While the t(8;21) translocation is one of the most recurrent chromosomal abnormalities in acute myeloid leukemia, prognostic studies have been hampered by the relatively few number of patients reported. We thus performed a large retrospective study in 161 adults and children with t(8;21) acute myeloid leukemia, all prospectively enrolled in 6 different trials conducted in France between 1987 and 1998 (median follow-up 4.9 years). Prognostic studies were performed in the 154 patients who achieved a complete remission. Individual data were registered, including sex, age, blood and marrow counts, extramedullary disease, and cytogenetics. The value of allogeneic stem cell transplantation versus chemotherapy as postremission therapy was evaluated according to the intent-to-treat principle. Estimated 5-year disease-free survival (DFS) and overall survival were 52% and 59%, respectively. Outcome was not significantly better in patients from the stem cell transplantation group (estimated 5-year DFS and survival, 56% vs 52% and 67% vs 57%; P = .55 and .64, respectively). White blood cell count (WBC) was the only identified prognostic factor. To further take into account the spontaneous differentiation potential of the leukemic clone, a WBC index was derived as the product of WBC by the ratio of marrow blast. This WBC index was a more powerful factor than the original WBC, allowing us to distinguish 3 subgroups of patients with different outcomes (low index, < 2.5; intermediate index, 2.5-20; high index, 20 or more). In multivariate analysis, the WBC index was the only prognostic factor for DFS (P = .003), complete remission duration (P = .002), and overall survival (P = .04).
Introduction
The t(8;21)(q22;q22) translocation is one of the most frequent balanced chromosomal translocations found in acute myeloid leukemia (AML), occurring in approximately 8% of patients with de novo AML1,2 while also found in patients with therapy-related AML.3 This translocation fuses theAML1 (CBFα2) gene located on chromosome 21 to theETO (MTG8) gene located on chromosome 8, resulting on a chimeric protein product. Fusion AML1-ETO transcripts may be evidenced by a specific reverse transcriptase–polymerase chain reaction assay, which provides a useful estimate of minimal residual disease during the course of the disease.4 The fusionAML1-ETO protein acts as a dominant repressor altering the physiologic role of the normal AML1-CBFβ core binding factor (CBF), which is a transcriptional complex involved in hematopoietic stem cell differentiation.5 6
AML carrying the t(8;21) translocation exhibits some specific characteristics. This AML subset is morphologically associated with the French-American-British AML-M2 subtype,7 with a potential spontaneous differentiation of the leukemic clone resulting in various degrees of abnormal granulocytic maturation with myeloid progenitors containing Auer rods, sometimes associated with notable marrow eosinophilia or mastocytosis.1,8,9 Cases with a higher degree of granulocytic maturation are characterized by a lower percentage of blast cells in the bone marrow, leading to classifying them as smoldering AML or even refractory anemia with excess of blast in transformation.10 It has recently been suggested that the t(8;21) rearrangement occurs at the level of an immature multipotent hematopoietic progenitor capable of differentiating into all myeloid lineages, including erythroid and megakaryocyte lineages, as well as into B cells.11 Additional oncogenic events leading to the disease development are thus very likely, even if not identified to date. Cytogenetically, the specific translocation may be associated with frequent losses of a sexual chromosome (LOS) or deletions of the long arm of the chromosome 9 (del(9q)).7Clinically, t(8;21) AML has been associated with the frequent development of extramedullary disease (granulocytic sarcomas)12 and with a high rate of complete remission (CR) and favorable outcome as compared with other AML subsets, at least in adult series.2 13
Not all patients with the disease, however, have such a good outcome. First, it has been observed that long-term disease-free survival (DFS) did not appear to be significantly increased in children with t(8;21) AML as compared with other AML subtypes.14 Secondly, a worse outcome has been reported in patients with a high initial white blood cell count (WBC) or absolute granulocyte count (AGC)15,16 as well as in patients with a phenotypical expression of the CD56 antigen,17,18 additional del(9q),19 or extramedullary disease at diagnosis.20 However, most of these bad-prognosis factors have not been strongly established to date. On the other hand, high rates of prolonged CR have been reported, especially by the Cancer and Leukemia Group B when using repeated cycles of high-dose cytarabine (HDAC) as postremission therapy,21 22 raising the question of allogeneic stem cell transplantation (SCT) value in first CR in these patients.
The aim of the present survey was (1) to investigate the prognostic factors for DFS and overall survival in a large population of patients with this disease after CR achievement, with a particular attention to WBC level and to spontaneous differentiation of the leukemic clone; and (2) to compare the results of allogeneic SCT in first CR with those of chemotherapy alone. Data from all patients with t(8;21) AML prospectively enrolled in 6 recent French multicenter AML trials between 1987 and 1998 were retrospectively reviewed. We found that the main and sole prognostic factor for outcome in multivariate analyses was a so-called “WBC index,” derived from the original WBC by adjustment for the spontaneous differentiation potential. We also report similar outcomes in patients treated with allogeneic SCT or chemotherapy alone on the intent-to-treat basis.
Patients and methods
Patient selection and review of data
All patients have been prospectively enrolled in 1 of the 6 following French multicenter trials between May 1987 and August 1998: LAME-91,23 ALFA-9000,24BGMT-87,25 BGMT-91,26GOELAM-01,27 and GOELAM-02.28 The LAME-91 study was a pediatric AML study, while the 5 other studies were adult AML studies. Between May 1987 and August 1998, 161 patients diagnosed with AML carrying the t(8;21) chromosomal translocation on standard karyotypic analysis at baseline entered 1 of these 6 trials. Molecular criteria including fluorescent in situ hybridization and reverse transcriptase–polymerase chain reaction were not considered for identification of these patients. A total of 154 (96%) of them achieving CR after the induction course have been retrospectively included in the present study. Given this very high CR rate (as usually reported in patients with this disease), we chose to not include the 7 failed patients in the prognostic study. Actually, 4 of them died early from infections during induction and only 3 had resistant disease. In these latter 3 patients, WBC and percentage of marrow blasts were 24.7, 6.6, 36.9 G/L (109/L) and 43%, 70%, and 63%, respectively.
A predefined set of data including demography, hematologic presentation, cytogenetics, postremission therapy, and outcome was collected for each patient, sent to a central coordinating center, and reviewed for consistency and completeness before analysis. Immunophenotyping of the leukemic cells was not retained due to the lack of consistent data in many cases. Follow-up observations extended through 1996 to 2000, depending of the trial, with a median follow-up of 6.5, 6.2, 10.9, 6.2, 3.5, and 3.3 years for the LAME-91, ALFA-9000, BGMT-87, BGMT-91, GOELAM-01, and GOELAM-02 study, respectively. Overall, the median follow-up was 4.9 years. For statistical analysis, outcome data were censored at 3.3 years, corresponding to the shorter median follow-up of the GEOLAM-02 study. Actually, only 10 relapses and 3 deaths were observed after this time. The present study was approved by the IRB, Hôpital Saint-Louis, Paris, France.
Induction therapy
Induction therapy varied among the 6 prospective trials considered. In the pediatric LAME-91 trial, all included patients received the same induction regimen comprising 12 mg/m2/d mitoxantrone for 5 days and 200 mg/m2/d cytarabine as continuous infusion for 7 days. In the adult ALFA-9000 trial, patients were randomized at inclusion to receive 1 of the 3 following reinforced induction regimens: Arm 1 consisted of 80 mg/m2/d daunorubicin for 3 days and 200 mg/m2/d cytarabine as continuous infusion for 7 days; arm 2 consisted of the same regimen followed at day 20 by a second induction course comprising 12 mg/m2/d mitoxantrone for 2 days and 500 mg/m2/12 h cytarabine as a 3-hour intravenous bolus infusion for 3 days; and arm 3 consisted of 80 mg/m2/d daunorubicin for 3 days and 500 mg/m2/d cytarabine as continuous infusion for 3 days, followed at day 8 by 12 mg/m2/d mitoxantrone for 2 days and 500 mg/m2/12 h cytarabine as a 3-hour intravenous bolus infusion for 3 days. In the adult BGMT-87 and BGMT-91 trials, all included patients received the same induction regimen comprising 60 mg/m2/d daunorubicin for 3 days and 100 mg/m2/d cytarabine as continuous infusion for 10 days. In the adult GOELAM-01 and GOELAM-02 trials, patients were randomized at inclusion to receive either 8 mg/m2/d idarubicin for 5 days or 200 mg/m2/d rubidazone for 4 days in combination with standard 200 mg/m2/d cytarabine as continuous infusion for 7 days. Standard National Cancer Institute CR criteria were similarly used in all these trials.29
Postremission therapy
The impact of postremission therapy on outcome was analyzed using the intent-to-treat principle (treatment allocated at CR achievement). Postremission therapy varied among the 6 prospective studies considered (Table 1). Differences concerned mainly the place of allogeneic SCT in first CR, the number of intensive postremission cycles of chemotherapy, the dosage of cytarabine within these intensive cycles, and the use of autologous SCT. The following definitions were used to classify postremission therapy: (1) Postremission cycles of chemotherapy were qualified as intensive if responsible for a median duration of induced neutropenia (number of days with a less than 0.5 G/L (109/L) absolute neutrophil count) of 2 weeks or more; and (2) HDAC was defined as cytarabine administered by 2.0 g/m2 or more bolus intravenous infusions for a total dose of 16 g/m2 or more, while intermediate-dose cytarabine (IDAC) was defined as cytarabine administered either by bolus or continuous intravenous infusions for a total dose of between 1.5 and 16 g/m2. Because of strong interactions related to study designs, treatment modalities mentioned above cannot be considered as independent variables. On one hand, few patients (n = 3) were allocated to receive 1 single intensive postremission cycle but containing HDAC. On the other hand, all patients allocated to receive an autologous SCT had previously received 1 HDAC cycle.
Design of postremission therapy
| LAME-9123 | |
| Intensive consolidation 1 | DNR, 40 mg/m2/d for 4 d |
| VP16, 100 mg/m2/d for 4 d | |
| AraC, 100 mg/m2/d for 4 d | |
| Allogeneic SCT after consolidation 1 | |
| Intensive consolidation 2 | Amsa, 150 mg/m2/d for 3 d |
| IDAC, 1000 mg/m2/12 h for 4 d | |
| L-ASPA, 6000 IU/m2/d for 2 d | |
| Maintenance or not* | |
| ALFA-900024 | |
| Mild consolidation 1 | Amsa, 90 mg/m2/d for 1 d |
| AraC, 120 mg/m2/d for 5 d | |
| No allogeneic SCT in first CR | |
| Intensive consolidation 2 | MTZ, 12 mg/m2/d for 3 d |
| VP16, 200 mg/m2/d for 3 d | |
| IDAC, 500 mg/m2/d for 6 d | |
| No maintenance | |
| BGMT-8725 and BGMT-9126 | |
| Mild consolidation 1 | DNR, 60 mg/m2/d for 2 d |
| AraC, 100 mg/m2/d for 7 d | |
| Allogeneic SCT after consolidation 1 | |
| Intensive consolidation 2 | DNR, 45 mg/m2/d for 3 d |
| HDAC, 3000 mg/m2/12 h for 4 d | |
| Autologous SCT or maintenance† | |
| GEOLAM-0127 and GEOLAM-0228 | |
| Allogeneic SCT before consolidation 1 | |
| Intensive consolidation 1 | IDA, 10 mg/m2/d for 2 d |
| or RBZ, 200 mg/m2/d for 2 d | |
| HDAC, 3000 mg/m2/12 h for 4 d | |
| Autologous SCT‡ or intensive consolidation 2 | Amsa, 150 mg/m2/d for 5 d |
| VP16, 100 mg/m2/d for 5 d | |
| No maintenance |
| LAME-9123 | |
| Intensive consolidation 1 | DNR, 40 mg/m2/d for 4 d |
| VP16, 100 mg/m2/d for 4 d | |
| AraC, 100 mg/m2/d for 4 d | |
| Allogeneic SCT after consolidation 1 | |
| Intensive consolidation 2 | Amsa, 150 mg/m2/d for 3 d |
| IDAC, 1000 mg/m2/12 h for 4 d | |
| L-ASPA, 6000 IU/m2/d for 2 d | |
| Maintenance or not* | |
| ALFA-900024 | |
| Mild consolidation 1 | Amsa, 90 mg/m2/d for 1 d |
| AraC, 120 mg/m2/d for 5 d | |
| No allogeneic SCT in first CR | |
| Intensive consolidation 2 | MTZ, 12 mg/m2/d for 3 d |
| VP16, 200 mg/m2/d for 3 d | |
| IDAC, 500 mg/m2/d for 6 d | |
| No maintenance | |
| BGMT-8725 and BGMT-9126 | |
| Mild consolidation 1 | DNR, 60 mg/m2/d for 2 d |
| AraC, 100 mg/m2/d for 7 d | |
| Allogeneic SCT after consolidation 1 | |
| Intensive consolidation 2 | DNR, 45 mg/m2/d for 3 d |
| HDAC, 3000 mg/m2/12 h for 4 d | |
| Autologous SCT or maintenance† | |
| GEOLAM-0127 and GEOLAM-0228 | |
| Allogeneic SCT before consolidation 1 | |
| Intensive consolidation 1 | IDA, 10 mg/m2/d for 2 d |
| or RBZ, 200 mg/m2/d for 2 d | |
| HDAC, 3000 mg/m2/12 h for 4 d | |
| Autologous SCT‡ or intensive consolidation 2 | Amsa, 150 mg/m2/d for 5 d |
| VP16, 100 mg/m2/d for 5 d | |
| No maintenance |
DNR indicates daunorubicin; VP16, etoposide; AraC, cytarabine; Amsa, amsacrine; L-ASPA, L-asparaginase; MTZ, mitoxantrone; IDA, idarubicin; and RBZ, rubidazone.
Randomization between maintenance therapy or not.
Randomization between autologous SCT or maintenance therapy in the BGMT-87 trial; autologous SCT for all patients in the BGMT-91 trial.
Randomization between autologous SCT and intensive consolidation 2 in the GOELAM-01 trial; intensive consolidation 2 for all patients in the GOELAM-02 trial.
Consequently, postremission therapy was first classified as follows: (1) allogeneic SCT (patients with an HLA-identical sibling enrolled in a study including allogeneic SCT in first CR) (ALLO; n = 37 patients); (2) 1 HDAC cycle followed by autologous SCT (HDAC-auto; n = 21 patients); (3) 2 intensive postremission cycles including 1 HDAC cycle (HDAC-2; n = 33 patients); (4) 2 intensive postremission cycles including 1 IDAC cycle but no HDAC (IDAC-2; n = 29 patients); and (5) 1 intensive IDAC cycle only (IDAC-1; n = 31 patients). The 3 patients treated with 1 HDAC cycle only were not included in this classification and thus not considered for the prognostic study. Because patients belonging to the subgroups HDAC-auto and HDAC-2 (all from the 2 GOELAM studies) have similar outcome (data not shown), the final classification retained to analyze the impact of postremission therapy was the following 4-group classification: (1) ALLO (n = 37 patients); (2) HDAC-2 (n = 54 patients including the 21 HDAC-auto patients); (3) IDAC-2 (n = 29 patients); and (4) IDAC-1 (n = 31 patients). In addition, the 37 patients from the ALLO group were also compared with the 114 patients from a CHEMO group including all patients from the HDAC-2, IDAC-2, and IDAC-1 groups.
Statistical methods
The duration of CR was calculated from the date of first CR until the date of first relapse. DFS was calculated from the date of first CR until the date of first relapse or the date of death in first CR. The Fisher exact test was used for binary variable comparison. The Mann-Whitney test was used for continuous variable comparison. Data on treatment failure were estimated by the Kaplan-Meier method30 and compared using the log-rank test.31 In multivariate analyses, outcome comparisons were adjusted with the Cox model32 and tested by the likelihood ratio test. P < .05 was considered to indicate statistical significance. All calculations were performed using the STATA software, version 7.0 (Stata, College Station, TX).
Results
Patient population
Outcome and prognostic factors for DFS, CR duration, and overall survival were evaluated in 154 (96%) of 161 patients with t(8;21) AML who achieved CR after induction therapy. Baseline characteristics of these 154 CR patients are indicated in Table2. The median age was relatively low (28 years), with 43 patients (28%) aged 15 years or less. A few patients had evidence of extramedullary disease, including granulocytic sarcoma and central nervous system involvement at diagnosis. Diagnostic procedures did not include, however, systematic computed tomography scans or systematic cerebrospinal fluid examination in all patients. Cytogenetic analysis showed obviously the t(8;21) translocation in all patients (inclusion criteria). Thirty-six percent had persistent normal metaphases. An associated LOS was observed in 54% of males and 29% of females (P = .004 by the Fisher exact test). A del(9q) was another associated recurrent abnormality, even if more rarely observed. Variant t(8;21) translocations were observed in 3 patients. Additional complex abnormalities were observed in 3 patients. Median WBC was 12 × 109/L, with only 10% of patients with a WBC of 41 × 109/L or more and 1% of patients with a WBC of 105 × 109/L or more at diagnosis. Partly because of the well-known partial spontaneous differentiation of the leukemic clone usually observed in some patients with the disease, many patients had a relatively low marrow blast percentage at diagnosis (25% of patients with 43% or less marrow blasts). Interestingly, at diagnosis, younger patients had a significantly higher WBC and higher marrow blast percentage than adults. The median WBC was 20 × 109/L in the 43 patients aged 15 years or less compared with 10 × 109/L in the 111 patients aged more than 15 years (P = .001 by the Mann-Whitney test). The median marrow blast percentage was 68% in the former group compared with 58% in the latter one (P = .01 by the Mann-Whitney test). Similar results were observed with an age cutoff at 25 years (P = .04 and .04 for WBC and marrow blasts percentage, respectively).
Patient characteristics
| No. of patients | 154 |
| Sex ratio, M/F | 97/57 |
| Median age, y (range) | 28 (3-63) |
| Study, no. of patients | 154 |
| LAME-91 | 45 |
| ALFA-9000 | 29 |
| BGMT-87 | 8 |
| BGMT-91 | 16 |
| GOELAM-01 | 29 |
| GOELAM-02 | 27 |
| Extramedullary disease, no. of patients | |
| Granulocytic sarcoma | 8 |
| Central nervous system involvement | 1 |
| Both | 3 |
| WBC, no. of patients | 154 |
| Median WBC (range) | 12 G/L (1.4-168) |
| 75th percentile upper limit | 24 G/L |
| 90th percentile upper limit | 41 G/L |
| 95th percentile upper limit | 67 G/L |
| 99th percentile upper limit | 105 G/L |
| Marrow blast percentage, no. of patients | 150 |
| Median marrow blast percentage, range | 60% (7%-100%) |
| 1st percentile lower limit | 20% |
| 5th percentile lower limit | 28% |
| 10th percentile lower limit | 31% |
| 25th percentile lower limit | 43% |
| Cytogenetics, no. of patients | 154 |
| AN/AA/information not available | 53/95/6 |
| Associated LOS | 67 |
| Associated del(9q) | 13 |
| Associated complex abnormalities | 3 |
| No. of patients | 154 |
| Sex ratio, M/F | 97/57 |
| Median age, y (range) | 28 (3-63) |
| Study, no. of patients | 154 |
| LAME-91 | 45 |
| ALFA-9000 | 29 |
| BGMT-87 | 8 |
| BGMT-91 | 16 |
| GOELAM-01 | 29 |
| GOELAM-02 | 27 |
| Extramedullary disease, no. of patients | |
| Granulocytic sarcoma | 8 |
| Central nervous system involvement | 1 |
| Both | 3 |
| WBC, no. of patients | 154 |
| Median WBC (range) | 12 G/L (1.4-168) |
| 75th percentile upper limit | 24 G/L |
| 90th percentile upper limit | 41 G/L |
| 95th percentile upper limit | 67 G/L |
| 99th percentile upper limit | 105 G/L |
| Marrow blast percentage, no. of patients | 150 |
| Median marrow blast percentage, range | 60% (7%-100%) |
| 1st percentile lower limit | 20% |
| 5th percentile lower limit | 28% |
| 10th percentile lower limit | 31% |
| 25th percentile lower limit | 43% |
| Cytogenetics, no. of patients | 154 |
| AN/AA/information not available | 53/95/6 |
| Associated LOS | 67 |
| Associated del(9q) | 13 |
| Associated complex abnormalities | 3 |
AN indicates simultaneous presence of normal and abnormal metaphases; AA, presence of abnormal metaphases only; G/L = 109/L.
Overall, the estimated 5-year DFS was 52% (95% confidence interval [CI], 44%-60%), and the estimated 5-year overall survival was 59% (95% CI, 50%-67%).
Prognostic factors
Demography.
In univariate analysis, age had no prognostic value for DFS when considered as a continuous variable (P = .31 using the univariable Cox model). DFS was similar in patients aged 15 years or less when compared with those aged more than 15 years (P = .78 by the log-rank test) as well as in those aged 25 years or less when compared with those aged more than 25 years (P = .44 by the log-rank test). Similar results were obtained when testing age as prognostic factor for CR duration (P = .26 and .18 for 15-year and 25-year age cutoffs, respectively, by the log-rank test) and overall survival (P = .13 and .14 for 15-year and 25-year age cutoffs, respectively, by the log-rank test). Gender had no prognostic value either for DFS or overall survival. The presence of extramedullary disease also was of no prognostic significance.
Marrow blast percentage, cytogenetics, and maturation index.
In univariate analysis, a trend for a longer DFS was observed in patients with a low marrow blast percentage (P = .09 using the univariable Cox model). A trend for a better outcome was also observed in the 53 AN patients (with persistent normal metaphases) as compared with the 95 AA patients (with 100% abnormal metaphases) (P = .08 and .08 for DFS and overall survival, respectively, by the log-rank test). In addition, a trend for lower marrow blast percentages was observed in the AN patients as compared with the AA patients (P = .11 by the Mann-Whitney test). Of note, the ratio of abnormal versus normal metaphases was very high in the 53 AN patients (median 0.88; range 0.31-0.97). As mentioned above, a low marrow blast percentage may be related to the partial differentiation of the leukemic clone but might also simply be related to early AML with partial marrow replacement. We thus derived a “maturation index” as the percentage of marrow blasts divided by the ratio of abnormal metaphases (maturation index = % of marrow blasts/[% of abnormal metaphases/100]). In univariate analysis, this maturation index significantly influenced DFS and CR duration when considered as a continuous variable (P = .03 and .02 for DFS and CR duration, respectively, using the univariable Cox model). Additional cytogenetic abnormalities had no influence on patient outcome either for DFS, CR duration, or overall survival. Outcome was similar in patients with or without associated LOS or del(9q).
White blood cell count and WBC index.
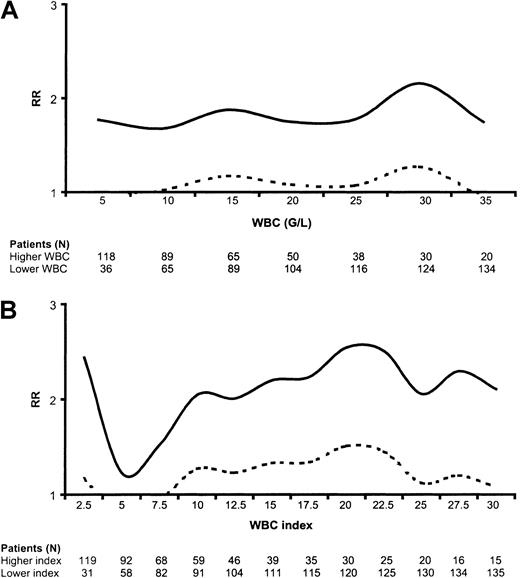
In univariate analysis, WBC had no prognostic impact on DFS when considered as a continuous variable (P = .14 using the univariable Cox model). We thus studied the prognostic impact of high WBC when using various WBC cutoffs. Figure1A indicates the variation of the relative risk in the higher-count as compared with the lower-count population as a function of the WBC cutoff values. We found that DFS was significantly shortened in high-count patients when using WBC cutoffs between 10 and 30 G/L (109/L) (Figure 1A). Similar significant results were observed for CR duration (not shown), while only nonsignificant trends were observed for overall survival at these various WBC cutoffs (not shown). Interestingly, we did not find any positive correlation between WBC and marrow blast percentage in a linear regression model.
Relative risk (RR) for disease-free survival.
(A) Risk according to WBC. Figure 1A indicates the variation of the relative risk in the higher WBC as compared to the lower WBC population as a function of the WBC cut-off values. (B) Risk according to WBC index. Figure 1B indicates the variation of the relative risk in the higher WBC index as compared to the lower WBC index population as a function of the WBC index cut-off values. Dashed curves represent the lower limit of the 95% confidence interval. A dashed curve upon the WBC or WBC index axis indicates thus the statistical significance of the cut-off (significantly worsened DFS in patients with higher WBC or WBC index); G/L = 109/L.
Relative risk (RR) for disease-free survival.
(A) Risk according to WBC. Figure 1A indicates the variation of the relative risk in the higher WBC as compared to the lower WBC population as a function of the WBC cut-off values. (B) Risk according to WBC index. Figure 1B indicates the variation of the relative risk in the higher WBC index as compared to the lower WBC index population as a function of the WBC index cut-off values. Dashed curves represent the lower limit of the 95% confidence interval. A dashed curve upon the WBC or WBC index axis indicates thus the statistical significance of the cut-off (significantly worsened DFS in patients with higher WBC or WBC index); G/L = 109/L.
To adjust the WBC on the differentiating capacity of the leukemic clone, we calculated a WBC index as the product of WBC by the ratio of marrow blasts (WBC index = WBC × [% of marrow blasts/100]). For instance, a patient with a WBC of 15 × 109/L and 100% marrow blasts had a WBC index of 15, while another patient with a WBC of 15 × 109/L and 50% marrow blasts had a WBC index of 7.5. This WBC index was available for 150 of the 154 patients. As observed for the WBC, the WBC index was significantly higher in younger than in older patients. The median WBC index was 13 in the 42 patients aged 15 years or less compared with 5.7 in the 108 patients aged more than 15 years (P = .0005 by the Mann-Whitney test). A similar result was observed with an age cutoff at 25 years (P = .02). As represented in Figure 1B, the WBC index had a more potent prognostic impact on DFS than the original nonadjusted WBC. DFS was significantly shortened in higher WBC index as compared with lower WBC index patients when using WBC index cutoffs between 10 and 27.5 (Figure 1B). The most efficient WBC index cutoff appeared to be 20. Interestingly, the WBC index also allowed identification of a subgroup of good-risk patients with a low WBC index below 2.5 (Figure 1B).
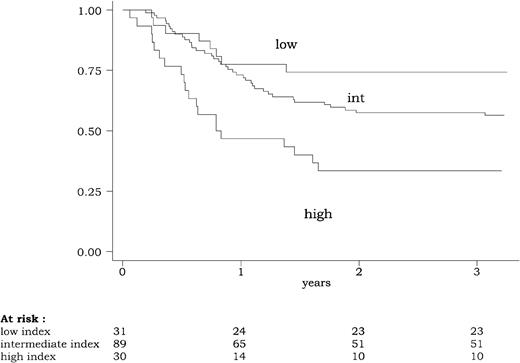
Using this 3-group classification (low WBC index, < 2.5; intermediate WBC index, 2.5-20; high WBC index, 20 or more), the WBC index had a strong prognostic impact on DFS, CR duration, and overall survival. DFS according to this powerful 3-group prognostic classification is represented in Figure2. Similar results were observed for CR duration and overall survival. At 3 years, estimated DFS was 74% (95% CI, 55%-86%) in patients with a low WBC index, 57% (95% CI, 46%-67%) in patients with an intermediate WBC index, and 33% (95% CI, 18%-50%) in patients with a high WBC index (P = .0015 by the log-rank test). At 3 years, estimated relapse rate was 20% (95% CI, 10%-40%) in patients with a low WBC index, 35% (95% CI, 25%-46%) in patients with an intermediate WBC index, and 61% (95% CI, 43%-79%) in patients with a high WBC index (P = .002 by the log-rank test). At 3 years, estimated overall survival was 74% (95% CI, 55%-86%) in patients with a low WBC index, 66% (95% CI, 55%-75%) in patients with an intermediate WBC index, and 47% (95% CI, 28%-63%) in patients with a high WBC index (P = .04 by the log-rank test).
Disease-free survival (DFS) according to the WBC index.
The WBC index, calculated as the product of WBC by the ratio of marrow blasts at diagnosis, was available for 150 of the 154 patients reaching a complete remission. DFS differed significantly among the three WBC index subgroups (low WBC index < 2.5; intermediate WBC index between 2.5 and 20; high WBC index of 20 or more) (P = .0015, by the log-rank test).
Disease-free survival (DFS) according to the WBC index.
The WBC index, calculated as the product of WBC by the ratio of marrow blasts at diagnosis, was available for 150 of the 154 patients reaching a complete remission. DFS differed significantly among the three WBC index subgroups (low WBC index < 2.5; intermediate WBC index between 2.5 and 20; high WBC index of 20 or more) (P = .0015, by the log-rank test).
Very similar results were obtained with a more sophisticated adjustment of the original WBC using the product of WBC by the maturation index (as defined above) and not simply by the ratio of marrow blasts (not shown). For practical reasons, we chose to further use the WBC index, which can be easily calculated in the clinical setting.
Postremission therapy.
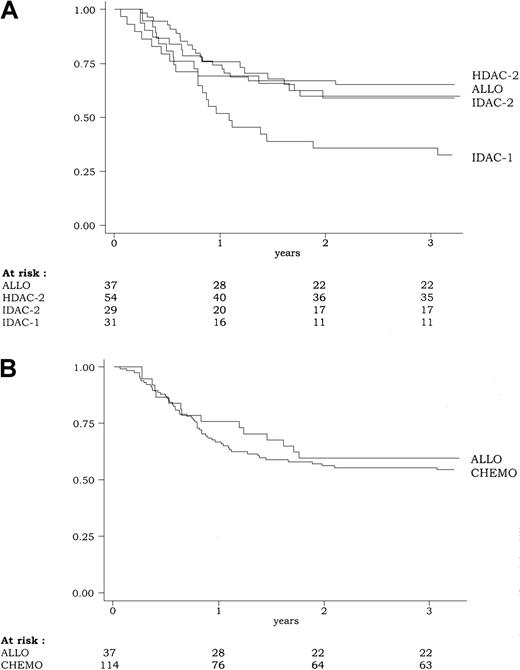
DFS according to the 4-group intent-to-treat classification of the postremission therapy (ALLO, HDAC-2, IDAC-2, IDAC-1) is shown in Figure3. In univariate analysis, DFS was significantly influenced by the postremission therapy (P = .03 by the log-rank test). At 3 years, estimated DFS was 59% (95% CI, 42%-73%), 65% (95% CI, 51%-76%), 59% (95% CI, 39%-74%), and 35% (95% CI, 19%-52%) in the ALLO, HDAC-2, IDAC-2, and IDAC-1 groups, respectively. Similar results were observed for the duration of CR and overall survival. At 3 years, estimated duration of CR was 75% (95% CI, 56%-89%), 67% (95% CI, 53%-78%), 71% (95% CI, 48%-85%), and 38% (95% CI, 21%-55%) in the ALLO, HDAC-2, IDAC-2, and IDAC-1 groups, respectively (P = .002 by the log-rank test). At 3 years, estimated overall survival was 68% (95% CI, 50%-80%), 72% (95% CI, 58%-82%), 72% (95% CI, 52%-85%), and 39% (95% CI, 22%-55%) in the ALLO, HDAC-2, IDAC-2, and IDAC-1 groups, respectively (P = .005 by the log-rank test). All these results were related to the worse outcome observed in the IDAC-1 group, while patients from ALLO, HDAC-2, and IDAC-2 had similar outcome.
Disease-free survival (DFS) according to the postremission therapy.
(A) Because of the poor outcome associated with the IDAC-1 group, DFS was significantly influenced by the postremission therapy (P = .03, by the log-rank test) (B). Overall, DFS was similar in patients from the CHEMO group (including HDAC-2, IDAC-2, and IDAC-1) as compared to those from the ALLO group (P = .55, by the log-rank test).
Disease-free survival (DFS) according to the postremission therapy.
(A) Because of the poor outcome associated with the IDAC-1 group, DFS was significantly influenced by the postremission therapy (P = .03, by the log-rank test) (B). Overall, DFS was similar in patients from the CHEMO group (including HDAC-2, IDAC-2, and IDAC-1) as compared to those from the ALLO group (P = .55, by the log-rank test).
Using the 2-group intent-to-treat classification of the postremission therapy (ALLO vs CHEMO), there was no significant difference in outcome between patients allocated to receive allogeneic transplantation in first CR and those allocated to receive chemotherapy either for DFS (P = .55 by the log-rank test) (Figure 3), CR duration (P = .10 by the log-rank test), and overall survival (P = .64 by the log-rank test). Estimated 5-year DFS was 56% versus 52% in the ALLO versus CHEMO group, respectively. Estimated 5-year survival was 56% versus 52% in the ALLO versus CHEMO group, respectively.
Multivariate analysis.
Given the results of univariate analyses, the Cox model was used to evaluate the prognostic value of the 5 following variables for DFS, CR duration, and overall survival: age (as a continuous variable), WBC index (as a 3-group variable using the 2.5/20 cutoffs), AN-AA classification, prospective study, and postremission therapy (as a 2-group intent-to-treat variable using the ALLO/CHEMO classification). Results are shown in Table 3. The WBC index appeared to be the only prognostic factor for DFS, CR duration, and survival.
Multivariate analyses for DFS, duration of CR, and overall survival
| . | P . | ||
|---|---|---|---|
| DFS . | CR duration . | Overall survival . | |
| Age, continuous variable | .16 | .35 | .10 |
| WBC index, 3-group classification | .003 | .002 | .04 |
| Hazard ratio (95% CI) | 1.9 (1.3-3.0) | 2.2 (1.3-3.7) | 1.6 (1.0-2.6) |
| AA-AN, 2-group classification | .50 | .75 | .38 |
| Six prospective studies3-150 | .48 | .99 | .55 |
| Postremission therapy, ALLO/CHEMO classification | .89 | .15 | .60 |
| . | P . | ||
|---|---|---|---|
| DFS . | CR duration . | Overall survival . | |
| Age, continuous variable | .16 | .35 | .10 |
| WBC index, 3-group classification | .003 | .002 | .04 |
| Hazard ratio (95% CI) | 1.9 (1.3-3.0) | 2.2 (1.3-3.7) | 1.6 (1.0-2.6) |
| AA-AN, 2-group classification | .50 | .75 | .38 |
| Six prospective studies3-150 | .48 | .99 | .55 |
| Postremission therapy, ALLO/CHEMO classification | .89 | .15 | .60 |
LAME-91, ALFA-9000, BGMT-87, BGMT-91, GOELAM-01, or GOELAM-02.
Even if interaction analysis was hindered by the relatively low numbers of patients within each subgroup, allogeneic SCT did not appear to confer a better outcome than chemotherapy even in high-risk patients from the high WBC index subgroup. DFS was similar in the ALLO group and the CHEMO group in patients either with a low WBC index (P = .84 by the log-rank test) or with an intermediate (P = .56 by the log-rank test) or high (P = .58 by the log-rank test) WBC index. Similar results were observed for CR duration and survival.
Discussion
In this study, which is the largest report dealing with patients with t(8;21) AML prospectively included in clinical trials, we found that the main and sole prognostic factor was related to morphologic presentation. The prognostic significance of high blood counts (either WBC or AGC) has already been reported in smaller patient populations.15,16 However, one important pitfall in interpreting these results is the relationship between WBC or AGC levels and the degree of spontaneous granulocytic maturation of t(8;21) leukemic clones, which is highly variable at the individual level.8-10 In other terms, some differences in the prognostic significance of a high WBC may exist when in patients with a high degree of maturation and low peripheral blood and marrow blast counts, as compared with patients with overt high blast counts. A high AGC itself, which has been correlated with poor survival in 43 patients,15 might include abnormal granulocytes deriving from leukemic precursors, as well as normal segmented cells. Because of anticipated difficulties in the multicenter evaluation of AGC in these patients without a central morphologic review, we rather choose to take into account the percentage of marrow blasts, which may represent another marker of leukemic maturation in this setting.10Actually, we observed a trend for a better outcome in patients with a low percentage of marrow blasts. This trend became statistically significant after adjustment on the ratio of abnormal metaphases. We thus decided to calculate a WBC index, derived from the original WBC as the product of the WBC by the ratio of marrow blasts. This adjustment of the original WBC on the maturation potential of the leukemic clone allowed us to establish the strong prognostic value of this WBC index in this disease and to propose a 3-subgroup WBC index prognostic classification. We are aware that the ratio of marrow blast itself is not a perfectly determined variable, subject to small variations including those related to the level of patient's erythropoiesis. We think, however, that the WBC index may represent a more simple and valid measurement than other morphologic markers such as AGC.
Based on the strong prognostic significance of this WBC index classification, it is thus tempting to consider that t(8;21) AML is not a single well-defined AML subset. Animal models have already evidenced that t(8;21) AML results from a multistep process, because theAML1-ETO fusion gene cannot fully represent the molecular pathogenesis of the disease. Transgenic mice with inducibleAML1-ETO expression do not develop leukemia despite a strong expression of AML1-ETO in their bone marrow.33In this model, additional nonidentified mutations are required for leukemogenesis.34 Even if the presence of a del(9q) has been associated with worse outcome in one study,19 usual additional chromosomal abnormalities do not appear to be very useful in identifying different t(8;21) AML subtypes with different prognosis. Tyrosine kinase receptor mutations have also been reported in AML. In the largest studies, internal tandem duplications of theFLT3 gene did not seem to be a frequent event in the t(8;21) subset.35,36 On the other hand, c-kit mutations have been observed with a particularly high incidence in CBF leukemias, such as t(8;21) and inv(16) AMLs.37 Given the future potential for therapeutic interventions with kinase inhibitors, it would be highly interesting to try to correlate the presence of tyrosine kinase receptor mutations with higher WBC index in patients with t(8;21) AML. Because a better outcome was observed in patients with a low WBC associated with a marked granulocytic maturation (those with a low WBC index), another manner to improve treatment results in these patients might be to combine chemotherapy and differentiation therapy with granulocyte colony-stimulating factor (G-CSF). On one hand, it has been demonstrated that G-CSF is capable of inducing in vitro and in vivo maturation of t(8;21) AML cells.38,39 On the other hand, despite the large number of randomized (and generally negative) G-CSF studies reported to date in AML patients,40 we do not know if there is any interaction between G-CSF therapy and the presence of the t(8;21) translocation.
Another important finding of the present study is the discrepancy in hematologic characteristics observed between children and adults with the disease. For unknown reasons, children had significantly higher WBC, higher marrow blast percentage, and higher WBC index at diagnosis than adults. This observation, which to our knowledge has never been reported, might be an explanation for worse outcome of t(8;21) AML in children than in adults.14 From a general point of view, one must keep in mind the strong prognostic impact of these hematologic characteristics when comparing the results reported in usually relatively small populations of patients with t(8;21) AML.
The second main result of the present study is the observation that allogeneic SCT in first CR did not significantly improve the outcome of patients with t(8;21) AML. This has been already reported by some investigators, although no single prospective AML trial had a sufficiently high statistical power to strongly confirm this statement. The present study is certainly not a single well-controlled prospective study, but all patients included were prospectively enrolled in modern clinical trials during the same time. The large number of patients included allows us to not recommend allogeneic SCT in first CR in patients with this disease. This recommendation is based on the results obtained in the whole population of t(8;21) AML patients studied. We did not observe any advantage (or disadvantage) of SCT within each of the 3 WBC index prognostic subgroups.
The Cancer and Leukemia Group B has recently emphasized the value of HDAC as consolidation therapy in patients with CBF leukemia.21 Furthermore, in a recent retrospective study of 50 patients with t(8;21) AML, longer DFS and survival were observed in patients receiving 3 HDAC cycles or more (19% relapses and a 5-year projected overall survival rate of 76%) as compared with those receiving only 1 HDAC cycle (62% relapses and a 5-year projected overall survival rate of 44%).22 The impact of HDAC and the number of postremission cycles cannot be readily analyzed in our study, because there were strong interactions between these 2 criteria in relation to protocol designs (only 3 patients receiving 1 single cycle containing HDAC). Nevertheless, we observed similar good outcomes in patients from the IDAC-2 group (2 postremission cycles with 1 cycle containing IDAC) and in those from the HDAC-2 group (2 postremission cycles with 1 cycle containing HDAC). It might be possible that the optimal postremission therapy in this disease must include multiple cycles of high-dose chemotherapy, including HDAC or not.
In conclusion, the value of the WBC index as main prognostic factor in patients with t(8;21) AML needs confirmatory results from other cooperative AML groups, and clinical studies are needed to further define the optimal treatment of high WBC index patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hervé Dombret, Département d'Hématologie, Hôpital Saint-Louis, 1, avenue Claude Vellefaux, 75010 Paris, France; e-mail:herve.dombret@sls.ap-hop-paris.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal