The transcription factor nuclear factor–κB (NF-κB) confers significant survival potential in a variety of tumors. Several established or novel anti–multiple myeloma (anti-MM) agents, such as dexamethasone, thalidomide, and proteasome inhibitors (PS-341), inhibit NF-κB activity as part of their diverse actions. However, studies to date have not delineated the effects of specific inhibition of NF-κB activity in MM. We therefore investigated the effect of SN50, a cell-permeable specific inhibitor of NF-κB nuclear translocation and activity, on MM cells. SN50 induced apoptosis in MM cell lines and patient cells; down-regulated expression of Bcl-2, A1, X-chromosome–linked inhibitor-of-apoptosis protein (XIAP), cellular inhibitor-of-apoptosis protein 1 (cIAP-1), cIAP-2, and survivin; up-regulated Bax; increased mitochondrial cytochromec release into the cytoplasm; and activated caspase-9 and caspase-3, but not caspase-8. We have previously demonstrated that tumor necrosis factor–α (TNF-α) is present locally in the bone marrow microenvironment and induces NF-κB–dependent up-regulation of adhesion molecules on both MM cells and bone marrow stromal cells, with resultant increased adhesion. In this study, TNF-α alone induced NF-κB nuclear translocation, cIAP-1 and cIAP-2 up-regulation, and MM cell proliferation; in contrast, SN50 pretreatment sensitized MM cells to TNF-α–induced apoptosis and cleavage of caspase-8 and caspase-3, similar to our previous finding of SN50-induced sensitization to apoptosis induced by the TNF-α family member TNF-related apoptosis-inducing ligand (TRAIL)/Apo2L. Moreover, SN50 inhibited TNF-α–induced expression of another NF-κB target gene, intercellular adhesion molecule–1. Although the p38 inhibitor PD169316 did not directly kill MM cells, it potentiated the apoptotic effect of SN50, suggesting an interaction between the p38 and NF-κB pathways. Our results therefore demonstrate that NF-κB activity in MM cells promotes tumor-cell survival and protects against apoptotic stimuli. These studies provide the framework for targeting NF-κB activity in novel biologically based therapies for MM.
Introduction
Multiple myeloma (MM), a presently incurable B-cell malignancy, affects 14 000 new patients in the United States annually and is the second most common hematologic malignancy.1-3 Combination chemotherapy offers initial response rates of 40% to 70% in MM patients,4 but refractoriness to these regimens eventually develops. High-dose chemotherapy with stem cell support has achieved higher response rates than conventional therapy, but few patients remain in long-term remission, highlighting the urgent need for novel therapeutic strategies.
The Rel/nuclear factor–κB (Rel/NF-κB) proteins comprise a family of transcription factors, which is conserved from Drosophilato humans and regulates a variety of physiological aspects of immune and inflammatory responses. These proteins bind to 10 base pair DNA sites (κB sites) as dimers and directly regulate gene transcription. NF-κB commonly refers to the p50-RelA heterodimer, which is the major Rel complex. In most cell types, NF-κB is present constitutively in the cytosol in a latent, inactive form where it is retained through its interaction with inhibitory IκB (inhibitor of NF-κB) proteins, masking its nuclear localization sequence. A variety of stimuli induce phosphorylation of IκB at 2 N-terminal Ser residues by the IκB kinase complex, followed by ubiquitination and degradation of IκB by the proteasome.5 Rel/NF-κB complex then enters the nucleus, binds to DNA, and activates transcription of target genes whose products include proteins mediating host immune responses, such as cytokines, chemokines, major histocompatibility complex molecules; proteins involved in antigen presentation; and receptors required for neutrophil adhesion and transmigration across blood vessel walls.5
NF-κB was originally identified as a B-cell nuclear factor, and NF-κB activity is required for proper regulation of B-cell homeostasis.6,7 Constitutive NF-κB activity is also present in some neurons,8 human thymocytes,9Sertoli cells,10 and photoreceptor cells.11Members of the NF-κB/Rel transcription–factor family are expressed constitutively during B-cell development, and they are further induced by mitogen activation.12 Conversely, activated B cells from mice harboring germline disruptions in individual NF-κB subunits exhibit increased sensitivity to apoptosis.13 There is also accumulating evidence that many tumor cells have constitutive NF-κB site-binding and transactivation activity.14-17 Consequently, NF-κB has emerged as a therapeutic target in a variety of neoplasias. For example, NF-κB is constitutively activated in primitive human acute myelogenous leukemia cells, and the proteasome inhibitor MG-132 inhibits IκB degradation and induces tumor cell apoptosis.18,19 Rel/NF-κB activity inhibits apoptosis in WEHI 231 immature B-lymphoma cells, whereas treatment with inhibitors of NF-κB induction, including the proteasome inhibitor N-tosyl-L-phenylalanine chloromethyl ketone and microinjection of glutathione-S-transferase–IκBα or an antibody to c-Rel, sensitizes these cells to anti–immunoglobulin M (anti-IgM)–induced apoptosis.20
The importance of NF-κB activity for survival of activated B cells, coupled with the constitutive activity of NF-κB in several malignancies, prompted us to investigate its role in MM pathophysiology. Moreover, several conventional or emerging anti-MM agents, such as dexamethasone, proteasome inhibitors, and thalidomide, possess anti–NF-κB activity.21-26 To date, however, the direct effects of specific NF-κB inhibition have not been studied in MM. We therefore evaluated the effects of SN50, a cell-permeable peptide that specifically inhibits NF-κB nuclear transport and activity, in MM cell lines and patient cells. As tumor necrosis factor–α (TNF-α) is secreted into the bone marrow microenvironment27 and confers resistance to MM cell apoptosis, we also investigated the effects of NF-κB inhibition on TNF-α–induced signaling. Our studies demonstrate that NF-κB activity promotes growth, survival, and drug resistance in MM cells and that these effects can be inhibited by NF-κB blockade. Importantly, they provide the framework for novel therapies targeting NF-κB in MM.
Materials and methods
MM cell lines and MM patient cells
Dexamethasone-sensitive MM.1S and dexamethasone-resistant MM.1R human MM cell lines were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL). RPMI-8226/S cells and its doxorubicin-resistant subline (Dox40) were a kind gift from Dr William Dalton (Lee Moffit Cancer Center, Tampa, FL). OCI-My5 cells were kindly provided by Dr H. A. Messner (Ontario Cancer Institute, Toronto, Ontario, Canada). ARH-77, HS-Sultan, and IM-9 Epstein-Barr virus (EBV)–transformed B-cell lines were obtained from the American Type Culture Collection (Manassas, VA). The MM-AS cell line has been established from an MM patient sample.28 Malignant plasma cells were freshly isolated from peripheral blood of a patient with plasma cell leukemia and were confirmed by flow cytometric analysis to be greater than 95% CD38+CD45RA−.
All cells were cultured in RPMI 1640 medium (GIBCO Laboratories, Grand Island NY) supplemented with 10% charcoal dextran–treated fetal bovine serum (Hyclone, Logan, UT) as well as L-glutamine, penicillin, and streptomycin (GIBCO).
Materials
The peptide SN50, consisting of the nuclear localization sequence of p50 (residues 360 to 369) fused to the hydrophobic region of the signal sequence of Kaposi fibroblast growth factor to provide cell permeability, specifically inhibits nuclear translocation of NF-κB.29-36 SN50M, a synthetic analog with a mutated nuclear localization sequence, is inactive and served as a negative control.29 Both peptides were purchased from BIOMOL (Plymouth Meeting, PA). TNF-related apoptosis-inducing ligand (TRAIL)/Apo2L was provided by Genentech (South San Francisco, CA) and PS-341 by Millennium (Cambridge, MA).
Other reagents were obtained as follows: p38 inhibitor PD169316 (Calbiochem, La Jolla, CA); mouse monoclonal antibodies for Bcl-2, BclxL, A1, Bax, and tubulin and polyclonal antibody for Mcl-1 (Santa Cruz Biotechnology, Santa Cruz, CA); human recombinant interleukin-6 (IL-6) and polyclonal antisera against cellular inhibitor-of-apoptosis protein 1 (cIAP-1), cIAP-2, and X-chromosome–linked IAP (XIAP) (R&D Systems, Minneapolis, MN); polyclonal antiserum against survivin (Oncogene Research, Cambridge, MA); 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dexamethasone, and doxorubicin (Sigma Chemical, St Louis, MO); a Complete (proprietary name) mixture of proteinase inhibitors, immunoglobulin-free normal horse serum and sodium dodecyl sulfate (SDS) (Life Technologies, Gaithersburg, MD); and the Enhanced Chemiluminescence (ECL) kit, which includes the peroxidase-labeled antimouse and antirabbit secondary antibodies (Amersham, Arlington Heights, IL).
Evaluation of NF-κB activity
The DNA-binding activity of NF-κB in MM.1S cells was quantified by enzyme linked immunosorbent assay (ELISA) by means of the Trans-AM NF-κB p65 Transcription Factor Assay Kit (Active Motif North America, Carlsbad, CA), according to the manufacturer's instructions. Briefly, MM.1S cells were cultured with or without SN50 (20 μM) for 1 hour, and then treated with or without TNF-α (50 ng/mL) for 4 hours. Nuclear extracts were prepared as previously described37and incubated in 96-well plates coated with immobilized oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′) containing a consensus (5′-GGGACTTTCC-3′) binding site for the p65 subunit of NF-κB. NF-κB binding to the target oligonucleotide was detected by incubation with primary antibody specific for the activated form of p65 (Active Motif North America), visualized by anti-IgG horseradish peroxidase conjugate and developing solution, and quantified at 450 nm with a reference wavelength of 655 nm. Background binding, obtained by incubation with a 2-nucleotide mutant oligonucleotide (5′-AGTTGAGGCCACTTTCCCAGGC-3′), was subtracted from the value obtained for binding to the consensus DNA sequence.
MTT colorimetric survival assay
The survival of MM cells was examined by means of the MTT colorimetric assay, as previously described.38 Cells were plated in 48-well plates at 70% to 80% confluence and then incubated for 18 hours with the indicated concentration of SN50. At the end of each treatment, cells were incubated with 1 mg/mL MTT for 4 hours at 37°C; a mixture of isopropanol and 1N HCl (23:2, vol/vol) was then added under vigorous pipetting to dissolve the formazan crystals. Dye absorbance in viable cells was measured at 570 nm, with 630 nm as a reference wavelength. Cell survival was estimated as a percentage of the value of untreated controls. All experiments were repeated at least 3 times, and each experimental condition was repeated in at least quadruplicate wells in each experiment.
Annexin V–propidium iodide staining
Detection of early apoptotic cells was performed by means of the annexin V–propidium iodide (PI) detection kit (Immunotech/Beckman Coulter, Miami, FL). Briefly, 106 MM cells were exposed for 4 hours to SN50 (20 M), washed with Dulbecco modified Eagle medium, incubated in the dark at 4°C with annexin V–fluorescein isothiocyanate (annexin V–FITC) and PI for 15 minutes, and then analyzed by dual-color flow cytometry. Cells that were annexin V–FITC–positive (with translocation of phosphatidylserine from the inner to the outer leaflet of the plasma membrane) and PI-negative (with intact cellular membrane) were considered early apoptotic cells. In another experiment, MM cells were preincubated with caspase inhibitors (pan-caspase inhibitor ZVAD-FMK, caspase-8 inhibitor IETD-FMK, caspase-3 inhibitor DEVD-FMK, and caspase-9 inhibitor LEHD-FMK; all used at 20 μM) for 1 hour prior to exposure to SN50 (20 μM).
Immunoblotting analysis
Immunoblotting analysis was performed as previously described.38 Briefly, cells were lysed for 30 minutes on ice in lysis buffer (50 mM Tris-HCl, pH 8, with 120 mM NaCl and 1% NP-40) supplemented with the Complete mixture of proteinase inhibitors. The samples were cleared by microcentrifugation (14 000 rpm, 30 minutes, 4°C) and assessed for protein concentration. We electrophoresed 30 μg protein per sample in a 12% SDS–polyacrylamide gel and electroblotted it onto nitrocellulose membranes. After 1-hour incubation in blocking solution (20% IgG-free normal horse serum, in phosphate-buffered saline [PBS]), the membranes were exposed overnight at 4°C to the primary antibody. Following washing in PBS, the respective secondary peroxidase-labeled antibody was applied at 1:10 000 dilution for 1 hour at room temperature. The proteins were visualized with the ECL technique.
Subcellular fractionation and cytochrome cdetection
MM.1S cells were treated with or without SN50 (20 μM) for 4 hours, washed in cold PBS once, harvested in 100 μL isotonic buffer (210 mM mannitol, 70 mM sucrose, 1 mM EDTA, and 10 mM Hepes, pH 7.5, supplemented with the Complete protease inhibitors cocktail) and homogenized with a Dounce homogenizer. Samples were centrifuged originally at 1000g to remove the nuclei, and subsequently at 10 000g for 30 minutes at 4°C to obtain the heavy membrane, mitochondria-enriched pellet. Both the mitochondria-enriched and the cytoplasm-enriched supernatant were assayed for the presence of cytochrome c by means of an ELISA,39 according to the manufacturer's instructions (R&D Systems).
Cleavage of caspases and poly(ADP-ribose) polymerase
The involvement of caspases in SN50-induced apoptosis in MM cells was studied by evaluating the levels of procaspase-8, procaspase-3, and procaspase-9, as well as the emergence of their cleaved active forms, by immunoblotting in lysates of cells treated with SN50 (20 μM) for 4, 8, and 16 hours. The cleavage of poly(ADP-ribose) polymerase (PARP), a well-known target of caspase activity, was also studied in the same lysates. Treatment with TRAIL/Apo2L served as a positive control for caspase activation, as previously reported.40
Statistical analysis
Statistical significance was examined by a 2-way analysis of variance, followed by a Duncan post hoc test. In all analyses,P < .05 was considered statistically significant.
Results
SN50 down-regulates constitutive and induced NF-κB activity in MM.1S
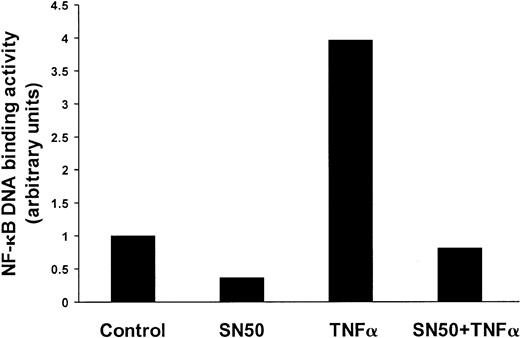
We first evaluated the ways in which the DNA-binding activity of NF-κB in MM cells was affected by SN50, a cell-permeable peptide derived from the nuclear localization sequence of p50, which inhibits the nuclear translocation of NF-κB.9 30 As seen in Figure 1, constitutive NF-κB DNA-binding activity in MM.1S cells was significantly inhibited by SN50. Moreover, SN50 also inhibited NF-κB activation induced by TNFα.
Effect of SN50 on NF-κB activity in MM cells.
SN50 down-regulates constitutive and induced NF-κB activity in MM cells. DNA-binding activity of the transcriptional factor NF-κB was quantified in MM.1S cells pretreated with or without SN50 (20 μM) for 1 hour, and then treated with or without TNF-α (50 ng/mL) for 4 hours. The DNA binding activity of NF-κB in MM.1S cells was quantified by ELISA with the use of the Trans-AM NF-κB p65 Transcription Factor Assay Kit, according to the manufacturer's instructions. Values (mean ± SD) were normalized for cellular protein content. Data are representative of 3 experiments. Constitutive NF-κB DNA-binding activity in MM.1S cells was significantly inhibited by SN50. Moreover, SN50 inhibited NF-κB activation induced by TNF-α.
Effect of SN50 on NF-κB activity in MM cells.
SN50 down-regulates constitutive and induced NF-κB activity in MM cells. DNA-binding activity of the transcriptional factor NF-κB was quantified in MM.1S cells pretreated with or without SN50 (20 μM) for 1 hour, and then treated with or without TNF-α (50 ng/mL) for 4 hours. The DNA binding activity of NF-κB in MM.1S cells was quantified by ELISA with the use of the Trans-AM NF-κB p65 Transcription Factor Assay Kit, according to the manufacturer's instructions. Values (mean ± SD) were normalized for cellular protein content. Data are representative of 3 experiments. Constitutive NF-κB DNA-binding activity in MM.1S cells was significantly inhibited by SN50. Moreover, SN50 inhibited NF-κB activation induced by TNF-α.
SN50 induces apoptosis in MM cells
We next investigated the effect of SN50 on MM cell survival by means of the MTT assay. As can be seen in Figure2A-B, SN50 induced concentration-dependent cell death in 7 of 9 cell lines, including MM cell lines resistant to dexamethasone or doxorubicin, EBV-transformed ARH-77 and IM-9 cells, and freshly isolated patient MM cells. In contrast, normal peripheral B cells were resistant to SN50-induced cell death. Annexin V–PI labeling revealed early externalization of phosphatidylserine in MM.1S cells treated with SN50 for 5 hours (Figure 2C-D), confirming that SN50 induced apoptosis. Mutation of 2 amino-acid residues in SN50, which results in loss of its NF-κB inhibitory activity, also abolished its anti-MM activity (Figure 2E).
Effect of NF-κB inhibition on apoptosis in MM cells.
NF-κB inhibition induces apoptosis in MM cells. (A) (B) Dose-response survival curves based on MTT colorimetric assay were generated for MM cells and healthy donor B cells cultured for 18 hours with SN50 (0 to 25 μM). Data shown (absorbance at 570 nm, mean ± SD) are representative of 3 experiments. Panel A shows dexamethasone-sensitive MM.1S (▴) and dexamethasone-resistant MM.1R (●) cells; RPMI-8226/S (▪) and its cytotoxic drug–resistant subline Dox40 (filled diamond) cells; and OCI-My5 (▵) and MM-AS (■) cells. Panel B shows EBV-transformed ARH-77 (▵) cells, HS Sultan (▴) cells, and IM-9 (■) cells; freshly isolated patient MM cells (▪); and healthy donor peripheral blood B cells (filled diamond). SN50 induced concentration-dependent cell death in 7 of 9 cell lines, including MM cell lines resistant to dexamethasone or doxorubicin, as well as in freshly isolated patient MM cells. In contrast, normal peripheral B cells were resistant to SN50-induced cell death. (C) (D) Annexin–PI staining of MM.1S cells cultured without (panel C) or with (panel D) SN50 (20 μM) for 4 hours revealed early externalization of phosphatidylserine in MM.1S cells treated with SN50 for 5 hours, confirming that SN50 induced apoptosis. (E) The proapoptotic activity of the NF-κB inhibitor SN50 (▴) was compared with its mutant (2 amino-acid residue difference), inactive analog SN50M (▪) in MM.1S cells. Data shown (absorbance at 570 nm, mean ± SD) are representative of 3 experiments. Loss of NF-κB inhibitory activity abolishes anti-MM activity.
Effect of NF-κB inhibition on apoptosis in MM cells.
NF-κB inhibition induces apoptosis in MM cells. (A) (B) Dose-response survival curves based on MTT colorimetric assay were generated for MM cells and healthy donor B cells cultured for 18 hours with SN50 (0 to 25 μM). Data shown (absorbance at 570 nm, mean ± SD) are representative of 3 experiments. Panel A shows dexamethasone-sensitive MM.1S (▴) and dexamethasone-resistant MM.1R (●) cells; RPMI-8226/S (▪) and its cytotoxic drug–resistant subline Dox40 (filled diamond) cells; and OCI-My5 (▵) and MM-AS (■) cells. Panel B shows EBV-transformed ARH-77 (▵) cells, HS Sultan (▴) cells, and IM-9 (■) cells; freshly isolated patient MM cells (▪); and healthy donor peripheral blood B cells (filled diamond). SN50 induced concentration-dependent cell death in 7 of 9 cell lines, including MM cell lines resistant to dexamethasone or doxorubicin, as well as in freshly isolated patient MM cells. In contrast, normal peripheral B cells were resistant to SN50-induced cell death. (C) (D) Annexin–PI staining of MM.1S cells cultured without (panel C) or with (panel D) SN50 (20 μM) for 4 hours revealed early externalization of phosphatidylserine in MM.1S cells treated with SN50 for 5 hours, confirming that SN50 induced apoptosis. (E) The proapoptotic activity of the NF-κB inhibitor SN50 (▴) was compared with its mutant (2 amino-acid residue difference), inactive analog SN50M (▪) in MM.1S cells. Data shown (absorbance at 570 nm, mean ± SD) are representative of 3 experiments. Loss of NF-κB inhibitory activity abolishes anti-MM activity.
SN50 decreases expression of apoptosis inhibitors
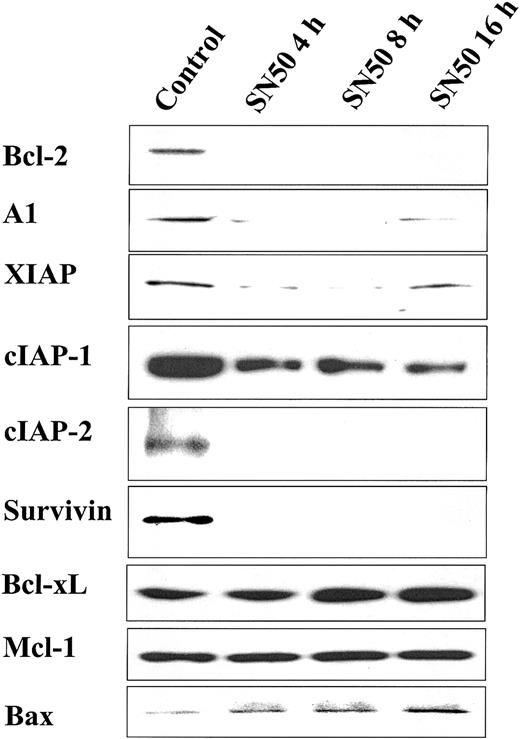
Having demonstrated that NF-κB inhibition with SN50 induces MM cell apoptosis, we next evaluated its effect on the level of protein expression of several apoptosis inhibitors. As can be seen in Figure3, SN50 rapidly decreased protein expression of Bcl-2, A1, XIAP, cIAP-1, cIAP-2, and survivin. In contrast, Mcl-1 and BclxL protein levels were not changed. Moreover, we found that SN50 up-regulated the expression of the proapoptotic Bax protein.
Effect of NF-κB inhibition on expression of apoptosis inhibitors.
NF-κB inhibition decreases expression of apoptosis inhibitors. Immunoblotting analysis for apoptosis regulators in cell lysates of MM.1S cells treated with SN50 (20 μM) for 0, 4, 8, or 16 hours. SN50 rapidly decreased protein expression of Bcl-2, A1, XIAP, cIAP-1, cIAP-2, and survivin. In contrast, Mcl-1 and BclxL protein levels were not changed. Moreover, SN50 up-regulated the expression of the proapoptotic Bax protein.
Effect of NF-κB inhibition on expression of apoptosis inhibitors.
NF-κB inhibition decreases expression of apoptosis inhibitors. Immunoblotting analysis for apoptosis regulators in cell lysates of MM.1S cells treated with SN50 (20 μM) for 0, 4, 8, or 16 hours. SN50 rapidly decreased protein expression of Bcl-2, A1, XIAP, cIAP-1, cIAP-2, and survivin. In contrast, Mcl-1 and BclxL protein levels were not changed. Moreover, SN50 up-regulated the expression of the proapoptotic Bax protein.
SN50 induces cytochrome c release from the mitochondria to the cytoplasm
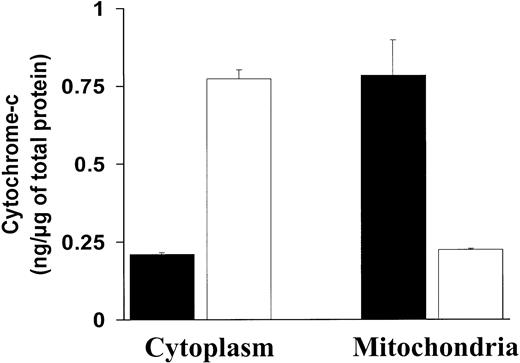
The above-mentioned effects of NF-κB inhibition on Bcl-2 family member expression (ie, increase of the proapoptotic Bax and decrease of the antiapoptotic Bcl-2 and A1 proteins) suggested that mitochondrial events could participate in the resulting induction of apoptosis. A major mechanism of mitochondrial regulation of apoptosis is via release of cytochrome c into the cytoplasm. We therefore assayed for cytochrome c in the cytoplasmic and mitochondrial fractions in MM.1S cells before and after treatment with SN50. As can be seen in Figure 4, treatment with SN50 induced a rapid decrease of cytochrome c in the mitochondrial fraction of MM.1S cells, associated with an increase of cytochrome cin the cytosolic fraction. These data suggest that cytochromec release is associated with MM cell apoptosis induced by NF-κB inhibition.
Effect of NF-κB inhibition on cytochrome
c release from the mitochondria to the cytoplasm.NF-κB inhibition induces cytochrome c release from the mitochondria to the cytoplasm. Cytochrome c protein levels (nanograms per microgram of total protein, mean ± SD) were measured in the cytoplasmic and mitochondrial fractions of MM.1S cells treated with (white bars) or without (black bars) SN50 (20 μM) for 4 hours. Treatment with SN50 induced a rapid decrease of cytochromec in the mitochondrial fraction of MM.1S cells, associated with an increase of cytochrome c in the cytosolic fraction.
Effect of NF-κB inhibition on cytochrome
c release from the mitochondria to the cytoplasm.NF-κB inhibition induces cytochrome c release from the mitochondria to the cytoplasm. Cytochrome c protein levels (nanograms per microgram of total protein, mean ± SD) were measured in the cytoplasmic and mitochondrial fractions of MM.1S cells treated with (white bars) or without (black bars) SN50 (20 μM) for 4 hours. Treatment with SN50 induced a rapid decrease of cytochromec in the mitochondrial fraction of MM.1S cells, associated with an increase of cytochrome c in the cytosolic fraction.
Activation of caspases by SN50
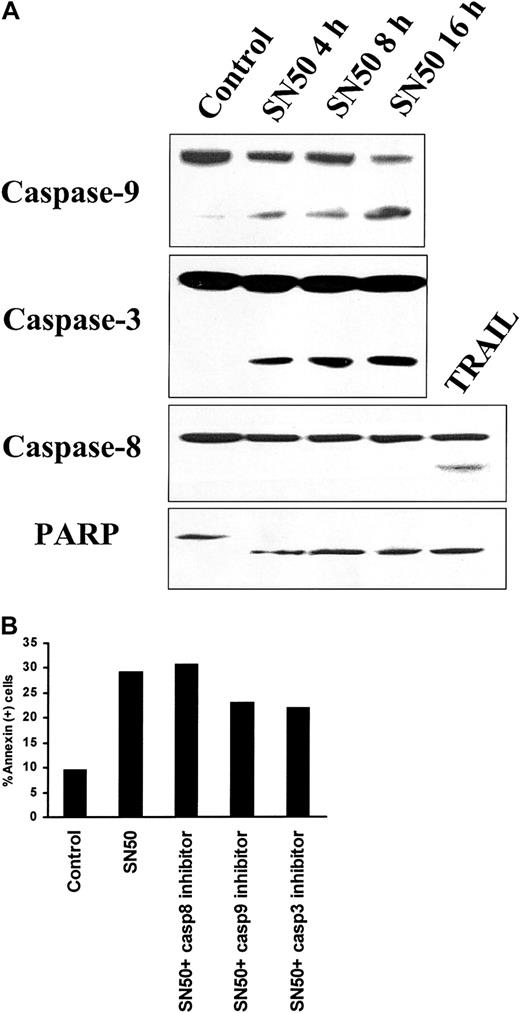
We next investigated the potential involvement of downstream caspases mediating SN50-induced MM cell apoptosis. As seen in Figure5A, treatment with SN50 induced cleavage of caspase-9, caspase-3, and PARP. In contrast, no cleavage of caspase-8 was detected upon SN50 treatment. TRAIL/Apo2L (300 ng/mL for 5 hours) induced caspase-8 cleavage, as in our prior study,40 and served as a positive control. To evaluate the functional involvement of caspases in SN50-induced apoptosis, we used specific inhibitors of caspase-3, caspase-8, and caspase-9. The caspase-9 inhibitor LEHD-FMK and the caspase-3 inhibitor DEVD-FMK partially blocked SN50-induced cell death, whereas the caspase-8 inhibitor IETD-FMK had no effect (Figure 5B). These data collectively suggest that SN50-induced apoptosis is mediated by the mitochondrial release of cytochrome c, triggering activation of caspase-9 and consequent caspase-3 activation. Caspase-8 is not involved, suggesting a mechanism distinct from death-receptor–induced cell death.
Activation of caspases by NF-κB inhibition.
(A) Immunoblotting analysis for caspase-9, caspase-3, and caspase-8, as well as PARP, was performed in lysates of MM.1S cells treated with SN50 (20 μM) for 0, 4, 8, or 16 hours. Treatment with SN50 induced cleavage of caspase-9, caspase-3, and PARP. In contrast, no cleavage of caspase-8 was detected upon SN50 treatment. TRAIL/Apo2L (300 ng/mL for 5 hours) induced caspase-8 cleavage, as in our prior studies,40 and served as a positive control. (B) Annexin V–PI staining was performed to quantify phosphatidylserine externalization in MM.1S cells treated with or without SN50 (20 μM) for 4 hours, in the presence or absence of specific inhibitors for caspase-3, caspase-8, and caspase-9. The caspase-9 inhibitor LEHD-FMK and the caspase-3 inhibitor DEVD-FMK partially blocked SN50-induced cell death, whereas the caspase-8 inhibitor IETD-FMK had no effect.
Activation of caspases by NF-κB inhibition.
(A) Immunoblotting analysis for caspase-9, caspase-3, and caspase-8, as well as PARP, was performed in lysates of MM.1S cells treated with SN50 (20 μM) for 0, 4, 8, or 16 hours. Treatment with SN50 induced cleavage of caspase-9, caspase-3, and PARP. In contrast, no cleavage of caspase-8 was detected upon SN50 treatment. TRAIL/Apo2L (300 ng/mL for 5 hours) induced caspase-8 cleavage, as in our prior studies,40 and served as a positive control. (B) Annexin V–PI staining was performed to quantify phosphatidylserine externalization in MM.1S cells treated with or without SN50 (20 μM) for 4 hours, in the presence or absence of specific inhibitors for caspase-3, caspase-8, and caspase-9. The caspase-9 inhibitor LEHD-FMK and the caspase-3 inhibitor DEVD-FMK partially blocked SN50-induced cell death, whereas the caspase-8 inhibitor IETD-FMK had no effect.
SN50 inhibits the stimulatory effect of IL-6 on MM.1S cells
Since IL-6 is a major growth factor for MM cells, we next evaluated the impact of SN50 on the stimulatory effect of IL-6 on MM cells. As seen in Figure 6A, IL-6 (50 ng/mL) increased the number of MM.1S cells (approximately 20% after 16 hours), whereas preincubation with SN50 (20 μM) abolished this effect. Moreover, our data demonstrate that IL-6 does not protect against SN50-induced cell death (Figure 6B), suggesting that NF-κB inhibition is not attenuated by IL-6.
Effect of NF-κB inhibition on the stimulatory effect of IL-6 on MM.1S cells.
NF-κB inhibition inhibits the stimulatory effect of IL-6 on MM.1S cells. SN50 overcomes IL-6–induced growth, as evidenced by MTT (panel A), and protection against apoptosis, as evidenced by PI staining (panel B), in MM.1S cells. Data shown (panel A, absorbance at 570 nm, mean ± SD) are representative of 3 experiments.
Effect of NF-κB inhibition on the stimulatory effect of IL-6 on MM.1S cells.
NF-κB inhibition inhibits the stimulatory effect of IL-6 on MM.1S cells. SN50 overcomes IL-6–induced growth, as evidenced by MTT (panel A), and protection against apoptosis, as evidenced by PI staining (panel B), in MM.1S cells. Data shown (panel A, absorbance at 570 nm, mean ± SD) are representative of 3 experiments.
Effect of SN50 on TNF-induced sequelae on MM cells
We have previously reported a small, but reproducible, stimulatory effect of TNF-α on MM cell proliferation.27 Here, we investigated the effect of NF-κB blockade on this response of MM cells to TNF-α. In MM.1S cells pretreated with a nontoxic concentration of SN50, TNF-α induced decreased, rather than increased, cell growth (Figure 7A). This decreased viability was due to MM cell apoptosis, as evidenced by cleavage and activation of caspase-8 and downstream caspase-3 (Figure7B). This ability of TNF-α to trigger death-receptor–mediated apoptosis in SN50-pretreated MM.1S cells was associated with down-regulation of expression of cIAP-1 and cIAP-2 caspase-8 inhibitory proteins. In contrast, TNF-α alone up-regulated cIAP-1 and cIAP-2 expression. These data confirm that TNF-α activates a proapoptotic pathway, via caspase-8 activation, as well as an antiapoptotic pathway, via NF-κB activation and up-regulation of cIAP-1 and cIAP-2, in MM cells. Our data further suggest that the balance between these 2 pathways is modulated by the level of NF-κB activation. Finally, we have also recently reported that the expression of another known NF-κB target, intercellular adhesion molecule–1 (ICAM-1), is up-regulated by TNF-α.27 As seen in Figure 7B, SN50 strongly inhibits this induction of ICAM-1 on MM.1S cells by TNF-α, further implicating NF-κB in the regulation of MM cell adhesion and interactions of MM cells in the bone marrow microenvironment.
Effect of NF-κB activity on TNF-α–induced signaling.
TNF-α–induced signaling is modulated by the activity of NF-κB. (A) Cell viability (mean ± SD) was assayed by MTT in MM.1S cells treated with or without TNF-α (50 ng/mL), in the presence or absence of SN50 (10 μM) for 18 hours. Data shown (absorbance at 570 nm, mean ± SD) are representative of 3 experiments. In MM.1S cells pretreated with SN50, TNF-α induced apoptosis, rather than cell growth. (B) This was evidenced by cleavage and activation of caspase-8 and downstream caspase-3 and was associated with down-regulation of expression of the caspase-8 inhibitory proteins cIAP-1 and cIAP-2 In contrast, TNF-α alone up-regulated cIAP-1 and cIAP-2 expression. Moreover, TNF-α up-regulated adhesion molecule intercellular adhesion molecule–1 (ICAM-1) expression, and SN50 blocked this effect.
Effect of NF-κB activity on TNF-α–induced signaling.
TNF-α–induced signaling is modulated by the activity of NF-κB. (A) Cell viability (mean ± SD) was assayed by MTT in MM.1S cells treated with or without TNF-α (50 ng/mL), in the presence or absence of SN50 (10 μM) for 18 hours. Data shown (absorbance at 570 nm, mean ± SD) are representative of 3 experiments. In MM.1S cells pretreated with SN50, TNF-α induced apoptosis, rather than cell growth. (B) This was evidenced by cleavage and activation of caspase-8 and downstream caspase-3 and was associated with down-regulation of expression of the caspase-8 inhibitory proteins cIAP-1 and cIAP-2 In contrast, TNF-α alone up-regulated cIAP-1 and cIAP-2 expression. Moreover, TNF-α up-regulated adhesion molecule intercellular adhesion molecule–1 (ICAM-1) expression, and SN50 blocked this effect.
The p38 inhibitor PD169316 sensitizes MM.1S cells to apoptosis induced by SN50
Since p38 kinase signaling regulates cell survival41and the activation of NF-κB,42 we next investigated the role of p38 in SN50-induced MM cell apoptosis. The p38 inhibitor PD169316 did not induce MM cell apoptosis, but did enhance the apoptotic effect of SN50, suggesting an interaction between the p38 and NF-κB pathways (Figure 8).
Effect of PD169316 on MM.1S-cell apoptosis induced by NF-κB inhibition.
The p38 inhibitor PD169316 sensitizes MM.1S cells to apoptosis induced by NF-κB inhibition. Cell death was assayed by MTT in MM.1S cells treated with a nontoxic concentration of the p38 inhibitor PD169316 (10 μM) and/or the NF-κB inhibitor SN50 (10 μM) for 18 hours. PD169316 did not induce MM cell apoptosis, but did enhance the apoptotic effect of SN50, suggesting an interaction between the p38 and NF-κB pathways. Data shown (mean ± SD) are representative of 3 experiments.
Effect of PD169316 on MM.1S-cell apoptosis induced by NF-κB inhibition.
The p38 inhibitor PD169316 sensitizes MM.1S cells to apoptosis induced by NF-κB inhibition. Cell death was assayed by MTT in MM.1S cells treated with a nontoxic concentration of the p38 inhibitor PD169316 (10 μM) and/or the NF-κB inhibitor SN50 (10 μM) for 18 hours. PD169316 did not induce MM cell apoptosis, but did enhance the apoptotic effect of SN50, suggesting an interaction between the p38 and NF-κB pathways. Data shown (mean ± SD) are representative of 3 experiments.
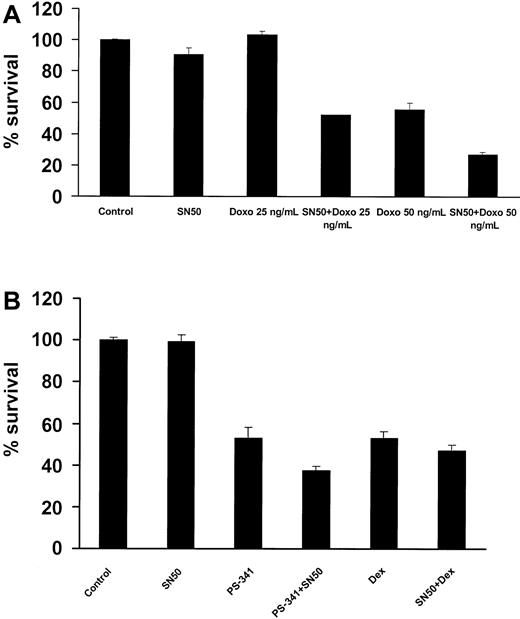
SN50 sensitizes MM cells to chemotherapy
The transcription factor NF-κB has been implicated in the regulation of cell sensitivity to chemotherapy in multiple model systems.43 44 We therefore investigated the effect of SN50 on chemotherapy-induced apoptosis in MM.1S cells. As seen in Figure9A, preincubation with a nontoxic concentration of the NF-κB inhibitor SN50 sensitized MM.1S cells to low concentrations of doxorubicin, with the percentage of MM cell survival as follows: 101.6% ± 1.9% with doxorubicin (25 ng/mL) versus 52.1% ± 0.2% with doxorubicin (25 ng/mL) plus SN50 (P < .016); 55.6% ± 4.0% with doxorubicin (50 ng/mL) versus 27.0% ± 1.4% with doxorubicin (50 ng/mL) plus SN50 (P < .005). These data suggest that inhibition of NF-κB strongly potentiates the anticancer effects of traditional anti-MM chemotherapy. We also evaluated the combined effects of SN50 with dexamethasone and the proteasome inhibitor PS-341 and found a modest potentiating effect (Figure 9B).
Effects of NF-κB inhibition combined with anti-MM agents.
(A) MM.1S cells were incubated with or without doxorubicin (doxo; 25 and 50 ng/mL). At 24 hours later, the cells were treated with or without the NF-κB inhibitor SN50 (10 μM) for 18 hours. Cell survival was assayed by MTT. Data shown (mean ± SD) are representative of 3 experiments. The NF-κB inhibitor strongly sensitized MM.1S cells to doxorubicin. (B) SN50 (10 μM) also increased, but to a lesser extent, the anti-MM effect of the proteasome inhibitor PS-341 (5 nM, 18 hours), and dexamethasone (dex; 1 μM, 48 hours).
Effects of NF-κB inhibition combined with anti-MM agents.
(A) MM.1S cells were incubated with or without doxorubicin (doxo; 25 and 50 ng/mL). At 24 hours later, the cells were treated with or without the NF-κB inhibitor SN50 (10 μM) for 18 hours. Cell survival was assayed by MTT. Data shown (mean ± SD) are representative of 3 experiments. The NF-κB inhibitor strongly sensitized MM.1S cells to doxorubicin. (B) SN50 (10 μM) also increased, but to a lesser extent, the anti-MM effect of the proteasome inhibitor PS-341 (5 nM, 18 hours), and dexamethasone (dex; 1 μM, 48 hours).
Discussion
In the present study, we investigated the effects of the NF-κB inhibitor SN50 on MM cell lines and patient cells, as well as on normal B lymphocytes. We found that SN50 inhibited constitutive and induced NF-κB activity and induced apoptosis in MM cell lines and patient cells, including those resistant to conventional therapies, but not in normal human B lymphocytes. SN50 down-regulated Bcl-2, A1, XIAP, cIAP-1, cIAP-2, and survivin protein levels, up-regulated Bax expression, induced release of mitochondrial cytochrome cinto the cytoplasm, and activated caspase-9 and caspase-3 but not caspase-8. SN50 also sensitized MM cells to TNF-α and doxorubicin and had a synergistic proapoptotic effect with a p38 inhibitor. These studies provide the framework for targeting NF-κB in MM therapies.
Several established or novel anti-MM agents, such as dexamethasone, thalidomide, proteasome inhibitors, and arsenic trioxide, have the ability to inhibit NF-κB activation.21-26 As a consequence, NF-κB inhibition may be at least a component of their antitumor activity. However, the exact contribution of NF-κB inhibition to their anti-MM activity has not been delineated. Moreover, the effect of specific NF-κB inhibition on MM cells is also unknown. We therefore used an inhibitor of the nuclear translocation of NF-κB to characterize the role of this transcription factor in MM cells.29 We found that the NF-κB inhibitor SN50 induced apoptosis in most MM cell lines, as well as in primary patient cells, but not in normal B lymphocytes. In previous studies of our panel of cell lines, the 2 SN50-resistant cell lines constitutively express the highest levels of various apoptosis inhibitors, including FLICE/caspase-8 inhibitory protein (FLIP), cIAP-1, cIAP-2, XIAP, and survivin.40
We then investigated the molecular mechanism of SN50-induced MM cell apoptosis. We found evidence of mitochondrial involvement, including Bcl-2 and A1 down-regulation, release of mitochondrial cytochrome c to the cytoplasm, and activation of caspase-9. Several members of the Bcl-2 family of apoptosis inhibitors are regulated by Rel/NF-κB transcription factors in various models, including Bcl-2 and A1.44-49 On the other hand, mitogen-activated B cells from mice lacking c-Rel or p50 expression are very sensitive to apoptosis and can be rescued by overexpression of Bcl-213 or A1.48 Moreover, A1 regulates the survival of macrophages in an NF-κB–dependent fashion by preserving mitochondrial integrity50 and suppresses apoptosis by inhibiting cytochrome c release and caspase-3 activation.44 In our model, the increase in Bax and the decrease in Bcl-2 and A1 protein levels shift the balance of Bcl2-A1/Bax toward apoptosis. Bax induces pore formation in the mitochondrial membrane and ultimately facilitates the release of cytochrome c into the cytoplasm,51 as in the present study. Cytochrome c binds the adaptor molecule Apaf-1 and forms the “apoptosome” that activates caspase-9, which cleaves and activates caspase-3.52 53 The role of caspase-9 and caspase-3 in our model is confirmed, since specific caspase-3 and caspase-9 inhibitors attenuated SN50-induced apoptosis.
The apoptosis inhibitor XIAP is NF-κB dependent in other models.35,54 Since XIAP inhibits caspase-9 activity, it appears that NF-κB inhibition stimulates caspase-9–dependent apoptosis in a dual manner: by stimulating the release of mitochondrial cytochrome c, leading to the formation of the apoptosome; and by down-regulating XIAP expression. Activated caspase-9 then activates caspase-3. In our study, the expression of survivin, another member of the IAP family, was also found to be NF-κB dependent in MM cells. Since caspase-3 can be inhibited by survivin,55 it appears that NF-κB also regulates MM cell apoptosis at the level of caspase-3 activity.
We recently demonstrated that TNF-α is secreted into the bone marrow microenvironment by MM cells and induces NF-κB–dependent alterations in adhesion molecule expression on both MM cells and bone marrow stromal cells, with resulting increased cell adhesion. This binding confers resistance to apoptosis and also triggers NF-κB–dependent secretion of IL-6. TNF-α also causes a modest, but reproducible, MM cell proliferation.27 In this study, we investigated the effect of NF-κB inhibition on the sequelae of TNF-α on MM.1S cells. In contrast to the moderate (approximately 25% increased) proliferation response to TNF-α monotherapy, TNF-α also induced a moderate reduction of survival (approximately 25% decrease) via activation of caspase-8 and caspase-3 when combined with a noncytotoxic concentration of SN50. These data suggest that TNF-α is not per se a growth factor for MM cells, but is rather a proapoptotic factor that activates caspase-8 and caspase-3. Our data further suggest that constitutive activity of NF-κB in MM cells abrogates the proapoptotic effect of TNF-α, owing to the potent up-regulation of cIAP-1 and cIAP-2, and induces modest proliferation. We have also recently reported that NF-κB inhibition increases the sensitivity of MM cells to another member of the TNF family, TRAIL/Apo2L.56 As was observed in this study, this sensitizing effect is associated with down-regulation of cIAP-1 and cIAP-2, members of the IAP family of antiapoptotic proteins that are recruited to the signaling complex of TNF receptor I via their interaction with TNF-receptor–associated factor 1 (TRAF1) and TRAF2, thereby exerting an inhibitory function on caspase-8 activation.57 The expression of cIAP-2 is also regulated by NF-κB in human leukemic cells.58 Moreover, overexpression of cIAP-1 and cIAP-2 can protect RelA-deficient cells, which are highly sensitive to TNF-α–induced apoptosis, by blocking the activation of caspase-8.57 Thus, cIAP-1 and/or cIAP-2 mediate, at least in part, the protective effect of NF-κB against apoptosis in MM cells.
These data collectively demonstrate a dual antiapoptotic effect of NF-κB in MM cells: first, at the level of caspase-8 in the death-receptor pathway and second, at the mitochondrial level. Our work further suggests that the death-receptor pathway is more sensitive to NF-κB inhibition, since lower concentrations of SN50 are sufficient to potentiate death-receptor–induced apoptosis in our model, in both this and prior studies.40 In contrast, mitochondrial dysfunction apparently requires complete abrogation of constitutive NF-κB activity. It is possible that this dual regulation corresponds to different conditions in vivo: complete abrogation of NF-κB activity may by itself be sufficient to induce MM cell apoptosis, whereas a partial block in NF-κB activity may require an extracellular stimulus in the form of a death ligand, such as TNF-α or TRAIL/Apo2L, to efficiently trigger cell death.
NF-κB confers protection against apoptosis induced by chemotherapy in several types of cells,44,57 and numerous tumor cells with elevated levels of NF-κB are resistant to apoptosis induced by chemotherapy and radiotherapy. Conversely, adenoviral delivery of a superrepressor form of IκBα sensitizes chemoresistant tumors to TNF-α and to the chemotherapeutic drug CPT-11, leading to tumor regression.59 In addition, proteasome inhibitors, such as PS-341, inhibit NF-κB by blocking the degradation of IκB, thereby increasing their sensitivity to doxorubicin.60 In this study, we similarly detected a potent sensitizing effect of SN50 on doxorubicin-induced apoptosis in MM cells, as well as more modest increments in apoptosis induced by the proteasome inhibitor PS-341 and dexamethasone. Finally, SN50 had a synergistic proapoptotic effect with p38 inhibition, suggesting cross-talk between these 2 signaling pathways in MM cells.
In summary, we have demonstrated that NF-κB protects MM cells from apoptosis both by maintaining mitochondrial stability via the expression of Bcl-2 and A1 and by attenuating death-receptor–induced apoptosis via the expression of cIAP-1 and cIAP-2. These studies delineate the role of NF-κB as an antiapoptotic factor in MM cells and suggest the potential utility of combining novel agents targeting NF-κB with standard anti-MM chemotherapeutics.
Supported by the Multiple Myeloma Research Foundation (N.M., C.S.M.); the Laurie Strauss Leukemia Foundation (N.M., C.S.M.); the Bailey Family Research Fund (N.M., C.S.M.); a National Institutes of Health Career Development Award (S.P.T.); and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth C. Anderson, Department of Adult Oncology, Dana Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: kenneth_anderson@dfci.harvard.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal