Abstract
Studies in mice suggest that the Ikaros (Ik) gene encodes several isoforms and is a critical regulator of hematolymphoid differentiation. Little is known on the role of Ikaros in human stem cell differentiation. Herein, the biological consequences of the forced expression of Ikaros 6 (Ik6) in human placental blood CD34+ progenitors are evaluated. Ik6 is one of the isoforms produced from the Ikaros premessenger RNA by alternative splicing and is thought to behave as a dominant negative isoform of the gene product because it lacks the DNA binding domain present in transcriptionally active isoforms. The results demonstrate that human cord blood CD34+ cells that express high levels of Ik6 as a result of retrovirally mediated gene transfer have a reduced capacity to produce lymphoid B cells in 2 independent assays: (1) in vitro reinitiation of human hematopoiesis during coculture with the MS-5 murine stromal cell line and (2) xenotransplantation in nonobese diabetic–severe combined immunodeficient mice. These results suggest that Ikaros plays an important role in stem cell commitment in humans and that the balance between the different isoforms is a key element of this regulatory system; they support the hypothesis that posttranscriptional events can participate in the control of human hematopoietic differentiation.
Introduction
Differentiation of hematopoietic stem cells is a complex but organized process by which the production of mature and functional cells of all lineages is tightly regulated. Regulatory processes involve both extracellular signals, such as cytokines, and intracellular molecules. The role of transcription factors in the control of hematopoietic cell fate is demonstrated both by the consequences of gene inactivation strategies in mice and by the involvement of these genes in numerous cases of human leukemias.1 2
Ikaros was first identified as a gene whose product associates with regulatory sequences that control the expression of genes that are typically expressed in lymphocytes.3,4 However, several lines of evidence suggest that Ikaros may act either as an activator or a repressor of transcription through complex mechanisms.5,6 The gene encodes several isoforms, as a result of alternative splicing of the premessenger RNA. Isoforms such as Ikaros 4 (Ik4), Ik6, Ik7, or Ik8 that lack the DNA binding domain encoded by exons 3, 4, and 5 cannot induce transcription and may thus act as dominant negative isoforms. Repression of transcription probably occurs in part through the association of excess molecules of short isoforms; with long isoforms such as Ik1, Ik2, or Ik3; or with structurally related molecules such as Aiolos or Helios, which possess the DNA binding domain and are able to activate DNA transcription. Heterodimerization or homodimerization of Ikaros isoforms results in different subcellular compartmentalization patterns of molecules,7 which in turn affects their biological function.
The consequences of gene inactivation showed that Ikaros plays a major role in lymphoid development,8,9 although more detailed analyses later demonstrated additional defects in the stem cell compartment.10 In addition, the arising of aggressive T-cell lymphomas and leukemias in animals heterozygous for a dominant negative mutation of the gene11 suggests (1) that the gene can act as a tumor suppressor gene and (2) that Ikaros probably plays a role through the balance of different isoforms produced by alternative splicing.3 Recent studies of the expression of the different Ikaros isoforms in normal murine hematopoietic progenitors12 provide additional arguments for this hypothesis. In humans, several groups have reported excesses of dominant negative isoforms, including Ik6, in cells obtained from patients diagnosed with poor-prognosis leukemias7,13-15; these are exciting observations because they suggest that leukemogenesis can result from dysregulated posttranscriptional events rather than from genomic rearrangements, which have rarely been described in the case of Ikaros.16
With the exception of studies performed on leukemic samples, most of what is known for Ikaros results from experiments performed in mice.17 The gene is highly conserved throughout species18 and plays a key role as a regulator of hematopoietic differentiation, both at the fetal and adult stages. To better understand the role of the different Ikaros isoforms in human postnatal hematopoietic stem cell differentiation, we undertook a series of experiments to evaluate the biological consequences of the forced expression of the Ik6 isoform in cord blood (CB) CD34+ progenitors; this experimental design has already been used to assess the role of a number of homeobox (HOX) genes and transcription factors, including one Ikaros isoform (Ik7), in murine or human hematopoietic cell development.19-26
Materials and methods
Cells and cell line
CB samples were obtained after informed consent. CD34+ cells were enriched from CB mononuclear fractions using immunoselection with the MACS technology according to the manufacturer's recommendations (Miltenyi Biotec, Bergish-Gladbach, Germany).
The human erythroleukemic and CD34+ cell line TF1 was cultured in RPMI, 10% fetal calf serum (Life Technologies, Cergy-Pontoise, France), in the presence of antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) (Biowhittaker, Emerainville, France) and granulocyte-macrophage colony-stimulating factor at 10 ng/mL (Leucomax, Novartis, and Schering Plough, Rueil Malmaison, France).
Construction of retroviral vectors
The Ik6 complementary DNA (cDNA) gene was cloned from the full-length human Ik1 cDNA27 using polymerase chain reaction and 2 pairs of oligonucleotides as follows: first pair: hIk start: TCCATGGATCCTGAGGACCATGGATGCTGA, and hIk2/7R: ACAGGCCCTTGTCCCCCACGACTCTGTCACTC to amplify exons 1 and 2; second pair: hIk 2/7F: AAGAGTGACAGAGTCGTGGGGGACAAGGGCCTGT, and hIk stop: CTCGAGAGCTTTAGCTCATGTGGAAGCG for amplification of exon 7.
Amplification products were pooled and amplified again with hIk start and hIk stop before cloning into pCR II using the TA cloning kit (Invitrogen, Groningen, The Netherlands). Amplifications were performed with the following program: a first cycle with 4 minutes at 94°C, 1 minute at 58°C, and 1 minute at 72°C, followed by 29 cycles with 1 minute at 94°C, 1 minute at 58°C, and 1 minute at 72°C; the last cycle ended with an extension of 7 minutes. The clone was sequenced to confirm that the gene was in frame and correctly oriented (ACT Gene, Evry, France). The Ik6 cDNA was extracted from pCR II and introduced upstream of the internal ribosomal entry site (IRES)–RevA sequence28 between BamHI and HindIII sites in the polylinker of a bicistronic retroviral vector: LZRS-RevA-EGFP.29 30 The vector encodes the enhanced green fluorescent protein (EGFP) downstream the IRES-RevA sequence that reinitiates translation from the full-length premessenger RNA.
LZRS-EGFP (control vector) or LZRS-Ik6-EGFP retroviral particles were produced by transient transfection of the helper-free retrovirus packaging cell line Phoenix30 by calcium phosphate coprecipitation using the Calphos Mammalian transfection kit (Clontech Laboratories, Ozyme, Montigny le Bretonneux, France). Selection of positive cells was performed in the presence of 1 μg/mL puromycin (Sigma, St Quentin Fallavier, France); the defective retroviral supernatant was then collected, passed though a 0.45 μm filter, aliquoted, and stored at −80°C until its use for transduction of target cells. Enough supernatant was produced for all described experiments, and viral titers were assayed in triplicate with the TF1 cell line. There were 1.2 × 105 viral particles per milliliter for LZRS-EGFP and 0.68 × 105 per milliliter for LZRS-Ik6-EGFP.
The defective retroviruses code for sequences situated between the 2 long terminal repeats (Ikaros-IRES-EGFP genes); sequences that are situated outside the long terminal repeats in the LZRS plasmid, including Epstein-Barr virus sequences and antibiotic resistance genes, are not present in the retroviral genome.
Transduction
To induce cycling, CB CD34+ cells were first stimulated for 60 hours in serum-free BIT medium (Stem Cell Technologies, Meylan, France) supplemented with a combination of cytokines that includes recombinant murine flt3 ligand (flt3-L;31 100 U/mL, a kind gift from DNAX Research Institute for Molecular and Cellular Biology, Palo Alto, CA), recombinant human (rh) Kit-L (ligand for c-kit) (stem cell factor, 100 ng/mL, a kind gift from Amgen, Thousand Oaks, CA), and recombinant human thrombopoietin (20 ng/mL, Stem Cell Technologies). Between 2 × 106 and 4 × 106 cells were transduced in 12.5 cm2 flasks coated with 50 μg/mL fibronectin (Chemicon, Euromedex, Souffelweyersheim, France), in the presence of 1.6 mL viral supernatant, and 4 μg/mL polybrene (Sigma). After 2 hours at 37°C, another 1.6 mL of fresh virus supernatant and 3.2 mL of medium and polybrene were added. Cells were cultured for the next 22 hours at 37°C. Cells were then washed and cultured in BIT medium and cytokines.
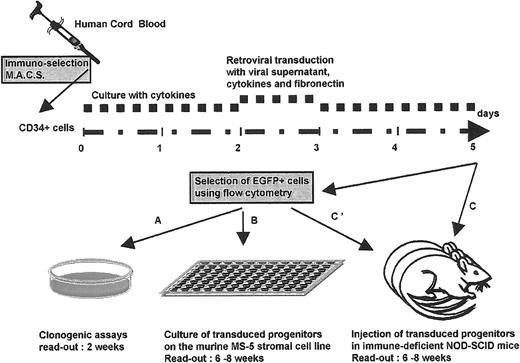
Forty-eight hours after the end of transduction, cells submitted to retroviral infection were evaluated for their hematopoietic potential either unfractionated or following fluorescence-activated cell sorting for EGFP positivity (FACS Vantage, Becton Dickinson, San Jose, CA) (Figure 1).
Experimental design.
Experimental design to assess the biological consequences of transducing human placental blood CD34+ cells, either with a test retroviral vector that encodes the Ik6 isoform, along with the EGFP marker gene, or with a control vector that encodes EGFP only.
Experimental design.
Experimental design to assess the biological consequences of transducing human placental blood CD34+ cells, either with a test retroviral vector that encodes the Ik6 isoform, along with the EGFP marker gene, or with a control vector that encodes EGFP only.
Clonogenic assays
A total of 250 transduced CD34+ purified cells were plated in triplicate in 0.5 mL methylcellulose (Methocult 4230, Stem Cell Technologies) supplemented with recombinant human hematopoietic growth factors: erythropoietin (2 U/mL, Eprex, Janssen-Cilag, Issy les Moulineaux, France), interleukin-3 (IL-3) (10 ng/mL, Amgen), IL-6 (10 ng/mL), granulocyte colony-stimulating factor (10 ng/mL, Amgen), and rhKit-L (100 ng/mL) in Iscoves modified Dulbecco medium (Biowhittaker). Granulocyte-macrophage colony-forming units (CFU-GMs) and erythroid burst-forming units (BFU-Es) were counted at day 14 according to published criteria.32
Ex vivo differentiation of transduced placental progenitors during coculture with the MS-5 murine stromal cell line
Cocultures of human hematopoietic progenitors with the MS-5 cell line were performed as previously described.33 Briefly, MS-5 cells34 were cultured in 24-well plates until they reached confluence. Sorted transduced CD34+ cells were then added at 104 cells per well in minimum essential medium (α-MEM) (Biowhittaker) supplemented with 10% human AB serum (Institut Jacques Boy, Reims, France), antibiotics, 5% fetal calf serum (Stem Cell Technologies), and 3 cytokines: rhIL-2 (5 ng/mL, Chiron Suresne, France), rhIL-15 (10 ng/mL, R & D Systems, Abingdon, United Kingdom), and rhKit-L (50 ng/mL, Amgen). Cells were maintained at 37°C in a fully humidified incubator with 5% CO2 and fed weekly by half medium change during 6 to 8 weeks. Cells were then collected, and their phenotype was assessed with flow cytometry.
Transplantation in nonobese diabetic severe combined immunodeficient mice
Conditions were reproduced from previously published works.33,35,36 Briefly, nonobese diabetic–severe combined immunodeficient (NOD-SCID) mice37 were obtained from Dr John Dick, Department of Molecular and Medical Genetics, Hospital for Sick Children, Toronto, Canada. Founders were bred at our animal facility. All animals were maintained in germ-free conditions. Animals 7 to 9 weeks old were used for xenotransplantation. Recipients were prepared with total body γ-irradiation at a dose of 3 Gy. Within 24 hours of irradiation, mice were injected with 0.05 × 106to 2 × 106 transduced or untransduced CD34+ cells via the tail vein. Animals were killed 6 to 9 weeks later; bone marrow cells were recovered from femora and tibiae by flushing. All cells were analyzed by flow cytometry for the expression of human antigens. The presence of human cells was detected with a PC5-conjugated murine monoclonal antibody (mAb) against human CD45; cells were simultaneously stained with phycoerythrin (PE)–conjugated murine mAbs that recognize different human hematopoietic differentiation antigens (CD19 and CD33) and evaluated for EGFP expression.
Flow cytometry analyses
Antibodies used in this study were murine mAb immunoglobulin G1-PC5 (phycoerythrin cyanin 5.1), CD45-PC5, CD45-PE, CD19-PE, CD10-PE, CD14-PE, CD33-PE, CD34-PE (Beckman-Coulter-Immunotech, Marseille, France), and immunoglobulin G1 PE (Becton Dickinson). Cells were analyzed with a FACSCalibur (Becton Dickinson). Dead cells were excluded for further analysis by gating out TO-PRO-3 iodide-positive cells (0.5 μM, Molecular Probes Interchim, Montluçon, France, analysis at 630 nm).
Western blot
A polyclonal rabbit antiserum against the C-terminal part of all Ikaros isoforms was produced in our animal facility by immunizing rabbits with an Ikaros C-terminal peptide (YSSHITRGEHRFHMS, Neosystem, Strasbourg, France) coupled to the KLH carrier protein. Animals received a first injection and at least 3 recall injections before blood collection. The antiserum was purified by affinity chromatography on the immobilized peptide using Affi-gel 10 (Bio-Rad, Ivry-sur-Seine, France). The antiserum was used for Western analysis at a 2 μg/mL concentration. For normalization of protein quantities, a rabbit anti-Grb2 antiserum (Santa Cruz Biotechnology, TEBU SA, Le Perray en Yvelines, France) was used at 200 ng/mL.
For Western blot analysis of primary cells, whole cell extracts were prepared by incubation during 15 minutes at 4°C in lysis buffer: 2.5 mM Tris-HCl, pH 7.4; 50 mM NaCl; 0.2 mM ethylenediaminetetraacetic acid; 10 mM MgCl2; 50 U/mL deoxyribonuclease in the presence of a protease inhibitor cocktail (Boehringer Mannheim, Meylan, France). After centrifugation for 10 minutes, the proteins in the supernatant were separated with 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to Immobilon P membrane (Millipore, St Quentin en Yvelines, France). Membranes were saturated with TBST (10 mM Tris, pH 7.4; 100 mM NaCl, and 0.1% Tween), 5% albumin, and then incubated with the specific antiserum for 1 hour; this was followed by incubation with 20 ng/mL peroxidase-conjugated goat anti–rabbit antiserum (Beckman-Coulter-Immunotech) and revealed with the enhanced chemiluminescence solution (Amersham-Pharmacia, Orsay, France).
Results
Expression of Ikaros proteins in transduced cells
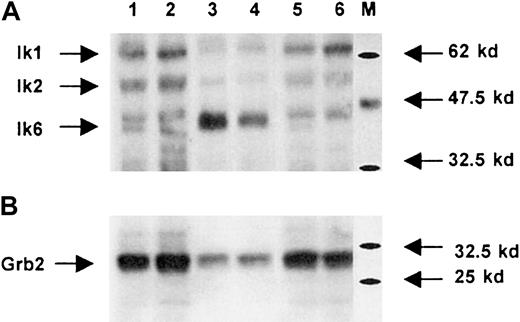
About 35% to 50% of CB CD34+ cells exposed to LZRS-EGFP retroviral supernatant and 15% to 35% of cells exposed to LZRS-Ik6-EGFP retroviral supernatant expressed EGFP 2 days after transduction. Proteins were extracted from transduced cells sorted as EGFP+ with flow cytometry. Western blot analysis with purified rabbit polyclonal antibodies demonstrated the presence of endogenous Ikaros isoforms. As previously reported,38isoforms that possess the DNA binding domain, particularly Ik1 and Ik2, were the most abundant; Ik6 was present at low levels in untransduced CD34+ cells or in cells transduced with the LZRS-EGFP retroviral vector; and Ik6 was overexpressed in cells transduced with the LZRS-Ik6-EGFP vector (Figure 2). These observations suggest that the retroviral system is efficient to induce forced expression of Ik6 and thus disturbs the balance between the different Ikaros isoforms.
Western blot analysis of wild-type and transduced cells.
Whole protein extracts obtained from CB CD34+ cells transduced with the LZRS-EGFP retroviral vector (lanes 1 and 2) or LZRS-Ik6-EGFP (lanes 3 and 4) and from untransduced CB CD34+ cells (lanes 5 and 6) are revealed with (A) an anti-Ikaros antiserum (see “Materials and methods”) or (B) an anti-Grb2 polyclonal antibody. M indicates protein markers. At least 3 isoforms of Ikaros (Ik1, Ik2, Ik6) are endogeneously produced; lanes 3 and 4 show that after retroviral transduction the relative abundance of the Ik6 isoform is greatly increased (note that for these 2 lanes the signal for Grb2 is slightly less than for other lanes).
Western blot analysis of wild-type and transduced cells.
Whole protein extracts obtained from CB CD34+ cells transduced with the LZRS-EGFP retroviral vector (lanes 1 and 2) or LZRS-Ik6-EGFP (lanes 3 and 4) and from untransduced CB CD34+ cells (lanes 5 and 6) are revealed with (A) an anti-Ikaros antiserum (see “Materials and methods”) or (B) an anti-Grb2 polyclonal antibody. M indicates protein markers. At least 3 isoforms of Ikaros (Ik1, Ik2, Ik6) are endogeneously produced; lanes 3 and 4 show that after retroviral transduction the relative abundance of the Ik6 isoform is greatly increased (note that for these 2 lanes the signal for Grb2 is slightly less than for other lanes).
Overexpression of the Ik6 isoform does not change the clonogenic capacity of transduced placental CD34+cells
Forty-eight hours after transduction, EGFP+ cells were selected using flow cytometry and plated in methylcellulose to assess the frequency of CFU-GMs and BFU-Es. Despite variability between samples, the clonogenic capacities of Ik6-expressing cells (41 ± 29 CFU-GMs and 98 ± 75 BFU-Es for 1000 plated cells, average ± SE) were not significantly different from the clonogenic capacities of control cells transduced with a retroviral vector that encodes only the EGFP gene (37 ± 33 CFU-GMs and 93 ± 99 BFU-Es for 1000 plated cells, n = 11, P > .05 with a Wilcoxon ttest for paired samples).
Overexpression of the Ik6 isoform impairs the ability of transduced human CB CD34+ cells to differentiate toward the B-cell lineage when cocultured with the MS-5 murine stromal cell line
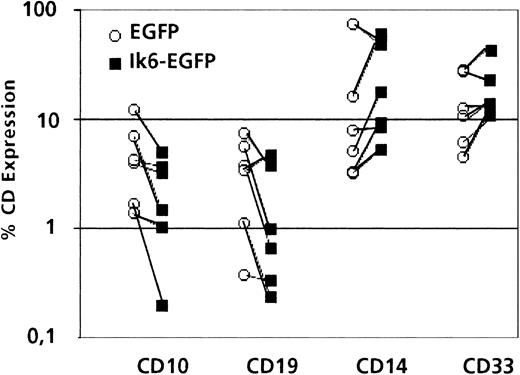
Typically, when cocultured with the MS-5 murine cell line33 or with other stromal cell lines,39human placental blood CD34+ progenitors give rise to a small percentage of B cells that can be identified through the expression of CD10 and CD19 after 6 to 8 weeks of culture. These cells are present in the “lymphocyte” gate characterized by low forward scatter and side scatter. When we analyzed 6 cultures initiated with sorted Ik6-expressing (EGFP+) cells, we observed in 5 cases a decreased percentage of CD10+ and CD19+ cells (B-lymphoid compartment) and an increased percentage of CD14+ and CD33+ cells (myeloid compartment) when compared with control cultures that were initiated with cells transduced with the LZRS-EGFP retroviral vector (Figure3). In addition, in the former, the small percentage of B-lymphoid cells that persisted was characterized by low levels of EGFP expression and was decreased when compared with myeloid cells that were identified in test cultures or to B-lymphoid cells that were present in control cultures (Figure4).
Comparison of lymphoid or myeloid antigen expression in transduced cells after in vitro differentiation.
Percentage of cells positive for CD10 or CD19 (B lineage) or CD14 or CD33 (myeloid lineage) in the “lymphocyte” gate of 6- to 8-week-old MS-5 cocultures, initiated with sorted EGFP+ placental CD34+ cells, transduced with LZRS-Ik6-EGFP or LZRS-EGFP (paired experiments). Five of 6 experiments showed a decrease of CD10+ or CD19+ cells and an increase of CD14+ and CD33+ cells in cultures initiated with CD34+ cells transduced with LZRS-Ik6-EGFP when compared with cultures initiated with LZRS-EGFP transduced CD34+ cells.
Comparison of lymphoid or myeloid antigen expression in transduced cells after in vitro differentiation.
Percentage of cells positive for CD10 or CD19 (B lineage) or CD14 or CD33 (myeloid lineage) in the “lymphocyte” gate of 6- to 8-week-old MS-5 cocultures, initiated with sorted EGFP+ placental CD34+ cells, transduced with LZRS-Ik6-EGFP or LZRS-EGFP (paired experiments). Five of 6 experiments showed a decrease of CD10+ or CD19+ cells and an increase of CD14+ and CD33+ cells in cultures initiated with CD34+ cells transduced with LZRS-Ik6-EGFP when compared with cultures initiated with LZRS-EGFP transduced CD34+ cells.
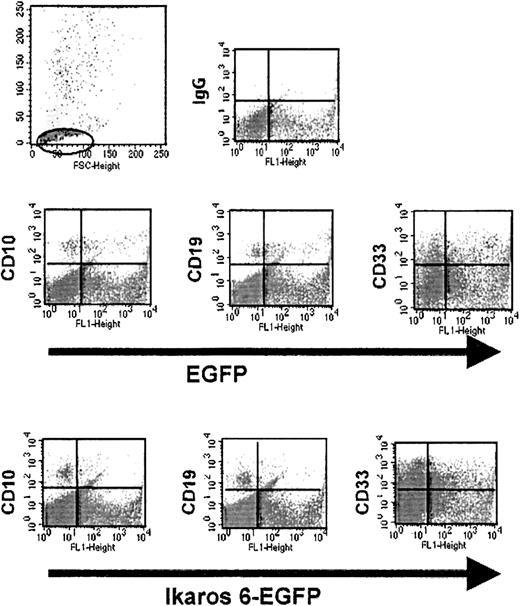
Flow cytometry analyses of human transduced hematopoietic cells after in vitro culture.
After 6 weeks, coculture with the MS-5 murine stromal cell line, initiated with sorted EGFP+ placental CD34+cells, transduced with LZRS-EGFP (top) or LZRS-Ik6-EGFP (bottom), were stained with antihuman mAbs against CD10, CD19, and CD33 conjugated with PE. In test cultures initiated with sorted CD34+ cells transduced with the LZRS-Ik6-EGFP retroviral vector, a small percentage of cells expressing CD10 and CD19 can be identified; residual B cells in these cultures expressed low levels of EGFP.
Flow cytometry analyses of human transduced hematopoietic cells after in vitro culture.
After 6 weeks, coculture with the MS-5 murine stromal cell line, initiated with sorted EGFP+ placental CD34+cells, transduced with LZRS-EGFP (top) or LZRS-Ik6-EGFP (bottom), were stained with antihuman mAbs against CD10, CD19, and CD33 conjugated with PE. In test cultures initiated with sorted CD34+ cells transduced with the LZRS-Ik6-EGFP retroviral vector, a small percentage of cells expressing CD10 and CD19 can be identified; residual B cells in these cultures expressed low levels of EGFP.
Overexpression of the Ik6 isoform impairs the ability of transduced human CB CD34+ cells to differentiate toward the B-cell lineage when injected into irradiated NOD-SCID mice
The NOD-SCID model33,35 36 was first validated in our laboratory by injecting between 0.05 × 106 and 0.5 × 106 freshly selected CB CD34+ cells (primary CD34+ cells) in the tail vein of irradiated animals. Figure 5 shows that engraftment occurred in 22 of 27 animals 6 to 9 weeks after injection when a threshold percentage of 0.1% CD45+ cells in the bone marrow was used to consider animals as engrafted; the rate of engraftment decreases to 13 of 27 when a threshold value of 1% CD45+ cells was used (CD45: 2.5%-62%). No clear dose-response effect was evidenced when animals were injected with cell numbers that fall in the following range of values: 3 of 5 animals injected with 0.05 × 106 CD34+ cells, 14 of 15 with 0.1 × 106, 2 of 4 with 0.2 × 106, or 3 of 3 with 0.5 × 106 engrafted. The threshold value of 1% CD45+ cells was used for all further experiments in which we addressed the question of the relative proportions of the B-lymphoid and myeloid cells, because it allows for reliable flow cytometry analyses. The percentage of CD19+cells was always superior to the percentage of CD33+ cells in 13 animals injected with primary CD34+ cells.
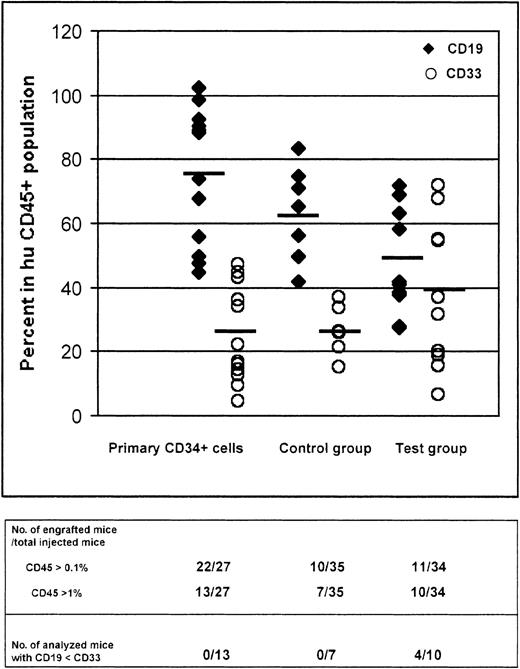
Percentages of CD19+ and CD33+cells 6 to 9 weeks after injection of CB CD34+ cells in 3 goups of NOD-SCID mice.
Percentage of cells expressing CD19 or CD33 recovered from the bone marrow of NOD-SCID mice that were killed 6 to 9 weeks after tail injection of fresh CD34+ CB cells (primary CD34+ cells that did not undergo amplification and transduction steps), or cultured CD34+ cells that underwent LZRF-EGFP transduction (control cells), or CD34+ cells tranduced with the LZRS-Ik6-EGFP retroviral vector (test cells). Detailed analysis of engraftment is provided in Tables 1 and2.
Percentages of CD19+ and CD33+cells 6 to 9 weeks after injection of CB CD34+ cells in 3 goups of NOD-SCID mice.
Percentage of cells expressing CD19 or CD33 recovered from the bone marrow of NOD-SCID mice that were killed 6 to 9 weeks after tail injection of fresh CD34+ CB cells (primary CD34+ cells that did not undergo amplification and transduction steps), or cultured CD34+ cells that underwent LZRF-EGFP transduction (control cells), or CD34+ cells tranduced with the LZRS-Ik6-EGFP retroviral vector (test cells). Detailed analysis of engraftment is provided in Tables 1 and2.
Then, several groups of sublethally irradiated animals were injected in parallel, with CD34+ cells transduced with LZRS-EGFP or LZRS-Ik6-EGFP (Table 1). In some experiments, sorted EGFP+ cells obtained from the 2 transduced cell populations were injected in mice. As previously mentioned, only mice that had more than 1% human CD45+cells 6 to 9 weeks after tail vein injection were further analyzed: 10 of 34 mice injected with cultured CD34+ cells transduced with LZRS-Ik6-EGFP engrafted, and 4 of 28 mice injected with cultured CD34+ cells transduced with LZRS-EGFP engrafted. The proportion of engrafted animals fell to 3 of 12 animals when injected with sorted EGFP+ LZRS-Ik6-EGFP transduced cells and 0 of 12 animals when injected with sorted EGFP+ LZRS-EGFP transduced cells; 7 of 14 animals and 3 of 7 animals had more than 1% detectable CD45+ cells in their bone marrow when mice were injected with the EGFP− fractions from both transduced cell populations, respectively.
Engraftment rate in different groups of NOD-SCID mice injected with different cell populations
| Cell population . | Primary CD34+cells . | LZRS-EGFP–transduced cells . | LZRS-Ik6-EGFP–transduced cells . | ||||
|---|---|---|---|---|---|---|---|
| Unsorted cells . | Sorted EGFP+ cells . | Sorted EGFP− cells . | Unsorted cells . | Sorted EGFP+ cells . | Sorted EGFP− cells . | ||
| No. of animals | 27 | 28 | 12 | 7 | 34 | 12 | 14 |
| CD45+ cells > 0.1% | 22 | 5 | 5 | 3 | 11 | 9 | 11 |
| CD45+cells > 1% | 13 | 4* | 0 | 3* | 10† | 3 | 7 |
| Cell population . | Primary CD34+cells . | LZRS-EGFP–transduced cells . | LZRS-Ik6-EGFP–transduced cells . | ||||
|---|---|---|---|---|---|---|---|
| Unsorted cells . | Sorted EGFP+ cells . | Sorted EGFP− cells . | Unsorted cells . | Sorted EGFP+ cells . | Sorted EGFP− cells . | ||
| No. of animals | 27 | 28 | 12 | 7 | 34 | 12 | 14 |
| CD45+ cells > 0.1% | 22 | 5 | 5 | 3 | 11 | 9 | 11 |
| CD45+cells > 1% | 13 | 4* | 0 | 3* | 10† | 3 | 7 |
The main end point for evaluating the consequences of forced expression of Ik6 was the relative proportions of CD19+ and CD33+ cells in engrafted animals. Thus, we analyzed and compared 10 animals that engrafted after injection of unsorted cultured CD34+ cells exposed to retrovirally mediated transduction with the LZRS-Ik6-EGFP vector (test group, CD45+ cells: 1.3%-51.4%) with 7 animals (control group, CD45+ cells: 3.23%-60.9%) that engrafted following injection of unsorted (4 animals) or sorted EGFP− (3 animals) CD34+cells exposed to transduction with the LZRS-EGFP vector (Figure 5). In addition, 3 animals injected with sorted EGFP+, CD34+ cells exposed to retrovirally mediated transduction with the LZRS-Ik6-EGFP vector engrafted and were further analyzed for their percentages of CD19+ and CD33+ cells; however, because the appropriate control is missing (all mice injected with sorted EGFP+, CD34+ cells exposed to retrovirally mediated transduction with the LZRS-EGFP vector had <1% CD45+ cells), data obtained in these 3 animals are not aggregated with the 10 other animals in the test group.
Four of 10 test animals were found to have a percentage of CD19+ cells lower than the percentage of CD33+cells; we never had a similar observation in animals engrafted with primary CD34+ cells or in animals from the control group. Thus, despite great heterogeneity between animals, the average percentage of CD19+ cells was lower in the test group (49%) than in the control group (63%), and the average percentage of CD33+ cells was higher in the test group (40%) than in the control group (26%). These results were analyzed with the Fisher exact t test, comparing the test group (10 evaluable animals) with all other engrafted animals (20 animals). The 2 groups were found to be statistically different (P = .021). Table2 details engraftment data for animals in the test and control groups. Of the 3 animals that engrafted following injection of sorted EGFP+ LZRS-Ik6-EGFP cells, 1 showed an altered pattern of human hematopoietic reconstitution, with a lower percentage of CD19+ cells than CD33+ cells (data not shown).
Analysis of engrafted mice
| Experiment* . | Nature . | Injected cells per animal . | Analysis of engraftment . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % EGFP . | Cell no. × 106 before†transduction . | Cell no. × 106 after‡transduction . | % CD45 . | % EGFP/ CD45 . | % 19+ . | % 33+ . | % EGFP/ CD19+ . | % EGFP/ CD33+ . | % CD19/ CD45 . | % CD33/ CD45 . | ||
| Control group | ||||||||||||
| B | EGFP | 58.60 | 0.25 | 1 | 3.23 | 0.96 | 2.7 | 1.2 | 0.97 | 0.43 | 83.59 | 37.15 |
| D | EGFP | 51 | 0.75 | 1.2 | 60.9 | 9.9 | 44.5 | 19.4 | 6.32 | 2.58 | 73.07 | 31.86 |
| D | EGFP | 51 | 0.75 | 1.2 | 7.02 | 3.83 | 2.93 | 1.51 | 1.63 | 0.91 | 41.74 | 21.51 |
| D | EGFP | 50 | 0.75 | 0.5 | 8.42 | 1.1 | 5.49 | 2.22 | 0.73 | 0.5 | 65.20 | 26.37 |
| C | EGFP− | 0 | 0.2 | 0.5 | 54.8 | 0 | 25.2 | 17 | 49.92 | 33.68 | ||
| C | EGFP− | 0 | 0.2 | 0.5 | 50.13 | 0 | 28.3 | 13 | 56.45 | 25.93 | ||
| E | EGFP− | 0 | 0.25 | 0.5 | 20.71 | 0 | 15.5 | 3.14 | 74.84 | 15.16 | ||
| Test group | ||||||||||||
| D | Ik6 | 18.80 | 0.45 | 2 | 5.32 | 1.61 | 2.06 | 1.01 | 0.79 | 0.29 | 38.72 | 18.98 |
| D | Ik6 | 18.80 | 0.45 | 2 | 1.27 | 0.1 | 0.74 | 0.4 | 0 | 0.1 | 58.27 | 31.50 |
| E | Ik6 | 15 | 0.1 | 1 | 10.65 | 0.84 | 7.34 | 0.69 | 0.72 | 0 | 68.92 | 6.48 |
| E | Ik6 | 15 | 0.1 | 1 | 51.36 | 9.38 | 32.46 | 10.28 | 6.14 | 2.21 | 63.20 | 20.02 |
| E | Ik6 | 15 | 0.1 | 1 | 11.46 | 6.21 | 8.23 | 1.81 | 5.1 | 0.1 | 71.82 | 15.79 |
| F | Ik6 | 21 | 0.35 | 0.8 | 2.8 | 0.1 | 1.15 | 1.04 | 0 | 0.1 | 41.07 | 37.14 |
| A2-153 | Ik6 | 16.3 | 0.09 | 0.5 | 11 | 0.7 | 4.59 | 6.07 | 0.28 | 0.44 | 41.73 | 55.18 |
| A2-153 | Ik6 | 16.3 | 0.09 | 0.5 | 19.6 | 0.36 | 7.39 | 13.26 | 0 | 0.4 | 37.70 | 67.65 |
| B2-153 | Ik6 | 18.50 | 0.15 | 0.4 | 11.13 | 0.94 | 3.05 | 6.1 | 0.1 | 0.8 | 27.40 | 54.81 |
| D2-153 | Ik6 | 18.80 | 0.45 | 2 | 4.13 | 0.23 | 1.15 | 2.97 | 0.1 | 0.1 | 27.85 | 71.91 |
| Experiment* . | Nature . | Injected cells per animal . | Analysis of engraftment . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % EGFP . | Cell no. × 106 before†transduction . | Cell no. × 106 after‡transduction . | % CD45 . | % EGFP/ CD45 . | % 19+ . | % 33+ . | % EGFP/ CD19+ . | % EGFP/ CD33+ . | % CD19/ CD45 . | % CD33/ CD45 . | ||
| Control group | ||||||||||||
| B | EGFP | 58.60 | 0.25 | 1 | 3.23 | 0.96 | 2.7 | 1.2 | 0.97 | 0.43 | 83.59 | 37.15 |
| D | EGFP | 51 | 0.75 | 1.2 | 60.9 | 9.9 | 44.5 | 19.4 | 6.32 | 2.58 | 73.07 | 31.86 |
| D | EGFP | 51 | 0.75 | 1.2 | 7.02 | 3.83 | 2.93 | 1.51 | 1.63 | 0.91 | 41.74 | 21.51 |
| D | EGFP | 50 | 0.75 | 0.5 | 8.42 | 1.1 | 5.49 | 2.22 | 0.73 | 0.5 | 65.20 | 26.37 |
| C | EGFP− | 0 | 0.2 | 0.5 | 54.8 | 0 | 25.2 | 17 | 49.92 | 33.68 | ||
| C | EGFP− | 0 | 0.2 | 0.5 | 50.13 | 0 | 28.3 | 13 | 56.45 | 25.93 | ||
| E | EGFP− | 0 | 0.25 | 0.5 | 20.71 | 0 | 15.5 | 3.14 | 74.84 | 15.16 | ||
| Test group | ||||||||||||
| D | Ik6 | 18.80 | 0.45 | 2 | 5.32 | 1.61 | 2.06 | 1.01 | 0.79 | 0.29 | 38.72 | 18.98 |
| D | Ik6 | 18.80 | 0.45 | 2 | 1.27 | 0.1 | 0.74 | 0.4 | 0 | 0.1 | 58.27 | 31.50 |
| E | Ik6 | 15 | 0.1 | 1 | 10.65 | 0.84 | 7.34 | 0.69 | 0.72 | 0 | 68.92 | 6.48 |
| E | Ik6 | 15 | 0.1 | 1 | 51.36 | 9.38 | 32.46 | 10.28 | 6.14 | 2.21 | 63.20 | 20.02 |
| E | Ik6 | 15 | 0.1 | 1 | 11.46 | 6.21 | 8.23 | 1.81 | 5.1 | 0.1 | 71.82 | 15.79 |
| F | Ik6 | 21 | 0.35 | 0.8 | 2.8 | 0.1 | 1.15 | 1.04 | 0 | 0.1 | 41.07 | 37.14 |
| A2-153 | Ik6 | 16.3 | 0.09 | 0.5 | 11 | 0.7 | 4.59 | 6.07 | 0.28 | 0.44 | 41.73 | 55.18 |
| A2-153 | Ik6 | 16.3 | 0.09 | 0.5 | 19.6 | 0.36 | 7.39 | 13.26 | 0 | 0.4 | 37.70 | 67.65 |
| B2-153 | Ik6 | 18.50 | 0.15 | 0.4 | 11.13 | 0.94 | 3.05 | 6.1 | 0.1 | 0.8 | 27.40 | 54.81 |
| D2-153 | Ik6 | 18.80 | 0.45 | 2 | 4.13 | 0.23 | 1.15 | 2.97 | 0.1 | 0.1 | 27.85 | 71.91 |
In the control group, 4 mice injected with cells submitted to retroviral transduction with the control LZRS-EGFP retroviral vector and 3 other animals (EGFP−) injected with the EGFP− fraction after the retroviral transduction process were analysed. In the test group, 2 different patterns of hematopoietic reconstitution can be distinguished: 6 animals had more CD19+ cells than CD33+ cells, as usually observed in NOD-SCID mice transplanted with uncultured, untransduced CD34+ cells, or in the control group; 4 animals (
) had more CD33+ cells than CD19+cells (Figure 5).
Different groups of animals, each group being engrafted with cells obtained from the same CB.
Equivalent number of fresh, uncultured cells.
Actual number of cells that were injected into mice, following the transduction procedure, and ex vivo expansion of cell numbers.
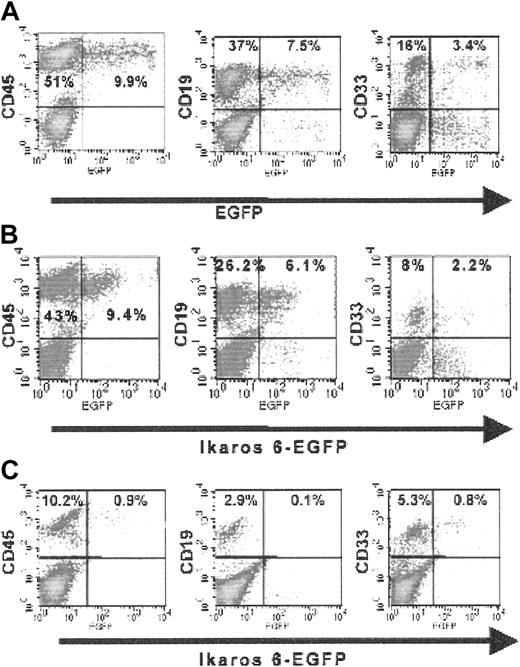
We analyzed EGFP expression in CD19+ and CD33+cells from engrafted animals. The percentages of EGFP+cells were variable. In the 4 test animals that had lower percentages of CD19+ cells than CD33+ cells, EGFP+ cells were present at low levels in both compartments; the percentage of EGFP+/CD19+cells was lower than the percentage of EGFP+/CD33+ in 3 of 4 animals, as illustrated in Figure 6, while control animals usually had more EGFP+/CD19+ cells than EGFP+/CD33+ cells (Table 2).
Flow cytometry analyses of human hematopoietic cells engrafted in NOD-SCID mice.
Six weeks after injection in a control animal injected with CD34+ cells transduced with the LZRS-EGFP retroviral vector (A), CD19+ cells are more abundant than CD33+cells and both populations express significant levels of the EGFP marker. The same pattern is observed in one of the test animals injected with the LZRS-Ik6-EGFP retroviral vector (B). In another test animal injected with CD34+ cells transduced with the LZRS-Ik6-EGFP retroviral vector (C), a different pattern is seen. A small percentage of cells expressing CD19 can be identified; only rare CD19+ cells express EGFP, in contrast with CD33+ cells recovered from the same animal. Similar numbers of events were acquired during flow cytometry analysis for all lines. For detailed analyses, see Table 2.
Flow cytometry analyses of human hematopoietic cells engrafted in NOD-SCID mice.
Six weeks after injection in a control animal injected with CD34+ cells transduced with the LZRS-EGFP retroviral vector (A), CD19+ cells are more abundant than CD33+cells and both populations express significant levels of the EGFP marker. The same pattern is observed in one of the test animals injected with the LZRS-Ik6-EGFP retroviral vector (B). In another test animal injected with CD34+ cells transduced with the LZRS-Ik6-EGFP retroviral vector (C), a different pattern is seen. A small percentage of cells expressing CD19 can be identified; only rare CD19+ cells express EGFP, in contrast with CD33+ cells recovered from the same animal. Similar numbers of events were acquired during flow cytometry analysis for all lines. For detailed analyses, see Table 2.
Discussion
We here report observations suggesting that we are able to overexpress an Ikaros isoform that lacks the DNA binding domain (Ik6) and thus disturbs the balance of endogenous Ikaros isoforms in primary human CB hematopoietic cells and progenitors. Our results suggest that retrovirally mediated gene transfer of the human Ik6 cDNA in CB CD34+ cells impairs the ability of transduced cells to differentiate in 2 independent models of human hematopoietic stem and progenitor cell differentiation. This conclusion is based on the evaluation of the percentage of CD19+ (B-lymphoid) cells in both differentiation assays; forced expression of Ik6 results in a decreased percentage of B cells and an increased percentage of myeloid cells identified as CD33+ cells.
Both the MS-5 coculture assay and the NOD-SCID model present technical limitations. In the MS-5 coculture assay, the percentage of CD10+/CD19+ B cells after several weeks of culture is low33; however, the combination of phenotype and forward scatter/side scatter allows for a reliable identification of this minor population, which we found to be decreased in 5 of 6 experiments when cultures were reinitiated with sorted Ik6 transduced cells. Analyses of different groups of NOD-SCID mice undergoing transplantation with different cell populations show (1) that engraftment with primary CD34+ cells occurs at similar rates than previously reported;36,40,41 (2) that engraftment with CD34+ cells exposed to a cytokine combination and retroviral transduction for 5 days occurs at lower rates, maybe as a consequence of myeloid- driven differentiation by Kit-L, flt3-L, and thrombopoietin; and (3) that retroviral integration occurs at low levels, especially in the most immature human hematopoietic progenitors, because mice undergoing transplantation with the sorted EGFP− fractions of cells submitted to the transduction procedure had higher rates of engraftment than mice undergoing transplantation with the sorted EGFP+ fractions. However, our goal was not to measure the frequency of NOD-SCID repopulating cells in fresh or cultured CB CD34+progenitors but, rather, to compare the relative percentages of CD19+ and CD33+ cells in engrafted mice after injection of different cell populations. To obtain reliable data when conducting these experiments, we chose to analyze only animals that had more than 1% human CD45+ cells in their marrow, a more stringent criteria than what has been used in most other reports. Notably, not all animals engrafted with cells overexpressing Ik6 displayed an altered pattern of differentiation: thus, observations obtained with the MS-5 assay that support the same interpretation are of the utmost importance. In addition, the heterogeneity in hematopoietic reconstitution of 10 animals undergoing transplantation with cells overexpressing Ik6 suggests that different populations of progenitors may exist and that forced expression of Ik6 in these different progenitor subsets may differently affect their repopulation ability. From this viewpoint, it is of interest that forced expression of Ik6 had no consequence on the clonogenic capacity of transduced cells. The differential effects of forced expression of murine Ik7 on 2 different pathways of in vitro human dendritic cell differentiation (decreased production of dendritic cells derived from lymphoid progenitors and no effect on dendritic cell differentiation from monocytes) further support this hypothesis.26
Analysis of EGFP expression in human cells recovered from engrafted NOD-SCID mice provides only limited information on the biological consequences of forced Ik6 expression. This is because gene expression following retrovirally mediated gene transfer is rarely stable over time and during human hematopoietic cell differentiation42 43; thus, the percentage of EGFP+ cells 6 to 9 weeks after transplantation probably does not reflect the actual frequency of transduced stem and progenitor cells that were infused into animals. Although numbers are too small for statistical analyses, it is however of interest that residual B cells that were detected in the MS-5 assay, and in 4 NOD-SCID mice with an altered pattern of hematopoietic reconstitution, were rarely positive for EGFP.
Despite its limitations, the NOD-SCID model provides additional information on in vitro assays and a different insight into human hematopoietic differentiation. There are only rare reports in which this assay has been used to study how the commitment of human progenitors to either the myeloid or the B-lymphoid compartment is controlled; Kapp et al44 have shown that extracellular factors, the combination of flt3-L and IL-7, are able to modulate the potential of NOD-SCID repopulating cells to differentiate toward the B-lymphoid lineage. Similarly to these observations, our data suggest that changes in intracellular regulators can also decrease the ability of human cells to differentiate toward the B-cell lineage.
Retrovirally mediated gene transfer has been used to evaluate the role of different murine and human transcription factors in hematopoietic stem and progenitor cell differentiation. Ectopic expression of the murine Hlx gene into 3 different murine cell lines resulted in changes in morphology, phenotype, and functional properties.45 Retrovirally mediated expression of NF-E2 in 2 murine hematopoietic cell lines resulted in reprogramming of the differentiation program of these erythroid and monocytic cell lines, respectively; when transduced into fetal liver cells, BFU-E formation was increased. The forced expression of different HOX genes in murine primary cells was reported on several occasions19-22; transplantation of HOXB3-expressing progenitors in myeloablated recipients negatively interfered with T- and B-cell differentiation while promoting the expansion of granulomacrophagic clonogenic progenitors20; overexpression of HOXB4 resulted in the expansion of the primitive progenitor cell population19,22; and forced expression of HOXA10 led to an imbalance in the respective proportions of committed progenitors and eventually to the appearance of leukemia in a proportion of the animals.21 In humans, retrovirally mediated transfer of the HOXC4 gene into peripheral blood CD34+ cells resulted in an increase in the frequency of CFU-GMs, BFU-Es, and long-term culture–initiating cells,23 while enforced expression of HOXB7 favored the development of myeloid clonogenic progenitors from the primitive stem cell compartment.24The forced expression of the Id gene25 into human CD34+ cells isolated from fetal liver or from postnatal thymus changed the balance between T-cell and natural killer cell production, as assessed in fetal thymic organ cultures and in natural killer cell liquid culture assay; interestingly, Id3 behaves as a repressor of transcription.
Together with previously published results,26 our observations confirm an important role for the relative expression of different Ikaros isoforms in human hematopoietic differentiation in in vitro and in vivo models. The balance between the different Ikaros isoforms via posttranscriptional regulation may actively participate in the control of stem cell commitment and differentiation, particularly at the point where the myeloid and lymphoid pathways diverge.
The authors thank Dr Françoise Pflumio (INSERM U474, Paris, France) for her help in establishing the NOD-SCID model and the MS-5 culture system and Patrick Gibier at the animal facility (INSERM U119) for his care of NOD-SCID mice. We also thank Rémy Galindo at the flow cytometry facility, Jean Michel Durey at the iconography facility, personnel at the radiation therapy department, and personnel at the cell therapy facility, all at Institut Paoli-Calmettes, for their support during the conduct of this work. The authors thank Laurent Humeau, Boris Calmels, and Patrice Dubreuil for critical reading of the manuscript.
Supported in part by Institut Paoli-Calmettes, grant no. 9903 from the Association pour la Recherche sur le Cancer to C.T., and a grant from the Fondation de France/Fondation contre la Leucémie to C.C.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cécile Tonnelle, Centre de Thérapie Cellulaire et Génique, Institut Paoli-Calmettes, CRLCC Provence-Alpes-Côte d'Azur; 232, Blvd Ste Marguerite 13273 Marseille CEDEX 9, France; e-mail:tonnellec@marseille.fnclcc.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal