A 44-year-old woman with a history of severe thrombotic manifestations presented with a markedly reduced activated protein C–sensitivity ratio (APC-SR). DNA sequencing of and around the regions encoding the APC cleavage sites in the factor Va molecule excluded the presence of the factor VLeiden mutation and of other known genetic mutations. No antiphospholipid antibodies were present in the patient's plasma and both prothrombin time and activated partial thromboplastin time were normal. The total immunoglobulin fraction was isolated from the patient's plasma and found to induce severe APC resistance when added to normal plasma and to factor V–deficient plasma supplemented with increasing concentrations of factor V. Immunoblotting and immunoprecipitation experiments with the total immunoglobulin fraction purified from the patient's plasma demonstrated that the antibody recognizes factor V, is polyclonal, and has conformational epitopes on the entire factor V molecule (heavy and light chains, and B region). Thus, the immunoglobulin fraction interferes with the anticoagulant pathway involving factor V. The inhibitor was isolated by sequential affinity chromatography on protein G–Sepharose and factor V–Sepharose. The isolated immunoglobulin fraction inhibited factor Va inactivation by APC because of impaired cleavage at Arg306 and Arg506 of the heavy chain of the cofactor. The isolated immunoglobulin fraction was also found to inhibit the cofactor effect of factor V for the inactivation of factor VIII by the APC/protein S complex. Our data provide for the first time the demonstration of an antifactor V antibody not related to the presence of antiphospholipid antibodies, which is responsible for thrombotic rather than hemorrhagic symptoms.
Introduction
Coagulation factor V circulates in plasma as a large single-chain protein with a Mr330 000.1,2 α-Thrombin cleaves single-chain factor V to give rise to the active cofactor (factor Va) and to 2 heavily glycosylated activation fragments. The factor Va resulting from α-thrombin cleavage of human factor V is composed of a heavy chain of Mr 105 000 containing the NH2-terminal part of the factor V molecule (A1-A2 domains) and a light chain of Mr 74 000 that is derived from the COOH-terminal end of the cofactor (A3-C1-C2 domains). Once formed, α-thrombin binds to the endothelial cell receptor thrombomodulin and initiates the protein C pathway leading to the formation of APC. Proteolytic cleavage of factor Va by APC is required for complete inactivation of the cofactor and arrest of its contribution to the procoagulant process.3Factor Va is inactivated by activated protein C (APC) following 3 cleavages of the heavy chain: Arg506, Arg306, and Arg679. Cleavage at Arg306 efficiently occurs on the membrane-bound cofactor and is responsible for complete inactivation of factor Va.3 Thus, irregularities in the mechanism of inactivation of factor Va by APC are associated with thrombotic episodes because of sustained prothrombin activation.
Individuals with a 1691G>A substitution in the factor V (F5) gene (resulting in an Arg506Gln mutation in the factor V molecule, factor VLeiden) have a poor anticoagulant response to APC (APC resistance), which is associated with a significant increase in risk for deep venous thrombosis (DVT) (7-fold for the heterozygous and 80-fold for the homozygous).4-8 APC resistance has been suggested to be the most common risk factor for developing DVT, most likely because factor VaLeiden is inactivated by APC at a slower rate than normal factor Va thus leading to prolonged thrombin generation.9 10
Antifactor V antibodies are relatively common and are developed usually after exposure of patients to topical hemostatic agents containing bovine α-thrombin, blood transfusion, or antibiotic administration (for complete review of the literature on antifactor V antibodies, see Knöbl and Lechner11 and Ortel12). The majority of the antifactor V antibodies thus far described result in hemorrhagic manifestations that can be mild to severe. Only 3 antifactor V antibodies have been demonstrated to cause thrombotic manifestations. However, these antibodies were not well characterized and, whereas 2 of them were associated with lupuslike symptoms,13,14 the third antifactor V antibody was associated with Sjögren syndrome.15 In the present manuscript, we present data from an individual with an antifactor V antibody that interferes with the anticoagulant pathway of blood coagulation.
Patient, materials, and methods
Case report
The patient is a 44-year-old woman who experienced DVT at the age of 25 during pregnancy. Histology of the placenta revealed diffuse thrombosis of microvilli. At the age of 30, the proposita developed recurrent idiopathic DVT. Since then, she experienced several DVT episodes in both legs and she developed postphlebitic syndrome. Administration of oral anticoagulant secondary prophylaxis (at international normalized ratio 2.0-3.0) prevented further recurrences in the proposita. The patient was referred to our Thrombosis Center in Padua in 1990. Because oral anticoagulant therapy had been stopped 6 months earlier, we performed an extensive coagulation screening including assays for antithrombin, protein C, and protein S, and tests for lupus anticoagulant and anticardiolipin antibodies (Table1). Tests for lupus anticoagulant as well as for anticardiolipin antibodies (IgG) were negative. Low concentrations of IgM anticardiolipin antibodies were detected in one occasion. From 1990, blood was collected yearly and coagulation tests repeated. Although the laboratory pattern for protein C and protein S has remained unchanged, both lupus anticoagulant and anticardiolipin antibodies (IgG and IgM) and, thereafter, anti-β2-glycoprotein I (IgG or IgM) and antiprothrombin antibodies, have always tested negative during 10 years of follow-up. In 1994, when the APC resistance assay became available, the patient's plasma was tested for APC resistance and the result was positive (strongly resistant). Genetic analyses failed to show the presence of factor VLeiden mutation or other mutations encoding for amino acids substitution at and around Arg306 and Arg679 of the factor V molecule. During the last 10 years the patient has been followed and studied extensively to assess the possible presence of an underlying autoimmune disorder. Neither clinical manifestations nor laboratory tests have been positive for systemic lupus erythematosus, lupuslike syndrome, or other connective tissue diseases. Assays for antiphospholipid antibodies (APAs) and lupus anticoagulant performed recently were still negative. Due to the previous history of recurrent venous thromboembolism and the persistent presence of the inhibitor, lifelong oral anticoagulant treatment has been assigned to the patient. Short courses of high-dose steroids have been given in the past in the attempt to eliminate the inhibitor without success. After the patient signed informed consent, blood from the patient was drawn yearly, centrifuged, and platelet-poor plasma stored in aliquots, at −80°C until use.
Main laboratory features of the patient's plasma
| . | Patient . | Normal values . |
|---|---|---|
| PTT | 13.3 s | 13.4 s |
| aPTT | 31.2 s | 30-40 s |
| Antithrombin activity | 116% | 80%-120% |
| Protein C antigen (ELISA) | 100% | 80%-120% |
| Protein C activity (amidolytic) | 98% | 70%-130% |
| Protein C activity (clotting) | 40% | 80%-120% |
| Protein C activity (clotting) in PC-deficient plasma reconstituted with 4 μg/mL PC isolated from patient's plasma | 100% | 80%-120% |
| Total protein S antigen | 90% | 70%-130% |
| Free protein S antigen | 88% | 70%-130% |
| Protein S clotting activity | 35% | 65%-135% |
| Protein S activity (clotting) in PS- deficient plasma reconstituted with 10 μg/mL PS isolated from patient's plasma | 90% | 65%-135% |
| Factor V antigen | 100% | 80%-120% |
| Factor V activity | 102% | 80%-120% |
| APC-SR | 1.14 | > 2.00 |
| Factor VLeiden mutation | Homozygous wild-type | Homozygous wild-type |
| . | Patient . | Normal values . |
|---|---|---|
| PTT | 13.3 s | 13.4 s |
| aPTT | 31.2 s | 30-40 s |
| Antithrombin activity | 116% | 80%-120% |
| Protein C antigen (ELISA) | 100% | 80%-120% |
| Protein C activity (amidolytic) | 98% | 70%-130% |
| Protein C activity (clotting) | 40% | 80%-120% |
| Protein C activity (clotting) in PC-deficient plasma reconstituted with 4 μg/mL PC isolated from patient's plasma | 100% | 80%-120% |
| Total protein S antigen | 90% | 70%-130% |
| Free protein S antigen | 88% | 70%-130% |
| Protein S clotting activity | 35% | 65%-135% |
| Protein S activity (clotting) in PS- deficient plasma reconstituted with 10 μg/mL PS isolated from patient's plasma | 90% | 65%-135% |
| Factor V antigen | 100% | 80%-120% |
| Factor V activity | 102% | 80%-120% |
| APC-SR | 1.14 | > 2.00 |
| Factor VLeiden mutation | Homozygous wild-type | Homozygous wild-type |
Materials and reagents
N-[2-Hydroxyethyl]piperazine-N′-2-ethanesulfonic acid (HEPES), Q-Sepharose fast flow, Sepharose CL-4B, bovine serum albumin (BSA), diisopropyl-fluorophosphate (DFP), antihuman IgG (whole molecule) coupled to horseradish peroxidase, a nonimmune human IgG preparation, and factor V–deficient plasma were purchased from Sigma (St Louis, MO). Antimouse IgG coupled to peroxidase was from Southern Biotechnology (Birmingham, AL). l-a-phosphatidylserine (PS) and l-a-phosphatidylcholine (PC) where from Avanti Polar Lipids (Alabaster, AL). Normal reference plasma and hirudin were from American Diagnostica (Greenwich, CT). The automated activated partial thromboplastin time (aPTT) reagent and the prothrombin time (PT) reagent (Simplastin Excel) were from Organon Technika (Durham, NC) and factor VIII–deficient plasma was from George King Biomedicals (Overland Park, KS). The chemiluminescent reagent ECL+ was from Amersham Pharmacia Biotech (Piscataway, NJ). Human APC, human factor Xa, human protein S, prothrombin, sheep antihuman factor V polyclonal antibody, α-thrombin, and the fluorescent α-thrombin inhibitor dansylarginine-N-(3-ethyl-1,5-pentanediyl)amide (DAPA) were from Haematologic Technologies (Essex Junction, VT). Human factor V was purified as previously described using an immunoaffinity column.16 The 2 monoclonal antibodies to human V (against the heavy and light chains of the cofactor) were a gift from Dr Kenneth G. Mann (University of Vermont, Burlington). Recombinant factor VIII preparations (Kogenate) were a gift from Dr Lisa Regan of Bayer (Berkeley, CA). The Kogenate concentrate was fractionated to extract factor VIII using a previously described method.17 Synthetic phospholipid vesicles composed of 75% PC and 25% PS (referred to as PCPS vesicles throughout this report) were prepared as previously described.18 The concentration of the PCPS vesicles within each mixture was determined by phosphorous assay.19 Prothrombinase activity was tested using purified reagents as described.3
APC-sensitivity assay
The APC-sensitivity ratio (APC-SR) is used to define an individual predisposition to thrombosis because of either genetic or acquired abnormalities. The APC-SR is defined by measuring the clotting time of plasma in the presence or absence of APC as described.4,5 20 The clotting time is registered and the ratio of clotting time in the presence of APC divided by the clotting time in the absence of APC is defined as the APC-SR. Normal APC-SR values are usually higher than 2.0. Any value below 2.0 is considered as APC resistance.
Detection of APAs and other routine laboratory determinations for lupus anticoagulant
For the determination of APAs, a polyvalent kit IgG, IgA, and IgM (Asserachrom APA, Stago, Asnieres, France), was used as described.21 The enzyme-linked immunosorbent assay (ELISA) kit contains cardiolipin, anionic phospholipid, and β2-glycoprotein I. Tests were considered positive if the values exceeded 15 phospholipid units (UPL)/mL and borderline when they were between 5 and 15 UPL/mL. ELISAs for the detection of anticardiolipin antibodies (Asserachrom Anticardiolipin IgG/IgM, Stago), anti-β2-glycoprotein I antibodies (Asserachrom anti-β2-glycoprotein I IgG/IgM, Stago) and antiprothrombin antibodies (Asserachrom antiprothrombin IgG/IgM, Stago) have also been performed using commercially available kits. For the detection of lupus anticoagulant, the guidelines recommended by the Subcommittee for Standardization of the International Society on Thrombosis and Hemostasis were followed,22 using a method reported elsewhere.21 Both PTT-based and dilute Russell viper venom time-based assays were performed to detect the presence of lupus anticoagulant as previously described.21
Protein C and protein S assays
Amidolytic and clotting activities of protein C were measured in plasma using Behrichrom protein C and protein C reagent kits, respectively (Dade-Behring, Milan, Italy), as previously described.23 In both methods, Protac was used to activate protein C. The amidolytic method measures the effect of APC on a specific chromogenic substrate (Chromozym-TH), whereas the clotting method measures the prolongation of the aPTT after activation of protein C present in the patient's plasma. For the determination of protein S activity, a commercially available kit, IL PS test (Instrumentation Laboratories, Milan, Italy) was used.24Protein S cofactor activity for APC is determined after activation of protein C present in the test mixture (including protein S–deficient plasma and diluted patient's plasma) by Protac. Functional tests for protein C and protein S were performed in patient's plasma or after reconstitution of protein C- and protein S–deficient plasmas with protein C and protein S, respectively, isolated from patient's plasma by immunoaffinity chromatography.
Protein C and total and free protein S antigen levels were determined as previously described.25,26 Factor V antigen was detected in patient's plasma by an ELISA as previously described.20 Factor V functional assay were performed on ACL 3000 plus (Instrumentation Laboratories) using the PT/fibrinogen reagent (Instrumentation Laboratories) and factor V–deficient plasma (Instrumentation Laboratories). Patient's plasma collected on many occasions was tested repeatedly during 10 years of follow-up using all the above-mentioned tests.
Preparation of the factor V–Sepharose 4 Fast Flow
Approximately 7 mL swollen activated Sepharose 4 Fast Flow (Amersham Pharmacia Biotech, Uppsala, Sweden) was washed as suggested by the manufacturer. Purified human factor V (∼30 mg) was extensively dialyzed against the coupling buffer, sodium citrate, pH 6.5, and added to the beads at 4°C. The percent coupling was monitored every 20 minutes by taking the optical density of the supernatant. Following a 2.5-hour incubation at 4°C the reaction was stopped. The coupling efficiency determined by the absorption at 280 nm of factor V remaining in the supernatant was 89%. The beads were further treated as described by the manufacturer and stored at 4°C in 20 mM Tris, 0.15M NaCl pH 7.4 (Tris-buffered saline [TBS]), and 0.01% NaN3.
Isolation of the factor V inhibitor
Two different separations were performed. For the APC-SR assays 5 mL plasma was applied to a 1-mL column of protein G–Sepharose (Sigma) in TBS. Following washing, the immunoglobulin fraction was eluted with 0.1 M glycine, 0.5 M NaCl, pH 3. Fractions were collected and neutralized in Tris pH 8.0. Tubes containing the patient's total immunoglobulin fraction were pooled, dialyzed, and used in the mixing experiments for the determination of the APC-SR as described.20
For the direct inhibition of the factor V inactivation by APC, a different procedure was used. Higher volumes of plasma containing the inhibitor were applied to a 5-mL column of protein G–Sepharose equilibrated in TBS (Amersham Pharmacia Biotech, Uppsala). Following washing, the column was eluted as described above with an acidic solution (pH ∼3). Approximately 100 mg total immunoglobulin isolated from patient's plasma was applied on a 7-mL human factor V–Sepharose column (27 mg factor V/7mL beads) equilibrated in the same buffer. Following washing with 20 mM Tris, 2.0 M NaCl, pH 7.4, elution was performed with either 3M NaSCN or an acidic solution (glycine, NaCl, pH ∼2.8). When elution was performed at low pH the fractions from the column were collected directly in tubes containing Tris buffer (pH 8.0) to neutralize the acidic solution. The absorbance at 280 nm of the fractions eluted from the antifactor V column was determined using an extinction coefficient of ε nm of 14. Protein containing fractions were immediately pooled, dialyzed against TBS, and used in the assays as inhibitors.
Measurement of inhibitory activity and clotting assays
Factor V cofactor activity was measured by a clotting assay using factor V-deficient plasma and standardized to the percent of control. The assay end point was determined by visualization of the fibrin clot. Factor V was incubated with the inhibitory immunoglobulin fraction eluted from the factor V column and was assayed directly in such a clotting assay. Control experiments included a commercial preparation of human nonimmune IgG, antibodies purified from normal plasma or buffer or both.
Inactivation of factor VIII
The effect of factor V on the inactivation of factor VIII was studied as previously described.27 Briefly, purified recombinant factor VIII (60 nM) was incubated with APC (10 nM) and protein S (100 nM) in the presence of PCPS vesicles (10 μM) at 37°C. At selected time intervals factor VIII activity was assayed as described by using a 2-stage clotting assay.27 When the effect of factor V was evaluated, the procofactor (± IgG) was preincubated with factor VIII in the presence of PCPS vesicles and protein S and APC was added to start the inactivation reaction.
Immunoprecipitation experiments
Human factor V (600 nM) was incubated with α-thrombin (15 nM) for 10 minutes at 37°C. The reaction was stopped with either DFP (2 mM) or hirudin (30 nM). Factor Va (600 nM) was incubated with the patient total immunoglobulin fraction in TBS, 5 mM Ca++ to final concentrations of 400 nM factor Va and 1 μM immunoglobulin fraction. Following a 30-minute incubation at room temperature the sample was split in half. EDTA (50 mM) was added to one solution, whereas the other solution was incubated with buffer in the absence of EDTA. Following a 10-minute incubation, protein G–Sepharose was added. The solutions were gently mixed and centrifuged for 10 minutes at 14 000g. The supernatant was discarded and the beads were washed 3 times with 2 M NaCl. Each time the supernatant was discarded. The final wash was performed in the presence of 2% sodium dodecyl sulfate (SDS). Following centrifugation, the supernatant was analyzed on a 5% to 15% SDS–polyacrylamide gel electrophoresis (PAGE). After transfer to nitrocellulose, factor V fragments were detected using monoclonal antibody αHVaHC#17 (2 μg/mL) that recognizes an epitope on factor Va heavy chain,9,10 monoclonal antibody αHFVaLC#9 (5 μg/mL) that recognizes an epitope on the light chain of factor Va,10 and a polyclonal antibody to factor V. Immunoreactive fragments were visualized with chemiluminescence. In some experiments, factor Va was treated with APC alone (20 nM) or with APC and PCPS (50 μM) prior to the addition of the EDTA and protein G–Sepharose.
Gel electrophoresis
The SDS-PAGE analyses were performed using 5% to15% and 8% to18% gradient gels according to the method of Laemmli.28Electroblotting was performing according to the method of Towbin et al.29 NH2-terminal sequencing from polyvinylidene difluoride (PVDF) membranes was performed as described3 in the analytical facility of Dr Alex Kurosky at the University of Texas Medical Branch at Galveston.
Results
Routine coagulation tests
The APC-SR of the patient was 1.14. This value is low and consistent with the presence of homozygous factor VLeidenmutation. Genetic analyses5 as well as plasma assays30 31 revealed that the patient has normal circulating factor V and has no mutation at and around the Arg506, Arg306, or Arg679 APC-cleavage sites. Further, the patient's factor V molecule has normal procoagulant activity and both PT and aPTT were within the normal ranges (Table 1). Total and free protein S antigen levels, protein C antigen level, and chromogenic activity were normal (Table 1). However, protein S and protein C anticoagulant activities appeared to be spuriously low in the aPTT- or PT-derived assays. After reconstitution of protein C- and protein S–deficient plasma with protein C and protein S isolated from patient's plasma, normal levels of anticoagulant activity were found. These data demonstrate that all known coagulation proteins involved in the APC pathway (ie, protein C, protein S, or factor V) isolated from the patient's plasma have normal activity when purified from the patient's plasma and tested in vitro with purified reagents. The severe APC resistance observed was most likely due to an inhibitor present in the plasma of the patient that could interfere with factor V inactivation by APC. No lupus anticoagulant or APAs have been detected in patient's plasma.
Effect of normal plasma on the patient's APC-SR
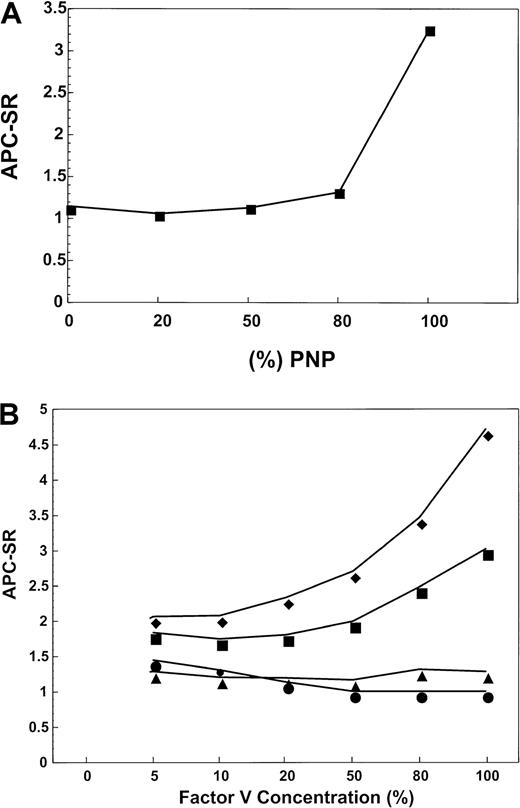
To ascertain if the patient's plasma contains an inhibitor that is able to disrupt the APC pathway in normal plasma, a mixing experiment was performed. The patient's plasma was mixed with increasing concentrations of pooled normal plasma (PNP) and tested for the APC-SR. Figure 1A demonstrates that the patient's plasma contains an inhibitor that impairs APC anticoagulant activity in normal plasma. The inhibitory activity of the patient's plasma appears to be very strong because the APC-SR of the mixtures remains the same (1.2-1.4) even at proportions 80:20 normal/patient plasma. These data demonstrate that the patient's plasma contains an inhibitor that is able to inhibit the anticoagulant pathway in normal plasma.
Effect of normal plasma on the patient's APC-SR.
(A) APC-SR in samples obtained by mixing the patient's plasma with PNP. For the determination of APC resistance, a standard method has been used.20 The amount of PNP is progressively increased in the mixtures from 0% to 100%. The APC-SR remained low and 20% of the patient's plasma in the mixture is still able to determine relevant APC resistance. This suggests the presence of a strong inhibitory effect of factor Va inactivation by APC. (B) Effect of the factor V concentration on APC-SR in samples obtained by mixing plasma under investigation with factor V-deficient plasma. When PNP (filled squares) is the source of factor V, as the concentration of the molecule increases, the APC-SR progressively rises within the normal ranges. Under similar experimental conditions, the patient's plasma (filled circles) displays a very low APC-SR, which is consistent with severe APC resistance. If PNP is supplemented with an immunoglobulin preparation derived from normal individuals (filled diamonds), the APC-SR increases with increasing factor V concentrations in the mixture as shown for PNP (filled squares). When a patient's immunoglobulin preparation is used to supplement PNP instead of immunoglobulin isolated from normal individuals, the APC-SR remains markedly low even at high (normal) concentration of factor V in the mixture (filled triangles).
Effect of normal plasma on the patient's APC-SR.
(A) APC-SR in samples obtained by mixing the patient's plasma with PNP. For the determination of APC resistance, a standard method has been used.20 The amount of PNP is progressively increased in the mixtures from 0% to 100%. The APC-SR remained low and 20% of the patient's plasma in the mixture is still able to determine relevant APC resistance. This suggests the presence of a strong inhibitory effect of factor Va inactivation by APC. (B) Effect of the factor V concentration on APC-SR in samples obtained by mixing plasma under investigation with factor V-deficient plasma. When PNP (filled squares) is the source of factor V, as the concentration of the molecule increases, the APC-SR progressively rises within the normal ranges. Under similar experimental conditions, the patient's plasma (filled circles) displays a very low APC-SR, which is consistent with severe APC resistance. If PNP is supplemented with an immunoglobulin preparation derived from normal individuals (filled diamonds), the APC-SR increases with increasing factor V concentrations in the mixture as shown for PNP (filled squares). When a patient's immunoglobulin preparation is used to supplement PNP instead of immunoglobulin isolated from normal individuals, the APC-SR remains markedly low even at high (normal) concentration of factor V in the mixture (filled triangles).
Properties of the isolated inhibitor
To ascertain the nature of the inhibitor, patient's plasma was loaded onto a protein G–Sepharose column. Before the protein G–Sepharose column, the APC-SR in patient's plasma was 1.11, whereas the patient's plasma that flowed through the column had a normal APC-SR value of 3.45. Control experiments demonstrated that similar treatment of normal plasma had no influence on the APC-SR values (before protein G–Sepharose, the APC-SR of normal plasma was 3.45, whereas after the passage through the column the value was 3.1). Overall these data demonstrate that the patient's plasma contains an immunoglobulin molecule that is responsible for the inhibition of the anticoagulant pathway and the resulting prothrombotic effect.
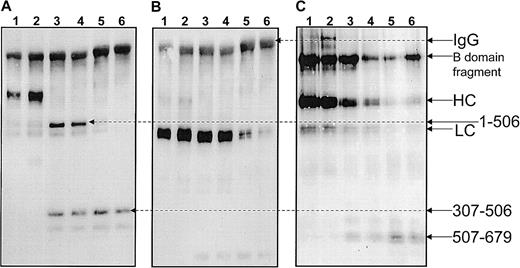
To test the hypothesis that the inhibitory antibody was directed against one of the proteins involved in the anticoagulant pathway (ie, factor V or APC or both) the specificity of the inhibitor was tested in a Western blot and was also assayed for its effect in normal plasma. Figure 2 demonstrates that the antibody only recognizes factor V under nonreducing conditions. The isolated immunoglobulin fraction from the patient's plasma did not recognize factor V under reducing conditions nor factor Va under either reducing or nonreducing conditions on a Western blot. Also, the antibody fraction from the patient's plasma did not recognize APC. These data demonstrate that the antifactor V antibody present in the patient's plasma most likely recognizes conformational epitopes on the procofactor.
Patient plasma contains an antifactor V antibody.
All components of prothrombinase (factor V, factor Va, factor Xa) as well as APC were analyzed nonreduced on a 5% to 15% SDS-PAGE followed by transfer to nitrocellulose (approximately 1.5 μg of each protein was applied per lane). The isolated total immunoglobulin fraction from the patient's plasma (eluted from the protein G–Sepharose column) was used as a source of primary antibody (at a concentration of 500 μg/mL) and staining of the proteins was accomplished with an antihuman immunoglobulin coupled to horseradish peroxidase (1:5000 dilution) and chemiluminescence. The lane assignment is indicated on the top of the figure. Control experiments were performed using a purified immunoglobulin fraction from normal plasma.
Patient plasma contains an antifactor V antibody.
All components of prothrombinase (factor V, factor Va, factor Xa) as well as APC were analyzed nonreduced on a 5% to 15% SDS-PAGE followed by transfer to nitrocellulose (approximately 1.5 μg of each protein was applied per lane). The isolated total immunoglobulin fraction from the patient's plasma (eluted from the protein G–Sepharose column) was used as a source of primary antibody (at a concentration of 500 μg/mL) and staining of the proteins was accomplished with an antihuman immunoglobulin coupled to horseradish peroxidase (1:5000 dilution) and chemiluminescence. The lane assignment is indicated on the top of the figure. Control experiments were performed using a purified immunoglobulin fraction from normal plasma.
The specificity of the inhibitor was further investigated by supplementing factor V–deficient plasma with PNP and purified immunoglobulin from either normal plasma (Figure 1B, filled diamonds) or from patient's plasma (Figure 1B, filled triangles). For comparison the factor V–deficient plasma was also supplemented with either normal plasma (Figure 1B, filled squares) or with patient's plasma (Figure1B, filled circles). No significant differences were observed between the APC-SR of the 4 mixtures at very low concentrations of factor V. However, the APC-SR of normal plasma supplemented with the patient's immunoglobulin fraction (Figure 1B, filled triangles) or the APC-SR in the patient's plasma (Figure 1B, filled circles) was consistently lower than that of normal plasma. Assuming a concentration of 20 nM factor V in normal plasma, the inhibition becomes apparent at about 20% factor V, which corresponds to approximately 4 nM. In fact, addition of patient's immunoglobulin to PNP is able to induce the same effect on the APC-SR as the effect observed with the patient's plasma (Figure 1B, filled triangles). Within physiologic levels of factor V, the inhibitory effect seems to be proportional to factor V concentrations. These data demonstrate that an increase in the concentration of factor V accentuates the inhibitory effect of the reconstituted plasma. The data strongly suggest that the patient's plasma contains an antifactor V antibody (immunoglobulin molecule) that strongly interferes with the ability of the molecule to be inactivated by APC.
To determine the location of the conformational epitope(s) recognized by the inhibitory antibody, an immunoprecipitation method was developed. Because the antibody recognizes conformational epitopes on factor V, experiments also need to be performed in the presence of EDTA (following the addition of α-thrombin) to verify the effect of the dissociation of the 2 chains of the cofactor on the specificity of the antibody's epitopes. Purified human factor Va was immunoprecipitated with the patient's immunoglobulin prior to and after the addition of EDTA. Purified human factor Va (400 nM) was also treated with APC (20 nM) (± PCPS vesicles, 50 μM) and immunoprecipitated with the patient's immunoglobulin prior to and after addition of EDTA. Because all fragments deriving from APC inactivation of factor Va (± PCPS vesicles) are well-characterized,3 these latter series of experiments were performed to identify some of the epitopes of the antibody on factor Va. At the end of the incubation all solutions were incubated with protein G–Sepharose and centrifuged. The data are presented in Figure 3. Panels A through C, lanes 1 through 4, demonstrate no differences in immunoprecipitation in the presence or absence of EDTA. Thus, the patient plasma contains antibodies directed against both chains of factor Va. It is noteworthy that all samples contain the human immunoglobulin molecule. In panels A and B of Figure 3 a band corresponding to a human IgG molecule is observed because the secondary antimouse molecule cross-reacts with the human immunoglobulin. In contrast, the secondary antisheep molecule does not appear to cross-react with the human immunoglobulin (Figure3C). Overall, the data demonstrate that the patient's plasma contains an antifactor V polyclonal antibody that has conformational epitopes on both the heavy and light chains of the cofactor (Figure 3A,B) as well as on the B region of the cofactor (Figure 3C).
Identification of the epitope(s) of the inhibitor.
Purified human factor V (600 nM) was incubated with 15 nM thrombin for 10 minutes at 37°C in TBS, 5 mM CaCl2, followed by the addition of 30 nM hirudin and treated with APC and PCPS. At the end of the incubation the samples were mixed with the total immunoglobulin fraction isolated from patient plasma. Immunoreactive fragments were precipitated with a solution containing protein G–Sepharose. Elution of fragments from the protein G–Sepharose beads was accomplished with a solution of 10% SDS and analysis of the fragments was performed on a 5% to 15% SDS-PAGE. Following transfer to nitrocellulose immunoreactive fragments were revealed with anti-factor V antibodies. (A) Staining with αHFVaHC#17, which recognizes an epitope on the heavy chain of factor Va between amino acid residues 307-506. (B) Staining with αHFVaLC#9, which recognizes an epitope on the light chain of the cofactor. (C) Staining with a polyclonal antihuman factor V; the antibody is directed against the whole factor V molecule and has epitopes on both chains of the cofactor as well as on the B region of the molecule. Lane 1, factor Va; lane 2, factor Va in the presence of EDTA prior to immunoprecipitation; lane 3, factor Va with APC; lane 4, factor Va with APC in the presence of EDTA prior to immunoprecipitation; lane 5, factor Va in the presence of APC and PCPS; lane 6, factor Va with APC and PCPS in the presence of EDTA prior to immunoprecipitation.
Identification of the epitope(s) of the inhibitor.
Purified human factor V (600 nM) was incubated with 15 nM thrombin for 10 minutes at 37°C in TBS, 5 mM CaCl2, followed by the addition of 30 nM hirudin and treated with APC and PCPS. At the end of the incubation the samples were mixed with the total immunoglobulin fraction isolated from patient plasma. Immunoreactive fragments were precipitated with a solution containing protein G–Sepharose. Elution of fragments from the protein G–Sepharose beads was accomplished with a solution of 10% SDS and analysis of the fragments was performed on a 5% to 15% SDS-PAGE. Following transfer to nitrocellulose immunoreactive fragments were revealed with anti-factor V antibodies. (A) Staining with αHFVaHC#17, which recognizes an epitope on the heavy chain of factor Va between amino acid residues 307-506. (B) Staining with αHFVaLC#9, which recognizes an epitope on the light chain of the cofactor. (C) Staining with a polyclonal antihuman factor V; the antibody is directed against the whole factor V molecule and has epitopes on both chains of the cofactor as well as on the B region of the molecule. Lane 1, factor Va; lane 2, factor Va in the presence of EDTA prior to immunoprecipitation; lane 3, factor Va with APC; lane 4, factor Va with APC in the presence of EDTA prior to immunoprecipitation; lane 5, factor Va in the presence of APC and PCPS; lane 6, factor Va with APC and PCPS in the presence of EDTA prior to immunoprecipitation.
Isolation of the inhibitor by affinity chromatography
To study the effect of the inhibitor on factor Va function, a monospecific polyclonal antibody is necessary because the inhibitory antifactor V population is most likely a minor portion of the total immunoglobulin fraction isolated by protein G–Sepharose. Thus, the inhibitor was isolated by sequential affinity chromatography using protein G–Sepharose and factor V–Sepharose. The inhibitory fraction contained within the material eluted from the protein G–Sepharose was subsequently subjected to affinity chromatography on factor V–Sepharose. The flow through of the latter column contained the majority of the patient's immunoglobulins. Following washing of the column with 2 M NaCl, elution was performed with either 3 M NaSCN or with a low pH buffer (pH 2.8).
Samples obtained following elution of the factor V column were dialyzed and analyzed by SDS-PAGE. The sample had the electrophoretic mobility of an IgG molecule (Mr 150 000) nonreduced (not shown). However, close examination of the gel revealed a doublet of Mr 150 000. Following transfer to a PVDF membrane and staining with an antihuman IgG (whole molecule), both bands of the doublet were visible. Further, on reduction 3 bands of Mrabout 100 000 (HC1), 50 000 (HC2), and 25 000 (LC) were detected. No band of Mr 150 000 remained following complete reduction, suggesting 2 populations of immunoglobulin molecules. An antihuman IgG molecule identified all 3 bands. It is noteworthy that although the bands of Mr50 000 and Mr 25 000 were readily visible on short exposures of the PVDF membrane to a film, the band of Mr100 000 was only observed following prolonged exposure of the PVDF membrane to the film. In addition, NH2-terminal amino sequence of the bands of Mr 50 000 and 25 000 fragments revealed complete identity with the heavy and light chains of an IgG molecule (not shown). NH2-terminal amino acid sequence of the third band of Mr 100 000 (HC1) was unsuccessful most likely because of a blocked NH2-terminal amino acid. However, the fact that an antihuman IgG molecule recognizes this band is indicative of a heavy chain of an abnormal immunoglobulin molecule. It is also possible that the Mr 100 000 band is a cross-reactive contaminating band in our preparation. Overall the data demonstrate that the patient's plasma contains 2 populations of antibodies (IgG) eluted from the factor V-Sepharose column, that are directed against factor V. One of these 2 antibodies has a Mr 100 000 heavy chain and a Mr 25 000 light chain, whereas the other antibody is a typical IgG molecule.
Analysis of the effect of the immunoglobulin fraction on the anticoagulant pathway involving factor V/Va and APC
The anticoagulant pathway involving factor V and APC relates to 2 different mechanisms. The first is the direct inactivation of the membrane-bound cofactor by APC. The second is more indirect and involves the cofactor effect of factor V for the inactivation of factor VIII by the APC/protein S complex.
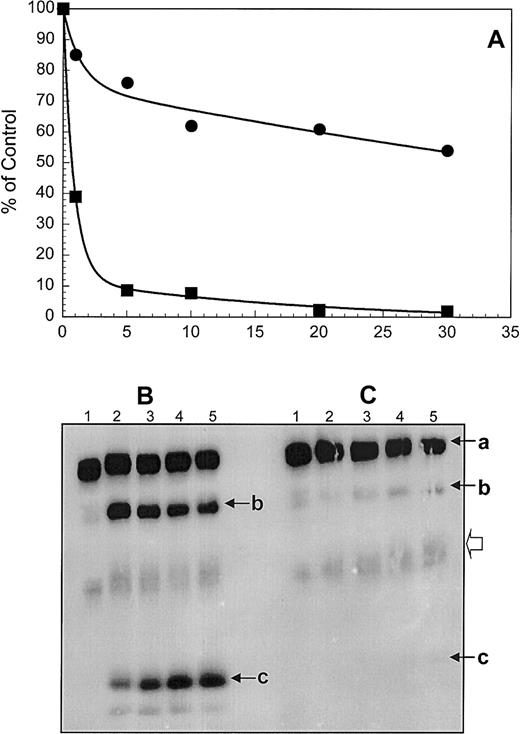
To ascertain the inhibitory potential of the purified monospecific antifactor V antibodies, the inhibition of the APC-mediated inactivation of membrane-bound factor Va was studied in a system using purified components. Factor Va activity was monitored with a clotting assay using factor V-deficient plasma. The results of the clotting assay are shown in Figure 4A. Under the conditions used, in the absence of the inhibitor, membrane-bound factor Va loses approximately 60% of its activity in 1 minute and more than 90% of cofactor activity is eliminated within the first 5 minutes of incubation with APC (Figure 4A, filled squares). The disappearance of cofactor activity coincides with cleavage of the heavy chain (a) at Arg506 and Arg306 and generation of fragments of Mr 75 000 (b) and 30 000 (c) (Figure 4B). At the end of the APC time course (Figure 4A, 30 minutes) no clotting activity remains. Incubation of factor Va with equimolar concentrations of the inhibitory molecule, prior to the addition of APC, results in a membrane-bound factor Va molecule that loses approximately 10% of its activity within 1 minute (Figure 4A, filled circles), whereas approximately 60% of its clotting activity remains following a 30-minute time period (Figure 4A, filled circles). The resulting activity profile demonstrates a continuous, slow decrease in activity, which persists following extensive incubation of membrane-bound factor Va with APC in the presence of the inhibitor (not shown). Gel electrophoresis analyses of samples taken during the time course of the inactivation of factor Va by APC in the presence of the inhibitor (Figure 4C) revealed slow cleavage of factor Va at both Arg306 and Arg506 (Figure 4C, lanes 4 and 5). The increase in density of the Mr 75 000 and 30 000 fragments is visible in lanes 4 and 5 (Figure 4C). There is also a slight increase in the concentration of a Mr 60 000 fragment (representing amino acid residues 307-679/709 of the heavy chain of the cofactor, Figure 4C, open arrowhead). Cleavage of the cofactor at Arg506 is membrane independent, whereas cleavage at Arg306 requires the presence of a membrane surface.2,3 Because both cleavages are delayed in the presence of the inhibitor, the data demonstrate that the inhibitor impairs efficient factor Va–APC interaction on the membrane surface and as a consequence proper inactivation of the cofactor. It is also possible that following binding to the inhibitor the conformation of the cofactor is altered, making the APC-cleavage sites of the heavy less accessible for APC. Because APC and factor Xa compete for the binding to factor Va,32 it is possible that the inhibitor may interfere with prothrombinase assembly. It is noteworthy that normal PT and aPTT values were observed in the patient (Table 1). To verify the effect of the antibody on the interaction of the cofactor with factor Xa, the purified immunoglobulin fraction was tested for direct inhibition of prothrombinase complex assembly and function. Under the conditions used (10 nM factor Xa, 4 nM factor Va, 8 nM IgG, and 20 μM PCPS) no inhibition of prothrombinase assembly and function was observed.
Inactivation of membrane-bound human factor Va by APC.
(A) Factor Va was made by incubating purified normal plasma factor V (50 nM) with α-thrombin (1 nM). The reaction was stopped with hirudin (2 nM). Following addition of phospholipid vesicles (50 μM) and normal APC (2.5 nM) the activity of factor Va was assessed in a clotting assay using factor V–deficient plasma (▪) at 1 minute, 5 minutes, 10 minutes, 15 minutes, 20 minutes, and 30 minutes following the addition of APC; (●) factor Va was incubated with the purified immunoglobulin inhibitor (64 nM) and the inactivation by APC was performed under similar experimental conditions as described for normal factor Va. (B) Some of the samples assayed for cofactor activity in panel A were also analyzed on a 5% to 15% linear gradient SDS-PAGE as described in “Patient, materials, and methods.” Lane 1, factor Va control, no APC; lanes 2 through 5, factor Va incubated with APC at 5, 10, 20, and 30 minutes, respectively. (C) Prior to the addition of APC and PCPS vesicles, factor Va was incubated with the purified immunoglobulin solution from the patient's plasma. The arrows indicate the heavy chain of factor Va (a), the Mr 75 000 intermediate (b), that results from cleavage at Arg506 of the heavy chain, and the Mr 30 000 fragment (c) that derives from cleavage of the Mr 75 000 intermediate at Arg306. The open arrowhead indicates cleavage at Arg306 first and generation of a fragment containing the region 307-6791709 of the heavy chain of the cofactor.
Inactivation of membrane-bound human factor Va by APC.
(A) Factor Va was made by incubating purified normal plasma factor V (50 nM) with α-thrombin (1 nM). The reaction was stopped with hirudin (2 nM). Following addition of phospholipid vesicles (50 μM) and normal APC (2.5 nM) the activity of factor Va was assessed in a clotting assay using factor V–deficient plasma (▪) at 1 minute, 5 minutes, 10 minutes, 15 minutes, 20 minutes, and 30 minutes following the addition of APC; (●) factor Va was incubated with the purified immunoglobulin inhibitor (64 nM) and the inactivation by APC was performed under similar experimental conditions as described for normal factor Va. (B) Some of the samples assayed for cofactor activity in panel A were also analyzed on a 5% to 15% linear gradient SDS-PAGE as described in “Patient, materials, and methods.” Lane 1, factor Va control, no APC; lanes 2 through 5, factor Va incubated with APC at 5, 10, 20, and 30 minutes, respectively. (C) Prior to the addition of APC and PCPS vesicles, factor Va was incubated with the purified immunoglobulin solution from the patient's plasma. The arrows indicate the heavy chain of factor Va (a), the Mr 75 000 intermediate (b), that results from cleavage at Arg506 of the heavy chain, and the Mr 30 000 fragment (c) that derives from cleavage of the Mr 75 000 intermediate at Arg306. The open arrowhead indicates cleavage at Arg306 first and generation of a fragment containing the region 307-6791709 of the heavy chain of the cofactor.
The effect of the antibody on the cofactor activity of factor V for the APC-mediated inactivation of factor VIII was next analyzed (Figure5). Factor V was found to enhance the inactivation rate of factor VIII by the APC/protein S complex by 2-fold as previously described27 33 (Figure 5, filled triangles) when compared to the inactivation of factor VIII in the absence of factor V (Figure 5, filled circles). The acceleration of the inactivation of factor VIII was inhibited by the immunoglobulin fraction eluted from the factor V-Sepharose column (Figure 5, filled diamonds). In contrast, under similar experimental conditions, a nonimmune human IgG commercial preparation had no inhibitory effect on the acceleration of factor VIII inactivation by the APC/protein S/factor V complex (Figure 5, filled inverted triangles). These data suggest another prothrombotic mechanism of the immunoglobulin fraction isolated from patient's plasma that could explain the APC resistance. Overall, the functional data indicate that the presence of the inhibitor in the patient's plasma is displayed in vivo by severe thrombotic manifestations.
Inactivation of membrane-bound human factor VIII by the APC/protein S complex.
Recombinant factor VIII (60 nM) was incubated with APC (5 nM) and protein S (100 nM) in the presence of PCPS vesicles (10 μM). At selected time intervals aliquots of the mixture were assayed for factor VIII activity as described27 using a 2-stage clotting assay (filled circles). Filled triangles represent the progress of the reaction in the presence of factor V (4 nM), and the filled diamonds represents the progress of the reaction in the presence of 8 nM inhibitory immunoglobulin fraction eluted from the factor V-Sepharose column. The filled inverted triangles represent the progress of the reaction in the presence of a commercially available preparation of nonimmune human IgG (8 nM). Incubation of recombinant factor VIII alone at 37°C did not result in any significant loss of cofactor function (filled squares). The error bars represent the average value found in 3 independent experiments.
Inactivation of membrane-bound human factor VIII by the APC/protein S complex.
Recombinant factor VIII (60 nM) was incubated with APC (5 nM) and protein S (100 nM) in the presence of PCPS vesicles (10 μM). At selected time intervals aliquots of the mixture were assayed for factor VIII activity as described27 using a 2-stage clotting assay (filled circles). Filled triangles represent the progress of the reaction in the presence of factor V (4 nM), and the filled diamonds represents the progress of the reaction in the presence of 8 nM inhibitory immunoglobulin fraction eluted from the factor V-Sepharose column. The filled inverted triangles represent the progress of the reaction in the presence of a commercially available preparation of nonimmune human IgG (8 nM). Incubation of recombinant factor VIII alone at 37°C did not result in any significant loss of cofactor function (filled squares). The error bars represent the average value found in 3 independent experiments.
Discussion
We have isolated and characterized an antifactor V antibody from plasma of a patient with recurrent thrombotic manifestations. Our data clearly demonstrate the presence of an antifactor V antibody that impairs proper inactivation of the molecule by APC. The antibody also interferes with the cofactor activity of factor V for the inactivation of factor VIII by the APC–protein S complex. Both, impaired inactivation of the cofactor by APC, as well as impaired inactivation of factor VIII, are most likely responsible for the strong APC resistance and the severe thrombotic manifestations observed in the patient plasma. To our knowledge, this the first time that an autoantibody that inhibits the factor V cofactor activity for the inactivation of factor VIII is reported, providing a physiologic relevance for this antithrombotic effect of factor V.27 33
This antibody appears to have occurred spontaneously because no diseases were identified during the follow-up of this patient nor were common causes for the development of antifactor V inhibitor such as exposure to plasma derivatives, antibiotics, or bovine thrombin preparation present. This antibody is not related to APAs because lupus anticoagulant and all other APAs have tested negative during 10 years of follow-up. The majority of the antifactor V antibodies described thus far in the literature have been found to produce a bleeding syndrome in the affected individuals.12 Only 3 cases of patients with antibodies to factor V have been associated with thrombotic events. However, 2 of the 3 cases had an autoimmune syndrome where lupuslike anticoagulant activity was demonstrated in association with anticardiolipin antibodies,13,14 whereas the antifactor V antibody in the third case was associated with Sjögren syndrome.15 It is interesting to note that in all 3 cases prolongation of the basal clotting time was present, suggesting an interference with the procoagulant function of factor V. It has been demonstrated in one case (Sjögren syndrome15) that although the antibody in vitro did not inhibit activated protein C activity toward factor Va, it was able to inhibit the procoagulant function of factor V. It is well established that the binding to a membrane surface is necessary for both normal procoagulant expression of factor Va cofactor activity as well as efficient down-regulation of its activity by APC.3 Thus, the latter 3 antibodies most likely inhibited the interaction of the cofactor with the membrane surface, resulting in both prolongation of clotting time and impaired APC inactivation. Two recent studies using recombinant factor V and factor V C2 domain have demonstrated that the majority of factor V inhibitors are antibodies directed to the C1-C2 domain of factor V and interfere with its binding to the PS-containing membranes.34 35 To our knowledge, this is the first report of an antifactor V antibody that directly inhibits the factor V–APC interaction inducing resistance of the cofactor to APC inactivation, without affecting the proper interaction of factor Va with the membrane surface.
It is still unknown how and when the patient described in our report first developed antifactor V antibodies. Antifactor V antibodies are known to develop in individuals secondary to blood transfusions, antibiotic exposure, or autoimmune disorders. However, antibodies to factor V have frequently been found in individuals exposed to topical thrombin36,37 or to bovine proteins (bovine thrombin or fibrin sealant) during major surgery.38-40 The isolated antifactor V antibody found in our patient has some peculiarity. It is not able to induce prolongation of the clotting time (no neutralizing effect), and because the concentration of factor V found in the patient's plasma was normal, it does not appear to interfere with the clearance of factor V. These characteristics make the antibody presented herein different from other antifactor V antibodies, which are often associated with bleeding manifestations and are directed against the C2 domain of the cofactor.34 35
Our experimental data strongly suggest that the antibody recognizes a conformational epitope on the factor V molecule. This concept is not new for neutralizing antibodies. Conformational epitopes were defined in autoantibodies to recoverin in cancer-associated retinopathy,41 in Graves disease where autoantibodies have been associated with a prionlike shift of a thyrotropin receptor A–subunit molecule,42,43 and in human autoimmune thyroiditis.44,45 Conformational epitopes only recognizing factor Va and not factor V were also developed in mice.46-48 Interestingly, the latter epitopes were only expressed on thrombin activation of the cofactor.48 The factor Va–APC interaction involves both chains of factor Va, whereas the B region of the procofactor alone appears to be involved in its anticoagulant effect.49 Our data suggest that the inhibitor developed in this patient is polyclonal with multiple epitopes all over the factor V molecule. The patient's plasma does not appear to have any other procoagulant defects. The presence of antifactor V antibodies with a selective effect on the inhibition of factor V interaction with APC represents a new mechanism responsible for APC resistance and possibly a new, though rare, acquired cause of thrombotic manifestations.
We wish to thank Dr Ken Mann from the University of Vermont for the monoclonal antibodies to human factor V and Dr Lisa Regan from Bayer Corporation for the recombinant factor VIII. We wish to thank Dr Alex Kurosky and Steve Smith from the University of Texas Medical Branch at Galveston, for amino acid sequencing, and Michael Ungham and Robert Dura for technical assistance.
Supported by start-up funds from the Department of Chemistry, Cleveland State University (M.K.), by a grant from the Department of Medical and Surgical Sciences, University of Padua (P.S.), and by grant HL34575 from the National Institutes of Health. Portions of this work were presented in abstract form at the XVIIth Congress of the International Society on Thrombosis and Hemostasis, Washington, DC, August 14-21 1999.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Kalafatis, Chemistry Department, Cleveland State University, Science Building, 2351 Euclid Ave, Cleveland, OH 44115; e-mail: m.kalafatis@csuohio.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal