The graft-versus-leukemia (GVL) effects and graft-versus-host disease (GVHD)–inducing activity of CD8 T cells was compared in murine recipients of wild-type (WT) or interferon γ (IFN-γ)–deficient (GKO) allogeneic donor cells. CD8 T cells (or CD4-depleted splenocytes) from GKO donor mice induced more severe GVHD in lethally irradiated allogeneic recipients compared to the same cell populations from WT donors. Consistent with GVHD severity, donor CD8 T-cell expansion in allogeneic recipients was augmented in the absence of IFN-γ. These results demonstrate that IFN-γ does not stimulate but instead down-modulates GVHD induced by donor CD8 T cells. Remarkably, antihost lymphohematopoietic reactions, including GVL effects against host leukemia/lymphoma cells, of CD8 T cells correlated inversely with their GVHD-inducing activity, and those of GKO donors were markedly weaker than those mediated by WT donor CD8 T cells. These data show for the first time that GVHD-inducing activity and GVL effects of allogeneic CD8 T cells can be separated by a single cytokine, IFN-γ.
Introduction
Allogeneic bone marrow transplantation (BMT) is an effective therapeutic approach for the treatment of otherwise fatal hematologic malignancies and nonmalignant hematopoietic disorders. Although reduced leukemic relapse rates resulting from graft-versus-leukemic (GVL) effects have been observed in patients receiving HLA antigen-mismatched marrow compared to HLA-identical transplants,1-5 the high incidences of graft-versus-host disease (GVHD) and GVHD-induced immunodeficiency present an enormous obstacle to HLA-mismatched BMT in humans.6-8 Thus, a major challenge is to separate beneficial GVL effects from GVHD. Although T-cell depletion of donor marrow can inhibit GVHD, and some studies have shown that GVL effects can be induced in recipients of allogeneic natural killer (NK) cells9-11 or T cell–depleted (TCD) allogeneic BMT,12,13 a strong association of leukemic relapse with TCD BMT has been proven in a number of animal studies and clinical trials.14-19 The high incidence of leukemic relapse associated with T-cell depletion indicates that in addition to GVHD, efficient GVL effects against certain leukemias are also largely dependent on donor T cells. Thus, methods that can selectively inhibit the GVHD-promoting activity of allogeneic T cells while preserving allogeneic T cell–mediated GVL effects would be highly beneficial in the use of allogeneic BMT for the treatment of leukemia.
Previous studies have shown that interferon-γ (IFN-γ) is required for the inhibition of CD4-dependent GVHD and preservation of GVL effects of donor CD8 T cells in allogeneic BMT recipients treated with a single dose of interleukin 12 (IL-12) at the time of transplantation.20-22 In the present study, we have investigated the role of IFN-γ in the GVHD- and GVL-associated alloreactivity of the donor CD8 T-cell subset in mice transplanted with CD4-depleted or purified CD8+ splenocytes from WT or IFN-γ–deficient allogeneic donors. Our results demonstrate that allogeneic CD8 T cells induce more severe systemic GVHD but weaker GVL/antihost lymphohematopoietic reactions if they are incapable of producing IFN-γ. Thus, GVHD- and GVL-associated alloresponses of CD8 T cells can be dissociated by an IFN-γ–dependent mechanism.
Materials and methods
Mice
Specific pathogen-free wild-type (WT) and IFN-γ knockout (GKO) mice on the BALB/c (BALB/c-Ifngtm1Ts, H-2d, KdIdDd) or C56BL/6 (C57BL/6-Ifngtm1Ts, H-2b, KbIbDb) backgrounds were purchased from the Jackson Laboratory (Bar Harbor, ME). WT C57BL/6 mice (B6, H-2b, KbIbDb) were purchased from the Frederick Cancer Research Facility (National Institutes of Health, Bethesda, MD). The 2C TCR transgenic mice on a C57BL/6 background were generously provided by Dr Dennis Y. Loh23 and were bred to the C57BL/6-Ifngtm1Ts mice to produce GKO 2C TCR transgenic progeny. Expression of the 2C TCR was detected by fluorescence-activated cell sorter (FACS) analysis using 1B2 anti-2C TCR monoclonal antibody (mAb; kindly provided by Dr Herman Eisen).24 IFN-γ genotyping was performed by tail DNA polymerase chain reaction (PCR) using primers for the endogenous (oIMR126 and oIMR127) or the targeted (oIMR128 and oIMR129) IFN-γ allele as described in the Jackson Laboratory Mice Database (www.jax.org). The sequences of these primers are: oIMR126, 5′-AGA AGT AAG TGG AAG GGC CCA GAA G-3′; oIMR127, 5′-AGG GAA ACT GGG AGA GGA GAA ATA T-3′; oIMR128, 5′-TCA GCG CAG GGG CGC CCG GTT CTT T-3′; oIMR129, 5′-ATC GAC AAG ACC GGC TTC CAT CCG A-3′. Mice were housed in sterilized microisolator cages and received autoclaved feed and drinking water.
Bone marrow transplantation
Sex- and age-matched B6 mice were lethally irradiated (9.75 Gy,137Cs source, 0.84 Gy/min) and reconstituted within 4 to 8 hours with 5 × 106 TCD allogeneic bone marrow cells (BMCs) and 7.5 to 10 × 106 CD4-depleted or 1 to 2.5 × 106 purified CD8+ spleen cells from WT or GKO BALB/c mice. Irradiated B6 mice transplanted with 5 × 106 TCD B6 BMCs were used as non-GVHD controls. In some experiments, 5 × 106 TCD B6 BMCs were also given to allogeneic recipients to provide a readout for lymphohematopoietic GVH reactions. In leukemia studies, recipients were additionally injected with 500 EL4 cells, a B6 T-cell leukemia/lymphoma cell line, as previously described.20 TCD was performed by incubating cells with anti-CD4 mAb (GK1.5) and anti-CD8 mAb (2.43) or with anti-CD4 only (for CD4 depletion), followed by rabbit complement, and the completeness of depletion was verified by FACS analysis as previously described.21,22 CD8+ splenocytes were purified using the MACS system (Miltenyi Biotec, Bergisch-Gladbach, Germany) according to the manufacturer's instructions. Briefly, mouse spleen cells were labeled with anti-CD8α (2.43)-coated magnetic microbeads and positively selected on a magnetic separation column. Aliquots of sorted CD8+ splenocytes were restained with fluorescein isothiocyanate (FITC)–conjugated anti-CD8β mAb (53-5.8; Pharmingen, San Diego, CA), and their purity was consistently greater than 97%. To avoid bias from cage-related effects, animals were randomized before and after BMT as described.25 Carcasses were saved in 10% formalin after death or euthanasia for autopsy. Tissues (spleen, liver, kidney, and lung) were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Necropsies and histologic analyses were performed on randomly chosen samples by observers who were unaware of which treatment group the carcasses belonged to, as previously described.26
Induction of GVH with 2C T cells was performed by intravenous injection of 10 × 106 BMCs and 12 × 106 splenocytes from WT or GKO 2C TCR transgenic B6 donors into lethally irradiated (8 Gy) BALB/c mice. Non-GVHD controls received similar numbers of BMCs and splenocytes from syngeneic donors. As described above, animals were randomized before and after BMT.
FACS analysis
For the measurement of donor T-cell expansion and chimerism, recipient white blood cells (WBCs) were stained for 30 minutes at 4°C with antihost H-2Kb mAb 5F1-FITC (Pharmingen) and phycoerythrin (PE)–conjugated anti-CD8 mAb (53-6.7, Pharmingen). To block nonspecific FcR binding of labeled antibodies, 10 μL of undiluted culture supernatant of 2.4G2 (rat antimouse FcγR mAb)27 was added to the first incubation. The expression of IFN-γ receptor on EL4 cells was measured by staining cells with rat antimouse CD119-FITC (GR20, Pharmingen). FITC-labeled and biotinylated mouse IgG2a mAb HOPC-1 and PE-labeled rat IgG2a mAb (Pharmingen) were used as nonstaining negative control antibodies. Cells were washed with FACS buffer (Hanks balanced salt solution containing 0.1% bovine serum albumin [BSA] and 0.1% NaN3) between each and following the last stain, and were analyzed on a FACScan (Becton Dickinson, Mountain View, CA).
Proliferation assay
EL4 cells (2 × 103/well) and WEHI-279 cells (1 × 104/well) were incubated in triplicate in 96-well plates with various concentrations of recombinant murine IFN-γ (Pharmingen) in RPMI medium supplemented with 10% fetal calf serum (FCS; Sigma, St Louis, MO), 4% nutrient mixture (l-glutamine, nonessential amino acids, sodium pyruvate, penicillin/streptomycin), 1% Hepes buffer, and 10 μM 2-mercaptoethanol. Cultures were pulsed with 1 μCi (0.037 MBq)3H-thymidine 36 hours after incubation, and harvested 12 hours later. 3H-thymidine uptake was counted on a Betaplate β counter (Wallac, Gaithersburg, MD) and data are presented as the mean ± SD (cpm) of triplicate samples. WEHI-279, a murine lymphoma cell line that is sensitive to an antiproliferative effect of IFN-γ, was used as an assay control.
The Nunc 10-mm tissue culture inserts (0.4 μm polycarbonate membrane, Nalge Nunc International, Roskilde, Denmark) were used to determine the effects of cytokines released by alloreactive T cells (ie, mixed lymphocyte reaction supernatants) on EL4 cell proliferation. BALB/c spleen cells (2 × 106 in 0.5 mL) or 0.5 mL media were added to each well of 24-well culture plates (Nalge Nunc International) containing 30 Gy-irradiated B6 spleen cells (2 × 106 in 0.5 mL). After 10-mm tissue culture inserts were placed into each well, 2.5 × 104 EL4 cells (1 mL) were added into each insert. EL4 cells were harvested on days 1, 2, 3, and 4 after incubation, and the number of viable EL4 cells in each well was counted by trypan blue exclusion. Three wells in each group were harvested at each time point and data are presented as the mean ± SD. The culture insert used in this study allows the permeation into the insert of cytokines produced by spleen cells, but blocks any spleen cell–mediated direct killing of EL4 cells.
Alloreactive cytotoxic T-lymphocyte assay
BALB/c spleen cells were cultured in triplicate in 96-well plates with irradiated B6 spleen cells (30 Gy), at a 1:1 ratio (8 × 105/well) in RPMI supplemented with 10% FCS, 4% nutrient mixture, 1% Hepes buffer, and 10 μM 2-mercaptoethanol for 5 days. Responder cells were mixed with 51Cr-labeled EL4 (target) cells in 96-well plates (8000 cells/well) at various ratios (50:1 to 0.78:1) and incubated for 4 hours. The supernatants were harvested and radioactivity was measured in an automatic gamma counter. The percent specific lysis was determined as follows: specific lysis (%) = [(cpm experimental − cpm background)/(cpm maximum − cpm background)] × 100%. Background cpm was taken as spontaneous release from target cells in the absence of responder cells, and maximum cpm as release by target cells treated with 0.5% Nonidet P-40.
Statistical analysis
Statistical analysis of survival data was performed with the log-rank test. The Student t test was used to determine the level of significance of differences in group means. AP < .05 was considered to be significant in both types of analysis.
Results
IFN-γ down-modulates CD8 T cell–mediated GVHD while mediating GVL effects of the CD8 subset
To determine the role of IFN-γ in GVHD- and GVL-associated alloresponses of allogeneic CD8 T cells, we compared these phenomena in lethally irradiated C57BL/6 (B6) mice receiving CD4-depleted spleen cells from WT or IFN-γ–deficient GKO BALB/c donors. B6 mice were lethally irradiated (9.75 Gy) and reconstituted with 5 × 106 TCD B6 BMCs (syngeneic controls) or with TCD BMCs (5 × 106) and CD4-depleted spleen cells (10 × 106) from WT or GKO BALB/c mice. Some recipients were injected with 500 EL4 cells (a B6 T-cell leukemia/lymphoma cell line) along with the BMT inoculum. It has been demonstrated that the GVL effect against EL4 cells is donor CD8+ cell dependent and CD4+ cell independent.20,28 Consistent with our previous finding that lethal acute GVHD in this strain combination is mostly CD4 dependent,22 most B6 mice injected with WT BALB/c CD4-depleted spleen cells survived long-term (Figure 1A). However, injection of a similar number of GKO BALB/c CD4-depleted spleen cells into B6 mice led to 60% mortality by 20 days (Figure 1A). Nonleukemic recipients of GKO BALB/c cells also showed more severe weight loss compared to nonleukemic recipients of WT BALB/c cells (Figure 1B). The body weight of most recipients of GKO BALB/c cells (4 of 5) decreased to less than 18 g by week 1 after BMT. However, only 1 of 5 mice that received WT BALB/c cells showed severe weight loss (< 18 g) by week 1 and the body weight of all surviving mice in this group recovered by week 2 after BMT. Because GVHD cannot be induced by donor spleen cells if both CD4 and CD8 T cells are depleted in this strain combination, these results indicate that donor-derived IFN-γ down-modulates systemic GVHD induced by allogeneic CD8 T cells.
Augmentation of GVHD is associated with reduction of GVL effects in B6 mice transplanted with CD4-depleted splenocytes from IFN-γ–deficient GKO BALB/c mice.
B6 mice were irradiated and transplanted with 5 × 106 B6 TCD BMC (▪, syngeneic), or 5 × 106 TCD BMC and 10 × 106 CD4-depleted splenocytes from WT (○, WT BALB) or GKO (●, GKO BALB) BALB/c donors. Leukemic recipients were also injected with 500 EL4 cells along with the BMT inoculum. Data shown are survival (A) and survivors' body weights (B) of nonleukemic recipients, and survival of leukemic recipients receiving 500 EL4 cells (C).
Augmentation of GVHD is associated with reduction of GVL effects in B6 mice transplanted with CD4-depleted splenocytes from IFN-γ–deficient GKO BALB/c mice.
B6 mice were irradiated and transplanted with 5 × 106 B6 TCD BMC (▪, syngeneic), or 5 × 106 TCD BMC and 10 × 106 CD4-depleted splenocytes from WT (○, WT BALB) or GKO (●, GKO BALB) BALB/c donors. Leukemic recipients were also injected with 500 EL4 cells along with the BMT inoculum. Data shown are survival (A) and survivors' body weights (B) of nonleukemic recipients, and survival of leukemic recipients receiving 500 EL4 cells (C).
Remarkably, donor-derived IFN-γ had an opposite effect on the GVL reactivity of donor CD8 T cells. In contrast to the exacerbating effect on GVHD, the absence of donor-derived IFN-γ diminished GVL effects of donor CD8 T cells. Administration of CD4-depleted WT but not GKO BALB/c spleen cells led to significant antileukemic effects (Figure 1C;P < .005 for WT BALB/c recipients compared to recipients of GKO BALB/c cells and to syngeneic BMT recipients). Autopsy analysis was performed in randomly selected carcasses without knowledge of which treatment the animals had received.20 Gross evidence for tumor, which was detected in all EL4 recipients receiving transplants with syngeneic cells, was only found in 1 of 7 recipients of WT allogeneic BMCs plus CD4-depleted spleen cells (Experiment [Exp] 1 in Table 1). In contrast, evidence for tumor at autopsy was observed in 4 of 7 leukemic recipients of GKO allogeneic BMCs plus CD4-depleted spleen cells. Furthermore, no histologic evidence for tumor (ie, tissue infiltration of leukemic cells) was detected in the spleen, liver, or kidney of leukemic recipients of WT BALB/c cells that showed lack of tumor at autopsy (data not shown).
Gross evidence for tumor at autopsy in B6 recipients of TCD BMCs plus CD4-depleted spleen cells from WT or GKO BALB/c mice
| Groups . | Tumor at autopsy (no. with tumor/total evaluated) . | |
|---|---|---|
| Exp 1 . | Exp 2 . | |
| Syngeneic BMT + 500 EL4 | 4/4 | 4/4 |
| WT BALB/c BMT + 500 EL4 | 1/7 | 0/7 |
| GKO BALB/c BMT + 500 EL4 | 4/7 | 3/7 |
| Nonleukemic recipients | 0/5 | 0/17 |
| Groups . | Tumor at autopsy (no. with tumor/total evaluated) . | |
|---|---|---|
| Exp 1 . | Exp 2 . | |
| Syngeneic BMT + 500 EL4 | 4/4 | 4/4 |
| WT BALB/c BMT + 500 EL4 | 1/7 | 0/7 |
| GKO BALB/c BMT + 500 EL4 | 4/7 | 3/7 |
| Nonleukemic recipients | 0/5 | 0/17 |
Allogeneic BMT recipient mice were transplanted with 5 × 106 TCD BMCs and 10 × 106 (Exp 1) or 7.5 × 106 (Exp 2) CD4-depleted spleen cells from WT or GKO BALB/c mice. B6 mice receiving 5 × 106 TCD B6 BMCs were used as syngeneic BMT controls. Leukemic recipients were additionally injected with 500 EL4 cells. Nonleukemic controls (nonleukemic recipients) are pooled animals from both syngeneic and allogeneic BMT groups.
Similar results were observed in a repeat experiment. To limit the potential for GVHD-associated mortality to interfere with the evaluation of GVL effects, B6 recipients in this experiment were injected with a reduced number (7.5 × 106) of BALB/c CD4-depleted spleen cells. As shown in Figure2A, with the exception of one nonleukemic recipient in each of the WT and GKO allogeneic BMT groups, nonleukemic mice survived for the duration of the experiment. However, the survival advantage against EL4 leukemia conferred by CD4-depleted spleen cells from GKO BALB/c mice was significantly less than that mediated by WT BALB/c CD4-depleted spleen cells (Figure 2B; P < .05 for leukemic recipients of WT BALB/c cells compared to leukemic recipients of GKO BALB/c cells). All recipients of WT BALB/c cells were protected from leukemia-associated lethality, whereas all syngeneic recipients died of leukemia by day 32 after BMT (P < .001). Although leukemic recipients of GKO BALB/c cells were also significantly protected from lethality compared to syngeneic recipients (P < .05), long-term survival was only achieved in less than 50% of these mice. Evidence for tumor at autopsy was found in 3 of 4 leukemic recipients of GKO BALB/c cells that died by 40 days after BMT (Exp 2 in Table 1). No tumor was detected in long-term surviving leukemic recipients of either GKO (3 mice) or WT (7 mice) BALB/c cells. Together, our results indicate that donor-derived IFN-γ contributes to the GVL effect of allogeneic CD8 T cells, while down-modulating CD8 T cell–mediated GVHD.
IFN-γ is required for the induction of optimal GVL effects.
B6 mice were irradiated and transplanted with 5 × 106 B6 TCD BMC (▪, syngeneic), or 5 × 106 TCD BMC and 7.5 × 106 CD4-depleted splenocytes from WT (○,WT BALB) or GKO (●, GKO BALB) BALB/c donors. Leukemic recipients were additionally injected with 500 EL4 cells along with the BMT inoculum. Survivals of nonleukemic (A) and leukemic (B) recipients are shown.
IFN-γ is required for the induction of optimal GVL effects.
B6 mice were irradiated and transplanted with 5 × 106 B6 TCD BMC (▪, syngeneic), or 5 × 106 TCD BMC and 7.5 × 106 CD4-depleted splenocytes from WT (○,WT BALB) or GKO (●, GKO BALB) BALB/c donors. Leukemic recipients were additionally injected with 500 EL4 cells along with the BMT inoculum. Survivals of nonleukemic (A) and leukemic (B) recipients are shown.
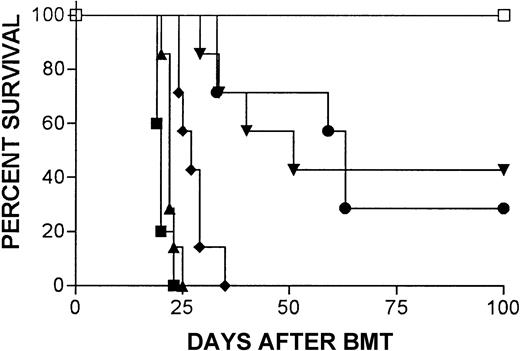
IFN-γ–deficient but not WT 2C T cells induce lethal acute GVHD in BALB/c mice
The 2C CTL clone was originally derived from an H-2bmouse immunized with H-2d cells, and the majority of T cells in 2C TCR transgenic mice are CD8+2CTCR+cells that specifically recognize Ld.24 Thus, transplantation of T cells from 2C TCR transgenic mice to H-2Ld+ (eg, BALB/c) mice provides a useful model to study GVH reactivity of CD8 T cells. We have previously shown that injection of 12 × 106 spleen cells along with BMCs from 2C TCR transgenic donors does not induce lethal acute GVHD in BALB/c mice,29 which is consistent with the finding that acute GVHD is largely CD4 dependent in most major histocompatibility complex–mismatched strain combinations in mice.28 30-32Remarkably, IFN-γ seems to be required for the absence of lethal GVHD in this 2C→BALB/c BMT model. As shown in Figure3, administration of 10 × 106 BMCs and 12 × 106 splenocytes from IFN-γ–deficient 2C donors caused severe acute GVHD, with 100% mortality by 20 days, whereas BALB/c mice receiving similar numbers of bone marrow and spleen cells from WT 2C mice survived long-term.
IFN-γ–deficient but not WT 2C T cells induce lethal acute GVHD in BALB/c mice.
Survival is shown for irradiated (8 Gy) BALB/c mice that were transplanted with 10 × 106 BMCs and 12 × 106 splenocytes from syngeneic donors (▪, syngeneic), WT (○, WT 2C) or GKO (●, GKO 2C) allogeneic 2C donors.
IFN-γ–deficient but not WT 2C T cells induce lethal acute GVHD in BALB/c mice.
Survival is shown for irradiated (8 Gy) BALB/c mice that were transplanted with 10 × 106 BMCs and 12 × 106 splenocytes from syngeneic donors (▪, syngeneic), WT (○, WT 2C) or GKO (●, GKO 2C) allogeneic 2C donors.
IFN-γ production by allogeneic CD8 T cells directs their alloreactivity toward GVHD or GVL effects in vivo
Our previous studies have demonstrated that the GVL effects against EL4 leukemia are dependent on donor CD8 T cells.20 28 To determine whether or not IFN-γ produced by the effector CD8 T cells plays a role in dissociating GVL effects from systemic GVHD, we compared GVHD versus GVL effects in lethally irradiated B6 mice receiving transplants with purified CD8+T cells (1 × 106) from GKO or WT BALB/c mice. B6 TCD BMCs (5 × 106) and GKO BALB/c TCD BMCs (5 × 106) were given to all allogeneic BMT recipients and 500 EL4 cells were injected into some groups. Coadministration of TCD B6 BMCs into these lethally irradiated recipients of allogeneic cells provided a marker for the evaluation of lymphohematopoietic GVH reactions (see below). Although GVHD death was not observed (during an observation period of > 100 days) in the recipients of either WT or GKO BALB/c CD8 T cells (Figure 4A), significant loss of body weight was again observed in nonleukemic recipients of GKO BALB/c (19.5 ± 0.9 g), but not in nonleukemic recipients of WT BALB/c (20.7 ± 1.1 g) cells at week 1 after BMT (P < .05). The average body weight of nonleukemic recipients of syngeneic cells at week 1 after BMT was 20.7 ± 0.9 g. However, GVL effects correlated inversely with the degree of GVHD-related weight loss. Despite the more severe GVHD, as indicated by weight loss in nonleukemic recipients of GKO BALB/c CD8 cells, the survival of leukemic recipients of GKO BALB/c CD8 cells was not significantly extended compared to that of syngeneic controls (P = .17). In contrast, the survival of leukemic mice transplanted with WT BALB/c CD8 cells was significantly prolonged (Figure 4A; P < .005 compared to leukemic recipients of syngeneic BMT or GKO BALB/c cells).
Allogeneic CD8 T cells induce more severe systemic GVHD but less marked GVL effects if they are incapable of IFN-γ production.
Survival is shown for nonleukemic and leukemic B6 recipients of 5 × 106 TCD B6 BMCs (syngeneic), or 5 × 106 TCD B6 BMCs plus 5 × 106 TCD GKO BALB/c BMCs and 1 × 106 (A) or 2.5 × 106(B) WT BALB/c CD8+ splenocytes (WT BALB), or 5 × 106 TCD B6 BMCs plus 5 × 106 TCD GKO BALB/c BMCs and 1 × 106 (A) or 2.5 × 106(B) GKO BALB/c CD8+ splenocytes (GKO BALB). Leukemic recipients were injected with 500 EL4 cells along with the BMT inoculum. Survival data from 2 independent experiments (panels A and B) are shown and the numbers of mice in each group of the 2 experiments are indicated inside parentheses (a and b are mouse numbers of the indicated group in experiment A and experiment B, respectively).
Allogeneic CD8 T cells induce more severe systemic GVHD but less marked GVL effects if they are incapable of IFN-γ production.
Survival is shown for nonleukemic and leukemic B6 recipients of 5 × 106 TCD B6 BMCs (syngeneic), or 5 × 106 TCD B6 BMCs plus 5 × 106 TCD GKO BALB/c BMCs and 1 × 106 (A) or 2.5 × 106(B) WT BALB/c CD8+ splenocytes (WT BALB), or 5 × 106 TCD B6 BMCs plus 5 × 106 TCD GKO BALB/c BMCs and 1 × 106 (A) or 2.5 × 106(B) GKO BALB/c CD8+ splenocytes (GKO BALB). Leukemic recipients were injected with 500 EL4 cells along with the BMT inoculum. Survival data from 2 independent experiments (panels A and B) are shown and the numbers of mice in each group of the 2 experiments are indicated inside parentheses (a and b are mouse numbers of the indicated group in experiment A and experiment B, respectively).
Consistent results were observed in a repeat experiment, in which allogeneic recipients were injected with a higher number (2.5 × 106) of WT or GKO BALB/c CD8 T cells. As shown in Figure 4B, 40% of nonleukemic recipients of GKO BALB/c CD8 cells died of GVHD, whereas all nonleukemic recipients of WT BALB/c CD8 cells survived long-term (> 100 days). In addition, the mean body weight of nonleukemic recipients of GKO BALB/c CD8 cells was significantly lower than that of nonleukemic recipients of WT BALB/c CD8 cells (19 ± 1.1 g versus 21 ± 1.2 g at week 1;P < .05). However, unlike GVHD, the potency of GKO BALB/c CD8 T cell–mediated GVL effects was not greater than that of WT BALB/c CD8 T cells (Figure 4B; P = .19). A significant delay in leukemic death was seen in leukemic recipients of both WT BALB/c (P < .0005) and GKO BALB/c (P < .005) CD8 cells compared to leukemic recipients of syngeneic BMT. Because most nonleukemic recipients (8 of 9) were surviving by the time when all leukemic recipients had died in recipients of GKO BALB/c CD8 cells, leukemia, but not GVHD, was the presumed cause of death in the leukemic group. Together, these results indicate that IFN-γ produced by donor CD8 T cells contributes to their GVL effects while mitigating their capacity to cause GVHD.
Discrepancy between systemic GVHD and antihost lymphohematopoietic alloreactivity in recipients of GKO BALB/c CD8 T cells
Previous studies in humans and animal models have shown that lymphohematopoietic GVH reactions that selectively eliminate host lymphohematopoietic cells, including lymphoma cells, can be induced without severe systemic GVHD in allogeneic BMT recipients.33-35 To determine whether or not the loss of GVL effects in recipients of GKO CD8 T cells was due to a reduced lymphohematopoietic GVH reaction, we compared donor CD8 T-cell expansion and levels of residual host hematopoietic cells in recipients of WT or GKO allogeneic BMT along with TCD host-type BMCs. Nonleukemic recipients of WT BALB/c or GKO BALB/c CD8 cells (these are the same mice shown in Figure 4A) were bled at weeks 5 and 9 after BMT, and the levels of donor CD8 T cells and surviving host cells in the recipient WBCs were determined by FACS analysis. Because these mice were injected with 5 × 106 TCD B6 BMCs along with allogeneic cells, lymphohematopoietic GVH reactions were assessed by measuring the preservation of host-type hematopoiesis. Consistent with the increased severity of systemic GVHD as shown by weight loss, the extent of GKO donor CD8 T-cell expansion was significantly greater than that of WT CD8 T cells in B6 recipients (Figure 5A). However, this greater expansion of GKO BALB/c CD8 T cells that was associated with significant loss of recipient body weight was associated with poor alloreactivity against host lymphohematopoietic cells. The levels of host (H-2Kb+) peripheral blood cells in mice receiving transplants with GKO BALB/c CD8 T cells were similar to and higher than those in the recipients of WT BALB/c CD8 T cells at weeks 5 and 9, respectively (Figure 5B). The difference was even more significant when comparing the levels of host-type cells in non–T-cell lineages (ie, when expanded donor T cells were excluded). As shown in Figure 5C, the percentages of host B cells (ie, percent of H-2Kb+CD19+ cells in the CD19+ cell population) in recipients of GKO BLAB/c CD8 T cells were markedly higher than that in recipients of WT CD8 T cells. These results indicate that allogeneic CD8 T cells induce more severe systemic GVHD but weaker antihost hematopoietic alloreactivity (and an associated reduction in GVL effects) if they are incapable of producing IFN-γ.
Discrepancy between donor T-cell expansion and antihost lymphohematopoietic alloreactivity in recipients of GKO BALB/c CD8 T cells.
WBCs were prepared from nonleukemic recipients of 5 × 106 TCD B6 BMCs plus 5 × 106 TCD GKO BALB/c BMCs and 1 × 106 purified CD8+splenocytes from WT (▪; n = 10) or GKO (■; n = 10) BALB/c mice at indicated times. Levels of donor CD8 T (5F1−CD8+) cells (A) and total host type (5F1+, ie, H-2Kb+) cells (B) in the WBCs, and percentages of host B cells (5F1+CD19+) in the B-cell (CD19+) population (C) were determined by FACS. Data are presented as group means (±SD). These mice are the same nonleukemic WT BALB and GKO BALB recipients shown in Figure 4A. *P < .05, **P < .01, ***P < .0001.
Discrepancy between donor T-cell expansion and antihost lymphohematopoietic alloreactivity in recipients of GKO BALB/c CD8 T cells.
WBCs were prepared from nonleukemic recipients of 5 × 106 TCD B6 BMCs plus 5 × 106 TCD GKO BALB/c BMCs and 1 × 106 purified CD8+splenocytes from WT (▪; n = 10) or GKO (■; n = 10) BALB/c mice at indicated times. Levels of donor CD8 T (5F1−CD8+) cells (A) and total host type (5F1+, ie, H-2Kb+) cells (B) in the WBCs, and percentages of host B cells (5F1+CD19+) in the B-cell (CD19+) population (C) were determined by FACS. Data are presented as group means (±SD). These mice are the same nonleukemic WT BALB and GKO BALB recipients shown in Figure 4A. *P < .05, **P < .01, ***P < .0001.
CD4−CD8− donor splenocytes are required for CD8 T cells to express maximal GVL effects
Although the GVL effect against EL4 in this model is donor CD8 T cell dependent, neither WT nor GKO CD8 T cells, when injected with GKO TCD BMCs, led to complete protection from leukemia induced by 500 EL4 cells (Figure 4), whereas complete protection was observed in recipients of this EL4 dose with CD4-depleted WT donor cells (Figures 1and 2). These results suggest that donor CD4−CD8− spleen cells or IFN-γ–producing BMCs or both play a role in the anti-EL4 GVL effect, which has been shown to be donor CD8 T-cell dependent.20,28 To address this possibility, we compared GVL effects in B6 recipients of 500 EL4 cells and 5 × 106 TCD WT BALB/c BMCs along with different populations of WT BALB/c splenocytes: (1) 8.5 × 106 CD4-depleted spleen cells (with 16.5% CD8+ cells); (2) 1.4 × 106 CD8+splenocytes; (3) 7.1 × 106 TCD (ie, CD4 and CD8 cell-depleted) splenocytes; or (4) 1.4 × 106CD8+ and 7.1 × 106 TCD splenocytes. Syngeneic controls were injected with 5 × 106 TCD B6 BMCs and 500 EL4 cells. Consistent with previous studies20,28 and the results described above (Figures 1and 2), GVL effects against EL4 cells were completely abolished by depletion of CD4+ and CD8+ splenocytes (P = .1 for the recipients of TCD BALB/c splenocytes compared to syngeneic controls), whereas they were preserved if only CD4+ splenocytes were depleted (P < .0005 for the recipients of CD4-depleted BALB/c splenocytes compared to syngeneic controls; Figure 6). Although leukemic mortality was delayed in mice receiving TCD BALB/c BMCs plus purified CD8+ BALB/c splenocytes compared to syngeneic controls (P < .005), all of these mice eventually succumbed to leukemia (Figure 6). However, the GVL effects were fully restored by adding TCD (CD4−CD8−) splenocytes back to purified CD8+ splenocytes. The potency of GVL effects in the recipients of a combination of CD8+ and TCD BALB/c splenocytes was significantly greater than that in mice receiving CD8+ BALB/c splenocytes only (P < .005), and was indistinguishable from that in recipients of CD4-depleted BALB/c splenocytes (P = .6; Figure 6). Thus, donor CD4− CD8− splenocytes, which do not mediate GVL effects when injected alone, act synergistically with CD8 T cells to augment the antileukemic alloreactivity of CD8 T cells. Furthermore, these results confirm our previous results20 28 that donor CD4 cells do not contribute to GVL effects in this model.
CD4−CD8− splenocytes act synergistically with CD8 T cells to augment GVL effects in vivo.
Survival is shown for mice that received 500 EL4 cells and 5 × 106 TCD WT BALB/c BMCs along with 8.5 × 106 CD4-depleted (●; n = 7), 1.4 × 106 CD8+ (♦; n = 7), 7.1 × 106 TCD (▴; n = 7), or 1.4 × 106 CD8+ and 7.1 × 106TCD (▾; n = 7) WT BALB/c splenocytes. Syngeneic controls receiving 5 × 106 TCD B6 BMCs (■; n = 5) or 5 × 106 B6 BMC plus 500 EL4 cells (▪; n = 5) are indicated. FACS analysis revealed that CD4-depleted splenocytes contained 16.5% CD8+ and 83.5% CD4−CD8− cells, so the actual numbers of BALB/c CD8+ and CD4−CD8−splenocytes given to the recipients of 8.5 × 106CD4-depleted BALB/c splenocytes were 1.4 × 106 and 7.1 × 106, respectively.
CD4−CD8− splenocytes act synergistically with CD8 T cells to augment GVL effects in vivo.
Survival is shown for mice that received 500 EL4 cells and 5 × 106 TCD WT BALB/c BMCs along with 8.5 × 106 CD4-depleted (●; n = 7), 1.4 × 106 CD8+ (♦; n = 7), 7.1 × 106 TCD (▴; n = 7), or 1.4 × 106 CD8+ and 7.1 × 106TCD (▾; n = 7) WT BALB/c splenocytes. Syngeneic controls receiving 5 × 106 TCD B6 BMCs (■; n = 5) or 5 × 106 B6 BMC plus 500 EL4 cells (▪; n = 5) are indicated. FACS analysis revealed that CD4-depleted splenocytes contained 16.5% CD8+ and 83.5% CD4−CD8− cells, so the actual numbers of BALB/c CD8+ and CD4−CD8−splenocytes given to the recipients of 8.5 × 106CD4-depleted BALB/c splenocytes were 1.4 × 106 and 7.1 × 106, respectively.
IFN-γ does not directly inhibit the proliferation of EL4 cells
Interferon-γ has been shown to mediate antitumor effects by directly inhibiting tumor cell growth and inducing T cell–mediated antitumor responses.36-41 To determine whether the reduced GVL effect in leukemic recipients of GKO allogeneic cells is due to the loss of direct inhibition of EL4 cell proliferation by donor-derived IFN-γ, we measured the susceptibility of EL4 cells to an IFN-γ–mediated antiproliferative effect. EL4 cells were incubated with varying concentrations of IFN-γ for 48 hours and cell proliferation was assessed by tritiated thymidine incorporation. Despite the expression of IFN-γ receptor on their surface (Figure7A), the proliferation of EL4 cells was not significantly inhibited by IFN-γ, while IFN-γ efficiently inhibited the growth of WEHI-279, an IFN-γ–susceptible murine lymphoma cell line (Figure 7B). Moreover, no suppression of EL4 proliferation was mediated by supernatants of BALB/c–anti-B6 mixed lymphocyte reactions, suggesting that cytokines released by alloreactive T cells are incapable of directly suppressing the growth of EL4 cells (Figure 7C).
EL4 cells are not susceptible to an IFN-γ–mediated antiproliferative effect.
(A) A FACS profile showing expression of IFN-γ receptor on EL4 cells. EL4 cells stained with anti-CD119 (IFN-γ receptor α chain) and isotype control mAb are presented as filled and open histograms, respectively. Results from one representative experiment of 3 are shown. (B) Proliferation of EL4 and WEHI-279 cells cultured in medium containing various concentrations of IFN-γ. Data are presented as the mean ± SD (cpm) of triplicate cultures in each culture condition. Results from one representative experiment of 3 are shown. (C) Lack of inhibitory activity on the proliferation of EL4 cells in allogeneic mixed lymphocyte reaction supernatants. EL4 cells were cultured inside tissue culture inserts placed in 24-well plates containing responders (BALB/c splenocytes) and stimulators (irradiated B6 splenocytes) (▪) or stimulators only (■) (see “Materials and methods”). Three wells from each group were harvested at each time point and the number of viable EL4 cells in each well was counted. Data are presented as the mean ± SD (cell number/well) of triplicate samples.
EL4 cells are not susceptible to an IFN-γ–mediated antiproliferative effect.
(A) A FACS profile showing expression of IFN-γ receptor on EL4 cells. EL4 cells stained with anti-CD119 (IFN-γ receptor α chain) and isotype control mAb are presented as filled and open histograms, respectively. Results from one representative experiment of 3 are shown. (B) Proliferation of EL4 and WEHI-279 cells cultured in medium containing various concentrations of IFN-γ. Data are presented as the mean ± SD (cpm) of triplicate cultures in each culture condition. Results from one representative experiment of 3 are shown. (C) Lack of inhibitory activity on the proliferation of EL4 cells in allogeneic mixed lymphocyte reaction supernatants. EL4 cells were cultured inside tissue culture inserts placed in 24-well plates containing responders (BALB/c splenocytes) and stimulators (irradiated B6 splenocytes) (▪) or stimulators only (■) (see “Materials and methods”). Three wells from each group were harvested at each time point and the number of viable EL4 cells in each well was counted. Data are presented as the mean ± SD (cell number/well) of triplicate samples.
IFN-γ up-regulates expression of Fas and major histocompatibility class I on EL4 cells and moderately increases the susceptibility of EL4 cells to the cytotoxicity of allogeneic CD8 T cells
Interferon-γ has been shown to augment the sensitivity of tumor cells to cytolytic T lymphocyte (CTL) activity by up-regulating surface expression of Fas and major histocompatibility complex (MHC) on tumor cells.42 43 To determine whether or not IFN-γ affects the expression of Fas and class I MHC on EL4 cells, we have analyzed the cell surface expression of these molecules on EL4 cells treated with IFN-γ in comparison with untreated EL4 cells. EL4 cells were incubated with IFN-γ at various concentrations (7 different concentrations from 0.78 to 50 ng/mL) for 12 to 14 hours. Both Fas and MHC class I expression were up-regulated on EL4 cells treated with IFN-γ at all concentrations compared to control EL4 cells incubated in IFN-γ–free medium (Figure 8A and data not shown). The peak expression for both molecules was observed on EL4 cells treated with IFN-γ in a concentration range of 6.25 to 12.5 ng/mL (Figure 8A). CTL assays revealed that EL4 cells are highly sensitive to the killing activity of both WT and GKO BALB/c CTLs (Figure 8B). Indeed, the levels of Fas and MHC class I expression were high even on untreated EL4 cells (Figure 8A). Consistently, the susceptibility of EL4 cells to alloreactive CTLs was only slightly increased by pretreatment with IFN-γ (Figure 8B).
Effect of IFN-γ on expression of Fas and MHC class I on EL4 cells and susceptibility of EL4 cells to the cytotoxicity of allogeneic CD8 T cells.
(A) IFN-γ up-regulates Fas and MHC class I expression on EL4 cells. Surface expression of Fas and MHC class I on EL4 cells cultured in the absence (dotted histograms) or the presence of IFN-γ (6.25 ng/mL; solid histograms) is shown. (B) Killing of EL4 cells by alloreactive CTLs. Spleen cells from WT or GKO BALB/c mice were stimulated with irradiated B6 splenocytes for 5 days, and the killing activity against untreated EL4 (○) or EL4 cells that were cultured with IFN-γ (6.25 or 12.5 ng/mL for 12-14 hours) (●) was measured by a 51Cr-release assay. Data are presented as the average (percent specific lysis) of triplicates. Results from one representative experiment of at least 3 independent experiments are shown.
Effect of IFN-γ on expression of Fas and MHC class I on EL4 cells and susceptibility of EL4 cells to the cytotoxicity of allogeneic CD8 T cells.
(A) IFN-γ up-regulates Fas and MHC class I expression on EL4 cells. Surface expression of Fas and MHC class I on EL4 cells cultured in the absence (dotted histograms) or the presence of IFN-γ (6.25 ng/mL; solid histograms) is shown. (B) Killing of EL4 cells by alloreactive CTLs. Spleen cells from WT or GKO BALB/c mice were stimulated with irradiated B6 splenocytes for 5 days, and the killing activity against untreated EL4 (○) or EL4 cells that were cultured with IFN-γ (6.25 or 12.5 ng/mL for 12-14 hours) (●) was measured by a 51Cr-release assay. Data are presented as the average (percent specific lysis) of triplicates. Results from one representative experiment of at least 3 independent experiments are shown.
Discussion
The data presented here demonstrate that IFN-γ produced by donor cells controls the alloresponses of donor CD8 T cells and determines whether they will mediate predominantly GVHD or lymphohematopoietic GVH reactions with associated GVL effects. Alloresponses mediated by GKO CD4-depleted (or purified CD8+) BALB/c splenocytes resulted in more severe GVHD (weight loss and mortality), but in reduced GVL effects in B6 recipients, compared to a similar cell population from WT BALB/c mice. In association with the increased severity of GVHD, the expansion of GKO donor CD8 T cells in vivo was significantly greater than that of WT donor CD8 T cells. However, the increased donor CD8 T-cell expansion was paradoxically associated with a reduction of lymphohematopoietic GVH reactions, which is able to mediate GVL effects without GVHD. It has been observed both in man and in animal models that a conversion from mixed chimerism to fully allogeneic donor chimerism can occur without clinical GVHD, demonstrating that lymphohematopoietic GVH reactions can be selectively preserved in allogeneic BMT recipients while the capacity to mediate tissue GVHD is suppressed.33-35 Such GVH reactions directed against host lymphohematopoietic cells can eliminate host leukemic cells and lead to long-term remissions in recipients of allogeneic BMT who have lymphomas.35 78 Results from the present study indicate that IFN-γ production by donor cells plays an important role in the induction of CD8 T cell–mediated lymphohematopoietic GVH reactions. Importantly, GVHD-inducing alloresponses of CD8 T cells display a differential response to IFN-γ, such that IFN-γ acts as an inhibitory cytokine.
The mechanism for the discrepancy between augmented systemic GVHD (identified as weight loss and mortality) and reduced lymphohematopoietic GVH reactions in the recipients of GKO BALB/c cells remains to be defined. It has been proposed that Th1 cytokines are critical for inducing acute GVHD,44 and a number of studies have shown that IFN-γ produced by activated alloreactive T cells plays an important role in the induction of acute GVHD.45-48 In contrast, both exogenously injected and endogenously produced IFN-γ has been shown to paradoxically inhibit GVHD,49-52 and lethal acute GVHD can be induced in the complete absence of IFN-γ.22 IFN-γ has been reported to play an important role in regulating the death of activated CD4 T cells.53-59 Consistently, Fas expression on donor T cells is required for IL-12–mediated inhibition of CD4-mediated GVHD, an IFN-γ–dependent effect.22,60 In the 2C BMT model, we have previously shown that WT 2C CD8 cells expanded greatly (about 15-fold) in BALB/c recipients at early times after transplantation. This expansion was followed by a rapid decline in 2C CD8 cell numbers (7- to 15-fold).29 Importantly, apoptosis was evident in a significant proportion of host antigen–activated 2C CD8 T cells in allogeneic transplant recipients, and the timing of the increase in apoptosis of 2C donor CD8 T cells coincided with the decrease in the numbers of 2C CD8 T cells in lethally irradiated BALB/c recipients of 2C splenocytes.29 It has been recently demonstrated that IFN-γ also contributes to the death phase of activated CD8 T cells.61 62 Thus, it is possible that the increased expansion of donor CD8 T cells and associated augmentation of GVHD in recipients of IFN-γ–deficient donor cells was due to decreased death of alloreactive CD8 T cells.
However, reduced death of alloreactive donor CD8 T cells alone cannot explain the opposing effects of IFN-γ on the 2 types of alloreactivity (ie, GVHD-inducing activity and lymphohematopoietic GVH response/GVL effects) mediated by CD8 T cells. Thus, other mechanisms must be involved. These may include effects of IFN-γ on both the GVL effector cells and the leukemic cells. Results of our ex vivo studies indicate that IFN-γ does not directly suppress the proliferation of EL4 cells (Figure 7), but that this cytokine might slightly increase the sensitivity of EL4 cells to the cytolytic activity of alloreactive CD8 T cells through up-regulation of MHC class I and Fas molecules (Figure 8). However, this small increase in sensitivity of EL4 cells to CTL-mediated killing seems insufficient to explain the observed differences in GVL effects of WT versus GKO CD8 T cells. Differences in the homing of alloreactive donor T cells in the presence and the absence of IFN-γ production might also contribute to the different outcomes observed for GKO and WT allogeneic BMT recipients. IFN-γ plays an important role in regulating chemokine production and thereby directing the tissue infiltration of activated, including alloantigen-primed, T cells.63-67 Studies using an immunogenic tumor model demonstrated that the failure of cytolytic effectors (“tumor-antigen”–specific CD8 T cells) to remain at the site of the tumor is a major limitation in the ability of CD8 T-cell responses to control tumor growth.68 Contact-dependent lysis is also critical for alloreactive CTLs to mediate GVL effects in allogeneic BMT recipients. It has been reported that IFN-γ contributes to alloreactive donor T-cell infiltrates in lymphoid tissues and lymphoid hypoplasia associated with GVHD,69 70suggesting that IFN-γ may direct alloresponses toward the lymphohematopoietic system rather than the parenchymal GVHD target tissues. Consistently, the present study showed that GKO donor CD8 T cells that induce more severe GVHD were less efficient in destroying host hematopoietic cells compared to WT donor CD8 T cells (Figure 5). Thus, it is possible that the reduction of GVL effects in mice receiving IFN-γ–deficient donor cells reflects a lack of sufficient contact between donor CD8 T cells and the leukemic cells within the lymphohematopoietic system in these mice, and that the increased GVHD is due to increased T-cell migration into the parenchymal GVHD target tissues.
Although the differentiation of cytotoxic CD8 T cells has been shown to require help from CD4 cells,71,72 the generation of alloreactive CD8 T cells can also be independent of CD4 help.73,74 The striking increase in GVHD mortality in recipients of GKO CD8 T cells or 2C cells demonstrates that this helper-independent CD8 subset is regulated by cell-autonomous IFN-γ production. Our previous studies have shown that the GVL effects of splenic T cells against EL4 can be completely eliminated by depleting CD8+ splenocytes, but are not affected by depleting CD4+ cells, indicating that such effects are CD8 dependent and CD4 independent.20,28 We have now observed that CD4−CD8− splenocytes are required for CD8+ cells to mediate an optimal GVL effect. It has recently been shown that the CD4-independent induction of cytotoxic CD8 T cells against allogeneic tumor cells is dependent on costimulation and can be inhibited by blocking the interaction of either CD40/CD40L or B7/CD28 between antigen-presenting cells (APCs) and CD8 T cells.74 However, it is unlikely that the synergistic effect of CD4−CD8− splenocytes observed in the present study was mediated by donor APCs included in the CD4−CD8− cell population, because donor BMCs that also contain APCs had no such effect (Figures 4 and 6). It is possible that efficient GVL effects may require the presence of both large quantities of IFN-γ and CD8 T cells, and the synergistic effects of CD4−CD8− splenocytes may reflect their capacity to produce IFN-γ. It has been reported that both NK and NKT cells are potential IFN-γ producers and play an important role in regulating alloresponses of T cells.75-77
The present study demonstrated that allogeneic CD8 T cells lacking the capacity for IFN-γ production induce more severe GVHD, but less potent antilymphohematopoietic GVH reactions and antileukemic effects than WT CD8 T cells. The findings suggest that global suppression of IFN-γ production should be avoided in the development of strategies for controlling GVHD in leukemic patients.
We thank Drs Markus Mapara and Yong-mi Kim for critical reading of the manuscript and Sharon Titus for her expert secretarial assistance.
Supported by National Institutes of Health grant RO1 CA79989, American Cancer Society grant IRG-87-007-13, and American Society for Blood and Marrow Transplantation/Orphan Medical New Investigator Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yong-Guang Yang, Bone Marrow Transplantation Section, Transplantation Biology Research Center, Massachusetts General Hospital, MGH East, Bldg 149-5102, 13th St, Boston, MA 02129; e-mail:yongguang.yang@tbrc.mgh.harvard.edu.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal