In clinical trials, the tyrosine kinase inhibitor STI571 has proven highly effective in reducing leukemic cell burden in chronic myeloid leukemia (CML). The overall sensitivity of CML CD34+ progenitor cells to STI571 and the degree to which cell death was dependent on cell cycle status were determined. Stem cells (Lin−CD34+) from the peripheral blood of patients with CML in chronic phase and from granulocyte–colony-stimulating factor–mobilized healthy donors were labeled with carboxy-fluorescein diacetate succinimidyl diester dye to enable high-resolution tracking of cell division. Then they were cultured for 3 days with and without growth factors ± STI571. After culture, the cells were separated by fluorescence-activated cell sorting into populations of viable quiescent versus cycling cells for genotyping. For healthy controls, in the presence of growth factors, STI571 affected neither cell cycle kinetics nor recovery of viable cells. In the absence of growth factors, normal cells were unable to divide. For CML samples, in the presence or absence of growth factors, the response to STI571 was variable. In the most sensitive cases, STI571 killed almost all dividing cells; however, a significant population of viable CD34+ cells was recovered in the undivided peak and confirmed to be part of the leukemic clone. STI571 also appeared to exhibit antiproliferative activity on the quiescent population. These studies confirm that CML stem cells remain viable in a quiescent state even in the presence of growth factors and STI571. Despite dramatic short-term responses in vivo, such in vitro insensitivity to STI571, in combination with its demonstrated antiproliferative activity, could translate into disease relapse after prolonged therapy.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disease characterized by the t(9;22) chromosome translocation that, in turn, creates the BCR-ABL oncogene.1-3 The fusion gene product is a p210 oncoprotein containing a constitutively active tyrosine kinase that confers certain growth advantages to the Philadelphia-positive (Ph+) clone compared with normal hematopoietic cells.4
We have demonstrated recently the existence of a population of rare, primitive, quiescent stem cells in all chronic-phase CML patient samples, whether derived from peripheral blood or bone marrow. These stem cells are predominantly Ph+, express high levels of CD34+ but lack the markers CD38, CD45RA, or CD71, and can spontaneously exit G0 to enter a continuously proliferating state, either in vitro or to produce Ph+progeny in immunocompromised mice in vivo.5 6
Many cancers are treated with relatively nonselective cytotoxic drugs that affect normal and malignant cells. Because most available chemotherapeutic agents show some degree of S-phase specificity, cells that are not actively dividing may prove resistant to such drugs. This raises the possibility that the quiescent leukemic cells we have identified in patients with CML are likely to survive standard chemotherapy regimens, and it may explain the clinical observation that, unlike acute myeloid leukemia, CML cannot be eradicated by chemotherapy alone.7 8
The recent development of a novel, molecularly targeted, anticancer agent has heralded a major breakthrough in leukemia therapy.9-11 STI571 (Glivec; Novartis Pharmaceuticals, Basel, Switzerland) is a signal transduction inhibitor that acts specifically on the p210BCR-ABL tyrosine kinase.9,10 In vitro, this agent selectively suppresses the growth of primary CML colony-forming cells and of BCR-ABL+ cell lines11,12 and can eradicate BCR-ABL+ tumors in nude mice.13 Phase 1 and 2 studies began in June 1998 and targeted advanced-phase CML, Ph+ acute leukemia, and chronic-phase CML refractory to, or intolerant of, interferon. To date, results appear far better than those achievable using other nontransplantation treatment modalities, suggesting that STI571 will prove to be a critical advance in the treatment of patients with CML.14-16
However, a note of caution should be taken from laboratory data generated by 3 independent groups. These investigators have shown that resistance to STI571 may be induced in human BCR-ABL+ cell lines and is frequently mediated by amplification and overexpression of the BCR-ABL gene, though overexpression of theMDR gene may also contribute in some instances.17-19 Similar data are now surfacing for patients in blast crisis who have relapses while still taking the drug.20 21 In this study we aimed to determine the sensitivity of CML CD34+ progenitor cells to STI571 and to assess to what degree the inhibitory effect of STI571 was dependent on cell cycle status and whether STI571 had antiproliferative activity on Ph+ stem cells.
Materials and methods
Cell samples
Fresh leukapheresis products from patients with chronic-phase CML (Table 1) or from healthy allogeneic donors were enriched for CD34+ cells by either StemSep (StemCell Technologies, Vancouver, BC, Canada) or Isolex (NEXELL International, Brussels, Belgium) systems. The cells were then cryopreserved in 10% dimethyl sulfoxide (Sigma Aldrich, United Kingdom) in ALBA (4.5% human albumin solution; Scottish National Blood Transfusion Service) and were stored in the vapor phase of liquid nitrogen until required. All human cell samples were obtained with informed consent.
Patient characteristics
| Patient code . | Starting WBC (× 109/L) . | Starting CD34 (%) . | CD34 enrichment (%) . |
|---|---|---|---|
| CML 1 | 181 | 0.5 | 46 |
| CML 2 | 234 | 5.5 | 78 |
| CML 3 | 518 | 5.3 | 98 |
| CML 4 | 741 | 3.4 | 99 |
| CML 5 | 206 | 19 | 96 |
| CML 6 | 410 | 3.3 | 58 |
| CML 7 | 701 | 14.4 | 85 |
| Patient code . | Starting WBC (× 109/L) . | Starting CD34 (%) . | CD34 enrichment (%) . |
|---|---|---|---|
| CML 1 | 181 | 0.5 | 46 |
| CML 2 | 234 | 5.5 | 78 |
| CML 3 | 518 | 5.3 | 98 |
| CML 4 | 741 | 3.4 | 99 |
| CML 5 | 206 | 19 | 96 |
| CML 6 | 410 | 3.3 | 58 |
| CML 7 | 701 | 14.4 | 85 |
All patient samples were collected at diagnosis before therapy. All patients had chronic-phase CML and t(9;22) and were BCR-ABL+.
Serum-free culture
Cells were recovered from liquid nitrogen and washed once in Dulbecco phosphate-buffered saline (PBS; Sigma) containing 2% fetal calf serum (PBS/2%; Life Technologies, Paisley, United Kingdom). For the time-course study, 5 × 104 CD34+cells were added to each well of a 24-well plate (Corning, Bucks, United Kingdom) in Iscoves modified Dulbecco medium (Sigma) supplemented with a serum substitute (BIT; StemCell), 40 μg/mL low-density lipoproteins (Sigma), and 10−4 M 2-mercaptoethanol (complete serum-free medium [SFM]), supplemented or not with 100 ng/mL recombinant human Flt3-ligand (Immunex Corporation, Seattle, WA) and Steel factor (Terry Fox Laboratory, Vancouver, BC, Canada), and with 20 ng/mL recombinant human interleukin-3 (IL-3) (Novartis, Basel, Switzerland), IL-6 (Cangene, Mississauga, ON, Canada), and granulocyte–colony-stimulating factor (Chugai Pharma, United Kingdom; abbreviated as 5 growth factors [GFs]). STI571 (a kind gift from Novartis) was added, or not, at concentrations from 1 μM to 10 μM. On days 3, 6, and 12, cells were harvested and viability was assessed by counting in a hemocytometer chamber slide in a 20% solution of trypan blue (Sigma).
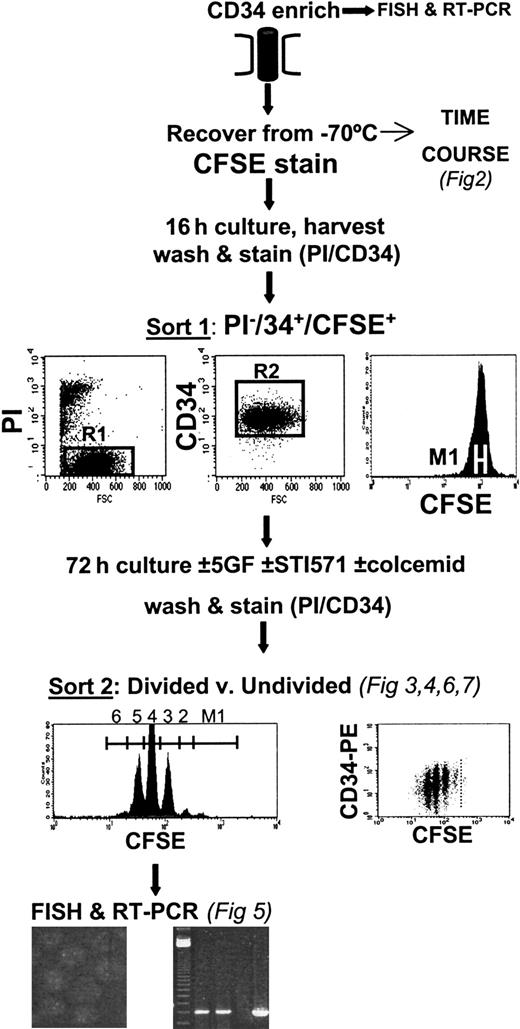
Flow cytometry and cell culture
As shown in the protocol in Figure1, CD34+-enriched cells were recovered from liquid nitrogen, washed once in PBS/2%, and stained with 1 μM carboxy-fluorescein diacetate succinimidyl diester (CFSE; Molecular Probes, Eugene, OR) as described in detail previously.5,6 22 Briefly, the labeled cells were then incubated overnight in SFM, with or without GFs. The next day, the cells were washed once in PBS/2% and labeled with anti-CD34–phycoerythrin (PE) (Becton Dickinson, Oxford, United Kingdom) and 1 μg/mL propidium iodide (PI; Sigma). Using a FACSVantage (Becton Dickinson), a homogenous subset of CD34+ CFSE+ PI− cells was sorted using a narrow fluorescence gate (36-40 channels wide using a 1024-channel log amplifier on FL1). These cells were then cultured in 8 experimental conditions for another 3 days in SFM, with and without GFs, with and without STI571 at 10 μM, and with and without 100 ng/mL Colcemid (Life Technologies). At the end of this time, all the cells were harvested, washed in PBS/2%, and stained with anti-CD34–PE and PI. Cells cultured in the presence of Colcemid were then used to establish the range of fluorescence exhibited by cells that had not divided during the 3-day postlabeling incubation. Cells were sorted into divided and undivided populations for each of the culture conditions described.
Experimental protocol.
After CFSE staining, CD34-enriched cells were cultured overnight before FACS to obtain viable (PI−), CD34+, homogeneously CFSE-stained cells. These cells were then cultured for 3 days in SFM, supplemented or not with GFs (see “Materials and methods”) and with or without the addition of 10 μM STI571. At the end of the culture period, cells were labeled with PI and CD34-PE, and viable divided versus undivided cells were isolated by FACS. These cell populations were then processed for FISH and RT-PCR to determine their genotype.
Experimental protocol.
After CFSE staining, CD34-enriched cells were cultured overnight before FACS to obtain viable (PI−), CD34+, homogeneously CFSE-stained cells. These cells were then cultured for 3 days in SFM, supplemented or not with GFs (see “Materials and methods”) and with or without the addition of 10 μM STI571. At the end of the culture period, cells were labeled with PI and CD34-PE, and viable divided versus undivided cells were isolated by FACS. These cell populations were then processed for FISH and RT-PCR to determine their genotype.
Recovery calculation
To measure the overall effect of STI571 on cell survival and to determine whether STI571 had demonstrable antiproliferative activity, the percentage recovery of viable CD34+ input cells was calculated for each division peak for cultures with and without GFs and with and without STI571 (Figure 1). The number of CD34+ cells used to establish each culture was first recorded. After the 3-day culture period, the total number of viable cells harvested from each culture condition was recorded, as were the percentages of total viable cells and of CD34+ cells found in the undivided fraction and in each division peak for all CFSE/CD34 dot-plots from the FACSVantage printout. Percentage recovery of input cells in each peak could then be calculated by dividing the absolute number of viable total cells or CD34+ cells in each peak on day 3, corrected for cell division, by the total number of input CD34+ cells and multiplying by 100%. The difference between plus and minus STI571 was then directly compared for each experiment.
Reverse transcription–polymerase chain reaction
Sorted cells were resuspended in guanidinium isothiocyanate lysis buffer (5 M GIT, 20 mM 1,4-diothioerythritol (DTT), 25 mM sodium citrate, pH 7.0, 0.05% Sarcosyl) before a 2-step (nested) reverse transcription–polymerase chain reaction (RT-PCR) was performed using an initial oligo (dT)-based primer and poly (A) tailing strategy.23 24 After electrophoresis of the amplified products, BCR-ABL and ABL-specific fragments were detected by Southern blotting using a cDNA probe for BCR-ABL (provided by J. Griffin, Dana Farber Cancer Institute, Boston, MA). Primer sets included, for ABL 1 and ABL 2, 5′TTCAGCGGCCAGTAGCATCTGACTT3′ and 5′GGTACCAGGAGTGTTTCTCCAGACTG3′ and, for BCR-ABL 1 and BCR-ABL 2, 5′CAGGGTGCACAGCCGCAACGGCAA3′ and 5′GTCCAGCGAGAAGGTTTTCCTTGGA3′.
Fluorescence in situ hybridization
Aliquots of approximately 5000 cells in 50 μL PBS/2% were centrifuged at 4000 rpm for 5 minutes in 0.2 mL tubes. The supernatant was carefully removed without disturbing the cell pellet before resuspension in 50 μL prewarmed (37°C) hypotonic solution (0.075 M potassium chloride). Aliquots were divided between duplicate wells of a previously poly-L-lysine (Sigma) coated multispot microscope slide (Hendley, Essex, United Kingdom). Cells were incubated for 20 minutes at room temperature before excess hypotonic solution was removed gently. Cell fixation was performed by the addition of 20 μL freshly prepared methanol:acetic acid (3:1) to each well and incubated at room temperature for 5 minutes. This fixation step was repeated, with final fixation in a Coplin jar, for a minimum of 5 minutes before air drying of the slide overnight. Slides were wrapped in parafilm and stored at −20°C until FISH was performed with the BCR/ABL1 S-FISH translocation DNA probe according to the manufacturer's instructions (Appligene Oncor, Middlesex, United Kingdom). Interphase nuclei were evaluated using a fluorescence microscope with a triple-band pass filter for DAPI, fluorescein isothiocyanate, and Texas red.
Statistics
Statistical analyses were performed using the Studentt test.
Results
Time course and titration of STI571
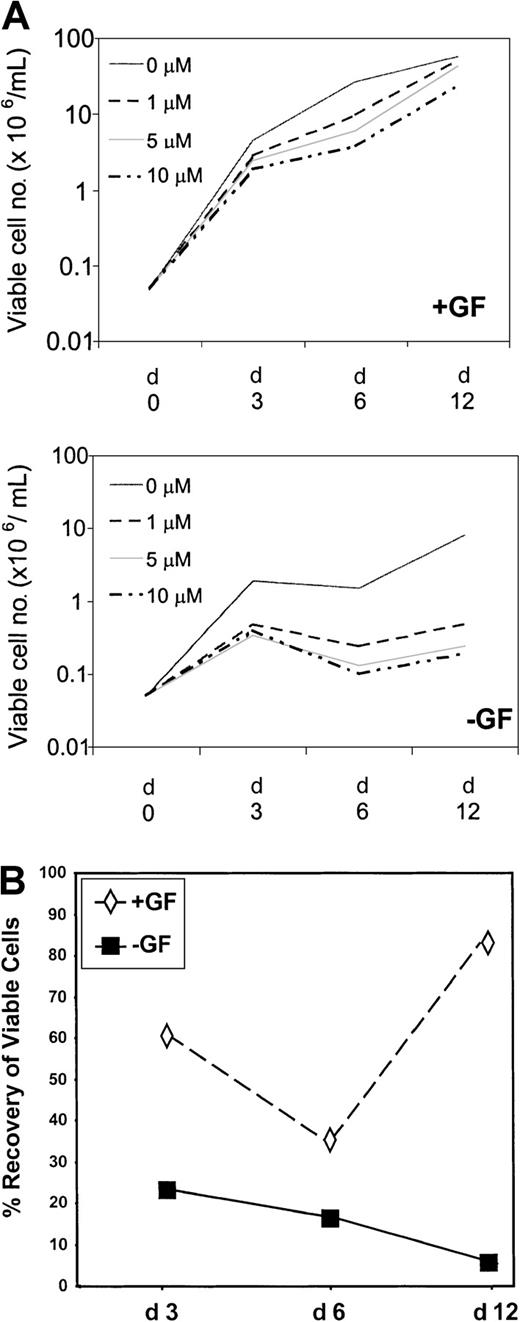
CD34+ cells, derived from 5 patients in chronic phase at diagnosis and screened by fluorescence in situ hybridization (FISH) for the presence of BCR-ABL, were established in liquid-phase, serum-free cultures. In the presence of GFs, mean total viable cell number increased by 91-, 538-, and 1167-fold on days 3, 6, and 12, respectively (Figure 2A, Table2). In the presence of STI571 at 1, 5, and 10 μM, respectively, total cell amplification by day 3 reached only 56-, 35-, and 50-fold; by day 6 it reached 192-, 123-, and 75-fold; and by day 12 it reached 1022-, 860-, and 478-fold. Maximum effect for STI571 was, therefore, observed on day 6, when overall viable cell recoveries were reduced to 36%, 23%, and 14% of control for 1, 5, and 10 μM STI571. However, by day 12, viable cell recoveries had significantly improved to 87% and 74% of control in the presence of 1 and 5 μM, respectively (P = .018;P = .048). An increase in cell recovery was also observed in the presence of 10 μM (41% vs 14%), but this did not reach statistical significance.
Time course and titration of STI571.
(A) CD34-enriched cells derived from 5 patients with chronic-phase CML were used to establish cultures in the presence (+GF) or absence (−GF) of GFs in SFM (see “Materials and methods”). STI571 was added on day 0 only, at concentrations ranging from 0 to 10 μM, as shown in the legend. On days 3, 6, and 12, triplicate wells for each condition were harvested, and viable cell counts were performed. Results represent the mean viable cell number ± SEM of triplicate measurements performed for each of 5 patient samples. (B) Recovery (compared with control) on days 3, 6, and 12 of cells cultured in the presence of STI571 at 1 μM (similar trend for 5 and 10 μM) and in the presence or the absence of growth factors.
Time course and titration of STI571.
(A) CD34-enriched cells derived from 5 patients with chronic-phase CML were used to establish cultures in the presence (+GF) or absence (−GF) of GFs in SFM (see “Materials and methods”). STI571 was added on day 0 only, at concentrations ranging from 0 to 10 μM, as shown in the legend. On days 3, 6, and 12, triplicate wells for each condition were harvested, and viable cell counts were performed. Results represent the mean viable cell number ± SEM of triplicate measurements performed for each of 5 patient samples. (B) Recovery (compared with control) on days 3, 6, and 12 of cells cultured in the presence of STI571 at 1 μM (similar trend for 5 and 10 μM) and in the presence or the absence of growth factors.
Cell amplification and recoveries in presence of increasing STI571 concentrations over time
| . | 0 μM STI571 . | 1 μM STI571 . | 5 μM STI571 . | 10 μM STI571 . | |||
|---|---|---|---|---|---|---|---|
| Fold amplification (mean) . | Fold amplification (mean) . | % Recovery (mean) . | Fold amplification (mean) . | % Recovery (mean) . | Fold amplification (mean) . | % Recovery (mean) . | |
| +GFs | |||||||
| Day 3 | 91 ± 36 | 56 ± 20 | 62 | 35 ± 13 | 39 | 50 ± 31 | 55 |
| Day 6 | 538 ± 204 | 192 ± 86 | 36 | 123 ± 62 | 23 | 75 ± 27 | 14 |
| Day 12 | 1167 ± 269 | 1022 ± 372 | 87 | 860 ± 342 | 74 | 478 ± 217 | 41 |
| −GFs | |||||||
| Day 3 | 38 ± 18 | 9 ± 3.1 | 24 | 7 ± 3.3 | 18 | 8 ± 5.1 | 21 |
| Day 6 | 30 ± 21 | 5 ± 1.6 | 17 | 3 ± 1.5 | 10 | 2 ± 1.0 | 7 |
| Day 12 | 162 ± 98 | 10 ± 6.8 | 6 | 5 ± 2.8 | 3 | 4 ± 1.5 | 2.5 |
| . | 0 μM STI571 . | 1 μM STI571 . | 5 μM STI571 . | 10 μM STI571 . | |||
|---|---|---|---|---|---|---|---|
| Fold amplification (mean) . | Fold amplification (mean) . | % Recovery (mean) . | Fold amplification (mean) . | % Recovery (mean) . | Fold amplification (mean) . | % Recovery (mean) . | |
| +GFs | |||||||
| Day 3 | 91 ± 36 | 56 ± 20 | 62 | 35 ± 13 | 39 | 50 ± 31 | 55 |
| Day 6 | 538 ± 204 | 192 ± 86 | 36 | 123 ± 62 | 23 | 75 ± 27 | 14 |
| Day 12 | 1167 ± 269 | 1022 ± 372 | 87 | 860 ± 342 | 74 | 478 ± 217 | 41 |
| −GFs | |||||||
| Day 3 | 38 ± 18 | 9 ± 3.1 | 24 | 7 ± 3.3 | 18 | 8 ± 5.1 | 21 |
| Day 6 | 30 ± 21 | 5 ± 1.6 | 17 | 3 ± 1.5 | 10 | 2 ± 1.0 | 7 |
| Day 12 | 162 ± 98 | 10 ± 6.8 | 6 | 5 ± 2.8 | 3 | 4 ± 1.5 | 2.5 |
Results shown represent mean ± SEM (n = 5) fold amplification based on input 5 × 104 cells per assay.
To establish the inherent sensitivity of primitive Ph+progenitor cells, parallel cultures were established in SFM without GFs, conditions under which only immature Ph+ progenitor cells can survive.25-27 In the absence of either GFs or STI571, mean total viable cell number increased by 38-, 30-, and 162-fold on days 3, 6, and 12, respectively (Figure 2, Table 2). The slight dip on day 6 is thought to reflect the death of cells that, though Ph+, are not fully growth factor independent. In the presence of 1, 5, and 10 μM STI571, amplification of viable cells declined to 9-, 7-, and 8-fold by day 3; to 5-, 3-, and 2-fold by day 6; and to 10-, 5-, and 4-fold by day 12. Maximum effect for STI571 was observed on day 12, when overall viable cell recoveries were only 6%, 3%, and 2.5% for 1, 5, and 10 μM STI571, respectively, compared with control. An apparent dose-response relationship was seen between days 6 and 12 with 1, 5, and 10 μM STI571. Between days 6 and 12, Ph+ cells appeared to proliferate once again, even in the presence of 10 μM STI571. Mean absolute number of viable cells increased by 2-fold at 1 μM and by 1.9-fold at both 5 and 10 μM STI571 with respect to a 5.4-fold increase in the absence of STI571 (P = NS). These data confirmed that a subset of Ph+ cells remains insensitive to STI571, whether cultured in the presence or absence of GFs.
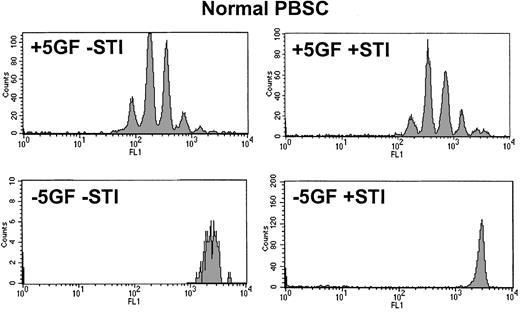
Quiescent Ph+CD34+ progenitor cells are insensitive to STI571
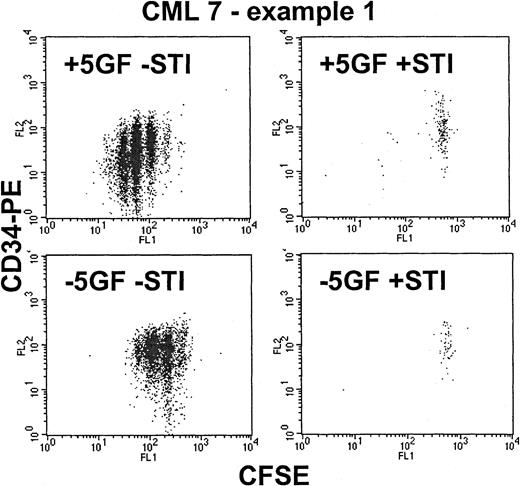
From the time-course experiments, a concentration of 10 μM STI571 was selected to achieve maximal STI571 inhibitory effect on Ph+ cells. In the absence of GFs, normal CD34+cells were unable to divide (Figure 3). In the presence of GFs, STI571 affected neither cell cycle kinetics nor the recovery of viable normal CD34+ cells. Although the addition of STI571 eradicated most dividing CML cells, in the presence or absence of GFs (Figure 4) a significant population of viable CD34+ cells was recovered in the undivided/quiescent peak in all patients. These quiescent cells were part of the leukemic clone, as shown by FISH or RT-PCR (Figure 5).
Day 3 CFSE profile for CD34+ cells derived from normal mobilized peripheral blood.
Upper histograms demonstrate that in the presence of added growth factors, normal CD34+ cells were stimulated to undergo 6 or fewer divisions by day 3 and that this pattern was not significantly altered by the addition of STI571. Lower histograms confirm that, in the absence of added growth factors, normal CD34+cells were unable to execute even a single division regardless of whether STI571 was present. PBSC indicates peripheral blood stem cells.
Day 3 CFSE profile for CD34+ cells derived from normal mobilized peripheral blood.
Upper histograms demonstrate that in the presence of added growth factors, normal CD34+ cells were stimulated to undergo 6 or fewer divisions by day 3 and that this pattern was not significantly altered by the addition of STI571. Lower histograms confirm that, in the absence of added growth factors, normal CD34+cells were unable to execute even a single division regardless of whether STI571 was present. PBSC indicates peripheral blood stem cells.
Effect of STI571 on proliferating Ph+CD34+ cells—CML 7, example 1.
These dot-plots show representative results for a CML sample that proved to be highly sensitive to STI571. As shown in the upper left dot-plot, in the presence of added growth factors, the CD34+ cells were stimulated to proliferate up to 6 times, with associated loss of CD34 expression induced by differentiation. The addition of STI571 (upper right) eradicated almost all the dividing cells, leaving behind only the nonproliferating quiescent fraction. In the absence of growth factors (lower left), the CD34+ cells demonstrated autonomous growth (compared with the normal control in Figure 3) with up to 4 divisions and with retention of CD34 expression. The addition of STI571 (lower right) once again eradicated all cells that entered cell division.
Effect of STI571 on proliferating Ph+CD34+ cells—CML 7, example 1.
These dot-plots show representative results for a CML sample that proved to be highly sensitive to STI571. As shown in the upper left dot-plot, in the presence of added growth factors, the CD34+ cells were stimulated to proliferate up to 6 times, with associated loss of CD34 expression induced by differentiation. The addition of STI571 (upper right) eradicated almost all the dividing cells, leaving behind only the nonproliferating quiescent fraction. In the absence of growth factors (lower left), the CD34+ cells demonstrated autonomous growth (compared with the normal control in Figure 3) with up to 4 divisions and with retention of CD34 expression. The addition of STI571 (lower right) once again eradicated all cells that entered cell division.
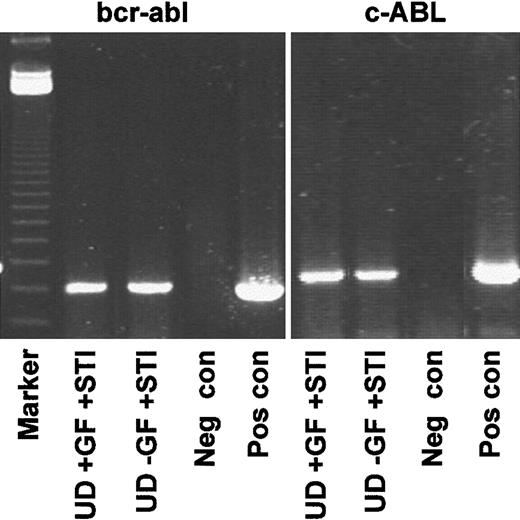
Genotyping of quiescent CD34+ cells resistant to STI571.
Representative RT-PCR (CML 3) demonstrating that the undivided population expressed transcripts for BCR-ABL, whether recovered from cultures with or without added growth factors. Lane 1, markers; lanes 2-5, BCR-ABL; lane 2, undivided cells from +GF culture with added STI571; lane 3, undivided cells from −GF culture with added STI571; lane 4, negative control (Neg con); lane 5, positive control (Pos con); lanes 6-9, c-ABL loading controls.
Genotyping of quiescent CD34+ cells resistant to STI571.
Representative RT-PCR (CML 3) demonstrating that the undivided population expressed transcripts for BCR-ABL, whether recovered from cultures with or without added growth factors. Lane 1, markers; lanes 2-5, BCR-ABL; lane 2, undivided cells from +GF culture with added STI571; lane 3, undivided cells from −GF culture with added STI571; lane 4, negative control (Neg con); lane 5, positive control (Pos con); lanes 6-9, c-ABL loading controls.
This response to STI571 was not observed in every patient with CML studied. In some patients, in the presence of GFs, a proportion of input cells was able to divide up to 4 times (Figure6) despite belonging to the Ph+ clone. Thus, sensitivity to STI571 of dividing CD34+ cells derived from different patients with newly diagnosed chronic phase CML is heterogeneous. In every patient, however, quiescent CD34+ Ph+ cells persisted, the viability of which had not been compromised by STI571.
Effect of STI571 on proliferating Ph+CD34+ cells—CML 2, example 2.
These dot-plots show representative results for a CML sample that proved to be relatively insensitive to STI571 despite more than 95% of the input cells being BCR-ABL+ by FISH. As shown in the upper left dot-plot, in the presence of added growth factors, the CD34+ cells were stimulated to proliferate up to 6 times. The addition of STI571 (upper right) reduced the number of division peaks to 4 and increased the proportion of cells in the undivided peak. In the absence of growth factors (lower left), the CD34+cells demonstrated autonomous growth with up to 3 divisions and with retention of CD34 expression. The addition of STI571 (lower right) reduced the division peaks to 2 with most cells found in the undivided fraction.
Effect of STI571 on proliferating Ph+CD34+ cells—CML 2, example 2.
These dot-plots show representative results for a CML sample that proved to be relatively insensitive to STI571 despite more than 95% of the input cells being BCR-ABL+ by FISH. As shown in the upper left dot-plot, in the presence of added growth factors, the CD34+ cells were stimulated to proliferate up to 6 times. The addition of STI571 (upper right) reduced the number of division peaks to 4 and increased the proportion of cells in the undivided peak. In the absence of growth factors (lower left), the CD34+cells demonstrated autonomous growth with up to 3 divisions and with retention of CD34 expression. The addition of STI571 (lower right) reduced the division peaks to 2 with most cells found in the undivided fraction.
Survival of quiescent Ph+CD34+ cells is not adversely affected by STI571, which may have antiproliferative activity on this population
For the 4 patient samples used for these experiments, overall cell expansions obtained on day 3 for the various culture conditions are shown in Table 3. As shown in Table4, in the presence of GFs, the mean proportion of input cells recovered in the undivided fraction was 17%, which was not significantly reduced by the presence of STI571 (16%). Furthermore, STI571 did not affect cell recovery until cells had executed 3 or more divisions. Overall recovery of input cells was 91% in the presence of GFs and 56% in the presence of GFs plus STI571.
Cell amplifications with and without growth factors, with and without 10 μM STI571
| Patient code . | Fold expansion . | |||
|---|---|---|---|---|
| GFs . | GFs/STI571 . | No GF . | No GF/STI571 . | |
| CML 2 | 11.5 | 3.5 | 1.3 | 0.2 |
| CML 3 | 6.6 | 2.8 | 1.6 | 0.4 |
| CML 6 | 0.5 | 0.24 | 0.3 | 0.2 |
| CML 7 | 4.3 | 0.2 | 1.2 | 0.1 |
| Mean | 3.8 | 1.1 | 1.0 | 0.2 |
| Patient code . | Fold expansion . | |||
|---|---|---|---|---|
| GFs . | GFs/STI571 . | No GF . | No GF/STI571 . | |
| CML 2 | 11.5 | 3.5 | 1.3 | 0.2 |
| CML 3 | 6.6 | 2.8 | 1.6 | 0.4 |
| CML 6 | 0.5 | 0.24 | 0.3 | 0.2 |
| CML 7 | 4.3 | 0.2 | 1.2 | 0.1 |
| Mean | 3.8 | 1.1 | 1.0 | 0.2 |
Viable CD34+ cell recoveries after successive cell divisions in the presence or absence of growth factors or STI571
| Conditions . | Recovery in M1 (mean %) . | Recovery in M2 (mean %) . | Recovery in M3 (mean %) . | Recovery in M4 (mean %) . | Recovery in M5 (mean %) . | Total recovery (mean %) . |
|---|---|---|---|---|---|---|
| Total viable cells | ||||||
| GFs | 17 | 13 | 26 | 28 | 6 | 91 |
| GFs/STI571 | 16 | 15 | 23 | 1.6 | 0.9 | 56 |
| No GFs | 14 | 21 | 10 | 1.4 | — | 47 |
| No GFs/STI571 | 11 | 9 | 2 | 0.2 | — | 22 |
| Total viable CD34+ cells | ||||||
| GFs | 11 | 13 | 28 | 28 | 6 | 85 |
| GFs/STI571 | 15 | 16 | 23 | 1.6 | 0.9 | 55 |
| No GFs | 13 | 25 | 11 | 1.4 | — | 51 |
| No GFs/STI571 | 11 | 10 | 2 | 0.2 | — | 24 |
| Conditions . | Recovery in M1 (mean %) . | Recovery in M2 (mean %) . | Recovery in M3 (mean %) . | Recovery in M4 (mean %) . | Recovery in M5 (mean %) . | Total recovery (mean %) . |
|---|---|---|---|---|---|---|
| Total viable cells | ||||||
| GFs | 17 | 13 | 26 | 28 | 6 | 91 |
| GFs/STI571 | 16 | 15 | 23 | 1.6 | 0.9 | 56 |
| No GFs | 14 | 21 | 10 | 1.4 | — | 47 |
| No GFs/STI571 | 11 | 9 | 2 | 0.2 | — | 22 |
| Total viable CD34+ cells | ||||||
| GFs | 11 | 13 | 28 | 28 | 6 | 85 |
| GFs/STI571 | 15 | 16 | 23 | 1.6 | 0.9 | 55 |
| No GFs | 13 | 25 | 11 | 1.4 | — | 51 |
| No GFs/STI571 | 11 | 10 | 2 | 0.2 | — | 24 |
Numbers represent mean (n = 4) percentage recovery for the number of cells in each division peak, corrected for the number of divisions, as a fraction of input cell numbers (see “Materials and methods”).
In the absence of GFs, approximately 14% of input cells were recovered in the undivided fraction and were not apparently affected by STI571 (11% recovery). However, under these conditions, the effect of STI571 on cycling cells was observed as soon as cells entered cell division (recovery in M2 = 21% −STI571 vs 9% +STI571). Overall recovery of input cells was reduced (47% for SFM alone and 22% for SFM +STI571) compared with cultures with GFs. For all 4 experimental arms, these results were mirrored exactly when recovery for CD34+cells, rather than total cells, was calculated (Table 4). An antiproliferative effect of STI571 could be demonstrated in CML 7. In duplicate assays, the percentage recovery of input cells found in the undivided quiescent fraction, in the presence of GFs, was significantly greater in the presence than in the absence of STI571 (15.6% ± 1.4% vs 3.2% ± 0.4%; P = .014). Antiproliferative activity of STI571 for this single sample was confirmed as the recovery of quiescent cells was increased by the addition of STI571, demonstrating that cells that had backed up in a nondividing state more than compensated for any cells lost through STI571 activity.
Ph+ cells capable of growth factor-independent proliferation retain high levels of CD34 expression
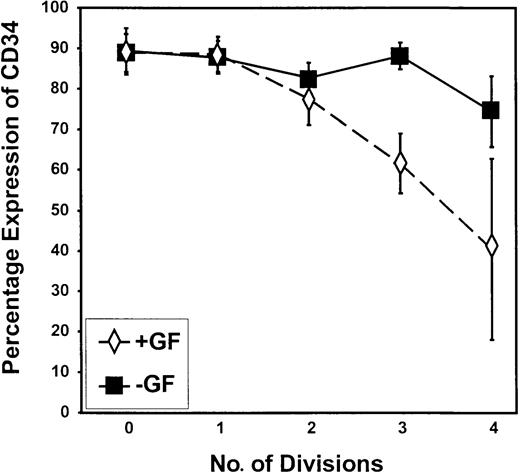
In this study, autonomous growth factor-independent proliferation was well demonstrated by the comparison of cell cycle kinetics for CD34+ progenitor cells from CML samples (Figures 4, 6) with that of normal peripheral blood stem cells (Figure 3) cultured without GFs. Cells capable of dividing up to 4 times in GF-free medium retained very high levels of CD34 expression compared with cells undergoing division in the presence of GFs (Figures 4, 6,7). The difference between the no GF and the GF-supplemented experimental arms was highly significant by division 3 (P = .009).
Retention of CD34 expression during proliferation in the absence of added growth factors.
The percentage of cells that remained CD34+ is shown on a peak-by-peak basis for divisions 0 to 4 for CML progenitor cells cultured in SFM in the presence (+GF) or absence (−GF) of GFs. As shown, the proportion of cells that retained CD34 expression was greater in the absence than in the presence of added growth factors after the second division.
Retention of CD34 expression during proliferation in the absence of added growth factors.
The percentage of cells that remained CD34+ is shown on a peak-by-peak basis for divisions 0 to 4 for CML progenitor cells cultured in SFM in the presence (+GF) or absence (−GF) of GFs. As shown, the proportion of cells that retained CD34 expression was greater in the absence than in the presence of added growth factors after the second division.
Discussion
Since the 1980s, when intensive chemotherapy trials were performed in chronic-phase CML and were unable to eradicate the Ph+clone, the presence of dormant leukemic stem cells has been suspected.7,8 However, alternative mechanisms, including possible antiapoptotic properties of BCR-ABL, have been proposed to explain the relative chemo-resistance of CML.28,29 More recently, the application of novel flow cytometric techniques has enabled us to demonstrate that quiescent leukemic stem cells do indeed exist in the blood and bone marrow of all patients with chronic-phase CML.5 6 With the introduction of STI571 for the treatment of CML, it was critical to establish its effects on the quiescent stem cell pool, an important target cell population for eradication to achieve cure of the disease.
Based on the results of the time-course experiments that showed a titration effect from 1 to 10 μM, this study used 10 μM STI571. Although 1 μM STI571 is the widely quoted inhibitory concentration and would approach the achievable drug level in patients' sera, this target concentration has often been determined based on observations of STI571 efficacy in colony-forming assays. In our liquid culture system in which we directly assess overall viability, the IC50 can be expected to be higher (> 1 μM) than that observed in colony-forming assays (0.5 μM).12 In the latter scenario, static effects of STI571 may result in apparent “kill” in that no colonies form; however, viable cells remain unaffected by drug activity.
Data from the time-course experiments initially raised the suspicion that the viability of at least a subset of growth factor-independent, Ph+ primary CML cells was unaffected by STI571. In the presence of growth factors, cells that survived to day 6 thereafter expanded by day 12, regardless of STI571 concentration. Deininger et al12 previously established the ability of STI571 to inhibit colony formation by CD34+ Ph+ primary CML cells even in the presence of exogenous growth factors. A similar trend of cell amplification in the presence of STI571 was observed for cells cultured in the absence of growth factors. Previous studies indicate that cells that exhibit such autonomous growth are likely to be primitive (CD34+ lineage−) and to express autocrine IL-3.25 If such cells are indeed spared by STI571, they would be anticipated to lead to disease resurgence during prolonged STI571 therapy.
The next series of experiments revealed that quiescent CD34+ Ph+ cells were highly insensitive to STI571, with recoveries of cells maintained alive and in G0equivalent in the presence versus the absence of 10 μM STI571. Furthermore, there was evidence that the response to STI571 was heterogeneous between samples, with the proliferating fraction in some samples completely eradicated by STI571 (eg, CML 7) and in others showing significant cell survival even to division 4 (eg, CML 2). In the absence of added growth factors, compared with the growth factor-supplemented arm, the proportion of input cells recovered per division peak declined as soon as the cells entered the first cell cycle. This implied that the GFs contributed an antiapoptotic effect in the presence of STI571.
As stated above, the proportion of input cells that remained quiescent and viable was not influenced by the addition of STI571. The obvious interpretation of this result was that the quiescent fraction exhibited an inherent insensitivity to STI571. Such insensitivity, however, is distinct from acquired resistance, as has been described in cell lines chronically exposed to the drug whereby resistance is mediated by such mechanisms as gene amplification,17,18,30 increased BCR-ABL protein without gene amplification,18 or reduced STI571 uptake through P-glycoprotein overexpression.19Progressive gene amplification and a single amino acid substitution has been found to confer resistance to STI571 in cells from patients with advanced-stage disease who undergo relapse after an initial response.30 Our analysis by FISH did not reveal gene amplification in the cells used in our study; nevertheless, drug efflux remains a potential explanation for quiescent stem cell insensitivity to STI571.
In addition to the inherent insensitivity of quiescent CD34+ Ph+ cells to STI571, it is possible that a second mechanism was operating. For example, maintenance of viable cells in an undivided state might have reflected ongoing STI571-induced apoptosis in combination with an antiproliferative effect of STI571 in preventing cells from entering cell division. Although antiproliferative activity could only be shown definitively for CML 7, it is likely to have played a part in the retention of cells in a quiescent state in all samples. Although it would have been desirable to definitively distinguish between the relative contributions of induction of apoptosis and antiproliferation to the overall effect of STI571, it was not practicable to do so in our current experimental set-up; thus, we can only conclude that both mechanisms were in operation. Indeed, the antiproliferative effect of STI571 had not been anticipated, but such antiproliferative activity would be in agreement with a recent report by Gesbert et al31 demonstrating that BCR-ABL+ cells exposed to STI571 resulted in recovery of the reversible, BCR-ABL+-induced down-regulation of p27, a key cell cycle regulator.
For Ph+ CD34+ cells cultured without growth factors, cells that survived and proliferated up to 4 times retained high levels of CD34 expression compared with cells undergoing division in the presence of growth factors. This implied either that as cells began to differentiate and lose CD34 expression, they were lost from the cultures, presumably through cell death, or that the absence of exogenous growth factors prompted self-renewal divisions with retention of CD34 expression. This finding may be explained by the differentiation-controlled autocrine expression of IL-3, which has been demonstrated previously.25 In those studies the level of autocrine IL-3 clearly fell as cells differentiated from the primitive (CD34+CD45RA/71−), through the intermediate (CD34+CD45RA/71+), and into the mature compartment (CD34−), and they may explain why, in this study, cell survival was dependent on retention of a primitive (and likely IL-3–expressing) phenotype.
As clinical trials with STI571 progress, it is anticipated that many patients will enter cytogenetic and possibly even molecular remission. However, to date, little is known regarding the efficacy of the drug in the longer term and whether surrogate markers of response, such as cytogenetic remission, will translate to prolonged survival. Moreover, our data suggest that quiescent hematopoietic stem cells are likely to survive STI571 monotherapy. It will be important to determine whether residual populations of quiescent Ph+ cells exist in treated patients. If so, adjuvant therapies are likely to prove important, either combining STI571 with other molecularly targeted therapy,32-34 with chemotherapy agents, or with immunotherapy.35 Recent in vitro studies, performed on primary CML cells or Ph+ cell lines, have demonstrated either additive or synergistic responses for a number of agents used in conjunction with STI571,36-38 and phase 1 combination clinical trials are actively pursued in a number of centers.
We thank the United Kingdom hematologists who contributed to our bank of CML samples. We thank Dr Allen Eaves and StemCell Technologies and Dr Connie Eaves and the Terry Fox Laboratories for their support. We also thank Novartis Pharmaceuticals for the generous gift of reagents and Professor Ian Franklin and Dr John Campbell for critically reviewing the manuscript.
Supported by The Sylvia Aitken Trust (S.M.G.) and by the UK Leukaemia Research Fund (H.G.J, T.L.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Susan M. Graham, Academic Transfusion Medicine Unit, Department of Medicine, Royal Infirmary, 10 Alexandra Parade, Glasgow G31 2ER, Scotland; e-mail: smg16a@clinmed.gla.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal