Abstract
Mature dendritic cells (DCs), in addition to providing costimulation, can define the Th1, in contrast to the Th2, nature of a T-cell response through the production of cytokines and chemokines. Because calcium signaling alone causes rapid DC maturation of both normal and transformed myeloid cells, it was evaluated whether calcium-mobilized DCs polarize T cells toward a Th1 or a Th2 phenotype. After human monocytes were cultured for 24 hours in serum-free medium and granulocyte-macrophage colony-stimulating factor to produce immature DCs, additional overnight culture with either calcium ionophore (CI) or interferon γ (IFN-γ), tumor necrosis factor-α (TNF-α), and soluble CD40L resulted in phenotypically mature DCs that produced interleukin-8 (IL-8) and displayed marked expression of CD80, CD86, CD40, CD54, CD83, DC-LAMP, and RelB. DCs matured by IFN-γ, TNF-α, and soluble CD40L were additionally distinguished by undetectable CD4 expression, marked secretion of IL-12, IL-6, and MIP-1β, and preferential ability to promote Th1/Tc1 characteristics during T-cell sensitization. In contrast, DCs matured by CI treatment were distinguished by CD4 expression, modest or absent levels of IL-12, IL-6, and MIP-1β, and preferential ability to promote Th2/Tc2 characteristics. Calcium signaling selectively antagonized IL-12 production by mature DCs activated with IFN-γ, TNF-α, and soluble CD40L. Although the activation of DCs by calcium signals is largely mediated through calcineurin phosphatase, the inhibition of IL-12 production by calcium signaling was independent of this enzyme. Naturally occurring calcium fluxes in immature DCs, therefore, negatively regulate Dc1 differentiation while promoting Dc2 characteristics and Th2/Tc2 polarization. Calcium-mobilized DCs may have clinical usefulness in treating disease states with excessive Th1/Tc1 activity, such as graft-versus-host disease or autoimmunity.
Introduction
Dendritic cells (DCs) are responsible for priming naive T lymphocytes.1-4 Immature DCs reside in peripheral tissues and sample environmental antigens. After exposure to “danger” signals or to various inflammatory signals, such as interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), or lipopolysaccharide, DCs terminate antigen acquisition and begin their maturation phase.5-7 Mature DCs with peptide antigens loaded in major histocompatibility complex (MHC) molecules then migrate to T-cell areas in lymph nodes, where they receive additional maturation signals from T cells through CD40L8-10—resulting in further increases in costimulatory molecule expression—and where they present antigens to CD4+ and CD8+ T cells.3 11
T-cell–dependent immune responses are often polarized in favor of T cells that secrete high levels of interferon γ (IFN-γ) (so-called Th1 cells) or cells that secrete high levels of IL-4, IL-5, and sometimes IL-10 (Th2 cells). This bias can have profound implications on whether the immune response will control or exacerbate a given type of infection, and it appears to play a role in effective antitumor responses in which Th1-type immunity appears desirable.12-14 Recently, DCs have been shown to deliver signals that steer Th1/Th2 development. It has been reported that plasmacytoid CD11b−11c− CD4+ DCs are considered DC2 and when they present antigens appear to favor Th2 development,15-17 whereas myeloid derived CD11b+11c+ CD33+ DCs are DC1 and steer Th1 development, suggesting that lineage itself may bias DC differentiation.16 However, other reports indicate that environmental signals delivered to DCs during maturation can also alter the polarization of the T-cell response, regardless of lineage.8,18-21 It has been shown that myeloid-derived DCs, when exposed to prostaglandin E2(PGE2), cholera toxin, or nematode-derived products, can bias T cells toward Th2 development.18-20 In contrast, myeloid DCs exposed to lipopolysaccharide or to viral-derived proteins during maturation favor Th1 development.8 21
The induction of Th2 responses by DC2 may play a critical role in several immunologically mediated disease states. DC2 may activate Th2 cells that can prevent or decrease graft-versus-host disease (GVHD),22,23 play a role in regulating autoimmunity,24,25 and be involved in preventing the rejection of solid organ transplants.26Cytokines such as granulocyte colony-stimulating factor (G-CSF) appear to increase DC2 precursor cells in the peripheral blood of patients undergoing allogeneic transplantation, which may result in decreased GVHD.27 28 It would be useful to explore the ability to regulate DC2 activity for the treatment of diseases thought to be mediated by excessive Th1 polarization, such as GVHD and other autoimmune diseases.
We have previously shown that calcium signaling results in the development of mature DCs29,30 that express high levels of costimulatory molecules and CD83 and that present antigens to naive T cells. Calcium signaling in myeloid precursor cells and chronic myeloid leukemia cells also induces activation into mature DCs.31,32 Many signal-transducing pathways, such as Fc receptor or complement receptor binding, lead to calcium fluxes within monocytes and macrophages33 through the calcium capacitance channel34-36 or from mobilization from intracellular stores. Calcium signaling, therefore, appears to be highly integrated into the regulation of DC activity. In the current study we sought to determine whether calcium signaling in mature myeloid-derived DCs differentially regulates a T-cell response toward Th1 or Th2 development. We report here that calcium signaling can promote mature DC2 that induces Th2/Tc2-type responses and that selectively antagonizes the induction of IL-12 production by DC1-promoting agents.
Materials and methods
Preparation of human peripheral blood mononuclear cell fractions
Healthy donors provided informed consent and underwent leukapheresis on a Fenwal CS3000 apparatus (Baxter, Deerfield, IL) to obtain a neutrophil-depleted fraction of peripheral blood mononuclear cell from 5 to 7 L blood.21 Then 4 to 10 × 109 peripheral blood mononuclear cells were applied to countercurrent centrifugal elutriation using a model J-6M centrifuge (Beckman Instruments, Palo Alto, CA) equipped with a JE-5.0 elutriation rotor operating at 1725g and 20°C.21Fractions were eluted with calcium/magnesium-free Hanks balanced salt solution (Ca++/Mg++-free HBSS buffer; Biofluids, Rockville, MD) with flow rates increasing from 120 to 200 mL/min to obtain lymphocyte-rich (120-140 mL/min and monocyte-rich (150-190 mL/min) fractions. Red blood cell contamination of the 120- to 140-lymphocyte–fraction was reduced by a Ficoll-Hypaque (Organon Teknika, Durham, NC) density-gradient separation. Elutriated fractions were cryopreserved in 10% dimethyl sulfoxide–90% pooled human AB serum until use.
Reagents and antibodies
Monoclonal antibodies used in cell surface analysis—including fluorescein (FITC)– or phycoerythrin (PE)–conjugated mouse anti–human CD3, CD4, CD11b, CD11c, CD14, CD20, CD56, CD80 (B7.1), and CD54 (ICAM-1)—and immunoglobulin G subclass-matched control antibodies were purchased from Becton Dickinson (Mountain View, CA). PE-conjugated mouse anti–human CD86 (B7.2) and purified mouse anti–human CD45RO, CD3, and CD28 were obtained from Pharmingen (San Diego, CA). Anti–human CD83 was purchased from Serotec (Raleigh, NC). Anti–DC-LAMP was obtained from Immunotech (Marseilles, France). Recombinant human cytokines IL-4, IL-12, granulocyte-macrophage CSF (GM-CSF), TNF-α, and IFN-γ were purchased from Pharmingen. Monocyte cultures were maintained in macrophage–serum-free medium (SFM) (Life Technologies, Grand Island, NY) supplemented with 50 ng/mL GM-CSF. Allogeneic T-cell stimulations were performed in RPMI 1640 medium supplemented with 5% pooled human AB serum (BioWhittaker, Walkersville, MD). Calcium ionophore, A23187, and cyclosporin A (CSA) were purchased from Sigma (St Louis, MO), and soluble CD40 ligand (CD40L) was the generous gift of the Immunex Corporation (Seattle, WA.)
Preparation of T-lymphocyte subsets
One hundred twenty to 140 lymphocyte-rich elutriation fractions were used to prepare CD4+ CD45RO− T cells using human T-cell subset columns (R&D Systems, Minneapolis, MN) by quantitative negative-depletion techniques, as recommended by the manufacturer. CD8+ T cells from HLA A2.1+donors were isolated through the same technique using the appropriate columns.
Monocyte cultures
One hundred fifty to 190 monocyte-rich fractions were used fresh or thawed and were plated at 1.5 × 106 cells/mL in either 24- or 48-well Costar cluster plates (Corning, Corning, NY). The cells were cultured in macrophage–monocyte medium supplemented with 50 ng/mL GM-CSF. After overnight culture (14-24 hours) in GM-CSF, the cells were matured as follows: either CI (150 ng/mL) alone was added to the culture for an additional 20 hours or IFN-γ (1000 U/mL) and TNF-α (10 ng/mL) were added and maturation was completed 6 hours later with the addition of CD40L at 1 μg/mL. Cells were cultured for an additional 14 hours. Activated DCs from both groups were harvested 40 hours after their initial placement in culture. Cells were harvested for coculture and fluorescence-activated cell sorter (FACS) analysis, and supernatants were harvested for enzyme-linked immunosorbent assay (ELISA) 14 to 24 hours after the addition of CI or CD40L. DCs were washed 2 to 3 times before coculture with T cells. In experiments in which IL-12 was added to DCs during activation with CI, the DCs were washed 3 times before coculture with T cells.
Cell surface FACS analysis
Elutriated fraction cells were analyzed by fluorescence multicolor flow cytometry (FACScan; Becton Dickinson, Mountain View, CA). Cells were stained at 4°C using Ca++/Mg++-free HBSS with 1% fetal bovine serum and 0.1% sodium azide as a diluent/wash buffer. Cells were preincubated with unconjugated goat γ-globulin (Sigma Chemical) for 10 minutes to block Fc receptor binding and, in most cases, were double stained with PE- and FITC-conjugated antibodies for 30 minutes. After washing with cold buffer, propidium iodide (PI) was added to distinguish viable from nonviable cells, and 3-color analysis was performed using a 5-W argon laser emitting 200 mW of 488-nm light. Simultaneous measurement of fluorescein, PE, and PI emission was made using 530, 575, and 650 filters with acquisition in logarithmic mode. High PI-staining, nonviable cell events were acquired, but they were eliminated from final analysis. For staining with anti-DC LAMP, the cells were permeabilized in Cytofix/Cytoperm (Pharmingen) solution, stained with anti-DC LAMP, and FITC-labeled with goat anti–mouse antibody.
Antigen uptake in immature dendritic cells
Cells from the monocyte-rich fractions of elutriation were cultured in SFM with GM-CSF as described above. At the time points of interest, the cells were pulsed with 1 mg/mL FITC-albumin (Sigma). After 2 hours of incubation at either 37°C or 4°C, the cells were washed 3 times with buffer and analyzed by flow cytometry. The difference in mean channel fluorescence between the groups at 37°C and 4°C was used as a measure of uptake by macropinocytosis. T and B cells were used as a negative control.
Evaluation of RelB expression
Dendritic cells were pelleted and lysed in a buffer containing 10 mM Tris (pH 7.4), 220 mM NaCl, 30 mM sodium phosphate, 50 mM NaF, 5 mM ZnCl2, 1% Triton X-100, and protease inhibitor mixture (Roche Molecular Biochemicals, Indianapolis, IN) supplemented with additional protease inhibitor α2-macroglobulin (1.25 U/mL; Roche Molecular Biochemicals). The extract was incubated on ice for 10 minutes and centrifuged at 500g for 15 minutes at 4°C; the supernatant constituted the whole cellular extract. Pelleted nuclei were extracted with 30 to 50 μL solution containing 10 mM Tris (pH 7.0), 220 mM NaCl, 30 mM sodium phosphate, 50 mM NaF, 5 mM ZnCl2, 0.05% Nonidet P40, and protease inhibitors at 4°C for 20 minutes with agitation. The extract was centrifuged at 15 000g for 15 minutes at 4°C; the supernatant constituted the nuclear extract. Protein concentrations were measured by using the Bio-Rad (Hercules, CA) protein assay using a bovine serum albumin standard. Protein (5-10 mg) was resolved by 10% tricine SDS-PAGE (Invitrogen, Carlsbad, CA) and transferred to Immobilon-P (Millipore, Bedford, MA). The membrane was probed with anti-relB antiserum 131937 and antiactin monoclonal antibody (Chemicon, Temecula, CA). Immunoreactive proteins were revealed with an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Arlington Heights, IL).
Allosensitization of CD4+ CD45RO−T cells
CD4+ CD45RO− T cells were cocultured with CI or IFN-γ–, TNF-α–, and CD40L-treated allogeneic antigen-presenting cells in 48-well flat bottom tissue culture plates at a ratio of 1 × 106 T cells to 1 × 105 antigen-presenting cells (ratio, 10:1) in the absence of any added cytokines. After 6 days, T cells were harvested and restimulated on anti-CD3, anti-CD28–coated 96-well plates at 1 × 105 cells/well. Supernatants were harvested 24 hours later and analyzed by ELISA.
ELISA assays
Capture and biotinylated detection antibodies and standards for IFN-γ, IL-4, IL-5, IL-6, IL-8, IL-10, and IL-12p70 ELISAs were purchased from Pharmingen and used according to the manufacturer's recommended protocols. Reagents for MIP-1β ELISA were purchased from R&D Systems and used according to the manufacturer's protocols.
Intracellular cytokine staining
After primary allosensitization as described above, T cells were harvested and restimulated with phorbol 12-myristate-13-acetate (50 ng/mL) and calcium ionophore (1 μg/mL) for 6 hours in the presence of monensin (2 μM). Cells were then harvested, fixed, and permeabilized in Cytofix/Cytoperm (Pharmingen) solution and stained with FITC–anti–IFN-γ according to the manufacturer's recommendations. Flow cytometric acquisition was performed as described above with the omission of PI gating.
CD8+ T-cell cocultures
Dendritic cells from healthy donors used in CD8+T-cell cocultures were pulsed with MART-1 peptide 27-35 at 50 ng/mL (National Cancer Institute, Bethesda, MD) 2 hours before harvest. They were harvested, washed with fresh media, and replated with lymphocytes at a T cell-to-DC ratio of 20:1, with recombinant human IL-2 IU/mL was added once. After 1 week, the T cells were harvested and restimulated with relevant (Mel624 A2+) and irrelevant control (Mel624 A2−, MW115) cell lines. Cell lines used were Mel 624 A2+, Mel 624 A2−, and MW115 and were obtained from Dr Steven A. Rosenberg (National Cancer Institute). Supernatants were harvested after 24 hours and analyzed by ELISA.
Results
Rapid development of immature dendritic cells from monocytes
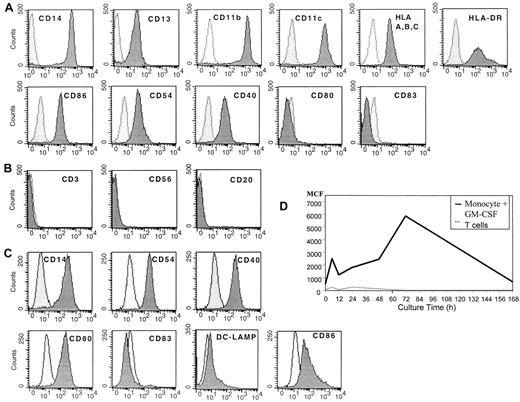
Before culture monocytes were initially CD14+, CD13+, CD11b+, and CD11c+, expressed MHC class 1 and class 2 molecules, and demonstrated some expression of CD86, CD54, and CD40, but they were CD80−and CD83− (Figure 1A). In addition, the cells lacked CD3, CD56, and CD20, suggesting that they were a relatively pure population of myeloid monocytes (Figure 1B). When these cells were cultured for 20 to 24 hours in SFM with GM-CSF, the cells had diminished expression of CD14 and increased expression of CD54 and CD40 (Figure 1C). The cells also expressed some CD80 and CD86 but had minimal expression of DC-LAMP or CD83 (Figure 1C). These DCs demonstrated increased uptake of antigen by macropinocytosis, as demonstrated by the uptake of FITC-labeled human albumin, suggesting they were immature DCs (Figure 1D).
Flow cytometric analysis of human peripheral blood monocytes obtained after leukapheresis and countercurrent centrifugation before and after cell culture.
(A) Fresh, uncultured monocytes. (B) Purity of monocytes prepared by combined leukapheresis elutriation. (C) Monocytes cultured in SFM in GM-CSF for 20 to 24 hours, then analyzed by flow cytometry. (D) Macropinocytosis by immature DCs prepared by culture in SFM and GM-CSF over time in culture. Results are representative of 3 experiments.
Flow cytometric analysis of human peripheral blood monocytes obtained after leukapheresis and countercurrent centrifugation before and after cell culture.
(A) Fresh, uncultured monocytes. (B) Purity of monocytes prepared by combined leukapheresis elutriation. (C) Monocytes cultured in SFM in GM-CSF for 20 to 24 hours, then analyzed by flow cytometry. (D) Macropinocytosis by immature DCs prepared by culture in SFM and GM-CSF over time in culture. Results are representative of 3 experiments.
Similar expression of CD83, RelB, and costimulatory molecules on mature dendritic cells
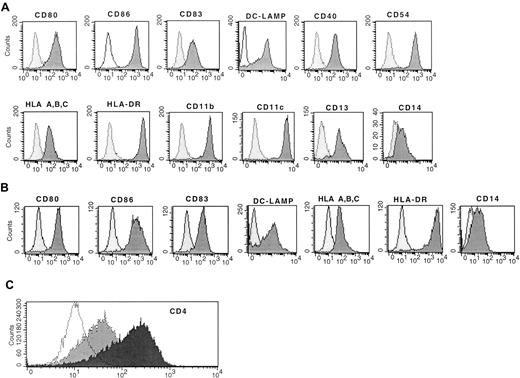
Immature DCs (monocytes cultured overnight in SFM and GM-CSF) treated with 150 ng/mL CI resulted in rapid increased expression of CD80, CD86, CD83, DC-LAMP, CD40, and CD54, which was evident within 20 hours of the addition of CI or 40 hours from initiation of the cultures (Figure 2A). In addition, there was increased expression of MHC class 1 and class 2 molecules (Figure 2A). There was also high-level expression of CD4+ (Figure 2C) and continued uniform expression of CD11b, CD11c, and CD13 with low CD14 expression (Figure 2A). This phenotype is consistent with that of mature DCs. Typically, cell yields of CI-treated DCs were approximately half those of plated cells for cryopreserved cells and 75% to 80% of plated cells for fresh monocytes, similar to the yields of immature DCs, suggesting the entire population acquired a mature DC phenotype, as we had previously demonstrated.29
Flow cytometric analysis of mature activated DCs.
(A) Expression of MHC class 1 class 2, costimulatory molecules, CD83, and DC-LAMP on immature DCs treated with CI alone. (B) Mature DCs treated with IFN-γ, TNF-α, and CD40L. (C) Expression of CD4 on mature DCs treated with CI (black) or IFN-γ, TNF-α, and CD40L (gray). The dotted histogram is the isotype control. Results are representative of 10 experiments.
Flow cytometric analysis of mature activated DCs.
(A) Expression of MHC class 1 class 2, costimulatory molecules, CD83, and DC-LAMP on immature DCs treated with CI alone. (B) Mature DCs treated with IFN-γ, TNF-α, and CD40L. (C) Expression of CD4 on mature DCs treated with CI (black) or IFN-γ, TNF-α, and CD40L (gray). The dotted histogram is the isotype control. Results are representative of 10 experiments.
Immature DCs (monocytes cultured in SFM and GM-CSF overnight) cultured with IFN-γ, TNF-α, and CD40L also demonstrated a mature DC phenotype similar to that of DCs activated with CI (Figure 2B). There was high-level expression of CD83, DC-LAMP, CD80, CD86, and MHC class 1 and class 2 molecules, with low expression of CD14 (Figure 2B). The expression of these cell surface molecules was noted also within 40 hours of culture (Figure 2B). Therefore, the kinetics of maturation was similar for CI-activated or for IFN-γ–, TNF-α–, and CD40L-activated DCs. Despite similar expression of CD83, DC-LAMP, CD80, and CD86, the IFN-γ ΤΝF-α, CD40L-treated DCs demonstrated minimal CD4 surface expression, which was in contrast to cells treated with CI (Figure 2).
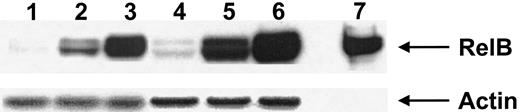
Transcription factors of the Rel/NFκB family, including p50, p52, and RelB, are expressed by and play an important role in the biologic function of DCs.38 Calcium-signaling agents have been shown to up-regulate RelB in mature DCs37; we therefore evaluated the expression of RelB by both calcium-activated DCs and those activated with IFN-γ, TNF-α, and CD40L. Both calcium-activated DCs and those activated with IFN-γ, TNF-α, and CD40L expressed similarly high levels of RelB (Figure3). This expression of RelB was much greater than that seen in immature DCs treated with GM-CSF (Figure 3).
Expression of RelB by activated DCs.
Nuclear cell extracts from 2 donors (donor 1 lanes 1-3, donor 2 lanes 4-6) were immunoblotted with anti-RelB antiserum 1319, as described in “Materials and methods.” Lanes 1 and 4, immature DCs (GM-CSF–treated); lanes 2 and 5, DCs activated with calcium ionophore; lanes 3 and 6, DCs activated with IFN-γ, TNF-α, and CD40L. Lane 7 represents the control for RelB expression. Antiactin was used to ensure similar levels of protein loading. Results are representative of a single experiment with 3 other experiments demonstrating similar results.
Expression of RelB by activated DCs.
Nuclear cell extracts from 2 donors (donor 1 lanes 1-3, donor 2 lanes 4-6) were immunoblotted with anti-RelB antiserum 1319, as described in “Materials and methods.” Lanes 1 and 4, immature DCs (GM-CSF–treated); lanes 2 and 5, DCs activated with calcium ionophore; lanes 3 and 6, DCs activated with IFN-γ, TNF-α, and CD40L. Lane 7 represents the control for RelB expression. Antiactin was used to ensure similar levels of protein loading. Results are representative of a single experiment with 3 other experiments demonstrating similar results.
Calcium signaling inhibits IL-12 production by dendritic cells
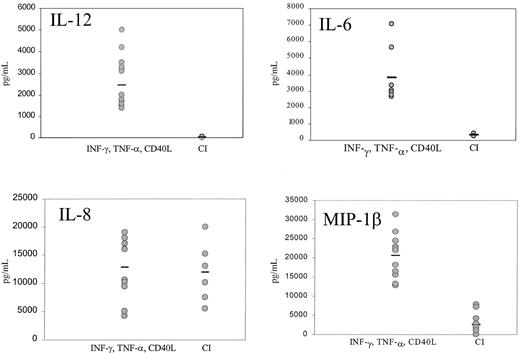
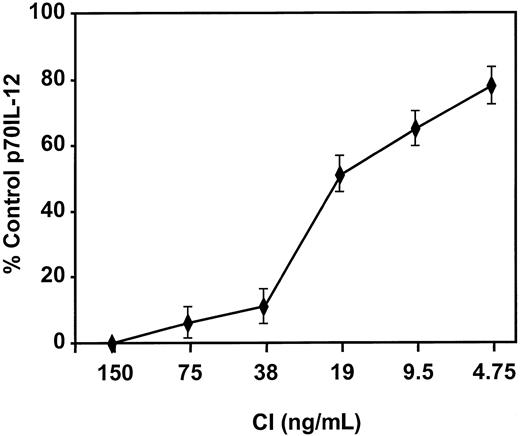
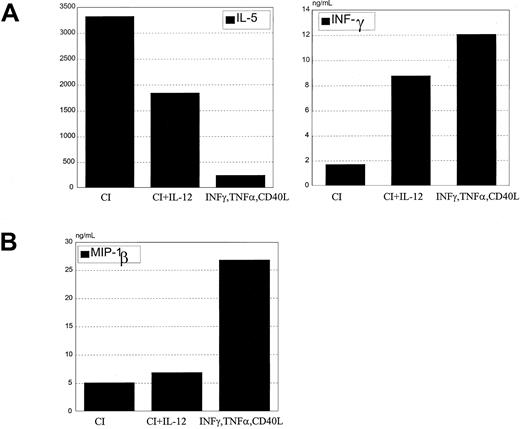
Mature DCs activated with CI produced high levels of IL-8 and low levels of MIP-1β, with no significant production of p70 IL-12 or IL-6 (Figure 4). Kinetic studies demonstrated no production of IL-12 from CI-activated DC at any point from 4 hours to 48 hours (not shown). In contrast, DCs activated with IFN-γ, TNF-α, and CD40L produced not only high quantities of IL-8 but high levels of p70 IL-12 (1000-5000 pg/mL), IL-6 (2500-7000 pg/mL), and MIP-1β (Figure 4). Calcium ionophore inhibited IL-12 production by DCs treated with IFN-γ, TNF-α, and CD40L (Figure5) in a dose-dependent fashion to concentrations as low as 9 ng/mL. CI added even as late as 6 hours after the activation of DCs with IFN-γ, TNF-α, and CD40L resulted in more than 50% inhibition of IL-12 production (not shown). In contrast, calcium signaling did not inhibit the production of MIP-1β by DCs treated with IFN-γ, TNF-α, and CD40L (not shown).
Production of cytokines by mature DCs.
IFN-γ–, TNF-α–, and CD40L-treated DCs or CI-treated mature DCs were cultured for 14 to 24 hours, the supernatants were harvested, and IL-12, IL-6, IL-8, and MIP-1β production were measured by ELISA. Each point represents a different experiment from 5 different donors. Bar represents the median production.
Production of cytokines by mature DCs.
IFN-γ–, TNF-α–, and CD40L-treated DCs or CI-treated mature DCs were cultured for 14 to 24 hours, the supernatants were harvested, and IL-12, IL-6, IL-8, and MIP-1β production were measured by ELISA. Each point represents a different experiment from 5 different donors. Bar represents the median production.
Calcium signaling inhibits IL-12 production by DCs.
DCs were activated with IFN-γ, TNF-α, and CD40L in the presence of the indicated concentrations of CI, and IL-12 p70 production was measured by ELISA. Levels of p70 IL-12 production by DCs activated with IFN-γ, TNF-α, and CD40L were 2000 to 3000 pg/mL. Results are based on triplicate cultures from a single representative experiment with SEM. Three other experiments demonstrated similar results.
Calcium signaling inhibits IL-12 production by DCs.
DCs were activated with IFN-γ, TNF-α, and CD40L in the presence of the indicated concentrations of CI, and IL-12 p70 production was measured by ELISA. Levels of p70 IL-12 production by DCs activated with IFN-γ, TNF-α, and CD40L were 2000 to 3000 pg/mL. Results are based on triplicate cultures from a single representative experiment with SEM. Three other experiments demonstrated similar results.
Calcium-signaling–induced inhibition of IL-12 production is not mediated through calcineurin phosphatase
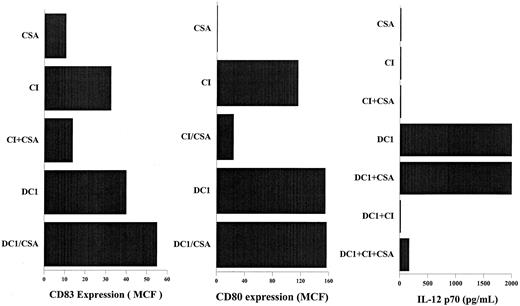
We have previously reported that calcium-signaling induced activation of DCs is mediated through the activation of calcineurin phosphatase and is inhibited by CSA.30,39 Addition of CSA to DCs activated with IFN-γ, TNF-α, and CD40L had no effect on the expression of CD83, CD80, or IL-12 secretion by the activated DCs (Figure 6). In contrast, DCs activated with calcium-signaling agents in the presence of CSA demonstrated significantly decreased expression of CD80 and CD83 (Figure 6). CSA also did not reverse the inhibition of IL-12 secretion by DCs activated with IFN-γ, TNF-α, and CD40L in the presence of CI (Figure 6), suggesting that the inhibition of IL-12 secretion by calcium-signaling agents is not mediated through calcineurin phosphatase, as is the activation of DCs by calcium-signaling agents.30 39
CSA inhibits DC activation by calcium ionophore but not the inhibition of IL-12 production caused by calcium ionophore.
DCs were activated as indicated for 40 hours in the presence or absence of 1 μM CSA. DC1, immature DCs treated with IFN-γ, TNF-α, and rhCD40L. CI, immature DCs treated with 150 ng/mL calcium ionophore, as described in “Materials and methods.” CD83 and CD80 expression were measured by flow cytometry using FITC- labeled anti–human CD83 and CD80, as described in “Materials and methods.” Secretion of p70 IL-12 was measured 24 hours after the activation of DCs using ELISA. Results are those of a representative experiment; 3 other experiments demonstrated similar results.
CSA inhibits DC activation by calcium ionophore but not the inhibition of IL-12 production caused by calcium ionophore.
DCs were activated as indicated for 40 hours in the presence or absence of 1 μM CSA. DC1, immature DCs treated with IFN-γ, TNF-α, and rhCD40L. CI, immature DCs treated with 150 ng/mL calcium ionophore, as described in “Materials and methods.” CD83 and CD80 expression were measured by flow cytometry using FITC- labeled anti–human CD83 and CD80, as described in “Materials and methods.” Secretion of p70 IL-12 was measured 24 hours after the activation of DCs using ELISA. Results are those of a representative experiment; 3 other experiments demonstrated similar results.
Calcium-signaling–activated dendritic cells induce Th2 cells
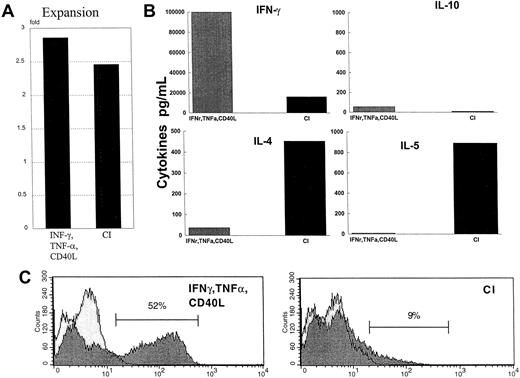
CI-activated DCs, when cocultured with naive CD45RA allogeneic CD4+ T cells, stimulated the T cells to proliferate significantly, as evidenced by a 3-fold expansion (Figure7A). This was similar to DCs activated with IFN-γ, TNF-α, and CD40L (Figure 7A). CI-activated DCs induced the CD4+ T cells to secrete high levels of IL-4 (400-800 pg/mL) and IL-5 (900-3,000 pg/mL), relatively low levels of IFN-γ, and no IL-10 (Figure 7B) when the T cells were restimulated. In contrast, DCs activated with IFN-γ, TNF-α, and CD40L stimulated high levels of IFN-γ production (60 000-100 000 pg/mL) by the CD4+ T cells, with minimal secretion of IL-4, IL-5, or IL-10 at restimulation (Figure 7B). In addition, intracellular cytokine analysis of IFN-γ demonstrated 52% (average, 51%; range, 47%-53%) of the CD4+ T cells cultured with DCs treated with IFN-γ, TNF-α, and CD40L-secreted IFN-γ, whereas only 9% (average, 11%; range, 6%-14%) of the CD4+ T cells induced by CI were positive for intracellular IFN-γ (Figure 7C). When DCs were activated with IFN-γ, TNF-α, and CD40L in the presence of calcium-signaling agents and then were cocultured with naive CD4+ T cells, the T cells secreted high levels of IL-4 and IL-5 with lower level production of IFN-γ (not shown).
Cytokine production by naive CD45RA CD4+ allogeneic T cells.
(A) CD4+ T cells were cocultured for 6 days with either IFN-γ–, TNF-α–, and CD40L-treated DCs or with CI-treated mature DCs. On day 6, the T cells were harvested, washed, and restimulated on plates coated with anti-CD3 and anti-CD28 for 24 hours. Supernatants were harvested and analyzed by ELISA for the production of IL-4, IL-5, IL-10, or IFN-γ. Results are those of a representative experiment of 6 different experiments. The letter A indicates the fold expansion from initial starting number of T cells. (B) Production of cytokines by restimulated CD4+ T cells. (C) Intracytoplasmic staining of IFN-γ by naive CD4+ T cells primed by IFN-γ–, TNF-α–, and CD40L-treated DCs or CI-treated mature DCs, as described in “Materials and methods.”
Cytokine production by naive CD45RA CD4+ allogeneic T cells.
(A) CD4+ T cells were cocultured for 6 days with either IFN-γ–, TNF-α–, and CD40L-treated DCs or with CI-treated mature DCs. On day 6, the T cells were harvested, washed, and restimulated on plates coated with anti-CD3 and anti-CD28 for 24 hours. Supernatants were harvested and analyzed by ELISA for the production of IL-4, IL-5, IL-10, or IFN-γ. Results are those of a representative experiment of 6 different experiments. The letter A indicates the fold expansion from initial starting number of T cells. (B) Production of cytokines by restimulated CD4+ T cells. (C) Intracytoplasmic staining of IFN-γ by naive CD4+ T cells primed by IFN-γ–, TNF-α–, and CD40L-treated DCs or CI-treated mature DCs, as described in “Materials and methods.”
Calcium-activated dendritic cells stimulate TC2 cells
MART-1 27-35 is an immunodominant peptide derived from MART-1/MelanA melanoma tumor antigen that is presented on the HLA-A2.1 binding domain.40 When CI-activated DCs from healthy donors were pulsed with MART-1 and were cocultured with autologous CD8+ T cells at restimulation with tumor cells expressing MART-1, the CD8+ T cells secreted predominantly IL-5 (Figure 8). In contrast, CD8+T cells sensitized with DCs activated with IFN-γ, TNF-α, and CD40L secreted high levels (6-10 ng/mL) of IFN-γ when the T cells were restimulated with melanoma cell lines (Figure 8). The amount of IFN-γ secreted by the MART-1–specific CD8+ T cells stimulated by peptide-pulsed IFN-γ–, TNF-α–, and CD40L-activated DCs was usually on the order of 10- to 20-fold greater release than CD8+ T cells generated by CI-pulsed DCs.
CI-activated DCs stimulate TC2 anti-MART CD8+ T cells.
Purified CD8+ T cells were cocultured with either IFN-γ–, TNF-α–, and CD40L-treated DCs or CI-treated mature DCs for 7 days. T cells were harvested, washed, and cocultured with melanoma cells expressing MART-1, 624mel A2+ (▨), 624mel MART-1+ A2− (▪), and non–MART-1 cell line MW115 (■) for 24 hours. Supernatants were then harvested and analyzed for IL-5 or IFN-γ production by ELISA. Results are the average of 3 different experiments with the SEM.
CI-activated DCs stimulate TC2 anti-MART CD8+ T cells.
Purified CD8+ T cells were cocultured with either IFN-γ–, TNF-α–, and CD40L-treated DCs or CI-treated mature DCs for 7 days. T cells were harvested, washed, and cocultured with melanoma cells expressing MART-1, 624mel A2+ (▨), 624mel MART-1+ A2− (▪), and non–MART-1 cell line MW115 (■) for 24 hours. Supernatants were then harvested and analyzed for IL-5 or IFN-γ production by ELISA. Results are the average of 3 different experiments with the SEM.
Calcium-activated dendritic cells retain DC2 activity in the presence of exogenous IL-12
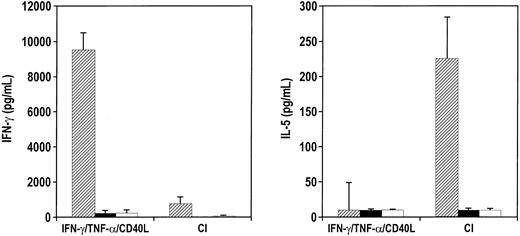
When exogenous IL-12 was added with calcium ionophore to DC cultures during maturation and was washed out before coculture with T cells, the cytokine secretion profile of naive allogeneic CD4+ T cells was characterized by increased IFN-γ and decreased IL-5 (Figure 9A). A corresponding shift in chemokine secretion was not seen. Despite IL-12 treatment, these DCs produced only low levels of MIP-1β that was not increased with the addition of IL-12 (Figure 9B).
IL-12 added to CI-activated DCs does not inhibit Th2 development.
(A) CI-activated DCs were cocultured with 1 ng/mL rhIL-12 for 24 hours, washed, and cocultured with allogeneic CD45RA CD4+ T cells for 6 days. T cells were harvested and restimulated with anti-CD3 and anti-CD28 for 24 hours, the supernatants were harvested, and IL-5 and IFN-γ production were measured by ELISA. (B) CI-activated DCs were treated with rhIL-12 for 24 hours, and MIP-1β production was measured by ELISA in the harvested supernatants. Results are representative of 5 different experiments.
IL-12 added to CI-activated DCs does not inhibit Th2 development.
(A) CI-activated DCs were cocultured with 1 ng/mL rhIL-12 for 24 hours, washed, and cocultured with allogeneic CD45RA CD4+ T cells for 6 days. T cells were harvested and restimulated with anti-CD3 and anti-CD28 for 24 hours, the supernatants were harvested, and IL-5 and IFN-γ production were measured by ELISA. (B) CI-activated DCs were treated with rhIL-12 for 24 hours, and MIP-1β production was measured by ELISA in the harvested supernatants. Results are representative of 5 different experiments.
Discussion
Multiple studies have suggested that monocytes can serve as a reservoir for human DCs.41-43 Monocytes cultured in vitro in serum-containing medium (eg, fetal calf serum or human serum) develop characteristics of immature DCs after 6 or 7 days.4,43,44 In contrast to such lengthy culture activation, monocytes can also acquire characteristics of DCs more rapidly.45 Randolph et al46 have shown, using an endothelial reverse-transmigration model, that monocytes crossing an endothelial monolayer and contacting particulate collagen matrix can acquire characteristics of DCs in as few as 2 days. It appears that this system may model monocyte extravasation, entry into peripheral tissues, and phagocytosis of particulate matter that forms part of the normal DC maturation process. Similarly, in the current study, monocytes cultured in SFM down-regulate their CD14 expression and acquire characteristics of immature DCs, such as increased ability to take up antigen within 40 hours of culture initiation. Because interstitial fluids, in which postextravasation DC precursors would find themselves, differ from serum in protein and ionic content, it may be possible that removal from serum is one of many cues that sensitize these cells to additional signals that trigger the rapid acquisition of mature DC characteristics. This would explain why we see more rapid differentiation and maturation from CD14+ precursors cultured in SFM than from similar treatments in serum-containing medium.
We have previously documented that calcium signaling in human monocytes results in the rapid development of immunologically activated DCs.29 30 These DCs express high levels of the costimulatory molecules B7.1 and B7.2 and high levels of the DC activation molecule CD83. The level of costimulatory molecule expression on DCs activated with CI was similar to that on DCs treated with IFN-γ, TNF-α, and CD40L. However, as shown in the current report, calcium-activated DCs secrete no IL-12 or IL-6 and minimal MIP-1β, and they stimulate both CD4+ and CD8+T cells to secrete IL-4 and IL-5 with little production of IFN-γ, consistent with T-cell polarization to Th2 and Tc2, respectively.
Other signaling agents such as cholera toxin,20 a nematode-derived phosphorylcholine glycoprotein,19 and PGE have been shown to activate DCs that direct Th2 development. Calcium signaling can be added to this list of agents that induce the maturation of myeloid-derived DCs that favor Th2 priming. Each of these agents has the ability to inhibit IL-12 production by DCs,19,20,47 cytokines critical for Th1 polarization.48 There are, however, some cytokine secretion differences among these agents. For example, calcium signaling induces DCs to secrete IL-8, whereas cholera toxin, which also induces DC maturation, does not.20 In addition, PGE2-activated DC2 secretes high levels of the chemokines MDC and TARC, but DCs activated with calcium-signaling agents do not secrete these Th2 chemokines (manuscript in preparation). This suggests that even among DC2 there can be subtle differences in cytokine or chemokine production that may appreciably modulate the developing immune response.
Several known signal receptor complexes can trigger calcium fluxes in monocytes and DCs.33,49 For instance, it has been shown that Fc receptor binding or complement receptor binding induces calcium fluxes within the cells.33 Calcium signaling has been shown by Sutterwala et al33 to diminish IL-12 production in monocytes; in the current study, calcium signaling inhibits IL-12 production by mature DCs. Hence, antigen-antibody complexes, or immune complexes binding to Fc or complement receptors and taken up on DCs, could be expected to favor Dc2 activity through the mobilization of intracellular calcium. Calcium signaling induced by monocyte chemoattractant protein-1 (MCP-1) in macrophages and monocytes49 also provides a mechanism by which to explain the ability of MCP-1 to inhibit IL-12 production and to induce T cells that secrete IL-4 when it is used as a vaccine adjuvant in the gastrointestinal tract.50 Therefore, calcium signaling may provide a common pathway for environmental pathogens and regulatory molecules to interact with activated DCs to regulate Th1 and Th2 cell development.
Plasmacytoid CD11b− CD11c− DCs express CD4,16 and the myeloid-derived CD11b+CD11c+ DCs activated with calcium-signaling agents in this study also express high levels of CD4 compared with myeloid-derived DCs activated with IFN-γ, TNF-α, and CD40L, which express minimal levels of CD4. CD4 interacts with MHC class 2 to stabilize the interaction between the APCs and the CD4+ T cells.51 52 The higher level expression of CD4 on DC2 may enable them to produce interactions between CD4+ T cells and MHC class 2+ B cells to induce antibody production. Similarly, CD4 expression may help DCs to better traffic to areas where MHC class 2+ B and T cells interact, such as germinal centers in lymph nodes.
Th2 and DC2 cells are thought to be involved in preventing GVHD in allogeneic transplantation,22,23,27 preventing autoimmunity,24,25 and preventing rejection in solid organ transplantation.26 The ability to activate monocytes into DC2 by simple ex vivo manipulation with calcium-signaling agents may make it possible to explore treating some of these diseases with DC2 vaccines. Calcium-signaling agents such as calcium ionophore may be more desirable than other DC2-activating agents such as cholera toxin and PGE2 because of the unavoidable antigenicity of cholera toxin and the variable effects of PGE2 on DC activation at different stages of maturation.53 54
The DC2 phenotype induced by calcium signaling is demonstrably stable because calcium-activated DCs exposed to exogenous IL-12 during maturation induce an increase in IFN-γ production by T cells, but there is still T-cell production of IL-4 and IL-5. Similarly, IL-12 does not increase secretion of the chemokine MIP-β by DCs, suggesting that IL-12 in the environment of maturing DCs may modulate Th2 cell responses, but the ultimate regulation of Th2 development may exist within the signals provided by mature DCs. This may account for the requirement for both anti–IL-4 and IL-12 to induce Th1 cell development.55
Transcription factors of the Rel/NF-κB family are expressed by and play an important role in the activation of DCs.38Although there was no difference in the expression of RelB between the DCs activated with calcium-signaling agents and those activated with IFN-γ, TNF-α, and CD40L, the signaling pathways used within the 2 types of DCs are different from those activated with calcium signaling using calcineurin phosphatase activation30,39 and those activated with IFN-γ–, TNF-α–, and CD40L-activated DCs using a calcineurin-independent pathway. Interestingly, the calcium-signaling inhibition of IL-12 production was not significantly associated with calcineurin phosphatase activation paralleling calcium-induced down-regulation of CD14 expression.30Calcium signaling can lead to the activation of arachidonic acid release and the production of prostaglandins such as E2,56,57 which has been shown to inhibit IL-12 production.47 Efforts to define the biochemical pathways leading to IL-12 inhibition are ongoing and have important clinical implications for potentially developing pharmacologic agents to suppress unwanted DC1 activity.
Signals other than IL-12 may be needed to induce a strong Th1 response. Th1 cells express the chemokine receptor CCR5 on their surfaces,58,59 and MIP-1β has been shown to be a chemokine that binds CCR5.59,60 Because high-level MIP-1β is produced by DCs activated with IFN-γ, this chemokine may play an essential role in attracting Th1 cells to be repetitively activated by DC1 to polarize the Th1 response. It has been shown that β-chemokine secretion by macrophage-derived DCs contributes directly to Th1 development.61 Therefore, compared with calcium signaling, differential MIP-1β production by DCs activated with IFN-γ, TNF-α, and CD40L may also play a role in the differential Th1 polarization seen between these 2 types of DCs.
Dendritic cells activated with INF-γ, TNF-α, and CD40L were extremely potent activators of Th1 and TC1 and induced extremely high levels of IFN-γ production by the T cells, which is consistent with the findings of Snijders et al62 and Vieira et al.47 Furthermore, the production of IL-12 by mature DCs may be limited to subsets of CD83+DCs,63 suggesting that mature DCs are indeed heterogeneous. Clearly, with multiple functional subsets of DCs, it may be possible to begin to define the relative roles of various DC subpopulations (DC1 vs DC2) on the development of antitumor CD4+ and CD8+ T-cell responses.12,13 64-66
In summary, calcium signaling in immature DCs results in the development of mature DCs that express high levels of CD83 and the costimulatory molecules, CD80 and CD86. These cells, when used as antigen-presenting cells, appear to favor the development of Th2 cells. Calcium signaling can also inhibit the production of IL-12 by DC1. Calcium signaling provides a common pathway for multiple environmental signals to be translated within DCs to induce Th2 development. DCs activated with calcium-signaling agents may have clinical usefulness in treating disease states with excessive Th1 activity.
We thank Dr Dupont Guerry for critical review of this manuscript. We thank the dedicated staff of the apheresis unit and the Cell Processing Center at the University of Pennsylvania. We also thank Kathy Pichak and Immunex for supplying rhCD40L.
Supported by American Cancer Society RPG-99-029-01.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Brian J. Czerniecki, Department of Surgery, University of Pennsylvania, 4 Silverstein, 3400 Spruce St, Philadelphia, PA 19104; e-mail: czerniec@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal