Nonirradiated bone marrow (BM) venules and sinusoids in murine skull support hematopoietic progenitor cell (HPC) rolling through constitutively expressed endothelial (P- and E-) selectins and VCAM-1. Using intravital microscopy, we tested whether host conditioning with total body irradiation (TBI) changes the molecular mechanisms by which murine HPCs from fetal livers (FL) interact with BM endothelial cells. Although a high dose of TBI did not affect the overall frequency of HPC rolling in BM microvessels, the underlying molecular mechanisms differed from those in nonirradiated BM. TBI induced VCAM-1 up-regulation in BM microvessels, whereas P-selectin expression was reduced and the low baseline level of E-selectin remained unchanged. Only the administration of anti–VCAM-1, but not anti–P- or –E-selectin monoclonal antibodies, decreased FL HPC rolling. Rolling was frequently followed by firm arrest (sticking), even in nonirradiated BM microvessels in which sticking was entirely pertussis toxin–insensitive—that is, Gαi-coupled signaling events (eg, through chemokines) were apparently not required. TBI increased the frequency of sticking FL HPC. This irradiation-induced additional sticking was reversed when FL HPCs were pretreated with pertussis toxin, suggesting that TBI induced elevated expression of a Gαi-protein–coupled chemotactic signal in the BM. This chemoattractant was probably distinct from SDF-1α because, unlike adult HPCs, FL HPCs (day 11 of gestation) responded poorly to SDF-1α in vitro. These results demonstrate that TBI induces profound changes in the expression of endothelial traffic molecules in the BM, and they indicate that FL HPCs can home to the BM in the absence of SDF-1α and other Gαi-protein–coupled signals.
Introduction
Bone marrow transplantation (BMT) has been increasingly used to replace the BM of patients with inborn errors of metabolism, malignancies, and chronic viral infections with hematopoietic progenitor cells (HPCs) from a healthy donor. BMT is also used to induce immunologic tolerance to other transplanted organs.1-3 In nearly all these clinical settings, recipients of donor HPCs must be preconditioned by total body irradiation (TBI) before receiving a BM graft.1 In patients receiving allografts, preconditioning prevents alloresponse against the graft by mature T and B cells. Animal studies also suggest that engraftment is more complete in irradiated recipients,4 5 presumably because of improved access for transplanted HPCs to supportive niches within the BM.
Outside the BM, irradiation increases leukocyte interaction with endothelium. This effect of TBI is caused by the induction of endothelial cell (EC) adhesion molecules, similar to changes seen during inflammation.6 However, HPC homing may not require the up-regulation of adhesion molecules above their constitutive levels7-9: some studies report a decrease in HPC accumulation in the BM shortly after irradiation,10,11even though others found increased expression of VCAM-1 on irradiated BM ECs.7 Whether TBI affects the expression of other endothelial surface adhesion molecules (eg, selectins) in BM has not been determined.
P- and E-selectin are constitutively expressed in normal BM, where they mediate HPCs rolling together with VCAM-1, which interacts with α4β1 (VLA-4) on HPCs.8 Strong adhesion through integrins is also required for rolling cells to arrest,12but the mechanisms of HPC arrest in normal and irradiated BM microvessels are unknown. Stimuli, such as chemokines, modify integrin affinity and avidity. Recent studies implicate the CXC chemokine stromal derived factor-1α (SDF-1α) and its receptor, CXCR4, in HPC trafficking to the BM.13-18 SDF-1α–mediated migration was required for homing and engraftment of human HPCs in NOD/SCID mice,16 and HPCs from CXCR4-deficient mice engrafted poorly in lethally irradiated syngenic recipients.17 18However, little is known about the precise mechanism(s) by which the CXCR4/SDF-1α pathway mediates the engraftment of FL HPCs.
Our study examines the effects of TBI on the expression of adhesion molecules and chemoattractants on BM ECs, and it explores the consequences of these effects for HPC adhesion in BM microvessels. We have shown previously that fetal liver (FL) HPCs from 11-day-old embryos roll in normal BM microvessels using the same adhesion pathways as murine HPC cell lines.8 FL cells, whose functional properties and adhesion molecules are largely similar to those of adult HPCs,19,20 not only seed the BM during late gestation but also efficiently repopulate the irradiated BM of adult recipients after adoptive transfer.21 Thus, FL is a relevant source of HPCs. We show that TBI did not change the overall frequency of FL HPC rolling in BM, but the molecular mechanisms mediating this process differed from those in nonirradiated BM. VCAM-1, but not P- and E-selectin, supported FL HPC rolling in irradiated BM. Indeed, irradiation caused the up-regulation of VCAM-1, but endothelial selectins were largely lost from the lumen of BM microvessels. However, unlike rolling, sticking was increased after irradiation because of the elevated expression of an unidentified G-protein–coupled signal probably distinct from SDF-1α.
Materials and methods
Antibodies and reagents
Monoclonal antibodies 9A9 and 5H1, which neutralize mouse E- and P-selectin, respectively, were provided by Dr B. Wolitzky (Hoffman LaRoche, Nutley, NJ); Mel-14 (anti–L-selectin), TIB213 (anti-CD11a), and M1/70 (anti-CD11b) were a gift from Dr E. Butcher (Stanford University, CA), and anti-CD44 monoclonal antibody (mAb) KM81 was a gift from Dr M. Siegelman (University of Texas Southwest Medical Center, Dallas). Anti–VCAM-1–producing hybridoma cells (MK 2.7) were obtained from ATCC (Manassas, VA). Monoclonal antibody was purified from culture supernatants on a high-performance liquid ion-exchange chromatography column (Bakerbond, J. T. Baker, Phillipsburg, NJ). Antihuman/mouse SDF-1 (clone 79014.111) was from R&D Systems (Minneapolis, MN).
For covalent protein coupling to fluorescent microspheres, mAbs to VCAM-1, P-selectin, E-selectin, and isotype controls were purchased from PharMingen (San Diego, CA). The following flow cytometry reagents were from PharMingen: anti-CD16/CD32 Fc block; phycoerythrin (PE)–conjugated or biotinylated anti–c-Kit; PE-conjugated mAbs to Ter119, Gr-1, B220, CD11b, Thy1.2, L-selectin, CD44, CD11a, CD11b, α4, α5, α4β7 complex, P-selectin glycoprotein ligand 1 (PSGL-1); fluorescein isothiocyanate (FITC)–conjugated mAb to CD34; isotype-matched controls; and CY-chrome–conjugated streptavidin.
Animals
Adult C57Bl6/J mice of both sexes were used. In some experiments, mice deficient in FucT-IV and FucT-VII (FucT−/−)22 were used as FL cell donors. Some animals were irradiated using a single dose (9 Gy) from a cesium source (Mark 1 Irradiator; JL Shepherd & Associates, San Fernando, CA). Animals were kept under VAF/SPF barrier conditions on standard laboratory chow and sterile water ad libitum. Experimental protocols were approved by the Standing Committees on Animals of Harvard Medical School and the Center for Blood Research.
Cells
HPCs were isolated from FLs generated by timed matings of FucT−/− or wild-type (WT) mice. Females were killed 11 days after observation of a vaginal plug. FL cell suspensions were prepared by 1-hour incubation at 37°C in 0.05% collagenase type 1 (Worthington Biochemical, Lakewood, NJ) followed by mechanical dissipation. For intravital microscopy (IVM) experiments, cells were labeled with 2′,7′-bis-(carboxyethyl)-5(and-6) carboxyfluorescein (BCECF; Molecular Probes, Eugene, OR). After staining for 30 minutes at 37°C (2.5 μg BCECF/107 cells), cells were washed and resuspended to 5 × 106/mL in RPMI 1640 (BioWhittaker, Walkersville, MD) containing 10% fetal calf serum (JRH Biosciences, Lenexa, KS). Cell viability was determined by trypan blue exclusion. In some experiments, FL HPCs were treated with pertussis toxin (PTX; CalBiochem, La Jolla, CA). Cells were resuspended to 107/mL in RPMI 1640 with 10% fetal calf serum (FCS) and 100 ng/mL PTX, incubated at 37°C for 2 hours, washed, resuspended to 5 × 106/mL, and used for IVM.
Flow cytometry
Surface expression of adhesion molecules on day 11 FL cells and adult BM HPCs was assessed by flow cytometry. Freshly harvested cells were resuspended to 0.5 × 106/mL in ice-cold PBS with 1% FCS. Anti-CD16/CD32 Fc block was added, and aliquots were labeled with specific or control mAbs using standard procedures. Stained cells were analyzed using a FACScan flow cytometer (Becton Dickinson).
Intravital microscopy
Mice were prepared for IVM of skull BM as described.8 HPC behavior was analyzed by determining the rolling fraction (RF), sticking fraction (SF), and sticking efficiency. RF was the number of interacting cells in each vessel per 100 cells passing through the same vessel; SF was the number of cells that arrested for 30 seconds or more per 100 rolling cells; and sticking efficiency was the percentage of firmly adherent (30 seconds or longer) cells in the total flux. For in vivo inhibition, 100 μg/mouse mAbs toVCAM-1, P- or E-selectin, or SDF-1α was injected 10 minutes before cell injection. Monoclonal antibodies to L-selectin, CD44, LFA-1, and Mac-1 were incubated with FL cells (100 μg/107 cells, 37°C) for 10 minutes before cell injection.
Covalent coupling of monoclonal antibodies to fluorescent microspheres
Nile red (NR; excitation/emission, 535 nm/575 nm) and yellow green (YG; 505 nm/515 nm) carboxylate-modified microspheres (1.0 μm diameter; Molecular Probes) were covalently labeled with mAbs; 35 μg protein in 2 mL 50 mM MES buffer (Sigma Chemical, St Louis, MO), pH 6.0, was added to 12.5 × 108 beads and incubated at room temperature for 15 minutes, and 2.4 mg 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (Molecular Probes) was added. The mixture was incubated on a rocker at pH 6.5 for 4 hours at room temperature. Next, 100 mM glycine (Fisher Scientific, Pittsburgh, PA) was added for 30 minutes at room temperature. The suspension was centrifuged (5000g, 20 minutes), and the pellet was washed 3 times in phosphate-buffered saline (PBS) and was resuspended in 500 μL PBS containing 1% bovine serum albumin (BSA; Sigma). To control for differences in nonspecific bead properties, NR and YG beads were conjugated to specific and control mAbs, and colors were swapped between experiments.
IVM studies with fluorescent beads in skull BM
Microspheres (5 × 107) of each color were sonicated and resuspended separately in 1 mL 0.01% Tween-20 (Fisher Scientific) in PBS. Nonspecific mAb-coated microspheres were injected intra-arterially, and microsphere accumulation in BM microvessels was videotaped 10 to 15 minutes later using rhodamine and FITC filters for NR and YG beads, respectively. After 15 minutes, when unbound microspheres were cleared from the circulation, specific mAb-conjugated microspheres of the second color were recorded in 8 to 14 fields of view in the same vascular bed. Specific binding was calculated as the number of bound specific mAb-conjugated microspheres divided by the number of bound nonspecific beads.
Accumulation of fluorescent beads in BM of long bones
NR and YG microspheres (7.5 × 107) coated with specific and control mAbs, respectively, were prepared as described above, mixed, and injected intra-arterially in anesthetized control or irradiated mice. An aliquot of the injection mixture was saved to control for differences in bead input using fluorescence-activated cell sorter (FACS) analysis. After 15 minutes, recipients were exsanguinated by cutting the abdominal vena cava and performing whole-body perfusion with ice-cold saline containing 1 U/mL heparin (Sigma). BM was harvested from femora and tibiae, single-cell suspensions were prepared, erythrocytes were lysed, and BM cells were used for duplicate cytospins. NR and YG microspheres were counted using epifluorescence microscopy. The bead accumulation ratio was calculated by dividing the number of specific mAb-coated beads by the number of control beads in the same field.
Chemotaxis assay
Chemotaxis assays were performed in 24-well plates with 5-μm pore size inserts (Costar, Cambridge, MA). Supernatant from the murine BM stroma cell line MS-5 with or without anti–SDF-1 mAb (50 μg/mL) or UltraCulture medium (BioWhittaker) with 1 to 500 ng/mL mouse SDF-1α (R&D Systems) was added to the lower chamber. FL cells or BM mononuclear cells in UltraCulture medium (5 × 106/mL) were loaded onto the inserts. Some cells were treated with PTX as described above. Cells migrating to the bottom well were collected after 3 hours and were counted by FACS after gating on c-Kit+ FL HPCs or Lin− CD34+ BM HPCs. In some experiments, migrated cells were instead plated in 0.9% methylcellulose (Sigma) with 15% FCS, 1% BSA, 0.13 mM β-mercaptoethanol (Sigma), 2.5 U/mL erythropoietin (Amgen, Thousand Oaks, CA), 35 ng/mL recombinant murine SCF (PeproTech, Rocky Hill, NJ), and 35 ng/mL recombinant murine IL-3 (PeproTech). Cultures were maintained at 37°C in 5% CO2. Colony-forming units were counted after 8 days.
Statistics
For comparison of 2 samples, a 2-tailed Student ttest was used. Multiple comparisons were performed by one-way analysis of variance with Bonferroni correction. Significance was set atP < .05.
Results
Effect of TBI on BM microvascular hemodynamics and barrier function
We measured the diameters and hemodynamics of BM microvessels in normal and irradiated mice. Mean blood flow velocity, wall shear rates, and shear stresses (Table 1) were significantly reduced after irradiation. The most prominent differences were observed in BM sinusoids and intermediate venules (described in detail in Mazo et al8), which were typically surrounded by a thick extravascular layer of hematopoietic tissue, suggesting that edematous swelling of the irradiated tissue might have compressed these microvessels (data not shown). Subsequent experiments revealed a massive post-TBI breakdown of endothelial barrier function as evidenced by extravasation of FITC-dextran, which diffused rapidly into BM cavities (Figure 1).
Dimensions and hemodynamic parameters of nonirradiated and irradiated BM microvessels
| Parameters . | Normal BM . | Irradiated BM . | P . |
|---|---|---|---|
| Diameter (μm) | 53.4 ± 6.5 (19-114) | 35.2 ± 4.4 (13-93) | .024 |
| Vrbc(μm/s) | 398.0 ± 72.3 (95-1385) | 156.7 ± 35.5 (28-606) | .0037 |
| Vblood(μm/s) | 387.0 ± 84.0 (92-1766) | 162.0 ± 35.0 (30-570) | .014 |
| WSR (s−1) | 65.7 ± 11.4 (9-283) | 36.8 ± 5.7 (9-125) | .026 |
| WSS (dyn/cm2) | 1.3 ± 0.1 (0.1-2.2) | 0.9 ± 0.1 (0.2-3.1) | .049 |
| Parameters . | Normal BM . | Irradiated BM . | P . |
|---|---|---|---|
| Diameter (μm) | 53.4 ± 6.5 (19-114) | 35.2 ± 4.4 (13-93) | .024 |
| Vrbc(μm/s) | 398.0 ± 72.3 (95-1385) | 156.7 ± 35.5 (28-606) | .0037 |
| Vblood(μm/s) | 387.0 ± 84.0 (92-1766) | 162.0 ± 35.0 (30-570) | .014 |
| WSR (s−1) | 65.7 ± 11.4 (9-283) | 36.8 ± 5.7 (9-125) | .026 |
| WSS (dyn/cm2) | 1.3 ± 0.1 (0.1-2.2) | 0.9 ± 0.1 (0.2-3.1) | .049 |
To visualize blood flow, murine red blood cells were fluorescence labeled ex vivo and injected into the carotid artery of anesthetized mice 48 hours after TBI (9 Gy). Fluorescent cells were recorded during their passage through the skull BM, and their velocity and luminal diameters were determined by off-line video analysis using a PC-based image analysis system.60 Data are presented as mean ± SEM. Ranges are given in parentheses.
Vrbc indicates red blood cell velocity; Vblood, mean blood flow velocity; WSR, wall shear rate; WSS, wall shear stress.
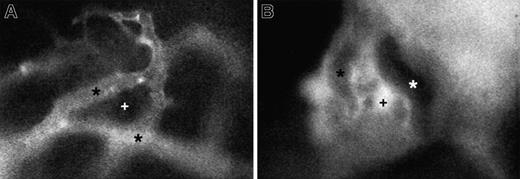
Representative micrographs of 150-kd FITC-dextran distribution in skull BM of a nonirradiated mouse and an animal 48 hours after irradiation.
(A) In normal BM, the high–molecular-weight plasma marker stays confined to the lumen of BM venules and sinusoids (*). There is no extravasation into extravascular BM cavities (+). (B) Irradiation-induced damage to BM endothelium causes a massive breakdown of endothelial barrier function, leading to the extravasation of FITC-dextran into BM cavities but not into adjacent solid bone tissue. Because of quenching of fluorescence by hemoglobin-rich erythrocytes, larger microvessels now appear darker than the surrounding extravascular compartment. Recordings in both animals were taken under identical conditions using a × 40 objective.
Representative micrographs of 150-kd FITC-dextran distribution in skull BM of a nonirradiated mouse and an animal 48 hours after irradiation.
(A) In normal BM, the high–molecular-weight plasma marker stays confined to the lumen of BM venules and sinusoids (*). There is no extravasation into extravascular BM cavities (+). (B) Irradiation-induced damage to BM endothelium causes a massive breakdown of endothelial barrier function, leading to the extravasation of FITC-dextran into BM cavities but not into adjacent solid bone tissue. Because of quenching of fluorescence by hemoglobin-rich erythrocytes, larger microvessels now appear darker than the surrounding extravascular compartment. Recordings in both animals were taken under identical conditions using a × 40 objective.
Surface expression of adhesion molecules on FL and BM HPC
Previous experiments8 show that the minimal number of cells required for one IVM experiment in mouse skull BM is approximately 7 × 106. We obtained a yield of less than approximately 2 × 105 BM CD34+ HPCs (which contain only a small fraction of lin− uncommitted progenitors) from one adult mouse. Thus, it is not feasible to obtain a sufficient number of adult BM HPCs for routine experiments in our model. Therefore, we resorted to isolating FL day 11 of gestation as a source of well-characterized, largely uncommitted HPCs.19,20 At this stage, FLs contained approximately 50% mononuclear cells expressing c-Kit (Figure2), a marker for embryonic HPCs.23 Up to 20% of c-Kit+ FL cells coexpressed CD34 (Table 2). Erythroid Ter119+ cells represented most of the c-Kit−CD34− population (not shown). Because it was difficult to separate c-Kit+ from c-Kit− FL cells without losing a large fraction of HPCs in the process, we used unseparated heterogeneous FL cells for in vivo experiments. Previous work has shown that the Ter119+ fraction is poorly labeled by BCECF and remains under the visualization threshold of our video camera. Consequently, approximately 90% of cells detected in vivo under fluorescence illumination were c-Kit+HPC.8
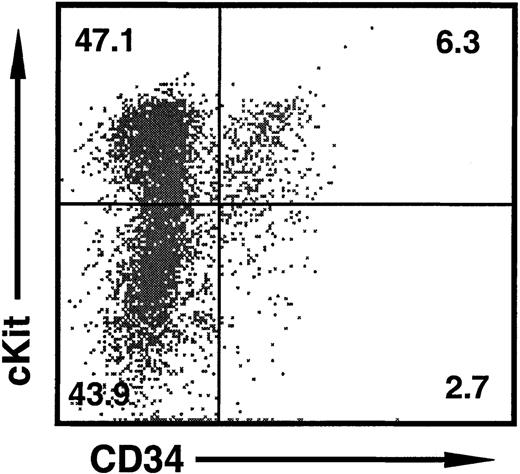
Representative 2-color dot plot of HPC markers on FL cells at day 11 of gestation.
Approximately half of all cells are c-Kit+ HPCs, of which 10% to 20% also express CD34. The c-Kit− population consists of Ter119+ erythroid cells (not shown). The percentage of cells in each quadrant is shown.
Representative 2-color dot plot of HPC markers on FL cells at day 11 of gestation.
Approximately half of all cells are c-Kit+ HPCs, of which 10% to 20% also express CD34. The c-Kit− population consists of Ter119+ erythroid cells (not shown). The percentage of cells in each quadrant is shown.
Repertoire of adhesion molecules on day 11 FL cells and BM HPCs
| Surface marker . | Adult BM CD34+ . | FL day 11 of gestation . | ||
|---|---|---|---|---|
| c-Kit+ CD34+ . | c-Kit+CD34− . | c-Kit+ . | ||
| L-selectin | 65.2 ± 2.5 | 11.6 ± 0.4‡ | 9.8 ± 2.0‡ | 8.2 ± 0.5‡ |
| (Mel-14) | (659 ± 88) | (269 ± 120) | (68 ± 13)† | (244 ± 18)* |
| CD44 | 92.2 ± 2.9 | 82.7 ± 5.7 | 95.7 ± 3.1 | 93.5 ± 0.8 |
| (IM7) | (1069 ± 246) | (1507 ± 38) | (967 ± 19) | (1158 ± 139) |
| CD11a | 71.3 ± 3.5 | 78.6 ± 5.4 | 80.1 ± 15.4 | 63.9 ± 9.4 |
| (2D7) | (462 ± 17) | (647 ± 137) | (348 ± 34) | (596 ± 131) |
| CD11b | 53.6 ± 14.5 | 58.5 ± 1.6 | 18.0 ± 4.2* | 27.3 ± 7.1 |
| (M1/70) | (1204 ± 294) | (730 ± 114) | (158 ± 31)† | (641 ± 139) |
| α4 | 54.0 ± 8.8 | 69.9 ± 4.7 | 99.4 ± 0.3† | 85.8 ± 1.7* |
| (R1-2) | (240 ± 26) | (447 ± 63)* | (599 ± 59)† | (535 ± 53)† |
| α5 | 57.5 ± 12.8 | 87.5 ± 7.7 | 97.6 ± 2.2* | 69.9 ± 11.9 |
| (5H10-27) | (333 ± 26) | (701 ± 26)‡ | (390 ± 138) | (570 ± 102) |
| α4β7 | 7.2 ± 2.6 | 2.2 ± 0.5 | 4.9 ± 2.3 | 0.8 ± 0.5 |
| (DATK 32) | (276 ± 43) | (309 ± 54) | (57 ± 14)* | (466 ± 84) |
| PSGL1 | 63.1 ± 3.5 | 49.9 ± 10.0 | 29.7 ± 8.6* | 36.3 ± 8.5* |
| (2PH1) | (794 ± 17) | (301 ± 26)‡ | (76 ± 14)‡ | (171 ± 22)‡ |
| Surface marker . | Adult BM CD34+ . | FL day 11 of gestation . | ||
|---|---|---|---|---|
| c-Kit+ CD34+ . | c-Kit+CD34− . | c-Kit+ . | ||
| L-selectin | 65.2 ± 2.5 | 11.6 ± 0.4‡ | 9.8 ± 2.0‡ | 8.2 ± 0.5‡ |
| (Mel-14) | (659 ± 88) | (269 ± 120) | (68 ± 13)† | (244 ± 18)* |
| CD44 | 92.2 ± 2.9 | 82.7 ± 5.7 | 95.7 ± 3.1 | 93.5 ± 0.8 |
| (IM7) | (1069 ± 246) | (1507 ± 38) | (967 ± 19) | (1158 ± 139) |
| CD11a | 71.3 ± 3.5 | 78.6 ± 5.4 | 80.1 ± 15.4 | 63.9 ± 9.4 |
| (2D7) | (462 ± 17) | (647 ± 137) | (348 ± 34) | (596 ± 131) |
| CD11b | 53.6 ± 14.5 | 58.5 ± 1.6 | 18.0 ± 4.2* | 27.3 ± 7.1 |
| (M1/70) | (1204 ± 294) | (730 ± 114) | (158 ± 31)† | (641 ± 139) |
| α4 | 54.0 ± 8.8 | 69.9 ± 4.7 | 99.4 ± 0.3† | 85.8 ± 1.7* |
| (R1-2) | (240 ± 26) | (447 ± 63)* | (599 ± 59)† | (535 ± 53)† |
| α5 | 57.5 ± 12.8 | 87.5 ± 7.7 | 97.6 ± 2.2* | 69.9 ± 11.9 |
| (5H10-27) | (333 ± 26) | (701 ± 26)‡ | (390 ± 138) | (570 ± 102) |
| α4β7 | 7.2 ± 2.6 | 2.2 ± 0.5 | 4.9 ± 2.3 | 0.8 ± 0.5 |
| (DATK 32) | (276 ± 43) | (309 ± 54) | (57 ± 14)* | (466 ± 84) |
| PSGL1 | 63.1 ± 3.5 | 49.9 ± 10.0 | 29.7 ± 8.6* | 36.3 ± 8.5* |
| (2PH1) | (794 ± 17) | (301 ± 26)‡ | (76 ± 14)‡ | (171 ± 22)‡ |
Cells were stained for HPC markers and adhesion molecules (names of mAbs used are shown in parentheses). Adult BM HPCs were defined as CD34+ cells and constituted 3% ± 1% of all BM cells. Day 11 FL HPC were defined as c-Kit+ (50% ± 3% of all day 11 FL cells) and were further subdivided into 2 groups: CD34+(9% ± 1%) and CD34− (41% ± 3%). Data are shown for both subsets individually and for all c-Kit+ cells. Results are shown as the mean percentage of marker-positive live cells. Numbers in parentheses represent mean fluorescence intensity. Data are shown as mean ± SEM of at least 3 independent experiments. For statistical comparison, results from FL HPCs were compared to BM-derived CD34+ HPCs.
P ≤ .05.
P ≤ .01.
P ≤ .005.
Although FL HPCs have potent BM homing abilities,24,25they may differ from adult BM HPCs, the standard source of HPCs in clinical settings. Therefore, we compared the expression pattern of adhesion molecules implicated in HPC trafficking on HPC subsets from day 11 FL and adult BM (Table 2). The c-Kit+CD34+ FL subset, containing most HPCs with long-term repopulating activity,20 was similar to that of BM HPCs, except the latter expressed lower levels of α4 and α5 integrins and higher levels of PSGL-1. The c-Kit+CD34− subset showed similar differences but also expressed lower levels of CD11b. Unlike CD34+ BM cells, most FL HPCs were devoid of L-selectin. However, the absence of L-selectin should not impact FL cell behavior: previous work has shown that L-selectin does not contribute to HPC adhesion in the BM.8Thus, despite quantitative differences, HPCs from day 11 FL and adult BM have a qualitatively similar repertoire of relevant traffic molecules, suggesting that FL cells can be used to probe BM microvessels for TBI-induced changes in endothelial adhesiveness.
TBI does not alter the frequency of HPC rolling in BM microvessels but does change the underlying molecular mechanisms
Skull BM microvessels constitutively support rolling of murine progenitor cell lines and day 11 FL HPC.8 Irradiation had no significant effect on the frequency of FL cell rolling irrespective of the time interval between irradiation and HPC injection (Table3). We next studied the molecular mechanisms of FL HPC rolling after TBI. As shown previously, nonirradiated BM microvessels support HPC rolling through P- and E-selectin and VCAM-1. All 3 molecules contribute to the overall rolling frequency and support rolling independently. Thus, day 11 FL HPCs roll at significantly lower frequency in the BM of P- and E-selectin–deficient mice than they do in WT BM, and rolling is further reduced by antibodies to VCAM-1.8 Consistent with these findings, neutralization of P- and E-selectin in WT mice reduced FL HPC rolling by 45.5% ± 18.1% (data not shown). Thus, day 11 FL HPCs interact readily with vascular selectins in normal BM, despite lower levels of PSGL-1 than on adult HPCs.
Effect of irradiation on FL HPC rolling in murine skull BM
| . | No. animals . | No. venules . | Rolling fraction (%) . |
|---|---|---|---|
| Normal BM | 12 | 56 | 18.9 ± 2.3 |
| 3 h after TBI | 11 | 50 | 18.6 ± 2.9 |
| 24 h after TBI | 3 | 9 | 16.8 ± 6.0 |
| 48 h after TBI | 24 | 109 | 23.0 ± 1.4 |
| . | No. animals . | No. venules . | Rolling fraction (%) . |
|---|---|---|---|
| Normal BM | 12 | 56 | 18.9 ± 2.3 |
| 3 h after TBI | 11 | 50 | 18.6 ± 2.9 |
| 24 h after TBI | 3 | 9 | 16.8 ± 6.0 |
| 48 h after TBI | 24 | 109 | 23.0 ± 1.4 |
Rolling fractions were defined as the percentage of interacting cells in all HPCs passing an individual venule or sinusoid during an observation period. Data are shown as mean ± SEM.
In contrast, mAbs to P- or E-selectin alone or in combination had no significant effect in irradiated BM 3 hours after TBI (Figure3A), even though irradiation induces selectin expression and leukocyte rolling in other tissues.6 Because irradiation-induced transcriptional effects may lead to E-selectin up-regulation after more than 3 hours,26 we also tested the roles of P- and E-selectin at 48 hours after irradiation. No significant effect of selectin inhibition was detected (Figure 3B). Anti–L-selectin treatment of FL HPCs did not affect rolling either, consistent with our previous findings8 and the fact that most FL HPCs were L-selectin−. We also tested the role of CD44, which is highly expressed on murine FL HPCs (Sanchez et al20 and Table 2). CD44 contributes to the homing of BM-derived HPCs to irradiated BM27 and mediates the rolling of activated T cells28 and human CD34+ BM cells29 on immobilized hyaluronate. However, a mAb that blocks CD44 binding to hyaluronate had no effect on FL HPC rolling in irradiated BM. In contrast, anti–VCAM-1 inhibited rolling at both time points, similar in magnitude to the effect of anti–VCAM-1 on FL HPC rolling in nonirradiated BM.8
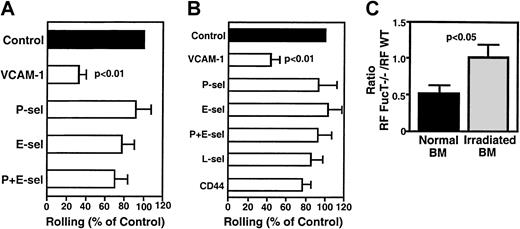
VCAM-1, but not P- or E-selectin, mediates rolling of FL HPCs in irradiated BM.
Effect of blocking mAbs (100 μg/mouse) on rolling frequency was assessed at 3 hours (A) or 48 hours (B) after irradiation. Rolling fractions after mAb treatment were normalized to rolling fractions determined in the same vessel before mAb application (control). Blocking mAb to VCAM-1 inhibited rolling by 67% at 3 hours and by 56% at 48 hours. (C) Rolling of FL HPCs of WT and FucT−/−mice in normal and irradiated BM. Mutant and WT FL cells were injected sequentially, and rolling was compared in the same venules. Data are presented as the ratio of rolling fractions of FucT−/−versus WT FL HPCs. Thus, a ratio of 1 indicates that both subsets rolled with equal frequency, whereas a ratio of 0.5 indicates that WT FL HPCs rolled twice as frequently as FucT−/− FL HPCs. Bars represent mean ± SEM.
VCAM-1, but not P- or E-selectin, mediates rolling of FL HPCs in irradiated BM.
Effect of blocking mAbs (100 μg/mouse) on rolling frequency was assessed at 3 hours (A) or 48 hours (B) after irradiation. Rolling fractions after mAb treatment were normalized to rolling fractions determined in the same vessel before mAb application (control). Blocking mAb to VCAM-1 inhibited rolling by 67% at 3 hours and by 56% at 48 hours. (C) Rolling of FL HPCs of WT and FucT−/−mice in normal and irradiated BM. Mutant and WT FL cells were injected sequentially, and rolling was compared in the same venules. Data are presented as the ratio of rolling fractions of FucT−/−versus WT FL HPCs. Thus, a ratio of 1 indicates that both subsets rolled with equal frequency, whereas a ratio of 0.5 indicates that WT FL HPCs rolled twice as frequently as FucT−/− FL HPCs. Bars represent mean ± SEM.
To confirm that TBI results in the loss of selectin-mediated rolling in the BM, we tested FL HPCs from FucT−/− mice genetically deficient in α1,3-fucosyltransferase IV (FucT-IV) and FucT-VII. Leukocytes in adult FucT−/− animals are devoid of selectin ligands.22 30 The rolling frequency of FucT−/− FL HPCs in nonirradiated BM was only half that of WT FL cells, confirming that FL HPCs interact with endothelial selectins in normal BM and indicating that FucT-IV and -VII are required for selectin ligand synthesis in FL HPCs. In contrast, in irradiated BM, FucT−/− and WT FL HPCs rolled equally, consistent with a post-TBI loss of endothelial selectin activity (Figure 3C).
Thus, endothelial selectins lose their ability to mediate HPC rolling, whereas VCAM-1 continues to initiate and maintain this process. However, rolling was not completely abolished after anti–VCAM-1 treatment, indicating that additional unknown adhesion pathway(s) are involved.
Up-regulation of VCAM-1, but not endothelial selectins, in irradiated BM microvessels
To explore the unexpected finding that selectins do not contribute to HPC rolling in irradiated BM, we analyzed endothelial surface adhesion molecule expression in live BM by using 2 sets of fluorescent beads, visualized by epifluorescence through rhodamine (NR beads) and FITC (YG beads) filters (Figure 4). One set of beads was coated with mAbs to VCAM-1, E-selectin, or P-selectin, whereas the other was conjugated to control mAb to evaluate background binding. Equivalent numbers of both sets were injected into recipient mice, and bead accumulation in skull BM vessels was recorded by IVM.
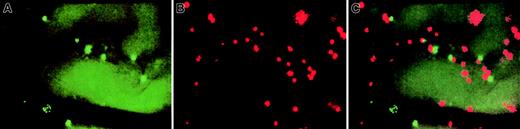
Representative intravital micrographs showing specific accumulation of anti–VCAM-1 beads and control beads in nonirradiated BM.
Equivalent numbers of control and specific mAb-coated beads were injected into the carotid artery, and beads bound to the luminal surface of the vessel wall were counted 10 to 15 minutes later using appropriate filters. (A) Accumulation of control beads (YG) in nonirradiated BM microvessels. (B) Binding of anti–VCAM-1–coated beads (NR) in nonirradiated BM microvessels (same field of view), providing evidence for constitutive expression of VCAM-1 on BM ECs. Because this field was recorded through a filter set optimized for red (rhodamine) fluorescence, the autofluorescence of extravascular tissue was much lower than in panel A, which was recorded through an FITC filter set. Thus, the outline of BM microvessels is undetectable in panel B. (C) Computer-generated overlay of panels A and B. The ratio of specific/nonspecific bead binding in this experiment was 3.7. The variability in apparent size of different beads was due, in part, to the blurring of individual beads located out of the focal plane and, in part, because some signal bleeding occurred when using SIT cameras to record intensely fluorescent particles. All scenes were recorded through a × 20 water immersion objective (Zeiss).
Representative intravital micrographs showing specific accumulation of anti–VCAM-1 beads and control beads in nonirradiated BM.
Equivalent numbers of control and specific mAb-coated beads were injected into the carotid artery, and beads bound to the luminal surface of the vessel wall were counted 10 to 15 minutes later using appropriate filters. (A) Accumulation of control beads (YG) in nonirradiated BM microvessels. (B) Binding of anti–VCAM-1–coated beads (NR) in nonirradiated BM microvessels (same field of view), providing evidence for constitutive expression of VCAM-1 on BM ECs. Because this field was recorded through a filter set optimized for red (rhodamine) fluorescence, the autofluorescence of extravascular tissue was much lower than in panel A, which was recorded through an FITC filter set. Thus, the outline of BM microvessels is undetectable in panel B. (C) Computer-generated overlay of panels A and B. The ratio of specific/nonspecific bead binding in this experiment was 3.7. The variability in apparent size of different beads was due, in part, to the blurring of individual beads located out of the focal plane and, in part, because some signal bleeding occurred when using SIT cameras to record intensely fluorescent particles. All scenes were recorded through a × 20 water immersion objective (Zeiss).
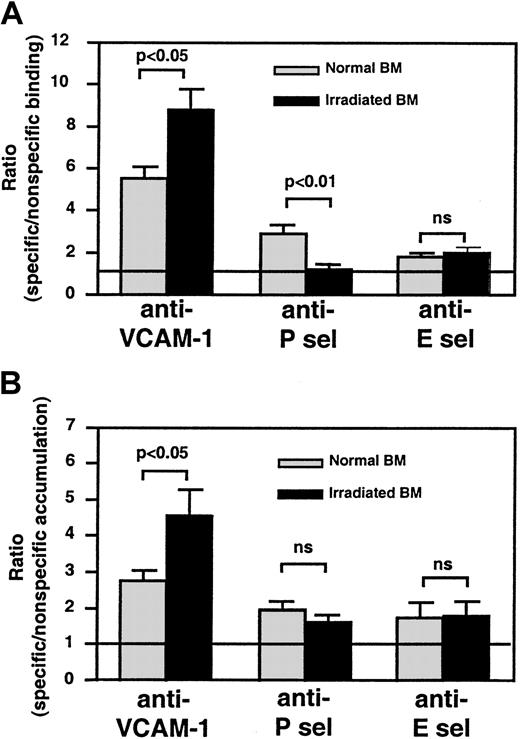
Specific beads to all 3 endothelial antigens bound more frequently in nonirradiated skull BM than beads coated with control mAb (Figure5A). After TBI, anti–VCAM-1–coated bead binding increased significantly (P < .05 vs nonirradiated), whereas anti–P-selectin bead binding was abolished (P < .01 vs nonirradiated). TBI made no difference in the low-level specific binding of anti–E-selectin–coated beads. The concentration of soluble serum P-selectin measured by enzyme-linked immunosorbent assay (ELISA) was similar before and 48 hours after TBI (not shown), excluding the possibility that high concentrations of soluble antigen interfered with anti–P-selectin bead binding. Thus, TBI causes the up-regulation of VCAM-1, little change in E-selectin (at least within the sensitivity limits of our bead assay), and, surprisingly, a dramatic reduction in P-selectin on the luminal surfaces of ECs in skull BM.
Semiquantitative fluorescence bead assay to assess changes in endothelial adhesion molecule expression in BM microvessels before and after TBI.
(A) IVM experiments were performed to quantitate the ratio of specific/nonspecific bead binding in skull BM microvessels, as described in Figure 4. The ratio in nonirradiated BM microvessels was always higher than 1, indicating constitutive expression of all 3 molecules. This was most apparent for VCAM-1 (P < .005) and, to a lesser extent, P-selectin (P < .01), whereas specific binding of anti–E-selectin–coated beads was weak but statistically significant (P < .05). Bead accumulation was analyzed in 8 to 14 fields of view in each experiment. (B) For tissue-specific accumulation of beads in long bones, equal numbers of specific and nonspecific mAb-coated beads were injected into irradiated and nonirradiated mice. Animals were exsanguinated 15 minutes later and were perfused with heparinized ice-cold saline to remove intravascular blood. BM from both femora and tibiae was harvested as described in “Materials and methods,” and a single-cell suspension was produced to generate cytospins. The ratio of specific/nonspecific beads in 20 fields of view per cytospin was determined by fluorescence microscopy and, if necessary, corrected for differences in the bead input ratio; thus, a ratio of 1 (represented by line) indicates no specific binding above background. Bars represent mean ± SEM. ns indicates nonsignificant.
Semiquantitative fluorescence bead assay to assess changes in endothelial adhesion molecule expression in BM microvessels before and after TBI.
(A) IVM experiments were performed to quantitate the ratio of specific/nonspecific bead binding in skull BM microvessels, as described in Figure 4. The ratio in nonirradiated BM microvessels was always higher than 1, indicating constitutive expression of all 3 molecules. This was most apparent for VCAM-1 (P < .005) and, to a lesser extent, P-selectin (P < .01), whereas specific binding of anti–E-selectin–coated beads was weak but statistically significant (P < .05). Bead accumulation was analyzed in 8 to 14 fields of view in each experiment. (B) For tissue-specific accumulation of beads in long bones, equal numbers of specific and nonspecific mAb-coated beads were injected into irradiated and nonirradiated mice. Animals were exsanguinated 15 minutes later and were perfused with heparinized ice-cold saline to remove intravascular blood. BM from both femora and tibiae was harvested as described in “Materials and methods,” and a single-cell suspension was produced to generate cytospins. The ratio of specific/nonspecific beads in 20 fields of view per cytospin was determined by fluorescence microscopy and, if necessary, corrected for differences in the bead input ratio; thus, a ratio of 1 (represented by line) indicates no specific binding above background. Bars represent mean ± SEM. ns indicates nonsignificant.
To test whether BM ECs in long bones respond differently to TBI than those in the skull, we performed a modified experiment by injecting equivalent numbers of specific and nonspecific beads into normal and irradiated recipients. Bead accumulation in femoral and tibial BM was determined by fluorescence microscopy in BM cytospins (Figure 5B). As in the skull, anti–VCAM-1–coated beads accumulated more efficiently after TBI in long bones. No difference in accumulation was seen with anti–E-selectin beads. In 4 of 5 experiments, irradiation resulted in decreased anti–P-selectin bead accumulation, but this tendency did not reach statistical significance (P = .09).
TBI increases HPC sticking, which is mostly mediated by VCAM-1
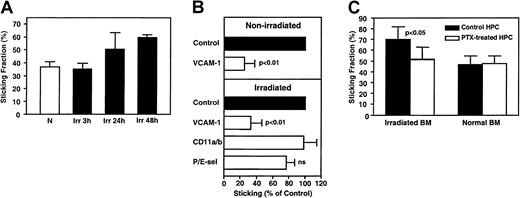
Although TBI did not affect rolling, it increased FL HPC sticking (Figure 6A). Administration of anti–VCAM-1 mAb had a similar and significant effect in nonirradiated and irradiated BM microvessels, reducing sticking fractions by 75% and 67%, respectively. Because rolling was also reduced by anti–VCAM-1 (Figure 3A-B), we conclude that most FL HPCs require VCAM-1 for sticking, even if they have alternative means to roll. Indeed, anti–VCAM-1 reduced the sticking efficiency (percentage of sticking HPCs in the total flux) in normal and irradiated BMs by 94.2% ± 3.2% and 89.4% ± 6.0%, respectively. Thus, VCAM-1 has a dual function in BM microvessels, supporting both rolling and firm arrest. Interestingly, there was no significant effect on HPC sticking by blocking β2 integrins (CD11a and CD11b) or P- and E-selectin function (Figure 6B).
Sticking of murine FL HPCs in nonirradiated and irradiated BM microvessels.
(A) TBI mediates increased HPC sticking. Sticking occurred at a considerable frequency in nonirradiated BM (N, sticking fraction 38.5% ± 3.8%), tended to be increased at 24 hours (Irr, 50.5% ± 12.7%, P > .05 vs N) and was significantly enhanced at 48 hours after TBI (60.3% ± 2.4%,P < .01 vs N). (B) Effect of blocking mAbs on FL HPC sticking. Sticking fractions (defined as percentage of rolling cells) after mAb treatment were normalized to sticking fractions determined in the same vessel before mAb application (control). (C) Only sticking in irradiated BM was partly inhibited by PTX. Cells (107/mL) were incubated with PTX (100 ng/mL) for 2 hours at 37°C. During the last 30 minutes of PTX incubation, treatment was combined with fluorescence staining. Treated cells were washed and used immediately for IVM. Data are presented as mean ± SEM. ns indicates nonsignificant.
Sticking of murine FL HPCs in nonirradiated and irradiated BM microvessels.
(A) TBI mediates increased HPC sticking. Sticking occurred at a considerable frequency in nonirradiated BM (N, sticking fraction 38.5% ± 3.8%), tended to be increased at 24 hours (Irr, 50.5% ± 12.7%, P > .05 vs N) and was significantly enhanced at 48 hours after TBI (60.3% ± 2.4%,P < .01 vs N). (B) Effect of blocking mAbs on FL HPC sticking. Sticking fractions (defined as percentage of rolling cells) after mAb treatment were normalized to sticking fractions determined in the same vessel before mAb application (control). (C) Only sticking in irradiated BM was partly inhibited by PTX. Cells (107/mL) were incubated with PTX (100 ng/mL) for 2 hours at 37°C. During the last 30 minutes of PTX incubation, treatment was combined with fluorescence staining. Treated cells were washed and used immediately for IVM. Data are presented as mean ± SEM. ns indicates nonsignificant.
TBI induces pertussis toxin–sensitive signals that stimulate FL HPC sticking
Our studies indicate that FL HPC sticking is largely mediated by VCAM-1 counter-receptors (most likely α4β1), which must be in a high-affinity state to mediate firm arrest.12 This suggests that irradiation causes BM to increase the production of an integrin activating factor(s), presumably chemokines, which signal through pertussis toxin (PTX)–sensitive Gαiprotein–coupled receptors.31 To test whether FL HPC sticking in BM depends on this mechanism, we compared HPC sticking fraction before and after PTX treatment in normal and irradiated BM (Figure 6C). Forty-eight hours after TBI, PTX treatment significantly attenuated sticking, though most FL HPCs continued to stick in a PTX-independent manner. Interestingly, HPC sticking in nonirradiated BM was equal to sticking of PTX-treated cells in irradiated BM and was not affected by PTX treatment.
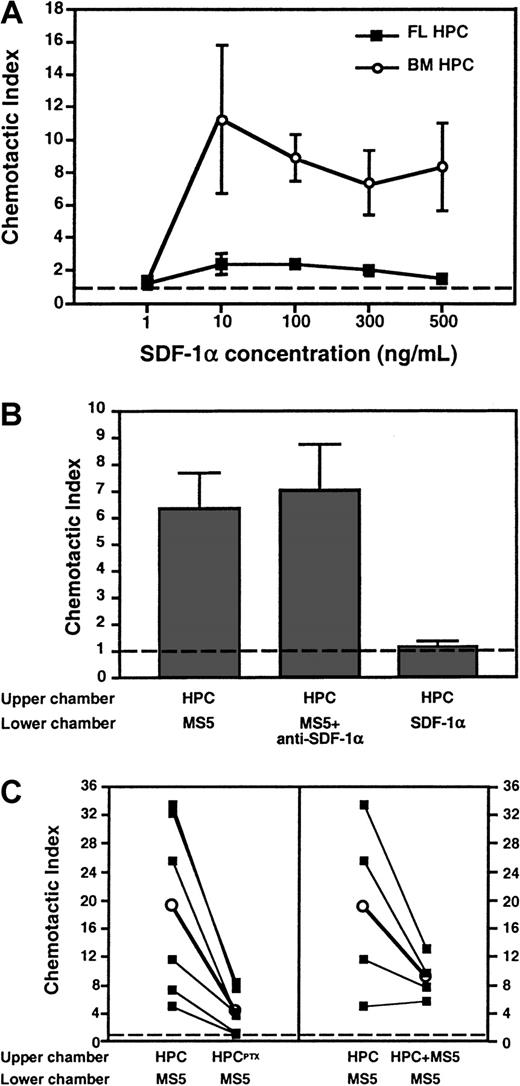
FL HPCs respond poorly to SDF-1α
The findings suggest that TBI causes the expression of a chemokine (or another Gαi protein–coupled signal) by BM cells, leading to increased PTX-sensitive sticking of FL HPCs in BM microvessels. One possible pathway is SDF-1α and its receptor, CXCR4, which have been implicated in the homing of human CD34+HPCs to BM of NOD/SCID mice.16 To determine whether day 11 FL HPCs respond to SDF-1α, we performed chemotaxis assays measuring the migration of FL HPCs to either recombinant SDF-1α or supernatant of MS5 BM stroma cells, which are known to secrete SDF-1α.13 32 Surprisingly, FL HPCs migrated poorly to recombinant SDF-1α, whereas adult BM-derived HPCs responded well to this chemokine (Figure 7A). When plated on methylcellulose, the few FL HPCs that migrated to SDF-1α failed to form colonies (Figure 7B). Similarly, though FL HPCs migrated avidly to the MS5 supernatant, the addition of a blocking anti–SDF-1α mAb did not reduce this migration. Moreover, when irradiated mice were treated with anti–SDF-1α, the sticking fraction of PTX-treated FL HPCs was 105% ± 10% of untreated FL HPCs (not shown). Thus, it is unlikely that SDF-1α triggered Gαi-independent FL HPC sticking.
Differential migration of FL HPCs and adult BM HPCs to SDF-1α in chemotaxis assays.
Migrated HPCs in the lower chamber of the assay system were quantitated (A and C) by flow cytometry gating on c-kit+cells (for FL HPC) or CD34+ cells (for BM HPC) or (B) by counting colony-forming units after plating of migrated cells in methylcellulose. (A) Dose-dependent chemotactic response of FL HPCs and BM HPCs to SDF-1α in the lower chamber. The chemotactic index was calculated as the number of migrated cells in chemokine-containing wells divided by the number of migrated cells in wells containing medium alone. The frequency of responsive FL HPCs was less than 0.2% of all input cells, whereas 15% to 18% of BM HPCs migrated toward SDF-1α at optimal chemokine concentration. (B) FL HPCs migrate avidly toward a chemotactic signal in supernatant from MS5 BM stroma cells distinct from SDF-1α. Undiluted MS5 supernatant or control medium was added to the lower chamber. Some experiments were performed in the presence of anti–SDF-1α (50 μg/mL). (C) Chemotactic response of FL HPC to MS5 supernatant is blocked after the treatment of HPCs with PTX (left panel) and is attenuated in the absence of a chemotactic gradient. Filled squares connected by lines represent individual experiments performed in parallel with the same batch of FL HPC. Empty circles represent the mean of 6 (left panel,P < .05) or 4 (right panel, P > .05) experiments. Broken line represents chemotaxis to control medium. Bars represent mean ± SEM.
Differential migration of FL HPCs and adult BM HPCs to SDF-1α in chemotaxis assays.
Migrated HPCs in the lower chamber of the assay system were quantitated (A and C) by flow cytometry gating on c-kit+cells (for FL HPC) or CD34+ cells (for BM HPC) or (B) by counting colony-forming units after plating of migrated cells in methylcellulose. (A) Dose-dependent chemotactic response of FL HPCs and BM HPCs to SDF-1α in the lower chamber. The chemotactic index was calculated as the number of migrated cells in chemokine-containing wells divided by the number of migrated cells in wells containing medium alone. The frequency of responsive FL HPCs was less than 0.2% of all input cells, whereas 15% to 18% of BM HPCs migrated toward SDF-1α at optimal chemokine concentration. (B) FL HPCs migrate avidly toward a chemotactic signal in supernatant from MS5 BM stroma cells distinct from SDF-1α. Undiluted MS5 supernatant or control medium was added to the lower chamber. Some experiments were performed in the presence of anti–SDF-1α (50 μg/mL). (C) Chemotactic response of FL HPC to MS5 supernatant is blocked after the treatment of HPCs with PTX (left panel) and is attenuated in the absence of a chemotactic gradient. Filled squares connected by lines represent individual experiments performed in parallel with the same batch of FL HPC. Empty circles represent the mean of 6 (left panel,P < .05) or 4 (right panel, P > .05) experiments. Broken line represents chemotaxis to control medium. Bars represent mean ± SEM.
BM stroma cells generate potent G-protein–coupled chemotactic and chemokinetic signals for FL HPC
Although FL HPCs responded to a potent chemoattractant in MS5 supernatant distinct from SDF-1α, this response was reduced after PTX treatment, indicating Gαi protein dependence (Figure7C, left panel). In the absence of a chemotactic gradient, with undiluted MS5 supernatant added in both wells (Figure 7C, right panel), FL HPC migration to the lower well was reduced (by 48%, on average). Although this tendency did not reach statistical significance, these results suggest that MS5 supernatant exerts chemotactic and chemokinetic effects on FL HPCs. PTX treatment abolished the chemokinetic component of FL HPC migration (not shown).
Discussion
The data presented here show that a single lethal dose of TBI causes profound changes in BM microvessels and alters the molecular interactions between microvascular ECs and circulating HPCs. To uncover these changes, we used an IVM technique we had used previously to examine HPC rolling in normal BM.8 Here, we modified our approach to approximate the clinical situation during BMT, which requires ablation of the recipient's resident BM. Adhesion and extravasation of transplanted HPCs in BM vessels is a critical first step for the success of BMT. Thus, we characterized HPC interactions with BM endothelium during the first 2 days after TBI. This period was chosen because experimental BMT in mouse models is typically performed during this time interval.4 33
Because adult BM-derived HPCs are not obtainable at sufficient quantities for IVM studies, we used FL HPCs, which have similar marrow repopulating capabilities.34 Injection of day 11 FL cells efficiently rescues lethally irradiated recipients for at least 5 weeks after TBI (I.B.M., unpublished data, March/April 1998). Although one recent study on the competitive repopulation of recipient BM by donor HPCs from FLs and adult BM detected long-term repopulating ability in day 12 but not day 11 FL HPCs,35 others found a long-term repopulating capacity of the c-Kit+CD34+ subset also in day 11 FLs.20 In our study, approximately 20% of c-Kit+ cells, which represent at least 90% of visualized FL cells,8 coexpressed CD34.
Although this approach enabled us to study a relatively pure population of primary murine HPCs, day 11 FL cells differ somewhat from postnatal HPCs.36 Our FACS analysis revealed that FL HPCs are nearly devoid of L-selectin and express less PSGL-1 but more α4β1 than adult CD34+ BM cells. Because little or no L-selectin ligands are expressed in BM microvessels,8 it is unlikely that the absence of L-selectin affected FL HPC adhesiveness. However, the differences in PSGL-1 and α4β1 suggest that FL HPCs might interact less with endothelial selectins and more with VCAM-1 than adult HPCs. Although this possibility exists, our previous and present results show that FL HPC rolling in nonirradiated BM depends equally on selectins and VCAM-1. Given that the main objective of this study was to assess the effects of TBI on selectin- and VCAM-1–mediated microvascular adhesiveness, FL HPCs seemed like a useful and relevant tool for this purpose.
By using this approach in irradiated mice, we made several novel observations on the effects of TBI. First, we found that TBI altered the composition of endothelial adhesion molecules mediating rolling; though VCAM-1 expression was up-regulated, contributions by selectins were greatly diminished. Second, TBI enhanced HPC arrest by triggering Gαi protein–coupled signaling in BM microvessels. Third, the irradiation-induced chemoattractant(s) appeared to be distinct from SDF-1α.
TBI also reduced blood flow and microvascular diameters in the BM and caused endothelial barrier breakdown. Several mechanisms might have contributed to these changes. Radiation-induced damage to ECs may lead to the exposure of subendothelial matrix and local platelet and leukocyte deposition, resulting in increased microvascular resistance. This effect may be exacerbated by vasoconstriction because of local cellular responses to radiation-induced and/or vasoactive mediators. In addition, the loss of vascular integrity and the ensuing edema likely increased the interstitial pressure, resulting in microvascular compression. Although these findings agree with microvascular changes in irradiated hamster cremaster muscle,37 studies in other tissues suggest that responses to irradiation vary. For example, irradiation increased rat pial and dermal vessel diameters and blood flow.38,39 Thus, microhemodynamic effects in any particular tissue cannot be extrapolated from observations in other organs. Nevertheless, if we assume that the observed effects in murine skull BM may occur in irradiated BM throughout a patient's body, this might result in significant hypoperfusion. Reduced blood flow to the BM may negatively influence the outcome of BMT because fewer blood-borne HPCs would reach that tissue. Indeed, short-term homing studies indicate that fewer HPCs accumulate in irradiated BM than in normal BM.10 11 Thus, modifications in treatment protocols may improve HPC engraftment and survival by minimizing the effects of TBI on blood flow in a patient's BM.
Microvascular responses to irradiation are commonly viewed as a form of inflammation associated with increased selectin-mediated rolling.6 However, P-selectin up-regulation was found in microvessels of irradiated gliomas, but not in surrounding brain vessels.40 Conversely, in irradiated skin, leukocyte rolling was only enhanced in normal microvessels but not in adjacent adenocarcinomas, indicating that irradiation has divergent effects on ECs in different tissues.39 The present findings in irradiated BM microvessels show that HPC rolling remains unchanged. Inflammatory responses may depend on the treatment regimen because the same dose of irradiation increased leukocyte rolling in rat mesentery only when given at a high dose rate.41 Thus, different treatment regimens with TBI might also exert distinct effects on BM microvessels. Because a single high dose of TBI used here induced severe microvascular dysfunction, it is unlikely that the unresponsiveness of rolling was caused by a suboptimal stimulus.
Our studies show that TBI profoundly altered the composition of adhesion molecules on BM ECs. Although it abolished P- and E-selectin–mediated rolling interactions, the contribution of VCAM-1 to HPC rolling was increased. Interestingly, although the anti–VCAM-1 blocking mAb MK2.7 used in this study efficiently blocks α4β1 binding,42,43 at least one third of FL HPCs rolled in irradiated BM in a selectin- and VCAM-1–independent fashion. One candidate, CD44, which has been implicated in rolling of lymphocytes28 and human HPC,29 was not responsible in this setting. Thus, the VCAM-1 independent HPC adhesion in irradiated BM probably involves adhesion pathways that have not been implicated previously in rolling.
Perhaps the most unexpected finding was the loss of selectin activity in irradiated BM. To confirm this, we evaluated the expression of luminal surface molecules on BM ECs. As expected,7-9 we found constitutive expression of P- and E-selectin and VCAM-1 in nonirradiated BM vessels using mAb-coated fluorescent beads. After TBI, specific binding of anti–P-selectin beads was lost, whereas anti–VCAM-1 bead accumulation increased. These findings are consistent with our functional studies on the post-TBI role of P-selectin and VCAM-1 and with previous reports that irradiation enhances VCAM-1.7 On the other hand, anti–E-selectin bead binding was equal before and after irradiation. Although this observation confirms a report that TBI does not alter E-selectin expression in the BM,44 it contrasts with our IVM studies, which failed to detect a significant contribution by E-selectin to HPC rolling after TBI, even though anti–E-selectin caused a significant reduction in rolling in normal BM.8 This discrepancy between E-selectin detectability and function after TBI remains unexplained.
Subsequent bead homing experiments revealed that BM ECs in the skull and in long bones react similarly to TBI. Only VCAM-1 was significantly up-regulated in long bones, whereas E-selectin remained unchanged. Anti–P-selectin beads accumulated less in long bones in 4 of 5 irradiated animals, though this tendency did not reach statistical significance. Taken together, these findings indicate that TBI alters BM microvessels throughout the body, resulting in reduced emphasis on selectins and enhanced contribution by VCAM-1 to HPC trafficking.
Because ELISA experiments showed that anti–P-selectin bead binding was not blocked by increased soluble P-selectin in the serum of irradiated mice, P-selectin loss after TBI could have been due to 3 (nonexclusive) reasons: reduction in overall expression, redistribution away from the lumen, or altered accessibility of the mAb epitope from microenvironmental changes (eg, glycosylation). All these mechanisms would be associated with a de facto loss of functional P-selectin in the vascular lumen.
This notion is also supported by experiments with FucT−/−FL HPCs. Mature FucT−/− leukocytes are devoid of selectin ligands22 and do not undergo selectin-dependent rolling.30 As expected, FucT−/− FL HPCs rolled significantly less than WT HPCs in normal BM microvessels in which P- and E-selectin are constitutively expressed, and this difference was abolished after TBI. These studies indicate that FucT-IV or FucT-VII is required for selectin ligand expression on FL HPCs, and they confirm the radiation-induced loss of selectin activity from BM ECs.
These findings necessitate a reconsideration of recent work in P- and E-selectin−/− mice.45 When lethally irradiated P- and E-selectin−/− mice were injected with small numbers of adult BM cells, they survived poorly compared with WT littermates, and treatment with anti–VCAM-1 decreased survival further. It was thought this difference resulted from the inability of circulating HPCs to roll on selectins in the irradiated mutant animals.45 Our new data question this interpretation, because selectin-mediated HPC rolling was also lost in the irradiated BM of WT mice. However, given that the expression of PSGL-1 was lower on day 11 FL HPCs than on adult BM HPCs, it is possible that adult HPCs are still able to interact with residual endothelial selectins after TBI, whereas selectin ligands on FL cells are too sparse to do so. Because adult HPCs also express lower levels of α4 integrins than FL HPCs, selectin deficiency might reduce homing to irradiated BM of the former, but not the latter—these 2 HPC populations may not benefit equivalently from TBI induced up-regulation of VCAM-1. Nevertheless, our findings suggest that the poor survival of P- and E-selectin−/− mice after BMT might involve mechanisms other than (or in addition to) reduced HPC rolling. For example, cross-linking of selectin ligands might trigger integrin activation.46 However, this mechanism seems unlikely here because blocking of P- and E-selectin did not affect firm adhesion. Alternatively, selectin-mediated signaling might be required for HPC engraftment or survival. For example, in vitro studies have shown that anti–E-selectin mAb blocks HPC chemotaxis toward SDF-1α,47 and PSGL-1 ligation affects HPC proliferation.48
TBI increased HPC arrest, which was inhibited by treatment with PTX. Thus, sticking was mediated by a TBI-induced G-protein–coupled signal that led to integrin activation. FL HPCs did not use β2 integrins to arrest, but sticking required VCAM-1, suggesting a role for α4β1. Interestingly, though anti–VCAM-1 only partially reduced FL HPC rolling in irradiated BM, most HPCs that continued to roll in a VCAM-1–independent fashion failed to stick. It is possible the subset of HPC that rolled without VCAM-1 had a reduced BM homing capacity a priori. However, VCAM-1 may contribute independently to rolling and firm arrest. Consequently, VCAM-1 inhibition reduced the overall sticking efficiency by 90% or more in normal and irradiated BM microvessels.
The mechanism of TBI-induced α4β1 activation remains to be identified. One possible candidate was SDF-1α, which has been implicated in the homing of human HPCs to the BM of NOD/SCID mice16 and in integrin-mediated arrest and transendothelial migration of HPCs in vitro.29,49,50Furthermore, irradiation was found to increase the production of SDF-1α in the BM microenvironment.51 However, though our results do not exclude a role for SDF-1α in the homing of HPCs from postnatal sources, they suggest that FL HPCs respond poorly to SDF-1α. Considering that PTX treatment reduced FL HPC sticking in irradiated BM by approximately 26%, whereas less than 0.2% of FL HPCs migrated to SDF-1α in vitro, it seems likely that another chemotactic agent was responsible for the TBI-induced FL HPC sticking. Indeed, the FL HPC response to a G-protein–coupled stimulus in the MS5 supernatant was not affected by anti–SDF-1α. Because SDF-1α is constitutively expressed in BM32 52 but G-protein–coupled chemoattractant activity for FL HPC was only apparent in irradiated BM, we propose that TBI up-regulates the expression of one or more unidentified chemoattractants distinct from SDF-1α that signal through G-protein–coupled receptor(s) on HPC.
Interestingly, sticking of essentially all FL HPCs in nonirradiated BM (and most HPCs after TBI) was insensitive to PTX. This agrees with recent studies that demonstrated the homing of PTX-treated adult HPCs in irradiated BM.53,54 One possible explanation for this could be a PTX-insensitive integrin-activating stimulus. Indeed, it has been reported that PTX treatment abolishes HPC chemotaxis to SDF-1α but not SDF-1α–induced integrin adhesion.54 However, such a Gαi-independent effect of SDF-1α can be ruled out here because the neutralization of SDF-1α failed to decrease the sticking of PTX-treated HPCs in irradiated BM. Another possibility is that integrins on day 11 FL HPC were in a high-affinity state a priori, thus making additional chemotactic signals unnecessary. Indeed, such a mechanism can mediate the sticking of activated T cells.55However, a recent study found that human FL HPCs have lower spontaneous β1-integrin–mediated adhesiveness for irradiated BM stroma than adult BM-derived HPCs.56 On the other hand, it is difficult to rule out stage-specific differences in HPC behavior36 because FL HPCs obtained at a later stage of gestation (day 12.5) are responsive to SDF-1α.57 It is possible that some FL HPCs acquire responsiveness to SDF-1α at this gestational age to prepare for their exodus from the FL to seed the fetal BM. However, the CXCR4-SDF-1α pathway is not essential for fetal BM development because BM is formed in CXCR4 or SDF-1α–deficient mice.58 59
In summary, we show that TBI severely reduces blood flow and barrier function of BM microvessels. TBI does not affect the frequency of FL HPC rolling, but the underlying molecular mechanisms differ from those in nonirradiated BM. Endothelial selectins are partially lost, whereas VCAM-1 is up-regulated. Additional as yet unknown adhesion pathways contribute to HPC rolling. Sticking was always VCAM-1 dependent and increased after TBI because of the induction of a G-protein–coupled signal in irradiated BM distinct from SDF-1α. Most FL HPCs arrested in normal and irradiated BM venules in a PTX-insensitive fashion, indicating that chemoattractant signaling through Gα proteins is not obligatory for the intravascular adhesion of HPCs in the BM.
We thank C. Bonafide, G. Cheng, and C. Schweitzer for expert technical help and J. Moore for editorial assistance. Thanks to L. Silberstein and W. Weninger for productive discussions and to D. Wagner for reagents to measure soluble P-selectin.
Supported by National Institute of Health grants HL56949 and HL15157. I.B.M. is a recipient of the Amy C. Potter Fellowship and Transfusion Biology and Medicine Training Grant T 32 HL66987.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ulrich H. von Andrian, Department of Pathology, The Center for Blood Research, 200 Longwood Ave, Boston, MA 02115; e-mail: uva@cbr.med.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal