Abstract
Use of oncoretroviral vectors in gene therapy for hemoglobinopathies has been impeded by low titer vectors, genetic instability, and poor expression. Fifteen self- inactivating (SIN) lentiviral vectors using 4 erythroid promoters in combination with 4 erythroid enhancers with or without the woodchuck hepatitis virus postregulatory element (WPRE) were generated using the enhanced green fluorescent protein as a reporter gene. Vectors with high erythroid-specific expression in cell lines were tested in primary human CD34+ cells and in vivo in the murine bone marrow (BM) transplantation model. Vectors containing the ankyrin-1 promoter showed high-level expression and stable proviral transmission. Two vectors containing the ankyrin-1 promoter and 2 erythroid enhancers (HS-40 plus GATA-1 or HS-40 plus 5-aminolevulinate synthase intron 8 [I8] enhancers) and WPRE expressed at levels higher than the HS2/β-promoter vector in bulk unilineage erythroid cultures and individual erythroid blast-forming units derived from human BM CD34+ cells. Sca1+/lineage− Ly5.1 mouse hematopoietic cells, transduced with these 2 ankyrin-1 promoter vectors, were injected into lethally irradiated Ly5.2 recipients. Eleven weeks after transplantation, high-level expression was seen from both vectors in blood (63%-89% of red blood cells) and erythroid cells in BM (70%-86% engraftment), compared with negligible expression in myeloid and lymphoid lineages in blood, BM, spleen, and thymus (0%-4%). The I8/HS-40–containing vector encoding a hybrid human β/γ-globin gene led to 43% to 113% human γ-globin expression/copy of the mouse α-globin gene. Thus, modular use of erythroid-specific enhancers/promoters and WPRE in SIN-lentiviral vectors led to identification of high-titer, stably transmitted vectors with high-level erythroid-specific expression for gene therapy of red cell diseases.
Introduction
Gene therapy for red blood cell (RBC) disorders, particularly for hemoglobinopathies, using retroviral vectors has been fraught with problems. Moloney murine leukemia virus (MLV) vectors are the most extensively studied oncoretroviral vectors. However, they do not transduce the nondividing hematopoietic stem cells. Exogenous genes in MLV vectors, driven by the viral long terminal repeat (LTR) promoter/enhancer, are expressed in a lineage-nonspecific manner. In addition, the MLV LTR interferes with the erythroid lineage–specific regulatory elements inserted in the vectors, resulting in unstable proviral transmission or poor transgene expression or both.1-3 Transcriptional interference from the LTR4-6 can be overcome by self-inactivating (SIN) vectors. In SIN vectors, the LTR promoter/enhancers are deleted on integration of the provirus.7 8 However, SIN-MLV vectors have shown limited applicability, because the deletion of the TATA box in them results in inefficient 3′ end processing of the viral RNA genome, resulting in low titer vectors.
Retroviral vectors are RNA-based vectors and, therefore, are restricted to incorporating complementary DNA (cDNA) forms of processed messenger RNA (mRNA). This results in low expression of intron-dependent genes, such as globin.9,10 Introns in the globin gene enhance transcription and allow proper 3′ end processing and export of globin transcripts into the cytoplasm.11-14 Retention of introns of the globin genes can be achieved by using vectors that contain the globin gene in reverse orientation to the viral transcriptional unit. However, this has not improved expression because these vectors have additional problems with antisense effects of the transcripts, low titers, and proviral instability.2Another way is to increase the expression of the globin cDNA that can be placed in sense orientation in the vector. Recently, the human and woodchuck hepatitis virus posttranscriptional regulatory elements (HPRE and WPRE) have been reported to replace the functions of the globin intron and improve expression of the globin cDNA.15 16
The recently developed lentiviral vectors (derived from the human immunodeficiency virus 1 [HIV-1])17 are attractive for globin gene therapy for several reasons. Lentiviral vectors have been shown to infect nondividing cells, including the quiescent hematopoietic stem cells.18,19 Lentiviral LTR transcription is tat dependent and lack of tat in the provirus results in very low level transcription from the LTR. The low-level LTR transcription is completely eliminated in SIN-lentiviral vectors,20,21 making them ideal for lineage-specific gene expression. Unlike SIN-MLV vectors, SIN-lentiviral vectors can be packaged without loss of titers.20-22
We therefore hypothesized that modular use of erythroid-specific enhancers and promoters and inclusion of the WPRE in SIN-lentiviral vectors could address issues of enhancer interference and intron independence of the globin gene and result in high-level erythroid-expressing cassettes for gene therapy of RBC disorders. We tested 4 erythroid enhancers with 4 erythroid promoters in different combinations with and without the WPRE, using green fluorescence protein (GFP) as the reporter gene. We observed a high-level, stable, and erythroid-lineage specific gene expression from vectors containing erythroid-specific elements. The highest levels of expression were seen with the ankyrin-1 promoter, in combination with 2 enhancers in tandem (the GATA-1 and HS-40 or the I8 and HS-40 enhancer pairs) in primary human cells as well as in mice 11 weeks after bone marrow transplantation (BMT), at levels higher than those observed with the HS2 enhancer/β-promoter vector. The I8/HS-40–containing vector encoding a hybrid human β/γ-globin gene led to 43% to 113% human γ-globin gene expression/copy of the mouse α-globin gene.
Materials and methods
Vector construction
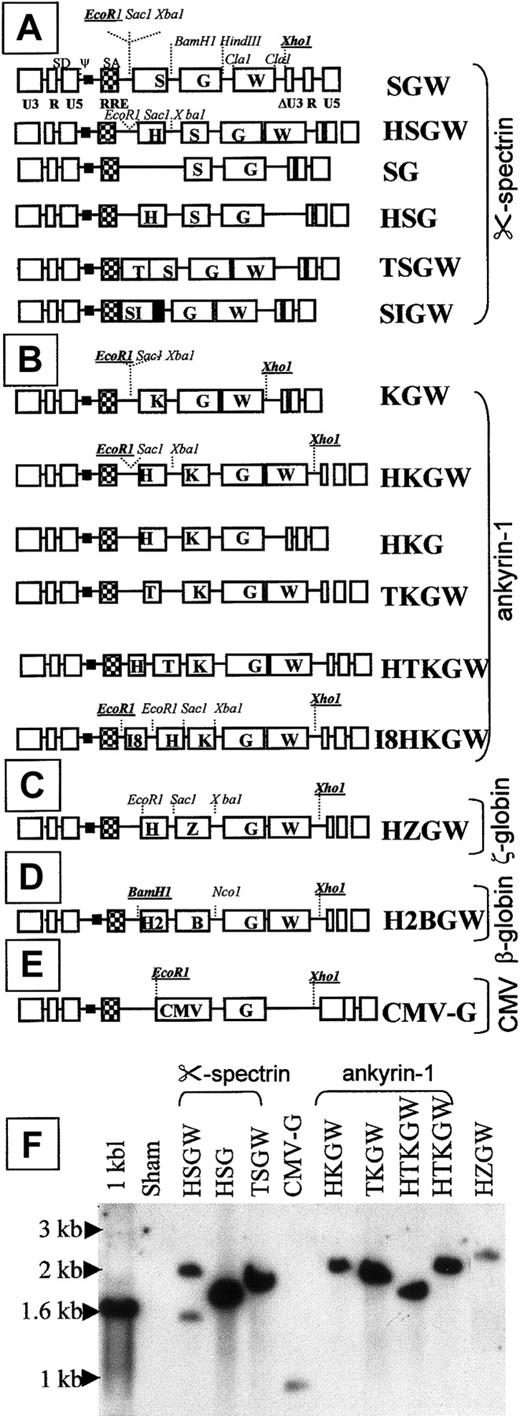
Fifteen lentiviral vectors were generated using the SIN-lentiviral vector, CS (a generous gift from Drs Miyoshi and Verma, Salk Institute, La Jolla, CA). The HS-40, GATA-1 autoregulatory element (ARE) and the erythroid 5-aminolevulinate synthase (eALAS) intron 8 enhancer element (I8) and the ζ-promoter element were obtained from sources previously described.23-25 The HS2/β promoter element was a generous gift of Dr Leboulch (Harvard University, Boston, MA). The ankyrin-1 and α-spectrin promoters were obtained by polymerase chain reaction (PCR) from human genomic DNA as follows. A 400-bp ankyrin-1 promoter was obtained using the following primers26,27: sense 5′AAATCTAGAGCGACTAAACCGGACTCCCTTTC3′ and antisense 5′AAAGGATCCGCCGGTCTTTCAGCAGGGGCCCGCCGAA3′. The full-length spectrin promoter was amplified from genomic DNA, representing 712-bp (−509 to +203) from the transcriptional start site, with ClaI and BamHI ends26 and inserted into pBS SK + plasmid to form pS1. A 372-bp α-spectrin promoter (without the first intron) modified with EcoRI, SacI, XbaI, and BamHI, NotI,HindIII ends was amplified using PCR from pS1 using the following sense and antisense primers, respectively: 5′-AAAGAATTCGAGCTCTCTAGAAAAAGCCAGGATAATTCG-3′ and 5′-AAAAAGCTTGCGGCCGCGGATCCTTTTCCTAAAGGTTTAGAATCTTGCTTGGTCCTAGAATCC-3′, digested by EcoRI-HindIII and subcloned in Puc19 to form pS2 plasmid. The antisense primer was designed to delete the first intron (+61 to +182) and to keep the first exon (+183 to +203) until the start codon. The sequence of the α-spectrin promoter region was verified by sequencing.
The cloning strategy for the α-spectrin series of vectors is described in detail. Important restriction sites used to clone in the various fragments are depicted in Figure1. Cloning strategies of the remaining vectors were very similar (details available on request).
SIN-lentiviral constructs.
Vector series driven by the α-spectrin promoter, S (A), the ankyrin-1 promoter, K (B), the ζ-globin promoter Z (C), the β-globin promoter, B (D), and the CMV promoter/enhancer (CMV) (E). RRE designates Rev-responsive element; SD, splice donor; SA, splice acceptor; and H, T, I8 represent the HS-40, GATA-1 ARE, and intron 8 enhancer from eALAS gene, respectively. W represents WPRE and G represents GFP. (F) Southern blot analysis of transduced 293A cell pools (restricted with EcoRI and XhoI, enzymes that flank the transcriptional unit and probed with GFP) shows appropriate single proviral bands in all (including H2BGW, not shown) except for HSGW that shows an additional smaller rearranged band of 1.5 kb. The expected size of the proviral bands ranged between 1.8 and 2.5 kb for all other erythroid vectors and 1.1 kb for the CMV-G vector.
SIN-lentiviral constructs.
Vector series driven by the α-spectrin promoter, S (A), the ankyrin-1 promoter, K (B), the ζ-globin promoter Z (C), the β-globin promoter, B (D), and the CMV promoter/enhancer (CMV) (E). RRE designates Rev-responsive element; SD, splice donor; SA, splice acceptor; and H, T, I8 represent the HS-40, GATA-1 ARE, and intron 8 enhancer from eALAS gene, respectively. W represents WPRE and G represents GFP. (F) Southern blot analysis of transduced 293A cell pools (restricted with EcoRI and XhoI, enzymes that flank the transcriptional unit and probed with GFP) shows appropriate single proviral bands in all (including H2BGW, not shown) except for HSGW that shows an additional smaller rearranged band of 1.5 kb. The expected size of the proviral bands ranged between 1.8 and 2.5 kb for all other erythroid vectors and 1.1 kb for the CMV-G vector.
The BamHI/NotI fragment containing the enhanced GFP cDNA (Clontech Laboratories, Palo Alto, CA) was inserted into the BamHI/NotI sites of pS2 downstream of the α-spectrin promoter resulting in pSG. The HS-40 from pHS40 z600-GH25 was cloned into pSG upstream of the α-spectrin promoter into the SacI-XbaI sites to generate pHSG. The pHSGW and pSGW were obtained by cloning theEcoRI-HindIII fragment of pHSG and pSG in theEcoRI HindIII sites of the pSK + WPRE B11.28 The pSIN-HSGW and pSIN-SGW lentiviral vectors were generated by cloning the EcoRI/XhoI fragment of the pHSGW and pSGW into the EcoRI/XhoI sites of CS plasmid. The pSIN-HSG and the pSIN-SG were obtained by removing WPRE cassette by ClaI enzyme. The pSIN-TSGW was derived from pSIN-HSGW by replacing the HS-40 fragment with the 200-bpEcoRI-XbaI fragment of the pSV0GATA plasmid.23 The 372-bp α-spectrin promoter was excised from the pSIN-SGW as a BamHI/BamHI fragment and replaced by a 712-bp fragment containing the α-spectrin with intron I excised from the pS1 plasmid promoter and cloned into the sameBamHI site to create the pSIN-S758GW vector.
The β/γ hybrid gene from the BGT50 construct, kindly provided by Dr James Ellis,29 has the β-globin promoter and gene with replacement of almost all of the coding sequences (exons 1-3) with those from the γ-globin gene. However, due to restraints of restriction enzyme sites, 3 of the 146 codons in this gene encode amino acids specific to the β-globin gene.29 These codons have no antisickling effect,29 although their effect on γ-globin function is unknown. Because the majority of the sequence encodes γ-globin, expression from this gene will be hereafter referred to as “human γ-globin” expression. The 3-kb β/γ hybrid gene was cloned upstream of WPRE and downstream of the I8 and HS-40 enhancer elements using XbaI andEcoRV sites, respectively, into the SIN-lentiviral vector to generate I8HβγW SIN-lentiviral vector (details of cloning are available on request).
Production and titration of vectors
Human kidney 293T cells were cotransfected with the packaging construct pCMVΔP8.91 (10 μg), VSV-G pMD.G (5 μg), and a vector construct (15 μg), induced with sodium butyrate (Sigma, St Louis, MO) and viral supernatant was collected and concentrated by ultracentrifugation.19 Viral titers were determined on 293A cells (American Type Culture Collection [ATCC], Rockville, MD) with serial dilutions of viral supernatant, followed by semiquantitative PCR analyses for DNA copy number 7 days later to amplify GFP. Lentiviral vector preparations were tested for the absence of replication-competent lentivirus (RCL) as described19and were found to be free of RCL. Briefly, peripheral blood mononuclear cells (PBMCs) were infected with 107 to 108vector particles/mL and a p24 enzyme-linked immunosorbent assay (ELISA) was performed using medium collected over a period of 3 weeks. Controls were run in parallel, using serial dilution of wild-type HIV-infected PBMCs efficiently at a concentration of 10−6 particles/mL.
DNA analysis
Genomic DNA from transduced cells (murine erythroleukemia [MEL] and 293A) was analyzed by semiquantitative PCR for GFP to determine the proviral copy number as described previously.16 Genomic DNA Southern blot was performed on MEL cells to test integrity of the provirus by digestion withBamHI and XhoI for H2BGW-transduced cells and with EcoRI and XhoI for all other vector-transduced cells. Digested DNA was gel fractionated on 0.8% agarose gels, blotted, and hybridized with 32P-labeled GFP cDNA probe.
RNA analyses
To compare human γ-globin expression to endogenous mouse α-globin RNA, RNAse protection assays (RPAs) were performed on total RNA extracted from transduced and differentiated MEL cells using the Riboquant In Vitro Transcription Kit (Pharmingen, San Diego, CA) according to the manufacturer's protocol. The linearized templates of plasmids protecting a 280-bp human γ-globin mRNA fragment and a 113-bp mouse α-globin coding sequence were used to transcribe32P-UTP–labeled RNA in vitro (unprotected probe sizes were 309 bp and 142 bp, respectively). Cellular RNA was extracted using RNA-stat and hybridized with an excess of 32P-UTP–labeled, in vitro transcribed RNA probe and then subjected to RNAse digestion. Protected fragments were resolved on a 6.5% polyacrylamide gel and quantified by phosphoimager analyses. A very small amount of the unprotected in vitro transcribed RNA (1/100 of the amount hybridized with cellular RNA) was loaded on the gel.
Quantification of mRNA levels
The level of the γ-globin mRNA was determined using a phosphoimager (Biorad, Hercules, CA). The relative amounts of emissions from the human γ-globin (hu-γ) RNA band and mouse α-globin (mu-α) RNA bands were calculated as percentages by the following formulas:
A single copy transgenic mouse RNA, derived from a founder expressing 20% hu-γ/total mouse β-globin from the HS3 enhancer/β-promoter was used as a positive control.29
Flow cytometry analyses
Fluorescence-activated cell sorter (FACS) analysis for GFP was performed on a FACS-Calibur and FACS-Vantage flow cytometer (Becton Dickinson, San Jose, CA). A ratio of the mean fluorescence intensity (MFI) of transduced cells and nontransduced cells was used to normalize GFP expression by FACS. Comparative analyses of fluorescence intensity were performed on the same FACS machine with regular calibration standards and constant voltage for each cell line. For analysis of lineage-specific GFP expression in mice, cells were stained with phycoerythrin (PE)–conjugated antimouse antibodies to CD3, B220, Gr-1, and Ter-119 (Pharmingen). Antimouse CD45.2PE (Ly5.1, Pharmingen) antibody was used for evaluation of donor-host chimerism in bone marrow (BM), spleen, and thymus. Human cell phenotyping was performed using PE-conjugated antihuman CD13 (Pharmingen) and glycophorin A (GlyA; Pharmingen) antibodies.
Cell lines and transduction
The 293T and 293A cells (ATCC) and MEL cells were maintained in Dulbecco modified Eagle medium (DMEM; Gibco, Grand Island, NY) supplemented with 10% horse serum (Omega Scientific, Tarzana, CA). MEL cells were induced to differentiate in DMEM, 10% fetal calf serum (FCS) containing 5 mM N, N-hexamethylene bisacetamide (HMBA, Sigma) and GFP expression determined by flow cytometry at day 4 of differentiation. The human K562 cells were maintained in RPMI (Gibco) supplemented with 10% FCS. All cell lines were transduced with serial dilutions of viral supernatant ranging from a multiplicity of infection (MOI) of 0.1 to 10, in the presence of 4 μg/mL polybrene (Sigma). On day 7 following transduction, cells were harvested and analyzed by FACS for GFP expression.
Hematopoietic cells and transduction
The CD34+ progenitor cells were isolated from normal human BM mononuclear cells using Mini-MACS columns (Miltenyi Biotech, Auburn, CA). Use of samples was approved by the Committee on Clinical Investigations at Children's Hospital Los Angeles. CD34+cells (5 × 104) were suspended in 100:l of X-vivo 15 medium (Biowhittaker, Walkersville, MD) containing the following recombinant cytokines: recombinant human (rh) interleukin (IL)-3 (rhIL-3; 10 ng/mL), rhIL-6 (10 ng/mL), rhFlt-3 (10 ng/mL), rh thrombopoeitin (rhTPO; 10 ng/mL), and rh stem cell factor (rhSCF; 25 ng/mL) and in 96-well plates coated with CH-296 fragment of recombinant fibronectin (Retronectin, Takara Shuzo, Otsu, Japan). Lentiviral supernatants at an MOI of 10 were added twice daily, 12 hours apart, after overnight prestimulation in the above medium. On day 2, transduced CD34+ cells were washed in phosphate-buffered saline (PBS) and used for in vitro assays.
Colony-forming unit assays
The CD34+ cells (500, 1000, and 1500) were plated in duplicate in methyl cellulose containing rhSCF (50 ng/mL), rhIL-3 (10 ng/mL), rhIL-6 (20 mg/mL), rh granulocyte-macrophage colony-stimulating factor (rhGM-CSF; 0.01 ng/mL), and rh erythropoietin (rhEpo; 3 U/mL) to support erythroid burst-forming units (BFU-Es) and the same combination of cytokines, but with 10 mg/mL rhGM-CSF without Epo, to support granulocyte-macrophage colony-forming units (CFU-GMs). BFU-Es and CFU-GMs were scored 14 days after plating, then harvested, washed with PBS and analyzed for GFP expression by FACS.
In vitro differentiation into unilineage erythroid and myeloid cells
The CD34+ cells were placed in erythroid culture conditions in basal BM medium (BBMM; IMDM based, supplemented with 25% FCS) with IL-3, GM-CSF, and Epo, as previously described30or in myeloid differentiation conditions (BBMM with huGM-CSF [10 ng/mL] huSCF [25 ng/mL] huIL-3 [10 ng/mL],) and huIL-6 [10 ng/mL]). Erythroid and myeloid cultures were harvested after 2 weeks and analyzed for GFP expression and erythroid or myeloid surface markers by FACS.
Transduction of murine BM and BMT
Bone marrow was harvested from C57Bl/LY5.1 mice (B/6.SJL-CD45a-Pep3b, Jackson Laboratory, Bar Harbor, ME) (CD45.1+) by flushing femurs and tibiae and sorted for Sca-1+ cells using the MACS Sca-1 multisort kit (Miltenyi Biotech). The Sca-1+ cells obtained were lineage-depleted by staining with a lineage cocktail of fluorescein isothiocyanate (FITC)–labeled antibodies to murine CD3, CD4, CD8, CD11b, Gr-1, and Ter-119 and PE-labeled antibody to Sca-1. The Sca+Lin− cells were sorted by FACS and were transduced in 1 mL Stemspan (Stemcell Technologies, Vancouver, BC, Canada) supplemented with 20% FCS containing 10 ng/mL of each of the following cytokines: muTPO, huFlt-3, muIL-3, huIL-6, muSCF, 4 U/mL hu-Epo, 10 mM dNTP, and 40 mg/mL low-density lipoprotein (LDL; Sigma). Cells were transduced with viral supernatants from the HTKGW, HI8KGW, or H2BGW lentiviral vectors 3 times at 12-hour intervals after overnight prestimulation, using similar concentrations of virus at 5 × 106 transmitted units (TU)/mL (MOI 100). On day 3, cells were washed twice with PBS and 15 000 hematopoietic cells/mouse were injected into the tail vein of 8- to 12-week-old male C57BL/6-ly5.2 (CD45.2+) mice (Jackson Laboratories), that had been irradiated with 12 Gy in 2 split doses of 6 Gy, 24 hours apart. Eleven weeks after BMT animals were killed and peripheral blood, BM, thymus, and spleen were harvested. In blood, RBCs were analyzed before RBC lysis for white blood cell (WBC) analyses. FACS analysis was performed for donor cells (CD45.2), GFP expression in different lineages (T cells, B cells, granulocytes, and RBCs), and DNA analysis to determine proviral copy number.
Results
Generation and titration of SIN-lentiviral vectors
To develop a high-expressing, erythroid-specific SIN-lentiviral vector, a series of 15 SIN-lentiviral vectors containing different modular combinations of erythroid promoters and enhancers were constructed (Figure 1A-E). The 4 erythroid promoters from the erythroid-specific genes ankyrin-1, α-spectrin, the ζ-globin (in the context of HS-40), and theβ-globin were tested. The cytomegalovirus (CMV) promoter vector served as a lineage-nonspecific control. The HS-40 enhancer used in these studies contains a single base pair mutation at the NFE2 site that results in derepression of the ζ-globinpromoter in adult erythroid cells and confers position-independent expression.25 Other erythroid enhancers used were the GATA-1 ARE, I8 from the eALAS gene, and HS2 from the β-globin locus control region (LCR). The α-spectrin and ankyrin-1 promoter series of vectors were tested with and without the WPRE.
Viral titers were determined by transducing 293A cells with serial dilutions of concentrated virus and determining the number of transmitted copies per cell by semiquantitative PCR. Due to extreme erythroid specificity of the vector cassettes, determination of titers by quantifying the proportion of GFP-expressing 293A cells was not possible. Vector titers ranged from 2.2 × 107 to 1.1 × 108 TU/mL, with a mean titer of 4.5 × 107 TU/mL, after a 100-fold concentration by ultracentrifugation. Thus, high-titer SIN-lentiviral vectors, containing various internal erythroid promoter/enhancers, could be obtained.
All vectors transmitted an intact provirus, except 2 vectors in the α-spectrin series, shown by the appropriate-sized single proviral bands in the genomic Southern blot analyses of the transduced 293A cells restricted with enzymes flanking the transcriptional cassette (Figure 1E). Two vectors driven by the α-spectrin promoter with (S) or without the α-spectrin intron (SI): the HSGW vector (Figure 1E) and SIGW vector (data not shown) (G denotes GFP, W denotes WPRE, and H denotes HS-40) transmitted a smaller provirus in addition to a normal-sized provirus.
Screening for vectors with strong erythroid activity in cell lines
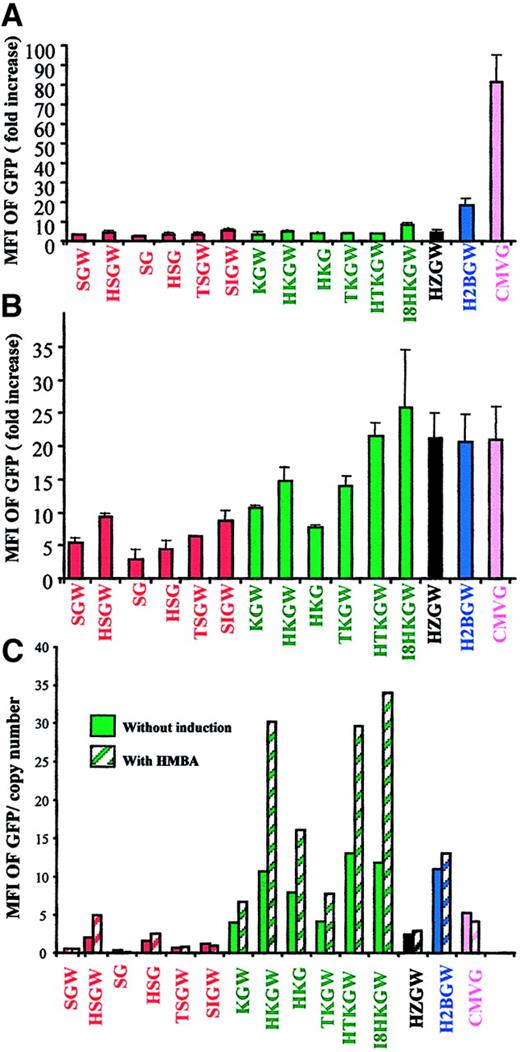
We first screened all SIN-lentiviral vectors in 6 different erythroid and nonerythroid cells lines (Figure 2A-C). Data from the nonerythroid human kidney 293A cells and the erythroid K562 cells (that express fetal-type globin) from 4 independent experiments is shown in panels A and B of Figure 2. K562 cells showed no remarkable difference in GFP expression after erythroid differentiation with hemin (40 μM) and sodium butyrate (100 μM) and, therefore, only data from undifferentiated cells are shown. Expression in MEL cells, which express adult-type globin on differentiation, was more clinically relevant and is shown before and after differentiation (Figure 2C). Because vectors expressing at high level in MEL cells were primarily of interest, semiquantitative PCR analysis was performed on transduced populations to normalize GFP expression level for proviral copy number. A representative experiment is shown in Figure 2C.
Expression of vectors in cell lines.
Fold increase in MFI of GFP in (A) the nonhematopoietic 293A cells, (B) the fetal hemoglobin-expressing K562 cells (mean ± SD, n = 4), and MFI/proviral copy number in (C) the adult-type hemoglobin-expressing MEL cells transduced with the SIN-lentiviral vectors. The ratio between MFI of transduced and nontransduced cells was used to determine the fold-increase in MFI (y-axes). The vectors are color-coded by promoters. MEL cell data are shown before (solid bars) and after (hatched bars) HMBA-induced differentiation after normalization for copy number from a representative experiment.
Expression of vectors in cell lines.
Fold increase in MFI of GFP in (A) the nonhematopoietic 293A cells, (B) the fetal hemoglobin-expressing K562 cells (mean ± SD, n = 4), and MFI/proviral copy number in (C) the adult-type hemoglobin-expressing MEL cells transduced with the SIN-lentiviral vectors. The ratio between MFI of transduced and nontransduced cells was used to determine the fold-increase in MFI (y-axes). The vectors are color-coded by promoters. MEL cell data are shown before (solid bars) and after (hatched bars) HMBA-induced differentiation after normalization for copy number from a representative experiment.
To favor single proviral integration/cell, all of these experiments were done at a relatively low MOI (MOI of 0.1 for the adherent 293A cells, and MOI of 1 for the nonadherent K562 and MEL cells) and the comparative FACS analyses were performed on cell pools with less than 30% transduction efficiency. The ratio between the MFI of GFP in transduced and nontransduced cells was used to quantify promoter/enhancer strength.
Although all 3 cell lines transduced by the control CMV lentiviral vector CMV-G expressed GFP (Figure 2A-C), expression from vectors containing erythroid elements was observed exclusively in K562 (Figure2B) and MEL cells (Figure 2C). In K562 and MEL cells, expression from vectors containing the ankyrin-1 promoter was higher (1.5-fold and 6-fold, respectively) than that from the α-spectrin promoter (HKG versus HSG and KGW versus SGW; Figure 2B,C). The expression was enhanced by 1.5- to 2.5- fold in the presence of the HS-40 enhancer (KGW versus HKGW, TKGW versus HTKGW). The inclusion of the WPRE increased the GFP expression by approximately 2-fold (HKGW versus HKG). The expression was greater in the presence of 2 erythroid enhancers in tandem, that is, the I8 and HS-40 (I8HKGW) or the HS-40 and GATA-1 enhancers (HTKGW), and these levels of expression were equal to or greater than those observed from vectors containing the HS2/β-promoter (H2BGW). Notably, the presence of any of the 3 enhancer elements (HS-40, I8, or GATA-1 ARE) in conjunction with the ankyrin-1 promoter remarkably increased expression in differentiated MEL cells (hatched bars, Figure 2C). Although HZGW expressed at very high levels in the fetal-globin–expressing K562 cells, it had lower expression in MEL cells, showing only partially derepression by the mutation in HS-40.
Overall, vectors containing the ankyrin-1 promoter expressed at highest levels in erythroid cell lines and the expression was inducible on MEL cell differentiation. The presence of 2 erythroid enhancers increased expression and inclusion of WPRE nearly doubled GFP expression.
Expression of erythroid SIN-lentiviral vectors in primary human BM cells
Although MEL and K562 cell lines were useful for an initial evaluation of transgene expression, comparison of transgene expression in primary human BM progenitors during their erythroid differentiation was more relevant. Therefore, the best 10 vectors from the initial screening were tested in primary cells. Human CD34+ cells from 4 different BM samples were transduced at an MOI of 10. This MOI favors single integration per cell in primary human CD34+cells in suspension cultures. A portion of transduced CD34+cells was plated for colony-forming assays and the remainder grown in liquid cultures for 2 weeks, in unilineage conditions favoring erythroid or myeloid differentiation. At 2 weeks, GFP-expressing colonies were scored and picked for FACS analyses. Cells in liquid culture were stained with PE-conjugated anti-GlyA or anti-CD13 antibodies and analyzed by FACS.
Table 1 shows that both erythroid and myeloid colonies transduced with CMV-G vector expressed GFP at a similar frequency. In contrast, with all other vectors expression of GFP was only observed in BFU-Es alone, demonstrating a particularly high lineage specificity of these vectors. Gene transfer, based on GFP expression, was similar in all vectors, ranging from 18% to 29%.
Percentage of GFP+ CFU derived from human BM CD34+ cells
| . | BFU-E . | CFU-GM . |
|---|---|---|
| CMV-G | 28.8 (± 1.7) | 25.6 (± 5.1) |
| HSGW | 19.1 (± 4.2) | 0 |
| TSGW | 18.1 (± 4.3) | 0 |
| HKGW | 24.6 (± 6) | 0 |
| TKGW | 28.8 (± 4.2) | 0 |
| HTKGW | 20.1 (± 5.3) | 0 |
| I8HKGW | 23.9 (± 9.8) | 0 |
| HZGW | 22.9 (± 7.9) | 0 |
| H2BGW | 23.7 (± 6.9) | 0 |
| . | BFU-E . | CFU-GM . |
|---|---|---|
| CMV-G | 28.8 (± 1.7) | 25.6 (± 5.1) |
| HSGW | 19.1 (± 4.2) | 0 |
| TSGW | 18.1 (± 4.3) | 0 |
| HKGW | 24.6 (± 6) | 0 |
| TKGW | 28.8 (± 4.2) | 0 |
| HTKGW | 20.1 (± 5.3) | 0 |
| I8HKGW | 23.9 (± 9.8) | 0 |
| HZGW | 22.9 (± 7.9) | 0 |
| H2BGW | 23.7 (± 6.9) | 0 |
Values obtained by counting with an inverted fluorescent microscope (means ± SD, n = 4).
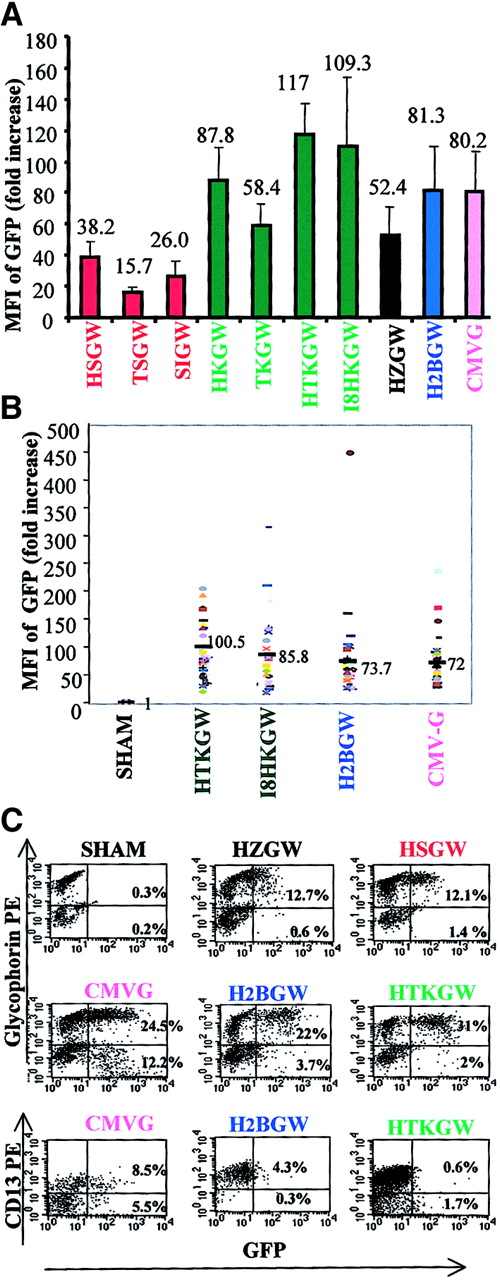
To compare the level of GFP expression from each vector, at least 10 randomly selected BFU-Es were pooled per experiment and were analyzed by FACS (Figure 3A, n = 4). The highest MFI (mean ± SD) was observed in BFU-Es transduced with the HTKGW vector and was significantly higher than expression in BFU-Es transduced with the H2BGW, CMV-G, or HKGW vectors (P < .05). This was closely followed by expression from the HI8KGW vector. Consistent with the cell line data, BFU-Es transduced with the α-spectrin promoter series expressed at much lower levels than BFU-Es transduced with ankyrin-1 promoter vectors. Expression from the vector containing the α-spectrin intron (SIGW) was higher than TSGW vector (without the intron and with the GATA-1 enhancer), and expression from the α-spectrin promoter vector containing HS-40 (HSGW) was higher despite the proviral rearrangements seen with the HSGW vector (Figure 1E). The vector containing the GATA-1 enhancer, TSGW, expressed at lower levels than the analogous HS-40 enhancer vector HSGW. Interestingly, the HZGW vector expressed at relatively high levels, showing more derepression of the ζ-promoter from the mutated HS-40 in primary human erythroid cells than in MEL cells, although the level of expression was still nearly half of the expression seen from the ankyrin-1 promoter vectors containing HS-40.
Expression of vectors in primary human cells.
The ratio between MFI of transduced and nontransduced cells was used to determine the fold-increase in MFI (y-axes). Fold increase in MFI of GFP in the progeny of primary human CD34+ cells (mean ± SD; n = 4) in (A) pooled transduced BFU-Es and (B) MFI from individual BFU-Es (40 BFU-E/vector). (C) FACS analyses of GFP expression (shown on the x-axes) in erythroid (GlyA+) and myeloid (CD13+) liquid culture differentiation (as described in “Materials and methods”) transduced with the key vectors. Myeloid and erythroid lineage staining is shown on the y-axes and the proportion of GFP+ cells annotated in the respective quadrants.
Expression of vectors in primary human cells.
The ratio between MFI of transduced and nontransduced cells was used to determine the fold-increase in MFI (y-axes). Fold increase in MFI of GFP in the progeny of primary human CD34+ cells (mean ± SD; n = 4) in (A) pooled transduced BFU-Es and (B) MFI from individual BFU-Es (40 BFU-E/vector). (C) FACS analyses of GFP expression (shown on the x-axes) in erythroid (GlyA+) and myeloid (CD13+) liquid culture differentiation (as described in “Materials and methods”) transduced with the key vectors. Myeloid and erythroid lineage staining is shown on the y-axes and the proportion of GFP+ cells annotated in the respective quadrants.
The NFE2 mutation HS-40 has been shown to allow position-independent expression of transgenes in adult erythroid cells.25Therefore, we studied gene expression at a clonal level, by analyzing 40 individual BFU-Es per vector by FACS in 4 individual experiments (Figure 3B). The level of GFP expression was variable in the presence of the HS-40 as well as the HS2 LCR element. This variability may be attributed to differences in maturation of individual BFU-Es or a relative lack of position independence. The mean MFI data on the individual BFU-Es closely paralleled that from pooled BFU-Es.
Panel C in Figure 3 shows a FACS analysis of myeloid and erythroid liquid cultures of the representative vectors. The results are consistent with the colony data. The CMV-G vector expressed in the myeloid as well as the erythroid progeny of CD34+ cells, whereas the erythroid vectors expressed only in the GlyA+cells. High levels of GFP expression were evident from the H2BGW, HTKGW, and CMV-G vectors and the MFI from ζ-globin (HZGW) and α-spectrin promoter vector (HSGW) was low.
In vivo activity of SIN-lentiviral vectors
We next studied the in vivo expression of the 3 best vectors, H2BGW, THKGW, and I8HKGW, in the murine BMT model. BM Sca+Lin− cells from Ly5.1 donor mice were transduced with the HTKGW, I8HKGW, and H2BGW vectors and transplanted into lethally irradiated Ly5.2 recipient mice. Eleven weeks after BMT, recipient mice were killed and blood, BM, spleen, and thymus were studied for GFP expression in the erythroid (TER119), myeloid (Gr1), T lymphocyte (CD3), and B lymphocyte (B220) lineages. The engraftment of the Sca+Lin− donor cells (Ly5.1) was high with a mean of 70% ± 9.7% (mean + SD) in BM and 85.9% ± 9% in peripheral blood. The expression of all 3 vectors was restricted to the erythroid lineage in BM, thymus, spleen, and peripheral blood in all mice (n = 8), consistent with results seen in cell lines and primary human cells.
Figure 4 shows data from 3 mice that had comparable BM engraftment (84%-92% donor cells) and similar proviral copy numbers (2-2.5 copies/cell). We observed a very high frequency of RBCs expressing GFP from H2BGW, THKGW, and I8HKGW vectors in peripheral blood, ranging from 63% to 87% (Figure 4A). After RBC lysis, GFP expression was analyzed in WBCs. Minimal GFP expression was seen in B, T, and myeloid cells (0.5%-3.7%) from all 3 vectors. Similar lineage specificity of GFP expression was observed in spleen, thymus, and BM cells, despite the presence of proviral DNA (data not shown).
Expression of vectors in murine cells in vivo.
FACS analyses of GFP expression in mice 11 weeks after BMT of Sca+/Lin− cells transduced with the H2BGW, I8HKGW, and HTKGW vectors. Representative mice with the relatively same donor cell engraftment and proviral copy number are shown. (A) Peripheral blood analyses of RBCs and WBCs (after RBC lysis) showing GFP expression (x-axes) in the erythroid, B cells, T cells, and granulocytes (y-axes). (B) BM analyses of erythroid cells in the same mice at progressively increasing stages of erythroid differentiation. Small (I—RBC), intermediate (II—intermediate normoblasts), and large cells (III—early normoblasts) were gated based on their FCS profile and Ter-119 expression. The percentages mentioned in different quadrants denote the proportion of cells present in that quadrant.
Expression of vectors in murine cells in vivo.
FACS analyses of GFP expression in mice 11 weeks after BMT of Sca+/Lin− cells transduced with the H2BGW, I8HKGW, and HTKGW vectors. Representative mice with the relatively same donor cell engraftment and proviral copy number are shown. (A) Peripheral blood analyses of RBCs and WBCs (after RBC lysis) showing GFP expression (x-axes) in the erythroid, B cells, T cells, and granulocytes (y-axes). (B) BM analyses of erythroid cells in the same mice at progressively increasing stages of erythroid differentiation. Small (I—RBC), intermediate (II—intermediate normoblasts), and large cells (III—early normoblasts) were gated based on their FCS profile and Ter-119 expression. The percentages mentioned in different quadrants denote the proportion of cells present in that quadrant.
We next analyzed GFP expression in BM erythroid cells at progressively increasing stages of erythroid differentiation, by gating on large (early normoblasts), intermediate (intermediate normoblasts), and small cells (RBCs), based on their forward cell scatter (FCS) profile and Ter-119 expression (Figure 4B, panels I-III). There was negligible expression of GFP in the TER119− population. With progressive erythroid maturation from early normoblasts (Figure 4, panel III) to mature RBCs (panel I), there were progressively increasing numbers of cells expressing GFP. These data confirm erythroid specificity and inducibility of all 3 constructs.
The MFI was higher in the more immature erythroid cells and decreased 2- to 3-fold with maturation with all 3 vectors, probably due to smaller cell size and shorter half-life of GFP in the transcriptionally inactive, enucleated RBCs. Progressive hemoglobinization with maturation may also result in quenching of GFP fluorescence because hemoglobin absorption and GFP emission wavelengths are similar. However, at all stages of maturation, the mean intensity from the ankyrin-1 promoter vectors (THKGW and I8HKGW) containing either of 2 enhancer element pairs is higher than that from the H2BGW vector.
Expression of human γ-globin by SIN-lentiviral vectors
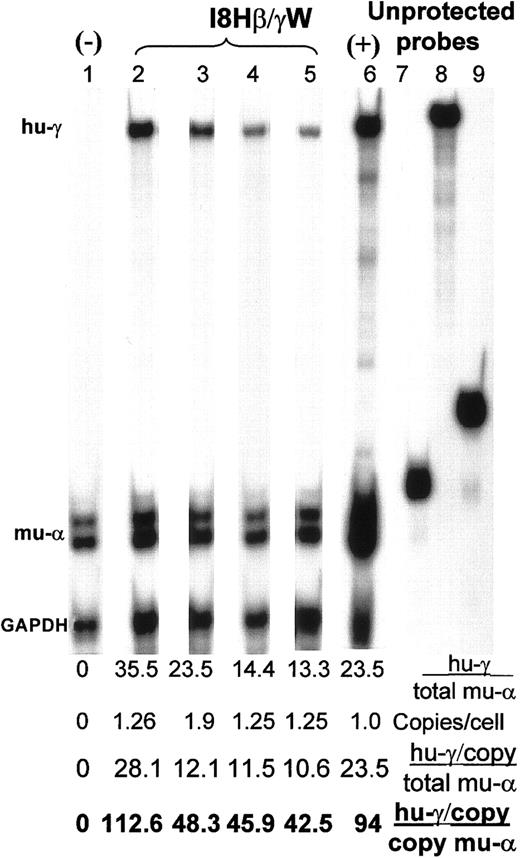
The MEL cells were transduced with the I8HβγW vector at low MOI (MOI 3-10) to obtain cell pools with approximately one copy per cell. Human γ-globin expression was analyzed in several unselected pools of cells. The proviral copy number of 3 cell pools, determined by semiquantitative PCR analyses, were 1.26, 1.9, and 1.25 copies/cell. Figure 5 shows an RNAse protection assay on the sham-transduced and I8HβγW-transduced MEL cells on differentiation. Human γ-globin expression/total murine α-globin in these pools was 35.5% (lane 2), 24% (lane 3), and 14% (the mean expression from duplicate samples in lanes 4 and 5), respectively. When normalized to single vector copy, this translates to 11% to 28% human γ-globin/total murine α-globin or 43% to 113% human γ-globin/copy of murine α-globin or 22% to 56% human γ-globin/murine α-globin allele.
Expression of human γ-globin in MEL cells.
RNAse protection assay showing murine α-globin (mu-α), human γ-globin (mu-γ), and GAPDH transcripts in MEL cells transduced with the I8Hβ/γW vector (containing the I8 and HS-40 enhancer elements and a β/γ hybrid gene) in lanes 2 to 5. Lanes 4 and 5 represent duplicate samples. Lane 1 shows sham-transduced MEL cells as a negative control for human γ-globin. Lane 6 represents the positive control derived from fetal liver RNA from a mouse transgenic for the β/γ hybrid gene. The transgenic founder of this mouse has been shown to express 20% human γ/total murine β-globin.29Lanes 7 to 9 represent in vitro transcribed unprotected probes (1/100 the amount used for protecting cellular RNA) concurrently loaded on the gel to show that the probe was in excess of the protected cellular RNA. The ratio of human γ-globin expression to murine α-globin (%), vector copy numbers, and expression normalized for copy number (expressed as hu-γ/total mu-α and hu-γ/copy of mu-α globin) are listed below each lane as a percentage.
Expression of human γ-globin in MEL cells.
RNAse protection assay showing murine α-globin (mu-α), human γ-globin (mu-γ), and GAPDH transcripts in MEL cells transduced with the I8Hβ/γW vector (containing the I8 and HS-40 enhancer elements and a β/γ hybrid gene) in lanes 2 to 5. Lanes 4 and 5 represent duplicate samples. Lane 1 shows sham-transduced MEL cells as a negative control for human γ-globin. Lane 6 represents the positive control derived from fetal liver RNA from a mouse transgenic for the β/γ hybrid gene. The transgenic founder of this mouse has been shown to express 20% human γ/total murine β-globin.29Lanes 7 to 9 represent in vitro transcribed unprotected probes (1/100 the amount used for protecting cellular RNA) concurrently loaded on the gel to show that the probe was in excess of the protected cellular RNA. The ratio of human γ-globin expression to murine α-globin (%), vector copy numbers, and expression normalized for copy number (expressed as hu-γ/total mu-α and hu-γ/copy of mu-α globin) are listed below each lane as a percentage.
Discussion
We tested SIN-lentiviral vectors in cell lines, in primary human cells, and in the murine BMT model. In all 3 systems (1) expression from vectors containing erythroid promoters and enhancers was highly lineage specific; (2) expression from the ankyrin-1 promoter vectors was higher than that from the α-spectrin promoter vectors; (3) expression was increased 2-fold with WPRE; and (4) expression was enhanced when 2 erythroid enhancers were inserted in tandem: HS-40 and GATA-1 enhancers or HS-40 and I8 enhancers.
High-level erythroid-specific expression in primary human and murine BM cells from SIN-lentiviral vectors could be obtained due to the absence of cis-acting influences from the HIV LTR promoter/enhancer. The lentiviral LTR is relatively inactive in the absence of tat allowing photoreceptor cell-specific expression, as has been previously reported in rat retina using the rhodopsin promoter.31However, these investigators later reported that an internal promoter placed within the lentiviral vector resulted in transcriptional interference that was overcome with deletion of the 3′ LTR enhancer in the SIN vector.21
The β-globin LCR is essential for high-level, position-independent, and copy number–dependent expression of the globin genes in transgenic mice, but it is too large to be incorporated into oncoretroviral vectors. Incorporation of mini-LCRs, containing only the core sequences, confers instability to the oncoretroviral vectors.2,32 Therefore, we adopted an alternative approach, using the smaller (∼400 bp) strong enhancer element located 40 kb upstream of the ζ-globin gene, in the α-globin gene cluster.25,33,34 HS-40 has been shown to be genetically stable in MLV vectors and enhances expression comparable to that of a single endogenous β-globingene.35-37 However, HS-40 lacks some of the properties of the LCR in that it does not confer position independence38and copy number dependence.39,40 A single base pair mutation of NF-E2/AP1 sequence motif converts HS-40 into an erythroid-specific LCR25 and results in expression of ζ-globin in adult RBCs in transgenic mice. We used this mutated HS-40 in all the vectors in this study and showed derepression of the ζ-globin promoter in primary adult BM-derived human cells, although the levels of expression were lower than those from the HS2/β-promoter vector. The assays in this study cannot conclusively address the issue of position independence with HS-40. We found 2- to 3-fold enhancement of expression with inclusion of the HS-40 enhancer and it was more effective when placed in tandem with GATA-1 or I8 enhancers. Two copies of HS-40 in tandem in the HTKGW vector did not improve expression further (data not shown). Placement of enhancers in tandem has not proven useful previously in globin vectors. Inhibition of enhancer function has been observed with tandem repeats of HS-40 or when HS-40 was placed in conjunction with the β-globin LCR elements.41
Replacement of the MLV LTR enhancer by the GATA-1 ARE enhancer has been previously reported to confer erythroid-specific expression to MLV vector cassette.23 GATA-1 ARE showed lower enhancement of expression than HS-40. However, if inserted in tandem with the HS-40, it enhanced expression levels to levels higher than the HS2/β-globin promoter vector. I8 of the eALAS gene contains a 239-bp strong erythroid-specific enhancer, shown to enhance expression of a heterologous thymidine kinase promoter in K562 cells 25-fold.24 Although the strength of I8 alone was not tested in these studies, when inserted in tandem with the HS-40, it resulted in expression levels comparable to the GATA-1 and HS-40 enhancers in tandem and at levels higher than the HS2/β-globin promoter.
The HS2 from the β-globin LCR is unique in that it behaves as a classic enhancer and a partial LCR42-44 and was used as a positive control vector, linked to the endogenous β-globin promoter45,46 along with the WPRE. It showed high-level erythroid-specific expression. However, inclusion of other hypersensitive sites or the mini-LCR, found previously to be unstable in oncoretroviral vectors, may enhance the expression further in lentiviral vectors, which have the capability to regulate packaging of unspliced viral genome. This ability is due to the presence ofrev/rev response element (RRE) RNA export element in HIV-based vectors that exports unspliced genomes into the cytoplasm. Indeed, stable transmission and high-level expression of β-globin has been recently reported when a large fragment of the micro-LCR is placed in lentiviral vectors.47 When several combinations and orientations of HS2, 3, and 4 were tested in oncoretroviral vectors, they were either unstable or showed very high variability in expression at a clonal level.1
The β-spectrin promoter results in relatively high level expression of the γ-globin gene in trangenic mice.48,49 We chose the α-spectrin promoter instead of the β-spectrin promoter because it is the most abundant protein of the RBC membrane skeleton and is synthesized in 3-fold excess relative to its dimerization partner, β-spectrin.50 To prevent vector instability, the first intron upstream of α-spectrin coding sequence was deleted in all vectors, except the SIGW vector. However, despite the abundance of α-spectrin in RBCs, the promoter was surprisingly weak and transmitted unstably in 2 of 5 vectors tested.
The human ankyrin-1 gene promoter was also chosen for similar reasons. Transgenic mice carrying the 272-bp core ankyrin-1 promoter fused with the γ-globin gene give rise to high expression in reticulocytes.51 All ankyrin-1 promoter vectors tested transmitted proviral DNA stably and showed high-level erythroid-specific expression.
The HPRE and WPRE have been reported to replace 2 of the known functions of β-globin intron, that is, facilitate 3′ end processing and improve mRNA export to the cytoplasm. The HPRE and WPRE stimulate expression of transgenes, inhibit splicing, and result in high-level expression of the otherwise intron-dependent β-globin cDNA.16,28 52 We observed a 2-fold increase in expression with the inclusion of WPRE.
The high level of erythroid-specific GFP expression is also translated to expression of therapeutic genes. We observed 43% to 113% human γ-globin expression from a single vector copy per copy of mouse α-globin in unselected pools of cells. May and colleagues47 reported a high level β-globin expression from the large micro-LCR–containing vector. However, their expression results cannot be accurately compared to the vectors used in this study because methods of quantification of mRNA differ in the 2 studies. Sabatino and coworkers have shown be 3% human γ-globin expression per mouse α-globin from the ankyrin promoter, in primary mice.53 Their vector, however, lacked the additional enhancer elements used in our study. We expect γ-globin expression levels to be higher from the γ-globin–encoding vector analogous to HTKGW, that shows the highest GFP expression in this study (the MFI of the HTKGW vector is nearly twice that of I8HKGW, Figure 4) and these studies are currently ongoing. We have also recently reported complete correction of erythrocytic protoporphyria in primary and secondary porphyria mice that received a transplant with cells transduced with the vector containing the HS-40 enhancer/ankyrin promoter driving the ferrochelatase gene.54
In summary, previous studies in gene therapy for hemoglobinopathies using oncoretroviral vectors using different erythroid regulatory elements have had problems with vector instability, interference from the viral LTR, low titer vectors of SIN-MLV vectors, and unpredictable expression of globin. Use of the SIN lentiviral vectors that lack LTR transcription allowed development of erythroid lineage-specific vectors. It also allowed comparisons of strengths of different promoter and enhancer combinations that result in stably transmitted high-level expression. Whether these vectors will resist transgene silencing in the long-term in vivo or require the use of insulator elements is currently under study. Further, vectors incorporating β- and γ-globin genes and cDNAs in these cassettes are also being studied. These studies form the basis for future vectors for gene therapy of hemoglobinopathies as well as other RBC disorders such as spherocytosis and erythropoietic porphyria.
We would like to thank Drs Inder Verma and H. Miyoshi for providing the SIN lentiviral vector backbone, Dr Philippe Leboulch for providing the HS2/β-promoter plasmid, and Drs Kohn, Nolta, and Epstein for helpful advice and comments on the manuscript.
Supported by the Association pour la Recherche sur le Cancer, Villejuif, France, the John Connell Gene Therapy Program, Children's Hospital Los Angeles, and the Sickle Scholar Award, University of Southern California Comprehensive Sickle Cell Center grant HL96-002B.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Punam Malik, Division of Hematology Oncology, Children's Hospital Los Angeles, MS 54, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail: pmalik@chla.usc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal