The CCAAT enhancer binding protein α (C/EBPα) transcription factor plays a critical role in granulocytopoiesis. Mice with a disruption of the C/EBPα gene demonstrate an early block in granulocytic differentiation, and disruption of C/EBPα function is a common theme in many types of human acute myelogenous leukemia, which is characterized by a block in myeloid development. To characterize further the nature of this block, we derived cell lines from the fetal liver of C/EBPα-deficient animals. These lines resembled morphologically the immature myeloid blasts observed in C/EBPα−/− fetal livers and did not express messenger RNA encoding early myeloid genes such as myeloperoxidase. Similarly, granulocytic markers such as Mac-1 and Gr-1 were not expressed; nor were erythroid and lymphoid surface antigens. Introduction of an inducible C/EBPα gene into the line revealed that conditional expression of C/EBPα induced the C/EBP family members C/EBPβ and C/EBPε and subsequent granulocyte differentiation. Similar results were obtained when C/EBPα−/− cells were stimulated with the cytokines interleukin-3 and granulocyte-macrophage colony-stimulating factor, but not with all-trans retinoic acid, supporting a model of at least 2 pathways leading to the differentiation of myeloid progenitors to granulocytes and implicating induction of other C/EBP family members in granulopoiesis.
Introduction
Recent findings have emphasized the role of lineage-specific transcription factors in regulating the differentiation of multipotential hematopoietic cells to specific lineages.1 2 Understanding the mechanisms of the differentiation of granulocytic cells is particularly relevant to understanding the mechanisms involved in the pathogenesis of acute myelogenous leukemia (AML), in which granulopoietic development is blocked at an early stage.
One transcription factor that has been shown to be especially critical in granulocyte development is C/EBPα. Although C/EBPα is expressed in many tissues, in the hematopoietic system it is specifically expressed in granulocytic cells only.3,4 C/EBPα is expressed in the earliest myeloid cells; subsequently, other C/EBP family members, such as C/EBPβ 3 and C/EBPε,4 are induced during granulocytic differentiation. C/EBPα regulates the promoters of a number of important granulocytic genes, including growth factor receptors such as the granulocyte colony-stimulating factor (G-CSF) receptor5 as well as primary granule proteins such as myeloperoxidase (MPO).6 Introduction of C/EBPα into bipotential human myeloid lines induces granulocytic and blocks monocytic differentiation,4 while expression of C/EBPα but not PU.1 into the murine myeloblast 32D line also leads to granulocytic maturation.7
Recent studies have demonstrated multiple mechanisms of inactivation of C/EBPα function in cells from human patients with AML. For example, a significant number of patients with the French-American-British M1 or M2 subtype harbor transdominant mutations in C/EBPα.8Patients with t(8;21) M2 AML do not harbor C/EBPα mutations, but the AML1/ETO fusion protein encoded by the translocation results in down-regulation of C/EBPα expression.9 Finally, in acute promyelocytic leukemia, C/EBPα is not down-regulated; nor can mutations in C/EBPα be detected.8,9 However, induction of the PML/RARα fusion protein encoded by the t(15;17) translocation in myeloid cell lines results in a marked decrease in C/EBPα DNA binding activity (H.S.R., unpublished results, June 2001), and treatment of acute promyelocytic leukemia cells with all-trans retinoic acid (ATRA) results in induction of first C/EBPβ 10,11 and then C/EBPε 12 as granulocytic differentiation is restored. In summary, these results not only further support the role of C/EBPα in granulocytic development but demonstrate how inactivation of C/EBPα can contribute to the granulocytic block characteristic of AML. Furthermore, they suggest that induction of C/EBPβ and C/EBPε may also play important roles in granulocytic differentiation.
A critical role for the function of C/EBPα in granulopoiesis was demonstrated in mice harboring a disruption of the C/EBPα gene.13 These mice show a selective early block in granulopoiesis, with the appearance of many myeloid blasts in fetal liver and peripheral blood.14 Other lineages, including macrophages, were not affected. These mice had a selective loss of granulocyte colony-forming units and interleukin-6 (IL-6) colony-forming units, which could be explained by the loss of expression of the G-CSF receptor and IL-6 receptor α (IL-6Rα).15,16 In addition, hematopoietic cells from these mice failed to express RNAs encoding primary or secondary neutrophil granule proteins, such as MPO or lactoferrin.16Further studies demonstrated that at least in vitro, restoration of granulocytic differentiation could be effected by administration of the cytokines IL-3 and granulocyte-macrophage CSF (GM-CSF).15These studies led us to propose a model in which at least 2 different molecular pathways, one involving C/EBPα, and one involving these growth factors, could induce granulocytic differentiation of multipotential cells.15
Further characterization of the nature of the granulocytic block in C/EBPα−/− mice has been limited by a number of technical problems. First, because C/EBPα regulates a number of liver genes involved in gluconeogenesis, the animals die shortly after birth from hypoglycemia.13 Therefore, studies of the C/EBPα granulocytic cells are largely limited to fetal liver cells and peripheral blood of newborn mice. In addition, such studies are limited by the heterogeneity of available hematopoietic cells from the fetal livers of these mice. Therefore, to characterize further the nature of the granulocytic block in cells lacking C/EBPα, we derived nontransformed cell lines from C/EBPα fetal livers. Such lines resemble in many aspects the myeloid blasts from C/EBPα−/− fetal livers. Furthermore, like C/EBPα−/− fetal liver cells, either introduction of C/EBPα expression or exposure to the cytokines IL-3 and GM-CSF induces expression of C/EBPβ and C/EBPε and subsequent granulocytic differentiation. In contrast, ATRA fails to induce these other C/EBP family members and, as is the case with C/EBPα−/− mice in vivo,14 fails to induce granulocytic differentiation in the absence of C/EBPα.
Materials and methods
Transduction of C/EBPα−/− fetal liver cells with HOX11
Fetal livers were isolated from pregnant C/EBPα+/− mice (day 16 of gestation). Fetal livers that had no mature granulocytes in Diff-Quick–stained (Allegiance Health Care Laboratory Products, Bedford, MA) touch preps were assumed to be C/EBPα−/−. Tail DNA was isolated to confirm the genotype by Southern blot analysis. Hematopoietic precursors were immortalized by infecting the cells with a retrovirus containing HOX11.17 Erythroid cells were lysed with ACK buffer, and hepatocytes were removed using a Falcon cell strainer. The remaining cells were cocultured overnight with the HOX11 retroviral producer cell line in the presence of 8 μg/mL polybrene in Iscoves modified Dulbecco medium (IMDM) supplemented with 20% heat-inactivated fetal calf serum, 1% WEHI-3B–conditioned medium as a source of IL-3,18 and 10% BHK-MKL as a source of stem cell factor (kindly provided by Schickwann Tsai and Ken Kaushansky). Twenty-four hours later, the supernatant containing the hematopoietic precursors was removed from the adherent producer cell line. These cells were cultured in fresh medium (as described above minus polybrene) for an additional 24 hours, at which time they were selected for retroviral integration with 400 μg/mL hygromycin B. In addition, culturing the cells in 400 μg/mL G418 eliminated any remaining producer cells; because the C/EBPα−/− mice were generated using a targeting construct containing G418 resistance, C/EBPα−/− cells were preferentially selected. After 2 months of continuous culture, individual clones were selected by limiting dilution. We were unable to isolate wild-type or heterozygous lines resembling the C/EBPα−/− lines.
Cell culture conditions and cytokine induction of differentiation
After establishment of C/EBPα−/− clonal lines, cells were grown in IMDM supplemented with 20% fetal calf serum, 1% WEHI-3B–conditioned medium, and 1% BHK-MKL–conditioned medium. To induce granulocytic differentiation with cytokines, both WEHI-3B– and BHK-MKL–conditioned medium were each increased to 10%, and murine GM-CSF (Genetics Institute, Cambridge, MA) was added to a final concentration of 15 ng/mL. Optimal morphologic differentiation was seen after 10 to 12 days. Other agents used in differentiation assays are as follows: recombinant human erythropoietin, 2 U/mL (Epogen, Amgen, Thousand Oaks, CA); recombinant human G-CSF, 2000 U/mL (Neupopgen, Amgen); 1.3 × 10−7 M tetradecanoyl phorbol acetate (TPA, Sigma, St Louis, MO); and 10−5 M ATRA (Sigma).
Southern blot analysis for genotyping
Cells were lysed 5 hours at 55°C in 10 mM Tris (pH 8), 100 mM NaCl, 10 mM ethylenediaminetetraacetic acid, 0.5% sodium dodecyl sulfate (SDS), and 100 μg/mL proteinase K. The DNA was extracted with phenol/chloroform and ethanol-precipitated. A total of 15 μg DNA was digested for 1 hour at 37°C with HincII and separated on a 0.8% agarose gel. DNA was transferred overnight in a solution of 0.4 M NaOH to a Biotrans (+) nylon membrane (ICN, Costa Mesa, CA) and immobilized with a Stratagene UV Stratalinker (La Jolla, CA). The membrane was prehybridized for 2 hours at 65°C in a 0.5 M NaPO4 (pH 7.2), 7% SDS, and 1% bovine serum albumin. Hybridization was performed overnight in the same solution using a random-primed labeled genomic fragment from the C/EBPα gene.13 The membrane was washed twice in 2 × SSC, 0.2% SDS at 65°C for 10 minutes followed by 2 washes in 0.2 × SSC, 0.2% SDS at 65 degrees for 10 minutes.
Analysis of expression of cell surface antigens
Monoclonal antibodies to CD34, Gr-1, c-Kit, Ter-119, Thy1.2, Sca-1, CD8a, CD3, CD4, and streptavidin-phycoerythrin were obtained from PharMingen (San Diego, CA) and to Mac-1 and B220 from Caltag (Burlingame, CA). A total of 5 × 105 cells were washed with phosphate-buffered saline (PBS) and resuspended in PBS/5% bovine serum albumin containing labeled antibodies at concentrations recommended by the manufacturer. The cells were incubated at 4°C for 1 hour, washed in PBS, and analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Establishment of stable cell lines harboring the C/EBPα-ER fusion gene
The stable cell line producing a retrovirus containing a C/EBPα–estrogen receptor (C/EBPα-ER) fusion construct has been previously described.7 19 We infected the 13α−/− line by cocultivation with the producer cells overnight in the presence of 8 μg/mL polybrene in the same growth medium as described above, except phenol red–free IMDM was used to reduce induction of fusion protein function. Twenty-four hours later, the transduced suspension cells were removed from the adherent producer cells. After another 24 hours, 400 μg/mL (active concentration) G418 was added to eliminate any remaining producer cells, and 1 μg/mL puromycin was added to select for retroviral integration. Independent clones were isolated via limiting dilution. Western blot analysis showed the presence of the C/EBPα-ER fusion protein in 15% of the puromycin-resistant clones. One representative clone, 10α−/−αER, was chosen for further study. For induction of functional C/EBPα protein, 10α−/−αER cells were incubated in medium containing 1.25 × 10−6 M β-estradiol, dissolved from a stock of 10 mM β-estradiol in ethanol. After 3 days, the cells were spun and resuspended in fresh β-estradiol–containing medium. Control cultures were treated with vehicle only.
Northern blot analysis
Total RNA was isolated from stably transfected cell line by guanidium extraction (Tri-Reagent, Molecular Research Center, Cincinnati, OH) or by extraction followed by cesium chloride gradients20 and blotted onto Biotrans (+) (ICN). Blots were washed 2 times at 65°C with 1 × SSC/0.5% SDS for 5 minutes, followed by 0.1 × SSC/0.5% SDS twice for 30 minutes. Expression of the MPO gene was detected by a random-primed labeled complementary DNA fragment16 and of the IL-6R by a 1.6-kilobaseSacI fragment of the murine IL-6R complementary DNA.21 As a loading control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) messenger RNA expression was determined with a random-primed labeled 1.3-kilobase PstI fragment of rat GAPDH complementary DNA.22
Western blot analysis of C/EBP proteins
The 13α−/− and 10α−/−αER cells were lysed by resuspending cell pellets in modified RIPA lysis buffer (50 mM Tris-HCl [pH 7.4], 1 mM ethyleneglycotetraacetic acid, 150 mM NaCl, 0.25% sodium deoxycholate, 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, and 1 μg/mL each of pepstatin A, leupeptin, and aprotinin). An equivalent amount of protein was separated on 10% SDS–polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA), blocked in 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween 20 (TBS-T) for 1 hour at room temperature, and then incubated with primary antibodies in TBS-T (with 5% nonfat dry milk) for 1 hour at room temperature. C/EBPα, C/EBPβ, and C/EBPε proteins were detected with rabbit C/EBPα polyclonal serum (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, cat. no. sc-61, recognizing amino acids 253 to 265 of rat C/EBPα); a mouse monoclonal C/EBPβ antibody (1:4000; Santa Cruz Biotechnology, cat. no. sc-7962X, recognizing amino acids 199 to 345 of human C/EBPβ); and a rabbit polyclonal C/EBPε antibody (1:5000; Santa Cruz Biotechnology, cat. no. sc-158X, recognizing a peptide mapping at the carboxyl-terminus of rat C/EBPε); respectively, followed by an antirabbit or antimouse immunoglobulin G–horseradish peroxidase–conjugated secondary antibody (Santa Cruz Biotechnology, cat. no. sc-2004 and sc-2055). In some experiments, C/EBPβ was detected with a polyclonal anti-C/EBPβ antibody (1:3000 dilution of Santa Cruz Biotechnology sc-150X, recognizing amino acids 258 to 276 of rat C/EBPβ), which gave results similar to that of the monoclonal antibody. The retinoic receptor α (RARα) antibody was a rabbit polyclonal antibody (1:4000; Santa Cruz Biotechnology, cat. no. sc-551X, recognizing amino acids 443 to 462 of human RARα1). A monoclonal antimouse β-tubulin antibody served as a loading control (Chemicon International, Temecula, CA).
Results
Establishment of early myeloid cell lines from C/EBPα−/− fetal liver
A number of methods have been employed to isolate nontransformed cell lines from mice with targeted disruption of specific hematopoietic genes. Our initial attempts involved culturing C/EBPα fetal liver cells from C/EBPα−/− mice in the presence of cytokines, such as stem cell factor, IL3, and/or GM-CSF. However, although such methods were recently used by several groups to isolate hematopoietic lines from PU.1-deficient animals,23 24 growth of C/EBPα−/− fetal liver cells under a variety of conditions only yielded mast cell cultures.
We therefore used a second strategy derived from the findings that the HOX11 homeobox-containing transcription factor can immortalize murine hematopoietic precursors.17 25 C/EBPα−/−fetal liver cells were incubated in the presence of HOX11 retrovirus producer lines, and antibiotic selection was used to isolate cells that both contained the C/EBPα knock-out targeting construct and the HOX11 retrovirus as described in “Materials and methods.” We were only able to obtain lines in the presence of low concentrations of stem cell factor and IL-3; culture of HOX11-transduced cells in the absence of added growth factor was unsuccessful. These lines demonstrated a disruption in both alleles of the C/EBPα gene identical to that observed in C/EBPα−/− knock-out mice (data not shown). The results obtained upon further analysis of several independent lines were similar to the ones presented with line 13α−/−described in detail below.
We have reported that the C/EBPα−/− fetal liver cells can be differentiated to morphologically mature granulocytes in vitro in the presence of IL-3 and GM-CSF. To analyze further the mechanism of C/EBPα-dependent and -independent granulocyte differentiation, we derived a cell line from the 13α−/− cells in which we could induce the expression of C/EBPα. Following retroviral transduction of 13α−/− cells with a retrovirus encoding a C/EBPα-ER fusion,7 independent clones were isolated by limiting dilution. Western blot analysis showed the presence of the C/EBPα-ER fusion protein in 15% of the puromycin-resistant clones (Figure 1 and data not shown). One representative clone, 10α−/−αER, was chosen for further study.
Expression of the C/EBPα-ER fusion protein in line 10-αER cells.
Western blot analysis using a polyclonal antibody recognizing C/EBPα was used to detect endogenous C/EBPα or transfected C/EBPα fusion proteins. Lane 1: A U937 line transfected with a metallothionein promoter C/EBPα construct4 as a positive control; lanes 2-4: lysates from fetal liver cells of C/EBPα−/− and C/EBPα+/− animals; lane 5: the 10α-1αER line in which a C/EBPα-ER fusion protein7 has been stably transfected; lane 6: the 13α−/− line. Shown on the right side are apparent molecular weight standards and, on the left, the position of migration of the C/EBPα-ER fusion and endogenous C/EBPα proteins. A number of smaller cross-reacting bands are observed in 10α-1αER; this has been observed previously in lines expressing this plasmid.7 9
Expression of the C/EBPα-ER fusion protein in line 10-αER cells.
Western blot analysis using a polyclonal antibody recognizing C/EBPα was used to detect endogenous C/EBPα or transfected C/EBPα fusion proteins. Lane 1: A U937 line transfected with a metallothionein promoter C/EBPα construct4 as a positive control; lanes 2-4: lysates from fetal liver cells of C/EBPα−/− and C/EBPα+/− animals; lane 5: the 10α-1αER line in which a C/EBPα-ER fusion protein7 has been stably transfected; lane 6: the 13α−/− line. Shown on the right side are apparent molecular weight standards and, on the left, the position of migration of the C/EBPα-ER fusion and endogenous C/EBPα proteins. A number of smaller cross-reacting bands are observed in 10α-1αER; this has been observed previously in lines expressing this plasmid.7 9
C/EBPα−/− cell lines resemble early myeloid blasts similar to those obtained from C/EBPα−/−mice
We first asked whether the 13α−/− cells resembled the early myeloid blasts observed in C/EBPα−/− fetal livers and peripheral blood13 by assessing both cell surface analysis (Figure 2) and morphology (Figure 3). The morphology of both types of cells was very similar, having large nuclei with prominent nucleoli and a relative absence of granules in the cytoplasm (Figure 3A,B).
The C/EBPα−/− and 10α−/−αER lines express cell surface markers similar to that of C/EBPα−/− fetal liver hematopoietic cells.
Cells were analyzed with monoclonal antibodies recognizing the stem cell markers Sca-1, c-Kit, and CD34, and myeloid markers Mac-1 and Gr-1. In each panel, the fluorescence with isotype control is shown as a dashed line and the specific antibody as a solid line along the x-axis (log scale) and relative number of cells on the y-axis. (A) C/EBPα−/− fetal liver hematopoietic cells; (B) 13α−/− cells; (C) 10α−/−αER cells.
The C/EBPα−/− and 10α−/−αER lines express cell surface markers similar to that of C/EBPα−/− fetal liver hematopoietic cells.
Cells were analyzed with monoclonal antibodies recognizing the stem cell markers Sca-1, c-Kit, and CD34, and myeloid markers Mac-1 and Gr-1. In each panel, the fluorescence with isotype control is shown as a dashed line and the specific antibody as a solid line along the x-axis (log scale) and relative number of cells on the y-axis. (A) C/EBPα−/− fetal liver hematopoietic cells; (B) 13α−/− cells; (C) 10α−/−αER cells.
The 13α−/− and 10α−/−αER lines can differentiate into granulocytic and monocytic cells.
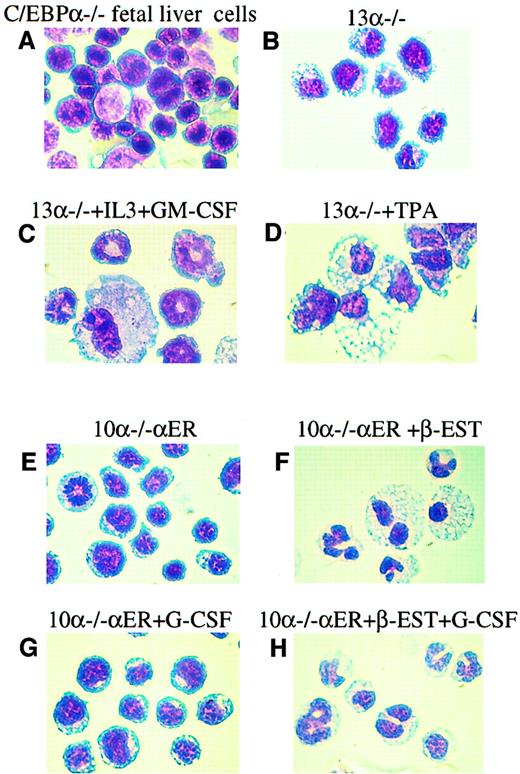
Diff Quick staining of representative cytocentrifuged populations from (A) C/EBPα−/− fetal livers; (B) 13α−/− cells; (C) 13α−/− cells following granulocytic differentiation induced by increased IL-3 and GM-CSF; (D) 13α−/− cells following macrophage differentiation induced by TPA; (E) line 10α−/−αER grown in vehicle only; (F) line 10α−/−αER 6 days after treatment with β-estradiol, which induces nuclear localization of the C/EBPα-ER fusion protein and C/EBPα function7 9; (G) line 10α−/−αER treated with G-CSF only; and (H) line 10α−/−αER treated with β-estradiol and G-CSF. Original magnification, × 100.
The 13α−/− and 10α−/−αER lines can differentiate into granulocytic and monocytic cells.
Diff Quick staining of representative cytocentrifuged populations from (A) C/EBPα−/− fetal livers; (B) 13α−/− cells; (C) 13α−/− cells following granulocytic differentiation induced by increased IL-3 and GM-CSF; (D) 13α−/− cells following macrophage differentiation induced by TPA; (E) line 10α−/−αER grown in vehicle only; (F) line 10α−/−αER 6 days after treatment with β-estradiol, which induces nuclear localization of the C/EBPα-ER fusion protein and C/EBPα function7 9; (G) line 10α−/−αER treated with G-CSF only; and (H) line 10α−/−αER treated with β-estradiol and G-CSF. Original magnification, × 100.
To assess further the characteristics of these cells, we compared surface expression of a number of hematopoietic cell surface markers to C/EBPα−/− fetal liver cells (Figure 2A) by flow cytometry. In terms of markers characteristic of early multipotential progenitors, 13α−/− cells expressed c-Kit but did not express murine CD34 (Figure 2B). The 13α−/− cells were negative for markers of more mature granulocytes, such as Gr-1 (Figure2B). Interestingly, 13α−/− cells were positive for Mac-1, which is highly expressed on mature granulocytes and neutrophils. However, several studies have suggested that Mac-1 can be expressed on early stem cells, is then down-regulated, and then again up-regulated upon terminal myeloid differentiation.26-28Similar results were observed with 10α−/−αER cells stained with these markers (Figure 2C). The 13α−/−cells, as well as 10α−/−αER, were negative for T- and B-cell markers and were negative for the panerythroid marker TER119 (data not shown). Therefore, the cells did not appear to have characteristics of stem cells or multilineage progenitors. We also measured the expression of the hematopoietic markers ER-MP12, ER-MP20, and ER-MP58, which recognize hematopoietic precursors and have previously been used as markers of monocytic differentiation.29-31 We could detect very low levels of ER-MP12, but not ER-MP20 or ER-MP58, on 13α−/− cells, consistent with an early myeloid phenotype (data not shown). In general, the pattern of surface expression of the 13α−/− cells was found to be similar to that of the hematopoietic cells from C/EBPα−/− fetal livers (Figure2A)13 and consistent with a very early myeloid phenotype.
C/EBPα−/− cells are bipotential; they can be specifically induced to either granulocytic or monocytic differentiation with different cytokines
Previously, we had demonstrated that immature myeloid blasts isolated from C/EBPα−/− fetal livers were blocked in granulocytic differentiation in vivo but could be induced to differentiate into granulocytic cells in vitro following treatment with IL-3 and/or GM-CSF.15 Therefore, we asked whether C/EBPα−/− lines could differentiate into mature granulocytes following exposure to higher concentrations of IL-3 and GM-CSF. Ten days after increasing the concentration of WEHI-3B–conditioned medium from 1% to 10% and supplementing the medium with 15 ng/mL recombinant murine GM-CSF, several of the clones, including 13α−/−, demonstrated marked granulocytic differentiation (Figure 3C) and an up-regulation of the granulocytic marker Gr-1 (data not shown). In addition, while 13α−/−cells expressed very low levels of the other C/EBP family members C/EBPβ and C/EBPε, induction of differentiation with IL-3 and GM-CSF resulted in marked increases in both within 48 days, and this increase in C/EBPβ and C/EBPε preceded the morphologic differentiation observed at 10 to 12 days (see below). Very little monocyte/macrophage differentiation was noted in the cultures; most of the cells resembled granulocytes, consistent with induction of Gr-1. Other putative granulocytic inducing agents, such as ATRA or G-CSF alone, failed to induce any differentiation of the cells.
In addition to granulocytic differentiation potential, we were interested in seeing whether these cells could differentiate into other cell types. Attempts to induce erythroid differentiation using erythropoietin in combination with other cytokines were unsuccessful, as were attempts to induce B-cell differentiation using combinations of IL-7 plus growth on stromal cell lines supporting B-cell development, perhaps due to low levels of C/EBPβ.32 In contrast, we were able to induce monocytic differentiation of the C/EBPα−/− cells. TPA is a potent inducer of monocytic differentiation in bipotential myeloid lines, such as HL-60,33 U937,34 and K562.35Therefore, we exposed the 13α−/− cells to 1.3 × 10−7 M TPA, a concentration that induces monocytic differentiation of these lines. After 4 days, the cells showed increased cytoplasm and demonstrated marked monocytic differentiation (Figure 3D), with no morphologic evidence for granulocytic differentiation. These results are consistent with the 13α−/− cells representing a very early bipotential myeloid precursor.
Induced expression of C/EBPα can restore granulocytic differentiation of C/EBPα−/− cells
The fact that increased IL-3 and GM-CSF can induce granulocytic differentiation of 13α−/− cells suggested that their immature granulocytic phenotype was a result of loss of C/EBPα only and not other genetic changes. The 10α−/−αER cells, when grown in the absence of β-estradiol, had a morphology (Figure3E) and surface antigen expression pattern (Figure 2C) similar to that of the parental line 13α−/− as well as to C/EBPα−/− cells derived from C/EBPα−/−fetal livers14 (Figures 2A and 3A). The 10α−/−αER cells were then tested for their ability to differentiate upon induction of functional C/EBPα protein; 1.25 × 10−7 M β-estradiol was added for induction. We have shown that under these conditions the fusion protein can be observed to translocate from the cytoplasm to the nucleus, accompanied by induction of C/EBPα DNA binding activity.9 After 6 days of treatment with β-estradiol, significant morphologic differentiation was seen in the 10α−/−αER cells (Figure 3F). In addition, the cells stained positive for nitroblue tetrazolium (data not shown). Differential counts revealed that, as opposed to the untreated culture, in which no differentiated cells were observed, we could detect 30% bands, 28% segmented granulocytic cells, and 23% monocytic cells in the culture. Therefore, these results indicated that expression of functional C/EBPα protein alone could induce myeloid differentiation of the C/EBPα−/−cells. While G-CSF by itself had no activity (Figure 3G), when used in combination with induction of exogenous C/EBPα expression, granulocytic differentiation was slightly enhanced, with an increase in segmented granulocytes to 42% (Figure 3H).
To assess further the induction of granulocytic differentiation, we also analyzed the expression of myeloid surface antigens in the 10α−/−αER line. In general, the pattern of surface marker expression in uninduced 10α−/−αER cells was similar to that observed in the parental 13α−/− line (Figure 2B-C). Both were CD34− and Gr-1− but Mac-1+. After induction of granulocytic differentiation following treatment with β-estradiol, 10α−/−αER cells demonstrated a dramatic up-regulation of the granulocytic marker Gr-1, similar to 13α−/− cells induced with IL-3 and GM-CSF (data not shown).
To demonstrate that expression of C/EBPα protein in the 10α−/−αER cells could up-regulate C/EBPα target genes, Northern blot analysis was performed before and after β-estradiol treatment. Uninduced 10α−/−αER cells are MPO- and IL-6Rα–negative. After induction, both of these genes are up-regulated (Figure 4A). A similar up-regulation of MPO RNA was noted in 13α−/− cells following granulocytic differentiation induced by IL-3 and GM-CSF (Figure 4B), while treatment of the 13α−/− cells with β-estradiol alone did not lead to differentiation (see below) or induction of IL-6R RNA (data not shown). In addition, like we observed for 13α−/− cells induced with IL-3 and GM-CSF, induction of differentiation of 10α−/−αER cells led to subsequent increases in C/EBPβ and C/EBPε (see below). In summary, expression of functional C/EBPα protein in C/EBPα−/− cells led to induction of a mature myeloid phenotype, as assessed by morphology, gene expression, and induction of cell surface antigens, demonstrating that the immature phenotype observed in C/EBPα−/− cells is a result of loss of C/EBPα alone.
Induction of myeloid gene expression following granulocytic differentiation in C/EBPα-expressing and nonexpressing cells.
(A) The 10α−/−αER cells were differentiated following induction of C/EBPα activity by treatment with β-estradiol for the number of days as indicated above each figure. The Northern blot demonstrates induction of MPO and IL-6Rα. The blot was hybridized to GAPDH (bottom panel) as a control for RNA loading and integrity. (B) The 13α−/− cells were induced with IL-3 and GM-CSF for the number of days as indicated. The Northern blot demonstrates induction of MPO RNA. The blot was hybridized with GAPDH as a loading control as indicated.
Induction of myeloid gene expression following granulocytic differentiation in C/EBPα-expressing and nonexpressing cells.
(A) The 10α−/−αER cells were differentiated following induction of C/EBPα activity by treatment with β-estradiol for the number of days as indicated above each figure. The Northern blot demonstrates induction of MPO and IL-6Rα. The blot was hybridized to GAPDH (bottom panel) as a control for RNA loading and integrity. (B) The 13α−/− cells were induced with IL-3 and GM-CSF for the number of days as indicated. The Northern blot demonstrates induction of MPO RNA. The blot was hybridized with GAPDH as a loading control as indicated.
C/EBPβ and C/EBPε are up-regulated in both C/EBPα-dependent and -independent granulocytic differentiation pathways but not by ATRA
As noted above, 13α−/− cells differentiated in response to restoration of C/EBPα expression. In addition, treatment with IL-3 and GM-CSF also led to granulocytic differentiation in the absence of C/EBPα in vitro, as we had previously observed with C/EBPα−/− fetal liver cells.15 However, treatment with ATRA failed to induce differentiation of C/EBPα−/− cells in vivo14 or in vitro15 and, also, as noted above, did not induce differentiation of 13α−/− cells. The availability of the 13α−/− line allowed us to begin to investigate the molecular mechanisms accounting for the ability of different conditions to induce granulocytic differentiation in the absence of C/EBPα. Increased expression of C/EBPβ by itself can induce marked granulocytic differentiation of multipotential cell lines,11 32D cl3 granulocytic cells,36 or primary human CD34+ cells.44 Induced expression of C/EBPε can also induce granulocytic differentiation of myeloid cell lines.12 37 Therefore, we asked whether ATRA, IL-3 plus GM-CSF, or induction of C/EBPα expression in 13α−/− cells could induce expression of C/EBPβ and/or C/EBPε. As shown in Figure 5A, treatment with ATRA, which did not induce granulocytic differentiation, failed to up-regulate either C/EBPβ or C/EBPε protein levels. This failure to respond to ATRA was not due to decreased or absent RARα levels, because RARα protein was easily detected in untreated 13α−/− cells and was not down-regulated by ATRA (Figure5A). In contrast, treatment of either 13α−/−cells (Figure 5B) or 10α−/−αER cells (data not shown) with IL-3 plus GM-CSF strongly up-regulated C/EBPβ and C/EBPε protein. In addition, induction of C/EBPα expression and granulocytic differentiation of 10α−/−αER cells following treatment with β-estradiol also strongly up-regulated C/EBPε protein, along with C/EBPβ to a lesser degree (Figure 6A). As a negative control for the induction of C/EBPα expression in 10α−/−αER cells, treatment of 13α−/−cells with β-estradiol did not induce the expression of C/EBPβ and C/EBPε and failed to induce granulocyte differentiation (Figure 6B). We conclude that conditions leading to granulocytic differentiation of C/EBPα−/− cells are associated with up-regulation of C/EBPβ and C/EBPε.
Induction of C/EBPβ and C/EBPε protein is associated with induction of C/EBPα-independent granulocytic differentiation but not with ATRA treatment.
C/EBP proteins were detected by Western blot analysis. A monoclonal antibody recognizing β-tubulin was used to control for levels of protein in each lane. Indicated above each blot are the days following induction of granulocytic differentiation with (A) ATRA and (B) IL-3 and GM-CSF. The blots in panel A were exposed longer than those in panel B to visualize C/EBPβ and C/EBPε proteins.
Induction of C/EBPβ and C/EBPε protein is associated with induction of C/EBPα-independent granulocytic differentiation but not with ATRA treatment.
C/EBP proteins were detected by Western blot analysis. A monoclonal antibody recognizing β-tubulin was used to control for levels of protein in each lane. Indicated above each blot are the days following induction of granulocytic differentiation with (A) ATRA and (B) IL-3 and GM-CSF. The blots in panel A were exposed longer than those in panel B to visualize C/EBPβ and C/EBPε proteins.
Up-regulation of C/EBPβ and C/EBPε protein is also associated with C/EBPα-induced granulopoiesis.
C/EBP proteins were detected by Western blot, and β-tubulin was used as a loading control. (A) Whole cell lysates were harvested before and 2, 4, and 6 days after induction of exogenous C/EBPα protein after β-estradiol treatment in 10α−/−αER cells. (B) Whole cell lysates from line 13α−/− before and after β-estradiol and G-CSF treatment. Days of treatment (2, 4, 6, and 8 days) are indicated. The blots in panel B were exposed longer than those in panel A to visualize C/EBPβ and C/EBPε proteins.
Up-regulation of C/EBPβ and C/EBPε protein is also associated with C/EBPα-induced granulopoiesis.
C/EBP proteins were detected by Western blot, and β-tubulin was used as a loading control. (A) Whole cell lysates were harvested before and 2, 4, and 6 days after induction of exogenous C/EBPα protein after β-estradiol treatment in 10α−/−αER cells. (B) Whole cell lysates from line 13α−/− before and after β-estradiol and G-CSF treatment. Days of treatment (2, 4, 6, and 8 days) are indicated. The blots in panel B were exposed longer than those in panel A to visualize C/EBPβ and C/EBPε proteins.
Discussion
A number of studies have implicated the important role of C/EBPα in granulocyte development, including analysis of patterns of expression,3,4 effects of induced expression in cell lines,4,7 knock-out studies,14,15 and studies implicating disruption of C/EBPα in human AML.8 9 A common theme of these studies is that C/EBPα is a key factor in induction of granulocyte differentiation and that loss of C/EBPα function in either knock-out animals or humans with AML leads to an early block in this process. To characterize further the nature of this block in granulocyte maturation, we have isolated and characterized novel myeloid lines derived from C/EBPα−/− fetal livers.
Analysis of these lines has led to additional insights into the nature of cells lacking C/EBPα, which we were unable to perform with cells from C/EBPα−/− mice due to the newborn lethality and/or the heterogeneous nature of fetal liver cells. First, we were unable to induce formation of erythroid or B cells from these lines, and these studies demonstrate that C/EBPα knock-out cells already represent a stage committed to the myeloid lineage. They do not appear to have erythroid, B-, or T-cell properties. This is consistent with the lack of abnormalities in any lineages other than granulocytes in C/EBPα−/− mice. Secondly, the C/EBPα−/−cells appear to be blocked at an early myeloid progenitor stage that is capable of efficient differentiation into monocytic/macrophage cells, consistent with the apparent normal production of peripheral blood monocytes and peritoneal macrophages in C/EBPα−/− mice in vivo.14 Thirdly, reintroduction of a functional C/EBPα protein into the deficient cells led to full granulocytic differentiation, indicating that the defects we observed were indeed a result of the loss of C/EBPα.
We have demonstrated that introduction of wild-type C/EBPα expression alone can induce differentiation of these cells, as assessed by morphology, induction of gene expression, and surface marker expression. This system will facilitate investigations of C/EBPα mutants defective in transactivation or interaction with other proteins.38 39 Such studies will help define whether such mutations affect the ability of C/EBPα to restore differentiation. In addition, these lines will also facilitate evaluation of the differentiation function of C/EBPα mutations found in human patients with AML.
Interestingly, we observed that certain combinations of cytokines (increased IL-3 plus GM-CSF) could induce granulocytic differentiation of C/EBPα cells. This finding was similar to that previously observed in in vitro culture of C/EBPα−/− fetal liver cells.15 Because we have only compared granulocytic cells induced by these 2 pathways (C/EBPα vs cytokine induction) in terms of morphology, surface marker expression, and induction of C/EBP proteins, it is still possible that they differ in important ways, and further functional studies will be required to determine if there are differences. In addition, as in cells derived from C/EBPα−/− mice, we did not observe significant differentiation in response to retinoic acid or G-CSF, consistent with other reports suggesting that these factors do not play a major role in granulocytic differentiation.40-42 The observation that IL-3 plus GM-CSF could induce differentiation is consistent with our previous model, in which we propose that there may be 2 distinct pathways leading to granulocytic differentiation of bipotential myeloid progenitors.15 One pathway, involving the expression of C/EBPα, is absolutely essential and nonredundant, at least at the newborn stage of life. In addition, conditional disruption of C/EBPα suggests that this requirement extends into the adult mouse.43 The other pathway, involving the cytokines IL-3 and GM-CSF, has thus far only been shown to work in in vitro culture systems. Although the mechanism of induction of granulocytic differentiation involving IL-3 and GM-CSF remains unknown, in these studies we demonstrate that “rescue” of C/EBPα−/−cells by either expression of exogenous C/EBPα or by cytokines is accompanied by expression of C/EBPβ and C/EBPε, while treatment with ATRA is not (Figure 5A). While it is possible that induction of C/EBPβ and C/EBPε are only markers of differentiation, the fact that expression of either one can induce granulocytic differentiation suggests that they play a deterministic role.11,12,36 37Future studies will be directed at determining whether induction of these other C/EBP family members is critical for granulocytic differentiation as well as the mechanisms involved in their up-regulation in response to expression of C/EBPα or stimulation by cytokines. The availability of the C/EBPα−/− cell lines described in this paper will greatly facilitate these mechanistic studies.
We thank S. Orkin for suggesting the use of HOX11 to immortalize C/EBPα knock-out cells; R. Hawley for providing HOX11 retrovirus–producing cell lines; P. Leenen and M. de Bruijn for ER-MP antibodies; members of the Tenen laboratory for their support; and M. Singleton and A. Lugay for assistance in preparation with the manuscript.
Supported by grants HL56745 and CA72009 (D.G.T.). E.N. is a Fellow of the Leukemia and Lymphoma Society.
P.Z. and E.N. are dual first authors of this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Daniel G. Tenen, Harvard Institutes of Medicine, Rm 954, 77 Ave Louis Pasteur, Boston, MA 02115; e-mail:dtenen@caregroup.harvard.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal