Activated platelets release biologically active compounds, which then recruit additional platelets into an evolving thrombus. We studied activation of αIIbβ3 and exposure of P-selectin on platelets recruited by releasates obtained from collagen-treated platelets and evaluated modifications in prothrombotic effects of releasates induced by platelet-erythrocyte interactions and aspirin treatment. Releasates from collagen-stimulated platelets induced αIIbβ3 activation and P-selectin exposure (monitored by flow cytometry using fluorescein isothiocyanate–PAC-1 and phycoerythrin-CD62 antibodies). These responses were markedly amplified by releasates from combined platelet-erythrocyte suspensions. This finding demonstrates a novel mechanism(s) by which erythrocytes intensify platelet aggregability and mediate increased platelet recruitment. Because P-selectin and αIIbβ3 are potential sites for platelet-leukocyte interactions, erythrocytes may also modulate leukocyte recruitment. Following aspirin ingestion both the recruiting capacity of platelet releasates and erythrocyte-induced amplification of platelet recruitment were down-regulated. These events represent an additional antithrombotic property of aspirin. We also examined the possibility that arachidonic acid, or eicosanoids derived therefrom, can induce a prothrombotic activity of erythrocytes. The TXA2-analog U46 619 and free arachidonate, but not PGI2 or 12-HETE, induced increases in cytosolic Ca++ and promoted phosphatidylserine (PS) exposure on a subpopulation of erythrocytes. PS exposure and increases in erythrocyte [Ca++]i are associated with enhanced procoagulant activity, increased endothelial adhesion, and reduced erythrocyte deformability. Our findings, therefore, suggest that TXA2 and arachidonic acid, derived from activated platelets, induce a prothrombotic phenotype on erythrocytes in proximity. We conclude that by these mechanisms, erythrocytes can actively contribute to platelet-driven thrombogenesis and microvascular occlusion.
Introduction
When platelets interact with the subendothelial matrix of an injured vessel, they become activated, release components of their intracellular granules, and generate metabolic products. These products include adenine nucleotides, eicosanoids, and serotonin.1 In turn, the platelet releasate functions as agonist for recruitment of additional platelets into the evolving thrombus.1-5
By using an experimental approach to evaluate platelet activation and recruitment independently, we previously demonstrated that cell-cell interactions between activated platelets and intact erythrocytes (RBCs) amplify both platelet activation and the proaggregatory capacity of cell-free releasates.2-4 In contrast, we found that platelet-neutrophil interactions down-regulate platelet reactivity in our system.5
One of the earliest events in platelet reactivity is activation of platelet αIIbβ3 integrin receptors. Such activation is a prerequisite for fibrinogen binding to platelets, which culminates in platelet aggregation.6 Moreover, αIIbβ3 plays an important role as a counterreceptor for fibrinogen bound to the activated Mac-1 integrin on leukocytes,7 8 thus contributing to adhesive processes between platelets and leukocytes.
P-selectin exposure is a measure of platelet-granule release on activated platelets.9 P-selectin interactions between platelets stabilize the initial αIIbβ3-fibrinogen interactions, thereby promoting formation of large, stable platelet aggregates.10 In addition, P-selectin is a ligand for P-selectin glycoprotein-1 (PSGP-1) on leukocytes. PSGP-1 promotes adhesion of leukocytes to P-selectin and leukocyte rolling on activated endothelium or platelets.11-14 Thus, platelet P-selectin and platelet αIIbβ3 may contribute to recruitment of both platelets and leukocytes into an evolving thrombus.10-15
Our previous studies of cell-cell interactions between activated platelets and erythrocytes indicated that biochemical communication between these cell types is initiated upon platelet activation, because, without a platelet agonist, erythrocytes cannot promote platelet activation or the recruiting activity of cell-free releasates.3 4 We, therefore, hypothesize that components of the platelet releasate act as extracellular mediators that bring about biochemical modifications generating prothrombotic activity on erythrocytes.
Among components of platelet releasates, eicosanoids are physiologically important for thrombus formation.1Moreover, metabolic interactions of erythrocytes with activated platelets modulate, within 1 minute of collagen addition, platelet arachidonate release and eicosanoid formation.3 This process promotes significant increases in platelet cyclooxygenase (TXA2) and lipoxygenase (12-HETE) metabolites. In the same time frame, in the presence of erythrocytes, extracellular free arachidonate increases 3- to 10-fold upon platelet stimulation with collagen.3
The effects of prostanoids have been examined in several cell types by studying agonist-induced changes in second messengers, including levels of cytosolic calcium ([Ca++]i).16 A rise in [Ca++]i is associated with loss of phospholipid asymmetry and exposure of phosphatidylserine (PS) on the erythrocyte membrane.17 This transforms the erythrocyte membrane to a procoagulant surface on which a prothrombinase complex can assemble, leading to thrombin generation.17-19
With the above in mind, we set the following aims: (1) to determine whether cell-free releasates from collagen-stimulated platelets act as agonists to induce αIIbβ3 activation and P-selectin exposure on the surface of recruited platelets; (2) to ascertain whether platelet interactions with intact erythrocytes affect the stimulatory capacity of releasates for αIIbβ3 activation and for P-selectin exposure on recruited platelets; (3) to establish whether aspirin ingestion affects ex vivo αIIbβ3 activation and P-selectin exposure during platelet recruitment; and (4) to test the hypothesis that, among the components of the activated platelet releasate, platelet-derived arachidonic acid (AA) or eicosanoids induce or mediate erythrocyte prothrombotic activity.
Our present data indicate that the releasate of collagen-stimulated platelets induces αIIbβ3 activation and P-selectin exposure on platelets under recruitment. These responses are markedly amplified in releasates from platelet-erythrocyte suspensions. This is a novel mechanism(s) by which erythrocytes amplify platelet aggregability and promote platelet as well as leukocyte recruitment to an evolving thrombus. Aspirin down-regulates the recruiting potency of platelet releasates and erythrocyte-induced amplification of platelet recruitment. Furthermore, we report novel activities of TXA2 and free arachidonate, inducing increases in cytosolic Ca++ and exposure of erythrocyte phosphatidylserine. These changes could contribute to erythrocyte enhancement of platelet reactivity2-4 and are associated with increased procoagulant activity of the erythrocyte membrane,17,18increased adhesiveness to endothelium,20-24 and reduced erythrocyte deformability.25,26 The changes in erythrocyte properties induced by TXA2 and AA would contribute to a prothrombotic state in a subpopulation of erythrocytes in close proximity to activated platelets, thus enhancing thrombus formation.17,18 26
Materials and methods
Materials
Fluorescence spectrophotometer RF-1501 with program pack for intracellular Ca++ measurements, constant temperature cell holder and stirrer (Shimadzu Corporation, Kyoto, Japan); flow cytometer XL-MCL and System II (3.0) software program (Izasa Coulter, Madrid, Spain); platelet aggregometer 540 (Chronolog, Havertown, PA); Eppendorf centrifuge 5417C (Eppendorf-Netheler-Hinz GmbH, Hamburg, Germany); fluorescein isothiocyanate (FITC)–labeled PAC-1 (Becton Dickinson, München, Germany); phycoerythrin (PE)–labeled CD62, FITC–Annexin-V (Cat. No. 2375; Immunotech, Marseille, France); PE–antiglycophorin-A (0.2 mg/mL; Pharmingen, San Diego, CA); Immunocheck beads (Izasa Coulter); collagen (1 g/mL; Nycomed Pharma GmbH, München, Germany); ionomycin (Calbiochem, Darmstadt, Germany); Ca++-ionophore A23 287, sodium arachidonate, U46 619, 12-HETE, PGI2, fura-2 am(Sigma-Aldrich, Madrid, Spain) were used. All other reagents were of analytical grade.
Blood cell collection
Citrate anticoagulated blood (129 mM; 9:1, vol:vol) was obtained from healthy donors after an overnight fast and collected in siliconized glass tubes (Vacutainer; Becton Dickinson). Volunteers had not taken any medication for at least 15 days prior to blood donation. In some instances, venipuncture of the same donors was carried out prior to and 2 hours following ingestion of 500 mg acetylsalicylic acid (ASA), after informed consent. The Internal Review Board of the Hospital Universitario La Fe approved our study involving humans.
Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were prepared by differential centrifugation (200g and 2500g, respectively, 15 minutes, 22°C). After removal of PRP, PPP, and buffy coat, 1 mL of erythrocytes was removed from the central area of the erythrocyte zone of each Vacutainer tube. The absence of platelets in the erythrocyte suspensions was verified by phase contact microscopy.
Measurement of cytosolic calcium in erythrocytes
Cytosolic Ca++ ([Ca++]i) in erythrocytes was measured by spectrofluorimetry. Fura-2am was dissolved in dimethyl sulfoxide at a concentration of 1 mM and stored in aliquots at −20°C. Isolated RBCs were washed twice with HEPES (123 mM NaCl, 5 mM KCL, 1 mM MgCl2, 10 mM glucose, 25 mM HEPES, 1 mM CaCl2, pH 7.4, 285 mOsm/L) (HEPES-A). Packed cells were diluted to 1% hematocrit with HEPES-A containing 0.05% bovine serum albumin (HEPES-B). RBCs were loaded with fura-2 am by incubation with 0.5 M fura-2 amfor 45 minutes at 37°C in the dark.27 Final dimethyl sulfoxide concentrations were always less than 0.1%. To remove extracellular fura-2 am, RBCs were diluted 1:10 with HEPES-A and washed twice (10 minutes, 300g) with the same buffer. The cells were suspended at 0.1% hematocrit and transferred to a quartz cuvette for fluorescence measurements, performed at 37°C under constant stirring (approximately 300 rpm), using excitation at 340 and 380 nm, and emission at 510 nm.28[Ca++]i was calculated according to Poenie et al.29 To verify that fluorescence increases were not due to fura-2 am de-esterification or leakage extracellularly, control experiments were performed with 1 mM Mn2+ in the cell suspension.30 We evaluated the effects of different concentrations of free AA, U46 619 (TXA2 analog), 12-HETE, prostacyclin (PGI2), or appropriate solvents on the erythrocyte [Ca++]i. Ionomycin (1 μM) was used as positive control because A23 187 interferes with fura-2am fluorescence measurements.
Because TXA2 is very labile, we used the U46 619 analog, a thromboxane receptor ligand in platelets as well as other human cells.16 The concentrations of AA and eicosanoids used did not induce cell lysis; U46 619 (0.5-1 μM) and AA (5-10 μM) aggregated washed platelets (not shown).
Measurement of platelet activation and recruitment
Platelet activation and recruitment were independently evaluated by using the activation-recruitment system, an in vitro dual cell system previously described.3-5 In the generating system, platelets alone (PRP) (2 × 108platelets/mL), platelet-erythrocyte mixtures (PRP + RBCs), or whole blood (WB) were preincubated (10 minutes, 37°C). Collagen (1 μg/mL) was added as primary platelet agonist; the tube contents were mixed by inversion (10 seconds) and rapidly centrifuged (13 000g; 50 seconds). The centrifugation step promotes cell-cell contact between platelets and other cells in the generating system and yields a cell-free, collagen-free releasate within 1 minute of collagen addition.3 In this releasate, biochemical studies can be performed to assess platelet activation by collagen in the generating system (eg, platelet granule release, TXA2 synthesis). In addition, an aliquot of this releasate was used as an agonist for recruitment of other platelets in the second cell system (assay system). This system was monitored by optical aggregometry in PRP3 or by flow cytometry of recruited platelets in which αIIbβ3 activation and P-selectin exposure were evaluated.
Flow cytometry
On recruited platelets.
FITC-labeled PAC-1, an antibody to the active conformation of the αIIbβ3 receptor,31 and PE-labeled CD62, which binds to exposed P-selectin, were used. To measure recruitment, we started with mixtures of 10 μL PRP (3.5 × 108 platelets/mL) and 40 μL 10 mM HEPES buffer (10 mM HEPES, 150 mM NaCl, 5 mM KCl, 1 mM MgSO4, 10 mM glucose, pH 7.4, 290 mOsm) (HEPES-C) plus saturating concentrations of antibodies. Cell-free releasates obtained from collagen-stimulated platelets (10 μL) were added to this mixture, and antibody reactions were allowed to complete in the resulting recruitment mixture (30 minutes, 20°C, undisturbed, dark).31 FITC–PAC-1 samples were quench-diluted with 500 μL HEPES-C, maintained at 4°C in the dark, and examined by flow cytometry the same day. For P-selectin determinations, 60 μL 2% paraformaldehyde was added to platelet samples following the 30-minute antibody incubation. Samples were then washed and suspended in 500 μL HEPES-C. Results are reported as percentage of platelets with bound PE-CD62 or FITC–PAC-1 in a total of 5000 events observed by flow cytometry.
Annexin-V binding to erythrocytes.
WB (5 μL) was diluted in 200 μL HEPES-C buffer in a polypropylene tube. Then, 13.4 μL of 25 mM CaCl2 in HEPES-C buffer was added, (final Ca++ concentration, 1.5 mM).32Small volumes (0.5-1.5 μL) of different concentrations of AA, U46 619 (the TXA2 mimetic), 12-HETE, PGI2, as well as appropriate solvent controls, were added. Ca++-ionophore A23 187 and ionomycin were used as positive controls. Samples were kept without stirring (5 minutes, 22°C). To each sample 5 μL FITC–annexin-V and 5 μL PE–antiglycophorin-A antibody were added (10 minutes, 4°C, undisturbed, dark). Samples were quench-diluted with 600 μL ice-cold HEPES-C (with 1.5 mM Ca++), kept at 4°C, and examined within 60 minutes by flow cytometry.32 Erythrocytes were identified by gating of forward and side scatter and as PE–antiglycophorin-A positive. Erythrocytes that exposed PS on the extracellular membrane leaflet were identified as both FITC–annexin-V positive and PE–antiglycophorin-A positive.
Data were collected by the System II software, and results were expressed as percentage of 250 000 events. To standardize, the flow cytometer was calibrated daily by using Immunocheck microbeads. Flow rate was adjusted to yield less than 3000 events/second by appropriately diluting samples with buffer. Integrity of the erythrocyte suspensions following treatment with the different agonists was established by lactate dehydrogenase (LDH) assays on cell supernatants.
Results and discussion
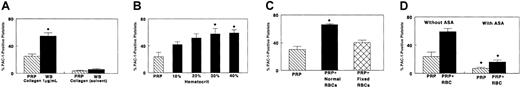
Releasates from collagen-stimulated PRP induced P-selectin exposure and αIIbβ3 activation in platelets under examination for recruitment. These effects were amplified when releasates derived from collagen-stimulated WB were tested. In contrast, supernatants obtained without collagen stimulation (buffer) had little or no effect (Figures 1A, 2A). Activation of αIIbβ3 provides a novel biochemical mechanism that mediates the effect of platelet releasates and is consistent with our previous reports of the proaggregatory properties of cell-free platelet releasates.3,4 33 Exposure of P-selectin verifies that platelet secretion is induced in the platelets targeted by releasates during the recruitment process. This process represents a “feed-forward” component of thrombus formation. Comparison of the effects of releasates from PRP and WB (Figures 1A,2A) indicates that cell-cell interactions are an integral aspect of the agonistic properties of platelet releasates. This finding led to further evaluation of the effects of platelet-erythrocyte interactions.
The platelet releasate acts as an agonist and induces P-selectin exposure on platelets tested for recruitment.
Effects of erythrocytes and aspirin ex vivo. PRP (2 × 108platelets/mL), PRP plus erythrocytes (RBCs), or whole blood (WB) were stimulated with collagen (1 μg/mL) and centrifuged to obtain a cell-free releasate within 1 minute. Aliquots of releasate were added as agonist to aspirin-free autologous PRP. P-selectin exposure induced by releasate was measured by flow cytometry of recruited platelets (“Materials and methods”). Releasates were obtained in duplicate for each data point, and duplicate flow cytometric measurements of each sample of releasate were performed. The 4 values so obtained from each donor were averaged; n represents the number of different volunteers studied. Data are mean ± SEM of percentage of platelets that bind PE–CD-62. (A) Effects of releasates from collagen-stimulated PRP, WB, and solvent controls (n = 15; *P < .001, PRP versus WB). (B) Relationship to hematocrit (n = 4; *P <.001, PRP versus PRP plus RBCs). (C) Normal versus glutaraldehyde-fixed RBCs, compared with PRP (hematocrit [Hct] 40%; n = 3; P < .001 normal RBCs versus PRP or fixed RBCs). (D) Effect of aspirin (n = 4; Hct 40%; before versus after ASA ingestion: *P < .01 for PRP, and *P < .0001 for PRP plus RBCs). Significance was determined with Student t test.
The platelet releasate acts as an agonist and induces P-selectin exposure on platelets tested for recruitment.
Effects of erythrocytes and aspirin ex vivo. PRP (2 × 108platelets/mL), PRP plus erythrocytes (RBCs), or whole blood (WB) were stimulated with collagen (1 μg/mL) and centrifuged to obtain a cell-free releasate within 1 minute. Aliquots of releasate were added as agonist to aspirin-free autologous PRP. P-selectin exposure induced by releasate was measured by flow cytometry of recruited platelets (“Materials and methods”). Releasates were obtained in duplicate for each data point, and duplicate flow cytometric measurements of each sample of releasate were performed. The 4 values so obtained from each donor were averaged; n represents the number of different volunteers studied. Data are mean ± SEM of percentage of platelets that bind PE–CD-62. (A) Effects of releasates from collagen-stimulated PRP, WB, and solvent controls (n = 15; *P < .001, PRP versus WB). (B) Relationship to hematocrit (n = 4; *P <.001, PRP versus PRP plus RBCs). (C) Normal versus glutaraldehyde-fixed RBCs, compared with PRP (hematocrit [Hct] 40%; n = 3; P < .001 normal RBCs versus PRP or fixed RBCs). (D) Effect of aspirin (n = 4; Hct 40%; before versus after ASA ingestion: *P < .01 for PRP, and *P < .0001 for PRP plus RBCs). Significance was determined with Student t test.
Stimulatory effect of platelet releasates for induction of αIIbβ3 activation in platelets tested for recruitment.
Role of erythrocytes and aspirin ex vivo. PRP (2 × 108platelets/mL), PRP plus RBCs, or WB were stimulated with collagen (1 μg/mL) and centrifuged to obtain a cell-free releasate within 1 minute. An aliquot of releasate was used as agonist for aspirin-free autologous PRP. Releasate-induced αIIbβ3 activation was monitored by flow cytometry with FITC–PAC-1 on recruiting platelets (“Materials and methods”). Releasates were obtained in duplicate for each data point, and duplicate flow cytometric measurements of each sample of releasate were performed. The 4 values so obtained from each donor were averaged; n represents the number of different volunteers studied. Data are mean ± SEM of percentage of platelets with bound PAC-1. (A) Effects of releasates from collagen-stimulated PRP, WB, and solvent controls (n = 22; *P < .001, PRP versus WB). (B) Relationship to hematocrit (n = 4; *P < .001). (C) Normal versus glutaraldehyde-fixed RBCs, compared with PRP (Hct 40%; n = 4;P < .001, normal RBCs versus PRP or fixed RBCs). (D) Effect of aspirin (n = 6; Hct 40%; before versus after ASA ingestion: *P < .005 for PRP, and *P < .001 for PRP plus RBCs). Significance was evaluated by Studentt test.
Stimulatory effect of platelet releasates for induction of αIIbβ3 activation in platelets tested for recruitment.
Role of erythrocytes and aspirin ex vivo. PRP (2 × 108platelets/mL), PRP plus RBCs, or WB were stimulated with collagen (1 μg/mL) and centrifuged to obtain a cell-free releasate within 1 minute. An aliquot of releasate was used as agonist for aspirin-free autologous PRP. Releasate-induced αIIbβ3 activation was monitored by flow cytometry with FITC–PAC-1 on recruiting platelets (“Materials and methods”). Releasates were obtained in duplicate for each data point, and duplicate flow cytometric measurements of each sample of releasate were performed. The 4 values so obtained from each donor were averaged; n represents the number of different volunteers studied. Data are mean ± SEM of percentage of platelets with bound PAC-1. (A) Effects of releasates from collagen-stimulated PRP, WB, and solvent controls (n = 22; *P < .001, PRP versus WB). (B) Relationship to hematocrit (n = 4; *P < .001). (C) Normal versus glutaraldehyde-fixed RBCs, compared with PRP (Hct 40%; n = 4;P < .001, normal RBCs versus PRP or fixed RBCs). (D) Effect of aspirin (n = 6; Hct 40%; before versus after ASA ingestion: *P < .005 for PRP, and *P < .001 for PRP plus RBCs). Significance was evaluated by Studentt test.
Releasates from platelet-erythrocyte mixtures enhanced P-selectin exposure and αIIbβ3 activation in a hematocrit-dependent manner (Figures 1B, 2B). This enhancing effect of erythrocytes on the agonistic capacity of releasates was also demonstrated by optical aggregometry (data not shown), similar to our previous reports.2-4 These effects are metabolic rather than physical because glutaraldehyde-fixed (ATP-depleted) erythrocytes have minimal effects as compared with metabolically active erythrocytes (Figures 1C, 2C). These metabolic processes do occur in the absence of lysis as verified by the absence of measurable LDH and hemoglobin release (not shown). Our experiments provide the first evidence for positive modulation of αIIbβ3 activation by erythrocytes during platelet recruitment. These data are of importance because αIIbβ3 activation is required for agonist-induced platelet aggregation.6 Increases in P-selectin exposure induced by erythrocytes on platelets being recruited may also promote formation of larger and more stable platelet aggregates in an evolving thrombus.10 In addition, by this mechanism, erythrocytes could modulate adhesion between platelets and leukocytes12 13 and thus recruit leukocytes to sites of vascular damage.
Increased P-selectin exposure has additional implications for thrombus evolution. We previously demonstrated inhibition of platelet reactivity by cell-cell interactions with neutrophils.5The inhibitory effect of neutrophils was amplified by P-selectin blockade with a specific antibody, suggesting a prothrombotic role for P-selectin in this setting.5 Moreover, P-selectin binding to monocytes can induce tissue factor activity34 and promote fibrin formation.15 This erythrocyte-promoted increase of P-selectin on recruited platelets constitutes an additional prothrombotic parameter. P-selectin binding to neutrophils is known to initiate activation and signal transduction by CD11b/CD18 (Mac-1),35 which promotes interactions with platelet αIIbβ3-bound fibrinogen.8 In this way, erythrocytes can modulate both leukocyte recruitment and fibrin formation.
When the action of releasates was examined 2 hours after ingestion of ASA, a significant reduction in αIIbβ3activation and α-granule release occurred, in the presence or absence of erythrocytes (Figures 1D, 2D). Therefore, ASA reduced the agonistic potency of platelet releasates, as previously reported.33There was no difference in response whether the target platelets were aspirin treated or not (data not shown). These results support previous observations by us and others that aspirin treatment does not directly affect αIIbβ3 activation or P-selectin exposure in stimulated platelets.36-38 Thus, down-regulation of the agonistic effects of platelet releasates by aspirin represents an additional mechanism for its antithrombotic action.
Data from the present (Figures 1, 2A) and previous studies2-4 indicate an absolute requirement for platelet activation to elicit the enhancing effect of erythrocytes on platelet reactivity. This finding indicates that erythrocytes may respond to one or more components of the platelet releasate.
Signaling by free AA and several of its eicosanoid metabolites was evaluated in this study. We measured cytosolic Ca++ levels and alterations in erythrocyte membrane lipid asymmetry (exposure of phosphatidylserine). This is a Ca++-dependent process in platelets and erythrocytes, with implications for hemostasis and thrombosis.17 18
Erythrocyte cytosolic Ca++ was measured at 1-second intervals for 600 seconds, and maximal [Ca++]i was determined. Mean basal [Ca++]i in erythrocytes was 34 nM (Figure3), in agreement with previous reports.30 Addition of the TXA2-analog U46 619 (0.5-2 μM) or sodium arachidonate (5-20 μM) to erythrocytes dose dependently increased maximal [Ca++]i (Figure 3). In contrast, cytosolic Ca++ was not modified by the endothelial COX-1 metabolite PGI2 (0.5-2 μM), or the platelet lipoxygenase product 12-HETE (0.1-1 μM) (not shown). This finding indicates specificity of the effects of different eicosanoids on erythrocyte [Ca++]i. For these experiments, the positive control (1 μM ionomycin) induced an increase in [Ca++]i of 105 nM (Figure 3, legend). This rise in [Ca++]i was similar to that observed after treatment of erythrocytes with AA or U46 619 (Figure 3), although the kinetics differed (Figure4). Arachidonate induced a rapid initial increase, followed by a slow rise during the 10-minute assay period (Figure 4A). The maximum increase of [Ca++]iwith U46 619 occurred at a later time (Figure 4B). Ionomycin produced, as expected, a virtually instantaneous rise in [Ca++]i (Figure 4C). These differences in kinetics of the [Ca++]i rise suggest that different signal transduction mechanisms are involved.
Effect of eicosanoids on erythrocyte cytosolic Ca++.
RBCs loaded with fura-2 am (0.1% hematocrit in HEPES buffer) were placed in a quartz cuvette with stirring (37°C, 300 rpm) for fluorescence measurements (“Materials and methods”). Fluorescence was determined with excitation wavelengths 340 and 380 nm and emission wavelength 510 nm in a spectrofluorimeter at 1-second intervals for 10 minutes. The maximum cytosolic Ca++concentration ([Ca++]i) in the erythrocytes was examined under basal conditions and following addition of the indicated concentrations of U46 619 (TXA2 analog), free-arachidonic acid, 12-HETE, PGI2, or solvents. Ionomycin (1 μM) served as positive controls (“Materials and methods”). U46 619 and free-AA dose dependently increased [Ca++]i of erythrocytes. In contrast, addition of PGI2 (0.5-2 μM) or 12-HETE (0.1-1 μM) did not increase [Ca++]i over solvent control (not shown). Ionomycin (1 μM) produced an immediate increase in [Ca++]i of 105 ± 10 nM (n = 15). Data are mean ± SEM of 6 to 20 determinations in different donors. *P < .001 for either U46 619 or AA versus control. Significance was determined by Student t test.
Effect of eicosanoids on erythrocyte cytosolic Ca++.
RBCs loaded with fura-2 am (0.1% hematocrit in HEPES buffer) were placed in a quartz cuvette with stirring (37°C, 300 rpm) for fluorescence measurements (“Materials and methods”). Fluorescence was determined with excitation wavelengths 340 and 380 nm and emission wavelength 510 nm in a spectrofluorimeter at 1-second intervals for 10 minutes. The maximum cytosolic Ca++concentration ([Ca++]i) in the erythrocytes was examined under basal conditions and following addition of the indicated concentrations of U46 619 (TXA2 analog), free-arachidonic acid, 12-HETE, PGI2, or solvents. Ionomycin (1 μM) served as positive controls (“Materials and methods”). U46 619 and free-AA dose dependently increased [Ca++]i of erythrocytes. In contrast, addition of PGI2 (0.5-2 μM) or 12-HETE (0.1-1 μM) did not increase [Ca++]i over solvent control (not shown). Ionomycin (1 μM) produced an immediate increase in [Ca++]i of 105 ± 10 nM (n = 15). Data are mean ± SEM of 6 to 20 determinations in different donors. *P < .001 for either U46 619 or AA versus control. Significance was determined by Student t test.
Kinetics of the effect of U46 619 and free AA on cytosolic Ca++ of erythrocytes.
RBCs loaded with fura-2 am (0.1% hematocrit in HEPES buffer) were placed in a quartz cuvette with stirring (37°C, 300 rpm) for fluorescence measurements (“Materials and methods”). Fluorescence was determined with excitation wavelengths 340 and 380 nm and 510 nm emission wavelength in a spectrofluorimeter. After determination of baseline [Ca++]i, U46 619 (1 μM), free AA (20 μM), ionomycin (1 μM), or solvent (controls) was added. [Ca++]i of erythrocytes was quantified at 1-second intervals for 10 minutes. The figure shows time-dependent [Ca++]i changes. Data are representative of 11 separate experiments in different donors. PGI2 (0.5-1 μM) or 12-HETE (0.1-1 μM) did not increase [Ca++]i over that of solvent control (not shown).
Kinetics of the effect of U46 619 and free AA on cytosolic Ca++ of erythrocytes.
RBCs loaded with fura-2 am (0.1% hematocrit in HEPES buffer) were placed in a quartz cuvette with stirring (37°C, 300 rpm) for fluorescence measurements (“Materials and methods”). Fluorescence was determined with excitation wavelengths 340 and 380 nm and 510 nm emission wavelength in a spectrofluorimeter. After determination of baseline [Ca++]i, U46 619 (1 μM), free AA (20 μM), ionomycin (1 μM), or solvent (controls) was added. [Ca++]i of erythrocytes was quantified at 1-second intervals for 10 minutes. The figure shows time-dependent [Ca++]i changes. Data are representative of 11 separate experiments in different donors. PGI2 (0.5-1 μM) or 12-HETE (0.1-1 μM) did not increase [Ca++]i over that of solvent control (not shown).
U46 619 is a TXA2-analog and a specific ligand for TXA2 receptors on different human cells,16whereas free AA (without enzymatic or nonenzymatic oxygenation) is a signaling molecule in different cell types.39 Because U46 619 and free AA increase cytosolic Ca++ in erythrocytes, our data demonstrate that TXA2 and free AA are involved in intercellular signaling between activated platelets and erythrocytes. This mechanism could contribute to the enhancing effect of erythrocytes on platelet reactivity3 4 (Figures 1, 2).
Our data adds additional support to the concept that erythrocytes are signaling cells, as previously suggested by other investigators,40-42 and further exemplified by thrombospondin-mediated erythrocyte adhesion of sickle erythrocytes by integrin-associated signal transduction mechanisms.24Interestingly, all substances tested thus far eliciting these effects (TXA2, free AA, PGE2, lysophosphatidic acid, and thrombospondin) are components of the releasate of activated platelets.24 40-42 This finding constitutes further support for an important role for regulated biochemical interactions between platelets and erythrocytes in (patho)physiology.
The increase of cytosolic Ca++ in erythrocytes is associated with disruption of phospholipid asymmetry and exposure of PS on the outer leaflet of the erythrocyte membrane, a modification with important implications for hemostasis and thrombosis.17 18We, therefore, examined the effect of different eicosanoids on PS exposure on erythrocytes in WB, using annexin-V binding measurements by flow cytometry.
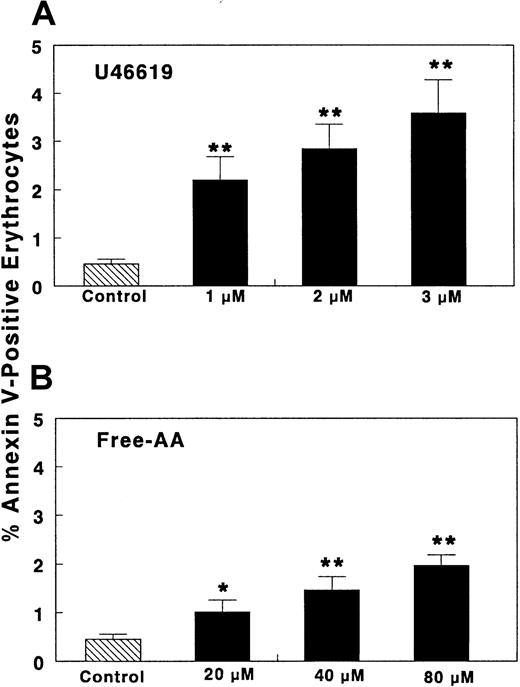
A small quantity (< 0.5%) of normal circulating erythrocytes exposes PS on their membrane (Figure 5), as reported previously.32,43-45 Addition of U46 619 (1-3 μM) dose dependently increased the number of erythrocytes binding annexin-V over resting levels to 2.2% to 3.6% (Figure 5). Sodium arachidonate (20-80 μM) also produced a significant increase (2- to 4-fold) over basal levels in the percentage of erythrocytes binding annexin-V (Figure 5). Lower AA concentrations did not produce an increase in annexin-V binding (not shown). The difference in AA concentrations that induced a rise in [Ca++]i(Figure 3) and annexin-V binding on erythrocytes (Figure 5) is likely due to the presence of albumin in diluted WB and to a higher extracellular Ca++ concentration in the annexin-V binding experiments, factors known to reduce availability of free fatty acid to act on the cells.39
Effect of eicosanoids on annexin-V binding to erythrocytes.
WB was diluted (1:40; vol/vol) with HEPES buffer and then CaCl2 was added (final Ca++ concentration, 1.5 mM) (“Materials and methods”). Different concentrations of either AA, U46 619, 12-HETE, PGI2, or appropriate solvent controls were added. Ca++-ionophore A23 187 and ionomycin were used as positive controls. Samples were kept without stirring (5 minutes, 22°C) and then FITC–annexin-V or PE–antiglycophorin-A antibodies were added. The samples were kept undisturbed (10 minutes, 4°C, dark) (“Materials and methods”). Samples were quench-diluted with ice-cold HEPES (with 1.5 mM Ca++) at 4°C and examined immediately by flow cytometry (“Materials and methods”). The TXA2-analog U46 619 and free AA dose dependently increased annexin-V binding to erythrocytes (n = 12). In the same time frame, Ca++-ionophore A23 187 (1 μM) induced annexin binding in 8.4% ± 1.03 erythrocytes (n = 17). Ionomycin 1 μM yielded PS exposure in 1.91% ± 0.19% of erythrocytes (n = 10). PGI2 (0.5-1 μM) or 12-HETE (0.1-1 μM) did not induce annexin-V binding (not shown). Results are expressed as mean ± SEM (%) of erythrocytes that bind annexin-V; n = number of different donors studied. *P < .05; **P < .001. Significance was determined by Studentt test.
Effect of eicosanoids on annexin-V binding to erythrocytes.
WB was diluted (1:40; vol/vol) with HEPES buffer and then CaCl2 was added (final Ca++ concentration, 1.5 mM) (“Materials and methods”). Different concentrations of either AA, U46 619, 12-HETE, PGI2, or appropriate solvent controls were added. Ca++-ionophore A23 187 and ionomycin were used as positive controls. Samples were kept without stirring (5 minutes, 22°C) and then FITC–annexin-V or PE–antiglycophorin-A antibodies were added. The samples were kept undisturbed (10 minutes, 4°C, dark) (“Materials and methods”). Samples were quench-diluted with ice-cold HEPES (with 1.5 mM Ca++) at 4°C and examined immediately by flow cytometry (“Materials and methods”). The TXA2-analog U46 619 and free AA dose dependently increased annexin-V binding to erythrocytes (n = 12). In the same time frame, Ca++-ionophore A23 187 (1 μM) induced annexin binding in 8.4% ± 1.03 erythrocytes (n = 17). Ionomycin 1 μM yielded PS exposure in 1.91% ± 0.19% of erythrocytes (n = 10). PGI2 (0.5-1 μM) or 12-HETE (0.1-1 μM) did not induce annexin-V binding (not shown). Results are expressed as mean ± SEM (%) of erythrocytes that bind annexin-V; n = number of different donors studied. *P < .05; **P < .001. Significance was determined by Studentt test.
PGI2 (0.5-2 μM) and 12-HETE (0.1-1 μM) did not increase annexin-V binding to erythrocytes (not shown). This paralleled the lack of effect on [Ca++]i. The positive control, Ca++-ionophore A23 187 (1 μM), is known to have a time-dependent effect.32 In the time frame of our study (5 minutes), A23 187 induced PS exposure in 8.4% of the erythrocytes in agreement with previous data.32 Ionomycin (1 μM, used as positive control for cytosolic Ca++ measurements because A23 187 interferes with fluorescence determinations) induced PS exposure in 1.91% ± 0.19% of erythrocytes (X ± SEM, n = 10). This ionomycin-induced PS exposure was similar in extent to that generated by1 μM U46 619 or 40 μM AA (Figure 5) as was the case for the increase in cytosolic Ca++ (Figure 3). However, the kinetics of the rise in cytosolic Ca++ differed among the 3 agonists (Figure 4), suggesting distinct biochemical mechanisms.
A novel aspect of our data is the demonstration that erythrocytes present PS on the outer leaflet of their membrane when exposed to components of the activated platelet releasate. This may be mediated by eicosanoid-induced increases in [Ca++]i, because treatment of erythrocytes with Ca++-ionophores induces PS exposure.20,32 The parallel effects on both [Ca++]i increases and PS exposure by ionomycin, U46 619, and AA support this mechanism. This finding is of pathophysiologic significance because erythrocytes exposing PS stimulate prothrombinase activity and enhance thrombin generation. This action occurs in patients with sickle cell disease (in which 2% to 3% of the erythrocytes expose PS)32,43 as well as β-thalassemia (in which 1.42% of erythrocytes expose PS).44 In our experiments a similar proportion of erythrocytes treated with U46 619 or AA expressed PS. In patients with sickle cell disease or β-thalassemia, this percentage of modified erythrocytes is sufficient to induce a large increase in erythrocyte procoagulant activity and thrombin generation.32 43-47Thus, procoagulant activity on the surface of erythrocytes in the microenvironment of an evolving thrombus can increase thrombin generation in vivo, thereby promoting further platelet activation and recruitment.
Another implication for thrombus formation on exposure of PS on erythrocytes is promotion of thrombospondin-mediated adhesive processes between PS-expressing erythrocytes and subendothelial matrix.20,21 The increase in PS-expressing erythrocytes facilitates close proximity of erythrocytes to platelets and endothelial cells, facilitating thrombus formation. These mechanisms are especially pertinent for the microcirculation, because the increase in [Ca++]i in erythrocytes by U46 619 and AA also reduces erythrocyte deformability,25 a phenomenon that promotes microvascular occlusion.26 Our results, therefore, suggest that a subpopulation of erythrocytes in proximity to activated platelets can acquire a prothrombotic phenotype that contributes to thrombin generation and microvascular occlusion.
We conclude that erythrocytes up-regulate the agonistic effects of platelet releasates on αIIbβ3 activation and secretion by recruited platelets. This effect contributes to platelet-platelet and platelet-leukocyte adhesive processes and recruitment that occur during thrombus formation. Reduction of the stimulatory capacity of combined cellular releasates by aspirin contributes to the clinical benefits of this compound as an antithrombotic modality because recruitment is the actual limiting step for formation of an occlusive thrombus.1 In addition, our results with U46 619 and free AA demonstrate for the first time that these components of platelet releasates induce PS exposure on the surface of normal erythrocytes because of increases in cytosolic calcium. These studies extend our concepts of the role of erythrocytes in thrombogenesis and support the hypothesis that erythrocytes participate actively as signaling cells in thrombogenesis.
We gratefully acknowledge the technical assistance of M. Carmen Insa, Amparo Garrido, and Pilar Ferriz.
Supported in part by grants FIS 98/0906 and FIS 01/1208 from the Fondo de Investigaciones Sanitarias (J.V., M.T.S., and J.A.), by a Merit Review grant from the Department of Veterans Affairs, and by grants HL-47073, HL-46403, and NS-41462 from the National Institutes of Health (M.J.B. and A.J.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Juana Vallés, Research Center, University Hospital La Fe, Avda Campanar, 21, 46009 Valencia, Spain; e-mail: valles_jua@gva.es.

![Fig. 1. The platelet releasate acts as an agonist and induces P-selectin exposure on platelets tested for recruitment. / Effects of erythrocytes and aspirin ex vivo. PRP (2 × 108platelets/mL), PRP plus erythrocytes (RBCs), or whole blood (WB) were stimulated with collagen (1 μg/mL) and centrifuged to obtain a cell-free releasate within 1 minute. Aliquots of releasate were added as agonist to aspirin-free autologous PRP. P-selectin exposure induced by releasate was measured by flow cytometry of recruited platelets (“Materials and methods”). Releasates were obtained in duplicate for each data point, and duplicate flow cytometric measurements of each sample of releasate were performed. The 4 values so obtained from each donor were averaged; n represents the number of different volunteers studied. Data are mean ± SEM of percentage of platelets that bind PE–CD-62. (A) Effects of releasates from collagen-stimulated PRP, WB, and solvent controls (n = 15; *P < .001, PRP versus WB). (B) Relationship to hematocrit (n = 4; *P <.001, PRP versus PRP plus RBCs). (C) Normal versus glutaraldehyde-fixed RBCs, compared with PRP (hematocrit [Hct] 40%; n = 3; P < .001 normal RBCs versus PRP or fixed RBCs). (D) Effect of aspirin (n = 4; Hct 40%; before versus after ASA ingestion: *P < .01 for PRP, and *P < .0001 for PRP plus RBCs). Significance was determined with Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/11/10.1182_blood.v99.11.3978/6/m_h81122629001.jpeg?Expires=1763779609&Signature=yFjKD3kTBlzwSN7IJrlRKIDHLr-5QVh5~cGXtOvdEdnZoIysZ8Whc8VotvjdXW7FwEgLYz1DaMB8Fl0f9bBd~g4V2zCMzpR1oqCtq1Ltj2kRN3LlxRfvilAnegh5FYN~jVOzjO4pxInUsMfl1UCyMq98c6iUQQ27BYgq0-q-czcg2RSsdwDvjGsjCCRTV9MwnDqYoONdrvkp3OD25KLC3vTlMN5PG9eVas3DcwbENm9ahIsE3jlnc5OFcyF2kV~U5R0KrfqboZTP6qlR3GD7MGURtGLa8CGBlC35YhvccXUzVFk-HYKDsA3fOsz5W7lXy4S5vf0Rz40TKGKx3o-GyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effect of eicosanoids on erythrocyte cytosolic Ca++. / RBCs loaded with fura-2 am (0.1% hematocrit in HEPES buffer) were placed in a quartz cuvette with stirring (37°C, 300 rpm) for fluorescence measurements (“Materials and methods”). Fluorescence was determined with excitation wavelengths 340 and 380 nm and emission wavelength 510 nm in a spectrofluorimeter at 1-second intervals for 10 minutes. The maximum cytosolic Ca++concentration ([Ca++]i) in the erythrocytes was examined under basal conditions and following addition of the indicated concentrations of U46 619 (TXA2 analog), free-arachidonic acid, 12-HETE, PGI2, or solvents. Ionomycin (1 μM) served as positive controls (“Materials and methods”). U46 619 and free-AA dose dependently increased [Ca++]i of erythrocytes. In contrast, addition of PGI2 (0.5-2 μM) or 12-HETE (0.1-1 μM) did not increase [Ca++]i over solvent control (not shown). Ionomycin (1 μM) produced an immediate increase in [Ca++]i of 105 ± 10 nM (n = 15). Data are mean ± SEM of 6 to 20 determinations in different donors. *P < .001 for either U46 619 or AA versus control. Significance was determined by Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/11/10.1182_blood.v99.11.3978/6/m_h81122629003.jpeg?Expires=1763779609&Signature=Zh2RDx6qi76kZIdc5hJPXpP33kNQYjl-NY2Q2jhHMyEAAY8R5M5SYufJpxrG3Uyga39MX5ROrTRPegnGESZd8qGY6UanTWPsbiJWtOtJkvcwcwQNMiZyHCaEoiT9VVuCMkk3PgwSl6Zd3KLsqlibPJG3WWP-EgFWyVbV9LCzqV5Fdd0zCb6mAWHbLMJw9QU2hNIlcr41~KPN2v2RWPDqcgJfm5wAzX9wwyM787Hqmx7dM4n4Apf6jsVQ0Mg6ppjcMiJevBrOx22qHiLZ4Clefe3u25DYoH0ELPnlIQbbrvHvWENRIyCEa9WiDEJG6RjC89vCdLqJLcCMJfjHVNC2TA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Kinetics of the effect of U46 619 and free AA on cytosolic Ca++ of erythrocytes. / RBCs loaded with fura-2 am (0.1% hematocrit in HEPES buffer) were placed in a quartz cuvette with stirring (37°C, 300 rpm) for fluorescence measurements (“Materials and methods”). Fluorescence was determined with excitation wavelengths 340 and 380 nm and 510 nm emission wavelength in a spectrofluorimeter. After determination of baseline [Ca++]i, U46 619 (1 μM), free AA (20 μM), ionomycin (1 μM), or solvent (controls) was added. [Ca++]i of erythrocytes was quantified at 1-second intervals for 10 minutes. The figure shows time-dependent [Ca++]i changes. Data are representative of 11 separate experiments in different donors. PGI2 (0.5-1 μM) or 12-HETE (0.1-1 μM) did not increase [Ca++]i over that of solvent control (not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/11/10.1182_blood.v99.11.3978/6/m_h81122629004.jpeg?Expires=1763779609&Signature=ElMkK9uI4BhPl5ppCmShznflqgOj4wQexc3MqDA0idxciXSDiW9OmnVDvRQ-9uQxTPzyJFuEg1Sy2obbqG03DDsR-ePvX82ZY8rRgugIZuozujwTMzKg0LvJ1wtjsyJPl-u2rdQYVSiyBdEmVHrfs-5-MVrxiF-SOx2y8iZAtGpcQLZjSES5Vaq9U6d-oAnHFgnxthvdUljxDKvXECZKqC7a2DhQJAKMtTL96RKc2QGq9Aj3aDLf2rekpn4GCGNht4~~1N~RW76kKXjf34nZwDlIyw4~dm6358~pPlipWcMUQw4aKWPFmm03fNGrI7oU5D9qj7-mD6EdfY9UEsmd2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal