Umbilical cord blood has been increasingly used as a source of hematopoietic stem cells. A major area of concern for the use of cord blood transplantation is the delay in myeloid and lymphoid recovery. To directly compare myeloid and lymphoid recovery using an animal model of bone marrow and cord blood as sources of stem cells, hematopoietic engraftment and immune recovery were studied following infusion of T-cell–depleted adult bone marrow or full-term fetal blood cells, as a model of cord blood in a murine allogeneic transplantation model (C57BL/6 [H-2b] → BALB/c [H-2d]). Allogeneic full-term fetal blood has poorer radioprotective capacity but greater long-term engraftment potential on a cell-to-cell basis compared with T-cell–depleted bone marrow. Allogeneic full-term fetal blood recipients had decreased absolute numbers of T, B, and dendritic cells compared with bone marrow recipients. Splenic T cells in allogeneic full-term fetal blood recipients proliferated poorly, were unable to generate cytotoxic effectors against third-party alloantigens in vitro, and failed to generate alloantigen-specific cytotoxic antibodies in vivo. In addition, reconstituting T cells in fetal blood recipients had decreased mouse T-cell receptorδ single-joint excision circles compared with bone marrow recipients. At a per-cell level, B cells from fetal blood recipients did not proliferate as well as those found in bone marrow recipients. These results suggest that full-term fetal blood can engraft allogeneic hosts across the major histocompatibility barrier with slower hematopoietic engraftment and impaired immune reconstitution.
Introduction
Umbilical cord blood has been increasingly utilized as a source of hematopoietic stem cells for allogeneic transplantation. In humans, immune reconstitution following cord blood transplantation has not yet been systematically studied. Infections have been a leading cause of morbidity and mortality,1-4 suggesting that mismatched unrelated cord blood may not have the potential to fully reconstitute immune function, especially in adult recipients. To better understand the possible defects in immune function, a murine model system was established in this study. Allogeneic bone marrow or full-term fetal blood recipients were compared in the 4 major cell components (T, B, natural killer [NK], and antigen-presenting cells).
The mechanisms underlying impaired immune function can be divided into the following 3 categories: (1) intrinsic defect of hematopoietic precursor cells (eg, severe combined immunodeficiency)5; (2) microenvironment abnormality (eg, thymic microenvironment abnormality in DiGeorge syndrome)6; (3) improper interaction between graft and microenvironment (eg, donor-recipient histoincompatibility-associated immune dysfunction7 and graft-versus-host disease [GVHD]–associated lymphoid hypoplasia8 9). In our present studies, allogeneic T-cell–depleted bone marrow served as a standard and a control for the microenvironment (host), and syngeneic full-term fetal blood as a control for full-term fetal blood transplantation (as a source of stem cells). These controls allowed the measurement of defects that are specific for recipients of allogeneic major histocompatability complex (MHC) mismatched full-term fetal blood.
Materials and methods
Animals
BALB/c (H-2d, Mls-2a, Mls-3a), C57BL/6 (H-2b, Mls-2b, Mls-3b), and C3H/HeJ (H-2k, Mls-2b, Mls-3b) mice were purchased from The Jackson Laboratories (Bar Harbor, ME).
Harvesting of full-term fetal blood cells
In order to collect the maximum amount of full-term fetal blood on the same day, the majority of C57BL/6 female mice were superovulated before coitus as described.10 Briefly, C57BL/6 female mice were injected intraperitoneally at 6 to 16 weeks of age with 5 IU pregnant mare serum gonadotropin (Sigma Chemical, St Louis, MO). They were injected 47 to 48 hours later with 5 IU human chorionic gonadotropin (Sigma Chemical) and placed with mature (2-month-old to 8-month-old) C57BL/6 males. Females were housed overnight with males and checked for vaginal plugs the following morning. The day of plug detection was considered day 1 after gestation. On day 20 after gestation, pregnant females were killed and all fetuses were delivered by Cesarean section. Fetuses were separated with placentas and dried on gauze to minimize the contamination of maternal blood before harvesting. Fetuses were then anesthetized by hypothermia, decapitated, and peripheral blood was harvested into complete RPMI-1640 medium. Fetal blood was pooled from all fetuses. Average nucleated cell number in fetal blood from one fetus is 3.9 × 105 (range, 2.6 × 105 to 6.4 × 105). Pregnant female mice give birth between day 20 and 22 after gestation, so fetal blood harvested from a day 20 fetus is considered full-term fetal blood and was used throughout the study as a surrogate for umbilical cord blood. The phenotypes of full-term fetal blood are shown in Table 1.
Phenotypic analyses of murine full-term fetal blood
| Percentage* . | Adult peripheral blood (n = 15) . | Adult T-cell– depleted bone marrow (n = 10) . | Full-term fetal blood (n = 12) . |
|---|---|---|---|
| Lymphocyte | 66.0 ± 4.2 | 25.8 ± 3.9† | 22.4 ± 4.9‡ |
| CD3+ | 30.4 ± 2.5 | 0.44 ± 0.23† | 2.6 ± 0.8‡ |
| CD4+ | 20.1 ± 1.5 | 0.14 ± 0.07† | 0.1 ± 0.0‡ |
| CD8+ | 10.1 ± 3.0 | 0.07 ± 0.03† | 0.9 ± 0.5‡ |
| B220+ | 60.9 ± 3.5 | 92.1 ± 0.5† | 62.1 ± 12.31-153 |
| NK1.1+CD3− | 3.5 ± 1.2 | 0.86 ± 0.15 | 16.7 ± 8.0‡,1-153 |
| Percentage* . | Adult peripheral blood (n = 15) . | Adult T-cell– depleted bone marrow (n = 10) . | Full-term fetal blood (n = 12) . |
|---|---|---|---|
| Lymphocyte | 66.0 ± 4.2 | 25.8 ± 3.9† | 22.4 ± 4.9‡ |
| CD3+ | 30.4 ± 2.5 | 0.44 ± 0.23† | 2.6 ± 0.8‡ |
| CD4+ | 20.1 ± 1.5 | 0.14 ± 0.07† | 0.1 ± 0.0‡ |
| CD8+ | 10.1 ± 3.0 | 0.07 ± 0.03† | 0.9 ± 0.5‡ |
| B220+ | 60.9 ± 3.5 | 92.1 ± 0.5† | 62.1 ± 12.31-153 |
| NK1.1+CD3− | 3.5 ± 1.2 | 0.86 ± 0.15 | 16.7 ± 8.0‡,1-153 |
Percentages among total white blood cells (CD45+) for lymphocytes; percentages among lymphocytes for other subsets.
P < .0001, compared with adult peripheral blood.
P < .0001, compared with both adult peripheral blood and T-cell–depleted bone marrow except indicated.
P < .0001, compared with adult T-cell–depleted bone marrow.
T-cell depletion from bone marrow
Bone marrow cells flushed out from femurs, tibias, and humeri were strained through 70 μm cell strainers (Becton Dickinson, Franklin Lakes, NJ). After washing, cells were resuspended in cytotoxicity medium (Cedarlane, Hornby, ON, Canada) at a concentration of 1 × 107/mL. Purified anti-Thy1.2 monoclonal antibody (0.1 μg per 1 × 107 cells) (Pharmingen, San Diego, CA) was added and mixed. After 60 minutes of incubation on ice, the cells were washed once and then suspended in cytotoxicity medium containing 1:10 Low-Tox-M Rabbit Complement (Cedarlane). The cells were incubated at 37°C for 60 minutes and washed twice before use. The final product contained less than 0.08% mature T cells, 0.11% interleukin-2 (IL-2)–producing cells and less than 0.01% interferon-γ (IFNγ)–producing cells.
Stem cell transplantation
BALB/c recipients, 9 to 12 weeks old at time of transplantation, were lethally irradiated (8.5 Gy), and were injected intraveneously via the tail vein with either: (1) graded doses of T-cell–depleted bone marrow cells from adult C57BL/6 mice, or (2) graded doses of full-term fetal blood cells. For the syngeneic full-term fetal blood control, lethally irradiated (10.5Gy) C57BL/6 mice were infused with 1 × 106 full-term fetal blood cells from C57BL/6 fetuses. All experiments were performed according to Duke University Institutional Animal Care and Use Committee (IACUC) guidelines. Mortality was recorded daily. Recipients were monitored daily for clinical signs of GVHD by the following parameters: body weight and extent of skin changes (hair loss and erythema) scored on a cumulative severity scale from 0 (none) to 8 (maximum): head 1, neck 1, back (1/3,2/3,3/3) 1-3, and front (1/3,2/3,2/3) 1-3 as described.11-14 Skin biopsies (from the ear) for histologic evidence of GVHD were routinely taken on days +30, +70, and +100 after transplantation and when mice were moribund.15
Flow cytometric analysis
Fifty microliters of blood or 1 × 106 cells were incubated with titrated antibodies for 15 minutes at room temperature in the dark. To lyse red cells in the whole blood samples, the stained adult whole blood was then processed in Multi-Q-Prep (Coulter, Miami, FL); the stained fetal blood was lysed with 1x FACS lysing solution (Becton Dickinson, San Jose, CA) and washed twice before analysis. The stained cells were analyzed using a Coulter EPICS XL-MCL flow cytometer equipped with System II software (Coulter). The antibodies used were fluorescein isothiocyanate (FITC)–conjugated anti-CD11b (M1/70), CD62L (MEL-14), goat anti–hamster IgG, H-2Db (CTDb), phycoerythrin (PE)–conjugated anti-CD3 (145-2C11), Gr-1 (RB6-8C5), Vβ3 (KJ25), Cy5PE-conjugated streptavidin, anti-CD3 (145-2C11) and their isotype controls from Pharmingen (San Diego, CA); FITC-conjugated anti-CD4 (CT-CD4), B220 (RA3-6B2), H-2Db (CTDb), IL-2 (JES6-5H3), interferon gamma (IFNγ) (XMG1.2), and PE-conjugated anti-CD4 (CT-CD4); CD8 (CT-CD8a), B220 (RA3-6B2), NK1.1 (PK136), tricolor(TC)–conjugated anti-CD4 (CT-CD4), CD8α (CT-CD8α), CD45 (YW62.3) and their isotype controls from Caltag (San Francisco, CA); and red 613–conjugated anti-CD4 (H129.19) from Life Technologies (Gaithersburg, MD); FITC-conjugated anti-BrdU (B44) (BDIS, San Jose, CA), and purified anti-CD11c (N418) (Harlan Bioproducts for Science, Indianapolis, IN).
Mixed lymphocyte reaction measured by [3H]thymidine incorporation
Responder spleen cells (2.5 × 105) were plated in flat-bottom 96-well culture plates with 5 × 105irradiated (20 Gy) spleen stimulator cells in a final volume of 200 μL. After 96 hours of incubation in 37°C and 5% CO2, cultures were pulsed with 1 μCi [3H]thymidine per well and harvested 16 hours later. Triplicate cultures were set up for every cell population tested. Culture medium was RPMI 1640 supplemented with 10% prescreened fetal calf serum, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, 100 U/mL penicillin, 100 μg/mL streptomycin. This medium was used for all subsequent in vitro studies.
Cytotoxic T lymphocyte assay
Graded numbers of responder cells were plated with 5 × 105 irradiated (20 Gy) stimulator cells. Plates were cultured in 37°C, 5% CO2 incubator for 112 hours. The cells were tested in situ for lysis of 51Cr-labeled 2-day Con A blast cells. After 4 hours of culture, supernatant was removed and counted in a gamma counter. In each experiment, cultures were tested in parallel for lysis of irrelevant targets to ensure antialloantigen-specific killing. The percentage of specific release was calculated as follows: ([experimental release − spontaneous release] / [maximal release − spontaneous release]) × 100 = % specific release.
Spontaneous release was obtained by incubating target cells with stimulator cells only. Maximal release was obtained after treatment with 1% Igepal CA-630 (Sigma).
Alloantigen-specific cytotoxic antibody assay
Target C3H/HeJ spleen cells were prepared at 1 × 106/mL in cytotoxicity medium (Cedarlane). Target cells (25 μL) and test serum samples (25 μL) were added to each well of round-bottom 96-well culture plates. After one hour of incubation at 4°C, wells were washed once and 50 μL per well of complement medium (1:12 dilution) (Cedarlane) was added. After an additional one hour of incubation at 37°C, 5 μL of 1% trypan blue (Life Technologies) per well was added. Three minutes later, we scored live versus dead cells in a hemocytometer. Cytotoxic index was calculated as follows: ([% dead cells {serum + complement}] − [% dead cells {complement alone}] / [100% − % dead cells {complement alone}]) × 100 = cytotoxic index.
Bromodeoxyuridine assay for detection of cellular proliferation
The assay was performed as described.16 Spleen cells (1.25 × 106 per well) were cultured with either a T-cell mitogen (immobilized anti-CD3; Pharmingen) or B-cell mitogens (anti-IgM, Jackson ImmunoResearch Laboratories, West Grove, PA; lipopolysaccharide [LPS], Sigma Chemical) in flat-bottom 48-well culture plates at 37°C and 5% CO2 for 48 hours. A final concentration of 30 μM of bromodeoxyuridine (BrdU) (Sigma Chemical) was added 24 hours before harvest. After culture, the cells were transferred into 6 mL tubes and washed once. The cells were then resuspended in 0.5 mL of FACS permeabilizing solution (BDIS) for fixation and permeabilization. After 3 hours of incubation at 4°C, cells were washed twice, stained with anti-BrdU antibody (10 μg/mL; BDIS) and anti–surface marker antibodies in the presence of DNAse I (Sigma) at a final concentration of 4 mg/mL for 30 minutes at room temperature. The stained cells were analyzed using a Coulter EPICS XL-MCL flow cytometer equipped with System II software (Coulter). This assay correlates well with proliferation assay measured by [3H]thymidine incorporation (r2 = 0.997 for T-cell response against phytohemagglutinin [PHA]; r2 = 0.990 for B-cell response against anti-IgM; data not shown).
Cardiac transplantation
The method of Fulmer et al,17 as modified separately by Judd and Trentin18 and Babany et al,19 was used for cardiac transplantation. Briefly, the dorsum of the pinna of an adult mouse was cleaned with 70% ethanol and a pouch 3 to 4 mm in diameter was prepared. The heart of a neonatal mouse (< 48 hours old) was placed in the pouch. Residual fluid was cleared from the pouch with a cotton swab after transplantation. Grafts were examined for contractions with a stereomicroscope at 10-fold magnification. Grafts resumed visual contraction between 6 to 7 days after transplantation. Rejection is defined as the point of cessation of visual cardiac activity. Grafts that were never observed to be contracting were considered surgical failure, and those data were excluded (none in this study). All grafts were monitored daily until day 60 after heart transplantation.
Quantification of mouse T-cell receptorδ excision circles by real-time polymerase chain reaction
Mouse T-cell receptor (TCR)δ signal joint excision circles (mTREC) are generated by a recombination event between pseudo J alpha and one of the 2 primary murine delta rec loci during TCR gene rearrangment in developing thymocytes. This recombination event generates a novel DNA sequence on an extrachromosomal DNA circle.20 Molecules of mTREC were quantitated by real-time polymerase chain reaction (PCR) using a standard curve of known number of molecules of mTREC, and specific primers and fluorescent probes as described (G.D.S. et al, manuscript in preparation, August 2001).21 22
For determination of molecules of mTREC per splenocyte or CD4+ or CD8+ splenocytes, single-cell suspensions of splenocytes were prepared by teasing the tissue through 70-μm cell strainers (Becton Dickinson Labware) using a 1 cc syringe plunger. Splenocytes were layered over Ficoll and centrifuged to remove red blood cells, and then separated into CD4+ and CD8+ populations using magnetic beads (Miltenyi Biotec, Auburn, CA). Cells were precisely counted using a hemacytometer and spun down for 2 minutes in a microfuge (12 000 rpm). Cell pellets were frozen at −80°C or immediately lysed in proteinase K (95 μg/mL; Boehringer Mannheim, Germany) at 10 000 cells per μL. Samples were run at 50 000 cell equivalents (5 μL) per PCR reaction.
An mTREC DNA standard for real-time PCR was generated by PCR-cloning a 482-base pair (bp) fragment of mTREC DNA from mouse thymus genomic DNA into pCR2 (Invitrogen, Carlsbad, CA). The plasmid was grown up inEscherichia coli and isolated by Maxi Prep (Qiagen, Valencia, CA). The absolute number of molecules of mTREC standard per 1 μg of plasmid DNA was determined and used to prepare stock dilutions of 107, 106, 105, 104, 103, and 102 molecules of mTREC per 5 μL (stored at −80°C). One vial of each dilution of standard was thawed immediately before use and run in duplicate to generate a standard curve for each real-time PCR run. All acceptable runs had curve fits (r2) of 0.995 and y intercepts of 45 ± 2.
Molecules of mTREC were quantitated by real-time quantitative PCR with the primers CATTGCCTTTGAACCAAGCTG and TTATGCACAGGGTGCAGGTG, and fluorescent probe FAM-CAGGGCAGGTTTTTGTAAAGGTGCTGCTCACTT-QSY (MegaBases, Evanston, IL). PCR reactions contain 0.5 U Platinum taq polymerase (Life Technologies), 3.5 mM MgCl2, 0.2 mM dNTPs, 500 nM each primer, 150 nM probe, and 1 × Blue-636 reference dye (MegaBases). Amplification conditions were 95°C for 5 minutes, then 40 cycles of 95°C for 30 seconds and 60°C for 1 minute. Genomic DNA samples were amplified and quantitated using an ABI Prism 7700 Sequence Detection System (PerkinElmer, Foster City, CA). Fluorescence of each reaction was detected at each cycle and used to determine molecules of mTREC using the standard curve by ABI Prism software. Samples were analyzed in duplicate. Molecules of mTRECs were determined in cell lysates equivalent to 50 000 cells.
Statistical analysis
Groupwide comparison was done by analysis of variance (ANOVA). Survival data were analyzed by log rank test. All statistical analyses were done using Statview software (SAS Institute, Cary, NC). It is considered not statistically significant if the Pvalue is more than .05.
Results
Engraftment
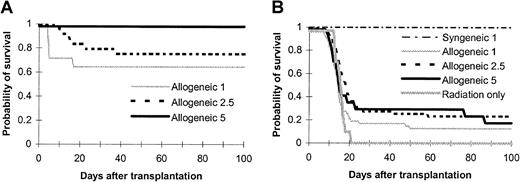
As demonstrated in Figure 1, all BALB/c mice that received lethal doses of radiation (8.5 Gy) died from bone marrow failure within 3 weeks after radiation (Figure 1B). Administration of 1 × 106 allogeneic full-term fetal blood cells from a 20-day-old C57BL/6 fetus rescued 13% of lethally irradiated BALB/c mice (Figure 1B; P < .05 compared with radiation-only control) that survived more than 100 days and remained healthy up to 200 days after transplantation. By increasing the cell numbers infused, there was not a statistical increase in the surviving rates (23% for 2.5 × 106, 18% for 5 × 106 on day +100, not significant between full-term fetal blood groups). The majority (> 70%) of the full-term fetal blood recipients died within the first 3 weeks. In contrast, allogeneic T-cell–depleted bone marrow cells protected the mice from lethal radiation in a dose-dependent manner (Figure 1A; 64% for 1 × 106, 79% for 2.5 × 106, 100% for 5 × 106 with the observation period of 100 days). A quantity of 1 × 106 syngeneic full-term fetal blood cells was also enough to rescue the recipients from lethal radiation (Figure 1B). These data suggest that allogeneic full-term fetal blood had a lower rate of engraftment compared with allogeneic T-cell–depleted bone marrow based on probability of survival. We next investigated the engraftment kinetics of full-term fetal blood stem cells compared with T-cell–depleted adult bone marrow stem cells in the allogeneic setting.
Ability of murine full-term fetal blood to reconstitute lethally irradiated adult murine recipients across major histocompatibility barrier.
Graded numbers of (A) T-cell–depleted bone marrow cells or (B) murine full-term fetal blood cells were transplanted into lethally irradiated (8.5 Gy) BALB/c recipients (full-term fetal blood: n = 47 for 1 × 106, n = 47 for 2.5 × 106, n = 17 for 5 × 106; T-cell–depleted bone marrow: n = 14 for 1 × 106, n = 24 for 2.5 × 106, n = 12 for 5 × 106). Controls included a group of mice receiving lethal radiation only (n = 10). Mortality was recorded daily. Data are probability of survival pooled from 2 to 6 independent experiments. Cell numbers in the legends were transplantation doses in millions. P < .05, allogeneic full-term fetal blood groups versus radiation only or versus T-cell–depleted bone marrow; P < .0001, between allogeneic and syngeneic full-term fetal blood groups; not significant, between different allogeneic full-term fetal blood groups; P < .05, between different T-cell–depleted bone marrow groups.
Ability of murine full-term fetal blood to reconstitute lethally irradiated adult murine recipients across major histocompatibility barrier.
Graded numbers of (A) T-cell–depleted bone marrow cells or (B) murine full-term fetal blood cells were transplanted into lethally irradiated (8.5 Gy) BALB/c recipients (full-term fetal blood: n = 47 for 1 × 106, n = 47 for 2.5 × 106, n = 17 for 5 × 106; T-cell–depleted bone marrow: n = 14 for 1 × 106, n = 24 for 2.5 × 106, n = 12 for 5 × 106). Controls included a group of mice receiving lethal radiation only (n = 10). Mortality was recorded daily. Data are probability of survival pooled from 2 to 6 independent experiments. Cell numbers in the legends were transplantation doses in millions. P < .05, allogeneic full-term fetal blood groups versus radiation only or versus T-cell–depleted bone marrow; P < .0001, between allogeneic and syngeneic full-term fetal blood groups; not significant, between different allogeneic full-term fetal blood groups; P < .05, between different T-cell–depleted bone marrow groups.
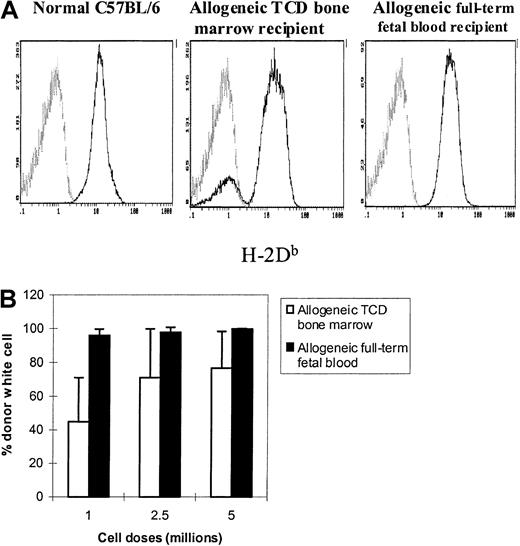
It is well known that T-cell depletion results in mixed chimerism in animal models when insufficient stem cells are infused. The level of mixed chimerism is related to the T-cell dose of the graft as well as to the total numbers of stem cells.23 We therefore tested the chimerism status of allogeneic fetal blood and bone marrow recipients. Figure 2A depicts one representative flow cytometric chimeric analysis. Full donor chimerism (> 95% H-2Db+ cells) was demonstrated in all allogeneic full-term fetal blood recipients, demonstrating the robust engraftment potential of these cells. The full-term fetal blood recipients that could survive the period of aplasia (see below) demonstrated superior engraftment as measured by their conversion to full donor chimerism. In contrast, the recipients of several doses (1 × 106-5 × 106) of T-cell–depleted bone marrow cells (Figure 2B) became mixed chimeras (a minimum of 1 × 107 T-cell–depleted bone marrow cells are needed for inducing full donor chimerism in this model). It is important to note that GVHD was not observed in either treatment group (data not shown).
Long-term engraftment potential of murine full-term fetal blood in MHC-mismatched adult recipients.
Peripheral blood was obtained from allogeneic full-term fetal blood recipients at least 100 days after transplantation. The whole blood samples were stained with FITC-conjugated anti-H-2Db(donor) and TC-conjugated anti-CD45 monoclonal antibodies simultaneously (see “Materials and methods”). The histograms were gated on CD45+ cells. The normal percent levels of H-2Db+ cells for C57BL/6 (positive) and BALB/c (negative) mice are 99.8% ± 0.1% and 0.09% ± 0.06%, respectively. (A) Representative histograms. The light gray lines represent recipient-derived cells. (B) Long-term engraftment potential of full-term fetal blood and T-cell–depleted bone marrow. Each group contained 3 to 9 animals. P < .05, allogeneic full-term fetal blood groups versus allogeneic T-cell–depleted bone marrow groups; not significant, allogeneic full-term fetal blood groups versus normal C57BL/6; P < .001, allogeneic full-term fetal blood groups versus normal BALB/c.
Long-term engraftment potential of murine full-term fetal blood in MHC-mismatched adult recipients.
Peripheral blood was obtained from allogeneic full-term fetal blood recipients at least 100 days after transplantation. The whole blood samples were stained with FITC-conjugated anti-H-2Db(donor) and TC-conjugated anti-CD45 monoclonal antibodies simultaneously (see “Materials and methods”). The histograms were gated on CD45+ cells. The normal percent levels of H-2Db+ cells for C57BL/6 (positive) and BALB/c (negative) mice are 99.8% ± 0.1% and 0.09% ± 0.06%, respectively. (A) Representative histograms. The light gray lines represent recipient-derived cells. (B) Long-term engraftment potential of full-term fetal blood and T-cell–depleted bone marrow. Each group contained 3 to 9 animals. P < .05, allogeneic full-term fetal blood groups versus allogeneic T-cell–depleted bone marrow groups; not significant, allogeneic full-term fetal blood groups versus normal C57BL/6; P < .001, allogeneic full-term fetal blood groups versus normal BALB/c.
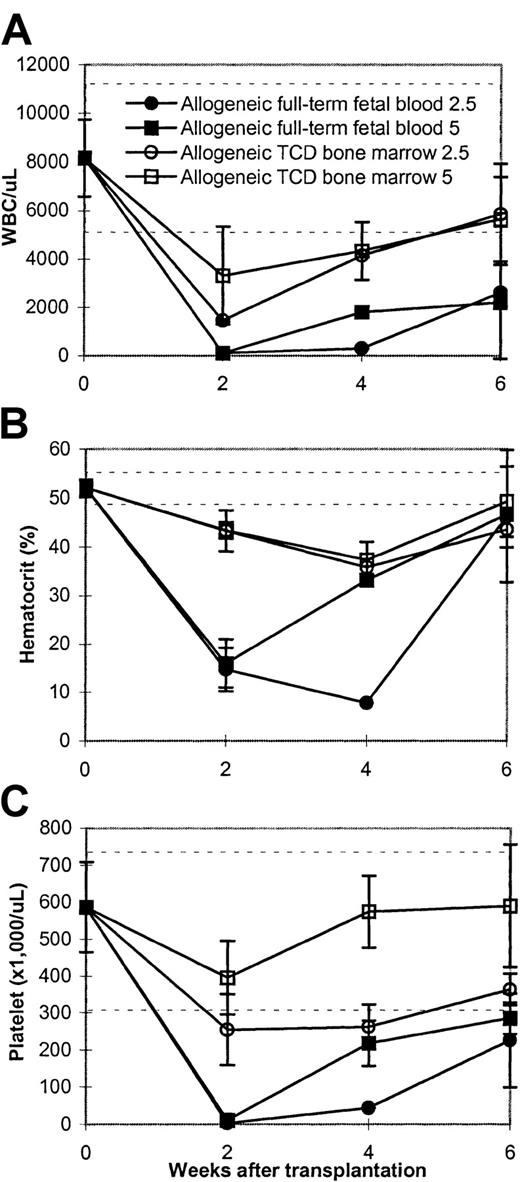
Many of the animals in the allogeneic full-term fetal blood group died from bleeding problems compared with the T-cell–depleted bone marrow group. We therefore monitored the engraftment of these animals by following the peripheral blood counts of both groups. The data (Figure3 and Figure4) demonstrated that the animals were dying because the allogeneic full-term fetal blood recipients became profoundly thrombocytopenic, anemic, and neutropenic compared with the bone marrow transplant controls. All animals had nadirs of all 3 lineages but whereas the T-cell–depleted bone marrow recipients recovered between 1 and 2 weeks, hematopoietic recovery in the full-term fetal blood recipients took significantly longer (Figure 3).
Kinetics of hematopoietic recovery in allogeneic full-term fetal blood recipients.
Lethally irradiated (8.5Gy) BALB/c mice were infused with full-term fetal blood from a 20-day-old C57BL/6 fetus. Peripheral blood was obtained using ethylenediaminetetracetic acid (EDTA) tubes every other week after transplantation. Data represent mean ± SD of 2 to 5 mice per timepoint. This is an experiment representative of 3 experiments. The dashed horizontal lines represent the lower and upper limits of normal. The values of blood counts in the mice receiving lethal radiation only on day +14 were as follows: WBC: 90/μL ± 38/μL blood; hematocrit: 14.5% ± 5.9%; platelet: 35.6/μL ± 20.7/μL blood. (A) White blood cells (WBC). (B) Hematocrit. (C) Platelet.
Kinetics of hematopoietic recovery in allogeneic full-term fetal blood recipients.
Lethally irradiated (8.5Gy) BALB/c mice were infused with full-term fetal blood from a 20-day-old C57BL/6 fetus. Peripheral blood was obtained using ethylenediaminetetracetic acid (EDTA) tubes every other week after transplantation. Data represent mean ± SD of 2 to 5 mice per timepoint. This is an experiment representative of 3 experiments. The dashed horizontal lines represent the lower and upper limits of normal. The values of blood counts in the mice receiving lethal radiation only on day +14 were as follows: WBC: 90/μL ± 38/μL blood; hematocrit: 14.5% ± 5.9%; platelet: 35.6/μL ± 20.7/μL blood. (A) White blood cells (WBC). (B) Hematocrit. (C) Platelet.
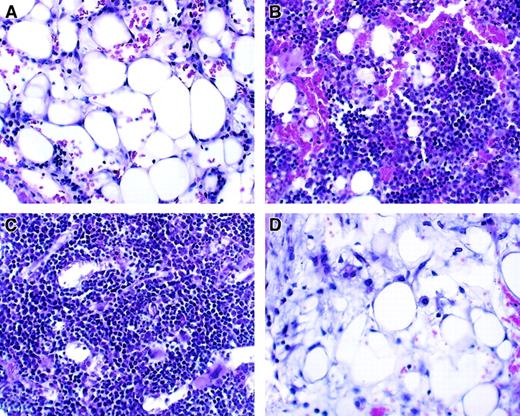
Histologic analysis of bone marrow from transplant recipients.
Hematoxylin and eosin staining; original magnification, ×100. Representative bone marrow changes in allogeneic full-term fetal blood recipients are shown. Bone barrow was obtained 14 to 15 days after treatment. (A) Radiation only. (B) Allogeneic T-cell–depleted bone marrow. (C) Allogeneic T-cell–depleted bone marrow plus T cells. (D) Allogeneic full-term fetal blood.
Histologic analysis of bone marrow from transplant recipients.
Hematoxylin and eosin staining; original magnification, ×100. Representative bone marrow changes in allogeneic full-term fetal blood recipients are shown. Bone barrow was obtained 14 to 15 days after treatment. (A) Radiation only. (B) Allogeneic T-cell–depleted bone marrow. (C) Allogeneic T-cell–depleted bone marrow plus T cells. (D) Allogeneic full-term fetal blood.
Absolute number of lymphocytes and lymphocyte subsets
Phenotypic analyses of peripheral blood from long-term survivors (200 days after stem cell transplantation) were performed. Table2 indicates that T cells (CD3+, CD3+4+, CD3+8+, CD4+62L+, and CD8+62L+) and B cells (B220+) were lower in allogeneic full-term fetal blood recipients compared with T-cell–depleted bone marrow recipients although the differences in CD3+8+ and CD8+62L+cells were not statistically significant. There was no difference in the frequency of NK cells (NK1.1+CD3−) compared with allogeneic T-cell–depleted bone marrow recipients, but the absolute numbers were lower.
Absolute counts of lymphocytes and lymphocyte subsets in peripheral blood
| Group . | Normal C57BL/6 (n = 15) . | Syngeneic full-term fetal blood (n = 5) . | Allogeneic T-cell–depleted bone marrow (n = 4) . | Allogeneic full-term fetal blood (n = 8) . |
|---|---|---|---|---|
| Total lymphocyte | 4243 ± 892 | 15 400 ± 3745* | 3051 ± 965 | 1534 ± 670*,†,‡ |
| CD3+ | 1363 ± 241 | 2682 ± 345* | 1454 ± 516 | 931 ± 201*,†,‡ |
| CD3+4+ | 773 ± 147 | 1540 ± 167* | 909 ± 274 | 605 ± 178*,†,‡ |
| CD3+8+ | 379 ± 69 | 1140 ± 198* | 488 ± 254 | 305 ± 104† |
| CD4+CD62L+ | 380 ± 57 | 433 ± 87 | 442 ± 132 | 146 ± 57*,†,‡ |
| CD8+CD62L+ | 187 ± 144 | 182 ± 113 | 112 ± 41 | 76 ± 56† |
| B220+ | 2912 ± 714 | 14 424 ± 4657* | 2352 ± 410 | 1024 ± 848*,†,‡ |
| NK1.1+CD3− | 152 ± 44 | 164 ± 69 | 43 ± 10 | 56 ± 21*,† |
| Group . | Normal C57BL/6 (n = 15) . | Syngeneic full-term fetal blood (n = 5) . | Allogeneic T-cell–depleted bone marrow (n = 4) . | Allogeneic full-term fetal blood (n = 8) . |
|---|---|---|---|---|
| Total lymphocyte | 4243 ± 892 | 15 400 ± 3745* | 3051 ± 965 | 1534 ± 670*,†,‡ |
| CD3+ | 1363 ± 241 | 2682 ± 345* | 1454 ± 516 | 931 ± 201*,†,‡ |
| CD3+4+ | 773 ± 147 | 1540 ± 167* | 909 ± 274 | 605 ± 178*,†,‡ |
| CD3+8+ | 379 ± 69 | 1140 ± 198* | 488 ± 254 | 305 ± 104† |
| CD4+CD62L+ | 380 ± 57 | 433 ± 87 | 442 ± 132 | 146 ± 57*,†,‡ |
| CD8+CD62L+ | 187 ± 144 | 182 ± 113 | 112 ± 41 | 76 ± 56† |
| B220+ | 2912 ± 714 | 14 424 ± 4657* | 2352 ± 410 | 1024 ± 848*,†,‡ |
| NK1.1+CD3− | 152 ± 44 | 164 ± 69 | 43 ± 10 | 56 ± 21*,† |
Peripheral blood was obtained 200 days after transplantation and analyzed by flow cytometric analysis. The values represent absolute counts per μL blood (mean ± SD), which were obtained by using Flow-Count fluorospheres (Coulter, Miami, FL).
P < .05, compared with normal C57BL/6 mice.
P < .05, compared with syngeneic full-term fetal blood recipients (C57BL/6 → C57BL/6).
P < .05, compared with allogeneic T-cell–depleted bone marrow recipients.
Measurement of in vivo responses: host-specific tolerance
To determine host antigen-specific alloreactive T-cell responses, hearts from C3H/HeJ (third party) and BALB/c (recipient) newborn mice were transplanted into left and right pouches, respectively, created in the pinnae of ears of transplant recipients between day 118 and day 145 after stem cell transplantation. Similar to age-compatible BALB/c mice and T-cell–depleted bone marrow recipients, allogeneic full-term fetal blood recipients accepted hearts of recipient origin for at least 60 days after heart transplantation (Figure5A; P < .01 compared with C57BL/6). All age-compatible C57BL/6 mice (positive control) rejected hearts of recipient origin between day 9 and day 11 after heart transplantation (Figure 5A).
Host-specific tolerance and delayed rejection of third-party hearts by murine adult full-term fetal blood recipients across major histocompatibility barrier.
Hearts from BALB/c (H-2d, recipient-strain heart [A]) and C3H/HeJ (H-2k, third-party heart [B]) newborn mice (< 48 hours old) were transplanted into pouches created in the pinnae of left and right ears of the mice, respectively. The cardiac transplantation was done between day 118 and day 145 after stem cell transplantation. Heart graft survival was assessed daily by visual evaluation of the presence or absence of heart contractions. Each group contained at least 4 mice. For recipient-strain graft (A), P < .01, C57BL/6 mice versus other groups. For third-party graft (B),P < .05, allogeneic T-cell–depleted bone marrow recipients versus other groups; P < .05, allogeneic full-term fetal blood recipients versus other groups.
Host-specific tolerance and delayed rejection of third-party hearts by murine adult full-term fetal blood recipients across major histocompatibility barrier.
Hearts from BALB/c (H-2d, recipient-strain heart [A]) and C3H/HeJ (H-2k, third-party heart [B]) newborn mice (< 48 hours old) were transplanted into pouches created in the pinnae of left and right ears of the mice, respectively. The cardiac transplantation was done between day 118 and day 145 after stem cell transplantation. Heart graft survival was assessed daily by visual evaluation of the presence or absence of heart contractions. Each group contained at least 4 mice. For recipient-strain graft (A), P < .01, C57BL/6 mice versus other groups. For third-party graft (B),P < .05, allogeneic T-cell–depleted bone marrow recipients versus other groups; P < .05, allogeneic full-term fetal blood recipients versus other groups.
Age-matched normal BALB/c, normal C57BL/6, and syngeneic full-term fetal blood control (C57BL/6 → C57BL/6) mice rejected third-party hearts (C3H/HeJ, H-2k) between day 9 and day 11 (median, 9 days) after heart transplantation (Figure 5B). Allogeneic T-cell–depleted bone marrow recipients rejected third-party hearts between day 14 and day 25 (median, 14.5 days; P < .05, compared with other groups). In contrast, third-party hearts were rejected following a significant delay in allogeneic full-term fetal blood recipients. However, all were eventually rejected between day 18 and day 30 (median, 23 days; P < .05 compared with other groups) after heart transplantation.
Decreased immune responses against alloantigens in vitro
Mice were challenged with third-party hearts (C3H/HeJ, H-2k) in vivo between day 118 and day 145 after stem cell transplantation. Forty-five to 85 days after the challenge, splenocyte and serum samples were taken for the following assays: proliferation, T-cell cytotoxicity, and cytotoxic antibody assays. Spleen cells were isolated and cultured with irradiated C3H/HeJ spleen cells for 112 hours for proliferation assays. As shown in Figure6A, allogeneic full-term fetal blood recipients had lower proliferative responses against third-party alloantigens compared with allogeneic T-cell–depleted bone marrow recipients (P < .05) or normal C57BL/6 mice (P < .01) respectively. In contrast, syngeneic full-term fetal blood recipients had increased proliferative responses (P < .0001).
Decreased immune responses against alloantigens in vitro of allogeneic full-term fetal blood recipients.
Mice were challenged with third-party newborn C3H/HeJ hearts in vivo 45 to 82 days before the assay. Spleen cells were isolated 200 days after transplantation and stimulated in vitro with irradiated C3H/HeJ spleen cells for proliferation and CTL assay. Sera were obtained on the same day for alloantigen-specific cytotoxic antibody assay. (A) Proliferation was determined by [3H]thymidine incorporation. (B) Cytotoxicity was assessed with51Cr-labeled 2-day Con A–activated C3H/HeJ spleen cells. Nonspecific release against irrelevant antigens (B10.D2) was performed to ensure the specificity of killing. (C) Third-party alloantigen-specific cytotoxic antibody was detected by microcytotoxicity assay. Two-day Con A–activated C3H/HeJ spleen cells were used as target cells. Dead cells were visualized by trypan blue. Each group contained 2 to 5 animals. *P < .05, compared with allogeneic T-cell–depleted bone marrow recipients.#P < .01, compared with normal C57BL/6 mice. TCD indicates T-cell–depleted.
Decreased immune responses against alloantigens in vitro of allogeneic full-term fetal blood recipients.
Mice were challenged with third-party newborn C3H/HeJ hearts in vivo 45 to 82 days before the assay. Spleen cells were isolated 200 days after transplantation and stimulated in vitro with irradiated C3H/HeJ spleen cells for proliferation and CTL assay. Sera were obtained on the same day for alloantigen-specific cytotoxic antibody assay. (A) Proliferation was determined by [3H]thymidine incorporation. (B) Cytotoxicity was assessed with51Cr-labeled 2-day Con A–activated C3H/HeJ spleen cells. Nonspecific release against irrelevant antigens (B10.D2) was performed to ensure the specificity of killing. (C) Third-party alloantigen-specific cytotoxic antibody was detected by microcytotoxicity assay. Two-day Con A–activated C3H/HeJ spleen cells were used as target cells. Dead cells were visualized by trypan blue. Each group contained 2 to 5 animals. *P < .05, compared with allogeneic T-cell–depleted bone marrow recipients.#P < .01, compared with normal C57BL/6 mice. TCD indicates T-cell–depleted.
For the cytotoxicity assays, spleen cells were isolated and cultured with irradiated C3H/HeJ spleen cells for 112 hours. Effector cells were assayed for cytotoxicity in standard 51Cr release assay using 2-day Con A–activated C3H/HeJ spleen cells. As shown in Figure6B, third-party alloantigen-specific cytotoxic T lymphocytes were elicited from normal C57BL/6, syngeneic full-term fetal blood and allogeneic T-cell–depleted bone marrow recipients, but not from allogeneic full-term fetal blood recipients (P < .01 in the ratio of 50:1 compared with other groups).
We next evaluated whether cytotoxic antibodies specific to C3H/HeJ were generated after in vivo C3H/HeJ challenge in a microcytotoxicity assay. As shown in Figure 6C, high titers of cytotoxic antibody were generated in normal C57BL/6 mice and syngeneic full-term fetal blood recipients, but not in allogeneic T-cell–depleted bone marrow or allogeneic full-term fetal blood recipients (P < .01, compared with normal C57BL/6).
Impaired B-cell proliferative responses against mitogen
B-cell proliferative responses were determined by BrdU incorporation assay. At a per-cell level, B cells in allogeneic full-term fetal blood recipients did not proliferate as well as those found in allogeneic T-cell–depleted bone marrow recipients, syngeneic full-term fetal blood recipients, and normal C57BL/6 mice (Table3, P < .05). In contrast, the proliferation responses of B cells from both allogeneic T-cell–depleted bone marrow and syngeneic full-term fetal blood recipients against either anti-IgM antibody or LPS were comparable with that from normal C57BL/6 mice (Table 3), demonstrating that B-cell proliferative defects were limited to allogeneic full-term fetal blood recipients.
B-cell proliferation potential assessed at a single cell level
| Groups . | % BrdU+ B cells . | |
|---|---|---|
| Anti-IgM . | LPS . | |
| Normal C57BL/6 | 30.5 ± 1.8 | 26.6 ± 3.4 |
| Syngeneic full-term fetal blood | 35.2 ± 6.6 | 31.1 ± 1.1 |
| Allogeneic T-cell–depleted bone marrow | 34.8 ± 5.3 | 33.0 ± 4.2 |
| Allogeneic full-term fetal blood | 22.5 ± 1.03-150 | 23.7 ± 1.23-150 |
| Groups . | % BrdU+ B cells . | |
|---|---|---|
| Anti-IgM . | LPS . | |
| Normal C57BL/6 | 30.5 ± 1.8 | 26.6 ± 3.4 |
| Syngeneic full-term fetal blood | 35.2 ± 6.6 | 31.1 ± 1.1 |
| Allogeneic T-cell–depleted bone marrow | 34.8 ± 5.3 | 33.0 ± 4.2 |
| Allogeneic full-term fetal blood | 22.5 ± 1.03-150 | 23.7 ± 1.23-150 |
Spleen cells were obtained 200 days after transplantation and activated by anti-IgM antibody and LPS. Proliferation of B cells was determined by incorporation of BrdU on B cells, which was measured by intracellular fluorescent detection of BrdU staining combined with B220 surface staining.
P < .05, compared with other groups.
Antigen-presenting cells
To evaluate the role of antigen-presenting cells on the impaired immune function found in allogeneic full-term fetal blood recipients, we measured the frequency of splenic dendritic cells24,25(CD11c+CD3−CD4−B220−Gr-1−), which are the most potent antigen-presenting cells.26 We also studied the ability of spleen cells to stimulate allogeneic T cells from normal BALB/c mice in mixed lymphocyte reaction (MLR). The frequency (data not shown) and the ability to present alloantigens (Figure 7B) of splenic dendritic cells were normal in allogeneic full-term fetal blood recipients. However, the absolute numbers of splenic dendritic cells were significantly lower in allogeneic full-term fetal blood recipients compared with T-cell–depleted bone marrow recipients (Figure7A, P < .01).
Antigen-presenting cells in allogeneic full-term fetal blood recipients.
Spleen cells were obtained between 148 and 258 days after transplantation for the following assays. (A) Absolute number of splenic dendritic cells. Spleen cells were stained with anti-CD11c, CD3, CD4, B220 and Gr-1 antibodies simutaneously. Splenic dendritic cells were defined as CD11c+CD3−CD4−B220−Gr-1−. (B) Functional analysis of antigen-presenting cells by MLR. Spleen cells were irradiated (20 Gy) and used as accessory cells to stimulate spleen cells from BALB/c mice (2.5 × 105/well). Proliferation was determined by incorporation of [3H]thymidine. Each group contained 5 animals.*P < .01, compared with other groups.
Antigen-presenting cells in allogeneic full-term fetal blood recipients.
Spleen cells were obtained between 148 and 258 days after transplantation for the following assays. (A) Absolute number of splenic dendritic cells. Spleen cells were stained with anti-CD11c, CD3, CD4, B220 and Gr-1 antibodies simutaneously. Splenic dendritic cells were defined as CD11c+CD3−CD4−B220−Gr-1−. (B) Functional analysis of antigen-presenting cells by MLR. Spleen cells were irradiated (20 Gy) and used as accessory cells to stimulate spleen cells from BALB/c mice (2.5 × 105/well). Proliferation was determined by incorporation of [3H]thymidine. Each group contained 5 animals.*P < .01, compared with other groups.
Measurement of T-cell receptor excision circle bearing recent thymic emigrants
Proper thymic function is an important component of immune recovery following stem cell transplantation.27 The T-cell receptor excision circle (TREC) assay is a powerful method to follow the emergence of T cells in humans undergoing stem cell transplantation.21 27 When T-cell precursors rearrange the TCRα chain locus the TCRδ locus is excised and forms a circular episome. These signal joint TRECs (sjTRECs) are detected in T cells that are recent thymic emigrants or long-lived naı̈ve cells. T cells that have divided in the periphery dilute out their no-replicated TRECs. Recently, the sjTREC assay in mice has been established by Sempowski et al (unpublished data, August 2001) and we have utilized this assay to test the T-cell recovery in the various groups of controls and experimental animals. This ability to quantitate mouse thymic emigrants is critical to follow T-cell immune reconstitution strategies. As can be appreciated from Table4, allogeneic full-term fetal blood recipients that have survived more than 100 days have a clear delay in reconstitution of mTREC-positive T cells compared with T-cell–depleted bone marrow–engrafted animals. These data are in agreement with the phenotypic data presented in Table 2.
mTREC levels in allogeneic full-term fetal blood recipients compared with T-cell–depleted bone marrow recipients
| Groups . | n . | mTRECs/100 000 cells . | |
|---|---|---|---|
| CD4 . | CD8 . | ||
| Normal C57BL/6 | 3 | 9860 ± 4878 | 8072 ± 1586 |
| Allogeneic T-cell–depleted bone marrow | 5 | 13 825 ± 3721 | 9604 ± 2771 |
| Allogeneic full-term fetal blood | 4 | 3092 ± 9024-150 | 2605 ± 2284-150 |
| Groups . | n . | mTRECs/100 000 cells . | |
|---|---|---|---|
| CD4 . | CD8 . | ||
| Normal C57BL/6 | 3 | 9860 ± 4878 | 8072 ± 1586 |
| Allogeneic T-cell–depleted bone marrow | 5 | 13 825 ± 3721 | 9604 ± 2771 |
| Allogeneic full-term fetal blood | 4 | 3092 ± 9024-150 | 2605 ± 2284-150 |
Spleen cells were obtained 200 days after transplantation. The methods for quantification of mTRECs can be found in “Materials and methods.”
mTRECs indicates mouse T-cell receptor signal joint excision circles.
P < .05, compared to other groups.
Discussion
In this study, we have compared the outcomes following transplantation of allogeneic T-cell–depleted adult bone marrow with full-term fetal blood. Studies of the clinical outcome following cord blood transplantation (CBT) in humans have demonstrated that myeloid engraftment and immune reconstitution is impaired.1 4 Since full-term fetal/cord blood in mice is not comparable with that found in humans because murine full-term fetal cord blood contains essentially no T cells (Table 1), we have compared murine full-term fetal blood with murine T-cell–depleted adult bone marrow cells. In these 2 clinical situations where T cells are not present, engraftment of myeloid and lymphoid cells can be observed and studied free from the confounding effects of GVHD.
The results demonstrate several interesting observations. First, in terms of hematopoietic engraftment, full-term fetal blood transplantation was not consistently able to protect the recipients from lethal irradiation when transplanted across a major histocompatibility complex (MHC) barrier (Figure 1). In full-term fetal blood, there is the suggestion of a relative paucity of radioprotective cells because when these cells are transplanted across an MHC barrier, the majority of recipients die from marrow aplasia (Figure 3 and Figure4). On the other hand, syngeneic fetal cells protect the host quite well (Figure 1). However, if the allogeneic recipient survives the prolonged period of aplasia, the engrafting cells are quite potent in that the recipients become fully donor chimeric rather than mixed chimeras (Figure 2). The results suggest that the murine full-term fetal blood cells have efficient long-term engrafting potential if the animal can be supported through the first several weeks following transplantation.
We also found a clear difference in the immune response between the ability of T-cell–depleted adult bone marrow compared with full-term fetal blood in both in vivo (Figure 5) and in vitro (Figure 6) responses. The impaired immune function in MHC mismatched adult murine recipients of full-term fetal blood was directly demonstrated in vivo through third-party heart transplantation (Figure 5).
Abnormal immune responses can be due to decreased numbers of total immunocompetent cells (quantitative defect). Phenotypic analysis of lymphocytes and lymphocyte subsets (Table 2) demonstrated that absolute numbers of lymphocyte subsets in allogeneic T-cell–depleted bone marrow recipients had recovered to the same level as those of normal C57BL/6 mice whereas allogeneic full-term fetal blood recipients had decreased numbers of total peripheral blood T, B, and splenic dendritic cells (Figure 7A) as well as CD4+62L+ T cells (including all naı̈ve and some “revertant” antigen-experienced CD4+ T cells). These data suggested that homeostasis of peripheral lymphocytes is normal in allogeneic T-cell–depleted bone marrow recipients but is not adequately maintained in recipients of allogeneic full-term fetal blood. The changes in immune recovery between T-cell–depleted bone marrow and full-term fetal blood may reflect the maturation status of the donor cells, numbers of lymphoid progenitors found in the graft, or the lack of proper niches for the full-term fetal blood cells to develop properly. However, at a per-cell level, T cells from allogeneic full-term fetal blood recipients proliferate and produce cytokines (IFNγ and IL-2) as well as those from T-cell–depleted bone marrow recipients (data not shown).
GVHD, which has been demonstrated by many investigators to interfere with the immune function,8,9,28 might possibly contribute to the impaired immune function observed in allogeneic fetal blood recipients. However, neither clinical nor histologic evidence of GVHD was found (data not shown). Donor-recipient histoincompatibility7 may explain the impaired immune function in both recipients of allogeneic full-term fetal blood and allogeneic T-cell–depleted bone marrow. It is unclear, however, why there is a more profound immune deficiency found in allogeneic full-term fetal blood recipients compared with that in allogeneic T-cell–depleted bone marrow recipients. Analysis of recent thymic emigrants from T-cell–depleted bone marrow compared with full-term fetal blood (Table 4) demonstrates that there are fewer mTRECs per 100 000 CD4+ or CD8+ cells in the spleen of allogeneic full-term fetal blood recipients. Decreased thymic output in allogeneic full-term fetal blood recipients may have resulted from fewer lymphoid precursors in fetal blood. The differences we have observed between T-cell–depleted bone marrow and full-term fetal blood may possibly reflect the contribution of very small numbers of mature T cells that are still present in T-cell–depleted grafts compared with fetal blood, which is devoid of any mature T cells. However, this assumption is not supported by the observation of higher mTREC-positive T cells in T-cell–depleted bone marrow recipients (Table 4).
Generation of specific antibody responses requires activation of B cells and T-cell help. B cells from allogeneic T-cell–depleted bone marrow and syngeneic full-term fetal blood recipients are normal in proliferation since they have comparable ability to proliferate with normal C57BL/6 mice (Table 3). In contrast, B cells from allogeneic full-term fetal blood recipients did not proliferate as well as those from other groups (Table 3), suggesting defective differentiation of B cells in allogeneic full-term fetal blood recipients, which may contribute to poor antibody production in these animals. In both allogeneic full-term fetal blood and allogeneic T-cell–depleted bone marrow recipients, normal or close-to-normal B-cell proliferation (Table 3), although unable to generate alloantigen-specific antibody in vivo (Figure 6C), suggests either a lack of T-cell help or inability to produce antibody by activated B cells.
Global impaired immune function may also be due to defective antigen-presenting cells (Figure 7A).29 However, a normal frequency of splenic dendritic cells (data not shown), which are the most potent antigen-presenting cells,26 along with the ability to initiate alloresponse (Figure 7B), imply that antigen-presenting cells from allogeneic full-term fetal blood recipients do not have an intrinsic defect.
Taken together, the mechanisms underlying the impaired immune function in allogeneic full-term fetal blood recipients include decreased absolute immunocompetent cells, indirect evidence of disturbance of T-cell homeostasis, reduced thymic output, and defects in B-cell antibody production. When combined, these differences contribute to the observed immune defects following allogeneic full-term fetal/cord blood engraftment. Understanding these differences will contribute to our understanding of CBT in our patients.
We would like to thank Dr Richard J. Rahija for assistance in collecting murine fetal blood, Dr Thaddeus V. Samulski for animal radiation, and Ms Julie Wilkinson for helpful discussion on flow cytometric techniques.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nelson J. Chao, Bone Marrow Transplantation Program, Duke University Medical Center, 2400 Pratt St, Suite 1100, Durham, NC 27705; e-mail: chao0002@mc.duke.edu.

![Fig. 5. Host-specific tolerance and delayed rejection of third-party hearts by murine adult full-term fetal blood recipients across major histocompatibility barrier. / Hearts from BALB/c (H-2d, recipient-strain heart [A]) and C3H/HeJ (H-2k, third-party heart [B]) newborn mice (< 48 hours old) were transplanted into pouches created in the pinnae of left and right ears of the mice, respectively. The cardiac transplantation was done between day 118 and day 145 after stem cell transplantation. Heart graft survival was assessed daily by visual evaluation of the presence or absence of heart contractions. Each group contained at least 4 mice. For recipient-strain graft (A), P < .01, C57BL/6 mice versus other groups. For third-party graft (B),P < .05, allogeneic T-cell–depleted bone marrow recipients versus other groups; P < .05, allogeneic full-term fetal blood recipients versus other groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.364/6/m_h80121925005.jpeg?Expires=1769197143&Signature=iynCRh3AisMXCkkRV30P~QlpgExW8VXYrI4F9pl56PDoj1lzlVc7flDe9NCsQB5Xr2o1BmTgEpzG9h2gUPvZHap8rHG-ft7rF8X~eMUd-92ElAiTNCPguv588iUGT91rtctKJ8TU7nmdVG9xEAFPcHiFh1PFMCct4cYpeFX37EZEBzLdHWf~AAl~9rfmVI88sMI56PYzDy~k9SuOnFaTQx7q9Y0peATGtss6-UU3gBbGAPZMNXzk53~hUw1SjElkm4o15iCX~ZgAy9ySxIUwYdZBMhWmh~m0a7cj8MUXiIgHIq8O9vovv3v0kzovWDhEKM3AOfeiwCcjREWQxfFdbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Decreased immune responses against alloantigens in vitro of allogeneic full-term fetal blood recipients. / Mice were challenged with third-party newborn C3H/HeJ hearts in vivo 45 to 82 days before the assay. Spleen cells were isolated 200 days after transplantation and stimulated in vitro with irradiated C3H/HeJ spleen cells for proliferation and CTL assay. Sera were obtained on the same day for alloantigen-specific cytotoxic antibody assay. (A) Proliferation was determined by [3H]thymidine incorporation. (B) Cytotoxicity was assessed with51Cr-labeled 2-day Con A–activated C3H/HeJ spleen cells. Nonspecific release against irrelevant antigens (B10.D2) was performed to ensure the specificity of killing. (C) Third-party alloantigen-specific cytotoxic antibody was detected by microcytotoxicity assay. Two-day Con A–activated C3H/HeJ spleen cells were used as target cells. Dead cells were visualized by trypan blue. Each group contained 2 to 5 animals. *P < .05, compared with allogeneic T-cell–depleted bone marrow recipients.#P < .01, compared with normal C57BL/6 mice. TCD indicates T-cell–depleted.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.364/6/m_h80121925006.jpeg?Expires=1769197143&Signature=Svd0-nd7lYujmEq4vv8htcUI15atTqHcwVGJmJ1STEYKdIfKbJlHVDxxSXtd6RjDHjggzfh7FDhqfekcP0xbvdbY17zsO2zj5n8wdBU2Y9uC7CswUTq5KsoBCoCzeGFmspxL15K2NOdO21C-mwcfjpGJbf0nef9pFquMOJpwx091pMAUaiOymEgBZ6FOAZV2r~1lvI1POaIUqSP9pjFTsYmHhGS6k3UR5p3ER1LG3tKRDoP-YGOp50t-inAjfj8HStj8pVeJ6LdVvUDd6FKnXLKBhUxCenoZeljQZQ9rKUe-fKqWWdgghWCGWzC32oS9NuEIP7MZ0VqU-MGybC1Q8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Antigen-presenting cells in allogeneic full-term fetal blood recipients. / Spleen cells were obtained between 148 and 258 days after transplantation for the following assays. (A) Absolute number of splenic dendritic cells. Spleen cells were stained with anti-CD11c, CD3, CD4, B220 and Gr-1 antibodies simutaneously. Splenic dendritic cells were defined as CD11c+CD3−CD4−B220−Gr-1−. (B) Functional analysis of antigen-presenting cells by MLR. Spleen cells were irradiated (20 Gy) and used as accessory cells to stimulate spleen cells from BALB/c mice (2.5 × 105/well). Proliferation was determined by incorporation of [3H]thymidine. Each group contained 5 animals.*P < .01, compared with other groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.364/6/m_h80121925007.jpeg?Expires=1769197143&Signature=EkkXhzpDO~PWBuZjpl9emvL7OegwrIar~yJOmxreGqExqwgghXiRPxy6-qnzAzxc1qzGLqOVmMOBA76kIKf1aU-lnLkNU-73zuDpiJ21y-qKO0p8BGvKIzFjFesCCNI7II53170SSR5UOkgXu4WrwUjVJRLWAdTyqY3LN8VKQiZC30Yroha7CuSzf~WqS-3OPMfN-C9WJs-qwedfd1E2UNNCDglj~yCoGK8rx09XM4F76vKuSdXOUxCn9zhkGPX9hABFxlcIrZUmJjHzqG8Ljfw6~GZpB-hK4GLF5o2I42cc1G1U7xiqzZz8Xd9vWTpYLKokbOUMrY371NbLcO2QRg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal