Within childhood T-cell acute lymphoblastic leukemia (T-ALL), patients with a cortical (CD1a+) immunophenotype have been identified as a subgroup with favorable outcome in the acute lymphoblastic leukemia–Berlin-Frankfurt-Münster (ALL-BFM), Cooperative study group for childhood acute lymphoblastic leukemia (COALL) and Pediatric Oncology Group studies. We investigated in leukemic samples of children with T-ALL (n = 81) whether the different in vivo therapy response could be linked to differential in vitro susceptibility to apoptotic cell death. The extent of dexamethasone- as well as doxorubicin-induced apoptosis, detected by annexin V staining, positively correlated with the expression levels of CD1a (Spearman correlation coefficient, rs = 0.3 and 0.4, respectively; P < .01). When compared to cortical T-ALL, mature (CD1a− , surface CD3+) T-ALL were significantly more resistant to doxorubicin, and immature, pro–/pre–T-ALL were more resistant to both drugs (P < .05). Apoptosis-related parameters (Bax, Bcl-2, CD95, and CD95-induced apoptosis) did not account for differential susceptibility to drug-induced apoptosis. By contrast, an interleukin 7–induced rescue of leukemic cells from spontaneous apoptosis, recently proposed to reflect distinct developmental stages and apoptotic programs in T-ALL, was highly associated with susceptibility to dexamethasone- but not doxorubicin-induced apoptosis (P < .001 versus P = .08). Analysis of clinical data showed that in vitro susceptibility to dexamethasone (but not to doxorubicin) closely correlated with early in vivo therapy response characterized by percentages of blast cells in bone marrow on day 15 (rs = −0.46, P = .001). Taken together, the in vitro assessment of drug-induced apoptosis revealed maturation-dependent differences within childhood T-ALL. The enhanced sensitivity to both drugs in cortical T-ALL might account for the better in vivo treatment response of this prognostically favorable T-ALL subgroup.
Introduction
Precursor T-cell acute lymphoblastic leukemia (T-ALL), which accounts for approximately 15% of all childhood acute leukemias, has been reported to have a worse prognosis than precursor B-cell ALL.1,2 However, within T-ALL a heterogeneous therapeutic response has been observed and, in recent years, immunophenotypic studies identified CD markers whose expression patterns distinguish prognostic subgroups in childhood T-ALL. T-ALL patients with a cortical (CD1a+) immunophenotype were identified as a subgroup with favorable outcome in the acute lymphoblastic leukemia–Berlin-Frankfurt-Münster (ALL-BFM) study,3 in the Cooperative study group for childhood acute lymphoblastic leukemia (COALL) study trial,4 and the Pediatric Oncology Group study.5 CD2+T-ALL had a better 5-year event-free survival compared to CD2− T-ALL in the Children's Cancer Study Group (United States) trial.6 Mature T-ALL subgroups, defined either by surface (s)CD3 expression alone7 or sCD3 expression on CD1a− cells,5 had a worse therapy outcome than more immature subtypes.
Because chemotherapeutic treatment might induce cell death by different apoptotic signaling pathways,8-10 it can be speculated that these immunophenotypically defined prognostic T-ALL subgroups differ in their susceptibility to chemotherapy-induced apoptosis. However, there are few experimental data on the in vitro accessibility to apoptotic programs within prognostic T-ALL subtypes. Within the Dutch Childhood Leukemia Study Group (DCLSG) study, positivity for peanut agglutinin binding as a prognostic favorable marker in T-ALL has been analyzed in context of immunophenotype and drug sensitivity in vitro.11 Peanut agglutinin binding did not correlate with maturation stage and in vitro prednisone sensitivity, although a correlation with prednisone response in vivo has been observed. Here, we investigated the apoptotic response to dexamethasone and doxorubicin in immunophenotypically defined T-ALL subtypes as well as a series of T-ALL cell lines. Leukemic cells were further examined with respect to several common apoptosis-related features: expression of Bax, Bcl-2, and CD95, extent of spontaneous apoptosis in vitro, and susceptibility to CD95-induced apoptosis. Interleukin 7 (IL-7)–mediated signaling, suggested to reflect distinct developmental stages and differences in the accessibility to apoptotic programs in childhood T-ALL,12 was used as a tool to investigate drug-induced apoptotic pathways in T-ALL. Finally, the relationship between in vitro sensitivity to drug-induced cell death and response to initial prednisone treatment and induction chemotherapy in vivo for childhood T-ALL cases enrolled in the ALL-BFM study trials was determined.
Patients, materials, and methods
Patient population
Leukemic samples at initial diagnosis from a consecutive series of children with T-ALL (n = 81) were investigated in the present study. Diagnosis of ALL was based on morphology as well as cytochemical and immunophenotypic features.13,14 Immunophenotyping was carried out on leukemic blasts isolated by standard Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation, and cell surface as well as intracytoplasmic antigens were detected by a panel of monoclonal antibodies either by direct or indirect immunofluorescence techniques as previously described.14,15 The criteria for marker positivity as well as the subclassification of T-ALL (pro–/pre–T-ALL, cortical T-ALL, and mature T-ALL) were adopted from the guidelines proposed by the European Group for the Immunological Characterization of Leukemias (EGIL).16 Seventeen samples were subclassified as pro–/pre– (CD1a− and sCD3−) T-ALL, 48 samples as cortical (CD1a+) T-ALL, and 16 samples as mature (CD1a−, sCD3+) T-ALL.
Freshly obtained leukemic blasts were purified either from bone marrow (BM) or peripheral blood (PB) patient samples by density gradient centrifugation. Viability of cells was always more than 90% as determined by trypan blue or propidium iodide (PI) (Sigma, Deisenhofen, Germany) exclusion. All samples contained more than 90% leukemic cells based on morphologic and immunophenotypic criteria. Most of the patients investigated were enrolled in the multicenter ALL-BFM study.2,17 Therapy of the patients in the first 14 days consisted of intrathecal methotrexate (on day 1), prednisone (daily, in rapidly increasing dose from day 1), vincristine and daunorubicin (on day 8), and asparaginase (on day 12).18 In the present in vitro study, glucocorticoids and anthracyclines were represented by dexamethasone and doxorubicin, respectively. The structurally related dexamethasone and prednisone19-21 as well as doxorubicin and daunorubicin20 22 reveal reportedly high levels of in vitro cross-resistance in acute leukemia.
Human leukemia cell lines of T-ALL origin (KE-37, MOLT-3, P12/Ichikawa, BE-13, Peer, Jurkat, PF-382, CCRF-CEM) used in the study were obtained from the DSM Cell Culture Bank (Braunschweig, Germany). All cultures were free of Mycoplasma contaminations as assessed byMycoplasma PCR Primer Set kit (Stratagene, Amsterdam, The Netherlands).
Cell culture
Leukemic cells were maintained in RPMI 1640 (Biochrom, Berlin, Germany) standard medium containing 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin and supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco BRL, Paisley, United Kingdom). To assess drug-induced apoptosis, 0.5 × 106leukemic cells/well were cultured in the presence or absence of doxorubicin or dexamethasone in 96-well microtiter plates (Nunc, Wiesbaden, Germany) in standard medium at 37°C for 24 hours in a humidified atmosphere of 5% CO2 in air. In the case of cell lines, 0.2 × 106 cells/well in 24-well microtiter plates (Nunc) were cultured. To investigate effects mediated by IL-7 or CD95, leukemic cells were cultured either in the presence of recombinant human IL-7 (25 ng/mL; Pharma Biotechnologie Hannover, Hannover, Germany) or agonistic CD95 antibody (1 μg/mL, clone CH-11, Beckman Coulter, Marseille, France) as previously described.12 23
Assessment of apoptosis
To determine the extent of apoptosis, cells were stained with fluorescein isothiocyanate (FITC)–conjugated annexin V and PI using the annexin V kit (Beckman Coulter) as previously described.12 23 Thereafter, samples were analyzed by flow cytometry (FACScan, Becton Dickinson, San Jose, CA) for the presence of viable (annexin V− and PI−), early apoptotic (annexin V+, PI−), and late apoptotic/secondary necrotic (annexin V− and PI+) cells.
The extent of apoptosis (N%) was quantified as percentage of annexin V+ cells as previously described.12,23 The extent of spontaneous apoptosis was calculated as difference of N% values in leukemic samples following and prior to culture in medium, ΔNspont.12 The extent of the drug-specific apoptosis (in percent) was assessed by the formula:
where the drug-induced apoptosis and the apoptosis in medium are the percentages of apoptotic cells in the presence and absence of the drugs, respectively. At least 10 000 cells were characterized by flow cytometry for apoptosis analysis. All tests were performed in doublets.
Assessment of IL-7 effects
As previously described, the rescue of leukemic cells from spontaneous apoptosis by IL-7 was evaluated as difference ΔNIL-7 between the extent of apoptosis after culturing in the absence and presence of IL-7.12
The extent of the drug-specific apoptosis in the presence of IL-7 (in percent) was calculated by the formula:
where the drug-induced apoptosisIL-7 and the apoptosis in mediumIL-7 are the percentages of apoptotic cells in the presence of IL-7 in the drug-treated and control samples, respectively.
Assessment of cell cycle distribution
To study cell cycle distributions, cells were fixed and stained as described elsewhere.12 Briefly, a pellet of 2 × 105 cells was fixed by adding 2 mL ice-cold 70% ethanol for at least one hour at 4°C. After washing, the cells were resuspended in 0.5 mL phosphate-buffered saline containing 50 μg/mL PI, pH 7.5. Following the treatment with 10 μL 10 mg/mL RNase (type I-A; Boehringer Mannheim, Mannheim, Germany) for 30 minutes at room temperature in the dark, the cells were stored at 4°C until flow cytometric analysis. Cell cycle analysis was carried out using either CellQuest (Becton Dickinson) or ModFit LT (Verity, Topsham, ME) software.
Assessment of CD95, Bcl-2, and Bax expression
Expression of the apoptosis-related molecules CD95, Bcl-2, and Bax was assessed as previously described.12 23 Briefly, surface CD95 expression was determined using the phycoerythrin-conjugated anti-CD95 monoclonal antibody (clone DX2, Pharmingen, San Diego, CA). To evaluate expression of the intracellular proteins Bcl-2 and Bax, leukemic cells were fixed and permeabilized using the fixation-permeabilization kit (Fix & Perm; An-der Grub, Kaumberg, Austria) as recommended by the manufacturer. Bcl-2 antigen was detected by the FITC-conjugated anti-Bcl-2 antibody clone 124 (Dako, Glostrup, Denmark). FITC-conjugated irrelevant mouse antibodies of the appropriate immunoglobulin isotype (Beckman Coulter) were used as negative controls. Bax has been detected by Bax-specific rabbit polyclonal antibodies raised against synthetic peptide sequences (I-19, Santa Cruz Biotechnology, Santa Cruz, CA). FITC-conjugated goat antirabbit serum (Medac, Hamburg, Germany) as a secondary staining reagent was used. For negative control, cells were stained with Bax antibody in the presence of the blocking synthetic peptide (provided by the manufacturer, Santa Cruz Biotechnology) at saturation conditions (10 μg peptide versus 1 μg antibody) as determined from peptide titration experiments (data not shown).
Immunofluorescence analysis was performed on a FACScan. The expression of antigens was quantified by flow cytometry in molecules of equivalent soluble fluorochrome (MESF) units using calibration beads as fluorescence standards (Dako) as previously described.12
Statistical analysis
Mean values are given as mean ± SEM or SD. Differences (P values) were evaluated using the 2-tailed nonparametric Mann-Whitney test for continuous variables and the Fisher exact test for categorical variables. Associations of protein expression levels, extent of spontaneous and chemotherapy-induced apoptosis, IL-7 rescue, as well as percentages of blast cells in BM were evaluated using bivariate nonparametric Spearman correlation statistics. Differences were considered significant for P < .05. Statistical analysis was performed using the SPSS software.
Results
In vitro susceptibility to drug-induced apoptosis in primary T-ALL
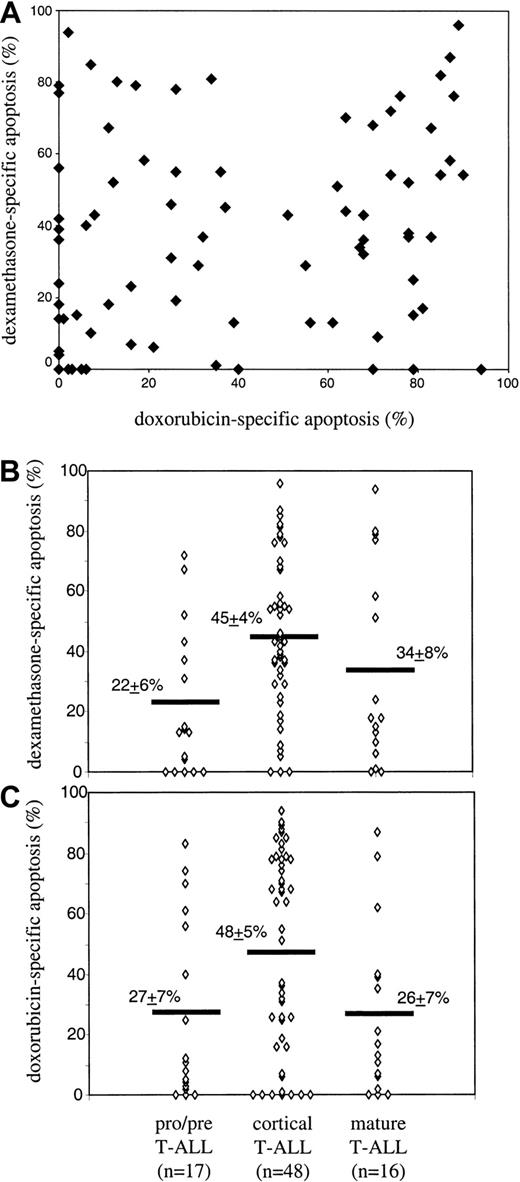
A series of 81 primary T-ALL cell samples were tested for susceptibility to dexamethasone- and doxorubicin-induced apoptosis. First, dose dependence of dexamethasone- and doxorubicin-induced apoptosis was analyzed in 12 and 47 T-ALL samples, respectively. In these experiments, in vitro incubation periods of 24 hours were found to be optimal because, after longer incubation periods, the cases with high rates of spontaneous or drug-induced apoptosis could not be evaluated. On the other hand, shorter periods were often not sufficient to induce apoptosis. In dexamethasone-treated cells, either dose-dependent apoptosis (n = 9) or total resistance (n = 3) in the whole concentration range (0.2-50 μM) has been observed. In the responding samples, 2 μM dexamethasone was sufficient to induce maximal drug response, and this concentration has been further used to characterize the whole T-ALL series. With respect to doxorubicin, almost all samples (45 of 47) disclosed dose-dependent apoptosis (mean percent of drug-specific apoptosis ± SEM: 11.5% ± 1.6%, 46.7% ± 4.5%, and 68.7% ± 4.1% at 0.2 μM, 1 μM, and 5 μM of doxorubicin, respectively). At 1 μM doxorubicin, the maximal heterogeneity between individual samples and, consequently, the best interindividual resolution has been observed (as also indicated by the highest value of SD: 31% at 1 μM doxorubicin versus 11% and 28% at 0.2 μM and 5 μM doxorubicin, respectively). Using these data, the whole series of T-ALL has been characterized by the response to the drugs at optimal concentrations. T-ALL samples disclosed highly heterogeneous response to both drugs (mean values of drug-specific apoptosis ± SEM: 39.2 ± 3.6 and 37.8 ± 3.1 for doxorubicin and dexamethasone, respectively; Figure1A). No clear-cut correlation between dexamethasone- and doxorubicin-specific apoptosis in individual T-ALL samples has been observed (Spearman correlation coefficient, rs = 0.22, P = .053; Figure 1A).
In vitro susceptibility to doxorubicin- and dexamethasone-induced apoptosis in T-ALL: absence of cross-reactivity and differential dependence on maturation stage.
Primary T-ALL cells were incubated in the presence of either doxorubicin (1 μM) or dexamethasone (2 μM) for 24 hours at 37°C. After incubation, apoptotic cells were detected by flow cytometry using annexin V/PI staining, and the extent of drug-specific apoptosis was calculated by the formula described in “Patients, materials, and methods.” (A) Dexamethasone- versus doxorubicin-specific apoptosis in individual T-ALL samples (Spearman correlation coefficient, rs = 0.22, P = .053). (B,C) Drug susceptibility in T-ALL subgroups. Subgroups were defined according to the EGIL criteria as pro–/pre– (CD1a−, sCD3−) T-ALL, cortical (CD1a+) T-ALL, and mature (CD1a−, sCD3+) T-ALL. Significant differences (Spearman analysis) were found: pro–/pre–T-ALL versus cortical (P = .002 and .02 for dexamethasone and doxorubicin) and cortical versus mature (P = .02 for doxorubicin).
In vitro susceptibility to doxorubicin- and dexamethasone-induced apoptosis in T-ALL: absence of cross-reactivity and differential dependence on maturation stage.
Primary T-ALL cells were incubated in the presence of either doxorubicin (1 μM) or dexamethasone (2 μM) for 24 hours at 37°C. After incubation, apoptotic cells were detected by flow cytometry using annexin V/PI staining, and the extent of drug-specific apoptosis was calculated by the formula described in “Patients, materials, and methods.” (A) Dexamethasone- versus doxorubicin-specific apoptosis in individual T-ALL samples (Spearman correlation coefficient, rs = 0.22, P = .053). (B,C) Drug susceptibility in T-ALL subgroups. Subgroups were defined according to the EGIL criteria as pro–/pre– (CD1a−, sCD3−) T-ALL, cortical (CD1a+) T-ALL, and mature (CD1a−, sCD3+) T-ALL. Significant differences (Spearman analysis) were found: pro–/pre–T-ALL versus cortical (P = .002 and .02 for dexamethasone and doxorubicin) and cortical versus mature (P = .02 for doxorubicin).
In vitro susceptibility to drug-induced apoptosis in context of immunophenotype and maturation stage
Within T-ALL, CD1a and sCD3 antigens are the most important immunophenotypic markers used to define cortical and mature T-ALL according to the EGIL classification.16 These markers were differently associated with the susceptibility to doxorubicin- and dexamethasone-induced cell death; both kinds of apoptosis correlated significantly with the expression levels of CD1a, whereas sCD3 expression was associated with the dexamethasone-induced apoptosis only (Table 1). Consequently, statistically significant differences have been observed between immunophenotypically defined T-ALL subgroups (Figure 1B,C). Cortical T-ALLs were more susceptible to doxorubicin-induced apoptosis as compared with immature pro–/pre–T-ALL (P = .02) and mature T-ALL (P = .02). Pro–/pre–T-ALL and mature T-ALL did not differ significantly in their susceptibility to doxorubicin-induced cell death (P = .92). With respect to dexamethasone-induced cell death, cortical T-ALL was also more susceptible compared with pro–/pre–T-ALL (P = .002). Mature T-ALL did not reveal significantly different susceptibility to dexamethasone-induced apoptosis as compared with pro–/pre–T-ALL (P = .23) and cortical T-ALL (P = .25).
In vitro susceptibility to dexamethasone- and doxorubicin-induced apoptosis in childhood T-ALL
| Bivariate correlation . | Dexamethasone-induced apoptosis . | Doxorubicin-induced apoptosis . | ||||
|---|---|---|---|---|---|---|
| rS . | P . | n . | rS . | P . | n . | |
| CD marker expression* | ||||||
| CD1a | 0.30 | .006 | 81 | 0.40 | .000 | 81 |
| CD2 | 0.19 | .089 | 81 | 0.26 | .020 | 81 |
| Surface CD3 | 0.26 | .018 | 81 | 0.08 | .455 | 81 |
| CD13 | −0.27 | .014 | 81 | −0.08 | .471 | 81 |
| CD33 | −0.16 | .149 | 81 | −0.08 | .479 | 81 |
| CD34 | −0.23 | .040 | 81 | −0.25 | .025 | 81 |
| Constitutive expression of apoptosis-related molecules† | ||||||
| Bcl-2 | −0.19 | .17 | 58 | −0.04 | .776 | 58 |
| Bax | 0.14 | .288 | 58 | −0.16 | .245 | 58 |
| Bcl-2/Bax ratio | −0.25 | .063 | 58 | 0.03 | .847 | 58 |
| CD95 | −0.21 | .139 | 51 | 0.04 | .758 | 51 |
| Functional in vitro apoptosis tests | ||||||
| Spontaneous apoptosis | 0.25 | .026 | 81 | 0.34 | .002 | 81 |
| IL-7 rescue | 0.40 | .000 | 77 | 0.20 | .084 | 77 |
| CD95 sensitivity | 0.03 | .829 | 51 | 0.11 | .443 | 51 |
| Bivariate correlation . | Dexamethasone-induced apoptosis . | Doxorubicin-induced apoptosis . | ||||
|---|---|---|---|---|---|---|
| rS . | P . | n . | rS . | P . | n . | |
| CD marker expression* | ||||||
| CD1a | 0.30 | .006 | 81 | 0.40 | .000 | 81 |
| CD2 | 0.19 | .089 | 81 | 0.26 | .020 | 81 |
| Surface CD3 | 0.26 | .018 | 81 | 0.08 | .455 | 81 |
| CD13 | −0.27 | .014 | 81 | −0.08 | .471 | 81 |
| CD33 | −0.16 | .149 | 81 | −0.08 | .479 | 81 |
| CD34 | −0.23 | .040 | 81 | −0.25 | .025 | 81 |
| Constitutive expression of apoptosis-related molecules† | ||||||
| Bcl-2 | −0.19 | .17 | 58 | −0.04 | .776 | 58 |
| Bax | 0.14 | .288 | 58 | −0.16 | .245 | 58 |
| Bcl-2/Bax ratio | −0.25 | .063 | 58 | 0.03 | .847 | 58 |
| CD95 | −0.21 | .139 | 51 | 0.04 | .758 | 51 |
| Functional in vitro apoptosis tests | ||||||
| Spontaneous apoptosis | 0.25 | .026 | 81 | 0.34 | .002 | 81 |
| IL-7 rescue | 0.40 | .000 | 77 | 0.20 | .084 | 77 |
| CD95 sensitivity | 0.03 | .829 | 51 | 0.11 | .443 | 51 |
Bivariate analyses (Spearman correlation coefficient rS) for maturation-dependent CD markers, apoptosis-related molecules, and functional in vitro apoptosis tests.
Percent of positive cells/sample.
In MESF (× 10−3).
In vitro susceptibility to drug-induced apoptosis in context of early cytoreduction in vivo
A poor in vivo response to initial glucocorticoid treatment (defined as > 1000/μL blasts in PB on day 8) has been shown to be a strong adverse prognostic parameter in the ALL-BFM trials.2,17 18 In our study, data on responses to initial therapy were available for 63 T-ALL samples, with the subtype distribution similar to that in the whole series of 81 patients: 13 pro–/pre–T-ALL, 38 cortical T-ALL, and 12 mature T-ALL. Good responders (36 of 63) showed a better susceptibility to dexamethasone and to doxorubicin than poor responders (27 of 63; mean percent of apoptosis ± SEM: 43% ± 4% versus 34% ± 6% and 49% ± 5% versus 34% ± 6% for dexamethasone and doxorubicin, respectively). However, only for doxorubicin did the difference between poor and good responder achieve a borderline significance (Mann-Whitney test, P = .052). For dexamethasone, statistical significance could be achieved by using 10% cutoff levels for drug-specific apoptosis: T-ALL cases with dexamethasone sensitivity higher than 10% were more often good responders to prednisone treatment (32 of 49) as compared to cases with the lower sensitivity (4 of 14) (95% CI for odd ratios, 1.3-17.3; Fisher exact test,P = .03).
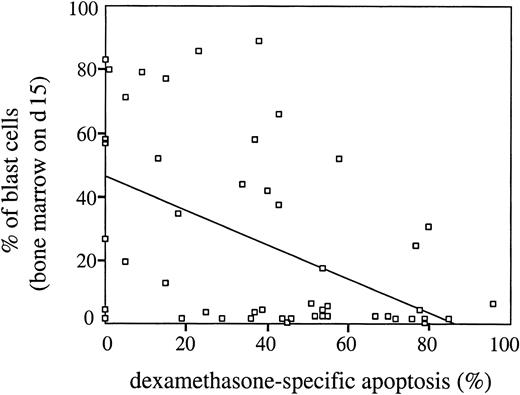
In addition to the good/poor prednisone response criterion in PB at day 8, early response to initial therapy can be characterized by percentage of blast cells in BM at day 15 (data available for 51 patients; distribution between T-ALL subgroups similar to that in the whole series of n = 63). As expected, percentages of blast cells in BM at day 15 were highly associated with prednisone response (Mann-Whitney test, P = .000). Interestingly, susceptibility to dexamethasone (but not to doxorubicin) correlated closely with the early cytoreduction characterized by the day 15 criterion (rs = −0.46, P = .001; Figure2).
Dexamethasone-specific apoptosis in vitro inversely correlates with percentages of blast cells in BM on day 15 of initial therapy in childhood T-ALL.
Primary T-ALL cells were incubated in the presence of dexamethasone (2 μM) for 24 hours at 37°C and the extent of drug-specific apoptosis was evaluated as described in “Patients, materials, and methods.” Spearman analysis: rs = −0.46, P = .001. Trend line was calculated using linear regression statistics.
Dexamethasone-specific apoptosis in vitro inversely correlates with percentages of blast cells in BM on day 15 of initial therapy in childhood T-ALL.
Primary T-ALL cells were incubated in the presence of dexamethasone (2 μM) for 24 hours at 37°C and the extent of drug-specific apoptosis was evaluated as described in “Patients, materials, and methods.” Spearman analysis: rs = −0.46, P = .001. Trend line was calculated using linear regression statistics.
In vitro susceptibility to drug-induced apoptosis in context of apoptosis-related parameters
To identify factors that might be responsible for the differential drug sensitivity in leukemic cells, we examined intracellular expression levels of apoptosis-regulating Bcl-2 and Bax proteins (n = 58) as well as surface expression and function of CD95 death receptor (n = 54). Furthermore, spontaneous apoptosis (n = 81) and cell rescue from spontaneous apoptosis by IL-7 (77 patients investigated; 4 samples could not be evaluated because of the absence of spontaneous apoptosis) were also examined. There were no statistically significant correlations between drug-induced cell death and Bcl-2 family–related as well as CD95-related parameters (Table 1). By contrast, there was a statistically significant association between the rate of spontaneous apoptosis in vitro with both doxorubicin- and dexamethasone-induced apoptosis (Table 1). Most interestingly, the extent of cell rescue from spontaneous apoptosis by IL-7 (IL-7 rescue) was highly associated with dexamethasone- but not with doxorubicin-induced apoptosis (Table 1). IL-7 rescue has been previously shown to correlate with the expression of CD1a and sCD3.12 Also in the present series, IL-7 rescue was significantly higher in CD1a+ versus CD1a− and in sCD3+ versus sCD3− T-ALL samples (Mann-Whitney test, P = .019 and .009, respectively). It was, therefore, possible that the observed association between dexamethasone and IL-7 effects in T-ALL was a reflection of the correlation between IL-7 rescue and immunophenotype. Therefore, we analyzed the relationship between dexamethasone-specific apoptosis and IL-7 rescue within CD1a+ or sCD3+ T-ALL as well as CD1a− or sCD3− T-ALL. Within these T-ALL subgroups, dexamethasone-induced apoptosis remained associated with IL-7 rescue (rs values between 0.31 and 0.44,P < .05).
Modulation of drug-induced apoptosis by IL-7
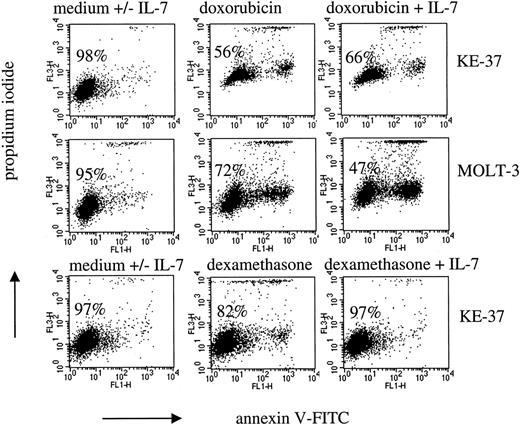
In T-ALL, IL-7–mediated signaling has been recently demonstrated to interfere with spontaneous apoptosis.12 In the present study, we investigated interference of IL-7 signaling with drug effects in T-ALL cell lines (Figure3) and primary T-ALL (Figure4).
Modulation of drug-induced apoptosis by IL-7 in T-ALL cell lines.
Leukemic cells were cultured with IL-7 (25 ng/mL) and either doxorubicin (1 μM and 0.04 μM in KE-37 and MOLT-3, respectively) for 24 hours or dexamethasone (2 μM) for 48 hours. Dot plots show flow cytometric analysis of cells stained with annexin V-FITC and PI. Percentages of viable cells (PI− and annexin V− cells) are indicated. Dot plots of cells in medium alone were identical to that of cells in the presence of IL-7 (not shown).
Modulation of drug-induced apoptosis by IL-7 in T-ALL cell lines.
Leukemic cells were cultured with IL-7 (25 ng/mL) and either doxorubicin (1 μM and 0.04 μM in KE-37 and MOLT-3, respectively) for 24 hours or dexamethasone (2 μM) for 48 hours. Dot plots show flow cytometric analysis of cells stained with annexin V-FITC and PI. Percentages of viable cells (PI− and annexin V− cells) are indicated. Dot plots of cells in medium alone were identical to that of cells in the presence of IL-7 (not shown).
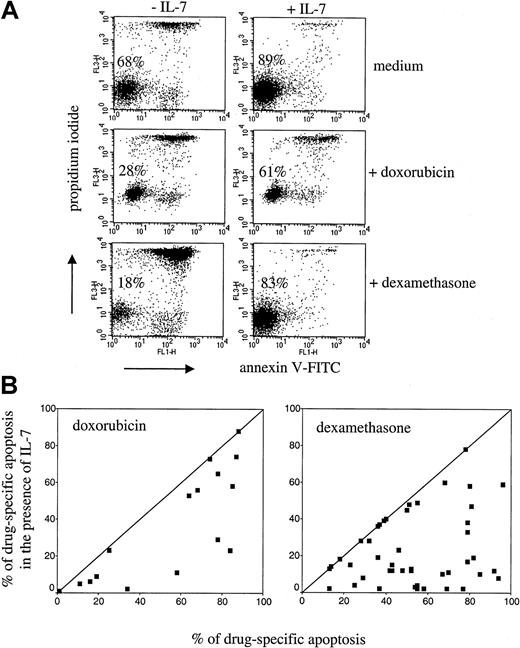
IL-7 inhibits drug-induced apoptosis in primary T-ALL cells.
Leukemic cells were treated with doxorubicin (1 μM) or dexamethasone (2 μM) in the presence or absence of IL-7 (25 ng/mL) for 24 hours. Dot plots (A) are examples of inhibition of spontaneous (in medium) and drug-induced apoptosis by IL-7. Percentages of viable cells (PI− and annexin V− cells) are indicated. At day 0, percentage of viable cells was 95% (not shown). Diagrams (B) show inhibition of doxorubicin- and dexamethasone-induced apoptosis by IL-7 investigated in 16 and 46 T-ALL samples, respectively. Each individual leukemic sample is presented as a point, positioned according to the value of drug-specific apoptosis in the absence (x-axis) and presence (y-axis) of IL-7.
IL-7 inhibits drug-induced apoptosis in primary T-ALL cells.
Leukemic cells were treated with doxorubicin (1 μM) or dexamethasone (2 μM) in the presence or absence of IL-7 (25 ng/mL) for 24 hours. Dot plots (A) are examples of inhibition of spontaneous (in medium) and drug-induced apoptosis by IL-7. Percentages of viable cells (PI− and annexin V− cells) are indicated. At day 0, percentage of viable cells was 95% (not shown). Diagrams (B) show inhibition of doxorubicin- and dexamethasone-induced apoptosis by IL-7 investigated in 16 and 46 T-ALL samples, respectively. Each individual leukemic sample is presented as a point, positioned according to the value of drug-specific apoptosis in the absence (x-axis) and presence (y-axis) of IL-7.
The T-ALL cell lines heterogeneously responded to doxorubicin (range of drug-specific apoptosis 12%-100%). In the majority of T-ALL cell lines, doxorubicin-induced apoptosis has not been influenced by coincubation with IL-7 (data not shown). However, IL-7 was able to inhibit apoptosis in KE-37 cells (Figure 3). Moreover, IL-7 enhanced doxorubicin-induced apoptosis (MOLT-3 cells, Figure 3), thus demonstrating a principal possibility of antiapoptotic and proapoptotic modulation of the drug-induced apoptosis by IL-7 in T-ALL.
With respect to dexamethasone, most of the cell lines were dexamethasone resistant, in striking difference to the primary T-ALL cells. In 2 cell lines only, P12/Ichikawa (data not shown) and KE-37 (Figure 3), low levels of apoptosis could be observed after extended incubation periods (48 hours). Furthermore, only these 2 cell lines were also susceptible to dexamethasone in terms of cell cycle perturbations (G0/G1 phase accumulation, data not shown). In both cell lines, IL-7 was able to inhibit dexamethasone-specific apoptosis but did not reverse cell accumulation in G0/G1 phase (Figure 3 and data not shown).
The effect of IL-7 on doxorubicin-induced apoptosis was investigated in 16 primary T-ALL samples (Figure 4). In 12 of the 16 samples examined, IL-7 was able to inhibit drug-induced apoptosis to different extents. Notably, we did not observe proapoptotic effects of IL-7 in primary leukemic cells. The effect of IL-7 on dexamethasone-induced apoptosis was studied in 46 primary T-ALLs. In most of the samples IL-7 strongly reduced dexamethasone-induced apoptosis (Figure 4). Similarly to the doxorubicin-treated cells, proapoptotic effects of IL-7 in the dexamethasone-treated primary leukemic cells have not been observed.
Discussion
Immunophenotypically defined T-ALL subgroups differ in their response to chemotherapy. Because these differences are supposed to be due to their distinct sensitivities to drug-induced apoptosis, we determined the in vitro susceptibility to dexamethasone- and doxorubicin-induced cell death of prognostic T-ALL subgroups. Furthermore, we addressed apoptotic mechanisms underlying chemotherapy-induced cell death in T-ALL.
CD1a+ T-ALL, previously shown as a prognostically favorable subgroup in 3 representative and independent clinical trials,3-5 disclosed in our study an increased in vitro susceptibility to both dexamethasone- and doxorubicin-induced cell death, compared with pro–/pre–T-ALL as well as mature T-ALL. The observed maturation-dependent difference may reflect a generally higher susceptibility to chemotherapy-induced cell death in cortical T-ALL. Thymocytes in the thymic cortex are extremely sensitive to glucocorticoid-induced cell death; even the physiologic concentrations achieved during a stress response can be sufficient to cause their apoptosis.24 Based on clinical data, Niehues et al4 suggested that cortical T-ALL cells may possess the higher apoptosis susceptibility characteristic of normal cortical thymocytes. Our study provides in vitro evidence supporting this hypothesis.
In context of clinical data, doxorubicin-induced apoptosis disclosed low levels of correlation with early cytoreduction parameters, thus indicating that in vitro anthracycline sensitivity reflects rather an overall than drug-specific susceptibility of leukemic cells to chemotherapeutic treatment in vivo. Application of a broad spectrum of anticancer drugs might be expected to improve the clinical impact of the in vitro apoptosis testing. However, a generally limited cell number in leukemia samples could hamper the effectiveness of this approach. With respect to dexamethasone-induced apoptosis, the observed relatively strong correlation with percentages of blast cells in BM at day 15 is a quite interesting observation, which points to a clinical relevance of this parameter. However, prednisone response in PB at day 8 (being strongly correlated with percentages of blast cells at day 15) revealed only a borderline correlation with the dexamethasone sensitivity. The reason for this difference is unknown, but it is tempting to speculate that it might reflect (time-dependent) differences of cytoreduction in BM and in PB during initial therapy.
Molecular mechanisms and factors responsible for differential sensitivity to drug treatment in ALL remain to be elucidated. Constitutive CD95 and Bcl-2 expression levels as well as CD95 function did not predict in vivo treatment response in childhood T-ALL.25 26 In the present study, we also did not observe associations between these parameters and the in vitro susceptibility to apoptosis, indicating that other intracellular checkpoints might be more important for resistance to chemotherapy-induced apoptosis in T-ALL.
Recently, we characterized childhood T-ALL cells with respect to their cytokine responsiveness in terms of modulation of spontaneous apoptosis in vitro.12 A strong inhibition of spontaneous apoptosis by coincubation with IL-7 was associated with CD1a and sCD3 expression and a better early cytoreduction in vivo. IL-7 rescue was suggested to be a surrogate marker reflecting differential survival factor dependence, apoptosis regulation, and treatment response in maturation-dependent T-ALL subgroups.12 In the current study, we investigated the effect of IL-7 on drug-induced apoptosis in childhood T-ALL. Recently, IL-7 has been reported to enhance cytotoxicity of cytarabine and thioguanine, and, in individual cases, to decrease cytotoxicity of prednisolone in ALL.27 In T-ALL cell lines, we also observed proapoptotic and antiapoptotic effects of IL-7; however, only an antiapoptotic effect of IL-7 was observed in primary T-ALL. This finding is in line with an up-regulation of Bcl-2 in IL-7–treated T-ALL cells as previously reported,12 28 and indicates that dexamethasone- and doxorubicin-induced apoptosis might use common checkpoints at the level of Bcl-2 protein family. On the other hand, the absence of correlation between susceptibilities to doxorubicin and dexamethasone in individual T-ALL samples points to largely differential pathways triggered by these drugs.
Glucocorticoids are principal agents in ALL therapy and identification of their signaling pathways in primary leukemic cells is of particular interest. In our study, dexamethasone and IL-7 effects were strongly associated in individual T-ALL samples. Given the previously observed association of the IL-7 rescue in vitro with glucocorticoid response in vivo,12 these data indicate that pathways triggered by IL-7 and glucocorticoids might be closely related in T-ALL cells. Glucocorticoids exert their biologic activity via ligation of glucocorticoid receptors (GRs), which may act directly as transcription factors. Steroid-inducible proapoptotic genes, however, have not yet been identified.24 Alternatively, glucocorticoid-activated GRs may exert considerable cross-reactivity with other transcription factors and modify their biologic activity.24 Cross-reaction of glucocorticoids with transcriptional regulators, responsible for the survival of target cells, would therefore result in induction of apoptosis. Transcription factor nuclear factor-κB (NF-κB) is a survival factor in different cellular systems, and, recently, we reported constitutive NF-κB activation as a common characteristic of childhood ALL cells.29,30 Cross-reaction between NF-κB and GR at the level of transcriptional regulation has been described in different systems.31-33 The possibility of interference of IL-7–mediated signaling and NF-κB activation has been recently demonstrated in normal precursor T cells.34-36Alternatively to NF-κB, transcription factors from the STAT family, involved in the principal pathway of IL-7 signaling,37,38as well as cdk inhibitor p27, recently shown to be involved in the IL-7–dependent apoptosis modulation in T-ALL,28 might be attractive targets for proapoptotic activity of glucocorticoids in T-ALL.
In vitro chemosensitivity testing by methyl-thiazol-tetrazolium (MTT) assay using a broad spectrum of cytotoxic drugs has proved to be a valuable prognostic marker for therapy response in childhood ALL.39,40 Using the MTT assay, a positive correlation between in vitro and in vivo response to prednisone treatment has been observed in childhood ALL.20 Within ALL, T-ALL cells were generally more resistant to a variety of cytotoxic drugs including glucocorticoids and anthracyclines as compared to common and pre-B-ALL.41-43 However, subtype-specific differences within T-ALL have not yet been reported. In our study, the annexin V–based testing provided useful information on maturation-dependent differences in the apoptotic behavior of leukemic cells within T-ALL and revealed significant in vitro–in vivo correlations. Thus, the flow cytometric annexin V method seems to be a valuable tool for the chemosensitivity assessment of acute leukemia cells. The long-term follow-up of the examined patients will show whether the investigated apoptosis parameters could also contribute to predict overall therapy outcome in childhood T-ALL.
In conclusion, our data show maturation-dependent differences in the in vitro susceptibility to dexamethasone and doxorubicin in childhood T-ALL. The enhanced sensitivity to both drugs in cortical T-ALL might account for the better in vivo treatment response of this prognostically favorable T-ALL subgroup. The in vitro assessment of apoptotic features of drug cytotoxicity provides further insights in chemotherapy-induced leukemic cell death, which might be useful for the development of specific tailored in vivo chemotherapy regimens in the future.
We would like to thank G. Czerwony, K. Ganzel, M. Martin, and K. Liebezeit for their excellent technical assistance. I. Krämer and C. Witt were most helpful with clinical data management. The cell samples included in this study were sent from various hospitals in Germany participating in the ongoing ALL-BFM trials. We thank all clinicians providing cell samples for our investigations.
Supported by grants of the “Deutsche José Carreras Leukämie-Stiftung” (JCLS 1998/NAT-3 to C.W.), the “Deutsche Leukämie-Forschungshilfe” (DLFH-98.04 to V.R.) and the “Monika Kutzner-Stiftung.”
C.W. and V.R. equally contributed to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Leonid Karawajew, Robert-Rössle-Clinic, Charité, Humboldt University of Berlin, Lindenberger Weg 80, 13125 Berlin, Germany; e-mail: karawajew@rrk-berlin.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal