphorylation. In addition, CO arrests SMCs at the G1/S transition of the cell cycle and selectively inhibits the expression of cyclin A while having no effect on the expression of cyclin D1 and E. Suppression of cyclin A expression by CO also results in the inhibition of cyclin A–associated kinase activity and cdk2 activity, independent of any changes in the level of cdk2 protein. Because cdk2 is a key regulator of both G1- and S-phase cell cycle progression,25 the ability of CO to block cdk2 activity may provide a potent mechanism by which CO inhibits SMC proliferation. Interestingly, the HO-1–catalyzed release of CO also up-regulates the expression of the cdk inhibitor, p21, suggesting that CO may block cdk2 activity via multiple mechanisms.26
In addition to directly blocking SMC growth, CO may also indirectly influence cell growth by regulating the release of growth factors. In this respect, Morita and Kourembanas28 have demonstrated in a coculture system that SMC-derived CO decreases the endothelial synthesis of the vascular mitogens endothelin-1 and PDGF. Moreover, studies in our laboratory and others found that CO inhibits platelet activation, thereby preventing the release of growth factors from platelet α granules.7 29 Thus, CO may exert its antiproliferative action on vascular SMC by several different pathways.
The capacity of growth factors to induce HO-1 and CO synthesis may be of pathophysiologic importance. After local injury of the blood vessel wall, growth factors are released from vascular cells and from activated platelets, and they stimulate the development of a neointima. However, the simultaneous induction of HO-1 and formation of CO by growth factors may limit the extent of SMC proliferation. In support of this proposal, a recent study found that inhibition of HO-1 activity by SnPP exacerbates intimal thickening following balloon injury.30 Alternatively, the induction of HO-1 by gene transfer or hemin administration blocks neointimal formation.26,30-33 In addition to regulating the growth of SMCs, CO may also preserve blood flow at sites of vascular damage by relaxing blood vessels and blocking platelet aggregation.7 11-14 Thus, the HO-1–catalyzed release of CO may provide an important adaptive mechanism to maintain homeostasis at sites of vascular injury.
In conclusion, the present study demonstrates that serum induces HO-1 gene expression in vascular SMC and that HO-1–catalyzed CO formation inhibits SMC growth. In addition, they show that the antiproliferative effect of CO is associated with a decrease in cyclin A expression and cdk2 activity. The ability of growth factors to induce CO synthesis may serve an important negative feedback role to limit SMC proliferation and the development of vascular disease. The HO-1/CO system represents a potentially new therapeutic target for modifying the vascular response to injury.
Supported in part by the National Heart, Lung, and Blood Institute grants HL59976, HL36045, and HL62467, and a grant from the American Heart Association. H.W. is an awardee of the Junior Faculty Scholar Award from the American Society of Hematology. W.D. is an Established Investigator of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William Durante, Houston VA Medical Center, Bldg 109, Rm 130, 2002 Holcombe Blvd, Houston, TX 77030; e-mail:wdurante@bcm.tmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal