Acute lymphoblastic leukemia cells from 19 children, including 7 who remain in first complete remission (CR1), were engrafted into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. High-level infiltration of bone marrow, spleen, and liver was observed, with variable infiltration of other organs. The immunophenotypes of xenografts were essentially unaltered compared with the original patient sample. In addition, sequencing of the entire p53 coding region revealed no mutations in 14 of 14 xenografts (10 from patients at diagnosis and 4 at relapse). Cells harvested from the spleens of engrafted mice readily transferred the leukemia to secondary and tertiary recipients. To correlate biologic characteristics of xenografts with clinical and prognostic features of the patients, the rates at which individual leukemia samples engrafted in NOD/SCID mice were analyzed. Differences in biologic correlates were encountered depending on stage of disease: a direct correlation was observed between the rate of engraftment and length of CR1 for samples harvested at relapse (r = 0.96; P = .002), but not diagnosis (r = 0.38; P = .40). In contrast, the in vivo responses of 6 xenografts to vincristine showed a direct correlation (r = 0.96; P = .002) between the length of CR1 and the rate at which the leukemia cell population recovered following vincristine treatment, regardless of whether the xenografts were derived from patients at diagnosis or relapse. This study supports previous findings that the NOD/SCID model of childhood ALL provides an accurate representation of the human disease and indicates that it may be of value to predict relapse and design alternative treatment strategies in a patient-specific manner.
Introduction
The inception and refinement of multiagent chemotherapy for childhood acute lymphoblastic leukemia (ALL) represents one of the most significant advances in the field of cancer treatment over the past 40 years.1 Current treatment protocols induce a complete remission in more than 95% of cases, and ultimately more than 70% of patients are cured.2 However, those patients who suffer a bone marrow relapse less than 12 months from diagnosis have only a 10% to 20% probability of long-term survival, with allogeneic bone marrow transplantation considered the best therapeutic option.3Early relapse of leukemia suggests primary resistance or rapidly acquired resistance to multiple agents, although the underlying mechanisms responsible for drug resistance in childhood ALL are poorly understood.
Advances in the clinical management of childhood ALL have been largely empirical and were not stimulated by significant improvements in experimental model systems to study the pathophysiology of the disease. For the most part, experimental studies have been carried out using childhood ALL cell lines, which are of limited relevance to the primary disease state. For example, the reported frequency of p53 mutations in childhood ALL at diagnosis and relapse is low, at around 2% and 19% of cases, respectively.4,5 However, the majority of childhood ALL cell lines that are used as experimental models of the disease express mutant p53,6 suggesting that defects in the p53 pathway may be a prerequisite for in vitro proliferation. An ideal model would mimic the proliferation and dissemination of leukemia cells that occur in the patient and behave in a similar fashion in response to treatment with chemotherapeutic drugs.
Until recently, attempts to propagate childhood ALL cells in various strains of immune-deficient mice have been problematic. In one large study of 681 patient bone marrow biopsy samples, only 104 (15.3%) successfully engrafted in severe combined immunodeficient (SCID) mice, which lack functional B and T cells.7 However, the nonobese diabetic/SCID (NOD/SCID) mouse, which has additional immunologic defects including low natural killer (NK) cell function and absence of circulating complement, has recently been shown to be highly receptive to engraftment of primary childhood ALL cells.8-10 Importantly, childhood ALL cells engrafted into NOD/SCID mice appear to retain the phenotypic and genotypic characteristics of the original patient sample.10Nevertheless, it is unclear to what extent the patients' clinical and prognostic characteristics predict the ability of their leukemias to engraft in NOD/SCID mice, and the responses of engrafted primary childhood ALL cells to chemotherapeutic drugs have not been assessed.
The aim of this study was to address relationships between the biologic characteristics of primary childhood ALL cells engrafted into NOD/SCID mice and the clinical and prognostic features of the patients. In all aspects studied, childhood ALL cells engrafted into NOD/SCID mice appear to provide an accurate representation of the human disease. Moreover, the NOD/SCID model reveals important biologic characteristics of childhood ALL that may ultimately be used to predict relapse and design novel treatment strategies.
Patients and methods
Leukemia cells
Leukemia cells used in this study were obtained from children presenting at Sydney Children's Hospital with ALL at initial diagnosis or bone marrow relapse. Children initially presenting with ALL were enrolled in Australia and New Zealand Children's Cancer Study Group (ANZCCSG) study VI (1992–1998) or study VII (1998 to the present day). ANZCCSG study VI patients were stratified into the following categories: low risk (age, 24-119 months; peripheral blood white cell count [WCC] < 10 000/μL, with no lymphoma features); average risk (age, 12-23 months and WCC < 10 000/μL; or age, 24-119 months, WCC 10 000-50 000/μL, and no lymphoma features); or high risk (age, > 119 months or WCC > 50 000/μL). Study VII patients were classified as standard risk (age, 12-119 months and WCC < 50 000/μL, or t(12;21) positive irrespective of WCC, and absence of t(9;22), t(4;11), t(1;19), and absence of hypodiploidy), or high risk (age, > 119 months or WCC > 50 000/μL and t(12;21) negative, or presence of t(9;22), t(4;11), t(1;19) or hypodiploidy, or central nervous system or testicular involvement). For the purposes of this analysis, study VI patients in the low- and average-risk categories were grouped as standard-risk. All experimental studies were approved by the appropriate institutional review committees.
Peripheral blood or bone marrow biopsy specimens were harvested as part of routine diagnosis and treatment protocols. Mononuclear cells were purified on a Ficoll density gradient (LymphoPrep, Nycomed, Sydney, Australia) and cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide (DMSO) until required. All cell preparations contained more than 85% leukemic blasts, as verified by morphologic and immunophenotype analysis. Prior to transplantation, cells were thawed rapidly in RPMI 1640 medium (Life Technologies, Gaithersburg, MD) containing 10% fetal bovine serum (FBS; Trace Biosciences, Castle Hill, Australia). After centrifugation at 250g for 5 minutes at 4°C, cells were resuspended in RPMI 1640 containing 10% FBS, and the number of viable cells estimated by exclusion of 0.2% trypan blue. Cells were recentrifuged, resuspended in ice-cold calcium- and magnesium-free phosphate-buffered saline (PBS), and placed on ice until inoculated into mice.
Engraftment of human leukemia cells into NOD/SCID mice
All experimental procedures involving NOD/SCID mice were approved by the University of New South Wales Animal Care and Ethics Committee. Female NOD/SCID (NOD/LtSz-scid/scid) mice aged 5 to 6 weeks were purchased from the Walter and Eliza Hall Institute (Melbourne, Australia), and housed in a specific pathogen-free environment for at least 1 week prior to inoculation with human leukemia cells. Mice were supplied with sterile food and water ad libitum. On the day of inoculation, mice received 250 cGy of total body irradiation at a dose rate of 325 cGy/min by parallel-opposed 4 MV x-rays.11
Immediately prior to inoculation, mice were warmed by infrared lamp, then inoculated by tail-vein injection with between 2.5 and 10 million leukemia cells in a maximum volume of 100 μL PBS. Mice were returned to their cages and examined daily for general well-being. Each mouse was monitored every 14 days for leukemia engraftment by staining approximately 50 μL peripheral blood taken from the tail vein with anti-CD45 (leukocyte common antigen, Ly-5) antibodies, fluorescein isothiocyanate (FITC)–conjugated antimurine and allophycocyanin-conjugated antihuman CD45 (BD Pharmingen, San Diego, CA). Following lysis of erythrocytes with ammonium chloride, samples were analyzed by multiparameter flow cytometry on a FACSCalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA). The proportion of human versus murine CD45+ cells was calculated, because this parameter has been shown to accurately reflect the overall leukemic burden.12 Our previous studies show that this method reliably detects 0.01% human CD45+ cells in murine peripheral blood.11 13
At the first indication of morbidity (weight loss, lethargy, ruffled fur), or no more than 28 weeks following inoculation, mice were killed by cervical dislocation. Cell suspensions of spleens and other organs were prepared by mincing the tissues and filtering through 70-μm cell strainers (BD Labware, Franklin Lakes, NJ). Bone marrow was collected by flushing femurs with RPMI 1640 containing 10% FBS. Mononuclear cells were purified by density gradient centrifugation, and cells were cryopreserved in FBS containing 10% DMSO. The proportions of human versus mouse CD45+ cells in bone marrow, peripheral blood, and minced tissues were quantified by multiparameter flow cytometry to ascertain the level of engraftment, as described above. Leukemia cells were considered to have successfully engrafted if at least a 2-fold expansion of the initial cell inoculum had occurred in the bone marrow (based on the estimate of approximately 8 × 107mononuclear cells being present in murine bone marrow),14or the proportion of human CD45+ cells in the peripheral blood reached 1%. For the experiments described in this study, the rate of engraftment was calculated as the number of days following transplantation for leukemia cells to disseminate and reach a proportion of 1% human CD45+ cells in the peripheral blood.
For use in secondary and tertiary transplantations, mononuclear cells were purified from the spleens of engrafted animals by density gradient centrifugation and cryopreserved. Prior to inoculation into groups of 4 mice, cells were thawed and processed exactly as described above for primary cells. For a comparison of rates of secondary and tertiary engraftment with the rate of primary engraftment, equal numbers of human leukemia cells were inoculated in each case.
Morphology and immunophenotype of engrafted cells
Human and murine peripheral blood smears were stained with Wright stain using standard procedures. Spleens and other organs were fixed in 10% neutral-buffered formalin for 24 hours, transferred to 70% (vol/vol) ethanol before being embedded in paraffin. Femurs were fixed in formalin, then decalcified for 48 hours in 0.38 M disodium EDTA (pH 7) before being transferred to 70% ethanol and embedded in paraffin. Glass slides with 3-μm sections were prepared and stained with hematoxylin and eosin.
Expression of a range of lineage-specific and differentiation markers on the surface of cells harvested from the spleens of engrafted mice was analyzed in the Flow Cytometry Laboratory (Department of Haematology, Prince of Wales Hospital) using standard procedures. Expression of specific markers was subsequently compared to the original patient specimen, which had been processed at the time of biopsy in the same laboratory. The antibodies included phycoerythrin (PE)-conjugated antihuman CD19, HLA-DR, CD22, CD8, CD14, CD11b, CD13, CD33, and FITC-conjugated antihuman CD10, CD2, CD3, CD4, CD7, CD34, and sIg, or the appropriate isotype control (BD Biosciences, San Jose, CA).
Genomic DNA extraction and sequencing of the p53 gene
Mononuclear cells were purified from the spleens of engrafted animals by density gradient centrifugation, and only preparations that consisted of more than 90% human CD45+cells were used. Genomic DNA was extracted using a Puregene DNA Isolation Kit (Gentra Systems, Minneapolis, MN) and quantified spectrophotometrically. Aliquots (200 ng) of DNA were used for polymerase chain reaction (PCR) amplification of the entire p53 coding region (exons 2-11) using Human p53 Amplimer Panels (Clontech Laboratories, Palo Alto, CA), according to the manufacturer's instructions. PCR products were generated to encompass exons 2-4 (637 bp), exons 5-6 (447 bp), exons 7-9 (783 bp), exon 10 (277 bp), and exon 11 (153 bp), and sequenced by dye-terminator chemistry following purification using a QIAquick PCR Purification Kit (Qiagen Pty, Clifton Hill, Australia). Sequences were compared to the published p53 sequence (GenBank accession number U94788). Control experiments demonstrated negligible contamination of PCR products with murine DNA (data not shown).
In vivo drug treatments
Groups of 10 mice were inoculated with 5 × 106mononuclear cells purified from the spleens of mice that had been previously engrafted with primary cells. Engraftment was monitored by flow cytometry as described above, and saline control or vincristine treatments were initiated when the proportion of human CD45+ cells in the peripheral blood reached an average of between 1% and 5%. At this point mice were randomized to receive vincristine (0.5 mg/kg intraperitoneally every 7 days for 4 weeks) or the equivalent volume of saline. The proportion of human CD45+ cells in the peripheral blood was monitored throughout the course of treatment. For statistical comparisons between xenografts and drug treatments, the event-free survival (EFS) was calculated from the initiation of vincristine or saline treatment. An event was defined a priori to be when the proportion of human CD45+ cells in the peripheral blood reached 25%, or when animals exhibited clinical signs of disease (weight loss, lethargy, ruffled fur) associated with high-level leukemic infiltration of bone marrow and spleen at necropsy.
Statistical analysis
All statistical analyses were carried out using StatView software version 5.0.1 (SAS Institute, Cary, NC). Correlations between NOD/SCID engraftment and patient characteristics were determined by regression analysis. For each analysis a Pearson correlation coefficient (r) was calculated, and a Fisher r toz transformation carried out to calculate a probability level (P) for the null hypothesis that the correlation is equal to zero. Quantitative variables were compared using the nonparametric Mann-Whitney U test or the Kruskall-Wallis test for multiple independent parameters. For all statistical tests, the level of significance was set to .05. All numerical data are presented ± SD.
Results
Engraftment of primary childhood ALL cells into NOD/SCID mice
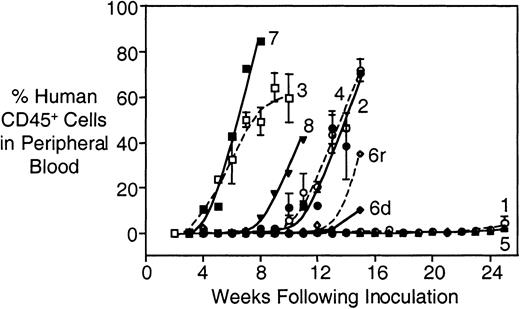
The characteristics and disease-specific details of the patients included in this study are shown in Table1. Cryopreserved cells were available from bone marrow biopsies of 18 patients (including one at both diagnosis and relapse), as well as peripheral blood from a single patient. Notably, 7 of 14 patients whose cells were harvested at diagnosis remain in first complete remission (CR1). Cells from all patients successfully engrafted into NOD/SCID mice, with 16 of 20 patient samples engrafting into 2 or more mice (Table2). The kinetics of engraftment for the samples from the first 8 patients is shown in Figure1. Engraftment was characterized by an initial lag phase, the length of which varied between patient samples, followed by an exponential increase in the proportion of human CD45+ cells in the peripheral blood. The experiment shown in Figure 1 was terminated at 25 weeks following inoculation; nevertheless, mice that received cells from patients 1 and 5 also showed evidence of engraftment at this time (Figure 1 and Table 2). In addition, cells obtained from patient 6 at both diagnosis (d) and relapse (r) successfully engrafted, with those from relapse engrafting approximately 1 week earlier than those obtained at diagnosis.
Patient clinical data
| Patient . | Age at diagnosis (mo)/sex . | ALL lineage . | Disease status at biopsy . | WCC at biopsy (× 109/L) . | Risk category . | Length of CR1 (mo) . | Survival after first relapse (mo) . | Current clinical status . |
|---|---|---|---|---|---|---|---|---|
| 1 | 26/M | B | Relapse 1 | 49.8 | Relapse | 65 | 75* | CR2 |
| 2 | 65/F | B | Relapse 3 | 1.7 | Relapse | 30 | 46 | DOD |
| 3 | 154/F | B | Diagnosis | 93.9 | High | 38 | 62* | CR2 |
| 4 | 105/M | Ph+, B | Diagnosis | 0.43 | High | 10 | 1 | DOD |
| 5 | 128/F | B | Diagnosis | 2.9 | High | 18* | — | CR1 |
| 6d | 55/F | B | Diagnosis | 221 | High | 37 | 22* | CR2 |
| 6r | 55/F | B | Relapse 1 | 8.2 | Relapse | 37 | 22* | CR2 |
| 7 | 88/M | Biphen | Diagnosis | 12.2 | Standard | 7 | 6 | DOD |
| 8 | 152/M | T | Relapse 1 | 67.4 | Relapse | 17 | 1 | DOD |
| 9 | 165/M | T | Diagnosis | 396 | High | 26* | — | CR1 |
| 10 | 48/M | B | Diagnosis | 51.0 | High | 26* | — | CR1 |
| 11 | 37/F | B | Diagnosis | 104 | High | 89* | — | CR1 |
| 12 | 45/M | B | Diagnosis | 28.4 | Standard | 39 | 63* | CR2 |
| 13 | 69/M | B | Diagnosis | 30.1 | Standard | 21* | — | CR1 |
| 14 | 51/F | B | Diagnosis | 14.8 | Standard | 22* | — | CR1 |
| 15 | 177/M | B | Diagnosis | 20.7 | High | 25 | 19 | DOD |
| 16 | 122/F | T | Diagnosis | 30.5 | High | 72* | — | CR1 |
| 17 | 107/F | B | Diagnosis | 97.3 | High | 25 | 1* | REL1 |
| 18 | 30/F | B | Relapse 1 | 7.9 | Relapse | 43 | 21* | CR2 |
| 19† | 194/M | B | Relapse 1 | 0.39 | Relapse | 3 | 7 | DOD |
| Patient . | Age at diagnosis (mo)/sex . | ALL lineage . | Disease status at biopsy . | WCC at biopsy (× 109/L) . | Risk category . | Length of CR1 (mo) . | Survival after first relapse (mo) . | Current clinical status . |
|---|---|---|---|---|---|---|---|---|
| 1 | 26/M | B | Relapse 1 | 49.8 | Relapse | 65 | 75* | CR2 |
| 2 | 65/F | B | Relapse 3 | 1.7 | Relapse | 30 | 46 | DOD |
| 3 | 154/F | B | Diagnosis | 93.9 | High | 38 | 62* | CR2 |
| 4 | 105/M | Ph+, B | Diagnosis | 0.43 | High | 10 | 1 | DOD |
| 5 | 128/F | B | Diagnosis | 2.9 | High | 18* | — | CR1 |
| 6d | 55/F | B | Diagnosis | 221 | High | 37 | 22* | CR2 |
| 6r | 55/F | B | Relapse 1 | 8.2 | Relapse | 37 | 22* | CR2 |
| 7 | 88/M | Biphen | Diagnosis | 12.2 | Standard | 7 | 6 | DOD |
| 8 | 152/M | T | Relapse 1 | 67.4 | Relapse | 17 | 1 | DOD |
| 9 | 165/M | T | Diagnosis | 396 | High | 26* | — | CR1 |
| 10 | 48/M | B | Diagnosis | 51.0 | High | 26* | — | CR1 |
| 11 | 37/F | B | Diagnosis | 104 | High | 89* | — | CR1 |
| 12 | 45/M | B | Diagnosis | 28.4 | Standard | 39 | 63* | CR2 |
| 13 | 69/M | B | Diagnosis | 30.1 | Standard | 21* | — | CR1 |
| 14 | 51/F | B | Diagnosis | 14.8 | Standard | 22* | — | CR1 |
| 15 | 177/M | B | Diagnosis | 20.7 | High | 25 | 19 | DOD |
| 16 | 122/F | T | Diagnosis | 30.5 | High | 72* | — | CR1 |
| 17 | 107/F | B | Diagnosis | 97.3 | High | 25 | 1* | REL1 |
| 18 | 30/F | B | Relapse 1 | 7.9 | Relapse | 43 | 21* | CR2 |
| 19† | 194/M | B | Relapse 1 | 0.39 | Relapse | 3 | 7 | DOD |
CR2 indicates alive in second complete remission; DOD, dead of disease; Ph+, Philadelphia chromosome positive ALL; biphen, biphenotypic; CR1, alive in first complete remission; REL1, first relapse.
No event (censored).
Peripheral blood sample.
Engraftment characteristics of patient samples in NOD/SCID mice
| Patient . | Cells inoculated (× 106) . | Mice engrafted . | Rate of engraftment (d ± SD) . | Time of death (d) . | Level of engraftment at death (% human CD45+ cells ± SD) . | ||
|---|---|---|---|---|---|---|---|
| Bone marrow . | Spleen . | Peripheral blood . | |||||
| 1 | 5.0 | 2/2 | 133 ± 31 | 173 | 26.2 ± 5.0 | 33.5 ± 13.6 | 4.6 ± 3.6 |
| 2 | 2.5 | 4/4 | 53 ± 5 | 107 | 97.6 ± 2.0 | 95.5 ± 3.2 | 68.3 ± 26.8 |
| 3 | 3.5 | 4/4 | 25 ± 4 | 72 | 99.4 ± 0.3 | 94.8 ± 5.2 | 69.8 ± 7.1 |
| 4 | 3.0 | 4/4 | 57 ± 5 | 107 | 94.3 ± 1.5 | 91.9 ± 3.3 | 76.2 ± 0.4 |
| 5 | 5.0 | 3/3 | 164 ± 12 | 167 | 88.6 ± 2.7 | 27.1 ± 29.7 | 1.7 ± 1.1 |
| 6d | 3.9 | 1/2 | 84 | 123 | 97.1 | 81.4 | 93.5 |
| 6r | 6.6 | 2/2 | 77 ± 4 | 117 | 87.7 ± 16.6 | 66.1 ± 10.0 | 32.4 ± 12.5 |
| 7 | 4.9 | 2/2 | 19 ± 5 | 75 | 99.9 | 99.6 | 90.4 ± 1.5 |
| 8 | 3.5 | 2/2 | 47 ± 4 | 98 | 97.9 ± 1.3 | 99.2 ± 1.0 | 72.7 ± 16.1 |
| 9 | 7.9 | 1/3 | 121 | 183 | 97.9 | 99.0 | 84.9 |
| 10 | 3.8 | 3/3 | 31 ± 10 | 68 | 97.9 ± 1.6 | 92.9 ± 1.7 | 51.3 ± 11.0 |
| 11 | 9.9 | 3/3 | 45 ± 8 | 116 | 83.6 ± 15.6 | 97.5 ± 1.2 | 72.6 ± 7.5 |
| 12 | 3.9 | 1/1 | 168 | 136 | 29.5 | 75.1 | 1.0 |
| 13 | 7.6 | 2/3 | 190* | 196 | 83.7 ± 19.1 | 2.1 ± 2.3 | 2.1 ± 2.9 |
| 14 | 4.6 | 3/3 | 89 ± 28 | 142 | 18.0 ± 15.1 | 74.3 ± 30.6 | 33.4 ± 38.0 |
| 15 | 7.5 | 1/2 | 182 | 180 | 78.7 | 1.7 | 1.0 |
| 16 | 8.0 | 3/3 | 55 ± 11 | 102 | 99.2 ± 0.5 | 97.7 ± 0.9 | 49.1 ± 11.9 |
| 17 | 4.0 | 3/3 | 64 ± 7 | 95 | 99.6 ± 0.3 | 99.0 ± 0.6 | 85.4 ± 14.2 |
| 18 | 6.1 | 3/3 | 108 ± 12 | 176 | 94.4 ± 1.8 | 99.7 ± 0.1 | 67.4 ± 17.8 |
| 19 | 4.7 | 2/2 | 29 ± 11 | 66 | 98.2 ± 0.1 | 85.2 ± 3.6 | 51.5 ± 33.2 |
| Patient . | Cells inoculated (× 106) . | Mice engrafted . | Rate of engraftment (d ± SD) . | Time of death (d) . | Level of engraftment at death (% human CD45+ cells ± SD) . | ||
|---|---|---|---|---|---|---|---|
| Bone marrow . | Spleen . | Peripheral blood . | |||||
| 1 | 5.0 | 2/2 | 133 ± 31 | 173 | 26.2 ± 5.0 | 33.5 ± 13.6 | 4.6 ± 3.6 |
| 2 | 2.5 | 4/4 | 53 ± 5 | 107 | 97.6 ± 2.0 | 95.5 ± 3.2 | 68.3 ± 26.8 |
| 3 | 3.5 | 4/4 | 25 ± 4 | 72 | 99.4 ± 0.3 | 94.8 ± 5.2 | 69.8 ± 7.1 |
| 4 | 3.0 | 4/4 | 57 ± 5 | 107 | 94.3 ± 1.5 | 91.9 ± 3.3 | 76.2 ± 0.4 |
| 5 | 5.0 | 3/3 | 164 ± 12 | 167 | 88.6 ± 2.7 | 27.1 ± 29.7 | 1.7 ± 1.1 |
| 6d | 3.9 | 1/2 | 84 | 123 | 97.1 | 81.4 | 93.5 |
| 6r | 6.6 | 2/2 | 77 ± 4 | 117 | 87.7 ± 16.6 | 66.1 ± 10.0 | 32.4 ± 12.5 |
| 7 | 4.9 | 2/2 | 19 ± 5 | 75 | 99.9 | 99.6 | 90.4 ± 1.5 |
| 8 | 3.5 | 2/2 | 47 ± 4 | 98 | 97.9 ± 1.3 | 99.2 ± 1.0 | 72.7 ± 16.1 |
| 9 | 7.9 | 1/3 | 121 | 183 | 97.9 | 99.0 | 84.9 |
| 10 | 3.8 | 3/3 | 31 ± 10 | 68 | 97.9 ± 1.6 | 92.9 ± 1.7 | 51.3 ± 11.0 |
| 11 | 9.9 | 3/3 | 45 ± 8 | 116 | 83.6 ± 15.6 | 97.5 ± 1.2 | 72.6 ± 7.5 |
| 12 | 3.9 | 1/1 | 168 | 136 | 29.5 | 75.1 | 1.0 |
| 13 | 7.6 | 2/3 | 190* | 196 | 83.7 ± 19.1 | 2.1 ± 2.3 | 2.1 ± 2.9 |
| 14 | 4.6 | 3/3 | 89 ± 28 | 142 | 18.0 ± 15.1 | 74.3 ± 30.6 | 33.4 ± 38.0 |
| 15 | 7.5 | 1/2 | 182 | 180 | 78.7 | 1.7 | 1.0 |
| 16 | 8.0 | 3/3 | 55 ± 11 | 102 | 99.2 ± 0.5 | 97.7 ± 0.9 | 49.1 ± 11.9 |
| 17 | 4.0 | 3/3 | 64 ± 7 | 95 | 99.6 ± 0.3 | 99.0 ± 0.6 | 85.4 ± 14.2 |
| 18 | 6.1 | 3/3 | 108 ± 12 | 176 | 94.4 ± 1.8 | 99.7 ± 0.1 | 67.4 ± 17.8 |
| 19 | 4.7 | 2/2 | 29 ± 11 | 66 | 98.2 ± 0.1 | 85.2 ± 3.6 | 51.5 ± 33.2 |
A single mouse reached 1% human CD45+ cells in peripheral blood.
Engraftment of primary childhood ALL cells from patients 1 through 8.
Mice were irradiated and inoculated with mononuclear cells from bone marrow biopsies. At weekly intervals the proportion of human CD45+ cells in the murine peripheral blood was estimated by multiparameter flow cytometry, as described in “Patients and methods.” Experiments were terminated at the first indication of morbidity, or at 25 weeks after inoculation. Numbers refer to patients (Table 1); 6d and 6r indicate patient 6 at diagnosis and relapse, respectively. All patient samples showed evidence of engraftment in the murine peripheral blood. The engraftment characteristics shown in this figure are representative of the entire cohort of 20 patient samples.
Engraftment of primary childhood ALL cells from patients 1 through 8.
Mice were irradiated and inoculated with mononuclear cells from bone marrow biopsies. At weekly intervals the proportion of human CD45+ cells in the murine peripheral blood was estimated by multiparameter flow cytometry, as described in “Patients and methods.” Experiments were terminated at the first indication of morbidity, or at 25 weeks after inoculation. Numbers refer to patients (Table 1); 6d and 6r indicate patient 6 at diagnosis and relapse, respectively. All patient samples showed evidence of engraftment in the murine peripheral blood. The engraftment characteristics shown in this figure are representative of the entire cohort of 20 patient samples.
Although we observed considerable interpatient variability in the rate of engraftment, there was marked concordance between mice within each cohort injected with individual patient samples (Table 2). For example, the 4 mice injected with cells from patient 2 reached 1% human CD45+ cells in the peripheral blood at 54, 46, 56, and 56 days after inoculation, whereas those that received cells from patient 3 took 21, 28, 21, and 28 days.
At harvest, bone marrow, spleens, and peripheral blood had reached high levels of human CD45+ cell involvement, with bone marrow and spleen murine CD45+ cells being almost entirely replaced with human leukocytes in the majority of cases (Table 2). In addition, the proportion of human CD45+ cells in the peripheral blood routinely exceeded 50% without significant morbidity (Table 2 and data not shown). However, morbidity and death were progressively encountered when the proportion of human CD45+ cells in the murine peripheral blood was allowed to exceed 90% (data not shown).
Evaluation of the extent to which the NOD/SCID model of childhood ALL recapitulates the human disease
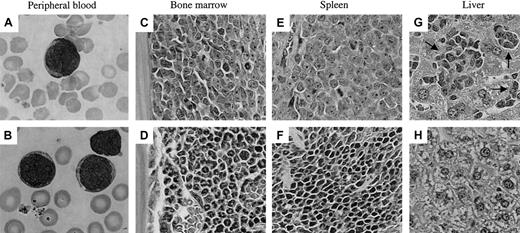
Experiments were undertaken to determine the extent to which childhood ALL cells engrafted in NOD/SCID mice reproduce the human disease. Figure 2A shows that the morphologic features of leukemic blasts in the mouse peripheral blood were identical to those in the original patient peripheral blood smear (Figure 2B). In addition, Figure 2 confirms the data shown in Table 2in which the mouse bone marrow (Figure 2C) and spleen (Figure 2E) were almost entirely replaced with leukemic blasts in comparison to normal tissues (Figure 2D,F). NOD/SCID mice engrafted with childhood ALL cells all presented with splenomegaly and infiltration of the liver (Figure2G and data not shown) compared to their nonengrafted counterparts (Figure 2H). Flow cytometric analysis of 7 mice engrafted with cells from separate patients showed variable leukemic infiltration of the kidneys (4 of 7), lungs (2 of 7), and meninges (5 of 7), which is consistent with clinical findings.15
Morphology of human leukemia cells in the murine peripheral blood and infiltrated tissues.
Murine (A) and original peripheral blood smear (B) from patient 17 showing similar morphology of leukemic blasts. Tissue sections of murine bone marrow (C,D), spleen (E,F), and liver (G,H) were prepared from mice engrafted with cells from patient 19 (C,E,G), and show replacement of the organ architecture with human leukemia cells compared with normal tissue (D,F,H). Arrows indicate foci of leukemic infiltration in the liver (G). Sections were prepared as described in “Patients and methods” and stained with hematoxylin and eosin. The levels of human cell engraftment (as quantified by the proportion of human versus mouse CD45+ cells) in the bone marrow, spleen, and liver of the mouse shown in panels C, E, and G were 98.5%, 92.6%, and 95.5%, respectively (data not shown). Original magnification × 1000 for panels A and B, × 200 for panels C through H.
Morphology of human leukemia cells in the murine peripheral blood and infiltrated tissues.
Murine (A) and original peripheral blood smear (B) from patient 17 showing similar morphology of leukemic blasts. Tissue sections of murine bone marrow (C,D), spleen (E,F), and liver (G,H) were prepared from mice engrafted with cells from patient 19 (C,E,G), and show replacement of the organ architecture with human leukemia cells compared with normal tissue (D,F,H). Arrows indicate foci of leukemic infiltration in the liver (G). Sections were prepared as described in “Patients and methods” and stained with hematoxylin and eosin. The levels of human cell engraftment (as quantified by the proportion of human versus mouse CD45+ cells) in the bone marrow, spleen, and liver of the mouse shown in panels C, E, and G were 98.5%, 92.6%, and 95.5%, respectively (data not shown). Original magnification × 1000 for panels A and B, × 200 for panels C through H.
The immunophenotypes of 13 of our xenografts were examined, 5 of which were derived from patients who remain in CR1 (Table 1). The data in Table 3 show that passage through NOD/SCID mice resulted in a change of a single cell surface antigen expression in 4 samples. Cells derived from patients 2 and 11 showed an increase in the proportion expressing CD34, whereas those from patient 14 exhibited a decrease. A reduction in the proportion of CD13-expressing cells was also observed for patient 7, a biphenotypic ALL. Cells from patients 2, 3, 7, 8, 10, 11, 17, and 19 have been harvested from the spleens of engrafted mice and transplanted into secondary and tertiary recipient mice (see below). These cells underwent no additional changes in immunophenotype (data not shown).
Cells staining positive (%) for expression of lineage-specific and differentiation markers before and after passage in NOD/SCID mice
| Patient . | MHC . | B-lineage . | T-lineage . | Monomyeloid . | Myeloid . | Progenitor . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA-DR . | CD19 . | CD10 . | CD22 . | sIg . | CD2 . | CD3 . | CD4 . | CD7 . | CD8 . | CD14 . | CD11b . | CD13 . | CD33 . | CD34 . | |
| 2P | 81 | 85 | 72 | 6 | 7 | 6 | 2 | 5 | < 1 | 5 | 2 | 6 | 2 | ||
| 2M | 95 | 93 | 95 | 89 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 86 | |
| 3P | 98 | 94 | 0 | 0 | 0 | 1 | 30 | 0 | |||||||
| 3M | 90 | 91 | 1 | 92 | 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 1 | 2 | 29 | |
| 4P | 99 | 99 | 99 | 99 | 1 | < 1 | < 1 | 2 | < 1 | < 1 | < 1 | < 1 | < 1 | 44 | |
| 4M | 94 | 94 | 96 | 94 | 2 | < 1 | < 1 | < 1 | < 1 | < 1 | 8 | < 1 | < 1 | 60 | |
| 5P | 92 | 95 | 92 | 94 | 1 | < 1 | 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 1 | 95 |
| 5M | 88 | 86 | 88 | 89 | |||||||||||
| 7P | 85 | 76 | 68 | 16 | 13 | 10 | 9 | 3 | 74 | 52 | 50 | < 1 | |||
| 7M | 94 | 91 | 86 | 96 | 1 | 1 | < 1 | < 1 | < 1 | < 1 | 62 | < 1 | 36 | < 1 | |
| 8P | 7 | < 1 | < 1 | 1 | < 1 | 98 | 98 | 29 | 97 | 93 | < 1 | < 1 | 88 | < 1 | < 1 |
| 8M | < 1 | < 1 | < 1 | < 1 | < 1 | 96 | 98 | 2 | 96 | 97 | < 1 | < 1 | 93 | < 1 | < 1 |
| 10P | 86 | 91 | 87 | 90 | 2 | 6 | 6 | 3 | 3 | < 1 | 3 | < 1 | < 1 | 87 | |
| 10M | 98 | 98 | 98 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 97 | ||
| 11P | 94 | 66 | 69 | < 1 | 2 | 2 | 2 | 1 | < 1 | < 1 | < 1 | 1 | < 1 | < 1 | |
| 11M | 96 | 94 | 96 | 83 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 1 | < 1 | < 1 | 40 |
| 14P | 97 | 96 | 69 | < 1 | < 1 | < 1 | < 1 | 4 | < 1 | 1 | 2 | 1 | 77 | ||
| 14M | 95 | 98 | 96 | 97 | < 1 | < 1 | < 1 | < 1 | < 1 | 2 | < 1 | < 1 | < 1 | < 1 | < 1 |
| 16P | 21 | 1 | 26 | 10 | < 1 | 67 | 84 | < 1 | 11 | 2 | < 1 | 3 | |||
| 16M | < 1 | < 1 | 42 | 2 | 86 | 5 | < 1 | 91 | 91 | < 1 | < 1 | < 1 | < 1 | 28 | |
| 17P | 94 | 92 | 85 | 2 | 7 | 6 | 3 | 3 | < 1 | 13 | < 1 | 1 | 62 | ||
| 17M | 97 | 93 | 87 | 95 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 94 | |
| 18P | 92 | 94 | 91 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 95 | ||
| 18M | 97 | 98 | 97 | 97 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 98 |
| 19P | 98 | 94 | 92 | 90 | < 1 | 6 | 5 | 1 | 5 | < 1 | 1 | < 1 | < 1 | 49 | |
| 19M | 73 | 70 | 72 | 61 | < 1 | < 1 | < 1 | < 1 | 1 | < 1 | < 1 | 11 | < 1 | < 1 | 70 |
| Patient . | MHC . | B-lineage . | T-lineage . | Monomyeloid . | Myeloid . | Progenitor . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA-DR . | CD19 . | CD10 . | CD22 . | sIg . | CD2 . | CD3 . | CD4 . | CD7 . | CD8 . | CD14 . | CD11b . | CD13 . | CD33 . | CD34 . | |
| 2P | 81 | 85 | 72 | 6 | 7 | 6 | 2 | 5 | < 1 | 5 | 2 | 6 | 2 | ||
| 2M | 95 | 93 | 95 | 89 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 86 | |
| 3P | 98 | 94 | 0 | 0 | 0 | 1 | 30 | 0 | |||||||
| 3M | 90 | 91 | 1 | 92 | 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 1 | 2 | 29 | |
| 4P | 99 | 99 | 99 | 99 | 1 | < 1 | < 1 | 2 | < 1 | < 1 | < 1 | < 1 | < 1 | 44 | |
| 4M | 94 | 94 | 96 | 94 | 2 | < 1 | < 1 | < 1 | < 1 | < 1 | 8 | < 1 | < 1 | 60 | |
| 5P | 92 | 95 | 92 | 94 | 1 | < 1 | 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 1 | 95 |
| 5M | 88 | 86 | 88 | 89 | |||||||||||
| 7P | 85 | 76 | 68 | 16 | 13 | 10 | 9 | 3 | 74 | 52 | 50 | < 1 | |||
| 7M | 94 | 91 | 86 | 96 | 1 | 1 | < 1 | < 1 | < 1 | < 1 | 62 | < 1 | 36 | < 1 | |
| 8P | 7 | < 1 | < 1 | 1 | < 1 | 98 | 98 | 29 | 97 | 93 | < 1 | < 1 | 88 | < 1 | < 1 |
| 8M | < 1 | < 1 | < 1 | < 1 | < 1 | 96 | 98 | 2 | 96 | 97 | < 1 | < 1 | 93 | < 1 | < 1 |
| 10P | 86 | 91 | 87 | 90 | 2 | 6 | 6 | 3 | 3 | < 1 | 3 | < 1 | < 1 | 87 | |
| 10M | 98 | 98 | 98 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 97 | ||
| 11P | 94 | 66 | 69 | < 1 | 2 | 2 | 2 | 1 | < 1 | < 1 | < 1 | 1 | < 1 | < 1 | |
| 11M | 96 | 94 | 96 | 83 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 1 | < 1 | < 1 | 40 |
| 14P | 97 | 96 | 69 | < 1 | < 1 | < 1 | < 1 | 4 | < 1 | 1 | 2 | 1 | 77 | ||
| 14M | 95 | 98 | 96 | 97 | < 1 | < 1 | < 1 | < 1 | < 1 | 2 | < 1 | < 1 | < 1 | < 1 | < 1 |
| 16P | 21 | 1 | 26 | 10 | < 1 | 67 | 84 | < 1 | 11 | 2 | < 1 | 3 | |||
| 16M | < 1 | < 1 | 42 | 2 | 86 | 5 | < 1 | 91 | 91 | < 1 | < 1 | < 1 | < 1 | 28 | |
| 17P | 94 | 92 | 85 | 2 | 7 | 6 | 3 | 3 | < 1 | 13 | < 1 | 1 | 62 | ||
| 17M | 97 | 93 | 87 | 95 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 94 | |
| 18P | 92 | 94 | 91 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 95 | ||
| 18M | 97 | 98 | 97 | 97 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | < 1 | 98 |
| 19P | 98 | 94 | 92 | 90 | < 1 | 6 | 5 | 1 | 5 | < 1 | 1 | < 1 | < 1 | 49 | |
| 19M | 73 | 70 | 72 | 61 | < 1 | < 1 | < 1 | < 1 | 1 | < 1 | < 1 | 11 | < 1 | < 1 | 70 |
MHC indicates major histocompatibility complex; P, patient biopsy sample; M, cells after passage through NOD/SCID mice.
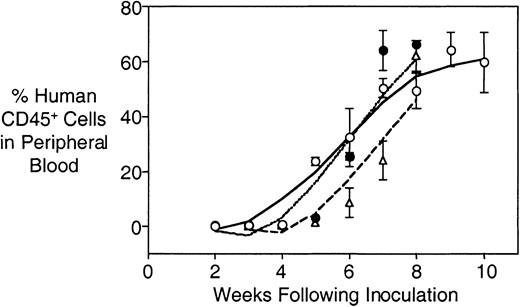
Serial transplants were carried out using cells harvested from spleens of engrafted mice to determine whether the leukemia could be transferred to secondary and tertiary recipients, and whether proliferation rates changed over repeated passaging. Cells from patient 3 were subjected to primary, secondary, and tertiary transplantation, without obvious changes in the kinetics of engraftment (Figure3). Data for 8 patients are shown in Table 4 and indicate that the rates of engraftment did not differ significantly from the primary transplant in 6 of 8 secondary transplants, and 3 of 4 tertiary transplants. However, the rate of engraftment was significantly faster for cells from patients 2 and 17.
Comparable rates of engraftment into primary, secondary, and tertiary recipient mice.
Primary leukemia cells from patient 3 were inoculated into a group of 4 irradiated mice, and engraftment monitored as described in “Patients and methods.” At death, spleens were minced and mononuclear cells purified by density gradient centrifugation and cryopreserved. Subsequently, cells were retrieved from cryostorage and 4 secondary recipient mice were inoculated and monitored for engraftment. When engrafted, the process of harvesting cells from spleens and inoculating tertiary recipient mice was repeated. Primary (open circles, solid line), secondary (closed circles, dotted line), and tertiary (open triangles, dashed line) recipient mice were inoculated with equal numbers of human leukemia cells (3.5 × 106). Individual data points are shown ± SD.
Comparable rates of engraftment into primary, secondary, and tertiary recipient mice.
Primary leukemia cells from patient 3 were inoculated into a group of 4 irradiated mice, and engraftment monitored as described in “Patients and methods.” At death, spleens were minced and mononuclear cells purified by density gradient centrifugation and cryopreserved. Subsequently, cells were retrieved from cryostorage and 4 secondary recipient mice were inoculated and monitored for engraftment. When engrafted, the process of harvesting cells from spleens and inoculating tertiary recipient mice was repeated. Primary (open circles, solid line), secondary (closed circles, dotted line), and tertiary (open triangles, dashed line) recipient mice were inoculated with equal numbers of human leukemia cells (3.5 × 106). Individual data points are shown ± SD.
Rates of primary, secondary, and tertiary engraftment
| Patient . | Rate of engraftment (d ± SD) . | |||
|---|---|---|---|---|
| Primary experiment 1 . | Primary experiment 2 . | Secondary . | Tertiary . | |
| 2 | 53 ± 5 | 42 ± 74-150 | ||
| 3 | 25 ± 4 | 26 ± 3 | 30 ± 4 | |
| 7 | 19 ± 5 | 20 ± 2 | 19 ± 2 | |
| 8 | 47 ± 4 | 48 ± 6 | 42 ± 5 | |
| 10 | 31 ± 10 | 33 ± 8 | 37 ± 11 | |
| 11 | 45 ± 8 | 46 ± 3 | ||
| 17 | 64 ± 7 | 58 ± 3 | 33 ± 44-150 | 38 ± 24-150 |
| 19 | 29 ± 11 | 20 ± 3 | 27 ± 4 | |
| Patient . | Rate of engraftment (d ± SD) . | |||
|---|---|---|---|---|
| Primary experiment 1 . | Primary experiment 2 . | Secondary . | Tertiary . | |
| 2 | 53 ± 5 | 42 ± 74-150 | ||
| 3 | 25 ± 4 | 26 ± 3 | 30 ± 4 | |
| 7 | 19 ± 5 | 20 ± 2 | 19 ± 2 | |
| 8 | 47 ± 4 | 48 ± 6 | 42 ± 5 | |
| 10 | 31 ± 10 | 33 ± 8 | 37 ± 11 | |
| 11 | 45 ± 8 | 46 ± 3 | ||
| 17 | 64 ± 7 | 58 ± 3 | 33 ± 44-150 | 38 ± 24-150 |
| 19 | 29 ± 11 | 20 ± 3 | 27 ± 4 | |
Significantly different from rate of primary engraftment (P < .05).
To verify that the differential engraftment rates shown in Table2 and Table 4 were not due to interexperiment variation, a second experiment was performed in which primary biopsy material from patients 8, 10, and 17 was inoculated into mice using identical conditions as for the initial experiment. The rates of engraftment of these leukemias were remarkably similar to their respective values for the first experiment (compare primary experiments 1 and 2 in Table 4). These data, along with the reproducible rates of secondary and tertiary engraftment shown in Table 4, provide compelling evidence that the rate of engraftment (as measured by dissemination of leukemia cells into the peripheral blood) is an inherent biologic property of individual leukemias.
To determine whether expression of mutant p53 is a prerequisite for the growth of childhood ALL cells in NOD/SCID mice, the entire p53 coding region (exons 2-11) was sequenced following PCR amplification of genomic DNA extracted from the spleens of engrafted animals. Sequence analysis of DNA extracted from xenografts derived from patients 2 to 4, 6d, 7, 8, 10 to 12, 14, 16 to 19 (10 at diagnosis and 4 at relapse) revealed no mutations compared to the published wild-type sequence (data not shown).
Factors influencing the rate of engraftment
No significant correlation was observed between the rate of engraftment and the number of cells from patients at diagnosis (r = 0.24; P = .40) or relapse (r = 0.49; P = .33) inoculated into NOD/SCID mice (Table 2). In an effort to gain a greater understanding of the NOD/SCID model of childhood ALL, the rate of engraftment was compared to clinical and prognostic features of the patients.
Peripheral blood WCC at diagnosis is a powerful prognostic indicator in childhood ALL.2 However, our study identified no correlation between the rate of engraftment and WCC in our cohort of patients at diagnosis (r = 0.032; P = .92) or relapse (r = 0.25; P = .75; Tables 1 and 2). Furthermore, while the median rate of engraftment for patients at relapse (65 days; range, 29-133 days) was marginally faster than for those at diagnosis (74 days; range, 19-190 days), the difference was not statistically significant (P = .62). Leukemias from patients at diagnosis who had been categorized as standard risk showed a trend to engraft at a slower (129 days; range, 19-190 days) rate than those from high-risk patients (median, 61 days; range, 25-182), although the difference was not statistically significant (P = .40), possibly due to insufficient sample size. Furthermore, the rate of engraftment was not significantly influenced by ALL subtype (eg, T versus B lineage) or age at diagnosis (Tables 1and 2). Taken together, these data indicate that the standard clinical and prognostic features of childhood ALL do not predict the extent or rate of leukemia engraftment in NOD/SCID mice.
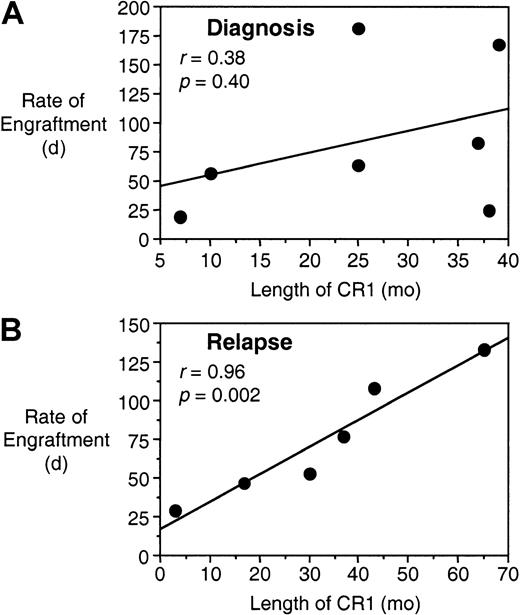
The length of CR1 for children with ALL is considered to be one of the most important prognostic factors for long-term survival after relapse.3 16 When the length of CR1 was compared to the rate of engraftment for each patient's biopsy sample, no significant correlation was encountered in the cohort of 7 patients whose bone marrow samples were harvested at diagnosis but who eventually underwent a relapse (r = 0.38; P = .40; Figure4A). However, in the group of 6 patients whose cells were harvested at relapse, there was a striking correlation between these 2 parameters (r = 0.96;P = .002; Figure 4B), indicating that a short CR1 predicts for rapid engraftment in the NOD/SCID mice. Moreover, of the 6 patients at relapse, the 3 whose cells engrafted most rapidly (patients 2, 8, and 19) died at 46, 1, and 7 months after relapse, respectively, whereas the 3 whose cells engrafted the slowest (patients 1, 6, and 18) remain alive at 75, 22, and 21 months, respectively (Tables 1 and2).
Rate of engraftment correlates with length of CR1 for leukemias harvested at relapse.
Data for the length of CR1 and the rate of engraftment are from Tables1 and 2, respectively, and are compared for those samples harvested from patients at diagnosis who eventually underwent a relapse (A), and samples harvested from patients at relapse (B).
Rate of engraftment correlates with length of CR1 for leukemias harvested at relapse.
Data for the length of CR1 and the rate of engraftment are from Tables1 and 2, respectively, and are compared for those samples harvested from patients at diagnosis who eventually underwent a relapse (A), and samples harvested from patients at relapse (B).
In vivo sensitivity to vincristine
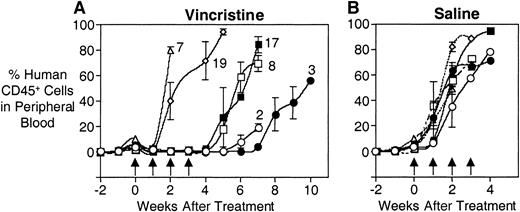
Experiments were performed to assess whether the in vivo responses to vincristine of a series of xenografts derived from patients at diagnosis (3, 7, and 17) or relapse (2, 8, and 19) reflected the patients' clinical experience. Patients 7, 8, and 19 relapsed within 2 years of diagnosis during their course of chemotherapy, whereas patients 2, 3, and 17 were in CR1 for more than 2 years, then suffered relapse at various intervals following completion of their treatment (Table 1). Figure 5A shows that leukemia cells from patients 2 and 3 took more than 6 weeks to reappear in the peripheral blood following the initiation of vincristine treatment. In contrast, cells from patients 7 and 19 essentially grew through the vincristine treatment. Xenografts derived from patients 8 and 17 showed an intermediate level of resistance in that they did not reappear in the peripheral blood until after the vincristine treatments had been completed, but did so at an accelerated rate compared to cells from patient 3. Saline-treated controls showed equivalent growth rates for all xenografts (Figure 5B).
In vivo responses of xenografts derived from patients 2, 3, 7, 8, 17, and 19 to vincristine.
Mice were inoculated with human leukemia cells, monitored for engraftment, and treated with vincristine (A) or saline control (B) as described in “Patients and methods.” During and following treatment, the leukemic burden was monitored by estimating the proportion of human CD45+ cells in murine peripheral blood. (A) The xenografts from patients 2 (open circles) and 3 (closed circles) took approximately 6 and 7 weeks, respectively, to reappear in the peripheral blood following the initiation of vincristine treatment, whereas xenografts from patients 7 (open triangles) and 19 (open diamonds) essentially grew through the vincristine. Xenografts from patients 8 (open squares) and 17 (closed squares) exhibited an intermediate response. (B) Xenografts exposed to saline exhibited equivalent growth rates. Arrows indicate vincristine or saline treatment times.
In vivo responses of xenografts derived from patients 2, 3, 7, 8, 17, and 19 to vincristine.
Mice were inoculated with human leukemia cells, monitored for engraftment, and treated with vincristine (A) or saline control (B) as described in “Patients and methods.” During and following treatment, the leukemic burden was monitored by estimating the proportion of human CD45+ cells in murine peripheral blood. (A) The xenografts from patients 2 (open circles) and 3 (closed circles) took approximately 6 and 7 weeks, respectively, to reappear in the peripheral blood following the initiation of vincristine treatment, whereas xenografts from patients 7 (open triangles) and 19 (open diamonds) essentially grew through the vincristine. Xenografts from patients 8 (open squares) and 17 (closed squares) exhibited an intermediate response. (B) Xenografts exposed to saline exhibited equivalent growth rates. Arrows indicate vincristine or saline treatment times.
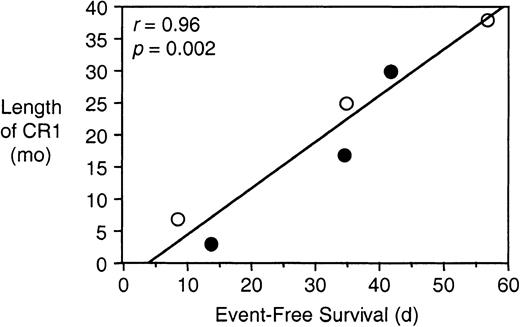
Table 5 summarizes the EFS following vincristine or saline control treatment of mice engrafted with cells from patients 2, 3, 7, 8, 17, and 19. Although there were no significant differences between the saline controls, the recovery times following vincristine treatment of xenografts derived from patients 2, 7, 8, 17, and 19 were significantly faster than that of patient 3, the most vincristine-sensitive xenograft. Moreover, although the responses of these xenografts to vincristine differed markedly, the differences appeared to reflect the clinical experience of the patients from whom they were derived (Table 1). Regression analysis revealed a significant correlation between the length of the patients' CR1 and the EFS of the mice following vincristine treatment (r = 0.96;P = .002; Figure 6). For the same group of patients there was no correlation between sensitivity to vincristine and the rate of engraftment (r = 0.33;P = .52; Tables 2 and 5).
In vivo responses to vincristine
| Patient . | EFS (d ± SD) . | |
|---|---|---|
| Saline control . | Vincristine . | |
| 2 | 12.6 ± 3.0 | 41.7 ± 7.55-150 |
| 3 | 7.3 ± 3.9 | 56.7 ± 5.3 |
| 7 | 7.5 ± 4.1 | 8.3 ± 4.15-150 |
| 8 | 6.1 ± 2.4 | 34.4 ± 3.85-150 |
| 17 | 8.5 ± 4.5 | 34.6 ± 4.75-150 |
| 19 | 5.1 ± 1.7 | 13.6 ± 7.95-150 |
| Patient . | EFS (d ± SD) . | |
|---|---|---|
| Saline control . | Vincristine . | |
| 2 | 12.6 ± 3.0 | 41.7 ± 7.55-150 |
| 3 | 7.3 ± 3.9 | 56.7 ± 5.3 |
| 7 | 7.5 ± 4.1 | 8.3 ± 4.15-150 |
| 8 | 6.1 ± 2.4 | 34.4 ± 3.85-150 |
| 17 | 8.5 ± 4.5 | 34.6 ± 4.75-150 |
| 19 | 5.1 ± 1.7 | 13.6 ± 7.95-150 |
Significantly different from patient 3 (P < .05).
In vivo sensitivity to vincristine correlates with length of CR1.
The EFS of NOD/SCID mice was quantified as the time taken for the leukemic population to reach 25% in the peripheral blood, or for the animals to show evidence of leukemia-related morbidity, following the initiation of vincristine treatment (Table 5). Data for length of CR1 are taken from Table 1. Data points are shown for patients whose cells were harvested at diagnosis (open circles) or relapse (closed circles).
In vivo sensitivity to vincristine correlates with length of CR1.
The EFS of NOD/SCID mice was quantified as the time taken for the leukemic population to reach 25% in the peripheral blood, or for the animals to show evidence of leukemia-related morbidity, following the initiation of vincristine treatment (Table 5). Data for length of CR1 are taken from Table 1. Data points are shown for patients whose cells were harvested at diagnosis (open circles) or relapse (closed circles).
Discussion
The highly efficient engraftment of childhood ALL cells reported in this study supports previous work identifying the NOD/SCID mouse as an ideal animal for xenotransplantation of normal and malignant human hematopoietic cells.8-10,12,14,17-21 The 20 leukemias engrafted in this study represent the largest series of childhood ALL patients reported to date, and 7 patients from whom xenografts have been developed remain in CR1. Previous studies have reported successful engraftment in NOD/SCID mice of cells from 7 of 7 T-lineage childhood ALL patients (4 of whom remained in CR1), or cells derived exclusively from patients with an unfavorable prognosis.8-10,19 We have also shown that efficient engraftment can be achieved using as few as 2.5 × 106 primary childhood ALL cells, in contrast to other studies that required a minimum inoculum of 107 cells for consistent and high level engraftment.8-10
Dialynas et al19 presented evidence that preconditioning NOD/SCID mice with fetal cord blood cells facilitates engraftment of T-lineage ALL from 8 of 8 patients, all of whom were high risk at diagnosis. In the absence of such preconditioning, we have demonstrated high-level engraftment of cells from 3 T-lineage ALL patients, 2 of whom remain in CR1. At this point it remains unknown whether the inclusion of fetal cord blood cells would have facilitated a more rapid engraftment of these cells in our study. However, based on a previous report,8 the high efficiency of engraftment and minimal variation between mice encountered in our experiments may be attributed to a well-defined preconditioning regimen of 250 cGy total body irradiation on the day of inoculation with primary ALL cells.11
Nijmeijer et al12 suggested that fluctuations in the murine leukocyte count may increase the variability encountered when monitoring the proportion of human leukemia cells in NOD/SCID peripheral blood, and that absolute numbers of human leukemia cells should be quantified. However, because of the relatively small variations encountered between mice within experimental groups, this factor does not appear to have been a confounding issue in our studies. Moreover, the presence of human CD45+ cells in the murine peripheral blood is not only an accurate indicator of leukemic burden,12 it also equates with the pathogenicity of the disease encountered both in the mice and in patients.
Our findings, that childhood ALL cells infiltrated the spleen and liver, with variable infiltration of kidneys, lungs, and meninges, are in agreement with previous reports using immune-deficient mice, as well as clinical observations.9,15,22 In addition, our data confirm previous studies that childhood ALL cells undergo minimal changes in immunophenotype when passaged through NOD/SCID or SCID mice,10,22 in contrast to studies with acute myelogenous leukemia.18 We have also shown that passage through NOD/SCID mice does not select cells that express mutant p53, in contrast to a majority of childhood ALL cell lines used for in vitro culture.6 Furthermore, secondary and tertiary transplantation is not associated with accelerated growth of the leukemias in 75% of our xenografts. Overall, our morphologic, immunophenotypic, biologic, and genetic data indicate that childhood ALL cells subjected to up to 3 passages in NOD/SCID mice provide a reliable and robust representation of the human disease. On a cautionary note, however, long-term passage in NOD/SCID mice may result in phenotypic and genotypic alterations from the original patient specimen.10 12 Because we routinely harvest more than 2.5 × 108 human leukemia cells from the spleen of an engrafted animal (data not shown), subsequent experiments can be carried out using early passage cells.
Previously, it was shown that ALL cells from individual pediatric and adult patients engrafted at different rates in NOD/SCID mice.8,10,12 The kinetics of engraftment of childhood ALL cells in our study closely resembles that reported for adult ALL,12 in which an initial lag phase is followed by exponential growth. This characteristic appears to represent different times required for cells to home to the bone marrow, proliferate to replace the normal architecture, and disseminate to other organs and peripheral blood (R.B.L. and N.L., unpublished observations, May 2001).
The exponential phase of leukemia growth in NOD/SCID mice was remarkably similar between samples from different patients (Figure 1). Because all of our leukemia samples engrafted, the patients' clinical and prognostic features were compared to the rate of engraftment (time taken from inoculation for leukemia cells to appear in the peripheral blood). Previous studies have not addressed such relationships.8-10 12 However, in our study the rate of engraftment did not correlate with standard features such as WCC, disease subtype or stage, risk stratification, or age at diagnosis, suggesting that alternative characteristics (eg, expression of homing/adhesion molecules) determine the engraftment rate.
In a study of 8 biopsy specimens, childhood B-lineage ALLs harvested at relapse from patients who relapsed within 13 months of diagnosis engrafted at a faster rate in SCID mice than those from patients who suffered a late relapse, whereas cells harvested from patients at diagnosis showed no evidence of engraftment.22Furthermore, multiple organ infiltration of childhood ALL in SCID mice may reflect a biologically more aggressive disease.7 In contrast, we and others have shown that the NOD/SCID mouse is much more receptive to engraftment of childhood ALL cells from all disease stages and risk categories.8-10 19 It remains unclear which, if any, of the identified additional immunologic defects in NOD/SCID compared to SCID mice facilitates this difference in engraftment of childhood ALL cells.
We have now identified, for the first time, a highly significant correlation between the length of CR1 and the rate of engraftment of samples obtained at relapse. Our finding that the same relationship was not apparent at diagnosis indicates that the rate of engraftment of childhood ALL cells in NOD/SCID mice is unlikely to be of value in predicting relapse. However, our results also indicate that childhood ALL at relapse is intrinsically different from that at diagnosis vis-à-vis their ability to engraft in NOD/SCID mice. Samples from patients at relapse appear to reflect their prior behavior in the patient, in that the characteristics determining the rapidity and aggressiveness of relapse are also revealed in the NOD/SCID mouse. In contrast, the rate of engraftment of cells from patients at diagnosis is not a prediction of their future behavior in the patient. The underlying cause for these differences may be that cells at relapse have been selected for replication/survival in the human hematopoietic system under conditions of extreme stress.
The ultimate aims of these experiments are to investigate the causes and improve the treatment of childhood ALL. The latter will require accurate evaluation of the in vivo responses of xenografts to established chemotherapeutic agents. Our study has demonstrated, for the first time, a striking correlation between the in vivo sensitivity to vincristine and the length of the patient's CR1, regardless of whether the xenografts were derived from patients at diagnosis or relapse. This correlation was not a reflection of differences in engraftment rates of the xenografts. The implications of these results are that (1) the responses to vincristine of samples derived from patients at relapse reflect their prior behavior in the patient, and (2) selection in NOD/SCID mice of cells derived from patients at diagnosis has resulted in a conversion to predict theirfuture behavior in the patient. The corollary is that the NOD/SCID model, when used in this context, may be of value to predict relapse and design alternative treatment strategies in a patient-specific manner.
Although our data agree with short-term in vitro assays that correlate vincristine sensitivity with treatment outcome,23 the latter do not provide a renewable source of leukemia cells for studies of mechanisms of acquired drug resistance and screening of novel agents, nor do they reflect the proliferation and dissemination of leukemia cells that occur in the patient.
In summary, we have shown efficient and high-level engraftment of primary childhood ALL cells from a broad range of patients. Evidence indicates that the NOD/SCID model provides a robust and reliable representation of the human disease, and that it also reflects the rapidity and aggressiveness of relapse as it occurs in the patient. Our data also suggest that the NOD/SCID model may be useful to predict relapse and optimize alternative therapeutic strategies tailored to specific patients. However, although the in vivo responses of our xenografts to vincristine are provocative, they require confirmation using a larger cohort of xenografts exposed to a greater variety of established chemotherapeutic agents.
The Children's Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and Sydney Children's Hospital. The authors wish to thank Diana Waldstein for assistance in immunophenotype analysis; Julie Wood for technical advice on the engraftment of primary leukemia cells into NOD/SCID mice; Eddie Kwan for initial purification and cryopreservation of bone marrow specimens; Cathryn Collins for irradiating the mice; and Laura Piras (CCIA), Scott Williams, and Sinden Medley (SCH) for providing clinical data.
Supported by the Perpetual Foundation, Baxter Charitable Foundation, and Ronald Geoffrey Arnott Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard Lock, Children's Cancer Institute Australia, PO Box 81, High St, Randwick, NSW 2031, Australia; e-mail:richard.lock@unsw.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal