Prolonged immunodeficiency after allogeneic bone marrow transplantation (BMT) causes significant morbidity and mortality from infection. This study examined in murine models the effects of interleukin-7 (IL-7) given to young and middle-aged (9-month-old) recipients of major histocompatibility complex (MHC)–matched or –mismatched allogeneic BMT. Although administration of IL-7 from day 0 to 14 after syngeneic BMT promoted lymphoid reconstitution, this regimen was ineffective after allogeneic BMT. However, IL-7 administration from day 14 (or 21) to 27 after allogeneic BMT accelerated restoration of the major lymphoid cell populations even in middle-aged recipients. This regimen significantly expanded donor-derived thymocytes and peripheral T cells, B-lineage cells in bone marrow and spleen, splenic natural killer (NK) cells, NK T cells, and monocytes and macrophages. Interestingly, although recipients treated with IL-7 had significant increases in CD4+ and CD8+ memory T-cell populations, increases in naive T cells were less profound. Most notable, however, were the observations that IL-7 treatment did not exacerbate graft-versus-host disease (GVHD) in recipients of an MHC-matched BMT, and would ameliorate GVHD in recipients of a MHC-mismatched BMT. Nonetheless, graft-versus-leukemia (GVL) activity (measured against 32Dp210 leukemia) remained intact. Although activated and memory CD4+ and CD8+ T cells normally express high levels of IL-7 receptor (IL-7R, CD127), activated and memory alloreactive donor-derived T cells from recipients of allogeneic BMT expressed little IL-7R. This might explain the failure of IL-7 administration to exacerbate GVHD. In conclusion, posttransplant IL-7 administration to recipients of an allogeneic BMT enhances lymphoid reconstitution without aggravating GVHD while preserving GVL.
Introduction
All recipients of an allogeneic bone marrow transplant (BMT) are subject to a varying period of T and B lymphocytopenia, which is associated with an increased susceptibility to infections, such as cytomegalovirus, herpes simplex virus, varicella zoster virus, fungi, and encapsulated bacteria as well as Epstein-Barr virus–induced lymphoproliferative disease. The severity and duration of this immunoincompetence is determined by a number of variables, including previous chemotherapy or radiation therapy, a lack of sustained transfer of donor immunity, recapitulation of immunologic ontology, thymic involution after puberty, donor/host histoincompatibility, graft-versus-host disease (GVHD), and graft rejection (reviewed in Parkman and Weinberg1). An inverse relationship between transplant recipient age and capacity to generate T lymphocytes (especially CD4+ T cells) has been found in several studies.2-8
Regeneration of T cells after allogeneic BMT can occur by at least 3 mechanisms: (1) a thymus-dependent pathway that involves positive and negative selection and represents a recapitulation of fetal T-cell ontogeny, (2) a thymus-independent pathway that is limited to the generation of small numbers of T cells from the bone marrow (BM) and intestines,9-12 and (3) a thymus-independent pathway that involves the peripheral expansion of mature donor T cells that were transferred with the stem cell graft.13-18 Data from human and murine studies demonstrate that T-cell reconstitution in older recipients of transplants depends more on the extrathymic pathways and results in slower reconstitution with fewer T cells (especially naive CD4+ T cells) and a diminished T-cell repertoire.13-16,18 19
Human interleukin-7 (IL-7) is a 25-kd glycoprotein that is secreted by fetal liver cells and stromal cells in the BM and thymus.20-22 The IL-7 receptor (IL-7R) consists of the IL-7Rα chain (CD127)23 and the common cytokine receptor γ chain24,25 and is expressed on early thymocytes, activated T cells, pre-B cells, and BM macrophages.23 IL-7 is a nonredundant cytokine for T- and B-cell development and mice deficient for IL-7 or IL-7Rα or mice treated with neutralizing anti-IL-7 antibodies all display severely impaired B- and T-cell development.26,27 In humans, a defect in the IL-7Rα chain28 or the common γ chain29 results in a severe combined immunodeficiency syndrome (SCID) with a complete lack of T cells.
Interleukin 7 plays a role in survival, differentiation, and proliferation of B- and T-cell precursors.30-34 IL-7 also promotes the survival of developing and mature T cells (possibly through the up-regulation of Bcl-2) in the thymus and the periphery.32,35-41 Administration of IL-7 to normal mice resulted in a marked increase in T and B cellularity in thymus, spleen, lymph nodes, and BM42,43and IL-7 administration to cyclophosphamide-treated or irradiated mice could improve T- and B-lymphocyte recovery.43,44 Bolotin and colleagues45 demonstrated that IL-7 administration after murine syngeneic BMT would increase thymic cellularity, peripheral CD4+ T-cell numbers, and B- and T-cell function. However, administration of IL-7 to recipients of allogeneic BMT had not been studied and was presumed to aggravate the development of graft-versus-host disease (GVHD) through its stimulating effects on T-cell activation and proliferation. Therefore, we studied the posttransplant administration of IL-7 in murine models for allogeneic BMT and analyzed its effects on immune reconstitution, GVHD, and graft-versus-leukemia (GVL).
Materials and methods
Cell lines and reagents
The murine IL-7–dependent immature B-cell line 2E8 was obtained from American Type Culture Collection (ATCC; Manassas, VA).46 The 32Dp210 (H-2k) is a myeloid leukemia cell line derived from 32Dc13 cells (of C3H/HeJ mouse origin) that were transfected with the p210 bcr/abloncogene.47 P815 (H-2d) is a mastocytoma cell line from DBA/2 mouse origin and YAC-1 mouse lymphoma cell line from A/Sn mouse origin (both were obtained from ATCC).
Antimurine CD16/CD32 FcR block (2.4G2) and all following fluorochrome-labeled antibodies against murine antigens were obtained from Pharmingen (San Diego, CA): Ly-9.1(30C7), CD5.1 (H11-86.1), CD3(145-2C11), CD4 (RM4-5), CD8a(53-6.7), CD8b.2(53-5.8), CD62L (MEL-14), CD122 (TM-B1), CD44 (IM7), CD19(1D3), CD45R/B220 (RA3-6B2), NK1.1-PE (PK136), Pan NK (DX5), CD11b (M1/70), CD25 (PC61), CD43 (S7), IgM-FITC (R6-60.2), T-cell receptor-β (TCR-β; H57-597), γδ-TCR (GL3), TCR-Vβ analysis kit, CD127 (B12-1), interferon-γ (IFN-γ; XMG1.2), tumor necrosis factor-α (TNF-α; MP6-XT22), IL-2 (JES6-5H4), IL-4(11B11), IL-10 (JES5-16E3); isotypic controls: rat IgG2a-κ (R35-95), rat IgG2a-λ (B39-4), rat IgG2b-(A95-1), rat IgG1-κ (R3-34), hamster IgG-group1-κ (A19-3), hamster IgG-group 2-λ (Ha4/8). Streptavidin-FITC and -phycoerythrin (PE) also were obtained from Pharmingen. Recombinant human IL-7 was kindly provided by Dr Michel Morre (Biotech Inflection Point, Gentilly, France) and recombinant murine IL-7 was obtained from R & D Systems (Minneapolis, MN). To confirm that the human recombinant IL-7 could stimulate murine cells, we performed thymidine incorporation proliferation assays with an IL-7–dependent murine pre-B cell line 2E8 and found that the human IL-7 used in our studies had a proliferative effect on murine cells that was equal to murine IL-7 (data not shown).
Tissue culture medium consisted of RPMI 1640 supplemented with 10% heat inactivated fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine (as well as 50 μM 2-mercaptoethanol for the culture of 32Dp210 cells and proliferation assays). Human IL-7 was obtained from R & D Systems. Concanavalin A (ConA) was obtained from Sigma (St Louis, MO).
Mice and BMT
Female C57BL/6J (B6, H-2b), C3FeB6F1/J([B6 × C3H]F1; H-2b/k), B10.BR (H-2k), and CBA/J (H-2k) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and used in BMT experiments when they were between 8 and 10 weeks of age or 9 months old (CBA/J). BMT protocols were approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee. The BM cells were removed aseptically from femurs and tibias. Donor BM was depleted of T cells by incubation with anti–Thy-1.2 for 30 minutes at 4°C followed by incubation with Low-TOX-M rabbit complement (Cedarlane Laboratories, Hornby, ON, Canada) for 1 hour at 37°C. Splenic T cells were obtained by purification over a nylon wool column followed by red cell removal with ammonium chloride red cell lysis buffer. Cells (5 × 106BM cells with or without splenic T cells and leukemia cells) were resuspended in Dulbecco modified essential medium (Life Technologies, Grand Island, NY) and transplanted by tail vein infusion (0.25 mL total volume) into lethally irradiated recipients on day 0. Prior to transplantation, on day 0 recipients received 1300 cGy total body irradiation (137Cs source) as split dose with 3 hours between doses (to reduce gastrointestinal toxicity). Mice were housed in sterilized microisolator cages and received normal chow and autoclaved hyperchlorinated drinking water (pH 3.0).
Administration of IL-7
We compared 2 routes of administration: (1) subcutaneous continuous administration by osmotic minipump, and (2) intraperitoneal injection. IL-7 was either given from day 14 to 28 (1 μg/d, subcutaneous pump) or day 21 to 27 (10 μg/d intraperitoneally) for immune reconstitution studies. For GVHD studies, IL-7 was either administered from day −1 to +13 (1 μg/d, subcutaneous pump), or day 14 to 28 (1 μg/d, subcutaneous pump). We used 14-day, miniosmotic pumps (Alzet, no. 2002; pump rate 0.5 μL/h; Palo Alto, CA). After methoxyflurane (Schering-Plough Animal Health, Union, NJ) anesthesia, pumps were implanted into animals through a small incision inferior to the scapula.
Assessment of GVHD
The severity of GVHD was assessed with a clinical GVHD scoring system as first described by Cooke and coworkers.48Briefly, ear-tagged animals in coded cages were individually scored every week for 5 clinical parameters on a scale from 0 to 2: weight loss, posture, activity, fur, and skin. A clinical GVHD index was generated by summation of the 5 criteria scores (0-10). Survival was monitored daily. Animals with scores of 5 or more were considered moribund and were humanely killed.
Leukemia induction and assessment of leukemic death versus death from GVHD
Animals received 32Dp210 cells intravenously on day 0 of BMT in a separate injection. Survival was monitored daily and the cause of death after BMT was determined by necropsy by our Veterinary Pathologist Dr Hai T. Nguyen (Cornell University, New York, NY) as previously described.49 50 Briefly, death from leukemia was characterized by hepatosplenomegaly and the presence of leukemic cells in liver and spleen on microscopic examination, whereas death from GVHD was defined as the absence of hepatosplenomegaly and leukemic cells in liver and spleen, and the presence of clinical symptoms of GVHD as assessed by our clinical GVHD scoring system at the time of death.
Cells and serum
As described before, splenic T cells were obtained by purification over a nylon wool column, which resulted in more than 75% purity. Splenocytes from recipients at day 14 after BMT, which were used in cytotoxicity assays, and proliferation assays, were first pretreated with ammonium chloride to remove red cells and percentages of CD4+ and CD8+ T cells from donor origin were subsequently determined by 2- and 3-color flow cytometry. Peripheral blood was obtained by retro-orbital or intracardiac puncture after general anesthesia with methoxyflurane.
Enzyme-linked immunosorbent assay
The murine TNF and human IL-7 enzyme-linked immunosorbent assay (ELISA) kits were obtained from R & D Systems and assays were performed according to the manufacturer's protocol.
Allogeneic stimulation
Splenocytes (2 × 106/mL), which were obtained from transplanted animals on day 14, were used as effectors against cell lines in cytotoxicity assays or incubated with 2000-cGy irradiated recipient splenocytes (1 × 106/mL) in 24-well plates for 5 days as stimulated cells for intracellular flow cytometric analysis.
51Cr release assays
Target cells were labeled with 100 μCi 51Cr at 2 × 106 cells/mL for 2 hours at 37°C and 5% CO2. After 3 washes, labeled targets were plated at 5 × 103 cells/well in U-bottomed plates (Costar, Cambridge, MA). Splenic T cells were added at various effector-to-target ratios in a final volume of 200 μL to 4 to 6 wells and incubated for 4 to 6 hours at 37°C and 5% CO2. Subsequently, 35 μL supernatant was removed from each well and counted in a gamma counter (Packard, Meriden, CT) to determine experimental release. Spontaneous release was obtained from wells receiving target cells and medium only, and total release was obtained from wells receiving 5% Triton X-100. The spontaneous release was less than 15% of the total release. Percent cytotoxicity was calculated by the following formula: percent toxicity = 100 × [(experimental release – spontaneous release)/(total release – spontaneous release)].
Proliferation assays
Splenocytes (4 × 105 cells/well; prepared as described above) were incubated for 5 days with irradiated (2000 cGy) (B6 × C3H)F1 splenocytes as stimulators (2 × 105cells/well) in 96-well plates and also splenocytes (4 × 105 cells/well) were stimulated with ConA (2.5 μg/mL as a final concentration) for 3 days. Cultures were pulsed during the final 18 hours with 1 μCi/well [3H]-thymidine and DNA was harvested on a Harvester 96 (Packard). Stimulation indices (SI) were calculated as the ratio of stimulated cells (cpm) over unstimulated cells (cpm).
Flow cytometric analysis
Splenocytes or thymocytes were washed in FACS buffer (phosphate-buffered saline [PBS]/2% bovine serum albumin [BSA]/0.1% azide) and 106 cells were incubated for 30 minutes at 4°C with CD16/CD32 FcR block. Subsequently, cells were incubated for 30 minutes at 4°C with primary antibody/antibodies and washed twice with FACS buffer. The stained cells were resuspended in FACS buffer and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) with CellQuest software.
Detection of alloreactive T-cell clones with intracellular cytokine staining
Briefly, cells were incubated for 12 to 15 hours (for secondary allogeneic stimulation with T cell–depleted [TCD], irradiated recipient cells) with Brefeldin A (10 μg/mL), harvested, washed, stained with primary (surface) fluorochrome (FITC, PerCP, and APC)-conjugated antibodies, fixed, and permeabilized with the Cytofix/Cytoperm kit (Pharmingen), and subsequently stained with secondary (intracellular cytokine) PE-conjugated antibody. FACS analysis was conducted by gating for the designated populations (O.A. and M.-A.P., manuscript in preparation). Flow cytometer and software were used as mentioned before.
Statistics
All values are expressed as mean ± SEM. The Mantel-Cox log-rank test was used for survival data and all other statistical analysis was performed with the nonparametric unpaired Mann-WhitneyU test. A P value less than .05 was considered statistically significant.
Results
IL-7 administration from day −1 to +13 increases thymocyte reconstitution after syngeneic BMT
In our initial studies we determined that continuous subcutaneous administration of IL-7 (1 μg/d from day −1 to +13) significantly increases thymocyte numbers at day +14 in 3-month-old CBA/J recipients of syngeneic CBA/J TCD BM compared to control mice that received PBS after transplantation (49 ± 6 × 106 versus 25 ± 3 × 106, P = .02; 4 mice/group). Further analysis of thymic populations revealed that CD4+CD8+ double-positive, CD4+single-positive, and CD8+ single-positive thymocytes were all significantly increased in IL-7–treated recipients. We found no significant increase in numbers of splenic B cells, T cells, or macrophages. However, these increases were not sustained because recipients of syngeneic TCD BM that received continuous subcutaneous IL-7 from day −1 to +13 were not significantly different from untreated recipients when analyzed at day +28 after BMT (data not shown).
IL-7 administration from day −1 to +13 has no effect on T- and B-cell reconstitution in recipients of allogeneic TCD BM
We chose 2 well-described murine allogeneic BMT models to test IL-7 administration after BMT: (1) B10.BR → CBA/J, a MHC-matched (H2k), but minor histocompatibility antigen-mismatched model for engraftment and GVHD,51,52 and (2) B6 (H-2b) → (B6 × C3H)F1 (H-2b/k), a parent → F1 MHC-mismatched model for engraftment, GVHD, and GVL.49 53 To assess the effects of IL-7 in middle-aged recipients of allogeneic BMT, 9-month-old CBA/J mice were also used as recipients. To determine the effect of IL-7 on GVL activity, some (B6 × C3H)F1 recipients were injected with 32Dp210 chronic myeloid leukemia (CML) cells at the time of BMT.
In our first experiments regarding allogeneic BMT we administered IL-7 continuously from day −1 to +13 and found at analysis on day +14 no effect on thymic cellularity and thymocyte subsets or splenic B-cell, T-cell, and monocyte/macrophage numbers (of donor or host origin) in CBA/J recipients of B10.BR TCD BM (data not shown). Also, continuous subcutaneous administration of IL-7 from day −1 to +13 did not result in significant changes in the numbers of thymocytes or splenic B and T cells (of donor or host origin) in recipients of allogeneic TCD BM when analyzed at day +28 (data not shown).
When we tested administration of IL-7 from day 0 to +42 (2.5 μg/d intraperitoneally), we were unable to detect by ELISA serum levels of human IL-7 in recipients of allogeneic TCD BM. This suggests that the prolonged administration of human recombinant IL-7 will result in the production of neutralizing antibodies, which is not unexpected because human IL-7 has only a 70% homology with murine IL-7. In contrast, we could demonstrate high levels of human IL-7 in all recipients of 2 weeks of continuous subcutaneous IL-7 administration (1 μg/d; either from day −1 to +13 or from day +14 to +28) or 1 week of intraperitoneal injections (10 μg/d; day +21 to +27). This suggests that these dosing schedules did not result in the induction of neutralizing antihuman IL-7 antibody production. In our next experiments we chose to administer IL-7 from day +14 (or +21) to +27, because (1) administration of IL-7 early after BMT seemed to be ineffective in recipients of an allogeneic BMT, and (2) prolonged administration of IL-7 was ineffective (probably due to neutralizing antibody formation).
Administration of IL-7 from day +21 to +27 enhances thymic reconstitution in 3- and 9-month-old recipients of allogeneic MHC-matched or MHC-mismatched TCD-BM
Administration of IL-7 (10 μg/d intraperitoneally from day +21 to +27) resulted in a remarkable enhancement of B- and T-cell reconstitution in 3-month-old CBA/J recipients of allogeneic B10.BR TCD BM. Analysis on day 28 demonstrated a significant increase in overall thymic cellularity, CD4+CD8+ double-positive thymocytes, and CD4+ and CD8+ single-positive thymocytes (Table 1). More than 98% of thymocytes were of donor origin. A specific analysis of double-negative CD4−CD8− thymocytes showed a significant increase in CD44+CD25− thymocytes (also termed: double negative 1 [DN1]), and CD44−CD25+ thymocytes (DN3). In similar experiments with continuous subcutaneous administration of IL-7 from day +14 to +28, we found also a significant increase in thymic cellularity and precursor populations (data not shown). These data suggest that posttransplant IL-7 administration has a stimulatory effect on thymic reconstitution and increases thymocyte populations including the earliest thymocyte precursors.
IL-7 administration enhances T- and B-cell reconstitution after allogeneic BMT
| . | Phenotype . | B10.BR → CBA (3 mo) . | B10.BR → CBA (9 mo) . | B6 → (B6 × C3H)F1 . | |||
|---|---|---|---|---|---|---|---|
| PBS . | IL-7 . | PBS . | IL-7 . | PBS . | IL-7 . | ||
| Thymocytes | |||||||
| Total number | 46.1 ± 7.1 | 80.7 ± 8.7 | 13.7 ± 2.2 | 31.0 ± 4.7 | 50.4 ± 5.8 | 75.3 ± 7.7 | |
| DN1 | CD44+ CD25−CD3− CD4−CD8− | 0.15 ± .06 | 0.23 ± .06 | .07 ± .01 | 0.1 ± .01 | 0.3 ± .03 | 0.8 ± 0.2 |
| DN2 | CD44+ CD25+ CD3−CD4−CD8− | 0.07 ± .03 | 0.07 ± .02 | .07 ± .03 | 0.04 ± .01 | 0.2 ± .02 | 0.2 ± .02 |
| DN3 | CD44− CD25+ CD3−CD4−CD8− | 0.4 ± .09 | 0.9 ± 0.1 | .07 ± .02 | 0.2 ± .05 | 0.6 ± 0.1 | 1.0 ± .06 |
| DP | CD4+CD8+ | 32.5 ± 5.2 | 65.9 ± 7.1 | 11.5 ± 1.9 | 27.0 ± 2.5 | 37.8 ± 4.7 | 55.4 ± 5.8 |
| CD4+ SP | CD4+CD8− | 3.6 ± .06 | 7.2 ± 0.9 | 1.2 ± 0.2 | 2.3 ± 0.3 | 7.8 ± 0.9 | 11.9 ± 0.3 |
| CD8+ SP | CD4−CD8+ | 1.5 ± 0.3 | 3.0 ± 0.4 | 0.4 ± .05 | 1.0 ± 1.1 | 2.1 ± 0.3 | 2.9 ± 0.2 |
| T cells (spleen) | |||||||
| T cells (donor) | CD3+Ly9.1− | 9.3 ± 2.5 | 20.7 ± 3.0 | 10.8 ± 1.6 | 20.6 ± 1.6 | 9.0 ± 1.0 | 22.4 ± 2.8 |
| T cells (host) | CD3+Ly9.1+ | 3.1 ± 0.4 | 5.0 ± 0.8 | 2.8 ± 0.6 | 4.2 ± 1.2 | 6.1 ± 0.6 | 18.7 ± 2.5 |
| Naive CD4+ | CD4+ CD44 (lo) CD62L (hi) | 0.6 ± 0.1 | 1.9 ± 0.3 | 0.8 ± 0.2 | 1.1 ± 0.2 | 4.2 ± 0.6 | 7.5 ± 0.8 |
| Activated CD4+ | CD4+ CD44 (hi) CD62L (hi) | 1.6 ± 0.3 | 4.4 ± 0.5 | 3.2 ± 0.4 | 5.9 ± 0.3 | 1.8 ± 0.2 | 4.8 ± 0.3 |
| Memory CD4+ | CD4+ CD44 (hi) CD62L (lo) | 6.2 ± 0.8 | 16.3 ± 1.0 | 5.8 ± 0.8 | 9.7 ± 0.7 | 3.7 ± 0.2 | 10.0 ± 0.6 |
| Naive CD8+ | CD8+ CD44 (lo) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.4 ± 0.1 | 1.2 ± 0.1 | 2.5 ± 0.2 |
| Act/Mem CD8+ | CD8+ CD44 (hi) | 2.7 ± 0.0 | 5.2 ± 0.8 | 6.1 ± 0.8 | 9.8 ± 0.7 | 2.2 ± 0.2 | 7.9 ± 0.8 |
| B cells | |||||||
| Pro-B (BM) | CD43+ CD45R+IgM− | 0.3 ± .05 | 0.8 ± 0.1 | 0.9 ± 0.2 | 3.3 ± 0.4 | 0.6 ± .06 | 0.7 ± 0.1 |
| Pre-B (BM) | CD43− CD45R+IgM− | 1.2 ± 0.3 | 5.0 ± 0.6 | 3.6 ± 0.6 | 10.7 ± 2.6 | 3.5 ± 0.3 | 9.5 ± 1.3 |
| Immature B (BM) | CD43− CD45R+IgM+ | 1.0 ± 0.2 | 1.9 ± 0.4 | 5.8 ± 1.0 | 10.7 ± 1.5 | 1.4 ± 0.1 | 2.7 ± 0.4 |
| Pro-B (spleen) | CD43+ CD45R+IgM− | 1.7 ± 0.6 | 14.6 ± 2.6 | 2.1 ± 0.3 | 12.9 ± 2.9 | 1.1 ± .09 | 4.8 ± 0.5 |
| Pre-B (spleen) | CD43− CD45R+IgM− | 3.9 ± 0.9 | 33.4 ± 3.8 | 10.4 ± 1.9 | 40.4 ± 4.3 | 2.9 ± 0.4 | 33.5 ± 5.1 |
| Immature B (spleen) | CD43− CD45R+IgM+ | 58.8 ± 8.0 | 108.0 ± 6.0 | 59.4 ± 11 | 122.6 ± 16 | 42.2 ± 5.2 | 122.5 ± 13 |
| CD19+ (donor) | CD19+Ly9.1− | 81.9 ± 13 | 186.0 ± 14 | 119.5 ± 9.3 | 233.2 ± 24 | 50.0 ± 5.3 | 169.6 ± 19 |
| CD19+ (host) | CD19+Ly9.1+ | 2.8 ± .05 | 4.6 ± 0.4 | 4.4 ± 0.5 | 7.4 ± 1.2 | 3.7 ± 0.4 | 11.6 ± 0.8 |
| Macrophages (spleen) | |||||||
| CD11b (donor) | CD11b+Ly9.1− | 8.3 ± 1.3 | 15.5 ± 2.0 | 36.8 ± 4.7 | 74.9 ± 6.0 | 8.9 ± 0.8 | 26.7 ± 1.5 |
| CD11b (host) | CD11b+Ly9.1+ | 0.8 ± 0.2 | 0.7 ± 0.1 | 2.9 ± 0.5 | 4.5 ± 0.7 | 2.2 ± 0.3 | 5.8 ± 0.6 |
| NK and NK T cells (spleen) | |||||||
| NK1.1 (donor) | NK1.1+Ly9.1− | 5.5 ± 1.2 | 11.3 ± 1.8 | 2.2 ± 0.2 | 3.6 ± 0.4 | 2.3 ± 0.3 | 5.2 ± 0.7 |
| NK1.1 (host) | NK1.1+Ly9.1+ | 1.9 ± 0.4 | 2.8 ± 0.8 | 0.6 ± 0.1 | 1.5 ± 0.3 | 0.9 ± .10 | 2.8 ± 0.3 |
| NK T | TCRαβ+NK1.1+ | 3.8 ± 0.5 | 3.0 ± 1.0 | 1.2 ± 0.2 | 2.2 ± 0.4 | 0.7 ± 0.1 | 2.8 ± 0.3 |
| Peripheral blood | |||||||
| WBCs | 3.3 ± .40 | 6.5 ± 0.6 | 2.2 ± .20 | 3.5 ± 0.2 | 4.0 ± 0.4 | 6.1 ± 1.0 | |
| Lymph | 2.5 ± 0.4 | 5.3 ± 0.6 | 1.3 ± 0.2 | 2.4 ± 0.1 | 2.7 ± 0.4 | 4.5 ± 0.7 | |
| . | Phenotype . | B10.BR → CBA (3 mo) . | B10.BR → CBA (9 mo) . | B6 → (B6 × C3H)F1 . | |||
|---|---|---|---|---|---|---|---|
| PBS . | IL-7 . | PBS . | IL-7 . | PBS . | IL-7 . | ||
| Thymocytes | |||||||
| Total number | 46.1 ± 7.1 | 80.7 ± 8.7 | 13.7 ± 2.2 | 31.0 ± 4.7 | 50.4 ± 5.8 | 75.3 ± 7.7 | |
| DN1 | CD44+ CD25−CD3− CD4−CD8− | 0.15 ± .06 | 0.23 ± .06 | .07 ± .01 | 0.1 ± .01 | 0.3 ± .03 | 0.8 ± 0.2 |
| DN2 | CD44+ CD25+ CD3−CD4−CD8− | 0.07 ± .03 | 0.07 ± .02 | .07 ± .03 | 0.04 ± .01 | 0.2 ± .02 | 0.2 ± .02 |
| DN3 | CD44− CD25+ CD3−CD4−CD8− | 0.4 ± .09 | 0.9 ± 0.1 | .07 ± .02 | 0.2 ± .05 | 0.6 ± 0.1 | 1.0 ± .06 |
| DP | CD4+CD8+ | 32.5 ± 5.2 | 65.9 ± 7.1 | 11.5 ± 1.9 | 27.0 ± 2.5 | 37.8 ± 4.7 | 55.4 ± 5.8 |
| CD4+ SP | CD4+CD8− | 3.6 ± .06 | 7.2 ± 0.9 | 1.2 ± 0.2 | 2.3 ± 0.3 | 7.8 ± 0.9 | 11.9 ± 0.3 |
| CD8+ SP | CD4−CD8+ | 1.5 ± 0.3 | 3.0 ± 0.4 | 0.4 ± .05 | 1.0 ± 1.1 | 2.1 ± 0.3 | 2.9 ± 0.2 |
| T cells (spleen) | |||||||
| T cells (donor) | CD3+Ly9.1− | 9.3 ± 2.5 | 20.7 ± 3.0 | 10.8 ± 1.6 | 20.6 ± 1.6 | 9.0 ± 1.0 | 22.4 ± 2.8 |
| T cells (host) | CD3+Ly9.1+ | 3.1 ± 0.4 | 5.0 ± 0.8 | 2.8 ± 0.6 | 4.2 ± 1.2 | 6.1 ± 0.6 | 18.7 ± 2.5 |
| Naive CD4+ | CD4+ CD44 (lo) CD62L (hi) | 0.6 ± 0.1 | 1.9 ± 0.3 | 0.8 ± 0.2 | 1.1 ± 0.2 | 4.2 ± 0.6 | 7.5 ± 0.8 |
| Activated CD4+ | CD4+ CD44 (hi) CD62L (hi) | 1.6 ± 0.3 | 4.4 ± 0.5 | 3.2 ± 0.4 | 5.9 ± 0.3 | 1.8 ± 0.2 | 4.8 ± 0.3 |
| Memory CD4+ | CD4+ CD44 (hi) CD62L (lo) | 6.2 ± 0.8 | 16.3 ± 1.0 | 5.8 ± 0.8 | 9.7 ± 0.7 | 3.7 ± 0.2 | 10.0 ± 0.6 |
| Naive CD8+ | CD8+ CD44 (lo) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.4 ± 0.1 | 1.2 ± 0.1 | 2.5 ± 0.2 |
| Act/Mem CD8+ | CD8+ CD44 (hi) | 2.7 ± 0.0 | 5.2 ± 0.8 | 6.1 ± 0.8 | 9.8 ± 0.7 | 2.2 ± 0.2 | 7.9 ± 0.8 |
| B cells | |||||||
| Pro-B (BM) | CD43+ CD45R+IgM− | 0.3 ± .05 | 0.8 ± 0.1 | 0.9 ± 0.2 | 3.3 ± 0.4 | 0.6 ± .06 | 0.7 ± 0.1 |
| Pre-B (BM) | CD43− CD45R+IgM− | 1.2 ± 0.3 | 5.0 ± 0.6 | 3.6 ± 0.6 | 10.7 ± 2.6 | 3.5 ± 0.3 | 9.5 ± 1.3 |
| Immature B (BM) | CD43− CD45R+IgM+ | 1.0 ± 0.2 | 1.9 ± 0.4 | 5.8 ± 1.0 | 10.7 ± 1.5 | 1.4 ± 0.1 | 2.7 ± 0.4 |
| Pro-B (spleen) | CD43+ CD45R+IgM− | 1.7 ± 0.6 | 14.6 ± 2.6 | 2.1 ± 0.3 | 12.9 ± 2.9 | 1.1 ± .09 | 4.8 ± 0.5 |
| Pre-B (spleen) | CD43− CD45R+IgM− | 3.9 ± 0.9 | 33.4 ± 3.8 | 10.4 ± 1.9 | 40.4 ± 4.3 | 2.9 ± 0.4 | 33.5 ± 5.1 |
| Immature B (spleen) | CD43− CD45R+IgM+ | 58.8 ± 8.0 | 108.0 ± 6.0 | 59.4 ± 11 | 122.6 ± 16 | 42.2 ± 5.2 | 122.5 ± 13 |
| CD19+ (donor) | CD19+Ly9.1− | 81.9 ± 13 | 186.0 ± 14 | 119.5 ± 9.3 | 233.2 ± 24 | 50.0 ± 5.3 | 169.6 ± 19 |
| CD19+ (host) | CD19+Ly9.1+ | 2.8 ± .05 | 4.6 ± 0.4 | 4.4 ± 0.5 | 7.4 ± 1.2 | 3.7 ± 0.4 | 11.6 ± 0.8 |
| Macrophages (spleen) | |||||||
| CD11b (donor) | CD11b+Ly9.1− | 8.3 ± 1.3 | 15.5 ± 2.0 | 36.8 ± 4.7 | 74.9 ± 6.0 | 8.9 ± 0.8 | 26.7 ± 1.5 |
| CD11b (host) | CD11b+Ly9.1+ | 0.8 ± 0.2 | 0.7 ± 0.1 | 2.9 ± 0.5 | 4.5 ± 0.7 | 2.2 ± 0.3 | 5.8 ± 0.6 |
| NK and NK T cells (spleen) | |||||||
| NK1.1 (donor) | NK1.1+Ly9.1− | 5.5 ± 1.2 | 11.3 ± 1.8 | 2.2 ± 0.2 | 3.6 ± 0.4 | 2.3 ± 0.3 | 5.2 ± 0.7 |
| NK1.1 (host) | NK1.1+Ly9.1+ | 1.9 ± 0.4 | 2.8 ± 0.8 | 0.6 ± 0.1 | 1.5 ± 0.3 | 0.9 ± .10 | 2.8 ± 0.3 |
| NK T | TCRαβ+NK1.1+ | 3.8 ± 0.5 | 3.0 ± 1.0 | 1.2 ± 0.2 | 2.2 ± 0.4 | 0.7 ± 0.1 | 2.8 ± 0.3 |
| Peripheral blood | |||||||
| WBCs | 3.3 ± .40 | 6.5 ± 0.6 | 2.2 ± .20 | 3.5 ± 0.2 | 4.0 ± 0.4 | 6.1 ± 1.0 | |
| Lymph | 2.5 ± 0.4 | 5.3 ± 0.6 | 1.3 ± 0.2 | 2.4 ± 0.1 | 2.7 ± 0.4 | 4.5 ± 0.7 | |
Lethally irradiated (1300 cGy) CBA/J recipients (3 and 9 months old) received transplants with B10.BR TCD BM (5 × 106) and lethally irradiated (1300 cGy) (B6 × C3H)F1 recipients received B6 TCD BM (5 × 106). Recipients received 10 μg/d IL-7 or PBS (control) by intraperitoneal injection from day 21 to day 27. Animals were humanely killed on day 28 and populations of cells were calculated from total cell counts and multicolor flow cytometric analyses. Donor or host origin was determined with anti-Ly9.1 or anti-CD5.1, which only reacts with leukocytes of host origin. Absolute lymphocyte counts were calculated from automated complete blood counts and differential counts on a blood smear. Values represent mean cell number (106) ± SE and each group contained 6 to 8 animals. All values in bold represent significantly increased cell numbers (P < .05) in IL-7–treated recipients compared to PBS-treated control recipients. Peripheral blood values represent cell count × 103/μL ± SE. DN indicates double-negative thymocytes; DP, double-positive thymocytes, SP, single-positive thymocytes; Act/Mem, activated + memory cells; WBCs, white blood cells; Lymph, lymphocytes.
We repeated these experiments with 9-month-old (so termed: middle-aged) CBA/J recipients of allogeneic B10.BR TCD BM to test the effects of posttransplant IL-7 in older recipients with greater pretransplant thymic involution. As expected, thymic cellularity at day +28 was less in 9-month-old recipients than in 3-month-old recipients. However, we found again a significant increase in thymic cellularity, CD44+CD25− DN1 thymocytes, CD4+CD8+ double-positive thymocytes, and CD4+ and CD8+ single-positive thymocytes in recipients treated with IL-7 (Table 1) and more than 95% of thymocytes were of donor origin (data not shown). We conclude that posttransplant IL-7 can enhance thymic reconstitution even in middle-aged recipients of an allogeneic BMT.
The same IL-7 administration schedule was also tested in a parent → F1 strain combination with MHC class I and II disparity. Analysis of thymic reconstitution at day +28 revealed a significant increase in thymic cellularity and thymocyte populations in recipients treated with IL-7 (Table 1). Again, more than 98% of thymocytes were of donor origin. These findings are similar to those in 3-month-old MHC-matched CBA/J recipients. We conclude that IL-7 administration can enhance thymic reconstitution in a parent → F1 BMT model despite MHC class I and II disparity.
Administration of IL-7 results in increased numbers of CD4+CD8dim thymocytes in recipients of an allogeneic BMT
A recent study by Brugnera and coworkers54 suggested that CD4+8+ double-positive thymocytes will go through a CD4+CD8dim stage before differentiating into CD4+ and CD8+single-positive cells. IL-7 was required for the development of CD8+ single-positive cells by promoting the survival of CD4+CD8dim cells, which are committed to develop into a CD8+ single-positive cell and whose TCR signals have ceased.
We analyzed the effects of IL-7 administration (10 μg/d intraperitoneally from day +21 to day +27) on these CD4+CD8dim thymocytes in thymi from recipients of an allogeneic BMT at day +28. We found in all 3 BMT models an increased percentage (and number) of donor-derived CD4+CD8dim thymocytes: 3-month-old CBA/J recipients (IL-7 treated 3.8% ± 0.2% and control 2.8% ± 0.1%;P = .002), 9-month-old CBA/J recipients (IL-7 treated 3.0% ± 0.2% and control 2.7% ± 0.2%; P = NS), and (B6 × C3H)F1 recipients (IL-7 treated 3.7% ± 0.2% and control 3.1% ± 0.2%; P = .02). However, as mentioned below, this increase in CD4+CD8dim thymocytes had no effect on the percentages (and ratio) of CD4+ and CD8+ single-positive thymocytes.
Administration of IL-7 from day +21 to +27 enhances splenic CD4+ and CD8+ T-cell reconstitution after allogeneic BMT
Analysis at day +28 of IL-7–treated allogeneic BMT recipients showed significantly increased numbers of donor-derived splenic CD3+ T cells independent of the age of the recipient or donor/host MHC disparity (Table 1). IL-7 increased the numbers of splenic CD4+ and CD8+ T cells equally well and no significant change in the CD4/CD8 ratio was noted in IL-7–treated recipients (data not shown). Further analysis by multicolor flow cytometry of donor-derived naive, activated, and memory CD4+ and CD8+ T cells demonstrated that IL-7 had a significantly greater effect on activated and memory T cells than on naive T cells. We also examined the effect of IL-7 treatment on CD8+CD122hi memory T cells and found again significantly increased numbers in the spleen on day 28 (data not shown).
Administration of IL-7 from day +21 to +27 enhances precursor and mature B-cell reconstitution in BM and spleen in recipients of an allogeneic BMT
To determine the effects of posttransplant IL-7 administration on B-cell reconstitution, we analyzed at day +28 the numbers of donor-derived precursor and mature B cells in BM and spleen. We found significant increases in precursor and mature B cells in spleen and BM of IL-7–treated recipients independent of the age of the recipient or the donor/host MHC disparity (Table 1). Repeat experiments with continuous subcutaneous administration of IL-7 from day +14 to day +28 had similar results (data not shown).
Administration of IL-7 from day +21 to +27 increases peripheral blood lymphocyte numbers and splenic monocyte, macrophage, NK, and NK T-cell numbers in allogeneic BMT recipients
We also performed complete peripheral blood counts at day +28 and found significantly increased white blood cell counts and absolute lymphocyte counts in IL-7–treated recipients (Table 1). All other counts, including platelets, absolute neutrophils, hemoglobin concentration, and hematocrit were not affected. Finally, we also detected at day +28 significant increases in the numbers of donor-derived splenic monocytes/macrophages, NK, and NK T cells (defined as NK1.1+TCRα/β+) in IL-7–treated recipients.
Posttransplant administration of IL-7 increases the proliferative capability of T cells, but has no effect on the T-cell repertoire
To assess the effects of posttransplant IL-7 treatment on T-cell function, we determined the proliferative capability of reconstituting splenic T cells at day +28 by stimulation with ConA. Figure 1A shows that splenic T cells from IL-7–treated recipients of allogeneic TCD BM displayed improved ConA-stimulated proliferation. We also analyzed the effects of posttransplant IL-7 treatment on the reconstitution of the T-cell repertoire as assessed by the expression of TCR-Vβ families. As shown in Figure 1B, we could not detect any apparent differences in TCR-Vβ expression between IL-7–treated recipients and PBS-treated control recipients.
IL-7 administration after BMT has no effect on the T-cell repertoire and increases the proliferative capability of T cells after BMT.
All CBA/J and (B6 × C3H)F1 recipients were transplanted as described in Table 1. IL-7 was administered from day 21 to day 27 by intraperitoneal injection (10 μg/d, panel A) or from day 14 to day 28 by subcutaneous osmotic pump (1 μg/d, panel B). Animals were humanely killed on day +28 and splenocytes were obtained for ConA proliferation assays (A) and for flow cytometric analysis of TCR-Vβ families (B). Splenocytes were cultured for 3 days with ConA (2.5 μg/mL) and [3H]-thymidine was added during the final 18 hours of culture. The mean thymidine incorporation in unstimulated splenocytes ranged from 2317 to 5141 cpm. Values represent means ± SE and each group contained 6 to 8 animals. The Vβ repertoire was determined in normal donor B10.BR mice, normal 3-month-old CBA mice, normal 9-month-old CBA/J mice, 9-month-old CBA/J BMT recipients (PBS treated; control), 9-month-old CBA/J BMT recipients (IL-7 treated).
IL-7 administration after BMT has no effect on the T-cell repertoire and increases the proliferative capability of T cells after BMT.
All CBA/J and (B6 × C3H)F1 recipients were transplanted as described in Table 1. IL-7 was administered from day 21 to day 27 by intraperitoneal injection (10 μg/d, panel A) or from day 14 to day 28 by subcutaneous osmotic pump (1 μg/d, panel B). Animals were humanely killed on day +28 and splenocytes were obtained for ConA proliferation assays (A) and for flow cytometric analysis of TCR-Vβ families (B). Splenocytes were cultured for 3 days with ConA (2.5 μg/mL) and [3H]-thymidine was added during the final 18 hours of culture. The mean thymidine incorporation in unstimulated splenocytes ranged from 2317 to 5141 cpm. Values represent means ± SE and each group contained 6 to 8 animals. The Vβ repertoire was determined in normal donor B10.BR mice, normal 3-month-old CBA mice, normal 9-month-old CBA/J mice, 9-month-old CBA/J BMT recipients (PBS treated; control), 9-month-old CBA/J BMT recipients (IL-7 treated).
Posttransplant IL-7 administration does not aggravate GVHD
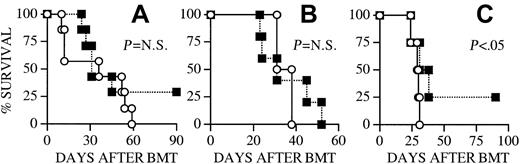
Apart from its effects on developing T cells, IL-7 can enhance in vitro proliferation and activation of mature T cells. This raises the possibility that IL-7 administration to recipients of an allogeneic BMT could have a stimulatory effect on mature T cells of donor origin, which play an important role in the development of GVHD. Therefore, IL-7 administration to recipients of an allogeneic BMT may aggravate GVHD. To analyze this potentially detrimental effect of posttransplant IL-7 administration we induced GVHD in our recipients by adding splenic donor T cells to the allogeneic TCD BM graft. The in vivo activation and proliferation of alloreactive donor T cells, which can induce GVHD, occurs primarily during the first 14 days after BMT.55Therefore we chose to administer IL-7 not only from day +14 to day +28, but also from day −1 to +13 to recipients of allogeneic TCD BM + T cells. We found that IL-7 administration did not aggravate the development of GVHD in any of our BMT models independent of MHC disparity or timing of IL-7 administration (Figure2) and, in contrast, resulted in significantly less GVHD in (B6 × C3H)F1 recipients (Figures 2C and5B). At the termination of the experiment (3 months after BMT) the 2 remaining animals in the IL-7 group of Figure 2C had significant GVHD with scores of 1 and 3, including significant weight loss. However, the 7 remaining animals in the IL-7 group in the experiment of Figure 5B had scores ranging from 0 to 2.5 (mean 0.8 ± 0.4) and were gaining weight, which suggests that these animals were recovering from acute GVHD.
IL-7 administration does not aggravate GVHD mortality in allogeneic BMT recipients.
(A) Lethally irradiated (1300 cGy) CBA/J recipients (3 months old) received transplants with B10.BR TCD BM cells (5 × 106) + splenic T cells (0.5 × 106). Osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day −1 to +13. (B) All CBA/J recipients (3 months old) underwent transplantation as described in panel A. Osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day +14 to day +28. (C) Lethally irradiated (1300 cGy) (B6 × C3H)F1 recipients underwent transplantation with B6 TCD BM cells (5 × 106) + splenic T cells (2 × 106). Osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day −1 to +13. Survival is depicted as a Kaplan-Meier curve. Each group contained 8 animals. ○ indicates PBS; ■, IL-7.
IL-7 administration does not aggravate GVHD mortality in allogeneic BMT recipients.
(A) Lethally irradiated (1300 cGy) CBA/J recipients (3 months old) received transplants with B10.BR TCD BM cells (5 × 106) + splenic T cells (0.5 × 106). Osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day −1 to +13. (B) All CBA/J recipients (3 months old) underwent transplantation as described in panel A. Osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day +14 to day +28. (C) Lethally irradiated (1300 cGy) (B6 × C3H)F1 recipients underwent transplantation with B6 TCD BM cells (5 × 106) + splenic T cells (2 × 106). Osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day −1 to +13. Survival is depicted as a Kaplan-Meier curve. Each group contained 8 animals. ○ indicates PBS; ■, IL-7.
Because of the critical importance of these results, we performed 12 allogeneic BMT experiments (involving a total of 158 recipients), which confirmed the representative data shown here. We never detected in any of these experiments an increase in GVHD morbidity or mortality due to IL-7 administration. More interestingly, IL-7 administration significantly decreased GVHD morbidity and mortality in every experiment with (B6 × C3H)F1 recipients, and overall survival from 3 independent experiments (with 3 months of follow-up) was 42% in the IL-7 group (10 of 24) compared to 21% in the control group (5 of 24).
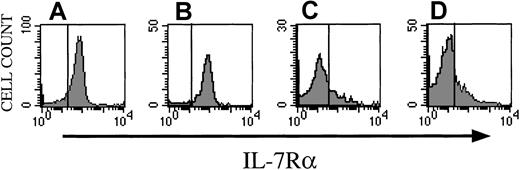
Activated/memory CD8+ donor T cells lose their IL-7 receptor during the development of GVHD
Naı̈ve and activated/memory CD8+ T cells in the spleen of normal mice express significant levels of IL-7Rα on their surface (Figure 3A,B). However, when we analyzed splenocytes at day +14 from recipients of allogeneic TCD BM + splenic T cells (which are developing GVHD), we found that the majority of donor-derived splenocytes, which are presumed to be the alloreactive GVHD-inducing effector cells, have an activated/memory phenotype and do not express IL-7Rα independent of IL-7 administration (Figure 3C,D). Similar data were obtained for alloreactive activated and memory CD4+ T cells (not shown). The loss of IL-7R expression on alloreactive donor T cells in vivo could explain why the administration of IL-7 does not aggravate GVHD.
Alloreactive donor T cells in recipients undergoing GVHD express no IL-7R.
Lethally irradiated (1300 cGy) CBA/J recipients (3 months old) received B10.BR TCD BM cells (5 × 106) + splenic T cells (0.5 × 106). Osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day −1 to day +13. All recipients were humanely killed on day 13 and spleens were harvested and processed for flow cytometric analysis. (A,B) Normal (untransplanted) B10BR splenocytes stained with CD8, CD44, CD122, and CD127 (IL-7Rα). Panel A shows CD127 expression on naive CD8 cells (CD8+, CD44−). Panel B shows CD127 expression on memory CD8 cells (CD8+, CD44+, CD122+). Panel C demonstrates CD127 expression on memory CD8+ T cells from PBS-treated recipients with GVHD. Panel D depicts CD127 expression on memory CD8+ T cells from IL-7–treated recipients with GVHD. The vertical lines represent the upper limit of the isotopic Ig controls.
Alloreactive donor T cells in recipients undergoing GVHD express no IL-7R.
Lethally irradiated (1300 cGy) CBA/J recipients (3 months old) received B10.BR TCD BM cells (5 × 106) + splenic T cells (0.5 × 106). Osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day −1 to day +13. All recipients were humanely killed on day 13 and spleens were harvested and processed for flow cytometric analysis. (A,B) Normal (untransplanted) B10BR splenocytes stained with CD8, CD44, CD122, and CD127 (IL-7Rα). Panel A shows CD127 expression on naive CD8 cells (CD8+, CD44−). Panel B shows CD127 expression on memory CD8 cells (CD8+, CD44+, CD122+). Panel C demonstrates CD127 expression on memory CD8+ T cells from PBS-treated recipients with GVHD. Panel D depicts CD127 expression on memory CD8+ T cells from IL-7–treated recipients with GVHD. The vertical lines represent the upper limit of the isotopic Ig controls.
IL-7 administration slightly diminishes the type I cytokine response in allogeneic BMT recipients
To further analyze the mechanism(s) by which IL-7 ameliorates GVHD in the (B6 × C3H)F1 recipients, we tested the expansion, cytolytic activity, intracytoplasmic cytokine secretion, and proliferative capability of splenic donor T cells from IL-7–treated recipients of allogeneic TCD BM + T cells. We first assessed the effects on in vivo donor T-cell expansion by analyzing the number of splenic donor T cells at day +14 in recipients of allogeneic TCD BM + T cells. As mentioned before, maximal donor T-cell expansion during the development of GVHD occurs in the spleen between day +10 and day +14.55-57 We found no significant difference in the in vivo donor T-cell expansion between IL-7–treated recipients and control recipients (Figure 4A). We then determined the intracytoplasmic expression of type 1 and 2 cytokines, including IL-2, IL-4, IL-10, TNF, and IFN, as well as cytolytic activity of these donor splenic T cells against host antigens and found slightly fewer T cells expressing type I inflammatory cytokines (TNF-α, IFN-γ, IL-2) in IL-7–treated than in control recipients (Figure 4B), whereas cytolytic activity was not decreased (Figure 4C). In addition to the expression of type 1 and 2 cytokines by alloreactive T cells from recipients of an allogeneic TCD BM + T cells, we also measured serum TNF levels at day +7 and day +14 in CBA/J recipients of allogeneic TCD BM + T cells and detected a moderate (but significant) difference between IL-7–treated and PBS-treated recipients on day +7 (261 ± 27 pg/mL, n = 8, versus 355 ± 19 pg/mL, n = 7, P = .04), but not on day +14 (583 ± 109 pg/mL, n = 8, versus 653 ± 207 pg/mL, n = 7,P = NS).
IL-7 administration does not stimulate alloreactive donor T cells in recipients undergoing GVHD.
Lethally irradiated (B6 × C3H)F1 or CBA/J recipients (1300 cGy) received transplants with B6 or B10.BR TCD BM (5 × 106) and splenic T cells (0.5 × 106) on day 0 as described in Table 1. Osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day −1 to day +13. All recipients were humanely killed on day 14 and spleens were harvested and processed for flow cytometric analysis and functional assays. (A) Numbers of splenic donor CD4+ and CD8+ T cells were calculated from total splenocyte counts and flow cytometric analyses with antibodies that recognize specifically host antigens (Ly9.1 and CD5.1) and T-cell antigens (CD4 and CD8). Values represent mean ± SE and each group contained 6 to 8 animals. (B) Intracellular cytokine expression of alloreactive T cells. Splenic B6 T cells were harvested on day +14 from IL-7– or PBS-treated recipients as described above and incubated with irradiated (20 Gy) (B6 × C3H)F1 splenic stimulator cells in 24-well plates for 5 days. Cells were harvested, and restimulated with TCD, irradiated (20 Gy) (B6 × C3H)F1 splenic stimulator cells for 16 hours. Brefeldin A (10 μg/mL) was added after the first hour of incubation. Intracellular cytokine expression in CD4 memory cells (CD4+, CD62L−, CD44+) was measured by flow cytometric analysis. Values represent mean ± SE and each group contained 3 animals. (C) Cytolytic activity of alloreactive T cells. Splenic B6 T cells were harvested on day +14 from IL-7– or PBS-treated recipients as described above and their cytotoxicity was determined against 32Dp210 and P815 (third party) cell lines in51Cr-release assays. (D) Alloreactive T-cell proliferation. Splenic T cells (4 × 105 cells/well) were incubated with irradiated (20 Gy) (B6 × C3H)F1 or CBA/J splenic stimulator cells (2 × 105 cells/well) in 96-well plates for 5 days and [3H]-thymidine was added during the final 20 hours of culture. The mean thymidine incorporation in unstimulated splenocytes ranged from 529 to 3336 cpm. Values represent means ± SE and each group contained 6 to 8 animals.
IL-7 administration does not stimulate alloreactive donor T cells in recipients undergoing GVHD.
Lethally irradiated (B6 × C3H)F1 or CBA/J recipients (1300 cGy) received transplants with B6 or B10.BR TCD BM (5 × 106) and splenic T cells (0.5 × 106) on day 0 as described in Table 1. Osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day −1 to day +13. All recipients were humanely killed on day 14 and spleens were harvested and processed for flow cytometric analysis and functional assays. (A) Numbers of splenic donor CD4+ and CD8+ T cells were calculated from total splenocyte counts and flow cytometric analyses with antibodies that recognize specifically host antigens (Ly9.1 and CD5.1) and T-cell antigens (CD4 and CD8). Values represent mean ± SE and each group contained 6 to 8 animals. (B) Intracellular cytokine expression of alloreactive T cells. Splenic B6 T cells were harvested on day +14 from IL-7– or PBS-treated recipients as described above and incubated with irradiated (20 Gy) (B6 × C3H)F1 splenic stimulator cells in 24-well plates for 5 days. Cells were harvested, and restimulated with TCD, irradiated (20 Gy) (B6 × C3H)F1 splenic stimulator cells for 16 hours. Brefeldin A (10 μg/mL) was added after the first hour of incubation. Intracellular cytokine expression in CD4 memory cells (CD4+, CD62L−, CD44+) was measured by flow cytometric analysis. Values represent mean ± SE and each group contained 3 animals. (C) Cytolytic activity of alloreactive T cells. Splenic B6 T cells were harvested on day +14 from IL-7– or PBS-treated recipients as described above and their cytotoxicity was determined against 32Dp210 and P815 (third party) cell lines in51Cr-release assays. (D) Alloreactive T-cell proliferation. Splenic T cells (4 × 105 cells/well) were incubated with irradiated (20 Gy) (B6 × C3H)F1 or CBA/J splenic stimulator cells (2 × 105 cells/well) in 96-well plates for 5 days and [3H]-thymidine was added during the final 20 hours of culture. The mean thymidine incorporation in unstimulated splenocytes ranged from 529 to 3336 cpm. Values represent means ± SE and each group contained 6 to 8 animals.
When we compared the antihost proliferative response of splenic donor T cells in a mixed lymphocyte reaction (MLR) against irradiated splenocytes of host origin, we found that IL-7 treatment did not aggravate the antihost proliferative activity of these cells (Figure4D).
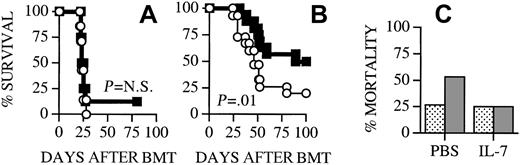
Posttransplant IL-7 administration does not diminish GVL activity
The GVHD and GVL activities are each thought to be primarily mediated by alloreactive donor T cells. We first wanted to analyze if IL-7 administration could have any effects on 32Dp210 leukemia (H-2k; C3H/HeJ background) growth in (B6 × C3H)F1 recipients of B6 TCD BM. Mortality from leukemia was unchanged in IL-7–treated recipients, which suggests that IL-7 exerts no effects on the host environment, donor BM graft, and leukemia cells, which influence leukemia growth or GVL activity (Figure 5A). To test the effects of IL-7 administration on GVL activity we administered IL-7 from day −1 to day +13 to (B6 × C3H)F1 recipients of allogeneic B6 TCD BM + T cells, which received 32Dp210 CML cells at the time of BMT. We found that IL-7–treated recipients had a significantly better overall survival (Figure 5B). All animals that died during the course of the experiment underwent an autopsy to determine the cause of death. The incidence of mortality from leukemia was identical in IL-7–treated recipients compared to control recipients, whereas the incidence of mortality from GVHD was significantly less in IL-7–treated recipients (Figure 5C). Therefore, IL-7 treatment did not seem to decrease GVL activity in these experiments. Moreover, these results confirmed our earlier results (Figure 2C), which indicated that IL-7 treatment could ameliorate the GVHD in (B6 × C3H)F1 recipients.
IL-7 administration does not diminish GVL activity.
(A) Lethally irradiated (1300 cGy) (B6 × C3H)F1 recipients were transplanted with B6 TCD BM cells (5 × 106) as described in Table 1. Recipients received 32Dp210 CML cells (5000) on day 0 and osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day −1 to day +13. Each group contained 8 animals. (B) All (B6 × C3H)F1 recipients were transplanted and treated with IL-7/PBS as described in panel A with the addition of B6 splenic T cells (0.5 × 106) on day 0. ○ indicates PBS; ■, IL-7. The cause of death (GVHD or leukemia) of all animals that died during the experiments shown in panel B was determined by necropsy and is shown in panel C. indicates tumor;
indicates tumor; , GVHD. Survival is depicted as a Kaplan-Meier curve and each group contained 14 animals.
, GVHD. Survival is depicted as a Kaplan-Meier curve and each group contained 14 animals.
IL-7 administration does not diminish GVL activity.
(A) Lethally irradiated (1300 cGy) (B6 × C3H)F1 recipients were transplanted with B6 TCD BM cells (5 × 106) as described in Table 1. Recipients received 32Dp210 CML cells (5000) on day 0 and osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day −1 to day +13. Each group contained 8 animals. (B) All (B6 × C3H)F1 recipients were transplanted and treated with IL-7/PBS as described in panel A with the addition of B6 splenic T cells (0.5 × 106) on day 0. ○ indicates PBS; ■, IL-7. The cause of death (GVHD or leukemia) of all animals that died during the experiments shown in panel B was determined by necropsy and is shown in panel C. indicates tumor;
indicates tumor; , GVHD. Survival is depicted as a Kaplan-Meier curve and each group contained 14 animals.
, GVHD. Survival is depicted as a Kaplan-Meier curve and each group contained 14 animals.
Discussion
Previous studies by Bolotin and coworkers45 and Abdul-Hai and colleagues58 59 have found in murine BMT models improved T- and B-cell reconstitution after IL-7 administration to recipients of syngeneic BMT. These studies did not address the effects of IL-7 in the 2 patient populations that have the greatest risk of prolonged immunodeficiency and opportunistic infections: recipients of an unrelated allogeneic BMT and adult recipients.
Fatal opportunistic infections after successful engraftment occur in 12% to 28% of unrelated HLA-matched transplant recipients and 4% to 15% of related HLA-matched transplant recipients.7,8,60-66 Several studies have shown that in unrelated HLA-matched transplant recipients the posttransplant mortality from infections is higher than from leukemic relapse.61,67-70 Recent data from the National Marrow Donor Program demonstrated that almost one third of all posttransplant mortality in recipients of an unrelated HLA-matched donor BMT for CML is due to infections.71 Adult recipients of a TCD unrelated BMT had prolonged and severe deficiencies in CD3+, CD4+, and CD8+ T cells when compared with pediatric recipients of TCD unrelated BMT.8These adult recipients had a significantly increased risk of life-threatening opportunistic infections that was associated with CD4+ T-cell populations less than 200/mm3. These profound differences in immune reconstitution between adult and pediatric transplant recipients suggest an age-related difference in T-cell development after transplantation.
To our knowledge, our study is the first to demonstrate in murine BMT models that IL-7 administration can enhance immune reconstitution in recipients of an allogeneic BMT and in middle-aged recipients. Although thymic involution in humans and mice is not fully comparable, it is encouraging that IL-7 administration could significantly enhance thymopoiesis in 9-month-old recipients that have a significantly decreased thymic cellularity at baseline (before transplant) compared to 3-month-old recipients (27.5 ± 6.6 × 106 cells versus 180 × 106 cells). Analysis of thymic differentiation in aged mice has shown a specific decline in the transition of DN1 to DN2 precursors, which has been associated with defective TCRβ-chain rearrangement and increased apoptosis of DN2 and DN3 cells.72 Treatment with IL-7 could reverse this age-associated defect in early thymocyte development in vitro and in vivo and result in increases in the numbers of DN1 and DN3 cells. These findings regarding thymopoiesis in normal aged mice are in full agreement with our studies in middle-aged recipients of an allogeneic BMT. Therefore, our data could be of great use for the development of clinical trials with IL-7 for those middle-aged patients, who are prone to benefit the most from an improvement of their posttransplant immune reconstitution.
Interleukin 7 might enhance posttransplant T-cell reconstitution in 2 different ways. It affects the development of thymic precursors as well as homeostasis of mature T cells in the periphery.30,41,45,73-75 In addition to its well-documented effects in the thymus on survival, differentiation, and proliferation on T-cell precursors, IL-7 could also promote thymic reconstitution through its effects on the common lymphoid precursor (CLP). Recently, Kondo and colleagues76 suggested that lymphopoiesis in mice depends on a CLP. The CLP is a rare precursor in the BM (± 0.02% of total BM cells), which gives rise to B, T, NK, and dendritic cells and is defined as an IL-7Rα+Lin-Thy1.1-Sca-1loc-Kitlocell in the BM.76-78 When CLPs were given in conjunction with hematopoietic stem cells to sublethally irradiatedRAG−/− mice, CLP-derived T and B cells appeared 7 to 10 days earlier (at week 2 after BMT) than stem cell–derived T and B cells. These CLP-derived T and B cells started to gradually decrease in numbers after 6 weeks indicating that their self-renewal is limited. Our data indicate that IL-7 administration can improve the reconstitution of the earliest thymic precursor (DN1) and this finding is in agreement with a stimulatory effect by IL-7 on the CLP.
Interleukin 7 has been proposed as a survival factor for naive cells in the periphery,41,75 whereas survival of memory cells is dependent on IL-15.41 In our studies, we detected in IL-7–treated recipients only a moderate increase in naive T cells, whereas activated and memory T cells were more profoundly increased. This “discrepancy” between increased numbers of memory T cells versus naive T cells could be due to the peripheral expansion of residual memory T cells in the BM graft, which had escaped T-cell depletion. However, we favor an alternative explanation based on recent findings regarding homeostatic T-cell proliferation in lymphopenic hosts.
The idea that peripheral T-cell homeostasis exists is supported by a number of observations: (1) T-cell numbers in spleen and lymph nodes are remarkably consistent depending on strain and age; (2) T-cell counts return to normal after insults, such as sublethal irradiation or viral infection, and mature T cells (in particular naive T cells) can detect decreased T-cell numbers and respond by dividing rapidly when transferred to T-cell–deficient mice75,79-81; and (3) TCR transgenic mice do not have increased numbers of T cells.75 Changes in the peripheral T-cell pool have no effect on thymocyte production. This would suggest that the naive T-cell pool is regulated through the homeostatic control of the loss of or expansion of naive T cells in the periphery.82
Several studies have demonstrated that naive CD8+ T cells in a lymphopenic host can temporarily acquire a memory phenotype during their homeostasis-driven expansion, but once the cellularity of the lymphoid compartment has been restored, they will regain the functional and phenotypic characteristics of naive T cells.83-85 We hypothesize that the T-cell reconstitution in our BMT recipients results in a temporary conversion of newly generated naive T cells to a memory phenotype once they encounter the lymphopenic posttransplant environment of the host. Therefore, the positive effects of IL-7 administration on posttransplant T-cell generation in the first weeks after BMT might only be reflected by an increase in the number of memory T cells. It will be of great interest to test this hypothesis further and study T-cell reconstitution after BMT sequentially to analyze if such a conversion and reversal of naive and memory phenotype occurs. We are currently analyzing the long-term effects of IL-7 on T-cell reconstitution in 3-month-old CBA/J recipients. In ongoing experiments we have observed that the populations of CD4+or CD8+ memory cells decrease from day +28 to day +84.
Levy and colleagues86 found that splenocytes (which contained alloreactive T cells) from B10.D2 → Balb/c BMT recipients in the first week after BMT could respond in vitro to IL-7. This suggested that IL-7 administration after transplantation could aggravate GVHD in recipients of allografts that contain donor T cells. However, we have tested this hypothesis extensively in 2 different GVHD models with different doses, routes, and intervals of administration of IL-7, and have not seen that IL-7 administration could aggravate GVHD. Our analyses of the underlying mechanisms for these unexpected results have revealed that alloreactive donor T cells lose their expression of IL-7Rα in vivo during the development of GVHD. We have found this loss of IL-7 receptor expression in vivo in both CD4+ and CD8+ donor T cells and in both GVHD models. These findings are in agreement with a recent study by Schluns and coworkers,87 who found that recently activated CD8+ T cells had decreased IL-7R expression, whereas naive and memory cells expressed the IL-7R. This suggested that CD8+ T cells down-regulate their IL-7R during activation and become less responsive to IL-7. We hypothesize that alloreactive T cells also down-regulate their IL-7R during stimulation and become unresponsive to the effects of IL-7 administration. This hypothesis is supported by our observations in recipients with GVHD that IL-7 administration had no effects on donor CD4+ and CD8+ T-cell expansion in vivo (Figure 4A).
In our model with a full MHC class I and II disparity, we found consistently that IL-7 could ameliorate GVHD. Our analyses of the underlying mechanisms of this interesting result demonstrated that in this model IL-7 administration results in fewer TNF-expressing alloreactive T cells. Moreover, we found recently that IL-7 administration to an MLR with alloreactive donor T cells (obtained from spleens of recipients with GVHD) as effectors and irradiated host cells as stimulators resulted in a decrease in the proliferative response (unpublished observations).
In conclusion, our data in clinically relevant murine models of allogeneic BMT suggest that the administration of IL-7 to patients who have received an allogeneic BMT could have a number of beneficial effects. First, it could result in decreased morbidity and mortality from posttransplant infections and Epstein-Barr virus lymphoproliferative disease. Second, it would allow for earlier (re)immunization against common pathogens after BMT. Third, it could allow earlier application of experimental tumor vaccination protocols after BMT. Fourth, our preliminary data raise the possibility that IL-7 in some cases could have an anti-GVHD effect while sparing GVL activity. Finally, IL-7 has the potential as a lymphoid growth factor in a variety of clinical settings to overcome lymphocytopenia or to stimulate lymphocyte regeneration, including autologous BMT, high-dose chemotherapy, acquired immunodeficiency syndrome, or vaccination (for infectious agents or tumors).
We would like to thank Drs Malcolm E. Moore, Keith B. Elkon, Richard J. O'Reilly, and Michel Morre for helpful discussions. We would like also to thank Dr Neil Lipman, Dr Hai Nguyen, and the staff at the Research Animal Resource Center (Memorial Sloan-Kettering Cancer Center and Weill Medical College of Cornell University) for their support.
Supported by translational research grants from the Leukemia and Lymphoma Society (to M.v.d.B. and B.E.R.) and a grant from the Byrne Fund (to M.v.d.B.). B.E.R. is the recipient of the Dermatology Foundation Career Development Award sponsored by Galderma Laboratories. M.v.d.B. is the recipient of a research award from the Amy Strelzer Manasevit Scholars Program for the study of posttransplant complications funded by The Marrow Foundation in cooperation with the National Marrow Donor Program, and a research award from the V Scholar Program of the V Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marcel R. M. van den Brink, Memorial Sloan-Kettering Cancer Center, Kettering 1118, Mailbox 111, 1275 York Ave, New York, NY 10021; e-mail address:m-van-den-brink@ski.mskcc.org.

![Fig. 1. IL-7 administration after BMT has no effect on the T-cell repertoire and increases the proliferative capability of T cells after BMT. / All CBA/J and (B6 × C3H)F1 recipients were transplanted as described in Table 1. IL-7 was administered from day 21 to day 27 by intraperitoneal injection (10 μg/d, panel A) or from day 14 to day 28 by subcutaneous osmotic pump (1 μg/d, panel B). Animals were humanely killed on day +28 and splenocytes were obtained for ConA proliferation assays (A) and for flow cytometric analysis of TCR-Vβ families (B). Splenocytes were cultured for 3 days with ConA (2.5 μg/mL) and [3H]-thymidine was added during the final 18 hours of culture. The mean thymidine incorporation in unstimulated splenocytes ranged from 2317 to 5141 cpm. Values represent means ± SE and each group contained 6 to 8 animals. The Vβ repertoire was determined in normal donor B10.BR mice, normal 3-month-old CBA mice, normal 9-month-old CBA/J mice, 9-month-old CBA/J BMT recipients (PBS treated; control), 9-month-old CBA/J BMT recipients (IL-7 treated).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2256/5/m_h81911605001.jpeg?Expires=1769095235&Signature=Rz2sLmun7IQXB1nvPplWeo2w8WhGDx9NfjD76O-RUzJTUSLcqEcUVOmx84jlUdM9nLTnjPiDCksecqLxR-AWemG~9V0QPJoKyll1YuJ-xlIajFA2Ees7vXeytG2OsWH8BwwMxzqQSmYYUl5uA5k5Pn30s-~n6ZFGBjPZyjpK4az94~VpMMtZXpjrrvOGHodevUJF5C2rV7W2ZEhVHsAQf~xaiq8H95mO08gIR4q5ynDenPrwFNMHh7BMkSqvRN1kjdfblgAN7vgFEQoRpYf0zKIxiVVVOkOcoubESRZoiHV4CHOTZndvvQXVJ0brKn-~4WUXx41s2SxS-AJPqQei8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. IL-7 administration does not stimulate alloreactive donor T cells in recipients undergoing GVHD. / Lethally irradiated (B6 × C3H)F1 or CBA/J recipients (1300 cGy) received transplants with B6 or B10.BR TCD BM (5 × 106) and splenic T cells (0.5 × 106) on day 0 as described in Table 1. Osmotic minipumps were used for continuous subcutaneous administration of 1 μg/d IL-7 or PBS (control) from day −1 to day +13. All recipients were humanely killed on day 14 and spleens were harvested and processed for flow cytometric analysis and functional assays. (A) Numbers of splenic donor CD4+ and CD8+ T cells were calculated from total splenocyte counts and flow cytometric analyses with antibodies that recognize specifically host antigens (Ly9.1 and CD5.1) and T-cell antigens (CD4 and CD8). Values represent mean ± SE and each group contained 6 to 8 animals. (B) Intracellular cytokine expression of alloreactive T cells. Splenic B6 T cells were harvested on day +14 from IL-7– or PBS-treated recipients as described above and incubated with irradiated (20 Gy) (B6 × C3H)F1 splenic stimulator cells in 24-well plates for 5 days. Cells were harvested, and restimulated with TCD, irradiated (20 Gy) (B6 × C3H)F1 splenic stimulator cells for 16 hours. Brefeldin A (10 μg/mL) was added after the first hour of incubation. Intracellular cytokine expression in CD4 memory cells (CD4+, CD62L−, CD44+) was measured by flow cytometric analysis. Values represent mean ± SE and each group contained 3 animals. (C) Cytolytic activity of alloreactive T cells. Splenic B6 T cells were harvested on day +14 from IL-7– or PBS-treated recipients as described above and their cytotoxicity was determined against 32Dp210 and P815 (third party) cell lines in51Cr-release assays. (D) Alloreactive T-cell proliferation. Splenic T cells (4 × 105 cells/well) were incubated with irradiated (20 Gy) (B6 × C3H)F1 or CBA/J splenic stimulator cells (2 × 105 cells/well) in 96-well plates for 5 days and [3H]-thymidine was added during the final 20 hours of culture. The mean thymidine incorporation in unstimulated splenocytes ranged from 529 to 3336 cpm. Values represent means ± SE and each group contained 6 to 8 animals.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2256/5/m_h81911605004.jpeg?Expires=1769095235&Signature=N2vRy4ER6nAqh1xUTK9eeMonMxURtN1Kq9stwHPtXj~QLqiw8gOd-HJmmBDkAafuBhnDKa~O8KbRPD9Rl2PYf5jXrGCODdlBstxGQ21-3Fp-f88Vl~gF8jIFxhbwvX00T~9kKfCXV7Axn5WUSuatIIlSZT9kgsxEI0B4eGfL-HhscqtLheft1rmJo7W99sGsvxiTVltiHUxhUAdXJrE94FWlp8UE4Vw1~eIB-2XRsoylIH0kVax8xNAVK5QrRmxlFYlnka5EsGyO~pEufHXk2qPW6sCq6mEnREiDksfF1k1SwE97M8anwcyC9MSe0BvFehIeo2hBsGJz35Uhehf2Zw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal