The transcription factor signal transducers and activators of transcription 5 (STAT5) is activated by numerous cytokines that orchestrate blood cell development. Multilineage peripheral blood cytopenias were observed in adult mice lacking both isoforms of STAT5 (STAT5A and STAT5B) as well as accelerated rates of apoptosis in the bone marrow. Although the hematopoietic stem cell (HSC) population was preserved in a number of these mice, the post-HSC progenitor populations were diminished and a marked reduction in functional progenitors (spleen colony-forming units) was detected. Competitive bone marrow transplantation studies in vivo revealed a profound impairment of repopulation potential of STAT5-null HSCs, leading to complete lack of contribution to the myeloid, erythroid, and lymphoid lineages. These abnormalities were associated with heightened proliferation activity in the HSC fraction, suggesting the action of homeostatic mechanisms to maintain sufficient levels of diverse blood cell types for viability. Thus, STAT5 normally sustains the robust hematopoietic reserve that contributes to host viability through crucial survival effects on early progenitor cells.
Introduction
Hematolymphoid development is a complex process controlled by multiple positive and negative regulatory systems to maintain homeostasis. This process begins with hematopoietic stem cells (HSCs), which possess a high proliferative capacity, a differentiative potential encompassing all hematopoietic lineages, and the ability to repopulate the hematopoietic system of a bone marrow–ablated animal for its lifespan. In response to unknown signals, HSCs undergo a differentiation program, yielding cells that have short-term repopulating activity. These cells subsequently give rise to multilineage progenitors that are restricted to either the myeloid (common myeloid progenitor, or CMP) or lymphoid (common lymphoid progenitor, or CLP) lineages.1,2 Lineage-specific differentiation of these pluripotent cells and further expansion produces mature cells of a given lineage.3
Cytokines that use class I cytokine receptors play an indispensable role in controlling hematopoietic development and function. Many of these cytokines act on the HSCs themselves, such as granulocyte colony-stimulating factor (G-CSF), interleukin-6 (IL-6), IL-11, thrombopoietin (TPO), and leukemia-inhibitory factor.4Others, such as erythropoietin, TPO, and G-CSF, act on lineage-committed progenitors exclusively or in addition to their earlier effects.5-7 How this family of receptors generates specificity in biologic outcomes while employing shared intracellular signaling pathways has been a point of considerable interest. The discovery of the JAK-STAT pathway appeared to offer one mechanism through which cytokine receptors could selectively up-regulate distinct target genes and be responsible for specific biologic behaviors. STATs are cytoplasmically located, latent transcription factors that dimerize on phosphorylation by an activated receptor complex, translocate into the nucleus, and increase the transcription of target genes by binding to specific DNA sequence motifs.8,9 Various genes have been shown to be up-regulated by STAT molecules, including those involved in cell cycle progression, antiapoptosis, and regulation of cytokine signaling.10
One member of the STAT family, STAT5, is activated by diverse cytokine receptors involved at multiple levels within the hematopoietic system. The widespread engagement of its 2 isoforms, STAT5A and STAT5B, has implicated STAT5 as a potentially important component of cytokine receptor signaling in this tissue. Cell culture studies have suggested a possible role for STAT5 in hematolymphoid development. For example, STAT5 was shown to be involved in erythropoietin-induced proliferation of TF-1 cells.11 STAT5 has also been implicated in both the IL-3–induced proliferation and G-CSF–induced differentiation in 32D cells.12 Surprisingly, therefore, mice deficient in either STAT5A13 or STAT5B14 were found to exhibit dramatic defects in specific nonhematopoietic tissues, but only subtle alterations in the regulation of hematopoietic cells.15-17 Moreover, mice deficient in both the STAT5A and STAT5B isoforms were reported to have no further defects in the production of mature blood cells of various lineages with the exception of decreased numbers of peripheral T cells.18 However, some evidence of additional hematologic dysregulation was evident in these animals, such as reduced bone marrow colony counts in vitro and notable extramedullary hematopoiesis.18,19 In addition, marked fetal anemia in vivo as well as defects in erythropoietin-dependent production and survival of fetal liver hematopoietic colonies in vitro were subsequently reported.20 Finally, an increase in apoptosis of cultured STAT5A/5B-deficient bone marrow progenitors in the presence of granulocyte-macrophage colony-stimulating factor was also described.21
In view of these provocative findings and the fact that compensatory mechanisms may mask the biologic action of a particular molecular component in vivo, we examined the hematologic status of STAT5A/5B-deficient mice in depth. Our studies uncovered an important role for STAT5 in hematopoiesis at an early progenitor stage in vivo. In these mice, marked impairment in hematopoietic potential affecting diverse blood lineages is linked to significant abnormalities in central and peripheral hematolymphoid tissues.
Materials and methods
Handling and characterization of mice
STAT5A/5B−/− mice18 were obtained from Dr James Ihle (St Jude Children's Research Hospital, Memphis, TN) and back-crossed onto a C57Bl/6 background at least 3 generations. Mice were housed in a pathogen-free rodent barrier facility and received mouse chow and acidified water ad libitum. All studies were performed on 8- to 10-week mice unless otherwise specified, and littermates were always used as wild-type controls. Peripheral blood was obtained via cardiac puncture, and EDTA-treated samples were used for complete blood counts (IDEXX Veterinary Service, Sacramento, CA). Bone marrow was harvested by flushing femurs and tibias into 6 mL phosphate-buffered saline (PBS) containing 2% fetal bovine serum, and cell counts were determined after ACK (NH4Cl) lysis by trypan blue exclusion.
Flow cytometry
All antibodies were obtained from Pharmingen (San Diego, CA), and the following clones were used: Sca-1 (E13-161.7), Ly-76 (Ter-119), Gr-1 (RB6-8C5), CD3 (145-2C11), CD41 (MWReg30), CD45.1 (A20), CD45.2 (104), B220 (RA3-6B2), and CD11b (M1/70). For blocking nonspecific binding to Fc receptors, purified antibody to CD16/CD32 (2.4G2) was used at 1:100 for 3 minutes. Subsequently, antibodies to surface markers of interest were used at 1:60 dilution. Apoptosis staining was performed by using AnnexinV-GFP22 (generously provided by Dr Joel Ernst, University of California, San Francisco, CA) in conjunction with antibodies to selected surface markers as well as one of 2 DNA dyes, ToPro (Molecular Probes, Eugene, OR) or 7-AAD (Pharmingen, San Diego, CA). DNA content was assessed by an initial incubation with antibodies to selected surface markers, followed by a 30-minute fixation and permeabilization step using 2% formaldehyde in H20, a 30-minute exposure to ToPro in the presence of 1 mg/mL RNase (Sigma, St Louis, MO), and fluorescence-activated cell sorter (FACS) analysis using a slow acquisition. All FACS analyses were performed by using a FACScalibur, and all sorting was performed by using a FACSvantage (Becton Dickinson).
Bone marrow transfer studies
Recipient mice were 8-week-old sex-matched C57Bl/6 obtained from Jackson Laboratories (Bar Harbor, ME). Recipients were γ-irradiated from a Cesium source in two 450-rad doses 4 to 5 hours apart. Spleen colony-forming unit (CFU-S) studies were performed as previously described.23 Briefly, whole bone marrow cells from donor mice were injected via tail vein, spleens were harvested 8 or 12 days after transfer, and macroscopic colonies were enumerated. Mice receiving the transplants were maintained on 2.5 mg/100 mL Sulfatrim Pediatric Suspension (Alpharma, Baltimore, MD). Competitive repopulation studies were performed as previously described.24 LindimSca-1+ tester cells (CD45.2+), derived from wild-type littermate or STAT5A/5B−/− whole bone marrow, were sorted by using antibodies to Sca-1 and Ter-119, Gr-1, CD3, B220, and CD11b. Competitor whole bone marrow from congenic B6.SLJ (CD45.1+) mice was harvested as above. Tester and competitor cells were mixed at various ratios (see “Inferior competitive repopulating capacity of STAT5A/5B-deficient HSCs”) and injected into irradiated recipients, prepared as above. After 8 to 10 weeks, chimeric mice were killed, and peripheral blood, spleen, thymus, and bone marrow were collected and analyzed by FACS for contribution of CD45.1+- and CD45.2+-derived cells to selected lineages.
Homing assay
Cell labeling with 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CSFE) was performed as described previously.25 Briefly, whole bone marrow from donor mice was labeled in PBS at a final concentration of 15 μM CSFE (Molecular Probes). After 12 minutes at 37°C, further dye uptake was prevented by adding a quarter volume of fetal bovine serum. Cells were washed twice with PBS, and 5 × 106 CFSE-labeled cells were injected into the tail vein of recipient mice that had been irradiated 18 hours before injection. Bone marrow was harvested 23 hours after injection, stained with lineage markers and Sca-1 as before, and the number of CFSE+ cells in each subset was enumerated by FACS.
Statistical analysis
Data are presented as mean ± SEM. Statistical significance was assessed by 2-sided Student t test.
Results
Adult STAT5A/5B-deficient mice exhibit cytopenias affecting multiple peripheral blood lineages
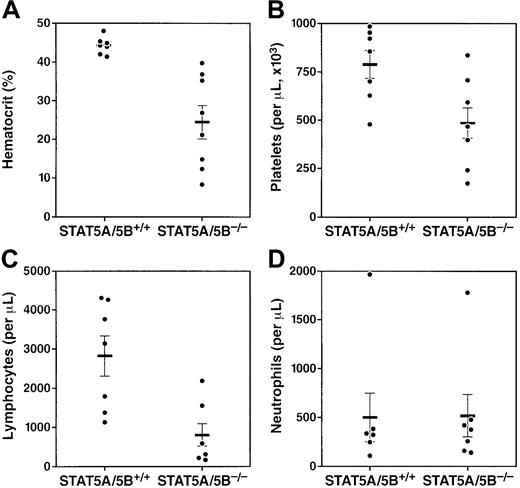
We characterized the peripheral blood compartment of adult STAT5A/5B-deficient mice and found significant abnormalities in multiple blood lineages. At 8 weeks of age, STAT5A/5B−/−mice exhibited significantly decreased numbers of erythrocytes in peripheral blood compared with wild-type littermate controls (Figure1A). Platelets, a second myeloid lineage, were also reduced in these mice, although this effect was less profound (Figure 1B). A marked decrease in lymphocyte number in peripheral blood was also observed (Figure 1C); both T cells and B cells were decreased in STAT5A/5B-deficient animals (data not shown). Finally, although no significant difference in peripheral neutrophil counts was detected (Figure 1D), we observed a significant reduction in mature neutrophils (Gr-1hi) in the bone marrow (see below). Thus, the absence of STAT5A/5B was associated with abnormalities in multiple blood cell lineages.
Multilineage cytopenias in adult STAT5A/5B-deficient mice.
Complete blood counts were performed on whole peripheral blood from STAT5A/5B−/− and STAT5A/5B+/+ mice. (A) Hematocrit (P < .001). (B) Platelets (P = .01). (C) Lymphocytes (P = .003). (D) Neutrophils (P = 1.0).
Multilineage cytopenias in adult STAT5A/5B-deficient mice.
Complete blood counts were performed on whole peripheral blood from STAT5A/5B−/− and STAT5A/5B+/+ mice. (A) Hematocrit (P < .001). (B) Platelets (P = .01). (C) Lymphocytes (P = .003). (D) Neutrophils (P = 1.0).
STAT5A/5B-deficient mice have hypocellular bone marrow and a defect within early progenitor cells
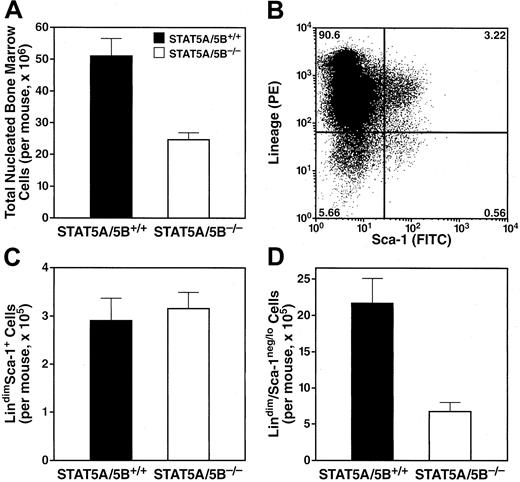
Such broad defects in peripheral blood cell numbers in STAT5A/5B-deficient mice could be due to accelerated peripheral consumption or destruction of mature cells, to a lowered capacity to produce mature cells, or to a combination of these 2 mechanisms. The multilineage character of the effects we observed suggested that a primary pathophysiologic defect might be in the bone marrow, where both unique and shared precursors for these cell types exist. Gross examination of bone marrow in STAT5A/5B-deficient mice revealed generalized hypocellularity compared with wild-type mice (Figure2A). This 2-fold decrease in total nucleated cells in the bone is consistent with the hypothesis that a central defect in the bone marrow is responsible for the pancytopenia observed in the periphery.
Decreased total nucleated cells in the bone marrow of STAT5A/5B-deficient mice.
Total nucleated cells were obtained from both hind legs, and cell counts were determined after red blood cell lysis. Subsetting was performed according to surface expression of lineage-defining markers and Sca-1. Absolute values were generated by multiplying gated percentages by total nucleated cell numbers. (A) Total nucleated cells (P = .002). (B) Representative FACS analysis to quantitate bone marrow subsets. (C) The lindimSca-1+compartment, containing HSCs (P = .7). (D) The lindimSca-1neg/lo compartment, containing post-HSC progenitors (P = .002). PE indicates phycoerythrin; FITC, fluorescein isothiocyanate.
Decreased total nucleated cells in the bone marrow of STAT5A/5B-deficient mice.
Total nucleated cells were obtained from both hind legs, and cell counts were determined after red blood cell lysis. Subsetting was performed according to surface expression of lineage-defining markers and Sca-1. Absolute values were generated by multiplying gated percentages by total nucleated cell numbers. (A) Total nucleated cells (P = .002). (B) Representative FACS analysis to quantitate bone marrow subsets. (C) The lindimSca-1+compartment, containing HSCs (P = .7). (D) The lindimSca-1neg/lo compartment, containing post-HSC progenitors (P = .002). PE indicates phycoerythrin; FITC, fluorescein isothiocyanate.
Various cytokines that trigger STAT5 activity are critical for the regulation of hematopoiesis at all levels of differentiation, including stem cells, multipotent progenitors, lineage-committed progenitors, and mature blood cells. We therefore sought to determine the specific developmental stage(s) in which the functional effects of STAT5 are manifested in the bone marrow. We used FACS analysis to subset bone marrow cells by expression of canonical surface lineage-defining markers (lin) and Sca-1 (Figure 2B). The HSCs, which are defined as cells that have both the capacity for self-renewal and the ability to reconstitute the multilineage hematopoietic system, are found within the lindimSca-1+ fraction.26 This population was increased as a percentage of total nucleated bone marrow cells in STAT5A/B-deleted mice compared with controls but was unchanged in absolute terms (Figure 2C). In contrast, the lindimSca-1neg/lo population, containing CLPs,2 CMPs,1 and oligopotent progenitors, was dramatically decreased in STAT5A/5B-deficient animals (Figure 2D). Also, we observed that the absolute number of mature neutrophils (Gr-1hi) in the bone marrow was decreased (wild type = 12.5 × 106 ± 4.1 per 2 hind legs and knock-out = 5.5 × 106 ± 1.6 per 2 hind legs) as well as progenitors for neutrophils (Gr-1int) (wild type = 10.4 × 106 ± 3.0 per 2 hind legs and knock-out = 5.2 × 106 ± 1.1 per 2 hind legs), erythrocytes (Ter119+) (wild type = 6.6 × 106 ± 0.96 per 2 hind legs and knock-out = 3.3 × 106 ± 0.79 per 2 hind legs), and B cells (B220+) (wild type = 12.6 × 106 ± 2.1 per 2 hind legs and knock-out = 5.0 × 106 ± 0.71 per 2 hind legs). Therefore, the absence of STAT5A/5B results in a marked decrease in lindimSca-1neg/lo cells as well as in specific lineage marker-positive cells in the bone marrow despite preservation of the earlier lindimSca-1+ HSC.
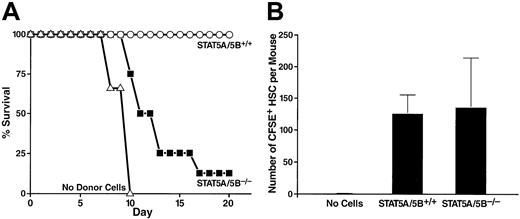
A number of short-term bone marrow transfer assays were performed to assess the functional capabilities of bone marrow from the STAT5A/5B-deficient mice. Lethally irradiated wild-type mice typically die of hematopoietic failure between 7 and 18 days after irradiation unless they are given new hematopoietic progenitors from a donor animal. Therefore, one functional assay determines the radioprotective ability of whole bone marrow from a donor mouse. A dose of 2.5 × 105 transplanted whole bone marrow cells from wild-type littermate control mice provided radioprotection to 100% of lethally irradiated recipient mice for 20 days (Figure3A). In contrast, the same dose of whole bone marrow from a STAT5A/5B-deficient donor provided radioprotection to only 12.5% of recipient mice through the same period (Figure 3A), demonstrating that these cells have markedly decreased reconstituting capacity as indicated by radioprotective effects. To determine whether this phenotype derives from defects in homing or in postengraftment expansion and hematopoiesis, irradiated recipients were injected with wild-type or STAT5A/5B-deficient whole bone marrow that had been labeled with the membrane dye CFSE. We found that the number of CFSE+ cells in the HSC-containing lindim/Sca-1+ fraction that had homed to the bone marrow after 23 hours was comparable when whole bone marrow from either STAT5A/5B-deficient or wild-type littermate donors was injected (Figure 3B).
Decreased short-term radioprotective ability of whole bone marrow despite preserved homing of HSCs from STAT5A/5B-deficient mice.
(A) Irradiated recipients received either 2.5 × 105STAT5A/5B+/+ whole bone marrow cells (n = 6), 2.5 × 105 STAT5A/5B−/− whole bone marrow cells (n = 8), or no donor cells (n = 3). Survival rates are shown as a Kaplan-Meyer plot. (B) Irradiated recipients received 5 × 106 CFSE-labeled whole bone marrow cells from a STAT5A/5B+/+ donor (n = 3) or a STAT5A/5B−/− donor (n = 3). Bone marrow was harvested 23 hours after transplantation, and the absolute number of CFSE+ cells within the HSC (lindim/Sca-1+) gate per mouse was determined.
Decreased short-term radioprotective ability of whole bone marrow despite preserved homing of HSCs from STAT5A/5B-deficient mice.
(A) Irradiated recipients received either 2.5 × 105STAT5A/5B+/+ whole bone marrow cells (n = 6), 2.5 × 105 STAT5A/5B−/− whole bone marrow cells (n = 8), or no donor cells (n = 3). Survival rates are shown as a Kaplan-Meyer plot. (B) Irradiated recipients received 5 × 106 CFSE-labeled whole bone marrow cells from a STAT5A/5B+/+ donor (n = 3) or a STAT5A/5B−/− donor (n = 3). Bone marrow was harvested 23 hours after transplantation, and the absolute number of CFSE+ cells within the HSC (lindim/Sca-1+) gate per mouse was determined.
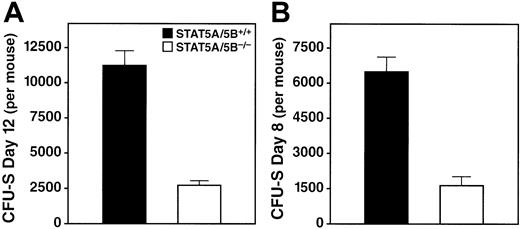
A second, direct, functional assay for progenitor cells is the in vivo CFU-S assay,23 in which macroscopic colonies in the spleens of irradiated recipients are counted 8 or 12 days after bone marrow transfer.26 We detected a pronounced reduction in CFU-S (day 12) colonies per donor in the STAT5A/5B-deleted mice relative to wild-type littermate controls (Figure4A), which indicates an abnormality in the early pluripotent progenitors. We observed a similarly dramatic decrease in CFU-S (day 8) per donor in STAT5A/5B−/− mice (Figure 4B), which indicates an abnormality in lineage-committed progenitors. Moreover, spleen colonies derived from STAT5A/5B-deficient donor cells were markedly smaller in size, both macroscopically and microscopically, compared with those produced by wild-type donor cells, reflecting that fewer progeny cells are produced per colony (data not shown). The reduction in both number and size of spleen colonies in these experiments indicates a marked functional impairment in both multilineage progenitors and lineage-committed progenitors in mice lacking STAT5A/5B.
Reduction in CFU-S activity in bone marrow from STAT5A/5B-deleted mice.
Whole bone marrow was obtained from the hind legs of donor mice, 7.5 × 104 (day 12) or 2 × 105 (day 8) cells were injected into irradiated recipients, and colonies were counted on the indicated days after transfer. Absolute CFU-S values were calculated by using the total nucleated cell counts from the donor mice. (A) CFU-S day 12 (P < .001). (B) CFU-S day 8 (P < .001).
Reduction in CFU-S activity in bone marrow from STAT5A/5B-deleted mice.
Whole bone marrow was obtained from the hind legs of donor mice, 7.5 × 104 (day 12) or 2 × 105 (day 8) cells were injected into irradiated recipients, and colonies were counted on the indicated days after transfer. Absolute CFU-S values were calculated by using the total nucleated cell counts from the donor mice. (A) CFU-S day 12 (P < .001). (B) CFU-S day 8 (P < .001).
Bone marrow deficiency involves increased apoptosis, rather than decreased proliferation
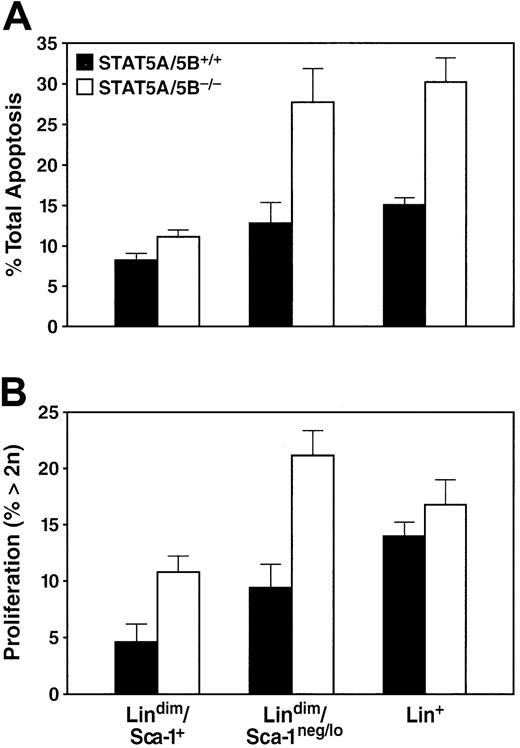
In principle, the cellular effects described might be caused by various mechanisms, including decreases in the rates of proliferation, survival, or differentiation of selected progenitor populations. We used cell labeling with Annexin-V in conjunction with a DNA dye, To-Pro, to measure rates of apoptosis in the bone marrow. The absence of STAT5A/5B was associated with an increase in the rate of apoptosis of unfractionated bone marrow (31.1% ± 2.65%) relative to wild type (17.6% ± 0.73%). This 2-fold increase in cell death was seen in both the lin+population as well as the lindimSca-1neg/lopopulation (Figure 5A), whereas the lindimSca-1+ compartment showed only a modest increase in the rate of apoptosis (Figure 5A). To measure proliferation, we used ToPro in conjunction with cell surface markers to quantitate DNA content in bone marrow by using DNA content more than 2n as an indirect measure of the proportion of cells undergoing cell cycle. An insignificant increase in the proportion of cells with DNA content more than 2n was observed among the unfractionated whole bone marrow population of STAT5A/5B-deficient bone marrow (17.2% ± 1.79%) in comparison to the wild-type marrow (13.4% ± 1.01%). Lin+ cells likewise showed little change in proliferation activity in STAT5A/5B-deleted mice (Figure 5B). In contrast, both the early lindimSca-1neg/loand the lindimSca-1+ subsets exhibited a 2-fold increase in the percentage of cells with DNA content more than 2n in STAT5A/5B-deficient mice (Figure 5B). Together, these findings provide evidence of globally decreased cellular survival (represented as increased apoptosis) in bone marrow cells in the absence of STAT5A/5B, with a concurrent increase in cellular proliferation among the least differentiated hematopoietic cells.
Increases in apoptosis and in the proportion of cells in S/G2/M in bone marrow of STAT5A/5B-deficient mice.
Whole bone marrow from the indicated mice was stained for apoptosis or DNA content measurements in conjunction with antibodies to lineage markers and Sca-1. (A) Percentage of apoptotic cells in the lin+ (P = .04), lindimSca-1neg/lo post-HSC progenitors (P = .03), and lindimSca-1+ HSC (P = .04) subsets in STAT5A/5B−/− and STAT5A/5B+/+ mice are shown. (B) Percentage of cells with more than 2n DNA content for the lin+(P = .2), lindimSca-1neg/lopost-HSC progenitors (P = .01), and lindimSca-1+ HSC (P = .005) subsets are shown.
Increases in apoptosis and in the proportion of cells in S/G2/M in bone marrow of STAT5A/5B-deficient mice.
Whole bone marrow from the indicated mice was stained for apoptosis or DNA content measurements in conjunction with antibodies to lineage markers and Sca-1. (A) Percentage of apoptotic cells in the lin+ (P = .04), lindimSca-1neg/lo post-HSC progenitors (P = .03), and lindimSca-1+ HSC (P = .04) subsets in STAT5A/5B−/− and STAT5A/5B+/+ mice are shown. (B) Percentage of cells with more than 2n DNA content for the lin+(P = .2), lindimSca-1neg/lopost-HSC progenitors (P = .01), and lindimSca-1+ HSC (P = .005) subsets are shown.
Inferior competitive repopulating capacity of STAT5A/5B-deficient HSCs
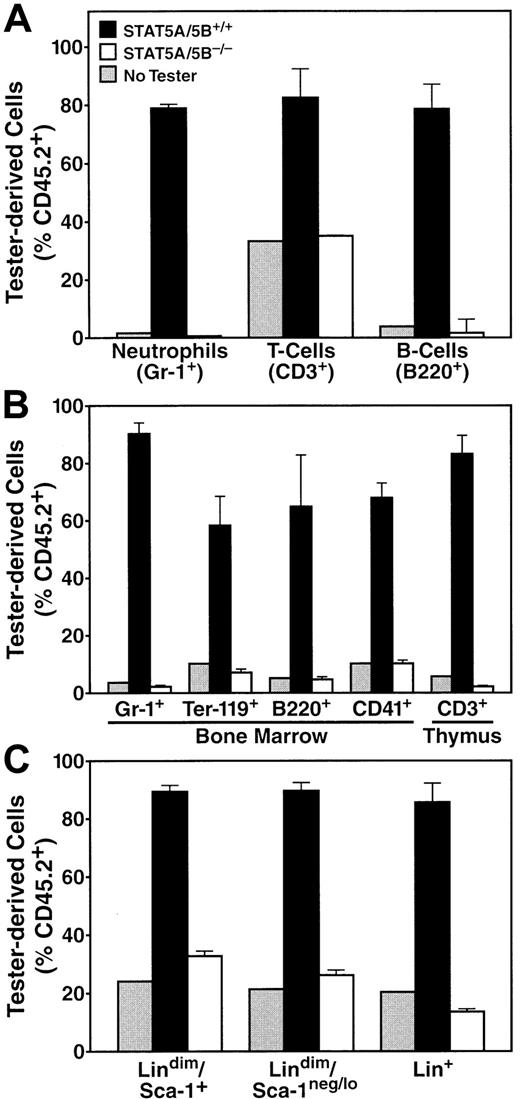
A rigorous functional test of stem cell fitness is the competitive repopulation assay, which measures the capacity of “tester” HSCs to reconstitute the hematopoietic system of irradiated recipient mice in direct competition with wild-type “competitor” bone marrow cells.26 27 For the representative experiment shown, we injected 2500 lindimSca-1+ tester cells from STAT5A/5B-deficient mice or wild-type littermate control mice, carrying the CD45.2 allele, together with 2 × 105 whole bone marrow competitor cells derived from congenic mice carrying the CD45.1 allele. An additional control group received 2 × 105whole bone marrow competitor cells alone to establish the level of ablation achieved by the irradiation regimen. After 10 weeks, peripheral blood, thymus, spleen, and bone marrow were harvested for analysis of hematopoietic lineages by using various antibodies to lineage markers and to the 2 alleles of CD45. At this ratio of input cells, which was weighted to favor tester cells, wild-type tester cells (CD45.2+) gave rise to approximately 80% of all cellular lineages in all tissues examined, including Gr-1+ (Figure6), and other specific lineages within peripheral blood, including T cells (CD3+) and B cells (B220+) (Figure 6A). As is commonly observed, residual radioresistant CD3+ T cells from the recipient mouse are detected in all groups, including those mice that received competitor cells alone (Figure 7A). Likewise, bone marrow cells representing the granulocyte (Gr-1+), B-cell (B220+), megakaryocyte (CD41+) lineages, and erythroid progenitors (Ter-119+), as well as thymocytes (CD3+), were predominantly CD45.2+ (Figure 7B). In contrast, at the same input doses, STAT5A/5B-deficient tester cells failed to give rise to significant numbers of cells of any lineage within peripheral blood, spleen, thymus, or bone marrow (Figures 6 and7 and data not shown); in no case was the CD45.2+ signal greater than that of the background observed in animals not receiving tester cells. Complete competitive failure was also observed in 2 other independent experiments with highly backcrossed donors (G4 and G7, respectively).
Absence of STAT5A/5B−/− contribution (CD45.2+) to granulocytes (Gr-1+) in peripheral blood after competitive repopulation.
Peripheral blood from irradiated recipients receiving competitor cells only (A), competitor cells plus STAT5A/5B+/+ tester cells (B), or competitor cells plus STAT5A/5B−/− tester cells (C) were analyzed 10 weeks after initiation of competitive repopulation assay. Peripheral blood was stained for CD45.1, CD45.2, and Gr-1 after red blood cell lysis and shown here are representative dot-plots portraying CD45.1 versus CD45.2 expression within the Gr-1+ gate.
Absence of STAT5A/5B−/− contribution (CD45.2+) to granulocytes (Gr-1+) in peripheral blood after competitive repopulation.
Peripheral blood from irradiated recipients receiving competitor cells only (A), competitor cells plus STAT5A/5B+/+ tester cells (B), or competitor cells plus STAT5A/5B−/− tester cells (C) were analyzed 10 weeks after initiation of competitive repopulation assay. Peripheral blood was stained for CD45.1, CD45.2, and Gr-1 after red blood cell lysis and shown here are representative dot-plots portraying CD45.1 versus CD45.2 expression within the Gr-1+ gate.
STAT5A/5B-deficient HSCs possess inferior competitive repopulating capacity.
Peripheral blood, thymus, and bone marrow were harvested from irradiated recipients 10 weeks after receiving either 2 × 105 competitor cells only (“No Tester,” n = 2), 2 × 105 competitor cells plus STAT5A/5B+/+ tester cells (“STAT5A/5B+/+Tester,” n = 3), or 2 × 105 competitor cells plus STAT5A/5B−/− tester cells (“STAT5A/5B−/−Tester,” n = 6). FACS analysis was performed by using lineage markers and CD45.1 and CD45.2, and the results for the indicated lineage-specific subsets are displayed. (A) Peripheral blood cells positive for Gr-1+ (P < .001), B220+ (P < .001), or CD3+(P = .002). (B) Cells from hematolymphoid organs, including bone marrow cells expressing for Gr-1 (P < .001), B220 (P = .001), Ter-119 (P = .001), or CD41 (P < .001) and thymocytes expressing CD3 (P < .001). (C) Bone marrow cells from the lin+ (P < .001), lindimSca-1neg/lo (P < .001), and lindimSca-1+ (P < .001) subsets; in this case CD45.2+ cells were scored according to CD45.1− phenotype.
STAT5A/5B-deficient HSCs possess inferior competitive repopulating capacity.
Peripheral blood, thymus, and bone marrow were harvested from irradiated recipients 10 weeks after receiving either 2 × 105 competitor cells only (“No Tester,” n = 2), 2 × 105 competitor cells plus STAT5A/5B+/+ tester cells (“STAT5A/5B+/+Tester,” n = 3), or 2 × 105 competitor cells plus STAT5A/5B−/− tester cells (“STAT5A/5B−/−Tester,” n = 6). FACS analysis was performed by using lineage markers and CD45.1 and CD45.2, and the results for the indicated lineage-specific subsets are displayed. (A) Peripheral blood cells positive for Gr-1+ (P < .001), B220+ (P < .001), or CD3+(P = .002). (B) Cells from hematolymphoid organs, including bone marrow cells expressing for Gr-1 (P < .001), B220 (P = .001), Ter-119 (P = .001), or CD41 (P < .001) and thymocytes expressing CD3 (P < .001). (C) Bone marrow cells from the lin+ (P < .001), lindimSca-1neg/lo (P < .001), and lindimSca-1+ (P < .001) subsets; in this case CD45.2+ cells were scored according to CD45.1− phenotype.
Finally, because STAT5A/5B-deficient mice exhibited broad hematopoietic deficiencies, we sought to identify the earliest stage of differentiation at which defects were evident in the competitive repopulation assay. We failed to detect lindimSca-1+ cells, lindimSca-1neg/lo cells, or lin+cells derived from STAT5A/5B-deficient cells at levels significantly above background (Figure 7C). Therefore, STAT5A/5B-deficient cells are inferior to wild-type cells in their ability to occupy the limited niche available for stem cells, early pluripotent cells, and oligopotent progenitor cells. The STAT5A/5B−/− HSCs thus failed to compete effectively with wild-type competitor cells in the same tissue environment in the production of progeny cells representing diverse hematolymphoid lineages. Overall, STAT5A/5B-deficient cells exhibit a profound defect in HSCs that is independent of both nonhematopoietic cell genotype and indirect effects mediated by mature cells, such as T cells.
Discussion
The transcription factor STAT5 is activated by multiple and diverse cytokines on binding to their cognate receptors, including several that act on the hematopoietic system. However, mice deficient for STAT5A, STAT5B, or both were reported to have surprisingly subtle deficiencies in hematolymphoid development, including reduction in peripheral T cells,13,14,18 impaired fetal erythropoiesis,20 and decreased survival of monocyte progenitors.21 Because numerous and complex regulatory pathways impinge on hematolymphoid development in vivo, we sought to define the hematologic features of STAT5A/5B-deficient mice to determine whether compensatory mechanisms may mask greater contributions of STAT5 in hematopoiesis in vivo.
The first phase of our characterization revealed multilineage effects in peripheral blood at steady state, including marked decreases in erythrocytes and reduced numbers of circulating platelets. We also observed significant lymphopenia affecting both T cells and B cells. Moreover, although there was no abnormality in levels of peripheral blood neutrophils, a substantial decrease in the pool of mature neutrophils in the bone marrow was observed. In addition, histologic analysis of whole long bones and spleen in STAT5A/5B-deficient mice revealed extramedullary hematopoiesis in the enlarged spleens that was nearly exclusively erythropoietic tissue, as well as an exaggeration of myelopoiesis and few erythropoietic cells within intramedullary tissue in bone (data not shown). Hematologic stress in the mouse characteristically induces a myelopoietic dominance within bone marrow sites and heightened erythropoiesis within extramedullary sites such as the spleen.28 Therefore our histologic findings may represent further evidence of hematopoietic stress in these mice. We note that our peripheral blood analysis of STAT5A/5B-deficient mice differs from the initial characterization of these mice.18One possible explanation is the age of the mice examined, because the influence of maturity has not been studied. Another possible factor is genetic background, because hematopoiesis is likely influenced by numerous strain-specific determinants. In any event, our findings indicate that there are defects in the circulating levels of 3 distinct blood lineages associated with signs of overall hematopoietic stress.
Multilineage cytopenia can be caused by hyperactive consumptive mechanisms broadly affecting peripheral blood cells, by multiple and independent defects affecting production or survival of individual cell types, or by a central bone marrow defect affecting progenitor cells that are common to multiple lineages. The scope of the blood cell abnormalities evident in STAT5A/5B−/− mice was most consistent with a central hematopoietic defect. Although STAT5A/5B-deficient mice possessed marked bone marrow hypocellularity overall, the cell population containing HSCs (lindimSca-1+) was preserved quantitatively, whereas cells of more restricted differentiation potential were profoundly reduced. Collectively, these findings based on cellular representation within the bone marrow strongly suggest that the absence of STAT5A/5B leads to a quantitative loss in the population of cells that contain the CMP,1 the CLP,2 oligopotent progenitors, and lineage-committed progenitors.
To complement the flow cytometry data, we performed several in vivo reconstitution assays. The short-term radioprotection assay showed a severe defect in the ability of whole bone marrow from STAT5A/5B-deficient mice to protect a recipient from radiation-induced hematopoietic failure. In addition, we found that the reduction in the functional capability of the bone marrow from STAT5A/5B-deficient mice was not due to decreased homing ability. We used a second assay, the CFU-S assay, to quantitate early progenitor cells on the basis of functional criteria. These experiments revealed a dramatic reduction of hematopoietic colonies derived from STAT5A/5B-deficient animals. These results support our earlier finding that there is a diminution of the population (lindimSca-1neg/lo) within the bone marrow reported to contain oligopotent or lineage-committed cells and demonstrate that STAT5A/5B is a regulator of the biology of early progenitor cells.
A decrease in the overall cell numbers in STAT5A/5B-deficient bone marrow could be caused by various mechanisms involving insensitivity of hematopoietic progenitors to one or more cytokines, including a reduction in survival half-life, a decrease in proliferation potential of progenitors, or an impaired execution of differentiation programs. We detected an increase in the rate of apoptosis in both the lin+ and lindimSca-1neg/lofractions of bone marrow in STAT5A/5B-deficient mice. Although these studies do not elucidate the precise mechanism of antiapoptosis, impaired regulation of Bcl-X20,21 or other antiapoptotic mediators may be operative. We also detected an increased proportion of bone marrow cells in the S/G2/M phases of the cell cycle, a feature present in both the HSC and post-HSC populations. We propose that this increase in the proportion of cycling cells represents part of a compensation mechanism seeking to counter relative ineffective hematopoiesis in STAT5A/5B-deficient mice. Alternatively, slowed rates of progression through cell cycle in vivo might also underlie our results, as prolonged in vitro doubling times have been reported for STAT5A/5B-deficient progenitors.21 Finally, progenitors from STAT5A/5B-deficient mice have been shown to differentiate fully to mature cells in vitro,21 implying that there is no absolute loss of differentiation potential of progenitor cells in the absence of STAT5A/5B. Overall, these findings provide evidence of globally decreased cellular survival in bone marrow cells in the absence of STAT5A/5B, with a concurrent increase in the proportion of cycling cells among the least differentiated hematopoietic cells.
To establish definitively the abnormalities in the stem cells of STAT5A/5B-deficient mice, we applied the most rigorous functional assay for stem cell fitness, the competitive repopulation assay.26 27 Cells from STAT5A/5B−/− animals were unable to give rise to significant numbers of cells of any lineage in any tissue examined, despite being introduced at a dose that yielded widespread reconstitution by STAT5A/5B+/+ cells. Additionally, these experiments addressed the cell autonomy of the STAT5A/5B-mediated defect in these cells. Because the STAT5A/5B−/− stem cells compete poorly with wild-type competitor cells in the same biologic environments, the functional defect evidently is independent of both nonhematopoietic cell genotype and indirect effects mediated by mature cells such as T cells.
Thus, these analyses of the role of STAT5 in hematopoiesis in vivo reveal that STAT5A/5B is an important positive factor that promotes HSC fitness and multilineage hematopoiesis. Relative insensitivity to cytokines such as G-CSF, TPO, growth hormone, IL-3, or others that act on progenitors could be responsible through either a reduction in the overall signal intensity or the loss of specific signals mediated by these cytokines. Bone marrow from STAT5A-deficient mice was shown to produce fewer in vitro colonies in the presence of flt3-ligand, a cytokine known to be important for hematopoietic progenitor homeostasis.29 At the cellular level, STAT5 may provide an antiapoptotic signal that lifts the threshold of survival in the context of internal and external apoptotic, antiapoptotic, proliferative, and differentiative signals. At the organismal level, this effect translates into lower viability of bone marrow hematopoietic cells, which likely results in fewer cells produced per stem cell that enter the differentiation program. In the context of lineage-specific defects shown in our analysis of post-HSC populations and in earlier reports regarding STAT5 deficiency, it remains unknown to what degree the cytopenias seen in these mice are attributable to the HSC, multilineage, or lineage-specific effects. In fact, evidence from some models in which stem cells or multilineage progenitors are affected30-32 suggests that defects at these stages alone may not induce mulitlineage cytopenias in some contexts. Nevertheless, our findings establish a novel and important role for STAT5 in the regulation of these early hematopoietic cells. This role is quantitative and nonessential, but genetic modifiers may control the degree of severity. These modifying loci could be responsible for multiple compensation mechanisms, such as extramedullary hematopoiesis and increases in bone marrow proliferation, which together allow the organism to achieve levels of hematopoietic production that are compatible with life but reduced nonetheless.
Further studies in these animals may promote better understanding of the molecular pathogenesis of some forms of bone marrow failure. Additionally, STAT5 may act in a similar manner in human hematopoiesis and would thus be an attractive target for therapy in hematologic settings. Cytokine therapy is often used to ameliorate the phenotypes of lineage-specific and multilineage cytopenias, but it may be associated with increased rates of leukemic transformation in some settings.33 Perhaps directed activation of specific cytokine-mediated intracellular signals would serve to increase blood cell production without a concomitant rise in the rate of transformation.
We thank Dr James Ihle for kindly providing STAT5A/5B−/− mice. We acknowledge the technical assistance of the Gladstone Flow Cytometry Core in the conduct of these experiments and the Laboratory Animal Resource Center animal care staff at the University of California at San Francisco. We also thank Dr Kevin Shannon and Stephen Chan for critical review of the manuscript, and Heather Gravois and John Carroll for their assistance in the preparation of this manuscript.
J.W.S. is supported by N.I.H. Immunology Training Grant AI07334 at the University of California, San Francisco. N.A. is supported by Damon-Runyon Fellowship 1548. B.H. is supported by P30 MH59037. This work was supported in part by N.I.H. grant GM54351 and the J. David Gladstone Institutes (M.A.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark A. Goldsmith, Gladstone Institute of Virology and Immunology, PO Box 419100, San Francisco, CA 9414-9100; e-mail:mgoldsmith@gladstone.ucsf.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal