Adenosine diphosphate (ADP) is a platelet agonist that causes platelet shape change and aggregation as well as generation of thromboxane A2, another platelet agonist, through its effects on P2Y1, P2Y12, and P2X1 receptors. It is now reported that both 2-propylthio-D-βγ-dichloromethylene adenosine 5′-triphosphate (AR-C67085), a P2Y12 receptor–selective antagonist, and adenosine-2′-phosphate-5′-phosphate (A2P5P), a P2Y1 receptor–selective antagonist, inhibited ADP-induced thromboxane A2 generation in a concentration-dependent manner, indicating that coactivation of the P2Y12 and P2Y1 receptors is essential for this event. SC49992, a fibrinogen receptor antagonist, blocked ADP-induced platelet aggregation and thromboxane A2 production in a concentration-dependent manner. Similarly, P2 receptor antagonists or SC49992 blocked ADP-induced arachidonic acid liberation. Whereas SC49992 blocked arachidonic acid–induced platelet aggregation, it failed to inhibit thromboxane A2 generation induced by arachidonic acid. Thus, ADP-induced arachidonic acid liberation, but not subsequent conversion to thromboxane A2, requires outside-in signaling through the fibrinogen receptor. The Fab fragment of ligand-induced binding site–6 (LIBS6) antibody, which induces a fibrinogen-binding site on the integrin αIIbβ3, caused both platelet aggregation and thromboxane A2 generation. Inhibitors of phosphoinositide 3-kinase, Syk, Src kinases, or protein tyrosine phosphatases inhibited platelet aggregation but not thromboxane A2 generation, indicating that these signaling molecules have no significant role in phospholipase A2 activation. In the presence of P2 receptor antagonists A2P5P or AR-C67085, LIBS6 failed to generate thromboxane A2, suggesting that inside-out signaling through ADP receptors is necessary for this event. It was concluded that both outside-in signaling from the fibrinogen receptor and inside-out signaling from the P2Y1 and P2Y12 receptors are necessary for phospholipase A2 activation, resulting in arachidonic acid liberation and thromboxane A2 generation.
Introduction
Adenosine diphosphate (ADP) is an important platelet agonist that plays a role in hemostasis and pathophysiological arterial thrombosis.1 ADP causes platelets to undergo shape change, release granule contents, and aggregate.2-4Upon exposure to activating agonists, such as ADP, platelets hydrolyze arachidonic acid from phospholipid and convert it into thromboxane A2 by sequential oxygenation via cyclo-oxygenase and thromboxane A2 synthase.5 The released thromboxane A2 acts as a positive feedback mediator in the activation and recruitment of more platelets to the primary hemostatic plug.6 ADP also causes adhesion of platelets to vitronectin or osteopontin, which might play an important role in anchoring platelets to disrupted atherosclerotic plaques and to the walls of the injured arteries.7 All the ADP-induced intracellular signaling events, viz, rapid influx of calcium, activation of phospholipase C, mobilization of calcium from intracellular stores, inhibition of stimulated adenylate cyclase, and activation of phospholipase A2,2,4 were initially proposed to occur through the activation of a single ADP receptor, designated P2T.8 We provided evidence for 3 receptors for ADP on human platelets9,10: the ionotropic P2X1 receptor mediating rapid influx of calcium, the P2Y1 receptor coupled to mobilization of calcium from intracellular stores through the activation of phospholipase C, and the P2TAC receptor coupled to the inhibition of adenylate cyclase. Several other recent studies11-15 also support this 3-receptor model. The ADP receptor coupled to the inhibition of adenylate cyclase has recently been cloned by 2 separate groups and has been designated the P2Y12 receptor.16,17 The 2-propylthio-D-βγ-difluoromethylene adenosine 5′-triphosphate (AR-C66096) and 2-propylthio-D-βγ-dichloromethylene adenosine 5′-triphosphate (AR-C67085) are selective competitive antagonists of the P2TAC (P2Y12) receptor,10,18whereas adenosine 3′-phosphate 5′-phosphosulfate (A3P5PS), adenosine 2′-phosphate 5′-phosphate (A2P5P), and adenosine 3′-phosphate 5′-phosphate (A3P5P), are selective competitive antagonists for the P2Y1 receptor.10,19 Using these selective antagonists, we have demonstrated the role of the P2Y1 receptor in ADP-induced platelet shape change.10 Furthermore, we20 and others14 15 have recently demonstrated that coactivation of the P2Y12 and P2Y1 receptors is essential for ADP-induced human platelet aggregation. The function of the P2X1 receptor on platelets is unknown.
The signaling events from agonist stimulation to fibrinogen receptor (integrin αIIbβ3) activation are termed inside-out signaling. The events that follow liganding of αIIbβ3 are termed outside-in signaling. The P2Y12 receptor and the P2Y1 receptor are coupled to the Giα221 and Gq10,20,22 guanosine 5′-triphosphate–binding protein signaling pathways. Thus, inside-out signaling here requires coactivation of Gi and Gq signaling pathways. Upon stimulation, the fibrinogen receptor binds fibrinogen and results in outside-in signaling. The events occurring during outside-in signaling include the 125-kd polypeptide focal adhesion kinase (PP125FAK) and Syk phosphorylations23 and phosphoinositide 3-kinase (PI-3 kinase)1activation.24
The nature of the P2 receptor subtypes involved in the production of thromboxane A2 has not been investigated. Here, we provide evidence that (1) ADP-induced generation of thromboxane A2requires coactivation of the P2Y12 and P2Y1 receptors, (2) outside-in signaling is not required for the conversion of arachidonic acid to thromboxane A2, and (3) both outside-in signaling through integrin αIIbβ3 and inside-out signaling from P2Y1 and P2Y12 receptors are necessary for arachidonic acid liberation and thromboxane A2 generation.
Materials and methods
Materials
A2P5P, serotonin, apyrase (type V; specific activity 4.2 U/mg protein; adenosine triphosphatease/ADPase = 1.4), fibrinogen (type I), bovine serum albumin (BSA) (lipidfree), and ADP were obtained from Sigma Chemical (St Louis, MO). Arachidonic acid, piceatannol, PP2, RK-682, and RWJ-60475 were purchased from Biomol (Plymouth Meeting, PA). Wortmannin and LY294002 were purchased from Calbiochem (San Diego, CA), and [3H]-arachidonic acid was purchased from Perkin-Elmer Life Sciences (Boston, MA). Epinephrine is a product of Research Biochemicals International (Natick, MA). AR-C67085 and AR-C66096 were gifts from Astra Research Laboratories (Loughborough, United Kingdom; formerly Fisons). SC49992 and SC57101 were gifts from Searle Research and Development (Skokie, IL). Thromboxane B2 enzyme immunoassay (EIA) kits were purchased from Amersham (Arlington Heights, IL). Anti–ligand-induced binding site antibody 6 (LIBS6) was the generous gift of Dr Mark Ginsberg (Scripps Research Institute, La Jolla, CA). The Fab fragment of LIBS6, free of Fc fragments, was prepared by a modification of a published method.25
Isolation of platelets
Blood was collected from informed healthy human volunteers in acid/citrate/dextrose as described.9 Platelet-rich plasma was obtained by centrifugation at 180g for 15 minutes at ambient temperature. Platelets, free of reticulocytes and other contaminants, were isolated from plasma by centrifugation at 800g for 15 minutes and resuspended in the final buffer consisting of 137 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 5 mM glucose, 10 mM Hepes (pH 7.4), 0.2% BSA, and 20 μg/mL apyrase. The platelet count was adjusted to 2 × 108 cells per milliliter. All experiments were performed in buffers with no added Ca++ unless otherwise noted.
Thromboxane A2 measurements and platelet aggregation
Washed human platelets (250 μL or 500 μL; 2 × 108/mL) were stimulated after adding fibrinogen (3 μM) with or without agonist in the presence or absence of other reagents at 37°C in an aggregometer while stirring at 900 rpm. At 3 minutes, the reaction was stopped by quickly freezing the sample in a dry ice–ethanol bath. After thawing at room temperature, the samples were centrifuged at 3000g for 10 minutes at 4°C to remove lysed platelets. The supernatants were diluted (1:10 or 1:50) with buffer and used to measure the content of thromboxane B2, the stable metabolite of thromboxane A2, by EIA according to the manufacturer's instructions.
Evaluation of phospholipase A2 activation
Phospholipase A2 activity was evaluated by measuring arachidonic acid plus metabolites by a procedure described previously.26 Platelet-rich plasma (PRP) was obtained by centrifugation at 180g for 15 minutes at ambient temperature. [3H]-arachidonic acid (specific activity: 98.6 Ci/mmol (3.6 TBq/mmol); 1 μCi/mL final concentration) was added to PRP and incubated for 30 minutes. Washed platelets were isolated from plasma by centrifugation at 800g for 15 minutes and resuspended in the final buffer consisting of 137 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 5 mM glucose, 10 mM Hepes (pH 7.4), 0.2% BSA (lipidfree), and 20 μg/mL apyrase. These platelets (500 μL; 2 × 108/mL) were stimulated with or without agonist in the presence or absence of other reagents at 37°C in an aggregometer while stirring at 900 rpm. At 3 minutes, the reaction was stopped with the addition of one-fourth volume of stopping solution containing 2% formaldehyde and 40 mM EDTA. Samples were collected and centrifuged at 5000g for 1 minute, and the supernatant was collected to measure the radioactivity with the use of a Wallac 1409 liquid scintillation counter (Gaithersburg, MD).
Platelet activation with Fab fragments of LIBS6 antibodies
Washed human platelets (2 × 108/mL)were incubated with LIBS6 Fab (0.6 or 1.5 μM) for 5 minutes at 37°C prior to the addition of fibrinogen (3 μM). The reaction was stopped 3 minutes after the addition of fibrinogen, and the thromboxane B2was measured as described above.
Results and discussion
Effect of P2 receptor–selective antagonists on ADP-induced thromboxane A2 generation
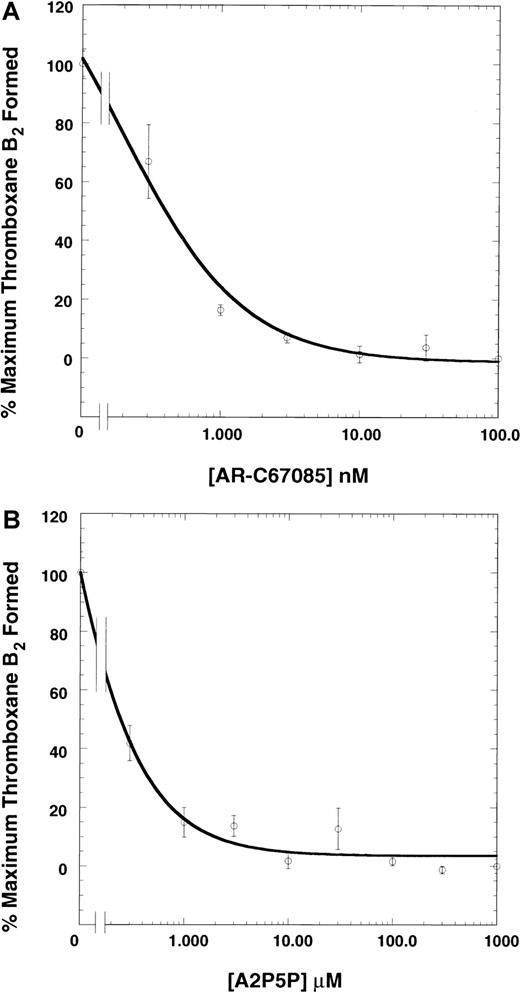
We investigated the role of the P2Y12 and P2Y1 receptors in ADP-induced generation of thromboxane A2 by blocking these receptors with the selective antagonists.9,10 18-20 We used 10 μM ADP, since this concentration was found to cause maximal thromboxane A2 generation (not shown). As shown in Figure1A, AR-C67085, a P2Y12 receptor selective–antagonist, inhibited ADP-induced thromboxane A2generation in a concentration-dependent manner. The maximum inhibition occurred with 300 nM AR-C67085. Similar results were obtained with another P2Y12 receptor antagonist, AR-C66096 (not shown). Both AR-C67085 and AR-C66096 completely blocked platelet aggregation induced by ADP. These data suggest that the P2Y12 receptor plays an essential role in ADP-induced thromboxane A2 generation. A2P5P, a P2Y1 receptor-selective antagonist, also blocked ADP-induced thromboxane A2 generation and platelet aggregation in a concentration-dependent manner, with the maximum effect seen at 300 μM (Figure 1B). These data suggest that the P2Y1 receptor activation is also essential for ADP-induced thromboxane A2generation.
Effect of P2 receptor antagonists on ADP-induced thromboxane A2 generation.
Human platelets were activated after adding fibrinogen (3 μM) with the ADP(10 μM) in the presence or absence of various concentration of AR-C67085 (panel A) or A2P5P (panel B), as indicated, at 37°C with stirring at 900 rpm. Reactions were stopped at 3 minutes after the addition of ADP by snap-freezing the samples. Thromboxane B2 was measured by means of an EIA as described in “Materials and methods.” The thromboxane B2 values were normalized to those in the absence of the antagonist (taken as 100%). The data are derived from at least 3 independent experiments. The curves represent hyperbolae that best fit the data with the use of the program Kaleidagraph (Synergy Software, Reading, PA).
Effect of P2 receptor antagonists on ADP-induced thromboxane A2 generation.
Human platelets were activated after adding fibrinogen (3 μM) with the ADP(10 μM) in the presence or absence of various concentration of AR-C67085 (panel A) or A2P5P (panel B), as indicated, at 37°C with stirring at 900 rpm. Reactions were stopped at 3 minutes after the addition of ADP by snap-freezing the samples. Thromboxane B2 was measured by means of an EIA as described in “Materials and methods.” The thromboxane B2 values were normalized to those in the absence of the antagonist (taken as 100%). The data are derived from at least 3 independent experiments. The curves represent hyperbolae that best fit the data with the use of the program Kaleidagraph (Synergy Software, Reading, PA).
Coactivation of Gq and Gi pathways in platelets has been shown to be required for ADP-induced platelet aggregation, whereas the Gq pathway is sufficient to cause platelet shape change.20 To investigate whether coactivation of Gq and Gi pathways is also required to cause generation of thromboxane A2, platelets were stimulated with serotonin and epinephrine, which stimulate the Gq and Gi pathways, respectively. Neither serotonin nor epinephrine alone caused any significant thromboxane A2 generation. Previously, we have shown that serotonin or epinephrine, when added alone, failed to cause platelet aggregation, although serotonin alone caused platelet shape change.20 However, after the addition of fibrinogen (3 μM), costimulation of platelets with serotonin and epinephrine caused thromboxane A2generation (4.23 ± 0.85 ng/108 platelets) comparable to that caused by ADP (4.43 ± 0.88 ng/108 platelets). These data suggest that coactivation of the Gq and Gi signaling pathways is essential for thromboxane A2 production in human platelets.
Effect of fibrinogen receptor antagonist on ADP-induced thromboxane A2 generation
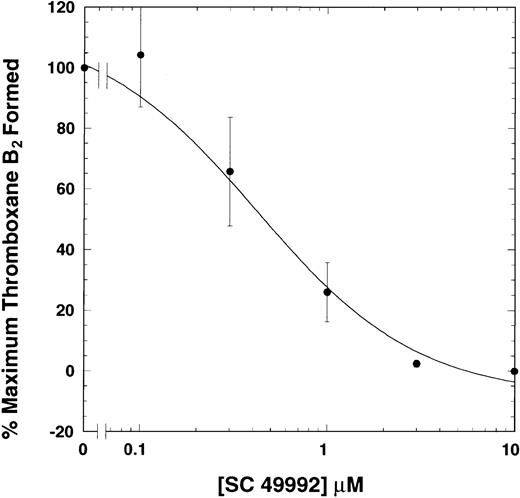
Since the ADP-induced platelet aggregation also requires coactivation of the P2Y1 and P2Y12 receptors, it is important to know whether ADP-induced thromboxane A2 production is an independent event requiring both Gi and Gq pathways or is dependent upon aggregation as well. The effect of aggregation on ADP-induced thromboxane A2 production was investigated by means of a fibrinogen receptor antagonist, SC49992. This antagonist blocks fibrinogen binding to αIIbβ3 and thus inhibits platelet aggregation.27 Platelets were stimulated with ADP (10 μM) in the presence of various concentrations of SC49992. This compound inhibited, in a concentration-dependent manner, both platelet aggregation (not shown) and thromboxane A2generation induced by ADP (Figure 2), indicating a strong correlation between ADP-induced platelet aggregation and thromboxane A2 generation. Thus, fibrinogen binding to its receptor and subsequent outside-in signaling significantly contribute to thromboxane A2production.
Effect of a fibrinogen receptor antagonist on ADP-induced generation of thromboxane A2.
Washed human platelets were activated with ADP (10 μM) plus fibrinogen at 37°C with stirring in the presence or absence of varying doses of SC49992, a fibrinogen receptor antagonist. Reaction in the absence of SC49992 is used as a control. Thromboxane B2was measured as described in Figure 1. The data are derived from at least 3 independent experiments in duplicate and analyzed as in Figure 1.
Effect of a fibrinogen receptor antagonist on ADP-induced generation of thromboxane A2.
Washed human platelets were activated with ADP (10 μM) plus fibrinogen at 37°C with stirring in the presence or absence of varying doses of SC49992, a fibrinogen receptor antagonist. Reaction in the absence of SC49992 is used as a control. Thromboxane B2was measured as described in Figure 1. The data are derived from at least 3 independent experiments in duplicate and analyzed as in Figure 1.
Previous studies have shown that ADP does not cause thromboxane A2 production in unstirred suspensions of platelets,28 which do not aggregate. It was argued that close cell-cell contact mediated by fibrinogen cross-linking is essential for ADP-induced thromboxane A2 production. Snake venom–derived proteins applaggin, echistatin, and trigramin, which block fibrinogen binding to its receptor, have been shown to block platelet aggregation and thromboxane production.29 30 Our results are consistent with these findings, providing additional evidence for the involvement of the fibrinogen binding to its receptor as an essential step in ADP-induced thromboxane A2production.
Effect of P2 receptor antagonists and αIIbβ3 antagonist on ADP-induced phospholipase A2 activation
The generation of thromboxane A2 involves release of arachidonic acid by activated phospholipase A2(PLA2) and subsequent conversion by cyclo-oxygenase pathway. To investigate whether the P2 receptor antagonists and SC49992 interfered with the liberation of arachidonic acid or its subsequent conversion to thromboxane A2, we quantitated [3H]-arachidonic acid plus metabolite liberation as a measure of PLA2 activation. [3H]-arachidonic acid–loaded human platelets were stimulated with ADP in the presence or absence of antagonists and the released label was measured. As shown in Figure 3, the P2 receptor antagonists, alone or in combination, inhibited the ADP-induced liberation of arachidonic acid plus metabolites. Furthermore, SC57101, a second fibrinogen receptor antagonist, also inhibited such release. These data indicate that the blockade of P2Y1 receptor, P2Y12 receptor, or fibrinogen receptor results in the inhibition of PLA2activation.
Effect of P2 receptor antagonists or SC57101 on ADP-induced arachidonic acid liberation.
Washed human platelets, loaded with [3H]-arachidonic acid, were activated with ADP (10 μM) plus fibrinogen at 37°C with stirring in the presence or absence of A3P5P, AR-C66096, or SC57101, a fibrinogen receptor antagonist. Arachidonic acid (AA) plus metabolites in the supernatant was measured as described in “Materials and methods.” The radioactivity in the unstimulated total platelets (0.5 mL) was taken as 100%, and the data were normalized to this value. The data are derived from at least 3 independent experiments in triplicate and analyzed as in Figure 1. NS indicates not statistically significant. *P < .05. **P < .025.
Effect of P2 receptor antagonists or SC57101 on ADP-induced arachidonic acid liberation.
Washed human platelets, loaded with [3H]-arachidonic acid, were activated with ADP (10 μM) plus fibrinogen at 37°C with stirring in the presence or absence of A3P5P, AR-C66096, or SC57101, a fibrinogen receptor antagonist. Arachidonic acid (AA) plus metabolites in the supernatant was measured as described in “Materials and methods.” The radioactivity in the unstimulated total platelets (0.5 mL) was taken as 100%, and the data were normalized to this value. The data are derived from at least 3 independent experiments in triplicate and analyzed as in Figure 1. NS indicates not statistically significant. *P < .05. **P < .025.
Effect of fibrinogen receptor antagonist on arachidonic acid–induced thromboxane A2 generation
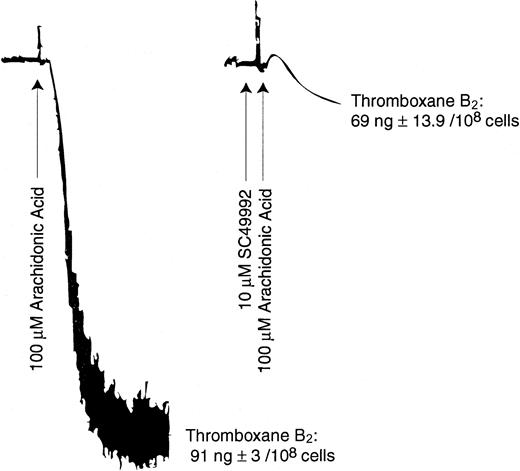
ADP-induced generation of thromboxane A2 requires liberation of arachidonic acid from platelet phospholipids. Arachidonic acid thus liberated is converted to thromboxane A2 through the cyclo-oxygenase pathway. We investigated the effect of SC49992 on arachidonic acid–induced platelet aggregation and thromboxane A2 generation. Platelets were stimulated with 100 μM arachidonic acid in the presence or absence of SC49992. As shown in Figure 4, although SC49992 inhibited arachidonic acid–induced aggregation, it failed to block thromboxane A2 generation induced by arachidonic acid. These data indicate that platelet aggregation is not required for conversion of arachidonic acid to thromboxane A2. Hence outside-in signaling through the fibrinogen receptor appears to be important for arachidonic acid liberation.
Effect of SC49992 on arachidonic acid–induced platelet aggregation and thromboxane A2 generation.
Human platelets were activated with 100 μM arachidonic acid at 37°C with stirring, in the presence or absence of 10 μM SC49992. Thromboxane B2 was measured as described in Figure 1. The data are derived from at least 3 independent experiments.
Effect of SC49992 on arachidonic acid–induced platelet aggregation and thromboxane A2 generation.
Human platelets were activated with 100 μM arachidonic acid at 37°C with stirring, in the presence or absence of 10 μM SC49992. Thromboxane B2 was measured as described in Figure 1. The data are derived from at least 3 independent experiments.
Effect of outside-in signaling on thromboxane A2generation
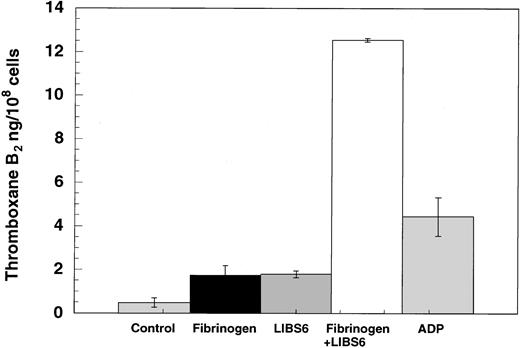
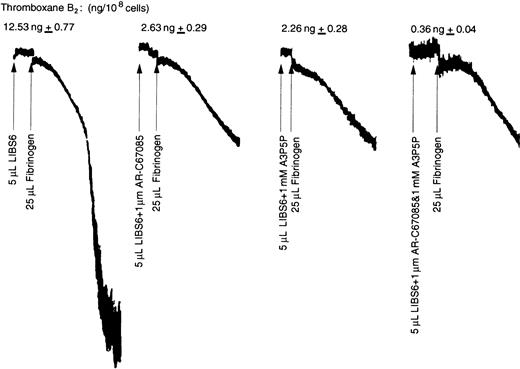
Since outside-in signaling appears to be essential for ADP-induced thromboxane A2 formation, we investigated whether outside-in signaling alone is sufficient for thromboxane A2generation or whether thromboxane A2 generation requires supplementation with inside-out signaling. Tyrosine phosphorylation of pp125FAK has previously been shown to require both outside-in signaling, through the fibrinogen receptor, and Gq signaling, through the agonist receptors.31 The LIBS6 antibody has been shown to induce the fibrinogen binding site by binding to β3 of αIIbβ3 and thereby cause outside-in signaling in the presence of fibrinogen.24,25 31 The Fab fragments of LIBS6 antibody were used in the thromboxane A2generation assay. As shown in Figure 5, addition to platelets of either fibrinogen or LIBS6 Fab generated small amounts of thromboxane A2. However, addition of fibrinogen to LIBS6 Fab–treated platelets resulted in highly potentiated thromboxane A2 generation. The extent of thromboxane A2 generated by outside-in signaling is larger than that by 10 μM ADP (12.5 ng versus 4.4 ng/108 cells). These data indicate that outside-in signaling is sufficient to cause arachidonic acid liberation and subsequent conversion to thromboxane A2.
Effect of LIBS6-induced platelet aggregation on thromboxane A2 generation.
Human platelets were treated with Fab fragments of LIBS6 Fab (600 nM) at 37°C with stirring for 5 minutes. Fibrinogen (3 μM) was then added, and stirring was continued for an additional 3 minutes. The reaction was stopped by snap-freezing, and thromboxane B2was measured as described in Figure 1. The results were from 3 independent experiments in duplicate.
Effect of LIBS6-induced platelet aggregation on thromboxane A2 generation.
Human platelets were treated with Fab fragments of LIBS6 Fab (600 nM) at 37°C with stirring for 5 minutes. Fibrinogen (3 μM) was then added, and stirring was continued for an additional 3 minutes. The reaction was stopped by snap-freezing, and thromboxane B2was measured as described in Figure 1. The results were from 3 independent experiments in duplicate.
PLA2 activation has been known to depend on mobilization of calcium ions from intracellular stores and phosphorylations.32 Since the selective P2Y1 receptor activation by ADP results in increases in cytosolic calcium,10 this pathway should have been sufficient to cause PLA2 activation if elevated cytosolic calcium alone were sufficient to cause PLA2 activation. Outside-in signaling might contribute to the phosphorylation events involved in PLA2 activation. It is also possible that integrin-mediated cytoskeletal rearrangement might contribute to the translocation of PLA2 to the phospholipid membrane and/or availability of the phospholipid substrates.
It has been suggested that agonist-induced thromboxane A2formation in citrated plasma is an artifact and does not occur under physiological conditions.1 In our hands, ADP-induced thromboxane A2 production was also inhibited by physiological concentrations of calcium (data not shown). However, under pathophysiological conditions, in the platelet plug microenvironment, calcium concentrations may be drastically lower than the physiological concentrations. Under these conditions, thromboxane A2 generation and subsequent secretion might be essential for strengthening the platelet plug. The use of aspirin as an antithrombotic drug substantiates the role of thromboxane A2 generation under pathophysiological conditions.33 Under normal physiological conditions, however, thromboxane A2 generation might be blocked by calcium ions, which is probably a regulatory mechanism to prevent unwanted generation of positive feedback regulators.
Role of ADP-induced inside-out signaling events in thromboxane A2 production
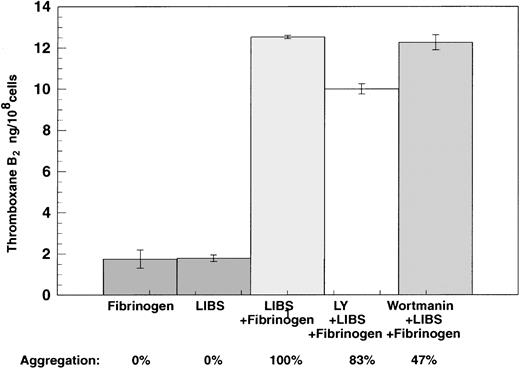
During the preparation of washed platelets, ADP is released from damaged cells and activated platelets. Furthermore, ADP is secreted during LIBS6-induced platelet aggregation.25 Hence, inside-out signaling from ADP receptors might contribute to LIBS-induced thromboxane A2 generation. To rule out this possibility, we investigated the effect of P2Y1 and P2Y12 receptor antagonists on LIBS6-induced thromboxane A2 generation. As shown in Figure 6, A2P5P or AR-C67085, when added together or alone, drastically inhibited LIBS6-induced thromboxane A2 generation. These data indicate that ADP-induced inside-out signaling through the P2Y1 and P2Y12 receptors is also essential for thromboxane A2 generation. The P2 receptor antagonists, however, did not have any effect on arachidonic acid–induced thromboxane A2 production (not shown). Hence both inside-out signaling events from Gq and Gi, and outside-in signaling from the fibrinogen receptor are required for phospholipase A2 activation.
Effect of P2 receptor antagonists on LIBS6-induced thromboxane A2 generation.
Platelets were activated with Fab fragments of the LIBS6 antibody (1.5 μM) at 37°C with stirring for 5 minutes. P2 receptor subtype–selective antagonist (1 μM AR-C67085 or 1 mM A2P5P) was added, as indicated, just prior to the addition of fibrinogen (3 μM), and stirring was continued for an additional 3 minutes. The reaction was stopped by snap-freezing, and thromboxane B2 was measured as described in Figure 1. The data were normalized to thromboxane B2 generated by LIBS6 and fibrinogen, taken as 100%. The results were from 2 independent experiments performed in duplicate.
Effect of P2 receptor antagonists on LIBS6-induced thromboxane A2 generation.
Platelets were activated with Fab fragments of the LIBS6 antibody (1.5 μM) at 37°C with stirring for 5 minutes. P2 receptor subtype–selective antagonist (1 μM AR-C67085 or 1 mM A2P5P) was added, as indicated, just prior to the addition of fibrinogen (3 μM), and stirring was continued for an additional 3 minutes. The reaction was stopped by snap-freezing, and thromboxane B2 was measured as described in Figure 1. The data were normalized to thromboxane B2 generated by LIBS6 and fibrinogen, taken as 100%. The results were from 2 independent experiments performed in duplicate.
The P2 receptor antagonists also blocked the second wave of aggregation induced by LIBS6 (Figure 6). Frelinger et al25 have shown that that the Fab fragments of anti-LIBS antibodies that induce aggregation also cause secretion. The LIBS-induced secretion and the second wave of aggregation are abolished when thromboxane synthesis was blocked.25 Thus, thromboxane A2 generated during LIBS-induced aggregation was responsible for the second wave of aggregation through the secretion of ADP. Thus, the outside-in signals leading to PLA2 activation are finally contributing to the platelet secretion.
Role of outside-in signaling events in thromboxane A2 generation
Previously we have demonstrated that PI-3 kinase is activated during both inside-out and outside-in signaling.24,34-36We investigated whether outside-in signaling causes thromboxane A2 production through a PI-3 kinase and potentially Akt/protein kinase B (PKB)–dependent pathway.36The platelets were preincubated with the PI-3 kinase inhibitors, wortmannin (20 nM) or LY294002 (10 μM), prior to the treatment with LIBS6 Fab and fibrinogen, and the effect of these compounds on thromboxane A2 generation was determined. Neither wortmannin nor LY294002 had any effect on LIBS Fab–induced thromboxane A2 generation (Figure 7), although these compounds inhibited ADP-induced platelet aggregation (not shown), as was shown previously.35 Thus LIBS-induced liberation of arachidonic acid liberation does not depend on PI-3 kinase activation.
Effect of PI-3 kinase inhibitors on LIBS6-induced thromboxane A2 generation.
Platelets activated with Fab fragments of the LIBS6 antibody after pretreatment with wortmannin (20 nM) or LY294002 (10 μM) at 37°C with stirring for 5 minutes. Fibrinogen was added at the end of the incubation, and stirring was continued for an additional 3 minutes. The reaction was stopped by snap-freezing, and thromboxane B2was estimated as described in Figure 1.
Effect of PI-3 kinase inhibitors on LIBS6-induced thromboxane A2 generation.
Platelets activated with Fab fragments of the LIBS6 antibody after pretreatment with wortmannin (20 nM) or LY294002 (10 μM) at 37°C with stirring for 5 minutes. Fibrinogen was added at the end of the incubation, and stirring was continued for an additional 3 minutes. The reaction was stopped by snap-freezing, and thromboxane B2was estimated as described in Figure 1.
Previous studies have implicated a Na+/H+exchanger in PLA2 activation by ADP.37-40Inhibitors of Na+/H+ exchanger blocked agonist-induced arachidonic acid liberation,37,38 and the alkalinization of the cytosol through the Na+/H+ exchanger required binding of fibrinogen to its receptor.37 Thus, Na+/H+ is very likely involved in LIBS6 Fab plus fibrinogen–induced PLA2 activation, arachidonic acid liberation, and thromboxane A2 generation.
Role of tyrosine kinases and phosphatases in ADP-induced thromboxane A2 generation
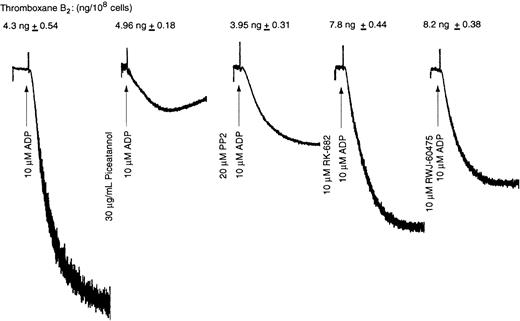
The outside-in signaling events include activation of Syk and tyrosine phosphorylations.23,41 To investigate whether Syk is involved in ADP-induced thromboxane A2 generation, we used piceatannol, a Syk inhibitor. Src kinases have been implicated in fibrinogen receptor activation.41 Hence, we used PP2, a selective Src kinase inhibitor, to investigate the role of Src kinases in ADP-induced thromboxane A2 generation. Piceatannol (10 μM) or PP2 (10 μM) inhibited ADP-induced platelet aggregation but had no significant effect on ADP-induced thromboxane A2generation (Figure 8). It appears that these kinases might inhibit platelet aggregation but do have a role in PLA2 activation.
Effect of protein tyrosine kinase and protein tyrosine phosphatase inhibitors on ADP-induced thromboxane A2generation.
Washed human platelets were activated with ADP (10 μM) plus fibrinogen at 37°C with stirring in the presence or absence of inhibitor (as labeled). Reaction in the absence of the inhibitor is used as a control. The aggregation tracings are representative of the data from 3 independent experiments, and the corresponding thromboxane B2 data were measured in experiments performed in duplicate (n = 3) and analyzed as in Figure 1.
Effect of protein tyrosine kinase and protein tyrosine phosphatase inhibitors on ADP-induced thromboxane A2generation.
Washed human platelets were activated with ADP (10 μM) plus fibrinogen at 37°C with stirring in the presence or absence of inhibitor (as labeled). Reaction in the absence of the inhibitor is used as a control. The aggregation tracings are representative of the data from 3 independent experiments, and the corresponding thromboxane B2 data were measured in experiments performed in duplicate (n = 3) and analyzed as in Figure 1.
As protein tyrosine phosphatases are activated downstream of fibrinogen binding to activated αIIbβ3, we investigated the effect of protein tyrosine phosphatase inhibitors RK-682 and RWJ-60475 on ADP-induced thromboxane A2generation. Although these tyrosine phosphatase inhibitors significantly inhibited ADP-induced platelet aggregation, they had no effect on ADP-induced thromboxane A2 generation (Figure 8). None of these inhibitors by themselves caused any thromboxane A2 generation (not shown). Hence, the residual aggregation and resultant outside-in signaling are sufficient to generate thromboxane A2 without an essential role for Syk, Src kinases, or protein tyrosine phosphatases.
Tyrosine phosphorylation of pp125FAK in platelets also has been shown to require coordinated signaling through occupied αIIbβ3 and agonist receptors.31 These separate pathways are postulated to converge and thus promote the cytoskeletal reorganization required for activation of FAK.31 Whether FAK plays any role in thromboxane A2 generation remains to be seen.
In conclusion, we have provided evidence that in human platelets, inside-out signaling events through the P2Y1 and P2Y12 receptors and outside-in signaling events via the fibrinogen receptor αIIbβ3 are necessary for arachidonic acid liberation and subsequent conversion to thromboxane A2. PI-3 kinases, Syk, Src kinases, or protein tyrosine phosphatases do not appear to be mediators of this pathway.
We thank Dr Mark Ginsberg, The Scripps Research Institute, La Jolla, CA, for providing LIBS6 antibodies.
Supported in part by research grants HL60683 and HL64943 (S.P.K.), and HL38622 (S.E.R.) from the National Institutes of Health and a postdoctoral fellowship from the American Heart Association Pennsylvania-Delaware affiliate (T.M.Q.). This work was performed during the tenure of an Established Investigator award in Thrombosis from American Heart Association and Genentech to S.P.K.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Satya P. Kunapuli, Department of Physiology, Temple University School of Medicine, 3420 N Broad St, Philadelphia PA 19140; e-mail: kunapuli@nimbus.temple.edu.

![Fig. 3. Effect of P2 receptor antagonists or SC57101 on ADP-induced arachidonic acid liberation. / Washed human platelets, loaded with [3H]-arachidonic acid, were activated with ADP (10 μM) plus fibrinogen at 37°C with stirring in the presence or absence of A3P5P, AR-C66096, or SC57101, a fibrinogen receptor antagonist. Arachidonic acid (AA) plus metabolites in the supernatant was measured as described in “Materials and methods.” The radioactivity in the unstimulated total platelets (0.5 mL) was taken as 100%, and the data were normalized to this value. The data are derived from at least 3 independent experiments in triplicate and analyzed as in Figure 1. NS indicates not statistically significant. *P < .05. **P < .025.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.193/6/m_h80121929003.jpeg?Expires=1769094382&Signature=EuzUw1txEZtUu5pQLhcU01T8hgzvu3Jmc5LhWRr2VgbCnzlQzNX3lwfww7f8cW3UnAI-pC-4En7Qj7U1K47dusZAxyRHTn2H1zRWk6z4d4piB1ALYI~kfmfctVjHQZnV6kt~-xQmla3dS2LINGre6VlfB1GS4FgWECIYL~hK5aQWxE8AErcDBDEEKQpeg~wGTuADWCwKZ0y~mIKrneKbT5H2E8ycF6kovFjX~fq~pV2~xtrHO~wLc0ujCyEVRVFcSzuVAh5eNnqtEM80vTbh-ynbmle8IVgm7UBVXCmOZpGCIN32oinajb4pnWZkjjC75LQjKH1t3X~ksUw7oowwKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal