Activation of endothelial cells by lipid oxidation products is a key event in the initiation and progression of the atherosclerotic lesion. Minimally modified low-density lipoprotein (MM-LDL) induces the expression of certain inflammatory molecules such as tissue factor (TF) in endothelial cells. This study examined intracellular signaling pathways leading to TF up-regulation by oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC), a biologically active component of MM-LDL. OxPAPC induced TF activity and protein expression in human umbilical vein endothelial cells (HUVECs). However, OxPAPC neither induced phosphorylation or degradation of IκBα nor DNA binding of nuclear factor-κB (NF-κB). Furthermore, OxPAPC-induced TF expression was not inhibited by overexpression of IκBα. These results strongly indicate that OxPAPC-induced TF expression is independent of the classical NF-κB pathway. However, OxPAPC stimulated phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 and expression of early growth response factor 1 (EGR-1). Inhibitors of mitogen-activated kinase/ERK (MEK) or protein kinase C (PKC) blocked elevation of both EGR-1 and TF. Furthermore, overexpression of NAB2, a corepressor of EGR-1, inhibited effects of OxPAPC. In addition, OxPAPC induced rapid and reversible elevation of free cytosolic Ca++ levels and nuclear factor of activated T cells (NFAT)/DNA binding. Induction of TF expression by OxPAPC was partially inhibited by cyclosporin A, known to block calcineurin, a Ca++-dependent phosphatase upstream of NFAT. Treatment of OxPAPC with phospholipase A2 destroyed its biologic activity and 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine was identified as one biologically active component of OxPAPC that induces TF expression. Together, the results demonstrate that OxPAPC induces TF expression in HUVECs through activation of PKC/ERK/EGR-1 and Ca++/calcineurin/NFAT pathways rather than by NF-κB–mediated transcription. Thus, oxidized phospholipids may contribute to inflammation by activating pathways alternative to the classical NF-κB pathway.
Introduction
Tissue factor (TF) is a cell surface receptor initiating blood coagulation,1 thereby promoting thrombotic events in atherosclerosis, sepsis, and cancer.2,3 Enhanced endothelial TF expression has been demonstrated in atherosclerotic plaques,4,5 a process that may account for thrombotic events associated with early and advanced atherosclerosis. TF expression in endothelial cells (ECs) can be induced by a variety of agonists, including inflammatory cytokines, angiogenic growth factors, infectious agents, and minimally modified low-density lipoprotein (MM-LDL).1,6 MM-LDL regulates TF expression at the level of transcription5; however, the signaling pathways and transcription factors involved in this process are not known. Some of the effects of MM-LDL can be mimicked by oxidation of 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC).7 Three biologically active components of oxidized PAPC (OxPAPC) have been structurally identified as 1-palmitoyl-2-oxovaleroyl-sn-glycero-3-phosphorylcholine (POVPC), 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine (PGPC),8 and 1-palmitoyl-2-epoxyisoprostane-sn-glycero-3-phosphorylcholine (PEIPC).9 Which of these components of MM-LDL is responsible for induction of TF is not known.
In contrast to interleukin-1 (IL-1) or tumor necrosis factor α (TNF-α), MM-LDL neither up-regulates E-selectin, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1), nor stimulates neutrophil binding to human ECs.7This suggests that classical inflammatory agonists and MM-LDL activate different signaling mechanisms. The major pathway induced by inflammatory cytokines activates transcription factors of the nuclear factor-κB (NF-κB) family.10 Whether NF-κB is activated by MM-LDL is a subject of controversy.6,11 In fact, it was shown that MM-LDL and some of its components were capable of down-regulating NF-κB–mediated transcription induced by inflammatory cytokines.12 Thus, the role of the NF-κB pathway in inflammatory activation of ECs by oxidized lipids requires further investigation.
Apart from NF-κB,13 transcription of the TFgene can be promoted by early growth response factor 1 (EGR-1) and nuclear factor of activated T cells (NFAT).14,15 Whereas inflammatory cytokines induce NF-κB as well as EGR-1, vascular endothelial growth factor (VEGF) triggers EGR-116 and NFAT15 to up-regulate TF. EGR-1 was shown to be important for transcription of the TF gene in rat vascular smooth muscle cells that had been treated with native or oxidized LDL.17 It has not been demonstrated whether EGR-1 and NFAT are activated in human ECs by oxidized phospholipids present in MM-LDL.
In the present study, we investigated signaling pathways and transcription factors mediating induction of TF expression in human ECs by biologically active oxidized phospholipids. We show that expression of TF is elevated by OxPAPC, and that this induction was mainly mediated by EGR-1– and NFAT-dependent transcription, but was independent of NF-κB activation. Upstream mechanisms activated by OxPAPC were elevation of cytosolic Ca++, activation of protein kinase C (PKC), and the mitogen-activated protein (MAP) kinase/extracellular signal-regulated kinase (ERK) kinase (MEK)/ERK MAP kinase cascade.
Materials and methods
Materials
Cyclosporin A was purchased from Novartis (Vienna, Austria); TNF-α from Genzyme (Cambridge, MA);l-α-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine, A23187, citrated pig plasma, and thromboplastin from Sigma Chemicals (St Louis, MO); and fura-2/am from Molecular Probes (Leiden, The Netherlands). PD98059, SB203580, and bisindolylmaleimide I (Bis I) were obtained from Calbiochem (La Jolla, CA). Polyclonal antibodies against phosphorylated forms of ERK1/2 and IκBα, nonphospho-ERK1/2, and LumiGLO were from New England Biolabs (Beverly, MA). Polyclonal anti–EGR-1, anti-NFATc (NFATc1), and anti-NFATp (NFATc2) anti-IκBα were from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal TF antibodies were a gift from Dr W. Ruf (Scripps Research Institute, La Jolla, CA). Peroxidase-conjugated secondary antibodies were purchased from Amersham Life Science (Amersham Place, Little Chalfont, United Kingdom). Immobilon-P transfer membranes were products of Millipore (Bedford, MA).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were cultured at 37°C and 5% CO2 in medium 199 containing 20% supplemented calf serum (SCS), 1 U/mL heparin, 50 μg/mL bovine endothelial cell growth supplement (Technoclone, Vienna, Austria), 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Experiments were performed using cells up to passage 5. In all experiments, before the addition of OxPAPC, HUVECs were incubated in medium 199 containing 1% SCS for 4 hours.
Lipid oxidation, preparation, and analysis of POVPC and PGPC
PAPC was oxidized by exposure of dry lipid to air for 72 hours. The extent of oxidation was monitored by positive ion electrospray mass spectrometry (ESI-MS) as described previously.8 POVPC and PGPC were prepared by ozonolysis of PAPC essentially as described.12 Purification was performed on a normal phase column (Hypersil, 3 μM, 4 × 150 mm) with UV detection at 270 nm. Fractions were collected and analyzed by ESI-MS. Lipids were stored at −70°C in chloroform and used not longer than 1 week after testing for purity. OxPAPC, POVPC, and PGPC preparations were shown negative for endotoxin by the Limulus amebocyte assay (Biowhittaker, Walkersville, MD).
Clotting assay
Confluent HUVECs grown in 6-well plates were incubated for 4 hours in medium 199/1% SCS before the addition of agonists. After 6 hours of stimulation with OxPAPC, cells were washed and scraped into 1 mL clotting buffer (10 mM sodium acetate, 7 mM sodium barbiturate, 130 mM NaCl, pH 7.4). The cells were pelleted in a microcentrifuge and resuspended in 200 μL clotting buffer. Clotting time was measured at 37°C after mixing equal volumes of sample, pig plasma, and 20 mM CaCl2. TF activity equivalents were determined from a standard curve obtained using rabbit brain thromboplastin.
Western blotting
After stimulation, HUVECs were lysed in Laemmli buffer and proteins separated by electrophoresis on 12% sodium dodecyl sulfate (SDS)–polyacrylamide gels. Proteins were blotted onto polyvinylidene difluoride (PVDF) membrane and, after blocking with 3% dry milk/0.1% Tween-20, incubated with primary antibodies in the same solution. Bound antibodies were detected by anti-IgG conjugated with peroxidase and subsequent chemiluminescent detection.
Measurements of intracellular free Ca++concentration
Analysis of NF-κB/DNA binding
Quantitation of p65 binding to its consensus oligonucleotide was performed using the enzyme-linked immunosorbent assay (ELISA)–based Trans-am NF-κB p65 kit (Active Motif, Rixensart, Belgium) according to the manufacturer's instructions. Specificity of p65 binding was confirmed by incubation of cell lysates with the immobilized NF-κB consensus probe in the presence of excess wild-type or mutated oligonucleotide.
Quantitation of NF-κB–mediated transcription
The HUVECs grown in 6-well dishes were cotransfected with pNF-κB-Luc (Stratagene, Amsterdam, The Netherlands, 0.5 μg/well) and pCMV-β (Clontech, Heidelberg, Germany, 0.3 μg/well) using Lipofectamine Plus reagent (Life Technologies, Lofer, Australia) according to the manufacturer's instructions. After 48 hours, the medium was changed to medium 199/10% SCS and cells stimulated with agonists. After 6 hours of incubation, the cells were washed with PBS, scraped into 0.1 M sodium phosphate, pH 7.8, and lysed by 3 freeze-thaw cycles. Luciferase activity was determined using adenosine triphosphate (ATP) andd-luciferin as substrates. Luminescence was measured in a Wallac 1420 Multilabel Counter (Wallac, Turku, Finland). β-Galactosidase activity was determined by spectrophotometric assay using chlorophenol red β-d-galactopyranoside as substrate. Optical density was read at 570 nm in a Wallac 1420 Multilabel Counter.
Transfections and fluorescence microscopy
The HUVECs were transiently transfected with a green fluorescence protein (GFP)–NFAT construct21 (kindly provided by L. Gerace, The Scripps Research Institute), using the Lipofectamine reagent (Life Technologies) according to the protocol published previously.14 One day after transfection cells were analyzed by fluorescence microscopy on a Nikon Diaphot TMD microscope equipped with a cooled charge-coupled device camera (Kappa, Gleichen, Germany). To induce nuclear import of GFP-NFAT, Ca++ ionophore A23187 (4 μM) or OxPAPC (125 μg/mL) was added to cells at 37°C for 30 minutes.
Nuclear extraction and electrophoretic mobility shift assays
Preparation of nuclear extracts and electrophoretic mobility shift assays (EMSAs) were performed as described previously,15 except that spermine/spermidine and polyvinylethanol were omitted from the extraction and binding buffers, respectively. Synthetic double-stranded nucleotides were labeled by filling in the overhangs with Klenow enzyme in the presence of [α-32P]dATP. Labeled probes were purified by polyacrylamide gel electrophoresis. The following oligonucleotides were used: 5′-aattATAAAATTTTCCAATGTAAAC-3′ (NFATp sequence −60 to −80 of the murine IL-4 promoter), 5′-aattCCGGAGTTTCCTACC-3′ (nucleotides −183 to −197 of the human TF promoter) and 5′-aattGGAGGCGGGGCAGGGGTGTGGAACTCG-3′ (Sp1 site from the human TF promoter). Each reaction contained 3 μg nuclear protein. To control for specificity of binding, 50-fold excess of unlabeled specific or unrelated (Sp1) oligonucleotide was added. For supershift, 4 μg antibody was added 15 minutes before addition of labeled oligonucleotide. Separation of DNA-protein complexes was performed in 5% nondenaturing polyacrylamide gels in 0.5 × Tris borate EDTA buffer (TBE).
Recombinant adenoviral constructs and infection
The generation of recombinant adenoviruses expressing NAB2 will be described elsewhere (M.L. and D.M., manuscript submitted). The IκBα-encoding adenovirus22 was from Rainer de Martin (Dept of Vascular Biology and Thrombosis Research, University of Vienna). HUVECs grown in 6-well plates were washed twice with phosphate-buffered saline (PBS) and incubated at a multiplicity of infection of 100 with recombinant adenovirus or control adenovirus in PBS. After 30 minutes, cells were washed with PBS and then cultured in normal medium. Twenty-four hours after infection, cells were incubated in 1% SCS medium for 4 hours followed by stimulation with OxPAPC for 6 hours.
Phospholipase A2 treatment and thin-layer chromatography of OxPAPC
OxPAPC has been treated with phospholipase A2(PLA2) as described previously9 with minor modifications. Briefly, 5 mg OxPAPC was dried, resuspended by vortexing in 1 mL PBS containing 5 mM CaCl2 and split into 2 portions. Then, 0.1 mL PBS/Ca++ containing 300 U Naja mossambica PLA2 was added to one tube, and the same amount of PBS/Ca++ to the other tube (mock-treated OxPAPC). After incubation for 1 hour at 36°C with periodic vortexing, lipids were extracted with 7 volumes of CHCl3/methanol (2:1) containing 0.01% butylated hydroxytoluene. After vortexing and centrifugation, lower phases were collected and 5 volumes of CHCl3 were added to the residual aqueous phases. After the next cycle of vortexing and centrifugation, lower organic phases were pooled, dried, redissolved in 250 μL CHCl3 and stored at −70°C until analysis of activity or separation by thin-layer chromatography (TLC).
Lipids were separated by TLC into phospholipids, neutral lipids, and fatty acids on Whatman 60K silica plates developed with hexane/ethyl ether/acetic acid (70:30:1). Standards were stained by iodine vapors, and plate areas corresponding to phospholipids and fatty acids were scraped off and extracted with CHCl3/methanol/H2O (25:50:20) for 40 minutes at room temperature with shaking. Supernatants obtained after centrifugation of silica gel suspension were evaporated, dissolved in CHCl3, and stored at −70°C until analysis.
Results
OxPAPC induces TF expression in HUVECs
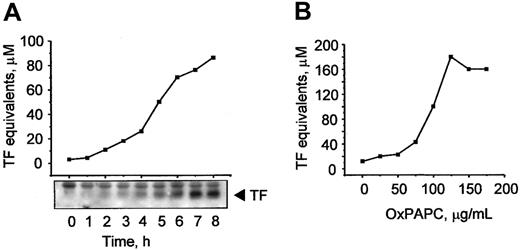
Previously, MM-LDL was shown to induce TF expression in ECs.6 It is known that some biologic effects of MM-LDL can be mimicked by OxPAPC, a component of MM-LDL.7 In this work, we tested whether OxPAPC was capable of inducing expression of TF in HUVECs. We found that OxPAPC elevated TF activity in a time-dependent manner (Figure 1A), whereas native phospholipid was inactive (not shown). The increase in activity paralleled increasing levels of TF protein, as demonstrated by Western blotting (Figure 1A, insert). The effects of OxPAPC were concentration dependent and reached apparent saturation at about 100 μg/mL (Figure 1B).
OxPAPC up-regulates TF activity and protein in HUVECs.
HUVECs were stimulated with 125 μg/mL OxPAPC for indicated time periods (A) or treated for 6 hours with the indicated concentrations of OxPAPC (B). TF activity was determined by clotting assay and the level of TF protein expression was analyzed by Western blotting. Data shown are representative of 2 or 3 independent experiments, in panels A and B, respectively.
OxPAPC up-regulates TF activity and protein in HUVECs.
HUVECs were stimulated with 125 μg/mL OxPAPC for indicated time periods (A) or treated for 6 hours with the indicated concentrations of OxPAPC (B). TF activity was determined by clotting assay and the level of TF protein expression was analyzed by Western blotting. Data shown are representative of 2 or 3 independent experiments, in panels A and B, respectively.
OxPAPC does not activate the NF-κB pathway
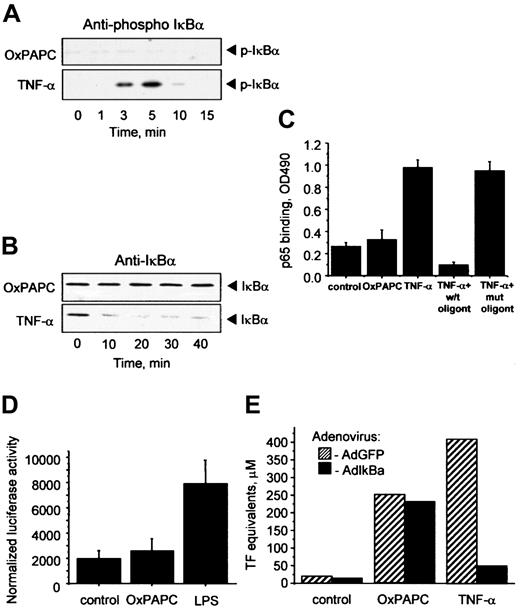
Inflammatory cytokines such as TNF-α or IL-1 elevate TF in ECs through activation of NF-κB–mediated transcription.23Important steps in this process are phosphorylation and degradation of the inhibitory IκBα subunit allowing translocation of active NF-κB to the nucleus. To address a possible involvement of the NF-κB pathway in OxPAPC-induced TF expression, we used 4 different approaches. First, we demonstrated by Western blotting that OxPAPC, in contrast to TNF-α, did not induce phosphorylation of IκBα (Figure2A). Furthermore, OxPAPC did not activate IκBα degradation, whereas TNF-α induced a rapid decrease in the levels of IκBα (Figure 2B). Second, OxPAPC did not induce binding of p65 to its consensus DNA (Figure 2C). Third, we have found that in contrast to lipopolysaccharide (LPS), OxPAPC did not stimulate a luciferase reporter construct bearing 5× NF-κB consensus site (Figure 2D). Fourth, we used an IκBα-expressing adenovirus (AdIκB) that has been described to block NF-κB activation.22 Overexpression of IκBα had no influence on the induction of TF by OxPAPC, but significantly inhibited the effect of TNF-α (Figure 2E). Together, these data strongly suggest that induction of TF expression by OxPAPC is independent of the classical NF-κB pathway in HUVECs.
Induction of TF by OxPAPC is independent of the NF-κB pathway.
HUVECs were treated with OxPAPC (125 μg/mL) or TNF-α (100 U/mL) for indicated times and then processed for Western blotting with antibodies to phospho-IκBα (A) or total IκBα (B). (C) HUVECs were stimulated with OxPAPC (125 μg/mL) or TNF-α (25 U/mL) for 1 hour. Cells were scraped and activated p65 was detected in cell lysates using an ELISA-based kit Trans-am NF-κB p65. The data are presented as means ± SD of triplicate measurements. Similar results were obtained in an independent experiment. (D) HUVECs were cotransfected with pNF-κB-Luc and pCMV-β and after 48 hours stimulated with OxPAPC (120 μg/mL) or LPS (250 ng/mL). After 6 hours the cells were scraped and analyzed for luciferase and β-galactosidase activities. The results are expressed as the ratio between luminescence units in luciferase assay and optical density in β-galactosidase assay. The data represent mean values ± SD. Similar results were obtained in an additional experiment. (E) HUVECs were infected either with control (AdGFP) or AdIκBα, and 24 hours after infection stimulated with OxPAPC (125 μg/mL) or TNF-α (100 U/mL) for 6 hours. TF activity was determined by clotting assay from duplicate wells. Similar results were obtained in an independent experiment.
Induction of TF by OxPAPC is independent of the NF-κB pathway.
HUVECs were treated with OxPAPC (125 μg/mL) or TNF-α (100 U/mL) for indicated times and then processed for Western blotting with antibodies to phospho-IκBα (A) or total IκBα (B). (C) HUVECs were stimulated with OxPAPC (125 μg/mL) or TNF-α (25 U/mL) for 1 hour. Cells were scraped and activated p65 was detected in cell lysates using an ELISA-based kit Trans-am NF-κB p65. The data are presented as means ± SD of triplicate measurements. Similar results were obtained in an independent experiment. (D) HUVECs were cotransfected with pNF-κB-Luc and pCMV-β and after 48 hours stimulated with OxPAPC (120 μg/mL) or LPS (250 ng/mL). After 6 hours the cells were scraped and analyzed for luciferase and β-galactosidase activities. The results are expressed as the ratio between luminescence units in luciferase assay and optical density in β-galactosidase assay. The data represent mean values ± SD. Similar results were obtained in an additional experiment. (E) HUVECs were infected either with control (AdGFP) or AdIκBα, and 24 hours after infection stimulated with OxPAPC (125 μg/mL) or TNF-α (100 U/mL) for 6 hours. TF activity was determined by clotting assay from duplicate wells. Similar results were obtained in an independent experiment.
OxPAPC activates ERK1/2 MAP kinases
Another mechanism of induction of gene transcription used by inflammatory cytokines is signal transduction through the MAP kinase cascades.24 Therefore, we examined whether OxPAPC could activate ERK1/2 MAP kinases. Western blotting with antibodies recognizing the active phosphorylated forms of ERK1/2 demonstrated rapid and sustained activation of these kinases after addition of OxPAPC or TNF-α (Figure 3). We also detected a slight activation of p38 MAP kinase; however, we could not detect activation of JNK by OxPAPC (data not shown).
OxPAPC induces ERK1/2 phosphorylation.
HUVECs were treated with OxPAPC (125 μg/mL) or TNF-α (100 U/mL) for indicated times. Cells were harvested and processed for Western blotting with antibodies to phospho-ERK1/2 or nonphosphorylated ERK1/2 to check for equal loading. The data are representative of 3 independent experiments.
OxPAPC induces ERK1/2 phosphorylation.
HUVECs were treated with OxPAPC (125 μg/mL) or TNF-α (100 U/mL) for indicated times. Cells were harvested and processed for Western blotting with antibodies to phospho-ERK1/2 or nonphosphorylated ERK1/2 to check for equal loading. The data are representative of 3 independent experiments.
OxPAPC induces expression of the transcription factor EGR-1 in a MEK/ERK-dependent manner
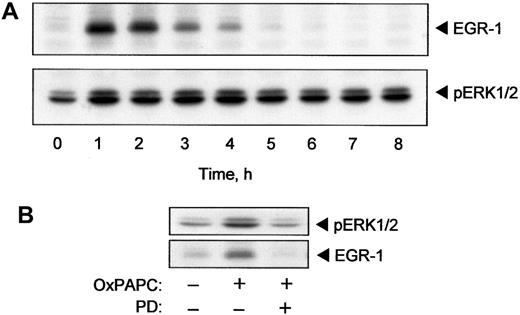
The TF promoter contains binding sites for the EGR-1 transcription factor.25 EGR-1 is required for induction of TF expression by several cytokines and growth factors including TNF-α and VEGF.16,26 It is known that ERK1/2 stimulates expression of EGR-1.27 Because we demonstrated activation of ERK1/2 by OxPAPC, we tested whether OxPAPC would also induce EGR-1. The data presented in Figure 4A demonstrate that OxPAPC induced rapid and transient elevation of EGR-1 protein. The maximum induction was reached after 60 minutes following treatment with OxPAPC and dropped to baseline levels within 5 hours. The time course (Figure 4A) indicated that elevation of EGR-1 preceded the rise of TF protein and activity levels (Figure 1A). The level of EGR-1 decreased after 2 hours of stimulation, whereas phosphorylation of ERK1/2 remained apparently constant for up to 8 hours after addition of OxPAPC (Figure 4A).
OxPAPC induces EGR-1 expression by an ERK1/2-dependent mechanism.
HUVECs were stimulated with 125 μg/mL OxPAPC for indicated times. Cells were collected and analyzed by Western blotting with anti–EGR-1 or anti–phospho-ERK1/2 antibody (A). (B) HUVECs were pretreated for 20 minutes with PD98059 (5 μM) and then stimulated with 125 μg/mL of OxPAPC for 15 minutes (ERK1/2) or 1 hour (EGR-1) in the presence of the inhibitor. Cells were scraped and processed for Western blotting with antibodies to phospho-ERK1/2 or EGR-1. Similar data were obtained in 3 independent experiments.
OxPAPC induces EGR-1 expression by an ERK1/2-dependent mechanism.
HUVECs were stimulated with 125 μg/mL OxPAPC for indicated times. Cells were collected and analyzed by Western blotting with anti–EGR-1 or anti–phospho-ERK1/2 antibody (A). (B) HUVECs were pretreated for 20 minutes with PD98059 (5 μM) and then stimulated with 125 μg/mL of OxPAPC for 15 minutes (ERK1/2) or 1 hour (EGR-1) in the presence of the inhibitor. Cells were scraped and processed for Western blotting with antibodies to phospho-ERK1/2 or EGR-1. Similar data were obtained in 3 independent experiments.
Furthermore, inhibition of MEK, which is upstream of ERK1/2, using a specific inhibitor (PD98059), significantly suppressed OxPAPC-induced phosphorylation of ERK1/2 and elevation of EGR-1 (Figure 4B). These results suggest that activation of ERK1/2 is necessary for induction of EGR-1 expression by OxPAPC.
Activation of ERK1/2 and elevation of EGR-1 are functionally important for induction of TF expression by OxPAPC
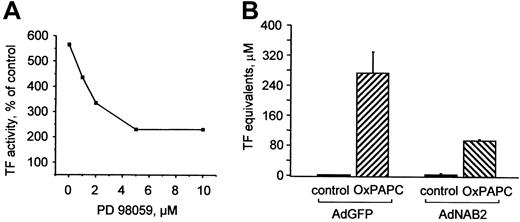
To elucidate the functional role of MAP kinases in induction of TF by OxPAPC, we pretreated cells with a MEK inhibitor (PD98059), which suppressed induction of TF expression by OxPAPC in a dose-dependent manner (Figure 5A). To address the role of EGR-1 in induction of TF by OxPAPC, HUVECs were infected with recombinant adenovirus to overexpress NAB2 (AdNAB2), a natural corepressor of EGR-1 capable of inhibiting its transcriptional activity.28 We found that overexpression of NAB2 in HUVECs significantly suppressed induction of TF by OxPAPC (Figure 5B). These results demonstrate that the MEK/ERK/EGR-1 pathway is critically involved in OxPAPC-induced expression of TF in HUVECs.
ERK1/2 and EGR-1 are important for OxPAPC-induced TF expression.
(A) HUVECs were pretreated for 20 minutes with indicated concentrations of the MEK inhibitor PD98059 and then stimulated with 125 μg/mL OxPAPC for 6 hours in the presence of the inhibitor. Cells were harvested and TF activity was determined by clotting assay. Data are expressed as percentage of activity in cells treated with vehicle (0.1% DMSO) only and are representative for 2 independent experiments. DMSO (0.1% final concentration) alone had no effect. (B) HUVECs were infected with either control (AdGFP) or NAB2-adenovirus (AdNAB2), and 24 hours after infection stimulated with OxPAPC (125 μg/mL) for 6 hours. After stimulation, TF activity was determined by clotting assay. Data are presented as means ± SD of 3 measurements. Similar results were obtained in another independent experiment.
ERK1/2 and EGR-1 are important for OxPAPC-induced TF expression.
(A) HUVECs were pretreated for 20 minutes with indicated concentrations of the MEK inhibitor PD98059 and then stimulated with 125 μg/mL OxPAPC for 6 hours in the presence of the inhibitor. Cells were harvested and TF activity was determined by clotting assay. Data are expressed as percentage of activity in cells treated with vehicle (0.1% DMSO) only and are representative for 2 independent experiments. DMSO (0.1% final concentration) alone had no effect. (B) HUVECs were infected with either control (AdGFP) or NAB2-adenovirus (AdNAB2), and 24 hours after infection stimulated with OxPAPC (125 μg/mL) for 6 hours. After stimulation, TF activity was determined by clotting assay. Data are presented as means ± SD of 3 measurements. Similar results were obtained in another independent experiment.
PKC is necessary for induction of ERK1/2, EGR-1, and TF by OxPAPC
The above data show that OxPAPC stimulates TF expression in HUVECs at least in part via activation of the ERK/EGR-1 pathway, which, in turn, may be stimulated by various upstream kinases, such as PKC.29 Treatment of ECs with the specific PKC inhibitor Bis I in a concentration-dependent manner inhibited TF activity induced by OxPAPC (Figure 6A). Furthermore, ERK1/2 and EGR-1 activation were also inhibited by Bis I (Figure 6B). These data indicate that PKC plays an important role in activation of ERK/EGR-1 signaling pathway and up-regulation of TF by OxPAPC.
OxPAPC-induced TF activity, ERK1/2 phosphorylation, and EGR-1 expression are PKC dependent.
(A) HUVECs were preincubated for 30 minutes with indicated concentrations of the PKC inhibitor Bis I and then stimulated with 120 μg/mL OxPAPC in the presence of the inhibitor for 6 hours. TF activity was determined by clotting assay. (B) HUVECs were treated with 125 μg/mL OxPAPC in the presence of 10 μM Bis I and after 15 minutes (ERK1/2) or 1 hour (EGR-1) harvested and analyzed by Western blotting with antibodies to phospho-ERK1/2 or EGR-1. Similar results were obtained in 2 additional experiments.
OxPAPC-induced TF activity, ERK1/2 phosphorylation, and EGR-1 expression are PKC dependent.
(A) HUVECs were preincubated for 30 minutes with indicated concentrations of the PKC inhibitor Bis I and then stimulated with 120 μg/mL OxPAPC in the presence of the inhibitor for 6 hours. TF activity was determined by clotting assay. (B) HUVECs were treated with 125 μg/mL OxPAPC in the presence of 10 μM Bis I and after 15 minutes (ERK1/2) or 1 hour (EGR-1) harvested and analyzed by Western blotting with antibodies to phospho-ERK1/2 or EGR-1. Similar results were obtained in 2 additional experiments.
OxPAPC elevates cytosolic Ca++ levels and induces nuclear translocation of NFAT
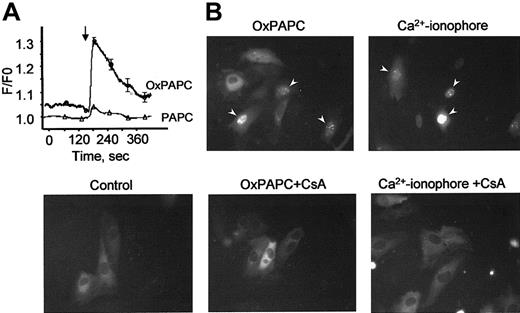
Phorbol esters, Ca++ ionophore, and VEGF are known to stimulate TF gene expression also through activation of the NFAT transcription factor.15,30 Because elevation of intracellular Ca++ levels is a prerequisite for stimulation of NFAT, we tested whether OxPAPC would induce a Ca++response in HUVECs. Using the fluorescent Ca++-sensitive probe fura-2, we demonstrate that OxPAPC, but not its nonoxidized precursor PAPC, induces rapid and transient elevation of Ca++ in the cytosol of HUVECs (Figure7A). To investigate whether OxPAPC is capable of inducing nuclear translocation of NFAT, we studied the intracellular distribution of NFAT tagged with GFP (NFAT-GFP) before and after OxPAPC-treatment. The NFAT-GFP in the transfected cells exhibits the same localization and nuclear translocation as described for endogenous NFAT.31 In unstimulated cells (control), NFAT-GFP is predominantly localized in the cytoplasm (Figure 7B). Addition of OxPAPC induced NFAT-GFP accumulation in cell nuclei within 30 minutes. A similar effect was exerted by calcium ionophore (A23187). Translocation of NFAT-GFP induced by both agonists was inhibited by cyclosporin A, known to suppress activation of NFAT by blocking its upstream phosphatase calcineurin30(Figure 7B).
OxPAPC up-regulates TF in HUVECs through Ca++/NFAT pathway.
(A) HUVECs grown on coverslips were loaded with fura-2 as described in “Materials and methods” and then stimulated with OxPAPC or its nonoxidized precursor (PAPC, both at 125 μg/mL). The arrow indicates the time of addition of the phospholipids. The data are mean values of 8 measurements. (B) HUVECs transfected with NFAT-GFP were stimulated with OxPAPC (125 μg/mL) or Ca++ ionophore (A23187, 4 μM). Where indicated, cells were preincubated with cyclosporin A (100 ng/mL) before stimulation with OxPAPC or Ca++ ionophore. Intracellular localization of NFAT-GFP was determined by fluorescent microscopy 30 minutes following stimulation. Similar results were obtained in an additional independent experiment.
OxPAPC up-regulates TF in HUVECs through Ca++/NFAT pathway.
(A) HUVECs grown on coverslips were loaded with fura-2 as described in “Materials and methods” and then stimulated with OxPAPC or its nonoxidized precursor (PAPC, both at 125 μg/mL). The arrow indicates the time of addition of the phospholipids. The data are mean values of 8 measurements. (B) HUVECs transfected with NFAT-GFP were stimulated with OxPAPC (125 μg/mL) or Ca++ ionophore (A23187, 4 μM). Where indicated, cells were preincubated with cyclosporin A (100 ng/mL) before stimulation with OxPAPC or Ca++ ionophore. Intracellular localization of NFAT-GFP was determined by fluorescent microscopy 30 minutes following stimulation. Similar results were obtained in an additional independent experiment.
OxPAPC induces NFAT/DNA binding activity
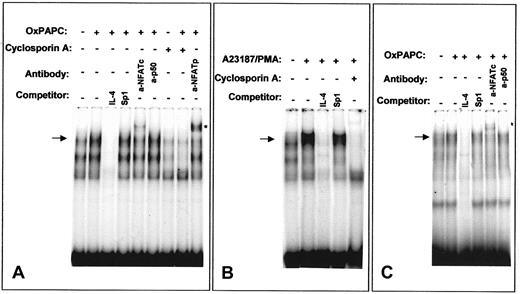
We further analyzed the ability of OxPAPC to activate NFAT by EMSA using an NFAT site of the murine IL-4 promoter that has been demonstrated to bind NFAT independently of AP-1.32 OxPAPC induced NFAT/DNA-binding activity in HUVECs (Figure8A). Similar DNA-protein complexes were formed after treatment of HUVECs with a combination of Ca++ionophore and phorbol ester (Figure 8B). In both cases, the bands were competed with excess of unlabeled specific oligonucleotide, but not by excess of an unlabeled probe for a Sp1 binding site. Moreover, cells pretreated with cyclosporin A failed to form indicated DNA-protein complexes independently whether OxPAPC or Ca++ionophore/phorbol myristate acetate (PMA) was used for stimulation (Figure 8A,B). Supershift experiments demonstrated the presence of NFATp as well as NFATc in the DNA-protein complexes induced by OxPAPC (Figure 8A). In contrast, incubation with an antibody against the NF-κB subunit p50 had no effect. These data correlate with previous studies showing the presence of NFATc (NFATc1) and NFATp (NFATc2) in HUVECs15 33 Performing EMSA using labeled oligonucleotide corresponding to the NFAT-binding site of human TF promoter produced results similar to those obtained with the NFAT-binding oligonucleotide from the IL-4 promoter (Figure8C).
OxPAPC induces NFAT/DNA-binding complexes.
Nuclear extracts from HUVECs stimulated for 40 minutes with OxPAPC (125 μg/mL) or A23187/PMA (2 μM and 10−7 M, respectively) were incubated with 32P-labeled NFAT probe of the murine IL-4 (A,B) or human TF (C) promoters and then separated by gel electrophoresis. Where indicated, cells were preincubated for 30 minutes with 500 ng/mL cyclosporin A before addition of OxPAPC or A23187/PMA. The mobility of the specific OxPAPC-induced/cyclosporin A–sensitive complex is indicated by an arrow. Supershifted band is labeled with an asterisk. The results are representative of 4 independent experiments.
OxPAPC induces NFAT/DNA-binding complexes.
Nuclear extracts from HUVECs stimulated for 40 minutes with OxPAPC (125 μg/mL) or A23187/PMA (2 μM and 10−7 M, respectively) were incubated with 32P-labeled NFAT probe of the murine IL-4 (A,B) or human TF (C) promoters and then separated by gel electrophoresis. Where indicated, cells were preincubated for 30 minutes with 500 ng/mL cyclosporin A before addition of OxPAPC or A23187/PMA. The mobility of the specific OxPAPC-induced/cyclosporin A–sensitive complex is indicated by an arrow. Supershifted band is labeled with an asterisk. The results are representative of 4 independent experiments.
Cyclosporin A inhibits induction of TF by OxPAPC
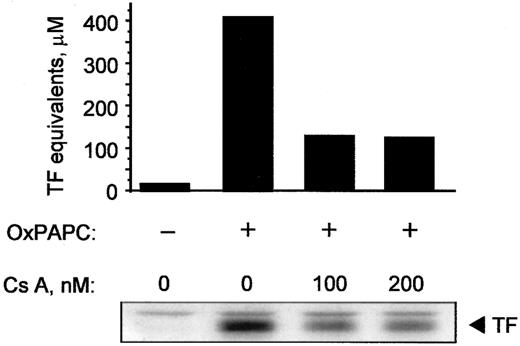
Because cyclosporin A inhibited activation of NFAT, we tested whether it would also block expression of TF induced by OxPAPC. Cyclosporin A decreased induction of TF activity as well as protein in OxPAPC-treated cells by 50% to 60% (Figure9). In addition, cyclosporin A blocked induction of TF by Ca++ ionophore; for both OxPAPC and Ca++ ionophore, maximal inhibition was observed at 100 ng/mL cyclosporin A (not shown). These data suggest that, in addition to EGR-1, activation of NFAT plays an important role in OxPAPC-induced TF expression in HUVECs.
Cyclosporin inhibits induction of TF activity and protein by OxPAPC.
HUVECs were pretreated with indicated concentrations of cyclosporin A for 20 minutes and then stimulated for 6 hours with 125 μg/mL OxPAPC in the presence of the inhibitor. Cells were scraped off and analyzed by clotting assay or Western blotting using antibodies to TF. Similar results were obtained in 2 additional experiments.
Cyclosporin inhibits induction of TF activity and protein by OxPAPC.
HUVECs were pretreated with indicated concentrations of cyclosporin A for 20 minutes and then stimulated for 6 hours with 125 μg/mL OxPAPC in the presence of the inhibitor. Cells were scraped off and analyzed by clotting assay or Western blotting using antibodies to TF. Similar results were obtained in 2 additional experiments.
PGPC is one biologically active component of OxPAPC responsible for TF up-regulation
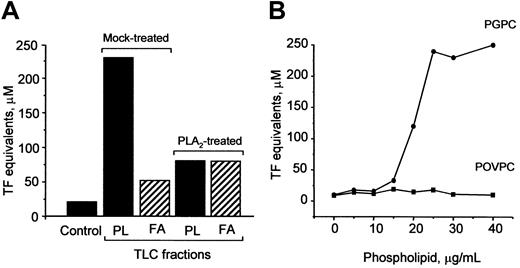
Oxidation of OxPAPC is known to generate a variety of products, such as oxidized phospholipids, lysophospholipids, and fatty acids.8 To determine which of these substances mediate induction of TF, we separated OxPAPC by TLC into fatty acid and phospholipid fractions and found that the activity was predominantly localized in the phospholipid fraction (Figure10A). Treatment of OxPAPC with PLA2 significantly reduced biologic activity (Figure 10A), strongly indicating that the fatty acid residue at the sn-2 position of oxidized phospholipids was important for the ability to induce TF expression. Furthermore, we tested the effect of 2 previously identified PAPC oxidation products, namely, POVPC and PGPC,8 on TF expression in HUVECs. We found that PGPC, but not POVPC, induced expression of TF in a concentration-dependent manner (Figure 10B).
PGPC is one biologically active component in OxPAPC.
(A) PLA2 or mock-treated OxPAPC was separated by TLC into phospholipids (PL) and fatty acids (FA). The fractions were eluted, evaporated, and used for stimulation of HUVECs. The lipid fractions were added in amounts equivalent to 150 μg/mL OxPAPC. After 6 hours, the cells were washed and analyzed in duplicate for TF activity by clotting assay. (B) HUVECs were stimulated with indicated concentrations of POVPC or PGPC for 6 hours. At the end of incubation, the cells were analyzed for TF activity. Similar results were obtained in 2 independent experiments.
PGPC is one biologically active component in OxPAPC.
(A) PLA2 or mock-treated OxPAPC was separated by TLC into phospholipids (PL) and fatty acids (FA). The fractions were eluted, evaporated, and used for stimulation of HUVECs. The lipid fractions were added in amounts equivalent to 150 μg/mL OxPAPC. After 6 hours, the cells were washed and analyzed in duplicate for TF activity by clotting assay. (B) HUVECs were stimulated with indicated concentrations of POVPC or PGPC for 6 hours. At the end of incubation, the cells were analyzed for TF activity. Similar results were obtained in 2 independent experiments.
Discussion
The inflammatory effects of MM-LDL can mainly be attributed to its oxidized phospholipid components.7 We used OxPAPC as a surrogate for MM-LDL to study signaling pathways and transcription factors mediating induction of TF expression in HUVECs. The induction of TF activity by OxPAPC paralleled with the increase in TF protein. These data are in good correlation with previous findings demonstrating transcriptional regulation of TF by MM-LDL in ECs5 and suggest that changes in protein expression, rather than in receptor activity, are responsible for the observed effects.
In addition, we demonstrate that transcriptional mechanisms mediating elevation of TF in OxPAPC-treated HUVECs differ from those activated by classical inflammatory mediators. The TF gene promoter contains consensus sites for Sp1,26 thought to be important for basal transcription, and for NF-κB, AP-1, EGR-1, and NFAT, which mediate regulation of inducible transcription of theTF gene.15,26 The NF-κB pathway is one important mechanism of transcriptional activation of the TFgene, used by the major inflammatory stimuli IL-1, TNF-α, and LPS.23 Whether the NF-κB pathway is activated in ECs by lipids in oxidized lipoproteins was not clear. Previous findings demonstrated that oxidized LDL was able to activate NF-κB in human coronary ECs34,35 and other cell types,36 whereas other studies demonstrated a lack of NF-κB activation by oxidized lipoproteins.11,37,38Here, we clearly demonstrate that OxPAPC, a component of oxidized LDL, does not activate the classical NF-κB pathway in HUVECs. Furthermore, inhibition of the NF-κB pathway by overexpression of IκΒα did not significantly change the degree of OxPAPC-induced elevation of TF. Thus, our data indicate that in HUVECs up-regulation of TF by OxPAPC is not mediated by the classical NF-κB pathway. In fact, we have previously shown that several components of OxPAPC are capable of inhibiting NF-κB–mediated transcription induced by LPS.12 Thus, whereas some OxPAPC components could be capable of activating the NF-κB pathway, inhibitory substances such as POVPC12 would block this effect, resulting in the net response shown in Figure 2.
Another transcription factor capable of binding to the TF promoter is EGR-1, which is critically involved in TF gene regulation by inflammatory cytokines, serum, VEGF, and phorbol esters.14,26 We have found that OxPAPC induced a rapid increase in EGR-1 levels in HUVECs. We also demonstrate a link between EGR-1 and TF expression, because overexpression of the EGR-1 corepressor NAB2 inhibited OxPAPC-induced TF expression. Recently, it was shown that EGR-1 and EGR-1–responsive genes are up-regulated in atherosclerotic lesions.39 However, the factors inducing EGR-1 expression in the vascular wall are not known. OxPAPC, which is present in atherosclerotic lesions,8 could very well be responsible for increased EGR-1 levels. EGR-1 is known to mediate induction of several growth factors and their receptors, including platelet-derived growth factor (PDGF)–A, PDGF-B, fibroblast growth factor 2, transforming growth factor β, and Flt-1.27 40Thus, activation of EGR-1–mediated transcription by OxPAPC could also promote cell migration, proliferation, and matrix synthesis and thus progression of the atherosclerotic plaque.
Analyzing signaling mechanisms induced by OxPAPC, we found that OxPAPC induced phosphorylation of ERK1/2. These data are consistent with previous findings of MAP kinase activation by oxidized LDL in other cell types.41,42 It is known that EGR-1 expression can be induced by ERK1/2 kinases, which stimulate a ternary complex factor, leading to induction of EGR-1transcription.27,43 We demonstrate here that the MEK/ERK signaling cascade is required for EGR-1 induction by OxPAPC. This mechanism is different from stress-induced EGR-1transcription, which was shown to be mediated by p38 MAP kinase and JNK, but shows striking similarities to TNF-α and VEGF-induced EGR-1 expression.16
Phosphorylation of ERK1/2 as well as elevation of EGR-1 and TF in OxPAPC-treated cells was suppressed by PD98059, known to block the upstream kinase MEK. These data indicate that activation of the MEK/ERK cascade is necessary for full induction of TF by OxPAPC. Various agonists can stimulate MEK/ERK kinases either through PKC-dependent or PKC-independent mechanisms.29 Taken together, our data show that PKC-MEK-ERK1/2-EGR-1 represents one pathway leading to induction of TF expression by OxPAPC.
In addition, the present study provides evidence for the first time that oxidized phospholipids are capable of inducing NFAT-mediated activation of ECs. NFAT was shown to participate in the regulation of TF expression by Ca++ ionophore, phorbol ester, and VEGF.15 30 Here we demonstrate that OxPAPC induces the typical mechanisms leading to NFAT activation, starting from elevation of cytosolic Ca++, next activation of calcineurin (as demonstrated by the inhibitory effect of cyclosporin A), and finally leading to nuclear translocation and DNA binding of NFAT. Furthermore, these data indicate that activation of NFAT by OxPAPC is functionally important for induction of TF, because both NFAT translocation and TF elevation were partially inhibited by cyclosporin A.
We also addressed the question with respect to the biologically active components in OxPAPC, which may be responsible for stimulation of TF expression. We found that the TF-inducing activity was associated mainly with the phospholipid fraction. The phospholipid nature of the major active principle was further confirmed by the loss of biologic activity after treatment of OxPAPC with PLA2. This result also indicates that the TF-inducing phospholipids were different from lysophosphatidylcholine. This conclusion is consistent with data demonstrating inhibition, rather than up-regulation, of TF in human monocytes by lysophosphatidylcholine.44
Two identified phospholipids, POVPC and PGPC, are present in OxPAPC and are capable of activating ECs.8 Both substances are generated by fragmentation of arachidonic acid in PAPC. POVPC contains a 5-carbon residue with an ω-aldehyde group at the sn-2 position, in contrast to the ω-carboxylic group in PGPC. Despite grossly similar chemical structure, these 2 compounds act on ECs in very different ways. Whereas PGPC stimulates binding of both neutrophils and monocytes to ECs as well as up-regulation of E-selectin and VCAM-1 expression,45 POVPC selectively induces monocyte binding due to up-regulation of a different set of cellular adhesion molecules and seems to inhibit the effects of PGPC.12,45 To study individual effects of these compounds, we compared the ability of POVPC and PGPC to induce TF expression. Although PGPC elevated TF approximately to the same maximal levels as OxPAPC, POVPC did not induce TF expression. Previously, we hypothesized that POVPC and PGPC may act through different types of membrane receptors12; our data demonstrating a dramatic difference between PGPC and POVPC in their ability to induce TF expression provide further support for this hypothesis.
Altogether, the data presented in this report are consistent with the following mechanism: OxPAPC activation of HUVECs elevates cytosolic Ca++ levels and activates PKC. Further steps include calcineurin-dependent activation and nuclear translocation of NFAT as well as parallel activation of ERK1/2, resulting in up-regulation of EGR-1. NFAT and EGR-1 bind to their respective sites in the TF promoter and stimulate transcription. Increased TF expression may promote local fibrin formation in the tissue in the course of the atherosclerotic process. In addition, NFAT and EGR-1 are known to regulate expression of other inflammatory genes. OxPAPC may thereby contribute to chronic inflammatory stimulation and thus to the progression of atherosclerosis by activation of signaling pathways in part distinct from the signals triggered by inflammatory cytokines. Our results contribute to the understanding of mechanisms of endothelial cell activation during chronic inflammatory processes, and thus may lead to novel strategies for therapeutic intervention.
We thank Dr Z. Zhegu and R. Ecker for preparation of HUVEC, G. Mitulovic for help with MS analysis of phospholipids, and Dr R. de Martin for providing the IκBα adenovirus.
Supported by the Austrian Science Foundation, project numbers P13954-MED (N.L.) and SFB-714, P-14586-PHA (W.F.G.), and by the ICP Program of the Austrian Federal Ministry for Education, Science and Culture. V.N.B. and D.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Norbert Leitinger, Department of Vascular Biology and Thrombosis Research, University of Vienna, Schwarzspanierstrasse 17, A-1090 Vienna, Austria; e-mail: norbert.leitinger@univie.ac.at.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal