Saliva of the hard tick and Lyme disease vector, Ixodes scapularis, has a repertoire of compounds that counteract host defenses. Following sequencing of an I scapularis salivary gland complementary DNA (cDNA) library, a clone with sequence homology to tissue factor pathway inhibitor (TFPI) was identified. This cDNA codes for a mature protein, herein called Ixolaris, with 140 amino acids containing 10 cysteines and 2 Kunitz-like domains. Recombinant Ixolaris was expressed in insect cells and shown to inhibit factor VIIa (FVIIa)/tissue factor (TF)–induced factor X (FX) activation with an inhibitory concentration of 50% (IC50) in the picomolar range. In nondenaturing gel, Ixolaris interacted stoichiometrically with FX and FXa but not FVIIa. Ixolaris behaves as a fast-and-tight ligand of the exosites of FXa and γ-carboxyglutamic acid domainless FXa (des-Gla-FXa), increasing its amidolytic activity. At high concentration, Ixolaris attenuates the amidolytic activity of FVIIa/TF; however, in the presence of DEGR-FX or DEGR-FXa (but not des-Gla-DEGR-FXa), Ixolaris becomes a tight inhibitor of FVIIa/TF as assessed by recombinant factor IX (BeneFIX) activation assays. This indicates that FX and FXa are scaffolds for Ixolaris in the inhibition of FVIIa/TF and implies that the Gla domain is necessary for FVIIa/TF/Ixolaris/FX(a) complex formation. Additionally, we show that Ixolaris blocks FXa generation by endothelial cells expressing TF. Ixolaris may be a useful tool to study the structural features of FVIIa, FX, and FXa, and an alternative anticoagulant in cardiovascular diseases.
Introduction
Following tissue injury, exposition of membrane-bound tissue factor (TF) is a crucial step in the initiation of blood coagulation.1 TF binds to blood coagulation factor VIIa (FVIIa), and the binary FVIIa/TF complex then activates factor X (FX) to factor Xa (FXa), leading to thrombin generation and fibrin formation.1 Initiation of blood coagulation by FVIIa/TF is under control of tissue factor pathway inhibitor (TFPI),2-5 a 34- to 43-kd multidomain protein with an acidic amino terminus, 3 typical Kunitz-type inhibitor domains, and a basic carboxy terminus.6,7 The second Kunitz domain binds and inhibits FXa,8 and the first Kunitz domain binds to FVIIa/TF through formation of a final quarternary inhibitory complex consisting of FVIIa/TF/TFPI/FXa.9-13 In addition to TFPI and other physiologic inhibitors of blood coagulation (eg, antithrombin III), a number of exogenous coagulation inhibitors from the salivary gland of blood-sucking invertebrates have been characterized,14 including nitrophorin-2 fromRhodnius prolixus15 and anophelin fromAnopheles albimanius,16,17 among others.14 Nitrophorin-2 inhibits the intrinsic FX activating complex,15 whereas anophelin is a cysteine-free molecule obtained by peptide synthesis16 and shown to tightly bind thrombin.17
Ticks, such as Ixodes scapularis, are ectoparasites that feed for several days with their mouthparts embedded in their vertebrate hosts. A number of pharmacologic properties have been described in I scapularis saliva including inhibitors of neutrophil function18 and complement activation,19 in addition to anti-inflammatory and immunosuppressive components.20 Antihemostatic compounds have also been molecularly characterized in the soft tickOrnithodoros moubata, including a platelet αIIbβ3-integrin antagonist21and inhibitors of blood coagulation such as ornithodorin, a thrombin inhibitor,22 and tick anticoagulant peptide, an FXa inhibitor.23 The presence of thrombin and FXa inhibitors seems to be a successful strategy evolutionarily developed by ticks to successfully feed on blood, because both molecules play an essential role in 2 key steps necessary for maintenance of the blood coagulation cascade24 25; however, an inhibitor of FVIIa/TF has not yet been molecularly characterized.
To understand the complexity of I scapularis saliva, with a primary focus on antihemostatic molecules, massive sequencing of a complementary DNA (cDNA) library of the salivary gland of I scapularis has been performed. Together with a complementary functional approach, a clone with sequence homology to TFPI has been expressed as an active molecule and its anticoagulant mechanism studied. The recombinant protein has the same properties found in saliva. This molecule, called Ixolaris, binds to FX in addition to FXa and is characterized here as a specific inhibitor of FVIIa/TF in the presence of zymogen or enzyme as scaffolds.
Materials and methods
Materials
Factor VIIa, TF (lipidated), and full-length TFPI (4900PC) were purchased from American Diagnostica (Greenwich, CT). FX, FXa, FXIa, thrombin, (5-[dimethylamino]-1-naphthalenesulfonyl) glutamylglycylarginylchloromethyl ketone (dansyl-Glu-Gly-Arg-CK or DEGR-CK), elastase, and activated protein C chromogenic substrate were purchased from Calbiochem (San Diego, CA). γ-Carboxyglutamic acid domainless FXa (Gla-FXa) and human DEGR-FXa (FVII/VIIa free; 0 U/mg FXa), and monoclonal antihuman FVIIa were obtained from Hematologic Technologies (Essex Junction, VT). Recombinant FIX (BeneFIX, 250 U) was acquired from Wyeth-Genetics Institute (Cambridge, MA). Goat antihuman FX affinity-purified IgG was from Enzyme Research Laboratories (South Bend, IN). Chromogenic substrates for FXa (N-benzoyl-L-isoleucyl-L-glutamyl-glycyl-L-arginine-p-nitroaniline hydrochloride, S2222), thrombin (H-D-phenylalanyl-L-pipecolyl-L-arginine-p-nitroaniline dihydrochloride, S2238), FVIIa (H-D-isoleucyl-L-prolyl-L-arginine-p-nitroaniline dihydrochloride, S2288), FXIa (L-pyroglutamyl-L-proplyl-L-arginine-p-nitroaniline dihydrochloride, S2366), and plasmin (H-D-valyl-L-leucyl-L-lysine-p-nitroaniline dihydrochloride, S2251) were purchased from Diapharma (Westchester, OH). Thrombomax with calcium reagent, trypsin, chymotrypsin, elastase, tryptase, and rabbit antigoat alkaline phosphatase (AP)–coupled secondary antibody was obtained from Sigma Chemical (St Louis, MO). Reptilase was obtained from Diagnostica Stago (Les Ulis, France). Precast gels were purchased from Invitrogen (San Diego, CA).
Ticks and tick saliva
Salivary gland cDNA construction
This was done as detailed before.19 Briefly, the Micro-FastTrack messenger RNA (mRNA) isolation kit (Invitrogen, San Diego, CA) was used to isolate the mRNA. The I scapularissalivary gland mRNA (200 ng) was reverse transcribed to cDNA followed by double-strand synthesis and ligated into a Lambda Triplex2 vector; the resulting ligation reaction was packed using Gigapack gold III from Stratagene/Biocrest (Cedar Creek, TN). The library obtained was plated by infecting log-phase XL1-blue cells. Randomly picked clones from this library were sequenced exactly as described before.19After identifying a cDNA with high similarity to TFPI following the Basic Local Alignment Search Tool X (BLASTX) program of the cDNA against the National Center for Biotechnological Information (NCBI) nonredundant database,27 an aliquot (∼100 ng) of Ixolaris polymerase chain reaction (PCR) sample was reamplified, and the entire cDNA was fully sequenced using custom primers.
Ixolaris expression vector
For expression of Ixolaris, full-length cDNA was used as a template to amplify only the cDNA that begins at the initial methionine and ends at the first stop codon. A Kozak consensus sequence (ANNATGG) was added and the DNA amplified (forward primer: 5′-AAA ATG GGC GCT GTT TCC TGC TTC-3′; reverse primer: 5′-GGA TGA TCA GTT AAT AGT GAC ATT TAC-3′) and cloned into the vector pIB/V5-His TOPO (Invitrogen, San Diego, CA) following manufacturer's specifications.
Expression of Ixolaris in insect cell line BTI-TN-5B1-4 (High Five)
High Five cells (Invitrogen), cultivated in High Five serum-free medium supplemented with 10 μg/mL gentamycin were used for transfection with Ixolaris and antisense (control) constructs plasmid as indicated by the manufacturer. After 2 days of incubation, the supernatant (5 mL) was collected and stored for further analysis.
Sequence analysis
Sequence similarity searches were performed using the BLAST27 program. Cleavage site predictions of the mature proteins used the SignalP28 program. Alignments of protein sequences were done with the ClustalW program version 1.7.29 The molar extinction coefficient of Ixolaris at 280 nm was obtained at http://www.mbshortcuts.com, yielding a value of ε280 nm = 20 260 M−1.cm−1; A 280 nm/cm (1 mg/mL) = 1.287. Other calculated parameters are: Mr, 15738.35; pI, 4.56. Detection of N-linked glycosylation sites was obtained athttp://molbio.info.nih.gov/molbio/gcglite.
Chromatographic procedures and purification of recombinant Ixolaris
Supernatants of transformed cells were filtrated with a 50-kd cutoff Centriprep filter (Millipore, Bedford, CA). The filtrate was concentrated 20-fold using a Centriprep (3-kd cutoff). One milliliter of concentrated supernatant (∼8 mg) was diluted to 5 mL with water and applied to a 5 × 100-mm Pharmacia MonoQ eluted with a gradient from 25 mM Hepes pH 7.2 to 1 M NaCl in the same buffer, for 1 hour, at 0.5 mL/min. Active fractions were pooled and applied to a 10 × 250-mm Vydac 218TP510 octadecyl-silica column (The Separation Group, Hesperia, CA) eluted at 1.5 mL/min with a 60-minute gradient from 10% to 80% acetonitrile in water containing 0.1% trifluoroacetic acid (TFA). Active fractions were pooled, diluted with water to 8 mL, and chromatographed again in a 2.1 × 250-mm Macrosphere octadecylsilica column (Altech, Deerfield, IL) eluted at 0.2 mL/min for 55 minutes, and at 0.1 mL/min afterward.
Polyacrylamide gel electrophoresis of recombinant Ixolaris
A sample of purified recombinant Ixolaris (0.25 μg) in the absence (native conditions) or presence of sodium dodecyl sulfate (SDS) and dithiothreitol (DTT; denaturing conditions) was loaded into a 12% NU-polyacrylamide gel electrophoresis (PAGE) gel (MOPS buffer). Gels were silver stained (Bio-Rad, Hercules, CA).
Estimation of Ixolaris concentration
Concentration of Ixolaris (corrected for ε 280 nm) was estimated by the area of absorbance at A280 nm (calibration with bovine serum albumin (BSA)) of the peak containing Ixolaris activity obtained in the last purification step.
Binding of Ixolaris to FX, FXa, and FVIIa
Ixolaris (20 nM) was preincubated with FX (20 nM), FXa (20 nM), or FVIIa (20 nM) in 5 mM Hepes, pH 7.4, for 10 minutes. The sample was loaded into 8% precast PAGE and proteins were then transferred to polyvinylidene difluoride (PVDF) membrane. Primary antibody (polyclonal antibody anti-FX, 10 μg/mL, or monoclonal antibody anti-FVII, 5 μg/mL) was incubated for 1 hour in Tris-buffered saline, with 0.05% Tween and 5% nonfat milk (TBS-T). After 3 washes with TBS-T, the membranes were incubated for 30 minutes with appropriate AP-coupled secondary antibody (1:10 000). Reactions were developed with Western Blue Stabilized substrate for AP (Promega, Madison, WI).
Kinetic assay of FXa production by FVIIa/TF
This was performed as described. Ixolaris was incubated for 15 minutes at 37°C with FX, followed by addition of FVIIa/TF (1 nM/0.2 pM) previously incubated for 15 minutes at 37°C in buffer containing 50 mM Hepes, 0.1 M NaCl, 5 mM CaCl2, 0.5% BSA, pH 7.4 (buffer A). Chromogenic substrate (S2222, 250 μM) hydrolysis was detected using a Versamax microplate enzyme-linked immunosorbent assay (ELISA) reader (Molecular Devices, Sunnyvale, CA) equipped with a microplate mixer and heating system.17Reactions were continuously recorded at 405 nm for 1 hour at 37°C. The total volume of the reactions was 200 μL. Care was taken to ensure that substrate was less than 20% hydrolyzed. To have an estimation of FXa production, smoothing of the raw data (readings every 12 seconds) was performed and the background (∼0.042 U OD/405 nm) discounted using Excel 2000 software (MicroSoft Excel Analysis Tools, Seattle, WA). Then, data were transformed as the Δ A405/min as described.9,10 Briefly, A405 nm absorbance readings at times 1.00, 1.12, 1.24 up to 59.36, 59.48, and 60.0 minutes were respectively subtracted from absorbance readings at times 0, 0.12, 0.24 up to 58.36, 58.48, and 59.0 minutes, enabling determination of FXa concentrations at each time point. The FXa concentrations were interpolated from a standard curve relating Δ A405/min and known FXa concentrations.9,10 Amidolytic activity by FVIIa/TF alone on S2222 alone was not detected. In some experiments, data were plotted as Vs/Vo against Ixolaris concentration, where Vs is the velocity of substrate hydrolysis in the presence of inhibitor and Vo in its absence.30 Enzymes, Ixolaris, and chromogenic substrate were diluted in buffer A.
Preparation of DEGR-FX and des-Gla-DEGR-Xa
Briefly, DEGR (40 μM) was added to des-Gla-FXa (2 μM) in 100 mM Tris, 100 mM NaCl, pH 7.4 and incubated overnight at room temperature.31 des-Gla-DEGR-FXa was extensively dialyzed against 1 liter TBS (3 times) and shown to be devoid of amidolytic activity with S2222. FX (2 μM) was incubated with DEGR (400 μM) as described above, dialyzed, and shown to be devoid of amidolytic activity (S2222) when incubated with FVIIa/TF. The rationale for using DEGR-FX is based on experiments showing that serine catalytic site mutated FX (FXs195A) has been used as an effective scaffold for NAPc2, a TFPI-like molecule from Ancylostoma caninum.32
FVIIa/TF amidolytic activity
Activation of FIX
This was performed as described by Komiyama et al.33 In all experiments, plasma- and albumin-free recombinant FIX34 was used (BeneFIX, 250 U diluted at 1 U/μL in distilled water). In a final volume of 25 μL, FVIIa (1 nM)/TF (1 nM) was incubated for 15 minutes with preformed Ixolaris/scaffold complexes followed by addition of recombinant FIX (0.02 U/μL, ∼1.2 μM). After 90 minutes at 37°C in a thermocycler, reactions were stopped by addition of Laemmli buffer and boiling. Proteins were separated by 4% to 12% NU-PAGE (MES buffer). Reactants were incubated in 50 mM Hepes, 100 mM NaCl , 5 mM CaCl2, 0.01% BSA. Gels were scanned (Scan Jet 4p) to perform band densitometry.
Human umbilical vein endothelial cell culture and generation of FXa by HUVECs
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (San Diego, CA). After trypsinization, cells were grown to confluence in 96-well plates in 200 μL endothelial cell basal medium-2 (EBM-2) supplemented with endothelial cell growth medium (EGM-2) in a humidified incubator at 37°C with 5% CO2. On the day of the experiment, EBM-2 was replaced by 200 μL fresh EBM-2, and lipopolysaccharide (LPS; 10 μg/mL) was added for 4 hours to induce TF expression as described.35Subsequently, cells were rinsed 3 times with Hepes buffer (10 mM Hepes, 135 mM NaCl, 4 mM KCl, 1 mM MgCl2, 4 mM CaCl2, 11 mM d-glucose, and 0.5% BSA). Hepes buffer was removed and a mixture of 180 μL containing FX (200 nM), and Ixolaris (0-4 nM) previously incubated at 37°C for 15 minutes was added to the cells. This was followed by addition of 20 μL FVIIa (1 nM, final concentration) to start reactions. After 30 minutes, 100 μL was removed and added to 100 μL S2222 (500 μM) diluted in buffer A. Absorbance readings at 405 nm were followed for 1 hour and FXa concentration was estimated using a standard curve.
Prothrombin time
Ixolaris (0-10 nM) was incubated with prewarmed (37°C) plasma (100 μL), followed by addition of 200 μL prewarmed Thrombomax with calcium reagent (Sigma). Clot formation was detected by visual inspection.
Specificity of Ixolaris
Ixolaris was preincubated with different enzymes, followed by addition of the appropriate chromogenic substrates as indicated in Table 1.
Specificity of Ixolaris to FVIIa/TF-induced FX activation
| Enzymes/substrates . | Residual activity (%) . |
|---|---|
| VIIa/TF (6.4 pM)/S2288 (1 mM) | 0 |
| Thrombin (125 pM)/S2238 (250 μM) | 103 ± 1.56 |
| Chymotrypsin (22.4 nM)/S2222 (250 μM) | 101.5 ± 0.09 |
| FXIa (58.7 pM)/S2256 (250 μM) | 89.2 ± 3.1 |
| Reptilase (0.3125 BU/mL)/S2238 (250 μM) | 98.5 ± 0.52 |
| Plasmin (5 mU/mL)/S2251 (250 μM) | 107.5 ± 0.75 |
| Elastase (250 pM)/substrate (500 μM) | 106 ± 8.02 |
| Tryptase (0.4 μg/mL)/S2222 (250 μM) | 101 ± 0.07 |
| Enzymes/substrates . | Residual activity (%) . |
|---|---|
| VIIa/TF (6.4 pM)/S2288 (1 mM) | 0 |
| Thrombin (125 pM)/S2238 (250 μM) | 103 ± 1.56 |
| Chymotrypsin (22.4 nM)/S2222 (250 μM) | 101.5 ± 0.09 |
| FXIa (58.7 pM)/S2256 (250 μM) | 89.2 ± 3.1 |
| Reptilase (0.3125 BU/mL)/S2238 (250 μM) | 98.5 ± 0.52 |
| Plasmin (5 mU/mL)/S2251 (250 μM) | 107.5 ± 0.75 |
| Elastase (250 pM)/substrate (500 μM) | 106 ± 8.02 |
| Tryptase (0.4 μg/mL)/S2222 (250 μM) | 101 ± 0.07 |
Ixolaris (32 nM, final concentration) was preincubated for 15 minutes at 37°C with the enzymes listed, followed by addition of the appropriate chromogenic substrate. Reactions were followed for 30 minutes at 37°C, and the effect of Ixolaris was estimated by setting the initial velocity obtained in the presence of enzyme alone (without inhibitor) as 100%.
Statistical analysis, curve fitting, and data handling
Data are presented as the mean ± SE, using SigmaPlot 5.0 Graphing software, curve fitting and statistical modes (Jandel Scientific, San Rafael, CA).
Results
A salivary gland cDNA library of the tick I scapularis was randomly cloned and sequenced, identifying a cDNA with high similarity to rabbit TFPI. The full-length nucleotide and deduced amino acid sequences of Ixodes TFPI-like protein are shown in Figure 1A. The translated protein has a short hydrophobic sequence of 25 amino acids typical of signal peptide, according to Signal P software for prediction of N-terminus of proteins.28 The mature protein, herein called Ixolaris, contains 140 amino acids (15.7 kd) including 10 cysteines, and a pI of 4.56. Ixolaris is similar to other members of the Kunitz family of proteins including human TFPI precursor (e value = 4e−14, P10646); lacunin from Manduca sexta(1e−12, AAF04457.1); hepatocyte growth factor pathway inhibitor (8e−12, AAF02490.1); inter-α-trypsin inhibitor (bikunin; 7e−11, P04365); amyloid-precursor-like protein (1e−11, CAA54906.1); and basic pancreatic trypsin inhibitor (aprotinin, 1e−05, 1510193A). For comparison, the alignment of Ixolaris and human TFPI sequences is shown (Figure1B). Figure 2 shows the predicted structure of Ixolaris assumed on the basis of the crystal structure of bovine pancreatic trypsin inhibitor.36
Nucleotide sequence and deduced amino acid sequence of Ixolaris.
(A) The nucleotides and amino acids are numbered from the translation starting site ATG. The signal peptide sequence is underlined. (B) Alignment of Ixolaris with partial human TFPI sequence.6Identical amino acids are shaded.
Nucleotide sequence and deduced amino acid sequence of Ixolaris.
(A) The nucleotides and amino acids are numbered from the translation starting site ATG. The signal peptide sequence is underlined. (B) Alignment of Ixolaris with partial human TFPI sequence.6Identical amino acids are shaded.
Predicted secondary folding structure for Ixolaris.
Disulfide bonds are assumed on the basis of the crystal structure of bovine pancreatic trypsin inhibitor.36 The charges of the amino acid side chains are shown. Predicted N-linked glycosylation sites are Asn65, Asn98, and Asn136.
Predicted secondary folding structure for Ixolaris.
Disulfide bonds are assumed on the basis of the crystal structure of bovine pancreatic trypsin inhibitor.36 The charges of the amino acid side chains are shown. Predicted N-linked glycosylation sites are Asn65, Asn98, and Asn136.
The Ixolaris sequence suggests the existence of a salivary anticoagulant directed toward the extrinsic pathway. Thus, we tested saliva of I scapularis separated by gel filtration in an assay measuring activation of FX by FVIIa/TF. Inhibition of FX activation was detected in fractions eluted between 11 and 17 minutes of retention time (not shown). To determine whether Ixolaris accounts for the inhibitory activity identified in the saliva, the full-length cDNA of Ixolaris was expressed in insect cells as described in “Materials and methods.” The concentrated supernatant was applied to a gel-filtration column under conditions identical to those described for saliva and the inhibitory activity was found with the same retention time (not shown). No activity was found with the supernatants of cells transfected with the control plasmid. These data indicate that Ixolaris and the salivary TFPI-like molecule have similar chromatographic properties on gel filtration.
To obtain larger amounts of purified Ixolaris, the supernatants of transfected cells were purified by 3 chromatographic steps (see “Materials and methods”). In the final purification step, one single peak at A280 nm was obtained and the corresponding fraction analyzed by PAGE under denaturing and nonreducing conditions. A major band of approximately 24 kd in addition to a minor component of about 15.5 kd were silver stained (not shown). Under reducing conditions, a band of about 27 kd and a minor component of about 15.5-kd were detected (not shown). PAGE of the sample under native conditions shows that only one major band has been stained (not shown).
The mechanism of inhibition of blood coagulation by Ixolaris was then studied. In these experiments, Ixolaris (0-10 nM) and FX (200 nM) were incubated at 37°C for 15 minutes, followed by addition of FVIIa (1 nM)/TF (0.2 pM) and chromogenic substrate for FXa (S2222). The progress curves were characterized by a lag phase, followed by significant hydrolytic increase between 5 and 20 minutes, and a linear augmentation thereafter showing accumulative production of FXa that was dose dependently inhibited by Ixolaris (Figure3A), or recombinant full-length clone human TFPI (Figure 3A, inset). To estimate the production of FXa at each minute, Δ405 nm absorbance/minute was calculated as described9,10 (see “Materials and methods”), and a linear increase of FXa production was observed. However, in the presence of Ixolaris, generation of FXa was immediately attenuated dose dependently (Figure 3B). TFPI also inhibited FXa generation in a concentration-dependent manner, although it behaved as a slow inhibitor in this assay (Figure 3B, inset), as previously reported.9Experiments were then performed at different concentrations of FX, ranging from 3 to 200 nM. Data were transformed according to Vs/Vo, where Vs and Vo are the velocity of FXa production in the presence and absence of inhibitor, respectively. Figure 3C shows that both Ixolaris and TFPI (inset) block FXa production at all FX concentrations tested. However, a linear increase of the inhibitory concentration of 50% (IC50) for Ixolaris (Figure 3D), but not for TFPI (inset), was obtained as a function of FX concentration. Accordingly, IC50 for Ixolaris increased from 30 pM at 3 nM FX to 420 pM at 200 nM FX (Figure 3D). In contrast, the IC50 for TFPI was 610 pM at 15 nM FX and 625 pM at 200 nM FX (inset). The remarkable effect of FX concentration in the IC50 for Ixolaris in this assay, but not for TFPI, suggested that both molecules operate by distinct mechanisms.
Inhibition of FVIIa/TF-induced FX activation by Ixolaris.
(A) Progress curves: FVIIa (1 nM)/TF (0.2 pM) was added to a mixture containing FX (200 nM), previously incubated with increasing concentrations of Ixolaris (a, 0 nM; b, 0.1 nM;c, 0.25 nM; d, 0.5 nM; e, 1 nM;f, 2.5 nM; g, 5 nM; h, 10 nM), or full-length clone TFPI (inset: a, 0 nM; b, 0.05 nM; c, 0.125 nM; d, 0.25 nM; e, 0.5 nM; f, 1.25 nM; g, 2.5 nM; h, 5 nM). Progress curves are representative experiments (n = 3). (B) Data generated in panel A were transformed as described in “Materials and methods” to estimate FXa production for Ixolaris (a, 0 nM;b, 0.1 nM; c, 0.25 nM; d, 0.5 nM;e, 1 nM; f, 2.5 nM; g, 5 nM;h, 10 nM), or TFPI (inset: a, 0 nM; b, 0.05 nM; c, 0.125 nM; d, 0.25 nM; e, 0.5 nM). (C) For determination of the IC50, Vs and Vo were plotted against Ixolaris concentration at different FX concentrations: (●) 3 nM; (▪) 15 nM; (▴) 40 nM; (▾) 75 nM; (○) 100 nM; (■) 140 nM); (▵) 175 nM; (▿) 200 nM (n = 3). Inset: Vs and Vo were plotted against TFPI concentration at different FX concentrations: (●) 15 nM; (▪) 40 nM; (▴) 75 nM; (▾) 200 nM (n = 3). Vs, inhibited velocity; Vo, control (uninhibited velocity). (D) IC50 for Ixolaris or TFPI (inset) at different concentrations of FX (n = 3).
Inhibition of FVIIa/TF-induced FX activation by Ixolaris.
(A) Progress curves: FVIIa (1 nM)/TF (0.2 pM) was added to a mixture containing FX (200 nM), previously incubated with increasing concentrations of Ixolaris (a, 0 nM; b, 0.1 nM;c, 0.25 nM; d, 0.5 nM; e, 1 nM;f, 2.5 nM; g, 5 nM; h, 10 nM), or full-length clone TFPI (inset: a, 0 nM; b, 0.05 nM; c, 0.125 nM; d, 0.25 nM; e, 0.5 nM; f, 1.25 nM; g, 2.5 nM; h, 5 nM). Progress curves are representative experiments (n = 3). (B) Data generated in panel A were transformed as described in “Materials and methods” to estimate FXa production for Ixolaris (a, 0 nM;b, 0.1 nM; c, 0.25 nM; d, 0.5 nM;e, 1 nM; f, 2.5 nM; g, 5 nM;h, 10 nM), or TFPI (inset: a, 0 nM; b, 0.05 nM; c, 0.125 nM; d, 0.25 nM; e, 0.5 nM). (C) For determination of the IC50, Vs and Vo were plotted against Ixolaris concentration at different FX concentrations: (●) 3 nM; (▪) 15 nM; (▴) 40 nM; (▾) 75 nM; (○) 100 nM; (■) 140 nM); (▵) 175 nM; (▿) 200 nM (n = 3). Inset: Vs and Vo were plotted against TFPI concentration at different FX concentrations: (●) 15 nM; (▪) 40 nM; (▴) 75 nM; (▾) 200 nM (n = 3). Vs, inhibited velocity; Vo, control (uninhibited velocity). (D) IC50 for Ixolaris or TFPI (inset) at different concentrations of FX (n = 3).
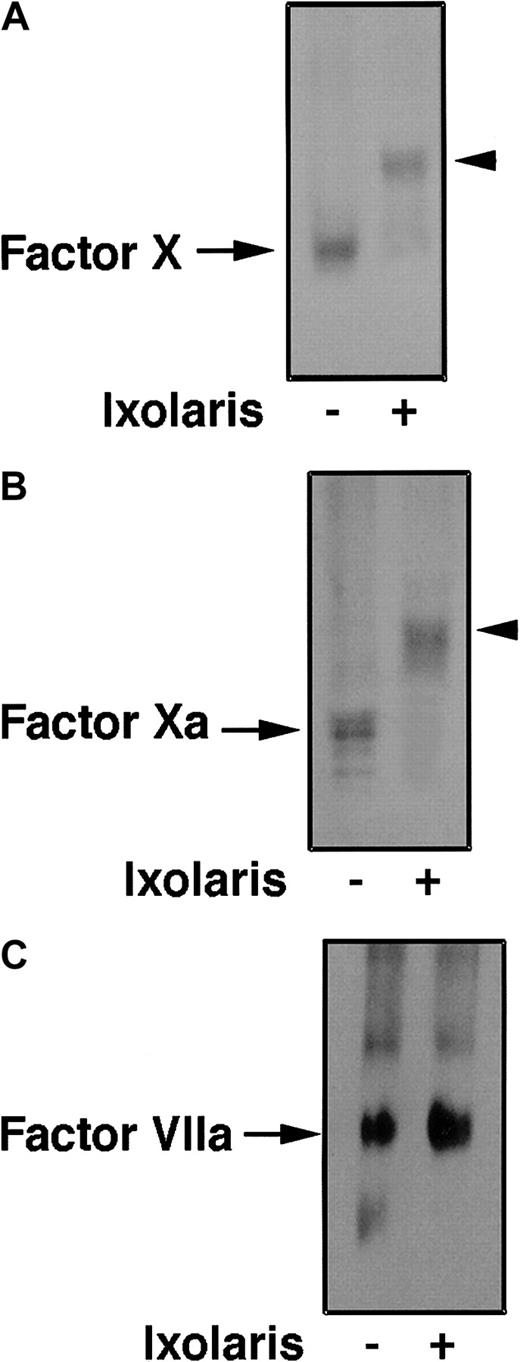
To detect Ixolaris binding to FX and FXa, reactants were incubated in equimolar concentrations (20 nM) for 15 minutes at 37°C followed by PAGE under nondenaturing conditions. Proteins were transferred to PVDF membrane. Experiments were performed using antihuman FX polyclonal antibodies. Results indicate complex formation between Ixolaris and FX (Figure 4A) and FXa (Figure 4B). On the other hand, complex formation between Ixolaris and FVIIa was not detected using antihuman FVIIa monoclonal antibody (Figure 4C), even when 10 times molar excess Ixolaris was used.
Ixolaris binds to FX and FXa, but not to FVIIa.
Ixolaris (20 nM) was preincubated with FX (20 nM), FXa (20 nM), or FVIIa (20 nM). PAGE of the sample under nondenaturing conditions was followed by transfer of proteins to PVDF membrane. FX, FXa, and FVIIa detection was performed using polyclonal anti-FX or monoclonal antibody anti-FVIIa, as described in “Materials and methods.” (A) Lane 1, FX; lane 2, FX plus Ixolaris. (B) Lane 1, FXa; lane 2, FXa plus Ixolaris. (C) Lane 1, FVIIa; lane 2, FVIIa plus Ixolaris. Each blotting is a representative experiment. Arrows indicate enzyme; arrowheads, enzyme-inhibitor complex.
Ixolaris binds to FX and FXa, but not to FVIIa.
Ixolaris (20 nM) was preincubated with FX (20 nM), FXa (20 nM), or FVIIa (20 nM). PAGE of the sample under nondenaturing conditions was followed by transfer of proteins to PVDF membrane. FX, FXa, and FVIIa detection was performed using polyclonal anti-FX or monoclonal antibody anti-FVIIa, as described in “Materials and methods.” (A) Lane 1, FX; lane 2, FX plus Ixolaris. (B) Lane 1, FXa; lane 2, FXa plus Ixolaris. (C) Lane 1, FVIIa; lane 2, FVIIa plus Ixolaris. Each blotting is a representative experiment. Arrows indicate enzyme; arrowheads, enzyme-inhibitor complex.
Human TFPI inhibits the catalytic site of FXa.8 To determine whether the binding of Ixolaris to FXa was accompanied by a change in the amidolytic activity of FXa, inhibitor, and chromogenic substrate (S2222) were incubated for 15 minutes at 37°C, followed by addition of FXa (125 pM). Ixolaris, at a concentration similar to the enzyme, instantaneously increased FXa activity (Figure5A) indicating that it is a tight-and-fast ligand of FXa, with an effective dose (ED50) of 159 ± 3.8 pM (Figure 5B). In contrast, full-length human TFPI behaved as a typical slow-binding inhibitor of FXa (Figure 5A), as reported.8 Similar results were obtained with des-Gla-FXa (Figure 5C) whose proteolytic activity increased 1.98-fold in the presence of Ixolaris (control, 0.4 nM des-Gla-FXa: 1.4 ± 0.12 U Vmax; 0.4 nM des-Gla-FXa plus 1.6 nM Ixolaris: 2.78 ± 0.1 units of Vmax; triplicate determination). Similar results were obtained for FXa but not for thrombin (Figure 5C).
Ixolaris increases the amidolytic activity of Fxa; effects of FX and des-Gla-FXa.
(A) Buffer (control; curve a), Ixolaris (0.8 nM, curve b), or human TFPI (20 nM, curve c) and chromogenic substrate (S2222, 250 μM) were incubated at 37°C for 15 minutes before addition of FXa (125 pM). (B) FXa (125 pM) and Ixolaris (0-1600 pM) were incubated at 37°C for 15 minutes followed by addition of S2222 (250 μM). Substrate hydrolysis was continuously recorded at 405 nm for 1 hour at 37°C. Inset shows progress curves of the effects of Ixolaris on FXa amidolytic activity: Ixolaris (a) 0 nM; (b) 40 pM; (c) 80 pM; (d) 160 pM; (e) 300 pM; (f) 600 pM; and (g) 1600 pM. (C) Ixolaris (1.6 nM) was incubated with FXa (125 pM), des-Gla-FXa (600 pM), or thrombin (150 pM), followed by addition of S2222, or S2238 for thrombin. Substrate hydrolysis was continuously recorded at 405 nm for 1 hour at 37°C. (D) Ixolaris (400 pM) was added to a mixture containing FXa (640 pM) and (▪) DEGR-FXa (0-3.2 nM), or (●) des-Gla-DEGR-FXa (0-3.2 nM), or (▴) DEGR-FX (0-3.2 nM). After a 15-minute incubation, reactions were initiated with S2222 (250 μM). Inset: Ixolaris was incubated with (▪) DEGR-FXa (0-3.2 nM), or (●) des-Gla-DEGR-FXa (0-3.2 nM), or (▴) DEGR-FX (0-3.2 nM), and S2222 (250 μM) for 15 minutes followed by addition of FXa (640 pM) (n = 3). Reactants were diluted in buffer A. Substrate hydrolysis was continuously recorded at 405 nm at 37°C.
Ixolaris increases the amidolytic activity of Fxa; effects of FX and des-Gla-FXa.
(A) Buffer (control; curve a), Ixolaris (0.8 nM, curve b), or human TFPI (20 nM, curve c) and chromogenic substrate (S2222, 250 μM) were incubated at 37°C for 15 minutes before addition of FXa (125 pM). (B) FXa (125 pM) and Ixolaris (0-1600 pM) were incubated at 37°C for 15 minutes followed by addition of S2222 (250 μM). Substrate hydrolysis was continuously recorded at 405 nm for 1 hour at 37°C. Inset shows progress curves of the effects of Ixolaris on FXa amidolytic activity: Ixolaris (a) 0 nM; (b) 40 pM; (c) 80 pM; (d) 160 pM; (e) 300 pM; (f) 600 pM; and (g) 1600 pM. (C) Ixolaris (1.6 nM) was incubated with FXa (125 pM), des-Gla-FXa (600 pM), or thrombin (150 pM), followed by addition of S2222, or S2238 for thrombin. Substrate hydrolysis was continuously recorded at 405 nm for 1 hour at 37°C. (D) Ixolaris (400 pM) was added to a mixture containing FXa (640 pM) and (▪) DEGR-FXa (0-3.2 nM), or (●) des-Gla-DEGR-FXa (0-3.2 nM), or (▴) DEGR-FX (0-3.2 nM). After a 15-minute incubation, reactions were initiated with S2222 (250 μM). Inset: Ixolaris was incubated with (▪) DEGR-FXa (0-3.2 nM), or (●) des-Gla-DEGR-FXa (0-3.2 nM), or (▴) DEGR-FX (0-3.2 nM), and S2222 (250 μM) for 15 minutes followed by addition of FXa (640 pM) (n = 3). Reactants were diluted in buffer A. Substrate hydrolysis was continuously recorded at 405 nm at 37°C.
Because both FXa and FX interact with Ixolaris as determined by native PAGE, we determined the relative affinities of Ixolaris for FX and FXa by studying the effects of FX derivatives on Ixolaris-mediated increase of FXa amidolytic activity. Figure 5D shows that when Ixolaris was added to and incubated with a preformed mixture of FXa and increasing concentrations of DEGR-FXa, or des-Gla-DEGR-FXa, followed by addition of S2222, increase of FXa amidolytic activity by Ixolaris was inhibited. However, this effect was not observed when Ixolaris was added to a mixture containing FXa and increasing concentrations of FX (up to 16 nM; see “Discussion”). When Ixolaris was preincubated with FX, DEGR-FXa, or des-Gla-DEGR-FXa and S2222, followed by addition of FXa, inhibition of Ixolaris-mediated increase of FXa amidolytic activity was attained in all cases with comparable IC50s(Figure 5D, inset; see “Discussion”).
It has been shown that human TFPI at high concentrations blocks the catalytic activity of FVIIa/TF, inhibition being remarkable in the presence of FXa.1 To test the effects of Ixolaris only, or Ixolaris in the presence of DEGR-FXa in the catalytic activity of FVIIa/TF, amidolytic assays were performed using chromogenic substrate (S2288). Figure 6A shows that Ixolaris at high concentrations (0-4.8 μM) dose dependently inhibits FVIIa/TF amidolytic activity. However, low concentrations of Ixolaris (5 nM) in the presence of DEGR-FXa (0-5 nM), effectively blocked FVIIa/TF amidolytic activity (Figure 6B). A transformation of the data as Vs/Vo yields an IC50 of 0.41 ± 0.04 nM; an almost complete (> 95%) inhibition was observed at DEGR-FXa concentration of 1 nM (Figure 6C). The finding that Ixolaris inhibits FVIIa/TF activity in an FXa-dependent manner was confirmed by increasing the concentration of Ixolaris (0-5 nM) at one concentration of DEGR-FXa (5 nM; Figure 6D); this result is compared with Ixolaris only (up to 4.8 μM; Figure 6D).
Inhibition of FVIIa/TF amidolytic activity by Ixolaris and DEGR-FXa/Ixolaris.
(A) Ixolaris (0-4.8 μM) was incubated with FVIIa/TF (1 nM) for 15 minutes at 37°C, followed by addition of S2288 (1 mM). Ixolaris:a, 0 μM; b, 0.48 μM; c, 1.6 μM;d, 4.8 μM. (B) Ixolaris (5 nM) was incubated with DEGR-FXa (a) 0 nM; (b) 0.1 nM; (c) 0.25 nM; (d) 0.5 nM; (e) 1 nM; (f) 2.5 nM; (g) 5 nM for 15 minutes at 37°C, followed by addition of FVIIa/TF (1 nM). Fifteen minutes later, S2288 (1 mM) was added. (C) Progress curves depicted in panel B are expressed as Vs/Vo, yielding a IC50 of 0.41 ± 0.04 nM in the presence of DEGR-FXa. DEGR-FXa did not affect FVIIa/TF amidolytic activity in the absence of Ixolaris (straight line). (D) In (●), DEGR-FXa (5 nM) was incubated with Ixolaris (0-5 nM) for 15 minutes, followed by addition of FVIIa/TF (1 nM). Reactions were initiated 15 minutes later by S2288 (1 mM). In (▪), Ixolaris without DEGR-FXa was incubated for 15 minutes with FVIIa/TF (1 nM) and reactions were initiated by S2288 (1 mM). In panels A through D, substrate hydrolysis was continuously recorded at 405 nm for 30 minutes at 37°C.
Inhibition of FVIIa/TF amidolytic activity by Ixolaris and DEGR-FXa/Ixolaris.
(A) Ixolaris (0-4.8 μM) was incubated with FVIIa/TF (1 nM) for 15 minutes at 37°C, followed by addition of S2288 (1 mM). Ixolaris:a, 0 μM; b, 0.48 μM; c, 1.6 μM;d, 4.8 μM. (B) Ixolaris (5 nM) was incubated with DEGR-FXa (a) 0 nM; (b) 0.1 nM; (c) 0.25 nM; (d) 0.5 nM; (e) 1 nM; (f) 2.5 nM; (g) 5 nM for 15 minutes at 37°C, followed by addition of FVIIa/TF (1 nM). Fifteen minutes later, S2288 (1 mM) was added. (C) Progress curves depicted in panel B are expressed as Vs/Vo, yielding a IC50 of 0.41 ± 0.04 nM in the presence of DEGR-FXa. DEGR-FXa did not affect FVIIa/TF amidolytic activity in the absence of Ixolaris (straight line). (D) In (●), DEGR-FXa (5 nM) was incubated with Ixolaris (0-5 nM) for 15 minutes, followed by addition of FVIIa/TF (1 nM). Reactions were initiated 15 minutes later by S2288 (1 mM). In (▪), Ixolaris without DEGR-FXa was incubated for 15 minutes with FVIIa/TF (1 nM) and reactions were initiated by S2288 (1 mM). In panels A through D, substrate hydrolysis was continuously recorded at 405 nm for 30 minutes at 37°C.
To study the effects of FX and FXa as scaffolds for Ixolaris in the inhibition of FVIIa/TF, FIX activation assays33 were performed. This assay is particular useful for these studies because FIX is a physiologic substrate for FVIIa/TF.24,25 To completely rule out the presence of contaminating coagulation factors that could operate as scaffolds for Ixolaris in this assay, experiments were performed with recombinant FIX34 (BeneFIX). FIX activation proceeds through two consecutive steps33 and is dependent on both catalytic site and FVIIa/TF exosite.37-40 In the first step, FIX (∼62 kd, apparent molecular weight) cleavage at the Arg145-Ala146 peptide bond generates the intermediate product termed FIXα heavy chain (FIXα HC; ∼44 kd) and a lower molecular weight product named FIXa light chain (FIXa LC; ∼20 kd). The activation peptide is cleaved from the heavy chain at the Arg180-Val181 bond (not detected), generating FIXaβ heavy chain (FIXa HC; ∼30 kd) that is biologically active. Figure7A shows that FIX was not activated when incubation with FVIIa/TF was not allowed to proceed (0 minutes incubation time; lane 1). However, after 90 minutes of FIX incubation with FVIIa/TF, bands corresponding to FIXα HC, FIXa HC, and FIXa LC were detected in the NU-PAGE gel (lane 2). Ixolaris (in the absence of DEGR-FXa), DEGR-FX, and DEGR-FXa (in the absence of Ixolaris) did not affect this pattern (not shown). On the other hand, Ixolaris in the presence of increasing concentrations of DEGR-FX (lanes 3-5) or DEGR-FXa (lanes 6-8) but not des-Gla-DEGR-FXa (lanes 9 and 10) caused a dose-dependent inhibition of FIX activation by FVIIa/TF (n = 8). Band densitometry for FIXa HC in the experiments of Figure7A was performed and the results are shown in Figure 7B.
FX and FXa, but not DEGR-FXa, are scaffolds for Ixolaris: recombinant FIX activation assays.
(A) Ixolaris (10 nM) was incubated with DEGR-FX derivatives (0-10 nM) for 15 minutes at 37°C followed by addition of FVIIa/TF (1 nM) and incubated for additional 15 minutes at 37°C. Reactions were initiated with recombinant FIX (BeneFIX, 1.2 μM). Ninety minutes later reactions were stopped with Laemmli buffer and proteins were separated by 4% to 12% SDS-PAGE. Lane 1, after addition of FVIIa/TF reactions were not allowed to proceed (0 minutes incubation time); lane 2, buffer (no inhibitor/no scaffold); lane 3, Ixolaris plus DEGR-FX (0.1 nM); lane 4, Ixolaris plus DEGR-FX (1 nM); lane 5, Ixolaris plus DEGR-FX (10 nM); lane 6, Ixolaris plus DEGR-FXa (0.1 nM); lane 7, Ixolaris plus DEGR-FXa (1 nM); lane 8, Ixolaris plus DEGR-FXa (10 nM); lane 9, Ixolaris plus des-Gla-DEGR-FXa (1 nM); lane 10, Ixolaris plus des-Gla-DEGR-FXa (10 nM). The bands correspond to (from the top): uncleaved FIX (FIX), the heavy chain of FIXα (FIXα HC), the heavy chain of FIXaβ (FIXa HC), and the light chain of FIXa (FIXa LC). The activation peptide is not detected. Typical gel is shown (n = 8). FX, FXa, and des-Gla-FXa only (tested up to 20 nM each), or Ixolaris (tested up to 200 nM) did not affect FIX activation by FVIIa/TF (not shown). (B) Results of the band densitometry for FIXa HC (A), showing inhibition of FVIIa/TF activity by Ixolaris/DEGR-FXa (●), Ixolaris/DEGR-FX (▪), but not Ixolaris/des-Gla-DEGR-FXa (▴).
FX and FXa, but not DEGR-FXa, are scaffolds for Ixolaris: recombinant FIX activation assays.
(A) Ixolaris (10 nM) was incubated with DEGR-FX derivatives (0-10 nM) for 15 minutes at 37°C followed by addition of FVIIa/TF (1 nM) and incubated for additional 15 minutes at 37°C. Reactions were initiated with recombinant FIX (BeneFIX, 1.2 μM). Ninety minutes later reactions were stopped with Laemmli buffer and proteins were separated by 4% to 12% SDS-PAGE. Lane 1, after addition of FVIIa/TF reactions were not allowed to proceed (0 minutes incubation time); lane 2, buffer (no inhibitor/no scaffold); lane 3, Ixolaris plus DEGR-FX (0.1 nM); lane 4, Ixolaris plus DEGR-FX (1 nM); lane 5, Ixolaris plus DEGR-FX (10 nM); lane 6, Ixolaris plus DEGR-FXa (0.1 nM); lane 7, Ixolaris plus DEGR-FXa (1 nM); lane 8, Ixolaris plus DEGR-FXa (10 nM); lane 9, Ixolaris plus des-Gla-DEGR-FXa (1 nM); lane 10, Ixolaris plus des-Gla-DEGR-FXa (10 nM). The bands correspond to (from the top): uncleaved FIX (FIX), the heavy chain of FIXα (FIXα HC), the heavy chain of FIXaβ (FIXa HC), and the light chain of FIXa (FIXa LC). The activation peptide is not detected. Typical gel is shown (n = 8). FX, FXa, and des-Gla-FXa only (tested up to 20 nM each), or Ixolaris (tested up to 200 nM) did not affect FIX activation by FVIIa/TF (not shown). (B) Results of the band densitometry for FIXa HC (A), showing inhibition of FVIIa/TF activity by Ixolaris/DEGR-FXa (●), Ixolaris/DEGR-FX (▪), but not Ixolaris/des-Gla-DEGR-FXa (▴).
In another set of experiments, we have attempted to characterize the kinetics of the interaction between FVIIa/TF and Ixolaris/FXa as slow or fast, by means of 2 independent approaches. Accordingly, Figure8A shows that when FVIIa/TF was added to a mixture containing preincubated Ixolaris/DEGR-FXa and S2288, a partial and typical slow-type inhibition was attained. We have also tested the macromolecular substrates FIX, in reactions initiated by FVIIa/TF. Accordingly, when FVIIa/TF was added to a mixture containing preincubated Ixolaris/DEGR-FXa and FIX, complete inhibition was observed (Figure 8B). Identical results were obtained with DEGR-FX (Figure 8C; see “Discussion”).
Kinetics of Ixolaris/FXa and FVIIa/TF interaction.
(A) Amidolytic assays. Ixolaris (10 nM) and DEGR-FXa (0-10 nM) were incubated for 15 minutes in the presence of S2288 (1 mM) followed by the addition of FVIIa/TF (1 nM). a, Ixolaris; b, Ixolaris plus 0.1 nM DEGR-FXa; c, Ixolaris plus 1 nM DEGR-FXa; d, Ixolaris plus 10 nM DEGR-FXa. (B,C) FIX activation assays: (B) Lane 1, buffer; lane 2, Ixolaris only (10 nM); or lane 3, Ixolaris/DEGR-FXa (10 nM each) were incubated for 15 minutes, followed by addition of FIX (1.2 μM). Five minutes later reaction was initiated by FVIIa/TF (1 nM) (except lane 1, which was not allowed to proceed) and followed as described in Figure7A. (C) Lane 1, buffer; lane 2, Ixolaris (10 nM); or lane 3, Ixolaris/DEGR-FX (10 nM each) were incubated for 15 minutes, followed by addition of FIX (1.2 μM). Five minutes later reaction was initiated by FVIIa/TF (1 nM) (except lane 1, which was not allowed to proceed) and followed as described in Figure 7A. In panels B and C reactions were stopped as described in Figure 7A. The bands correspond to (from the top): uncleaved FIX (FIX), the heavy chain of FIXα (FIXα HC), the heavy chain of FIXaβ (FIXa HC), and the light chain of FIXa (FIXa LC). The activation peptide is not detected. Typical gels are shown (n = 3).
Kinetics of Ixolaris/FXa and FVIIa/TF interaction.
(A) Amidolytic assays. Ixolaris (10 nM) and DEGR-FXa (0-10 nM) were incubated for 15 minutes in the presence of S2288 (1 mM) followed by the addition of FVIIa/TF (1 nM). a, Ixolaris; b, Ixolaris plus 0.1 nM DEGR-FXa; c, Ixolaris plus 1 nM DEGR-FXa; d, Ixolaris plus 10 nM DEGR-FXa. (B,C) FIX activation assays: (B) Lane 1, buffer; lane 2, Ixolaris only (10 nM); or lane 3, Ixolaris/DEGR-FXa (10 nM each) were incubated for 15 minutes, followed by addition of FIX (1.2 μM). Five minutes later reaction was initiated by FVIIa/TF (1 nM) (except lane 1, which was not allowed to proceed) and followed as described in Figure7A. (C) Lane 1, buffer; lane 2, Ixolaris (10 nM); or lane 3, Ixolaris/DEGR-FX (10 nM each) were incubated for 15 minutes, followed by addition of FIX (1.2 μM). Five minutes later reaction was initiated by FVIIa/TF (1 nM) (except lane 1, which was not allowed to proceed) and followed as described in Figure 7A. In panels B and C reactions were stopped as described in Figure 7A. The bands correspond to (from the top): uncleaved FIX (FIX), the heavy chain of FIXα (FIXα HC), the heavy chain of FIXaβ (FIXa HC), and the light chain of FIXa (FIXa LC). The activation peptide is not detected. Typical gels are shown (n = 3).
In an attempt to study the effects of Ixolaris in a cell-mediated initiation of blood coagulation,35 HUVECs were stimulated to express TF after 4 hours of incubation with LPS. Then a mixture containing FX (200 nM) and Ixolaris (0-4 nM) previously incubated for 15 minutes was added to the cells, followed by addition of FVIIa (1 nM). After 30 minutes, an aliquot was taken to measure FXa using chromogenic substrate for FXa (S2222). Figure9 shows that Ixolaris dose dependently inhibits FXa production, with an IC50 of about 500 pM.
Ixolaris inhibits FXa generated by LPS-stimulated HUVECs.
A mixture (180 μL) containing FX (200 nM) and Ixolaris (0-4 nM) that have previously incubated at 37°C for 15 minutes was added to confluent LPS-stimulated HUVECs. This was followed by addition of FVIIa (1 nM) to start reactions. After 30 minutes, 100 μL was removed and added to a 96-well plate containing 100 μL S2222 (500 μM) diluted in 50 mM Hepes, 100 mM NaCl, 50 mM EDTA, BSA 0.5%, pH 7.4. Absorbance reading at 405 nm (substrate hydrolysis) was continuously recorded for 1 hour and FXa concentration was estimated using a standard curve, using known concentrations of FXa. Appropriate controls were run in parallel: HUVECs that have not been exposed to LPS, FXa concentration is 68.4 ± 8.7 pM. LPS-exposed HUVECs in the absence of FVIIa, FXa concentration is 78.08 ± 6.59 pM. Data are the mean ± SE of triplicate experiments. Experiments were performed with HUVECs in the second or third passages.
Ixolaris inhibits FXa generated by LPS-stimulated HUVECs.
A mixture (180 μL) containing FX (200 nM) and Ixolaris (0-4 nM) that have previously incubated at 37°C for 15 minutes was added to confluent LPS-stimulated HUVECs. This was followed by addition of FVIIa (1 nM) to start reactions. After 30 minutes, 100 μL was removed and added to a 96-well plate containing 100 μL S2222 (500 μM) diluted in 50 mM Hepes, 100 mM NaCl, 50 mM EDTA, BSA 0.5%, pH 7.4. Absorbance reading at 405 nm (substrate hydrolysis) was continuously recorded for 1 hour and FXa concentration was estimated using a standard curve, using known concentrations of FXa. Appropriate controls were run in parallel: HUVECs that have not been exposed to LPS, FXa concentration is 68.4 ± 8.7 pM. LPS-exposed HUVECs in the absence of FVIIa, FXa concentration is 78.08 ± 6.59 pM. Data are the mean ± SE of triplicate experiments. Experiments were performed with HUVECs in the second or third passages.
Table 1 shows that Ixolaris is a specific inhibitor for FVIIa/TF, and does not affect the catalytic activity of other enzymes. We have also tested the effects of Ixolaris on the prothrombin time and no inhibition was detected (control, 12.6 ± 0.3 seconds; Ixolaris up to 10 nM, 12.3 ± 0.3 seconds). Figure10 shows a model proposing the mechanism of action of Ixolaris (see figure legends).
Working hypothesis for the mechanism of action of Ixolaris.
Initially, the (second) Kunitz domain of Ixolaris binds to exosite of FXa,45-47 or to an FX putative “pro-exosite” (not shown), leading to the formation of a stable Ixolaris/FXa complex. Then, the scaffold using presumably a similar docking mechanism as the natural substrate, FX (not shown)37 or the product FXa,38 interacts with the exosite formed by FVIIa and TF.39,40 This interaction allows the (first) Kunitz domain of Ixolaris/FXa to dock into the active site of FVIIa/TF, with subsequent assembling of a tightly bound quaternary inhibitory complex composed of FVIIa/TF/Ixolaris/FXa. The scaffold Gla-domain may participate in complex formation mediating Ixolaris/FXa interaction with FVIIa/TF,37-40 and with the membrane.50
Working hypothesis for the mechanism of action of Ixolaris.
Initially, the (second) Kunitz domain of Ixolaris binds to exosite of FXa,45-47 or to an FX putative “pro-exosite” (not shown), leading to the formation of a stable Ixolaris/FXa complex. Then, the scaffold using presumably a similar docking mechanism as the natural substrate, FX (not shown)37 or the product FXa,38 interacts with the exosite formed by FVIIa and TF.39,40 This interaction allows the (first) Kunitz domain of Ixolaris/FXa to dock into the active site of FVIIa/TF, with subsequent assembling of a tightly bound quaternary inhibitory complex composed of FVIIa/TF/Ixolaris/FXa. The scaffold Gla-domain may participate in complex formation mediating Ixolaris/FXa interaction with FVIIa/TF,37-40 and with the membrane.50
Discussion
The recombinant tick salivary protein, called Ixolaris in this paper, potently inhibits FVIIa/TF-induced FX activation with an IC50 in the picomolar range. Ixolaris is functionally and structurally distinct from its endogenous counterpart, human TFPI1; although the 6 cysteines that characterize the first Kunitz domain of TFPI1 are conserved in Ixolaris, only 4 of 6 cysteines present in the second Kunitz domain of human TFPI are present.6,7 Also, whereas the sixth and the first cysteines that, respectively, terminate and initiate the first and second Kunitz domains in human TFPI are separated by 20 amino acids,6,7 only 7 amino acids separate the corresponding cysteines in Ixolaris. Additionally, the Kunitz-type domain 2 in Ixolaris is unusual by containing 4 additional amino acids between the fourth and fifth cysteine residues, making this loop longer than most Kunitz-type family members. Also, the presumed P1reactive-site residue of the first domain in Ixolaris is Glu, whereas Lys occupies this position in TFPI.6,7 Finally, Ixolaris has a short and basic carboxy terminus but, unlike TFPI, it has only 14 amino acids where the positively charged amino acids are not organized as a cluster. In human TFPI, this basic carboxy terminus has been consistently shown to increase its anticoagulant activity41and to shorten its half-life.42 43 The Ixolaris cDNA also encodes 3 putative N-linked glycosylation sites, at Asn65, Asn98, and Asn136. Consistent with a calculated mass of 15.7 kd for the carbohydrate-free protein, we could detect a band of about 15.5 kd in the gels loaded with recombinant Ixolaris; however, an intense smear was observed in PAGE of Ixolaris at a molecular weight range of about 24 kd. Accordingly, it is likely that these Asn residues are indeed glycosylated, and this is the most abundant form (> 95%) of the secreted recombinant molecule. It remains to be determined whether both forms of Ixolaris are equally effective as anticoagulants.
Ixolaris and TFPI are unrelated in many functional aspects. Ixolaris is a highly specific inhibitor that, unlike human TFPI, does not inhibit trypsin or chymotrypsin.44 Ixolaris immediately increases the amidolytic activity of FXa toward chromogenic substrates (Figure 5A); therefore, it behaves as a fast ligand of FXa, in contrast to TFPI, a typical FXa slow-binding inhibitor.8 Additionally, Ixolaris binds to des-Gla-FXa and to a tripeptidyl chloromethylketone covalently occupied catalytic site of FXa (DEGR-FXa), 2 properties not shared by TFPI.2,10 This indicates that Ixolaris binds at a site distinct from the active center of FXa, or exosite,45-47and implies that γ-carboxyglutamic acid residues are not involved in the interaction between enzyme and inhibitor. Remarkably, Ixolaris also binds to FX in addition to FXa, presumably through its second Kunitz domain that is atypical in the sense that it contains only 4 cysteines. Such a pattern of cysteines may confer more flexibility to this domain,48 and may have evolved to interact with FXa exosite,45-47 or with FX putative “pro-exosite.”49 Accordingly, although relative affinities of Ixolaris for FX, Fxa, and des-Gla-FXa are similar (Figure5D, inset), it is unknown whether Ixolaris binding sites in the zymogen or enzyme are identical. Concerning the kinetics of the interaction, competition experiments indicate that Ixolaris is a fast-ligand for both FXa and des-Gla-FXa, but a slow-ligand for FX (Figure 5D). It is conceivable to suggest that the structural features of FX require an induced fit during the association phase with Ixolaris. Of interest, it has been recently shown that NAPc2, a TFPI-like molecule from A caninum also binds to FX, with an association kinetics 5 to 10 lower rates than those measured with FXa.32
It has been shown that TFPI at high concentrations (μM range) inhibits FVIIa/TF amidolytic activity, inhibition being remarkable in the presence of FXa.1,2 Figure 6A shows that Ixolaris at high concentrations also blocks the amidolytic activity of FVIIa/TF. This indicates that the inhibitor interacts directly with the active site of the enzyme or sterically prevents access of the substrate to the active site. As described for TFPI,6,7 we suggest that Ixolaris interaction with FVIIa/TF occurs via its first Kunitz domain that is typical11 for catalytic site recognition. Because this effect occurs at [I] >>> [E], Ixolaris does not behave as a tight inhibitor of FVIIa/TF, a contention that is in accordance with no complex formation being detected between Ixolaris and FVIIa in native gel experiments (Figure 4). Remarkably, in the presence of Ixolaris/DEGR-FXa, a stoichiometric blockade of FVIIa/TF amidolytic activity was achieved at [I] ∼ [E] (Figure6). It is also clear from the FIX activation assays that both FX zymogen (FX) and enzyme (FXa) operate as efficient scaffolds for the inhibitor, with formation of a tight complex composed by FVIIa/TF/Ixolaris/FX(a). The finding that Ixolaris binds to the zymogen, and this complex inhibits FVIIa/TF, is one of the most remarkable observations of this paper and it clearly distinguishes this inhibitor from TFPI.1 2 This inhibitory strategy seems to be especially effective because Ixolaris/FX may inhibit FVIIa/TF in vivo before and independently of FXa production.
Although the true Ki of Ixolaris/scaffolds for FVIIa/TF and the type of inhibition (competitive versus noncompetitive) remain to be determined, relative affinities are comparable as determined in Figure7. On the other hand, des-Gla-DEGR-FXa was shown to be a completely ineffective scaffold in both amidolytic (not shown) and FIX activation assays (Figure 7). This indicates that the anionic Gla domain of FX(a), known to play a crucial role in the interaction of FXa with procoagulant membrane surface50 and TF,39is directly involved in Ixolaris/FX(a) interactions with FVIIa/TF complex.
To study the kinetics of Ixolaris/FXa interaction with FVIIa/TF, 2 independent and complementary approaches have been used. Accordingly, when FVIIa/TF was used to initiate reactions containing Ixolaris/FXa and small chromogenic substrate, a typical slow-type inhibition was attained (Figure 8A). It is likely that the interaction of the (first) Kunitz domain of Ixolaris/FXa with FVIIa/TF requires an induced fit to dock productively to the enzyme catalytic site. However, and notably, production of FXa was immediately blocked when FVIIa/TF was added to a mixture containing Ixolaris/FX and FX (Figure 3B), characterizing a fast-type inhibition. On the other hand, TFPI was shown in the experiments described here (Figure 3B, inset) and elsewhere,9 to behave as a slow-type inhibitor in similar experimental conditions. Of interest, our results have also demonstrated that FIXa generation by FVIIa/TF was completely prevented when enzyme/cofactor was added to a mixture containing Ixolaris/FX(a) and FIX (Figure 8B,C). Although FIX activation assays do not allow us to estimate FVIIa/TF inhibition by Ixolaris/scaffold in the first seconds of the assay, these results suggest that enzyme activity was blocked very shortly after the initiation of the reactions. Accordingly, it is plausible to suggest that interactions of Ixolaris/FX(a) with FVIIa/TF can be better described as fast when macromolecular (FX or FIX), instead of small chromogenic substrates (S2288), are appropriately used to determine the kinetics of the interaction involving enzyme and inhibitor. Presumably, this step is mediated by the exosite formed by FVIIa/TF39 and the exosite recognition domains from both FX and FXa37,38; this interaction is strengthened by the docking of Ixolaris to the catalytic site of FVIIa/TF (Figure 8A), as described for bifunctional protein.51,52 Of interest, it has been recently proposed that the exosite formed by FVIIa/TF37-39 plays a predominant role in determining the affinity and kinetics of the interaction with FX40 (Km ∼200 nM).24,40 Because the affinity of Ixolaris/FX for FVIIa/TF is significantly higher (pM range), it should operate as an effective anticoagulant in vivo even if only a fraction of FX has bound Ixolaris. In fact, due to the high affinity interaction, inhibitor may form a stable complex with the zymogen in the blood; this inhibitory complex is effective under conditions such as coagulation initiated by HUVEC-expressing TF (Figure 9). Similar conclusions have been obtained for NAPc2 that differ from Ixolaris, however, in a number of structural and kinetic aspects.32,53 In fact, NAPc2 is an 84-amino acid non-Kunitz molecule with an FXa cleavage site32 that is absent in Ixolaris. Finally, novel molecules that block initiation of blood coagulation, such as Ixolaris, may be useful as inhibitors to prevent or ameliorate a number of pathologic conditions leading to cardiovascular diseases provoked or amplified by abnormal expression of TFPI.54-62
We are grateful to Drs Robert W. Gwadz, Thomas J. Kindt, and Louis H. Miller for encouragement and support. The authors thank Brenda Rae Marshall for editorial support.
Prepublished online as Blood First Edition paper, April 17, 2002; DOI 10.1182/blood-2001-12-0237.
This work was presented at the 42nd Annual Meeting of the American Society of Hematology, December 1-5, 2000, San Francisco, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
José M. C. Ribeiro, SME, LPD, NIAID, NIH, 4 Center Dr, Rm 4/126, MSC 0425, Bethesda, MD 20892-0425; e-mail:jribeiro@nih.gov.










This feature is available to Subscribers Only
Sign In or Create an Account Close Modal