Abstract
During the cell transformation processes leading to erythroleukemia, erythroid progenitors often become erythropoietin (Epo)-independent for their proliferation. The biochemical events that could lead an erythroleukemic cell to growth factor–independence were investigated using spi-1 transgenic poerythroblasts. Spi-1/PU.1 is a myeloid and B-cell transcription factor of the ETS family and is activated by insertional mutagenesis during Friend erythroleukemia. Its overexpression in proerythroblasts induces their differentiation arrest without altering their erythropoietin requirement for proliferation (HS1 cells). At a later step, genetic alterations most probably occur allowingspi-1 transgenic poerythroblasts to proliferate in the absence of erythropoietin (HS2 cells). The signaling transduction pathways in HS1 and HS2 proerythroblasts were analyzed. The authors have previously shown that the Jak/STAT pathway was not activated in Epo-independent cells, but remained sensitive to Epo stimulation. In the present study, it is shown that the Epo-independent proliferation of HS2 cells requires active phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways. In these cells, PI3K was constitutively associated with the molecular adapters Grb2 and Gab1, and with the phosphatases SHP-2 and SHIP. Moreover, PI3K activity was correlated with the constitutive phosphorylation of serine-threonine protein kinase (AKT) in HS2 cells. Lastly, a constitutive activation of the MAPKs extracellular signal-regulated kinases (ERK1/2) in HS2 cells was observed that occurs in a PI3K-independent manner, but depends strictly on the activity of the protein kinase C (PKC). These results suggest that constitutive activations of PI3K/AKT and PKC/MAPK pathways can act in synergy to lead a proerythroblast to proliferate without Epo.
Introduction
Erythropoietin (Epo) is involved in the survival, proliferation, and differentiation of erythroid progenitor cells.1 The binding of Epo to its receptor (EpoR) induces the activation of a variety of downstream signaling pathways such as the Jak2/STAT5, the Shc/Ras/MAPK, and the PI3K/AKT (serine-threonine protein kinase) signaling cascades. Upon Epo stimulation the EpoR-associated Janus kinase 2 (Jak2)2 is activated and phosphorylates the transcriptional activator STAT5. Activation of this pathway could be involved in both proliferation3 and differentiation of the proerythroblast.4 The activation of the MAPKs' extracellular signal-related kinases (ERKs)5appears mainly involved in the proliferation of the erythroid cells, whereas activation of the stress-activated protein kinase (JNK/SAPK)6 and the p38 MAPK7 would be associated with Epo-induced differentiation.8 A third signal transduction pathway activated by Epo involves the PI3K. The p85 regulatory subunit of the PI3K becomes associated through its SH2 domains to a phosphorylated tyrosine of the EpoR,3 and the PI3K can induce activation of the cell survival protein AKT/PKB.9 p85 also associates with several tyrosine-phosphorylated molecular adapters such as the insulin receptor substrate IRS-2 and the tyrosine-phosphorylated Grb2-associated binding proteins Gab110 and Gab2.11 Indeed, association of Gab1 and/or Gab2 to p85 has been observed during stimulation of hematopoietic receptors such as EpoR,12thrombopoietin receptor, T-cell and B-cell receptors,13Kit,14 interleukin-3 (IL-3),15 and IL-6 receptors stimulated by their respective cytokines. In this way, Gab1 acts as a molecular adapter that mediates signaling from cytokine receptors to ERK MAPKs.16 17
Growth factor–independent proliferation is frequently observed during the cell transformation process. In erythroid cells, several mechanisms lead to Epo independence, such as alterations of the EpoR, autocriny, and apoptosis resistance. Indeed, the EpoR can be constitutively activated either by interaction with the env protein encoded by the spleen focus forming virus (SFFV) component of the Friend virus18 or by a point mutation in the exoplasmic domain of the EpoR.19 In murine F-MuLV–induced erythroleukemias20,21 as well as in human erythroid leukemic cells,22 autocrine Epo production constitutively activates the EpoR. Moreover, the Epo-independent growth of the HCD57 murine erythroid cell line has been related to a constitutive activation of both PKB/AKT and STAT5 proteins.9
A well-known model to study the Epo-independent proliferation of the proerythroblast is Friend disease.23 SFFV induces the proliferation and differentiation of proerythroblasts as a consequence of EpoR constitutive activation by the viral glycoprotein gp55. Later on, malignant immature erythroid cells blocked in their differentiation develop in the infected animals.23Emergence of malignant proerythroblasts is correlated to the transcriptional activation of the ETS gene spi-1/PU.1 and to the extinction of the normal p53 gene expression. The pathology developed by transgenic mice that overexpress spi-1revealed that ectopic expression of spi-1 in the proerythroblast is responsible for the differentiation arrest of this cell.24 Indeed, the spi-1 transgenic mice develop a multistep erythroleukemic process characterized at first by the proliferation of proerythroblasts arrested in their differentiation and dependent on Epo for their proliferation. This initial step (the so-called HS1 step) is followed by the development of proerythroblastic cells that become Epo independent and tumorigenic (HS2 step). Thus, this model appears suitable to identify molecular events involved in Epo proliferative signals in a proerythroblast (HS1) in which differentiation pathways are failing. Moreover, analysis of the intracellular signaling pathways in both HS1 and HS2 proerythroblasts appears appropriate to search for anomalies that could account for the Epo-independent proliferation of the proerythroblast.
The current data indicate that deregulation of the intracellular signaling pathways in the HS2 cells involve the PI3K pathway by a mechanism leading to AKT activation. We show a constitutive association to the p85 subunit of the PI3K with Grb2 and with several tyrosine-phosphorylated proteins, in particular the molecular adapter Gab1, the tyrosine phosphatase SHP-2 and the inositol phosphatase SHIP. In addition, a constitutive activation of the MAPKs ERK1 and ERK2 is detected which depends on the activities of the protein kinase C (PKC). Taken together, our data suggest that activation of the signaling pathways involving the PI3K and the MAPKs ERKs could lead to Epo-independence in the proliferating spi-1 transgenic proerythroblasts.
Materials and methods
Cell lines and stimulation
HS1 cells (663) and HS2 cells (606, 622, 601, 921, 931) have been previously described.24 They were grown in alpha minimum essential medium (αMEM) (Gibco, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS; Gibco) and 2 U/mL recombinant human Epo for HS1 cells.
For proliferation assay, the cells were plated at 2 × 105 cells/mL. LY294002 (PI3K inhibitor; Sigma, St Louis, MO), U0126 (MEK inhibitor; Promega, Madison, WI) or GF 109203X (Bisindolylmaleimide I, PKC inhibitor; Calbiochem, La Jolla, CA) were added at a concentration of 10 μM, 20 μM and 10 or 20 μM, respectively. Cell viability was monitored by 0.2% of trypan blue exclusion staining (Sigma).
For Epo stimulation, the cells were washed 3 times in culture medium without serum and Epo, resuspended at 1 × 106 cells/mL in αMEM and cultured without Epo and serum for 4 hours at 37°C. Then the cells were stimulated either 30 minutes (AKT activation) or 10 minutes (EpoR phosphorylation, MAPK activation) with 10% of FCS and/or 10 U/mL Epo.
Antibodies
Anti-p85 and anti-EpoR were previously described.12Two types of anti-Gab2 were used: a polyclonal antibody prepared against the 534-676 amino acids of the human Gab2 protein and the anti-Gab2 provided by Dr Benjamin G. Neel (Boston, MA). The other antibodies were purchased from the following sources: PY-99 antiphosphotyrosine (sc-7020), anti-Grb2 (sc-255), and anti–SHP-2 (sc-280) from Santa Cruz Biotechnology (Santa Cruz, CA); anti–IRS-2 (06-506) and anti-Gab1 (06-579) from Upstate Biotechnology (Lake Placid, NY); anti–phospho-AKT (9271S), anti-AKT (9272), anti–phospho-p44/p42 MAPK (9106S), and anti-p44/42 MAPK (9102) from New England Biolabs (Beverly, MA); anti-Shc (S68020) from Transduction Laboratory (Lexington, KY). Anti-SHIP (P2C2) was a gift from Dr L. Rohrschneider (Fred Hutchinson Cancer Research Center, Seattle, WA). Anti-p110α and p110β were provided by Dr Serge Roche (Montpellier, France).
Immunoprecipitation
Cells were lysed at 2 × 107 cells/mL in ice-cold lysis buffer (10 mM Tris pH 7.4, 150 mM NaCl, 5 mM EDTA, 20 mM NaF, 25 mM β-glycerophosphate, 1 mM Na pyrophosphate, 10% glycerol, 1% NP40, 1 mM Na3VO4, 1x protease inhibitors; Roche). Lysates were centrifuged at 18 000g for 15 minutes at 4°C. The supernatants were incubated with specific antibodies for 2 hours at 4°C. The immunoprecipitates bound to protein G sepharose (Amersham) were washed with 1% NP40 lysis buffer. The proteins were eluted in sodium dodecyl sulfate (SDS) sample loading buffer (62 mM Tris pH 6.8, 2% SDS, 10% glycerol, 0,1% bromophenol blue, 50 mM dithiothreitol [DTT]) before electrophoresis.
Western blotting
For direct Western blotting, 1 × 107 cells/100 μL SDS sample loading buffer were lysed by sonication. Two 106 equivalent cells or immunoprecipitated proteins were resolved by SDS–polyacrylamide gel electrophoresis (PAGE) using 8%, 12%, or 20% polyacrylamide gels. After blotting on Hybond nitrocellulose membrane (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom), the membranes were blocked with 5% nonfat dry milk in phosphate-buffered saline–0.1% Tween 20 (PBS-Tween) and incubated overnight at 4°C with specific antibodies. After washing in PBS-Tween, membranes were incubated with horseradish peroxidase (HRP)–conjugated secondary antibodies and extensively washed in PBS-Tween. The proteins were visualized by the enhanced chemiluminescence Western blotting detection system (Amersham).
EpoR complementary DNA sequencing
Total cellular RNAs were extracted from HS1 and HS2 cells as previously described.24 Complementary DNAs (cDNAs) were synthesized with SuperScript II reverse transcriptase (Gibco BRL) and amplified by polymerase chain reaction (PCR) with Goldstar DNA polymerase (Eurogentec) and the following oligonucleotide primers: ATG-REpo (5′-TGAGCTTCCTGAAGCTAGG-3′), REpo-1 (5′-AGTCACGAAAGTCATGTAGC-3′), REpo-1R (5′-AGCGGCACAAAACTCGATG-3′), REpo-2 (5′-CACATCCGATATGAAGTGGA-3′), REpo-2R (5′-GGACCTCCACCCTTTGTG-3′), REpo-3 (5′-AGAAGATCTGGCCTGGCAT-3′), REpo-3R (5′-CACAGCTGGAAGTTACCCT-3′), REpo-4 (5′-GGTATTGGATAAGTGGTTGC-3′), REpo-4R (5′-GACGGGCAGGAAGATGTTT-3′), and STOP-REpo (5′-GACGTTGTAGGCTGGAGTC-3′). PCR products were subjected to sequencing analysis with Abi Prism 310 Genetic Analyzer (PerkinElmer).
MAPK assay
MAPK activity was detected in complexes immunoprecipitated with 2 μg anti-ERK1 antibody (sc-94, Santa Cruz) in an in vitro assay using 0.5 mg/mL myelin basic protein (MBP; Sigma) and 0.4625 MBq (12.5 μCi) [γ-32P]ATP in MAPK buffer (12.5 mM MOPS pH 7.5, 12.5 mM β glycerophosphate, 7.5 mM MgCl2, 0.5 mM ethyleneglycotetraacetic acid [EGTA], 0.5 mM NaF, 10 μM adenosine triphosphate [ATP], 1 mM DTT) for 30 minutes at 30°C. The reaction was stopped with 40% tricyclic antidepressant (TCA) on ice and 10 μL supernatant was spotted on phosphocellulose paper in duplicate. Filters were washed in 0.5% phosphoric acid and the amount of phosphorylated MBP was measured in a liquid scintillation counter.
PI3K assay
PI3K activity was measured in a final volume of 100 μL containing 50mM Tris-HCl, pH 7.4, 1.5 mM DTT, 100 mM NaCl, 0.5 mM ethylenediaminetetraacetic acid (EDTA), 5 mM MgCl2, 100 μM ATP, 0.925 MBq (25 μCi) of [γ-32P] ATP (11.1 × 1013 [3000 Ci/mmol], NEN Life Science Product, Paris, France), exogenous lipid vesicles (100 μM phosphatidylinositol 4,5-bisphosphate plus 200 μM phosphatidylserine, prepared by sonication in 50 mM Tris-HCl, pH 7.4), and p85 immunoprecipitates. After 10 minutes at 37°C, reactions were stopped by adding a mixture of chloroform/methanol (vol/vol). Lipids were extracted as described,25 separated by thin-layer chromatography (TLC; Merck) using chloroform/acetone/methanol/acetic acid/water (80/30/26/24/14 vol/vol) and the radioactive spots corresponding to 32P-PIP3 were visualized by a Phosphorimager (Molecular Dynamics).
Results
The EpoR is not mutated and its phosphorylation is not altered in HS2 proerythroblasts
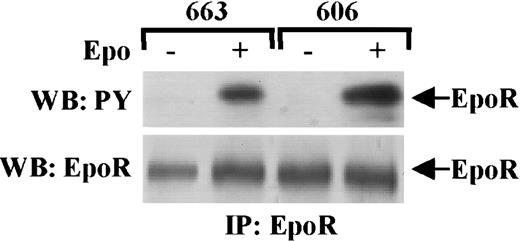
As an Arg 129 mutation is known to constitutively activate the EpoR,19 we first investigated whether mutations were detectable in EpoR that could contribute to Epo independence of the HS2 proerythroblasts. From the 606 HS2 cells, the EpoR cDNA was amplified by reverse transcriptase (RT)–PCR and its nucleotidic sequence was analyzed. The EpoR was wild type. Next, we compared in HS1 cells and HS2 cells the protein level and posttranslational processing of the EpoR by immunoprecipitating the EpoR in extracts from 663 and 606 cells grown either in normal conditions or after Epo stimulation. EpoR immunoprecipitates were analyzed by Western blotting (Figure1) with antiphosphotyrosine antibodies and then reprobed with anti-EpoR antibody. The EpoR was expressed at the same levels in HS1 and HS2 cells and identically phosphorylated in both Epo-stimulated cell extracts.
EpoR is not constitutively activated in HS2 cells.
HS1 cells (663) and HS2 cells (606) were stimulated or not stimulated with 10 U/mL Epo for 10 minutes. Cell lysates were immunoprecipitated (IP) with anti-EpoR antibodies and analyzed by Western blotting (WB) with anti-PY antibodies (upper panel) or with anti-EpoR antibodies (lower panel). Arrows point to phosphorylated EpoR (upper panel) and EpoR (lower panel).
EpoR is not constitutively activated in HS2 cells.
HS1 cells (663) and HS2 cells (606) were stimulated or not stimulated with 10 U/mL Epo for 10 minutes. Cell lysates were immunoprecipitated (IP) with anti-EpoR antibodies and analyzed by Western blotting (WB) with anti-PY antibodies (upper panel) or with anti-EpoR antibodies (lower panel). Arrows point to phosphorylated EpoR (upper panel) and EpoR (lower panel).
PI3K pathway is involved in HS1 and HS2 cell proliferation
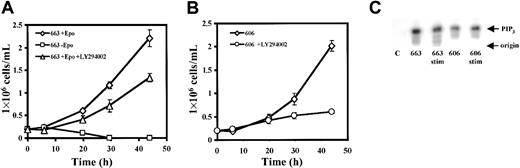
Our previous studies showed that STAT5 activity is normally induced by Epo stimulation in the Epo-independent HS2 cells, but remains inactive when cells are grown in the absence of Epo.26 Then, we focused on other signaling pathways regulated by Epo in erythroid cells. First, we examined the contribution of PI3K to the survival and proliferation of HS1 and HS2 cells by analyzing the effect of the PI3K inhibitor LY29400227 on cell growth (Figure2A). Compared with the arrest of the 663 cell proliferation following Epo withdrawal from the culture medium, 10 μM LY294002 treatment induced a 45% inhibition of the Epo-dependent HS1 cell growth (Figure 2A). Increasing LY294002 doses did not induce a higher inhibition (data not shown). This suggested that in addition to the PI3K pathway, another Epo-dependent signaling event was important for 663 HS1 cell growth. Addition of 10 μM LY294002 in culture medium of the 606 HS2 cells (Figure 2B) induced an 80% decrease of cell growth. Moreover, an enzyme activity able to convert a major PI3K substrate, the phosphatidylinositol 4,5-bisphosphate into phosphatidylinositol 3,4,5-trisphosphate could be detected in the proteins associated with the p85 regulatory subunit of the PI3K in anti-p85 immunoprecipitates from HS1 and HS2 cell extracts (Figure 2C). Altogether these data show that the PI3K activity is involved in the growth control of Epo-dependent and Epo-independent spi-1transgenic proerythroblasts.
An active PI3K is required for the proliferation of HS1 and HS2 cells.
663 HS1 cells (A) or 606 HS2 cells (B) were cultured for the indicated times in the presence or absence of 10 μM LY294002. The means and standard deviations were determined from 4 experiments. (C) 663 HS1 cells were either grown in the presence of 2 U/mL (lane 663) or starved in Epo and serum for 4 hours before being stimulated for 30 minutes with 10 U/mL of Epo (lane 663stim). 606 HS2 cells were either grown with serum (lane 606) or stimulated for 30 minutes with 10 U/mL Epo and serum after 4 hours of serum starvation (lane 606stim). Anti-p85 immunoprecipitates from cell lysates were tested for PI3K activity in an in vitro PI3K assay using the phosphatidylinositol 4,5-bisphosphate as substrate. A control (lane C) is the PI3K activity in cell extracts immunoprecipitated with a nonimmune serum. The PIP3 was separated by thin-layer chromatography and visualized by a Phosphorimager.
An active PI3K is required for the proliferation of HS1 and HS2 cells.
663 HS1 cells (A) or 606 HS2 cells (B) were cultured for the indicated times in the presence or absence of 10 μM LY294002. The means and standard deviations were determined from 4 experiments. (C) 663 HS1 cells were either grown in the presence of 2 U/mL (lane 663) or starved in Epo and serum for 4 hours before being stimulated for 30 minutes with 10 U/mL of Epo (lane 663stim). 606 HS2 cells were either grown with serum (lane 606) or stimulated for 30 minutes with 10 U/mL Epo and serum after 4 hours of serum starvation (lane 606stim). Anti-p85 immunoprecipitates from cell lysates were tested for PI3K activity in an in vitro PI3K assay using the phosphatidylinositol 4,5-bisphosphate as substrate. A control (lane C) is the PI3K activity in cell extracts immunoprecipitated with a nonimmune serum. The PIP3 was separated by thin-layer chromatography and visualized by a Phosphorimager.
The p85 subunit of the PI3K is constitutively associated with tyrosine-phosphorylated proteins in HS2 proerythroblasts
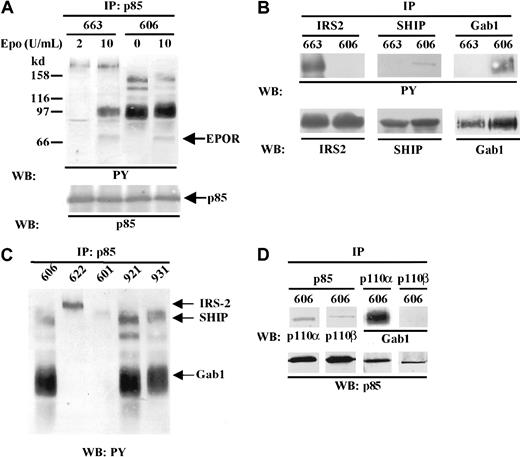
Our data indicated that an active PI3K pathway was decisive in the proliferation of HS2 cells. Because PI3K activation most generally requires the binding of the p85 regulatory subunit to tyrosine-phosphorylated proteins, we sought to determine whether p85 was associated with the same tyrosine-phosphorylated partners in HS1 and HS2 cells. Anti-p85 immunoprecipitates from cell lysates were analyzed by SDS-PAGE and immunoblotting with an antiphosphotyrosine antibody (Figure 3A). We identified tyrosine-phosphorylated proteins that coimmunoprecipitated with p85 by reprobing the immunoblot with antibodies reactive with the presumed proteins (Figure 3B). In 663 HS1 proerythroblasts under unstimulated conditions, 2 faint bands corresponding to proteins of 180 kd and 85 kd molecular masses were detected. The 180-kd protein corresponded to the IRS-2 adapter.28 The nature of the 85-kd protein remains questionable. Indeed it could be a PI3K-associated tyrosine-phosphorylated protein that comigrates with the regulatory subunit of the PI3K or the p85 itself. After Epo stimulation, 2 additional tyrosine-phosphorylated proteins characterized as the adapter Gab1 (97 kd)29 and the EpoR (70 kd) were evident. When the same analysis was performed in 606 HS2 proerythroblasts, several tyrosine-phosphorylated proteins appeared bound to p85. The most evident protein was Gab1. The 140-kd protein was identified as the SHIP inositol phosphatase (Figure 3B) (for review see Rohrschneider et al30). The 2 bands around 120 kd to 110 kd remain unidentified. The pattern of p85 tyrosine-phosphorylated partners was not changed when 606 HS2 cells were stimulated by Epo (10 U/mL), except the additional detection of the tyrosine-phosphorylated form of the EpoR. It should be observed that IRS-2 phosphorylation was not detectable in 606 HS2 cell extracts even after Epo stimulation (Figure 3A,B). Altogether, these data reveal that in Epo-independent proerythroblasts, tyrosine-phosphorylated proteins known to be involved in Epo signaling such as Gab1 and SHIP are constitutively associated with the PI3K.
Constitutive association of tyrosine-phosphorylated proteins with p85/PI3K in HS2 cells.
The position of molecular weight markers are indicated on left and the positions of the different proteins are indicated on right. (A) 663 HS1 cells were either grown in the presence of 2 U/mL Epo or starved in Epo and serum for 4 hours before being stimulated for 30 minutes by 10 U/mL Epo. 606 HS2 cells were grown either with serum or stimulated for 30 minutes with 10 U/mL Epo and serum after 4 hours of serum starvation. Proteins immunoprecipitated from cell lysates (IP) with anti-p85 antibodies were separated by SDS-PAGE and immunoblotted (WB) with anti-PY antibodies. (B) Cell lysates from unstimulated 663 HS1 and 606 HS2 cells were immunoprecipitated with anti–IRS-2, anti-SHIP, anti-Gab1, or anti-p85 antibodies (IP) and analyzed by Western blotting (WB) with antiphosphotyrosine (PY) antibodies or with anti–IRS-2, anti-SHIP, or anti-Gab1 antibodies. (C) Various unstimulated HS2 cell lines were subjected to immunoprecipitation (IP) with anti-p85 antibodies and tyrosine-phosphorylated proteins were detected by Western blotting (WB) with anti-PY antibodies. (D) 606 HS2 cell lysates were immunoprecipitated (IP) with anti-p85, anti-p110α, and anti-p110β antibodies. The immunoprecipitates were subjected to immunoblotting (WB) with anti-p110α, anti-p110β, and anti-Gab1 antibodies (upper panel) or with anti-p85 antibodies (lower panel).
Constitutive association of tyrosine-phosphorylated proteins with p85/PI3K in HS2 cells.
The position of molecular weight markers are indicated on left and the positions of the different proteins are indicated on right. (A) 663 HS1 cells were either grown in the presence of 2 U/mL Epo or starved in Epo and serum for 4 hours before being stimulated for 30 minutes by 10 U/mL Epo. 606 HS2 cells were grown either with serum or stimulated for 30 minutes with 10 U/mL Epo and serum after 4 hours of serum starvation. Proteins immunoprecipitated from cell lysates (IP) with anti-p85 antibodies were separated by SDS-PAGE and immunoblotted (WB) with anti-PY antibodies. (B) Cell lysates from unstimulated 663 HS1 and 606 HS2 cells were immunoprecipitated with anti–IRS-2, anti-SHIP, anti-Gab1, or anti-p85 antibodies (IP) and analyzed by Western blotting (WB) with antiphosphotyrosine (PY) antibodies or with anti–IRS-2, anti-SHIP, or anti-Gab1 antibodies. (C) Various unstimulated HS2 cell lines were subjected to immunoprecipitation (IP) with anti-p85 antibodies and tyrosine-phosphorylated proteins were detected by Western blotting (WB) with anti-PY antibodies. (D) 606 HS2 cell lysates were immunoprecipitated (IP) with anti-p85, anti-p110α, and anti-p110β antibodies. The immunoprecipitates were subjected to immunoblotting (WB) with anti-p110α, anti-p110β, and anti-Gab1 antibodies (upper panel) or with anti-p85 antibodies (lower panel).
Such a study was extended to 4 other HS2 cell lines. In Figure 3C, the patterns of tyrosine-phosphorylated proteins associated to p85 were compared in the 606, 622, 601, 921, and 931 HS2 proerythroblasts cultured in the absence of Epo. Two cell lines (921 and 931) presented a pattern of phosphorylated proteins identical to the one described for 606 cells. In contrast, in 2 HS2 cell lines (622 and 601) the pattern of tyrosine-phosphorylated proteins looked like the one characterized in 663 HS1 cells, with, in particular, the presence of the tyrosine-phosphorylated IRS-2. Interestingly, we previously reported that the 622 and 601 HS2 cell lines were autocrine for Epo.24 This discrepancy between Epo-productive and Epo-unproductive HS2 cells was not abolished when 606 HS2 cells were Epo stimulated (Figure 3A). This suggests that the constitutive association of p85 to SHIP and Gab1 could be correlated to the ability of the proerythroblast to grow in the absence of Epo.
Because the 606 HS2 cell proliferation was dependent on an active PI3K, we tried to identify the nature of the catalytic subunit of the PI3K. Three p110 isoforms (p110α, p110β, p110δ) of the class IA PI3Ks are known.31 Because the p110α and p110β isoforms are ubiquitously expressed, whereas p110δ has a tissue distribution restricted to leukocytes,32 we investigated whether p110α and/or p110β were recruited by p85. Anti-p85 immunoprecipitates from 606 cell lysates were subjected to immunoblotting with anti-p110α or p110β (Figure 3D). Both p110α and p110β were detected in p85 immunoprecipitates. However, when we looked for the presence of Gab1 in the p110 immunoprecipitates, only p110α appeared associated to Gab1 (Figure 3D), whereas reprobing the membranes with anti-p85 antibody showed that p85 was detected at the same level in either anti-p110α or anti-p110β immunoprecipitates. Thus, Gab1 appeared mainly associated with a p85/p110α complex.
Grb2 is associated with p85 and participitates in the PI3K/Gab1 complex with SHP-2 and Shc
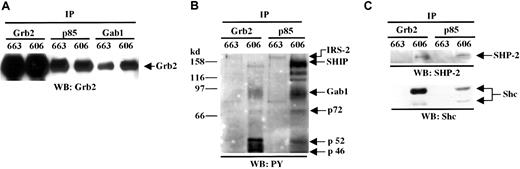
Gab1 has been identified as the Grb2-associated binder protein 1.10 Thus, we investigated whether Grb2 (for review see Buday33) was associated to both p85 and Gab1 in the HS1 and HS2 proerythroblasts. Anti-Grb2, anti-Gab1, and anti-p85 immunoprecipitates from 606 and 663 lysates were immunoblotted with anti-Grb2 (Figure 4A). The 26-kd Grb2 was found to be associated with both p85 and Gab1 in 663 HS1 cells as well as in 606 HS2 cells. Moreover, comparative analysis of the tyrosine-phosphorylated proteins associated with either Grb2 or p85 in 606 HS2 cells revealed that in addition to SHIP and Gab1, 72-kd, 52-kd, and 46-kd proteins (Figure 4B) were present in both types of complexes. The 72-kd protein was identified as the SHP-2 tyrosine phosphatase and the 52-kd and 46-kd proteins as the Shc adapters after reprobing the membrane with anti–SHP-2 antibodies and anti-Shc antibodies (Figure 4C). Therefore, in the 606 cells, Grb2 is associated with Shc and SHP2 as well as p85, SHIP, and Gab1 in the absence of EpoR stimulation.
Grb2, SHP-2, and Shc are associated with the PI3K complex in HS2 cells.
(A) Protein extracts from 663 HS1 and 606 HS2 cells were immunoprecipitated (IP) with anti-Grb2, anti-p85, and anti-Gab1 antibodies and analyzed by immunoblotting (WB) with anti-Grb2 antibody. The position of Grb2 is indicated. (B) Proteins immunoprecipitated from 663 HS1 and 606 HS2 cell lysates with anti-Grb2 or anti-p85 antibodies (IP) were analyzed by immunoblotting (WB) with anti-PY antibodies. (C) Proteins immunoprecipitated from 663 HS1 and 606 HS2 cell lysates with anti-Grb2 or anti-p85 antibodies (IP) were analyzed by immunoblotting (WB) with anti–SHP-2 and Shc antibodies. The positions of the phosphorylated proteins were indicated.
Grb2, SHP-2, and Shc are associated with the PI3K complex in HS2 cells.
(A) Protein extracts from 663 HS1 and 606 HS2 cells were immunoprecipitated (IP) with anti-Grb2, anti-p85, and anti-Gab1 antibodies and analyzed by immunoblotting (WB) with anti-Grb2 antibody. The position of Grb2 is indicated. (B) Proteins immunoprecipitated from 663 HS1 and 606 HS2 cell lysates with anti-Grb2 or anti-p85 antibodies (IP) were analyzed by immunoblotting (WB) with anti-PY antibodies. (C) Proteins immunoprecipitated from 663 HS1 and 606 HS2 cell lysates with anti-Grb2 or anti-p85 antibodies (IP) were analyzed by immunoblotting (WB) with anti–SHP-2 and Shc antibodies. The positions of the phosphorylated proteins were indicated.
AKT is constitutively phosphorylated in Epo-independent HS2 cells
The survival protein AKT is a major downstream effector of the PI3K. In various Epo-independent erythroleukemic cell lines, AKT appears constitutively activated.9 34 To investigate whether the survival of HS2 cells was correlated to AKT constitutive activation, antibodies specific for AKT phosphorylated on serine 473 were used to analyze AKT phosphorylation. In Figure5A, AKT appeared phosphorylated in the 663 HS1 cells grown under normal conditions (2 U/mL of Epo). A very low level of phosphorylated AKT remained detectable in Epo and serum-starved cells but stimulation of these cells by 10 U/mL Epo for 30 minutes was sufficient to induce an optimal level of AKT phosphorylation. A 10 μM LY294002 treatment of these cells abolished this Epo-induced AKT activation. Western blot analyses with anti-AKT antibody showed similar levels of AKT protein in all samples. Thus, in the proliferating Epo-dependent HS1 proerythroblasts, AKT activation appeared as a cellular response to the cell stimulation by Epo and was PI3K dependent. In contrast, phosphorylation of AKT was detected in the absence of Epo in 606 HS2 cells as well as in serum-starved HS2 cells (Figure 5B). Moreover, addition of Epo (2 U/mL) in culture medium or stimulation of serum-starved HS2 cells by 10 U/mL Epo did not induce a significant change in the AKT phosphorylation level. This AKT phosphorylation was strictly dependent on a PI3K activity because it was abolished by treatment of cells with LY294002. These data indicate that the PI3K/AKT pathway is constitutively activated in HS2 cells, suggesting that AKT activation could participate in their survival and proliferation.
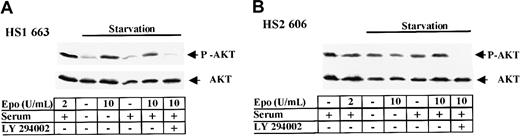
AKT is constitutively phosphorylated in HS2 cells.
(A) 663 HS1 cells were either grown in the presence of 2 U/mL Epo and 10% serum or starved in Epo and serum for 4 hours before being stimulated with 10 U/mL Epo and/or serum. 10 μM LY294002 was added during the last starvation hour. (B) 606 HS2 cells were either grown in the presence or absence of 2 U/mL Epo and 10% serum or starved in Epo and serum for 4 hours before being hyperstimulated by 10 U/mL Epo and/or serum. 10 μM LY294002 was added during the last starvation hour. Cell lysates were subjected to immunoblotting with anti–phospho-AKT (A, B upper panels) and anti-AKT (A, B lower panels) antibodies. The positions of phosphorylated AKT (P-AKT) and AKT were indicated.
AKT is constitutively phosphorylated in HS2 cells.
(A) 663 HS1 cells were either grown in the presence of 2 U/mL Epo and 10% serum or starved in Epo and serum for 4 hours before being stimulated with 10 U/mL Epo and/or serum. 10 μM LY294002 was added during the last starvation hour. (B) 606 HS2 cells were either grown in the presence or absence of 2 U/mL Epo and 10% serum or starved in Epo and serum for 4 hours before being hyperstimulated by 10 U/mL Epo and/or serum. 10 μM LY294002 was added during the last starvation hour. Cell lysates were subjected to immunoblotting with anti–phospho-AKT (A, B upper panels) and anti-AKT (A, B lower panels) antibodies. The positions of phosphorylated AKT (P-AKT) and AKT were indicated.
ERK1/2 MAPK pathway is constitutively activated in HS2 cells
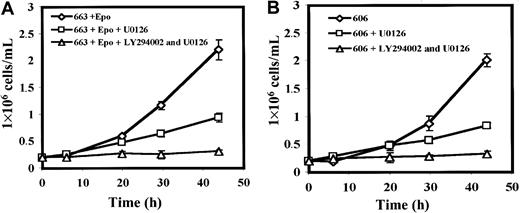
The MAPK pathway is activated during the erythroid proliferation induced by Epo.35,36 This activation can be mediated by PI3K activation in association with Grb237 and Gab1. At first, we examined whether MAPKs play a role in the proliferation of HS1 and HS2 cells by testing the effects of the MAPK inhibitor U012638 on cell growth. The presence of 20 μM U0126 in culture medium decreased by more than 65% the proliferation of HS1 cells and the growth inhibition was complete when both 20 μM U0126 and 10 μM of LY294002 were added (Figure6A). The same inhibitory effect of U0126 either alone or in conjunction with LY294002 was observed on the proliferation of the HS2 cells (Figure 6B). These data indicate that MAPKs activities in addition to PI3K activities were required for the growth of HS1 and HS2 cells.
Active MAPKs are required for HS1 and HS2 cell proliferation.
(A) 663 HS1 cells and 606 HS2 cells (B) were treated or not treated with 20 μM of the MEK kinase inhibitor U0126 and/or 10 μM of the PI3K inhibitor LY294002 for the indicated times. The means and standard deviations were determined from 4 experiments.
Active MAPKs are required for HS1 and HS2 cell proliferation.
(A) 663 HS1 cells and 606 HS2 cells (B) were treated or not treated with 20 μM of the MEK kinase inhibitor U0126 and/or 10 μM of the PI3K inhibitor LY294002 for the indicated times. The means and standard deviations were determined from 4 experiments.
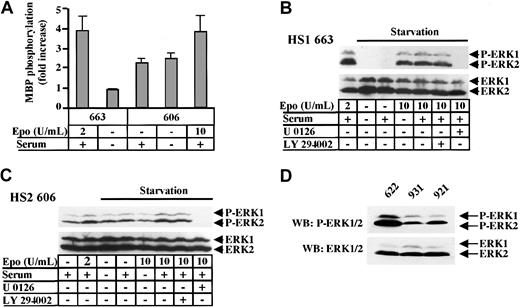
To ascertain the role of MAPKs in cell proliferation, we searched for MAPK activity in extracts from HS1 and HS2 cells grown either in normal culture conditions or in Epo and/or serum-starvation conditions. MAPK activity was measured in anti–ERK1 immunoprecipitates using myelin basic peptide (MBP) as a substrate. MAPK activity was detected in extracts from HS1 cells stimulated by Epo (Figure7A). In extracts from HS2 cells, MAPK activity appeared evident even when cells were serum starved. Nevertheless, an increase in MAPK activity could be observed when HS2 cells were starved in serum before being stimulated by 10 U/mL Epo, revealing that an Epo-dependent activation of MAPKs can occur in addition to the constitutive MAPK activity.
ERK1 and ERK2 are constitutively phosphorylated in HS2 cells.
(A) 663 HS1 cells were either grown in the presence of 2 U/mL Epo and 10% serum or starved in Epo and serum for 4 hours. 606 HS2 cells were either grown in the presence of 10% serum or starved in serum for 4 hours or starved and then stimulated with 10 U/mL Epo and serum for 30 minutes. ERK1 was immunoprecipitated from cell extracts and an in vitro kinase assay was performed using the MBP peptide as substrate. The means and standard deviations were determined from 3 experiments. (B) 663 HS1 cells were either grown in the presence of 2 U/mL Epo and 10% serum or starved in Epo and serum for 4 hours before being stimulated with 10 U/mL Epo and/or 10% serum. A quantity of μM of LY294002 or 20 μM of U0126 was added for the last hour of starvation. (C) 606 HS2 cells were either grown in the presence or absence of 2 U/mL Epo and 10% serum or starved in Epo and serum for 4 hours before being stimulated with 10 U/mL Epo and/or 10% serum. They were treated with 10 μM LY294002 or 20 μM U0126 for the last hour of starvation. Cell lysates were immunoblotted with anti–phospho-ERK1/2 (B, C upper panels) and anti-ERK1/2 (B, C lower panels) antibodies. (D) Protein extracts of 622, 921, and 931 unstimulated HS2 cell lines were immunoblotted with anti–phospho-ERK1/2 (upper panel) and anti-ERK1/2 (lower panel) antibodies. The positions of phosphorylated ERK1 and ERK2 (P-ERK1, P-ERK2) and ERK1 and ERK2 were indicated.
ERK1 and ERK2 are constitutively phosphorylated in HS2 cells.
(A) 663 HS1 cells were either grown in the presence of 2 U/mL Epo and 10% serum or starved in Epo and serum for 4 hours. 606 HS2 cells were either grown in the presence of 10% serum or starved in serum for 4 hours or starved and then stimulated with 10 U/mL Epo and serum for 30 minutes. ERK1 was immunoprecipitated from cell extracts and an in vitro kinase assay was performed using the MBP peptide as substrate. The means and standard deviations were determined from 3 experiments. (B) 663 HS1 cells were either grown in the presence of 2 U/mL Epo and 10% serum or starved in Epo and serum for 4 hours before being stimulated with 10 U/mL Epo and/or 10% serum. A quantity of μM of LY294002 or 20 μM of U0126 was added for the last hour of starvation. (C) 606 HS2 cells were either grown in the presence or absence of 2 U/mL Epo and 10% serum or starved in Epo and serum for 4 hours before being stimulated with 10 U/mL Epo and/or 10% serum. They were treated with 10 μM LY294002 or 20 μM U0126 for the last hour of starvation. Cell lysates were immunoblotted with anti–phospho-ERK1/2 (B, C upper panels) and anti-ERK1/2 (B, C lower panels) antibodies. (D) Protein extracts of 622, 921, and 931 unstimulated HS2 cell lines were immunoblotted with anti–phospho-ERK1/2 (upper panel) and anti-ERK1/2 (lower panel) antibodies. The positions of phosphorylated ERK1 and ERK2 (P-ERK1, P-ERK2) and ERK1 and ERK2 were indicated.
Then, we examined the activation state of ERK1 and ERK2 in HS1 and HS2 cells by analyzing their phosphorylation (for review see Garrington and Johnson39). Serum- and Epo-starved 663 HS1 cells and serum-starved 606 HS2 cells were stimulated for 10 minutes with serum and/or Epo. The active phosphorylated forms of ERK1/2 proteins were detected in cell protein extracts by Western blotting with specific antibodies. In 663 HS1 cells (Figure 7B) grown in medium containing serum and 2 U/mL Epo, ERK1/2 were phosphorylated. After starvation, Epo (10 U/mL) appeared sufficient to stimulate ERK1/2, whereas serum did not. Treatment of the cells with the PI3K inhibitor LY294002 was without effect on the MAPK phosphorylation induced by Epo, whereas the MEK inhibitor U0126 impeded activation of ERK1 and ERK2. These data indicate that in HS1 cells, the activation of the MAPK pathway was strictly dependent on Epo and did not require PI3K activity. In 606 HS2 cells (Figure 7C), the detection of ERK1/2 phosphorylated forms was possible in unstimulated growth conditions as well as in serum-starvation growth conditions. Again, the treatment of HS2 cells by U0127 abolished the ERK1/2 phosphorylation, whereas LY294002 remains inactive. Figure 7D shows that ERK1/2 constitutive phosphorylation was detected in other HS2 cell lines (921 and 931 cells). In the 622 cells, which were autocrine for Epo, the MAPK phosphorylation level appeared higher than in cells that were not autocrine, most probably because of additional Epo-induced signaling pathways. These results were consistent with the MAPK activities detected in the in vitro MAPK assay (Figure 7A) as well as with the faint increase in ERK1/2 phosphorylation level observed in Epo-stimulated 606 cells (Figure 7C, Figure 9B). Thus, we can conclude that the proliferation of HS2 cells is correlated to a constitutive activation of ERK1 and ERK2 in a PI3K-independent manner.
PKCs induce MAPK constitutive activation in spi-1transgenic proerythroblasts
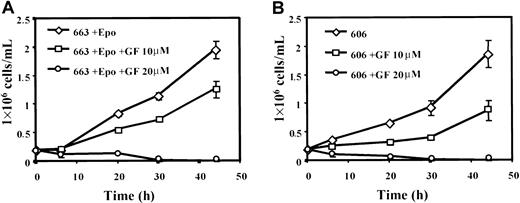
Because PKC isoforms may represent essential elements in the signaling pathways of some cytokines and in particular in the Epo signaling pathway,40 the proliferation of HS1 and HS2 cells was examined in the presence of bisindolylmaleimide (GF 109203X), a selective inhibitor of PKCs. Addition of GF 109203X (10 μM) in culture medium reduced the growth of 663 cells (Figure8A) and 606 cells (Figure 8B) by 40%. Thus, PKC activity plays a role in the proliferation of HS1 and HS2 proerythroblastic cells. Because MAPK activation by PKCs has been reported in primary erythroid cells41 and in SFFV-infected cells,42 we analyzed whether MAPK phosphorylation was altered in HS1 and HS2 cells grown in the presence of GF 109203X. Data in Figure 9A indicate that the level of ERK1/2 phosphorylation was significantly reduced in serum-starved/Epo-starved HS1 cells treated with GF 109203X and then stimulated by 10 U/mL Epo and serum. This result indicates that ERK1/2 phosphorylation was PKC dependent in HS1 cells. No ERK1/2 phosphorylated forms could be detected in HS2 cells grown in the presence of GF 109203X (Figure 9B) and their activation level was strongly reduced in Epo-stimulated GF 109203X-treated HS2 cells. These data demonstrate that the constitutive activation of MAPKs in HS2 cells is strictly determined by a PKC-dependent mechanism. The residual activation of MAPKs detected in both HS1 and HS2 cells treated by GF 109203X and then stimulated by Epo most probably reflects the partial inhibition of cell proliferation (40%) induced by the GF 109203X dose (10 μM) used in these experiments. Altogether, our data demonstrate that the constitutive activation of MAPKs in the Epo-independent HS2 cells is strictly determined by a PKC-dependent mechanism.
Active PKCs are required for HS1 and HS2 cell proliferation.
663 HS1 cells (A) and 606 HS2 cells (B) were treated or not treated with 10 μM or 20 μM of the PKC inhibitor GF 109203X (GF) for the indicated times. The means and standard deviations were determined from 3 experiments.
Active PKCs are required for HS1 and HS2 cell proliferation.
663 HS1 cells (A) and 606 HS2 cells (B) were treated or not treated with 10 μM or 20 μM of the PKC inhibitor GF 109203X (GF) for the indicated times. The means and standard deviations were determined from 3 experiments.
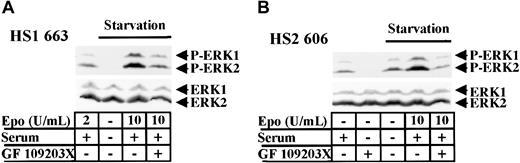
GF 109203X inhibits the ERK1/2 constitutive phosphorylation in HS2 cells.
(A) 663 HS1 cells were either grown in the presence of 2 U/mL Epo and 10% serum or starved in Epo and serum for 4 hours before being stimulated with 10 U/mL Epo and 10% serum or not. A quantity of 10 μM GF 109203X was added for the last hour of starvation. (B) 606 HS2 cells were either grown in the absence of Epo and 10% serum or starved in Epo and serum for 4 hours before being stimulated with 10 U/mL Epo and 10% serum or not. They were treated with 10 μM GF 109203X for the last hour of starvation. Cell lysates were immunoblotted with anti–phospho-ERK1/2 (A, B upper panels) and anti-ERK1/2 (A, B lower panels) antibodies. The positions of phosphorylated ERK1 and ERK2 (P-ERK1, P-ERK2) and ERK1 and ERK2 were indicated.
GF 109203X inhibits the ERK1/2 constitutive phosphorylation in HS2 cells.
(A) 663 HS1 cells were either grown in the presence of 2 U/mL Epo and 10% serum or starved in Epo and serum for 4 hours before being stimulated with 10 U/mL Epo and 10% serum or not. A quantity of 10 μM GF 109203X was added for the last hour of starvation. (B) 606 HS2 cells were either grown in the absence of Epo and 10% serum or starved in Epo and serum for 4 hours before being stimulated with 10 U/mL Epo and 10% serum or not. They were treated with 10 μM GF 109203X for the last hour of starvation. Cell lysates were immunoblotted with anti–phospho-ERK1/2 (A, B upper panels) and anti-ERK1/2 (A, B lower panels) antibodies. The positions of phosphorylated ERK1 and ERK2 (P-ERK1, P-ERK2) and ERK1 and ERK2 were indicated.
Discussion
In the present study we have investigated the intracellular signal transduction pathways in Epo-independent erythroid leukemic cells whose differentiation is arrested as a consequence of the ectopic expression of the proto-oncogene spi-1.
The balance between growth control, survival, and terminal differentiation of erythroid progenitor cells is tightly controlled by Epo. The intracellular mechanisms that coordinate this balance in a normal erythroid cell are not well understood due to the cross talk between the signaling pathways involved in both proliferation and differentiation. Alterations of this balance are hallmarks of the erythroleukemic cells and can lead to the loss of Epo dependence. During the multistep erythroleukemic process developed by the spi-1 transgenic mice, 2 populations of proerythroblasts arrested in their differentiation process can be successively isolated.24 The early population is strictly dependent on Epo to proliferate, whereas the later population is able to grow without Epo. Thus, these 2 cell types appear suitable for the investigation of the molecular events that allow a proerythroblast prone to proliferate to escape Epo control.
At first, the hypothesis that a mutational activation of the EpoR could be responsible for Epo independence has been tested. Indeed, activating mutations as the mutation R129C in the extracellular domain of the EpoR19 or the truncation of the EpoR C-terminus region are known to trigger the proliferative function of the EpoR.43However, we did not find alteration in the primary structure of the EpoR in the 606 HS2 cells. In addition, we observed that the EpoR is in an inactivate form in the absence of Epo, although it can be normally phosphorylated in response to Epo stimulation. Thus, an indirect activation of the EpoR by transmembrane or intracytoplasmic signaling could be discarded. Autocriny is another process related to Epo independence of the erythroleukemic cells. We screened 12 HS2 cell lines for Epo autocriny (Moreau-Gachelin et al24 and data not shown). Only 2 HS2 cell lines (601,622) produced Epo, suggesting that Epo autocriny remains a limited mechanism for Epo independence in the spi-1 transgenic proerythroblasts. We also turned down the possibility that a growth factor hypersensitivity rather than a growth factor independence may be sufficient to support the proliferation of HS2 cells with regard to the presence of growth factors in serum. Indeed, no differences in the survival and proliferation of HS2 cells could be observed when cells were grown in a serum-free medium (data not shown).
Intracellular signal transduction pathways have also been investigated. We already reported that the Jak2/STAT5 pathway was at rest in HS2 cells as a direct consequence of an inactive EpoR,26excluding that anomalies in this pathway could lead to a proliferative signal in the HS2 cells.
PI3K is involved in the regulation of many signaling events involved in mitogenesis and apoptosis.44 The inhibition ofspi-1 transgenic proerythroblast growth by the PI3K inhibitor LY294002 demonstrates that PI3K is involved in the proliferation of these cells. This effect is more sensitive in HS2 cells than in HS1 cells most probably because additional signaling pathways from EpoR as the JAK/STAT activation are functional in HS1 cells, whereas they are at rest in HS2 cells. Nevertheless, it remains partial even at 40 μM LY294002 (data not shown), demonstrating that other signaling pathways besides the PI3K pathway synergize in inducingspi-1 transgenic proerythroblasts to proliferate.
The PI3K activity induced by Epo is known to depend on binding of the p85 subunit to EpoR or to molecular adapters of the IRS family, IRS2 and Gab1.12,28,29 Indeed, the activation of PI3K during the proliferative response of HS1 cells to Epo stimulation involves these processes because EpoR, IRS2, and Gab1 are tyrosine phosphorylated and bound to p85. The association of p85 with IRS2 was detectable before Epo stimulation. An explanation is that the Epo level in normal cell growth conditions induces a residual tyrosine phosphorylation of IRS2 sufficient to provide docking sites for p85. In contrast, association of Gab1 is only detectable after Epo stimulation, suggesting that association of Gab1 with p85 is determined by a sustained phosphorylation of the EpoR. Gab2 can also be detected as a p85-associated protein that comigrates with Gab1 (data not shown). However, compared with Gab1, Gab2 appears as a minor component in the p85 complex. Thus, as described in other Epo-dependent cell lines,12,29 Gab1 participates in Epo signaling in HS1 proliferation. In HS2 cells, the most evident protein associated with p85 is Gab1. Although EpoR is at rest and Gab1 is not overexpressed in these cells, Gab1 is highly tyrosine phosphorylated. Thus, Gab1 may be more efficiently recruited by p85 in HS2 cells, leading to an increase in PI3K-Gab1 complexes potentially involved in cellular proliferative signals. Among the proteins associated with p85 in HS2 cells, besides Gab1, we identified various SH2-containing proteins as the adapter Shc, the tyrosine phosphatase SHP-2, and the inositol phosphatase SHIP, all of them described as partners of Gab1 in Epo-stimulated cells.12 The same tyrosine-phosphorylated protein pattern was identified in a pull-down experiment from HS2 cell extracts using a GST fusion protein containing only the p85 amino and carboxy terminal SH2 domains (data not shown). This data further substantiate that PI3K activation in HS2 cells is related to the interaction of p85 by its SH2 domains with the tyrosine-phosphorylated signaling proteins. Strikingly, this constitutive PI3K/Gab1/Grb2/SHP-2/Shc/SHIP association is not detected in HS2 cells that produce Epo (601 and 622 cells). In these cells only IRS-2 and Gab1 are associated with p85 in the same way as Epo-dependent HS1 cells. These data suggest that a selection process leading to emergence of Epo-independent proerythroblasts during thespi-1 transgenic erythroleukemic process could involve some biochemical events such as the constitutive phosphorylation and association of signaling molecules such as Gab1, Grb2, SHP-2, Shc, and SHIP with PI3K. Which are the kinases or phosphatases responsible for such a constitutive phosphorylation of signaling proteins and the genetic events involved in activation of such kinases or phosphatases are opened questions.
It has been recently shown that AKT is activated by phosphorylation on 2 residues (Thr 308 and Ser 473) in response to Epo stimulation and that constitutive activation of AKT is associated with an apoptosis resistance process in the Epo-independent HCD57 murine erythroid cells.9 Moreover, AKT activation has been reported in SFFV-infected erythroleukemic cells, in which EpoR is constitutively activated by the viral gp55,34 suggesting that AKT activation could explain that these cells did not undergo apoptosis. In HS1 cells grown in the presence of Epo, we observed that AKT was phosphorylated and that this activation was determined by Epo and mediated by PI3K. These data indicate that AKT is a key factor in the Epo-induced antiapoptotic effect during proliferation of these cells. In HS2 cells, AKT phosphorylated at Ser 473 was detectable in the absence of Epo. We also observed the AKT phosphorylation by using an antibody that recognizes the Thr 308–phosphorylated form of AKT (data not shown). Moreover, AKT phosphorylation was inhibited when cells were treated with LY294002 at 10 μM at which dosage HS2 cell proliferation was abolished. This PI3K-dependent phosphorylation of AKT agrees with the constitutive binding of p85 to SHIP in HS2 cells. SHIP is a phosphatidylinositol phosphatase that generates the phosphatidylinositol 3,4-bisphosphate identified as the main activator of AKT.45 Thus, our data strongly suggest that the survival of Epo-independent spi-1 transgenic proerythroblasts involves the constitutive activation of the antiapoptotic PI3K/AKT pathway. An attractive prospect will be to identify the downstream target substrates of AKT that prevent the apoptotic process in HS2 proerythroblasts in the absence of Epo.
Some data argue for a role of the MAPK ERKs as well as JNK and p38 in the regulation of growth, survival, and differentiation of erythroid cells.36 In the spi-1 transgenic proerythroblasts we failed to detect activated forms of both p38 MAPK and Jun kinases in HS2 cells (data not shown). In contrast, we found that the MAPKs ERK1/2 were constitutively activated in HS2 cells and that this process was involved in cell proliferation. ERK1/2 phosphorylation can either occur through successive activations of upstream MAPK/ERK-activating kinase (MEK) and Raf-1 serine/threonine kinases induced by the Ras GTP-binding activity46 or through mediation by PKC/Raf-1 pathway.47 In particular, ligand stimulation of EpoR induces the activation of the MAPK cascade through the Ras signaling pathway.48 A constitutive activation of the ERK kinase pathway has been correlated to the proliferation of HCD57 erythroleukemic cells infected by SFFV.49 The same authors have recently shown that PKC activation leading to MAPK activation is sufficient to induce the proliferation of these HCD57 cells whether they were stimulated by Epo or infected by SFFV.42 Here, we demonstrated that the Epo-controlled proliferation of the HS1 spi-1 transgenic proerythroblasts depended in part on ERK1 and ERK2 activities. In addition, we showed that ERK phosphorylation was determined by PKC activities and did not require PI3K activity. This result provides strong evidence that the proliferation of Epo-dependent HS1 cells involves the stimulation of MAPKs through a signaling pathway dependent on PKC activities. A PKC-dependent signaling cascade leading to MAPK activation is also involved in the proliferation and differentiation of fetal liver erythroid progenitor cells.41 However, this cascade is initiated by the recruitment of the p85 subunit of PI3K to the EpoR stimulated by its ligand. This suggests that the role of PI3K in the PKC/MAPK signaling pathway would be related to a differentiation process rather than to a proliferation process in the erythroid lineage. The Epo-independent proliferation of HS2 cells is correlated with the constitutive activation of MAPK in a PI3K-independent manner. The PKC inhibitor strongly reduces cell growth and completely abrogates the constitutive activation of MAPK. Thus, the constitutive activation of the PKC/MAPK pathway appears as a second cellular process altered during growth factor–independent proliferation of spi-1 transgenic proerythroblasts. This raises the question of the nature of the molecular mechanisms leading to activation of the PKC/MAPK pathway in these cells.
Together, our results allow us to consider that cellular selection events during the leukemic progression of the spi-1transgenic proerythroblasts lead to dysregulation of 2 signaling pathways: the PI3K/AKT pathway and the PKC/MAPK pathway. This study shows that such cellular selection events involve signaling alterations similar to those initiated during infection of the proerythroblast by the Friend virus. Further studies to determine the molecular mechanisms responsible for alterations in the PI3K and MAPK pathways may provide novel insights into the nature of oncogenic events that lead a proerythroblast to malignancy.
We are grateful to Dr L. Rohrschneider for the gift of SHIP antibodies, to Dr Serge Roche for the gift of anti-p110α and p110β antibodies, to Dr Benjamin G. Neel for the gift of anti-Gab2 antibody, to Dr Jacques Bertoglio for the gift of the expression vector for GST-SH2/PI3K recombinant protein, and to Cilag (Levallois-Perret, France) for the gift of recombinant human Epo. We are grateful to Nicole Denis, Marie Bourgeois, and Philippe Martin-Hardy for excellent technical assistance. We thank Laurent Delva, Christelle Guillouf, Jean de Gunzburg, and Jacques Camonis for valuable discussions. We also thank Sylvie Gisselbrecht for helpful comments on the manuscript.
Supported by grants from the Institut National de la Santé Et de la Recherche Médicale, the Association pour la Recherche sur la Cancer, the Ligue de Recherche contre le Cancer, and the Institut Curie.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Françoise Moreau-Gachelin, Inserm U528, Institut Curie-Section Recherche, 26 rue d'Ulm, 75005 Paris, France; e-mail: framoreau@curie.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal