In this multicenter retrospective study, the outcomes of 234 patients with myelodysplastic syndrome (MDS) who underwent transplantation between 1995 and 1999 from HLA-identical siblings were analyzed according to the hematopoietic stem cell source used, that is, bone marrow (BM, n = 132) or granulocyte colony-stimulating factor–mobilized peripheral blood progenitor cells (PBPCs, n = 102). There were 69 cases of refractory anemia (RA), 86 RA with excess blasts (RAEB), 75 RAEB in transformation (RAEB-t), and 4 unclassified MDS at diagnosis. The International Prognostic Scoring System was intermediate-2 or high in 104 of the 158 available scores. Multivariate analyses focused on transplantation-related mortality (TRM), 2-year treatment failure incidence, and survival. Use of PBPCs reduced the median duration of neutropenia and thrombocytopenia by 4 and 12 days, respectively. The incidence of acute GVHD was similar whatever the graft type used. Chronic GVHD was more likely to have occurred with PBPCs (odds ratio [OR], 1.62; 95% confidence interval [CI], 0.87-3.02). Two-year TRM was significantly reduced with PBPCs (relative risk [RR], 0.33; 95% CI, 0.15-0.73; P < .007), except for patients who had either RA or high-risk cytogenetics. The 2-year treatment failure incidence was significantly decreased with PBPCs, from 38% to 13% (RR, 0.22; 95% CI, 0.10-0.48;P < .001). Estimate of the 2-year event-free survival was 50% with PBPCs versus 39% with BM. In multivariate analysis, the outcome was significantly improved with PBPCs (RR, 0.27; 95% CI, 0.13-0.52; P < .001), except for patients with either RA or high-risk cytogenetics. In conclusion, PBPCs might be preferred for allogeneic transplantation in MDS patients at high risk for relapse on the basis of morphologic criteria because the use of this hematopoietic stem cell was associated with lower treatment failure incidence and improved survival.
Introduction
Myelodysplastic syndromes (MDS) represent a heterogeneous group of clonal disorders characterized by ineffective hematopoiesis and the propensity to progress to acute myeloid leukemia.1 Assessment of prognosis in patients with newly diagnosed and untreated MDS is mainly based on the number of severe cytopenia, the percentage of marrow blasts, and the cytogenetic characteristics.2 According to the International Prognostic Scoring System (IPSS), patients in the intermediate-2 or high-risk subgroup have a median survival ranging from 5 to 14 months, whereas median survival ranges from 3.5 to 5.7 years in the low or intermediate-1 risk subgroup.2 Allogeneic stem cell transplantation (SCT) is a curative approach that can be proposed if an HLA-matched donor is available and the patient is younger than 55 years of age.3,4 However, transplantation-related mortality (TRM) is high, ranging from 37% to 68% in the largest studies reporting on SCT from HLA-identical siblings.3,4 Moreover, approximately one third of the patients will have relapses, with a risk of recurrence mainly depending on the percentage of marrow blasts and the presence of cytogenetic abnormalities before transplantation.3-5 Because of these high TRM and relapse rates, the disease-free survival is usually below 50%.3,4Recipient age, time interval from diagnosis to transplantation, number of severe cytopenia, cytogenetic abnormalities, percentage of marrow blasts, disease stage, and French-American-British (FAB) classification at transplantation, as well as the intensity of the conditioning regimen and the use of T-cell depletion, are the main predictors of posttransplantation outcome.3-12
The impact of the hematopoietic stem cell source used for transplantation has never been investigated in the setting of allogeneic SCT for MDS. In chronic myeloid and acute leukemia, the use of granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood progenitor cells (PBPCs) has been associated with faster hematopoietic recovery when compared with bone marrow.13-20 In some studies, it has also been shown that the incidence of chronic graft-versus-host disease (GVHD) was significantly increased with the use of PBPCs.14-16,20-24Regarding other usual end points, data from prospective and retrospective series are controversial. Therefore, it has not yet been clearly demonstrated that the use of PBPCs has a significant advantage over bone marrow in terms of TRM, treatment failure, or survival.14-25
In this retrospective, multicenter study, we assessed the impact of using PBPCs rather than bone marrow (BM) as a source of hematopoietic stem cells on the outcome of patients receiving HLA-identical sibling transplants for MDS.
Patients, materials, and methods
Patient and transplantation characteristics
Included in the study were 234 patients, from 72 centers, with a primary diagnosis of either refractory anemia with or without ring sideroblasts (RA+RARS, n = 67 + 2; BM, 44; PBPC, 25), RA with excess of blasts (RAEB, n = 86; BM, 40; PBPC, 46), RAEB in transformation (RAEB-t, n = 75; BM, 44; PBPC, 31), or unclassified MDS (n = 4, all in the BM group).26 Patients with chronic myelomonocytic leukemia and those who had evolved into secondary acute leukemia after diagnosis were excluded. Only patients who underwent transplantation between January 1995 and December 1999 with HLA-identical siblings were included because there was no allogeneic PBPC transplantation recorded before 1995 in the European Group for Blood and Marrow Transplantation registry. Patients who received BM together with PBPCs or PBPCs alone for a second transplantation were not considered. Therefore, the graft was BM hematopoietic stem cells in 132 patients and G-CSF–mobilized PBPCs in 102 patients. Main characteristics of the patients are summarized in Table 1, according to the hematopoietic stem cell source used, and the IPSS score at diagnosis. Median recipient age at transplantation was 41 years for the BM group (range, 4-59 years) and 47 years for the PBPC group (range, 12-60 years;P < .001). Regarding the patients for whom the IPSS score was available (n = 155), the percentages of patients with intermediate-2 or high scores were not significantly different between the BM and PBPC groups (66% in both groups). Thirty-three patients had evolved toward a worse prognostic FAB group after diagnosis, from RA to RAEB (n = 9), RAEB to RAEB-t (n = 20), or unclassified MDS to RAEB or RAEB-t (n = 4). Therefore, disease morphology according to the FAB classification was also considered before high-dose chemotherapy—if complete remission (CR) was achieved—or transplantation if the patient did not receive such treatment or was no longer in CR. At this “last disease evaluation,” 60 patients had RA (BM, 41; PBPC, 19; 31% vs 19%; P = .04), 77 patients had RAEB (BM, 40; PBPC, 37;P = NS), and 97 patients had RAEB-t (BM, 51; PBPC, 46;P = NS). There were significantly more patients with RA at last disease evaluation in the BM group than in the PBPC group (31% vs 19%; P = .04). One hundred three patients did not receive high-dose (ie, remission-induction) chemotherapy before SCT, and they accounted for the “untreated group” (RA, 46%; RAEB, 32%; RAEB-t, 22%). At last disease evaluation, significantly more patients with RA (78%) than patients with RAEB (43%) or RAEB-t (24%) underwent transplantation without any prior chemotherapy (P < .001). One hundred thirty-one patients received high-dose chemotherapy before SCT, of whom 70 patients (RA, 3%; RAEB, 30%; RAEB-t, 67%) achieved a first hematologic CR. The remaining 61 patients accounted for the “treated, not in CR” group because they were either in partial response or they had stable or progressive disease after chemotherapy (RA, 18%; RAEB, 38%; RAEB-t, 44%). Cytogenetic data were available in 155 patients. High-risk karyotypes (ie, chromosome 7 or multiple abnormalities) were more frequently observed in patients with “treated, not in CR” stage MDS (39% vs 20%; P < .016). Characteristics of the transplantation procedures are listed in Table 1 according to the hematopoietic stem cell source used and the IPSS score at diagnosis.
Patient characteristics and transplantation procedures according to graft type and IPSS
| Covariates . | BM . | PBPC . | P . | Low + Int-1 . | Int-2 + High . | NA . | P . |
|---|---|---|---|---|---|---|---|
| Entire group | 132 | 102 | 54 | 104 | 76 | ||
| Recipient sex | |||||||
| Male | 70 | 62 | NS | 32 | 51 | 49 | NS |
| Female | 62 | 40 | 22 | 53 | 27 | ||
| Age at SCT, y | |||||||
| 35 or younger | 42 | 16 | .004 | 12 | 28 | 18 | NS |
| Older than 35 | 90 | 86 | 42 | 76 | 58 | ||
| Time from diagnosis to SCT | |||||||
| 6 months or less | 68 | 55 | NS | 15 | 65 | 31 | < .001 |
| More than 6 months | 64 | 47 | 39 | 39 | 45 | ||
| FAB classification at diagnosis | |||||||
| RA | 44 | 25 | .08 | 40 | 11 | 18 | < .001 |
| RAEB | 40 | 46 | 11 | 44 | 31 | ||
| RAEB-t | 44 | 31 | 3 | 46 | 26 | ||
| Unclassified | 4 | 0 | 0 | 3 | 1 | ||
| Last disease evaluation | |||||||
| RA | 41 | 19 | NS | 34 | 9 | 17 | < .001 |
| RAEB | 40 | 37 | 13 | 38 | 26 | ||
| RAEB-t | 51 | 46 | 7 | 59 | 31 | ||
| Disease stage at SCT | |||||||
| Complete remission | 42 | 28 | NS | 2 | 40 | 28 | < .001 |
| Untreated | 57 | 46 | 49 | 36 | 18 | ||
| Treated, not in CR | 33 | 28 | 3 | 28 | 30 | ||
| Marrow blasts, less than 10% | 102 | 70 | NS | 45 | 74 | 53 | NS |
| Marrow blasts, 10% or more | 22 | 24 | 9 | 26 | 11 | ||
| Cytogenetics | |||||||
| High-risk cytogenetics | 24 | 14 | NS | 1 | 33 | 4 | < .001 |
| Other cytogenetics | 65 | 52 | 51 | 52 | 14 | ||
| Missing | 43 | 36 | 2 | 19 | 58 | ||
| Donor sex | |||||||
| Male | 74 | 64 | NS | 37 | 61 | 40 | NS |
| Female | 58 | 38 | 17 | 43 | 36 | ||
| Donor/recipient sex match | |||||||
| Female-male | 27 | 20 | NS | 10 | 17 | 20 | NS |
| Other combinations | 105 | 82 | 44 | 87 | 56 | ||
| Nucleated cell dose infused/kg | |||||||
| Less than 3 × 108/kg | 45 | 1 | < .001 | 14 | 24 | 8 | NS |
| 3 × 108/kg or more | 44 | 58 | 19 | 42 | 41 | ||
| Total body irradiation | |||||||
| No | 60 | 69 | < .001 | 30 | 51 | 48 | NS |
| Yes | 71 | 33 | 24 | 53 | 27 | ||
| T-cell depletion | |||||||
| No | 106 | 75 | NS | 35 | 79 | 67 | NS |
| Yes | 26 | 27 | 19 | 25 | 9 | ||
| GVHD prophylaxis | |||||||
| CsA alone | 7 | 5 | NS | 7 | 3 | 2 | NS |
| CsA + corticosteroids | 0 | 1 | 1 | 0 | 0 | ||
| CsA + MTX | 62 | 38 | 40 | 22 | 38 | ||
| CsA + MTX + corticosteroids | 9 | 6 | 10 | 4 | 1 | ||
| With anti–T-cell serotherapy | 1 | 3 | 3 | 1 | 0 | ||
| Other | 2 | 0 | 0 | 1 | 1 | ||
| Missing | 25 | 21 | 18 | 4 | 24 |
| Covariates . | BM . | PBPC . | P . | Low + Int-1 . | Int-2 + High . | NA . | P . |
|---|---|---|---|---|---|---|---|
| Entire group | 132 | 102 | 54 | 104 | 76 | ||
| Recipient sex | |||||||
| Male | 70 | 62 | NS | 32 | 51 | 49 | NS |
| Female | 62 | 40 | 22 | 53 | 27 | ||
| Age at SCT, y | |||||||
| 35 or younger | 42 | 16 | .004 | 12 | 28 | 18 | NS |
| Older than 35 | 90 | 86 | 42 | 76 | 58 | ||
| Time from diagnosis to SCT | |||||||
| 6 months or less | 68 | 55 | NS | 15 | 65 | 31 | < .001 |
| More than 6 months | 64 | 47 | 39 | 39 | 45 | ||
| FAB classification at diagnosis | |||||||
| RA | 44 | 25 | .08 | 40 | 11 | 18 | < .001 |
| RAEB | 40 | 46 | 11 | 44 | 31 | ||
| RAEB-t | 44 | 31 | 3 | 46 | 26 | ||
| Unclassified | 4 | 0 | 0 | 3 | 1 | ||
| Last disease evaluation | |||||||
| RA | 41 | 19 | NS | 34 | 9 | 17 | < .001 |
| RAEB | 40 | 37 | 13 | 38 | 26 | ||
| RAEB-t | 51 | 46 | 7 | 59 | 31 | ||
| Disease stage at SCT | |||||||
| Complete remission | 42 | 28 | NS | 2 | 40 | 28 | < .001 |
| Untreated | 57 | 46 | 49 | 36 | 18 | ||
| Treated, not in CR | 33 | 28 | 3 | 28 | 30 | ||
| Marrow blasts, less than 10% | 102 | 70 | NS | 45 | 74 | 53 | NS |
| Marrow blasts, 10% or more | 22 | 24 | 9 | 26 | 11 | ||
| Cytogenetics | |||||||
| High-risk cytogenetics | 24 | 14 | NS | 1 | 33 | 4 | < .001 |
| Other cytogenetics | 65 | 52 | 51 | 52 | 14 | ||
| Missing | 43 | 36 | 2 | 19 | 58 | ||
| Donor sex | |||||||
| Male | 74 | 64 | NS | 37 | 61 | 40 | NS |
| Female | 58 | 38 | 17 | 43 | 36 | ||
| Donor/recipient sex match | |||||||
| Female-male | 27 | 20 | NS | 10 | 17 | 20 | NS |
| Other combinations | 105 | 82 | 44 | 87 | 56 | ||
| Nucleated cell dose infused/kg | |||||||
| Less than 3 × 108/kg | 45 | 1 | < .001 | 14 | 24 | 8 | NS |
| 3 × 108/kg or more | 44 | 58 | 19 | 42 | 41 | ||
| Total body irradiation | |||||||
| No | 60 | 69 | < .001 | 30 | 51 | 48 | NS |
| Yes | 71 | 33 | 24 | 53 | 27 | ||
| T-cell depletion | |||||||
| No | 106 | 75 | NS | 35 | 79 | 67 | NS |
| Yes | 26 | 27 | 19 | 25 | 9 | ||
| GVHD prophylaxis | |||||||
| CsA alone | 7 | 5 | NS | 7 | 3 | 2 | NS |
| CsA + corticosteroids | 0 | 1 | 1 | 0 | 0 | ||
| CsA + MTX | 62 | 38 | 40 | 22 | 38 | ||
| CsA + MTX + corticosteroids | 9 | 6 | 10 | 4 | 1 | ||
| With anti–T-cell serotherapy | 1 | 3 | 3 | 1 | 0 | ||
| Other | 2 | 0 | 0 | 1 | 1 | ||
| Missing | 25 | 21 | 18 | 4 | 24 |
CsA indicates cyclosporin A; MTX, methotrexate.
P values for Low + Int-1 vs Int-2 + High IPSS.
End point definitions
End points were assessed on the date of last patient contact and were analyzed on January 1, 2001. Median follow-up time from transplantation was 1 year for the overall group (60 patients with a minimum follow-up of 2 years after transplantation), without any significant difference between the BM (median, 396 days; range, 14 days to 4.7 years) and the PBPC (median, 359 days; range, 8 days to 4.7 years) groups. Median follow-up time for the patients alive at last news was 517 days in the PBPC group (range, 51 days to 4.7 years) and 728 days in the BM group (range, 71 days to 4.7 years). Analysis focused on hematopoietic recovery, acute and chronic GVHD, TRM, treatment failure incidence (TFI), event-free survival (EFS), and overall survival (OS). The date of neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count above 0.5 × 109/L. The date of platelet recovery was defined as the first of 7 consecutive days with a platelet count higher than 50 × 109/L without transfusion. Acute and chronic GVHD were graded according to the Seattle criteria.27 28Analysis of chronic GVHD included patients who had neutrophil recovery and survived longer than 90 days from transplantation; cases were coded as absent, limited, or extensive. Assessment of TRM was performed by day 100 (early TRM) and by 2 years after transplantation (late TRM). Relapsed disease and refractory disease were defined according to cytologic criteria and both were considered treatment failures. Second transplantation for graft failure or rejection, treatment failure and death were the events defined to estimate the event-free survival (EFS). All deaths were considered when the OS was estimated.
Statistical methods
The probabilities of hematopoietic recovery, GVHD, TRM, TFI, EFS, and OS were estimated from the time of transplantation, according to the Kaplan-Meier product limit method.29 Groups were compared using the 2-tailed log-rank test. P-spline method and penalized Cox model were used to define the most appropriate cut-off value(s) for continuous covariates.30 In addition to the source of hematopoietic stem cell used for transplantation, the following covariates were analyzed in univariate analysis: recipient age at transplantation (continuous covariate, 35 years and younger vs older than 35 years, and 50 years and younger vs older than 50 years), time interval between diagnosis and transplantation (continuous covariate, and 6 months and less vs more than 6 months), recipient sex, disease morphology at diagnosis and at last disease evaluation (RA vs RAEB vs RAEB-t), IPSS score at diagnosis (low + intermediate-1 vs intermediate-2 + high, low vs others, high vs others), cytogenetics (high-risk karyotypes vs other karyotypes), marrow blast percentage before transplantation (continuous covariate, less than 10% vs 10% or more), disease stage at transplantation (CR vs untreated disease vs treated but not in CR), donor sex, recipient–donor sex match, conditioning regimen (with vs without total body irradiation), T-cell depletion, and speed of neutrophil recovery (continuous covariate, and 14 days or less vs more than 14 days, and 21 days or less vs more than 21 days). For multivariate analysis, covariates found significant at P < .10 in univariate analysis were introduced in the Cox proportional hazards models31 and were selected through a stepwise procedure. Graft type was held in the model at each step. Potential interactions between the covariate graft type and the other covariates were tested adding cross-product terms to the model. Grade II-IV acute GVHD was introduced in the final models—for TRM, TFI, EFS, and OS—as a time-dependent covariate. Departure from the proportional hazards assumption was assessed using methods based on partial residuals and a graphical approach.32 If the proportional hazards assumption did not hold for a covariate, stratified or extended Cox models with time-dependent covariates were used. When groups were compared according to continuous covariates, the Mann-Whitney U test or Kruskal-Wallis one-way analysis of variance on ranks test were used for differences in medians. According to the group sizes, χ2 analysis or Fisher exact test was used to compare categorical covariates. S Plus 2000 Professional 3 (Mathsoft, Seattle, WA) was used for all statistical analysis.
Results
Hematopoietic recovery
Neutrophil recovery occurred in all but 14 patients (7 in the BM group and 7 in the PBPC group; P = .60). Median time to reach an absolute neutrophil count above 0.5 × 109/L was 14 days in the PBPC group (range, 7-43 days) compared with 18 days in the BM group (range, 9-36 days;P < .001). The use of PBPCs resulted in a higher probability to reach the neutrophil recovery end point by day 14 (RR, 4.07; 95% CI, 2.21-7.48) and by day 21. In a multivariate Cox model, the use of PBPCs was the most powerful predictor of early neutrophil recovery by day 14 (RR, 4.28; 95% CI, 2.53-7.24) and day 21. Data about the time required for platelet recovery were available in 164 patients (PBPC, n = 80; BM, n = 84). Median time to achieve a platelet count above 50 × 109/L was 16 days in the PBPC group (range, 11-211 days) compared with 28 days in the BM group (range, 15-258 days). In multivariate analysis, the use of PBPCs remained associated with an improved platelet recovery by day 30 (RR, 3.21; 95% CI, 2.15-4.80).
Acute GVHD
Regarding the overall group of patients, the incidence of grade II-IV acute GVHD was not significantly different when comparing the PBPC and BM groups (Kaplan-Meier estimates, 35% with PBPC vs 31% with BM; RR, 1.11; 95% CI, 0.68-1.83). Furthermore, no significant difference was observed between the PBPC and BM groups when patients who received unmanipulated (Kaplan-Meier estimates, 43% with PBPC vs 33% with BM; RR, 1.37; 95% CI, 0.80-2.34) or T-cell–depleted grafts (Kaplan-Meier estimates, 13% with PBPC vs 24% with BM; RR, 0.49; 95% CI, 0.12-1.96) were separately analyzed. The occurrence of grade II-IV acute GVHD was affected by the speed of neutrophil recovery, with a plot of spline fit term showing an increased risk for acute GVHD with neutrophil recovery achieved during the first 3 weeks after transplantation. Patients who achieved neutrophil recovery before day 22 had an estimated grade II-IV acute GVHD incidence of 37% compared with 21% when neutrophil recovery was achieved later (RR, 2.07; 95% CI, 0.98-4.37; P = .055). Regarding grade III-IV acute GVHD incidence, no significant difference was observed between the PBPC and BM groups (Kaplan-Meier estimates, 16% with PBPC vs 14% with BM; RR, 1.15; 95% CI, 0.56-2.37). In multivariate analyses, the hematopoietic stem cell source used for transplantation did not significantly affect the incidence of grade II-IV (RR, 1.10; 95% CI, 0.66-1.86) or grade III-IV acute GVHD (RR, 0.91; 95% CI, 0.46-1.98).
Chronic GVHD
Data concerning chronic GVHD were available in 167 of the 182 (92%) evaluable patients. Chronic GVHD occurred in 58% of the patients in the PBPC group (62% for unmanipulated PBPCs) and in 46% of those who received BM grafts (51% for unmanipulated BM) (OR, 1.62; 95% CI, 0.87-3.02). Of note, patients who achieved neutrophil recovery before day 22 more frequently acquired chronic GVHD than those who reached the neutrophil recovery end point later (OR, 2.38; 95% CI, 1.13-4.98). Regardless of whether posttransplantation covariates were introduced in the logistic regression model, the use of PBPCs was not significantly associated with an increased risk for chronic GVHD in multivariate analysis (without post-SCT covariates: OR, 1.17; 95% CI, 0.59-2.31) (with post-SCT covariates: OR, 0.99; 95% CI, 0.45-2.15). Extensive chronic GVHD was observed in 14% of the patients in the PBPC group (16% for unmanipulated PBPCs) compared with 23% of those in the BM group (25% for unmanipulated BM) (OR, 0.55; 95% CI, 0.24-1.25).
Early transplantation-related mortality
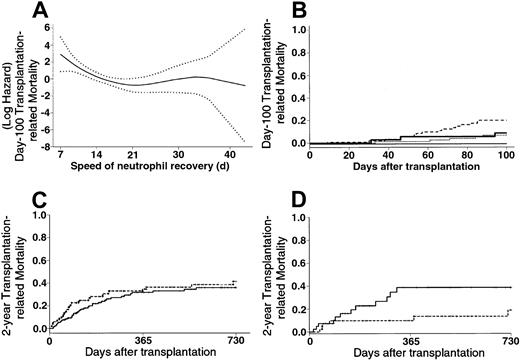
The use of PBPCs was associated with a 10% increased early TRM when considering the overall group of patients in univariate analysis (Kaplan-Meier estimates: 23% with PBPCs vs 13% with BM; RR, 1.82; 95% CI, 0.97-3.40). Early neutrophil recovery was associated with increased early TRM, especially when PBPCs were used (Figure1A-B). Regarding patients who acquired grade II-IV acute GVHD, those who experienced neutrophil recovery during the first 2 weeks after transplantation had a significantly higher early TRM rate than those who experienced neutrophil recovery after day 14 (Kaplan-Meier estimates, 46% vs 11%; RR, 5.31; 95% CI, 1.86-15.10). After adjusting for other significant covariates in a multivariate Cox model, the use of PBPCs was not significantly associated with increased early TRM (Table2).
TRM.
(A) Evolution of the early TRM risk according to the speed of neutrophil recovery (···· indicates 95% confidence interval of the risk). An increasing risk for transplantation-related death was observed when neutrophil recovery was rapidly achieved, that is, before day 22. (B) Patients who received BM grafts had early TRM of 7% and 9% if they reached an absolute neutrophil count greater than or equal to .5 × 109/L before day 22 (· · · ·) and thereafter ( ), respectively. Patients who received PBPCs had early TRM of 21% and 0% if they had reached an absolute neutrophil count greater than or equal to .5 × 109/L before day 22 (---) and thereafter (—), respectively. (C) Patients who received BM grafts (
), respectively. Patients who received PBPCs had early TRM of 21% and 0% if they had reached an absolute neutrophil count greater than or equal to .5 × 109/L before day 22 (---) and thereafter (—), respectively. (C) Patients who received BM grafts ( ) had late TRM of 36% compared with 42% for those who received PBPCs (---) (P = .32). (D) Patients with RAEB or RAEB-t at last disease evaluation and no high-risk cytogenetics (n = 82) had a 2-year TRM of 19% with PBPCs (---) compared with 39% with BM (
) had late TRM of 36% compared with 42% for those who received PBPCs (---) (P = .32). (D) Patients with RAEB or RAEB-t at last disease evaluation and no high-risk cytogenetics (n = 82) had a 2-year TRM of 19% with PBPCs (---) compared with 39% with BM ( ) (P < .05).
) (P < .05).
TRM.
(A) Evolution of the early TRM risk according to the speed of neutrophil recovery (···· indicates 95% confidence interval of the risk). An increasing risk for transplantation-related death was observed when neutrophil recovery was rapidly achieved, that is, before day 22. (B) Patients who received BM grafts had early TRM of 7% and 9% if they reached an absolute neutrophil count greater than or equal to .5 × 109/L before day 22 (· · · ·) and thereafter ( ), respectively. Patients who received PBPCs had early TRM of 21% and 0% if they had reached an absolute neutrophil count greater than or equal to .5 × 109/L before day 22 (---) and thereafter (—), respectively. (C) Patients who received BM grafts (
), respectively. Patients who received PBPCs had early TRM of 21% and 0% if they had reached an absolute neutrophil count greater than or equal to .5 × 109/L before day 22 (---) and thereafter (—), respectively. (C) Patients who received BM grafts ( ) had late TRM of 36% compared with 42% for those who received PBPCs (---) (P = .32). (D) Patients with RAEB or RAEB-t at last disease evaluation and no high-risk cytogenetics (n = 82) had a 2-year TRM of 19% with PBPCs (---) compared with 39% with BM (
) had late TRM of 36% compared with 42% for those who received PBPCs (---) (P = .32). (D) Patients with RAEB or RAEB-t at last disease evaluation and no high-risk cytogenetics (n = 82) had a 2-year TRM of 19% with PBPCs (---) compared with 39% with BM ( ) (P < .05).
) (P < .05).
Transplantation-related mortality, treatment failures, and survival: multivariate analyses
| Covariates . | Relative risk . | 95% CI . | P . |
|---|---|---|---|
| Cox models fitted without posttransplantation covariates | |||
| Early TRM | |||
| PBPC | 1.63 | 0.87 -3.08 | .13 |
| Recipient age 35 or younger | 0.33 | 0.11 -0.93 | .036 |
| T-cell depletion | 0.34 | 0.12 -0.95 | .039 |
| Late TRM | |||
| PBPC | 0.48 | 0.23 -1.00 | .05 |
| Recipient age 35 or younger | 0.23 | 0.09 -0.60 | .002 |
| RA at last disease evaluation | 0.47 | 0.19 -1.13 | .092 |
| High-risk cytogenetics | 1.91 | 1.02 -3.58 | .044 |
| Interaction PBPC × RA at last evaluation | 6.66 | 1.78 -24.83 | .002 |
| Treatment failure | |||
| PBPC | 0.22 | 0.10 -0.48 | < .001 |
| RA at last disease evaluation | 0.19 | 0.07 -0.55 | .002 |
| Treated, not in CR MDS | 2.12 | 1.15 -3.91 | .016 |
| Event-free survival | |||
| PBPC | 0.27 | 0.13 -0.52 | < .001 |
| Recipient age 35 or younger | 0.42 | 0.24 -0.76 | .004 |
| RA at last disease evaluation | 0.41 | 0.21 -0.78 | < .007 |
| Treated, not in CR MDS | 1.86 | 1.13 -3.06 | .016 |
| High-risk cytogenetics | 1.17 | 0.62 -2.19 | .63 |
| Interaction PBPC × RA at last evaluation | 5.47 | 1.84 -16.22 | .002 |
| Interaction PBPC × high-risk cytogenetics | 3.07 | 1.10 -8.63 | .033 |
| Cox models fitted with posttransplantation covariates | |||
| Early TRM | |||
| PBPC | 1.59 | 0.69 -3.62 | .27 |
| ANC ≥ 0.5 × 109/L before day 15 | 2.62 | 1.14 -6.03 | .024 |
| Grade II-IV acute GVHD | 4.63 | 1.93 -11.12 | < .001 |
| Late TRM | |||
| PBPC | 0.33 | 0.15 -0.73 | < .007 |
| Recipient age 35 or younger | 0.26 | 0.11 -0.63 | < .003 |
| RA at last disease evaluation | 0.47 | 0.20 -1.11 | .084 |
| High-risk cytogenetics | 0.93 | 0.37 -2.31 | .87 |
| Acute GVHD | 2.69 | 1.44 -5.00 | < .002 |
| Interaction PBPC × RA at last evaluation | 6.66 | 1.78 -24.83 | < .005 |
| Interaction PBPC × high-risk cytogenetics | 4.35 | 1.20 -15.71 | .025 |
| Event-free survival | |||
| PBPC | 0.17 | 0.08 -0.37 | < .001 |
| Recipient age 35 or younger | 0.49 | 0.28 -0.85 | .011 |
| RA at last disease evaluation | 0.42 | 0.24 -0.76 | < .004 |
| High-risk cytogenetics | 0.96 | 0.54 -1.70 | .88 |
| Treated, not in CR MDS | 2.15 | 1.37 -3.40 | < .001 |
| Grade II-IV acute GVHD | 0.82 | 0.44 -1.55 | .55 |
| Interaction PBPC × RA at last evaluation | 5.20 | 1.68 -16.05 | .004 |
| Interaction PBPC × high-risk cytogenetics | 4.24 | 1.57 -11.47 | .004 |
| Interaction PBPC × acute GVHD | 3.29 | 1.21 -8.93 | .019 |
| Covariates . | Relative risk . | 95% CI . | P . |
|---|---|---|---|
| Cox models fitted without posttransplantation covariates | |||
| Early TRM | |||
| PBPC | 1.63 | 0.87 -3.08 | .13 |
| Recipient age 35 or younger | 0.33 | 0.11 -0.93 | .036 |
| T-cell depletion | 0.34 | 0.12 -0.95 | .039 |
| Late TRM | |||
| PBPC | 0.48 | 0.23 -1.00 | .05 |
| Recipient age 35 or younger | 0.23 | 0.09 -0.60 | .002 |
| RA at last disease evaluation | 0.47 | 0.19 -1.13 | .092 |
| High-risk cytogenetics | 1.91 | 1.02 -3.58 | .044 |
| Interaction PBPC × RA at last evaluation | 6.66 | 1.78 -24.83 | .002 |
| Treatment failure | |||
| PBPC | 0.22 | 0.10 -0.48 | < .001 |
| RA at last disease evaluation | 0.19 | 0.07 -0.55 | .002 |
| Treated, not in CR MDS | 2.12 | 1.15 -3.91 | .016 |
| Event-free survival | |||
| PBPC | 0.27 | 0.13 -0.52 | < .001 |
| Recipient age 35 or younger | 0.42 | 0.24 -0.76 | .004 |
| RA at last disease evaluation | 0.41 | 0.21 -0.78 | < .007 |
| Treated, not in CR MDS | 1.86 | 1.13 -3.06 | .016 |
| High-risk cytogenetics | 1.17 | 0.62 -2.19 | .63 |
| Interaction PBPC × RA at last evaluation | 5.47 | 1.84 -16.22 | .002 |
| Interaction PBPC × high-risk cytogenetics | 3.07 | 1.10 -8.63 | .033 |
| Cox models fitted with posttransplantation covariates | |||
| Early TRM | |||
| PBPC | 1.59 | 0.69 -3.62 | .27 |
| ANC ≥ 0.5 × 109/L before day 15 | 2.62 | 1.14 -6.03 | .024 |
| Grade II-IV acute GVHD | 4.63 | 1.93 -11.12 | < .001 |
| Late TRM | |||
| PBPC | 0.33 | 0.15 -0.73 | < .007 |
| Recipient age 35 or younger | 0.26 | 0.11 -0.63 | < .003 |
| RA at last disease evaluation | 0.47 | 0.20 -1.11 | .084 |
| High-risk cytogenetics | 0.93 | 0.37 -2.31 | .87 |
| Acute GVHD | 2.69 | 1.44 -5.00 | < .002 |
| Interaction PBPC × RA at last evaluation | 6.66 | 1.78 -24.83 | < .005 |
| Interaction PBPC × high-risk cytogenetics | 4.35 | 1.20 -15.71 | .025 |
| Event-free survival | |||
| PBPC | 0.17 | 0.08 -0.37 | < .001 |
| Recipient age 35 or younger | 0.49 | 0.28 -0.85 | .011 |
| RA at last disease evaluation | 0.42 | 0.24 -0.76 | < .004 |
| High-risk cytogenetics | 0.96 | 0.54 -1.70 | .88 |
| Treated, not in CR MDS | 2.15 | 1.37 -3.40 | < .001 |
| Grade II-IV acute GVHD | 0.82 | 0.44 -1.55 | .55 |
| Interaction PBPC × RA at last evaluation | 5.20 | 1.68 -16.05 | .004 |
| Interaction PBPC × high-risk cytogenetics | 4.24 | 1.57 -11.47 | .004 |
| Interaction PBPC × acute GVHD | 3.29 | 1.21 -8.93 | .019 |
ANC indicates absolute neutrophil count.
Late transplantation-related mortality
No significant difference was observed between the PBPC and the BM groups regarding late TRM (Kaplan-Meier estimates, 42% with PBPC vs 36% with BM; RR, 1.26; 95% CI, 0.80-1.99; Figure 1C) in univariate analysis. Predictors of late TRM in univariate analysis are listed in Table 3. In a first multivariate Cox model in which no interaction or posttransplantation covariates were tested, the use of PBPCs had no significant influence on late TRM (without cytogenetics: RR, 1.05; 95% CI, 0.66-1.67;P = .82) (with cytogenetics, RR, 0.89; 95% CI, 0.49-1.61;P = .70). In a second model testing interactions, the use of PBPCs was associated with significantly lower late TRM, except if patients had RA and received PBPCs (Table 2). In a final model including posttransplantation covariates, the use of PBPCs remained associated with lower late TRM, except for 2 distinct groups of patients—those who had high-risk cytogenetics and those with RA at last disease evaluation (Table 2). The increased late TRM observed in patients with RA or high-risk cytogenetics who received PBPCs was mainly caused by an increase in early TRM (Kaplan-Meier estimates, 32% vs 10% with BM; P = .03 for RA; 45% vs 9% with BM;P = .013 for cytogenetics). There was no significant difference between the PBPC and BM groups regarding late TRM of patients with RA or high-risk cytogenetics who did not die of transplantation-related causes during the first 100 days (P = .36 for RA; P = .95 for cytogenetics). Regarding the causes of transplantation-related death according to the hematopoietic stem cell source used, the mortality rate related to acute GVHD was significantly higher in the PBPC group (Kaplan-Meier estimates, 16% vs 8% with BM; P = .029). This difference was mainly observed in patients with RAEB-t (Kaplan-Meier estimates, 19% vs 2% with BM; P = .013). In addition, more patients who underwent transplantation with PBPCs died of the consequences of hepatic veno-occlusive disease (Kaplan-Meier estimates, 6% vs 2% with BM; P = .07). This difference was mainly observed in patients with RAEB-t (Kaplan-Meier estimates, 9% vs 0% with BM; P = .037), patients with MDS “treated, not in CR” (Kaplan-Meier estimates, 11% vs 0% with BM;P = .024), or high-risk cytogenetics (Kaplan-Meier estimates, 21% vs 0% with BM; P = .024). Patients with high-risk cytogenetics in the PBPC group had an increased cumulative incidence of deaths related to fungal infections than those observed in the BM group (Kaplan-Meier estimates, 54% vs 10% with BM;P = .06). Finally, a significantly higher incidence of lethal hemorrhage was observed in RA patients in the PBPC group (Kaplan-Meier estimates, 23% vs 3% with BM;P = .013).
Transplantation-related mortality, treatment failures, and survival: univariate analyses
| Covariates . | Overall group . | Bone marrow . | PBPC . | P . | |||
|---|---|---|---|---|---|---|---|
| KM, % . | P . | KM, % . | P . | KM, % . | P . | ||
| Late TRM | |||||||
| Recipient age, y | < .001 | .004 | .058 | ||||
| 35 or younger | 14 | 14 | 15 | NS | |||
| Older than 35 | 46 | 46 | 46 | NS | |||
| Last disease evaluation | NS | NS | NS | ||||
| RA | 38 | 31 | 51 | .022 | |||
| Others | 39 | 38 | 39 | NS | |||
| Cytogenetics | .037 | NS | .003 | ||||
| High-risk | 49 | 39 | 77 | .07 | |||
| Others | 33 | 38 | 27 | NS | |||
| IPSS at diagnosis | NS | NS | NS | ||||
| High | 36 | 35 | 37 | NS | |||
| Others | 43 | 33 | 54 | .09 | |||
| Disease stage at SCT | .039 | NS | NS | ||||
| Treated, no CR | 50 | 51 | 49 | NS | |||
| Others | 35 | 32 | 39 | NS | |||
| Treatment failures | |||||||
| Last disease evaluation | .01 | .002 | NS | ||||
| RA | 13 | 15 | 0 | NS | |||
| RAEB | 28 | 41 | 16 | .026 | |||
| RAEB-t | 38 | 55 | 14 | < .001 | |||
| Cytogenetics | .07 | NS | .038 | ||||
| High-risk | 41 | 41 | 32 | NS | |||
| Others | 21 | 32 | 6 | .03 | |||
| IPSS at diagnosis | < .001 | .014 | NS | ||||
| High | 42 | 54 | 13 | NS | |||
| Others | 23 | 31 | 7 | .003 | |||
| Disease stage at SCT | .004 | .021 | .085 | ||||
| Untreated | 15 | 21 | 7 | .07 | |||
| CR | 32 | 43 | 12 | .016 | |||
| Treated, no CR | 50 | 66 | 29 | .08 | |||
| Event-free survival | |||||||
| Recipient age, y | < .01 | .015 | NS | ||||
| 35 or younger | 61 | 57 | 71 | NS | |||
| Older than 35 | 37 | 30 | 46 | NS | |||
| Last disease evaluation | NS | .01 | NS | ||||
| RA | 52 | 56 | 49 | NS | |||
| RAEB | 43 | 30 | 56 | .019 | |||
| RAEB-T | 39 | 32 | 47 | NS | |||
| Cytogenetics | < .001 | NS | < .001 | ||||
| High-risk | 30 | 36 | 16 | NS | |||
| Others | 52 | 41 | 67 | .015 | |||
| IPSS at diagnosis | NS | NS | NS | ||||
| Low + Int-1 | 43 | 46 | 46 | NS | |||
| Int-2 + High | 44 | 36 | 57 | NS | |||
| Disease stage at SCT | < .004 | .021 | .085 | ||||
| Untreated | 15 | 21 | 7 | .07 | |||
| CR | 32 | 43 | 12 | .016 | |||
| Treated, no CR | 50 | 66 | 29 | .08 | |||
| Covariates . | Overall group . | Bone marrow . | PBPC . | P . | |||
|---|---|---|---|---|---|---|---|
| KM, % . | P . | KM, % . | P . | KM, % . | P . | ||
| Late TRM | |||||||
| Recipient age, y | < .001 | .004 | .058 | ||||
| 35 or younger | 14 | 14 | 15 | NS | |||
| Older than 35 | 46 | 46 | 46 | NS | |||
| Last disease evaluation | NS | NS | NS | ||||
| RA | 38 | 31 | 51 | .022 | |||
| Others | 39 | 38 | 39 | NS | |||
| Cytogenetics | .037 | NS | .003 | ||||
| High-risk | 49 | 39 | 77 | .07 | |||
| Others | 33 | 38 | 27 | NS | |||
| IPSS at diagnosis | NS | NS | NS | ||||
| High | 36 | 35 | 37 | NS | |||
| Others | 43 | 33 | 54 | .09 | |||
| Disease stage at SCT | .039 | NS | NS | ||||
| Treated, no CR | 50 | 51 | 49 | NS | |||
| Others | 35 | 32 | 39 | NS | |||
| Treatment failures | |||||||
| Last disease evaluation | .01 | .002 | NS | ||||
| RA | 13 | 15 | 0 | NS | |||
| RAEB | 28 | 41 | 16 | .026 | |||
| RAEB-t | 38 | 55 | 14 | < .001 | |||
| Cytogenetics | .07 | NS | .038 | ||||
| High-risk | 41 | 41 | 32 | NS | |||
| Others | 21 | 32 | 6 | .03 | |||
| IPSS at diagnosis | < .001 | .014 | NS | ||||
| High | 42 | 54 | 13 | NS | |||
| Others | 23 | 31 | 7 | .003 | |||
| Disease stage at SCT | .004 | .021 | .085 | ||||
| Untreated | 15 | 21 | 7 | .07 | |||
| CR | 32 | 43 | 12 | .016 | |||
| Treated, no CR | 50 | 66 | 29 | .08 | |||
| Event-free survival | |||||||
| Recipient age, y | < .01 | .015 | NS | ||||
| 35 or younger | 61 | 57 | 71 | NS | |||
| Older than 35 | 37 | 30 | 46 | NS | |||
| Last disease evaluation | NS | .01 | NS | ||||
| RA | 52 | 56 | 49 | NS | |||
| RAEB | 43 | 30 | 56 | .019 | |||
| RAEB-T | 39 | 32 | 47 | NS | |||
| Cytogenetics | < .001 | NS | < .001 | ||||
| High-risk | 30 | 36 | 16 | NS | |||
| Others | 52 | 41 | 67 | .015 | |||
| IPSS at diagnosis | NS | NS | NS | ||||
| Low + Int-1 | 43 | 46 | 46 | NS | |||
| Int-2 + High | 44 | 36 | 57 | NS | |||
| Disease stage at SCT | < .004 | .021 | .085 | ||||
| Untreated | 15 | 21 | 7 | .07 | |||
| CR | 32 | 43 | 12 | .016 | |||
| Treated, no CR | 50 | 66 | 29 | .08 | |||
p1 indicates comparisons within one group (PBPC or BM) between estimates of each group defined by the value of the tested covariate; p2, comparisons between the BM and the PBPC groups for each estimates of one covariate; and KM, Kaplan-Meier.
Treatment failure incidence
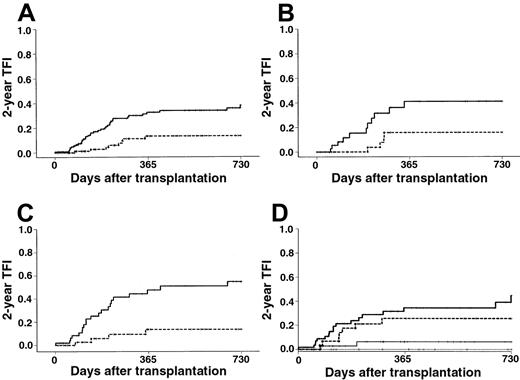
Forty-four patients had refractory or relapsing disease after transplantation. The median time from graft infusion to disease recurrence was 145 days in the BM group versus 229 days in the PBPC group. All but 3 treatment failures were diagnosed during the first year after transplantation. The overall 2-year estimated TFI was dramatically lower in the PBPC group than in the BM group (Kaplan-Meier estimates, 13% vs 38%; RR, 0.29; 95% CI, 0.13-0.62; Figure2A). The TFI was decreased in all MDS categories with the use of PBPCs, whatever the disease morphology at last evaluation and disease stage at time of transplantation were. Estimated reduction rates of the 2-year TFI with PBPCs was 15%, 25%, and 41% for patients with RA, RAEB, and RAEB-t, respectively (Table 3; Figure 2B-C). Estimated reduction rates of the 2-year TFI with PBPCs in patients with untreated MDS, MDS in CR, and MDS not in CR after remission-induction chemotherapy were 14%, 31%, and 37%, respectively. Whereas the high IPSS score was associated with a significant increase of the TFI in the BM group, it had no significant effect on this end point in the PBPC group (Figure 2D). The protective effect of PBPCs was more marked in the group for whom grafts were not T-cell depleted (Kaplan-Meier estimates, 14% vs 41%; RR, 0.28; 95% CI, 0.12-0.68) than in the one for whom T-cell depletion was performed (Kaplan-Meier estimates, 12% vs 28%; RR, 0.32; 95% CI, 0.06-1.60). In multivariate analysis, the use of PBPCs was the most significant predictor of a low TFI (Table 2). This finding remained unchanged when cytogenetics was included in the multivariate Cox model (RR, 0.19; 95% CI, 0.07-0.57; P < .003) or when only patients who received unmanipulated grafts were considered (RR, 0.20; 95% CI, 0.08-0.49; P < .001). Regarding patients who acquired chronic GVHD, estimate of the TFI was slightly, but not significantly, lower in the PBPC group (Kaplan-Meier estimates, 15% vs 30%; RR, 0.46; 95% CI, 0.16-1.32). Considering patients who did not acquire chronic GVHD, this difference became significant in univariate (Kaplan-Meier estimates, 5% with PBPC vs 38% with BM; RR, 0.11; 95% CI, 0.01-0.82) and multivariate (RR, 0.24; 95% CI, 0.10-0.55;P < .001) analyses.
TFI.
(A) The 2-year TFI was 38% in the BM group ( ) versus 13% in the PBPC group (---) (P < .001). (B) The 2-year TFI in patients with RAEB at last evaluation was 41% with BM (
) versus 13% in the PBPC group (---) (P < .001). (B) The 2-year TFI in patients with RAEB at last evaluation was 41% with BM ( ) versus 16% with PBPCs (---) (P = .026). (C) The 2-year TFI in patients with RAEB-t at last evaluation was 55% with BM (
) versus 16% with PBPCs (---) (P = .026). (C) The 2-year TFI in patients with RAEB-t at last evaluation was 55% with BM ( ) versus 14% with PBPCs (---) (P < .001). (D) The 2-year TFI in patients with intermediate-2 or high IPSS score was 6% with PBPCs (—) versus 45% with BM (
) versus 14% with PBPCs (---) (P < .001). (D) The 2-year TFI in patients with intermediate-2 or high IPSS score was 6% with PBPCs (—) versus 45% with BM ( ) (P < .003). The 2-year TFI in patients with low or intermediate-1 IPSS score was 0% with PBPCs (····) versus 26% with BM (---) (P = .046).
) (P < .003). The 2-year TFI in patients with low or intermediate-1 IPSS score was 0% with PBPCs (····) versus 26% with BM (---) (P = .046).
TFI.
(A) The 2-year TFI was 38% in the BM group ( ) versus 13% in the PBPC group (---) (P < .001). (B) The 2-year TFI in patients with RAEB at last evaluation was 41% with BM (
) versus 13% in the PBPC group (---) (P < .001). (B) The 2-year TFI in patients with RAEB at last evaluation was 41% with BM ( ) versus 16% with PBPCs (---) (P = .026). (C) The 2-year TFI in patients with RAEB-t at last evaluation was 55% with BM (
) versus 16% with PBPCs (---) (P = .026). (C) The 2-year TFI in patients with RAEB-t at last evaluation was 55% with BM ( ) versus 14% with PBPCs (---) (P < .001). (D) The 2-year TFI in patients with intermediate-2 or high IPSS score was 6% with PBPCs (—) versus 45% with BM (
) versus 14% with PBPCs (---) (P < .001). (D) The 2-year TFI in patients with intermediate-2 or high IPSS score was 6% with PBPCs (—) versus 45% with BM ( ) (P < .003). The 2-year TFI in patients with low or intermediate-1 IPSS score was 0% with PBPCs (····) versus 26% with BM (---) (P = .046).
) (P < .003). The 2-year TFI in patients with low or intermediate-1 IPSS score was 0% with PBPCs (····) versus 26% with BM (---) (P = .046).
Event-free survival
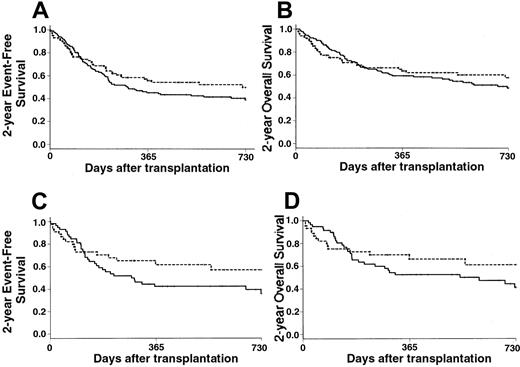
Estimates of the 2-year EFS rates were 50% in the PBPC group and 39% in the BM group (RR, 0.79; 95% CI, 0.54-1.14) (Figure3A). However, the proportional hazards assumption did not hold for the covariate graft type, leading to consideration of it as a time-dependent covariate with 2 time intervals—from day 0 to day 90 after transplantation and from day 91 thereafter. In that way, the use of PBPCs had a significant impact only on the 2-year EFS after day 90 (RR, 0.52; 95% CI, 0.32-0.86), whereas it had no significant impact on outcome during the first 90 days after transplantation (RR, 1.50; 95% CI, 0.85-2.66). Predictors of 2-year EFS in univariate analysis are listed in Table 3. In a first multivariate Cox model that did not include posttransplantation covariates, the use of PBPCs was associated with improved 2-year EFS, except for patients who had either RA or high-risk cytogenetics (Table 2). When considering the subgroup of patients who received unmanipulated grafts, the use of PBPCs remained associated with an improved 2-year EFS (RR, 0.25; 95% CI, 0.11-0.57;P < .001). In the extended Cox model in which posttransplantation covariates were introduced, the use of PBPCs remained significantly associated with improved outcome, with the exception of patients who had RA at last disease evaluation, high-risk cytogenetics, or grade II-IV acute GVHD (Table 2).
Survival.
(A) The 2-year EFS of patients who received PBPCs (---) was 50% versus 39% of those who received BM grafts (—) (P = .20). (B) The 2-year OS was 58% in the PBPC group (---) versus 49% in the BM group (—) (P = .40). (C) Patients with intermediate-2 or high IPSS scores had a 2-year EFS of 57% with PBPC (---) versus 36% with BM (—) (P = .15). (D) Patients with intermediate-2 or high IPSS scores had a 2-year OS of 61% with PBPCs (---) versus 46% with BM (—) (P = .13).
Survival.
(A) The 2-year EFS of patients who received PBPCs (---) was 50% versus 39% of those who received BM grafts (—) (P = .20). (B) The 2-year OS was 58% in the PBPC group (---) versus 49% in the BM group (—) (P = .40). (C) Patients with intermediate-2 or high IPSS scores had a 2-year EFS of 57% with PBPC (---) versus 36% with BM (—) (P = .15). (D) Patients with intermediate-2 or high IPSS scores had a 2-year OS of 61% with PBPCs (---) versus 46% with BM (—) (P = .13).
Overall survival
Estimates of the 2-year OS rates were 58% in the PBPC group and 49% in the BM group (RR, 0.89; 95% CI, 0.60-1.34) (Figure3B). Patients with RA, RAEB, and RAEB-t had 2-year OS rates of 49%, 68%, and 53%, respectively with PBPCs compared with 66%, 46%, and 37% with BM, respectively. Patients with low or intermediate-1 IPSS scores had 2-year OS rates of 46% with PBPCs and 59% with BM. Patients with intermediate-2 or high IPSS scores had 2-year OS rates of 61% with PBPCs compared with 41% with BM (Figure 3C). When considering disease stage at transplantation, 2-year OS was improved in all 3 categories when PBPCs were used, but the differences did not reach significance. As for the EFS, multivariate analysis confirmed the beneficial effect of PBPCs (RR, 0.23; 95% CI, 0.11-0.49;P < .001), except for patients with RA (RR of the interaction, 8.85; 95% CI, 2.73-28.70; P < .001) or high-risk cytogenetics (RR of the interaction, 3.67; 95% CI, 1.19-11.28; P = .023). When considering the group of patients who received unmanipulated grafts, PBPCs remained associated with an improved outcome (RR, 0.24; 95% CI, 0.10-0.55;P < .001), with the exception of patients who had RA (RR, 4.47; 95% CI, 0.91-21.90; P = .06) or high-risk cytogenetics (RR, 10.09; 95% CI, 2.78-36.65;P < .001).
Discussion
This is the first large multicenter study comparing G-CSF–mobilized PBPCs with BM as a source of hematopoietic stem cells for allogeneic transplantation in a specific disease. In this retrospective analysis, the use of PBPCs was associated with a dramatic decrease of the TFI, despite a higher proportion of patients with RA (low relapse-risk disease) in the BM group. The TFI observed in the BM group was similar to the one reported in previous series,3,4 indicating that the differences between the PBPC and BM groups were, a priori, not caused by recruitment bias. Because treatment failures occurred during the first year after transplantation in more than 90% of the patients, it is unlikely that, regarding TFI, the difference observed between the PBPC and BM groups will be modified with a longer posttransplantation follow-up time in the PBPC group. Adjusting for disease stage and morphology (FAB classification) and for cytogenetic characteristics, graft type appeared to be the most powerful predictor of treatment failure by 2 years after transplantation. The low TFI observed with PBPC is in agreement with the results of 2 prospective studies that enrolled significant numbers of patients with advanced-stage leukemia.14,19 It suggests that a “graft-versus-MDS” effect exists and that it could be enhanced by the use of G-CSF–mobilized PBPCs. It is well recognized that chronic GVHD is one of the key factors limiting disease recurrence after allogeneic bone marrow transplantation in disorders such as chronic myeloid leukemia.33 As in most reports comparing PBPCs with BM, there was a trend toward more chronic GVHD among patients who received PBPC. This finding could account for the overall 25% estimated reduction of the TFI in the PBPC group. However, a dramatic reduction of the TFI was also observed in patients who did not acquire chronic GVHD after allogeneic PBPC transplantation. These results suggest that the use of PBPCs instead of BM grafts might be an efficient way to prevent posttransplantation disease recurrence in patients with high relapse-risk MDS.
As in most previous studies, the use of PBPCs was associated with faster hematopoietic recovery than the use of BM grafts.13-23 It has already been shown that PBPC products contained at least twice as many CD34+ cells per kilogram of recipient body weight as BM grafts. This “cell dose effect” could explain the earlier hematopoietic recovery observed with PBPCs.14,15,18,19,23 We were unable to assess whether the speed of hematopoietic recovery was correlated with the nucleated cell and CD34+ cell doses infused because information about these parameters was lacking for a large number of patients. Unexpectedly, early neutrophil recovery was not associated with improved outcome, especially when patients received PBPCs. It has already been shown in allogeneic PBPC transplantation that infusions of high CD34+ cell doses are associated with an increased risk for acute GVHD.34 Moreover, early neutrophil recovery after allogeneic bone marrow transplantation has been associated with an increased risk for acute GVHD.35 In this study, a higher incidence of grade II-IV acute GVHD was observed in patients who had early neutrophil recovery, especially when PBPCs were used. In fact, acute GVHD in patients who received PBPCs was associated with a 10% increase in early TRM when compared with the estimated rate in patients who received bone marrow and had this complication. The significantly higher rate of acute GVHD-related mortality in patients who received PBPCs was in agreement with the hypothesis that patients who had early neutrophil recovery were more likely to die as a result of the transplantation procedure during the first 100 days. Furthermore, patients who had early neutrophil recovery and survived more than 3 months after transplantation more frequently acquired chronic GVHD. Therefore, because of an increased risk for acute and chronic GVHD, early neutrophil recovery might be predictive of a worse outcome after first allogeneic PBPC transplantation.
The higher TRM rate observed with PBPCs in patients with RA or high-risk cytogenetics was not expected. It has already been shown that patients with high-risk cytogenetics had a worse outcome because of a higher relapse incidence, but no significant increase of the TRM was reported for these patients.5,8,36 The fact that the unexpected differences were mainly caused by an increase in early TRM could mean that these patients were highly selected—that is, they were at high risk for transplantation-related death because of older age, worse performance status, or pre-existing organ dysfunction. Patients in the PBPC group were older than those who received BM grafts (for high-risk cytogenetics: median age, 48.5 years vs 42 years;P = .04) (for RA: median age, 46 years vs 40 years;P = .10). The higher incidence of death related to hepatic veno-occlusive disease and fungal infection for patients with high-risk cytogenetics who underwent PBPC transplantation also supports that hypothesis. In addition, patients with RA who underwent PBPC transplantation had a longer time interval between diagnosis and SCT (more than 6 months: 84% with PBPCs vs 62% with BM;P = .09) and a higher incidence of lethal hemorrhage. These 2 points could be 2 indirect arguments in favor of a higher rate of posttransfusion hemochromatosis and immunization, complications already known to affect the outcome of patients who undergo transplantation for RA. Although we cannot definitely rule out that the increased late TRM was more likely attributed to patient characteristics than to disease or chance, an interaction study has highlighted that patients without RA or high-risk cytogenetics (n = 82) had a lower risk for late TRM with PBPCs. Indeed, excluding patients with high-risk cytogenetics, our results were concordant with those reported in the retrospective IBMTR study, which suggested that the use of PBPCs was associated with lower TRM in patients with advanced-stage leukemia.16 Given the potential for confounding factors and recruitment bias, results dealing with TRM in retrospective comparisons between PBPCs and BM should be cautiously interpreted because they might underestimate the benefit of using PBPCs.
Regarding young patients with low IPSS score MDS or RA, given that their median expected survival is more than 10 years without transplantation,2 standard allogeneic transplantation procedures, with a TRM of approximately 40% whatever the hematopoietic stem cell source used, do not seem adequate. New strategies have to be assessed to decrease this unacceptably high TRM rate. Because these patients are at low risk for relapse, non–myelo-ablative conditioning regimens might offer a new approach combining lower TRM and improved outcome.
We thank the following physicians for referring their patients to the EBMT Chronic Leukemia Working Party registry: Fibbe WE (Leiden, Netherlands), Greinix HT (Vienna, Austria), Ortega JJ (Barcelona, Spain), Fauser AA (Idar-Oberstein, Germany), Vitek A (Prague, Czech Republic), Gratwohl A (Basel, Switzerland), Kuentz M (Créteil, France), Russel NH (Nottingham, United Kingdom), Zander A (Hamburg, Germany), Parker A (Glasgow, United Kingdom), Ferrant A (Brussels, Belgium), Brinch L (Oslo, Norway), Conde E (Santander, Spain), Wachowiak J (Poznan, Poland), Mariani G (Palermo, Italy), Arcese W (Roma, Italy), Gahrton G (Huddinge, Sweden), Powles R (Sutton, United Kingdom), Ball SE (London, United Kingdom), Lange A (Wroclaw, Poland), Chapuis B (Geneva, Switzerland), Caballero D (Salamanca, Spain), Sanz MA (Valencia, Spain), Fassas A (Thessaloniki, Greece), Amadori S (Roma, Italy), Beguin Y (Liège, Belgium), Selleslag D (Brugge, Belgium), Lenhoff S (Lund, Sweden), Apperley JF (London, United Kingdom), Ifrah N (Angers, France), Buzyn A (Paris, France), Milpied N (Nantes, France), Michallet M (Lyons, France), Boiron JM (Pessac, France), Gorin NC (Paris, France), Arnold R (Berlin, Germany), Beelen D (Hamburg, Germany), McCann S (Dublin, Ireland), Barbui T (Bergamo, Italy), Pihkala U (Helsinki, Finland), Remes K (Turku, Finland), Hamblin T (Bournemouth, United Kingdom), Rodriguez Fernandez JM (Sevilla, Spain), Van den Berg H (Amsterdam, Netherlands), Milligan DW (Birmingham, United Kingdom), Harhalakis N (Athens, Greece), Pogliani EM (Monza, Italy), Coser P (Bolzano, Italy), Giustolisi R (Catania, Italy), Scanni A (Milano, Italy), Urban C (Graz, Austria), Schroyens W (Antwerp, Belgium), Haas R (Düsseldorf, Germany).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Philippe Guardiola, Service de Greffe de Moelle Trèfle 3, Fédération d'Hématologie, Hôpital Saint-Louis, 1 avenue Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail: phguardiol@aol.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal