A critical role for the endothelium of yolk sac and dorsal aorta has been shown in embryonic hematopoiesis. A stromal cell line derived from yolk sac, YSCL-72, has been chosen to search for a novel molecule associated with embryonic hematopoiesis. Analysis between YSCL-72 and an adult aorta-derived endothelial cell line, EOMA, demonstrated that activated leukocyte cell adhesion molecule (ALCAM, or CD166) was specifically expressed in YSCL-72 but not in EOMA. Immunohistochemical study showed that ALCAM was expressed in the endothelium of yolk sac and dorsal aorta but not in adult aorta. ALCAM-transfected EOMA cells supported development of hematopoietic progenitor cells compared with vector-transfected EOMA cells, suggesting that ALCAM appeared to be crucial for hematopoiesis. In addition, ALCAM was found to be involved in capillary tube formation and hemangioblast differentiation. Taken together with these findings, ALCAM is highly associated not only with embryonic hematopoiesis but also vasculoangiogenesis.
Introduction
The interaction between the stromal and hematopoietic cells plays an important role in proliferation and differentiation of hematopoietic stem cells and progenitors. Several groups have investigated the potential functions of stromal cells derived from various hematopoietic sites. However, because of the complexity of these cell-to-cell interactions, the molecular mechanisms by which stromal and hematopoietic cells interact with each other remain obscure. In contrast to the complicated morphology of bone marrow hematopoietic regions, the type of stromal cells surrounding hematopoietic cells in the yolk sac and dorsal aorta is purely endothelial. Thus, analyses of the interactions between embryonic stromal endothelial cells and hematopoietic stem/progenitor cells can shed light on new molecules that are associated with hematopoiesis during embryonic development.
Accumulating evidence suggests that the endothelium of both the dorsal aorta in the AGM (aorta-gonad-mesonephros) region and the yolk sac is involved in hematopoiesis, particularly in the development of hematopoietic stem cells. It has been reported that small clusters of CD34+ hematopoietic cells attached to the endothelium of dorsal aorta and the yolk sac in human and mouse embryos, consistent with the importance of endothelial cells in the support of hematopoietic stem cell or progenitor cell development.1-4In addition, air/liquid organ cultures of AGM regions demonstrated expansion of hematopoietic stem cells,5 indicating that this region, which contained endothelial and hematopoietic cell types, was involved with the amplification and/or maintenance of pluripotent hematopoietic cells. From these findings, we hypothesized that hematopoietic stem cells are expanded on endothelial cells and novel factors produced from endothelial cells would support expansion of these stem cells.
Yolk sac blood islands also contain endothelial cells surrounding hematopoietic cells. However, the existence of yolk sac–derived hematopoietic stem cells able to repopulate adult irradiated mice is still controversial. Yoder and colleagues6 showed that hematopoietic stem cells derived from the yolk sac could repopulate recipient mice when these cells were first injected into fetal livers in newborns, suggesting that hematopoietic stem cells in yolk sac are somehow different from those in AGM, fetal liver, or bone marrow. One possible explanation of these experiments was that embryonic hematopoietic stem cells found in yolk sac need a suitable microenvironment (ie, the fetal liver stroma) to adjust to the adult hematopoietic environment. These findings also suggest that the endothelium in yolk sac might have an important role in hematopoietic stem cell development, and Fennie et al clearly demonstrated that CD34+ yolk sac–derived endothelial cell lines, YSCL-72 and YSCL-71, extensively supported hematopoiesis in vitro.7 In addition, we have shown that YSCL-72 has modest ability to maintain hematopoietic stem cells.4 These results led us to attempt to find a novel factor in yolk sac endothelium involved in the maintenance of hematopoietic stem or progenitor cells.
Activated leukocyte cell adhesion molecule (ALCAM, or CD166), also known as KG-CAM, neurolin, and BEN/DM-GRASP/SC1, is a cell surface immunoglobulin superfamily member that is involved with homophilic adhesion as well as binding to CD6.8-14 BEN was reported to be a marker in the developing chick central and peripheral nervous system and was also expressed on hematopoietic progenitor cells.15 Recently, the human homolog of ALCAM, hematopoietic cell antigen (HCA), was isolated by Uchida et al,16 and this protein was detected on the most primitive subset of hematopoietic stem cells as well as on myeloid progenitors in bone marrow. Because of its known adhesive activity, HCA was speculated to play a role in the homophilic binding of hematopoietic progenitor cells, perhaps to the stromal cell.17 Here we support this conjecture by demonstrating that mouse ALCAM18 was found to be expressed in yolk sac–derived endothelial cell line, YSCL-72, which was known to support the development of hematopoietic stem cells and progenitors. To examine a functional role of ALCAM in hematopoiesis and vasculoangiogenesis in vitro, ALCAM+ hematopoietic cells and endothelial cells derived from AGM or yolk sac were investigated. These results suggested the involvement of ALCAM not only in maintenance of hematopoietic stem cells or expansion of progenitor cells but also in endothelial tube formation. Accordingly, these findings imply that ALCAM might be a key adhesion molecule involved with the development of hematopoietic stem cells and endothelial progenitors.
Materials and methods
Production of monoclonal antibodies
The monoclonal antibodies were produced by inoculating YSCL-72 cells into rat, and the hybridoma supernatants were screened for the presence of YSCL-72 cell–specific and the absence of EOMA (an adult aorta-derived endothelial cell line) cell–specific monoclonal antibodies by fluorescence-activated cell sorting (FACS) (Becton Dickinson, San Jose, CA). Selected 7 monoclonal antibodies (3F3, 5C5, 5C5/44, 5D5, 761, 17C6, 19B8) were subsequently used for staining on embryo sections, and 17C6 was found to stain yolk sac endothelium.
Cloning of ALCAM
Messenger RNA was isolated from YSCL-72 cells with a Fast Track 2.0 messenger RNA Isolation Kit (Invitrogen, Carlsbad, CA). Oligo (dT)-primed complementary DNAs (cDNAs), which were size-selected above 1.5 kilobases, were inserted directionally into theXhoI-NotI sites of the expression vector, pRK-5 (Becton Dickinson). Purified plasmid cDNA from 40 pools of 105 clones per pool were electroporated into COS7 cells. Two days postelectroporation, transfected cells were labeled with 17C6 monoclonal antibody and were plated on the panning plate, which was coated with goat antirat immunoglobulin G (IgG) (heavy and light chain) (Caltag Laboratories, South San Francisco, CA). Following 3 successive rounds of electroporation and plasmid recoveries from the cells attached to the plate, 800 colonies picked from a single positive pool produced a single positive clone. Sequence analysis revealed that the predicted sequence for 17C6 antibody was 100% homologous to that of murine ALCAM (CD166).18
Cells lines
E11 yolk sac–derived endothelial cell lines, YSCL-72 and YSCL-71, were maintained in HAVA medium as described elsewhere.4 Mouse EOMA was maintained in Dulbecco modified Eagle medium/10% fetal bovine serum (JRH Bioscience, Lenexa, KS), and ALCAM-transfected EOMA cell lines (GM2, GM10, and vector alone) were adjusted to HAVA medium containing 600 μg/mL G418 (Gibco, Grand Island, NY). OP9 stromal cell line is maintained in a modified minimum essential media (α-MEM) (Gibco) supplemented with 20% fetal bovine serum.
Mice and antibodies
Timed pregnant mice (C57BL/6) or 2- to 3-month-old male mice (C57BL/6) were purchased from Harlan Sprague Dawley Laboratory (Dallas, TX) or Japan SLC (Shizuoka, Japan). Congenic C57BL6, Ly5.2 male mice were purchased from the National Cancer Institute (Frederick, MD). Mice were killed by cervical dislocation.
The antibodies used for fetal liver stem cell sorting were all obtained from Pharmingen (San Diego, CA). Biotinylated antibodies for lineage cocktail contain anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti-B220 (6B2), anti–Gr-1 (8C5), and antierythroid (TER119). Other antibodies include phycoerythrin (PE)-conjugated anti–Sca-1 antibody (E13) and fluorescein (FITC)-conjugated anti–c-Kit antibody (2B8). Secondary reagent includes allophycoerythrin-labeled streptavidin (Caltag Laboratories) and FITC-labeled donkey antirabbit IgG (Jackson ImmunoResearch, West Grove, PA). The antibodies used for isolation of hematopoietic progenitor cells from AGM or yolk sac were rabbit–anti-CD34 polyclonal antibody, PE-conjugated anti–c-Kit antibody, and biotinylated anti-ALCAM antibody. The antibodies used for isolation of endothelial precursor cells from AGM or yolk sac were PE-conjugated anti–Flk-1 antibody (Avas12α1) (Pharmingen), FITC-conjugated anti-CD31 antibody (platelet endothelial cell adhesion molecule-1 [PECAM-1]) (MEC13.3) (Pharmingen), and biotinylated anti-ALCAM antibody. Isotype-matched rat immunoglobulins were obtained from Pharmingen.
Isolation of fetal liver hematopoietic stem cells and long-term reconstitution assay
Mouse fetal liver stem cells were isolated from E13.5 as described previously.4 Purified liver stem cells (Lin−Sca-1+c-Kit+) were cultured with irradiated stromal cells for 7 or 25 days. Cultured cells were trypsinized, and one third of culture (corresponding to 1000 stem cells) or one sixth of culture (corresponding to 500 stem cells) was transplanted into the recipients as described elsewhere.4All mice used as donors for long-term reconstitution were Ly5.1, and C57Bl/6-Ly5.2 recipient mice were lethally irradiated with 10.50 Gy (1050 rad) as a single dose from 137Cs source. Freshly isolated stem cells or progenitor cells cultured on stromal cells were injected into the tail vein along with 1 × 106 whole bone marrow cells from Ly5.2 congenic source for radioprotection. Groups of 6 or 8 mice were injected for each cell type. For analysis of reconstitution, mice were bled from the retro-orbital sinus and assayed for the presence of Ly5.1+cells 12 weeks after transplantation as previously described.4
Isolation of hematopoietic progenitors from AGM and yolk sac and differentiation of hematopoietic cells on stromal cells
E10.5 AGM and yolk sacs were dissected out, and single cells were obtained as previously reported.19 Cells were fractionated for CD34+c-Kit+ cells, and they were further divided on the basis of the expression of ALCAM by FACS. Sorted cells were cultured with irradiated stromal cells (GM2 or vector alone) for 7 days. Cells were trypsinized, and adherent cells including stromal cells were eliminated after passing through a G10 column (Amersham Pharmacia Biotech, Uppsala, Sweden). Numbers of nonadherent cells were scored, and the frequency of c-Kit+ cells was analyzed by FACS. Harvested 1000 nonadherent cells were subsequently cultured for 7 days with methylcellulose medium (Methocult M3234, StemCell Technologies, Vancouver, BC) containing 100 ng/mL stem cell factor (SCF) (R & D Systems, Minneapolis, MN), 100 ng/mL interleukin-3 (IL-3) (R & D Systems), 100 ng/mL IL-6 (a gift from Dr T. Sudo, Toray Industries, Kamakura, Japan), and 2 U/mL erythropoietin (Epo) (a gift from Snow-Brand Milk Products). The number of colonies containing more than 50 cells was scored on day 7, and morphologies of colonies (G, granulocyte colonies; GM, granulocyte-macrophage; M, macrophage; E, erythroid burst; GEM, granulocyte-erythroid-macrophage; and GEMM, granulocyte-erythroid-macrophage-megakaryocyte) were evaluated under the microscope.
Isolation of endothelial progenitors from AGM and yolk sac and differentiation of endothelial progenitors on OP9 cells
E10.5 AGM– and yolk sac–derived cells were obtained as described above. A total of 500 Flk-1+PECAM-1+ALCAM+ or Flk-1+PECAM-1+ ALCAM− cells from yolk sac or AGM were plated on OP9 cells and cultured with 10 ng/mL vascular endothelial growth factor (VEGF) (PeproTech, London, United Kingdom) for 10 days. Cultured cells were fixed with 4% paraformaldehyde and stained with anti–PECAM-1 antibody. For the differentiation of Flk-1+PECAM-1+ALCAM+ or Flk-1+PECAM-1+ ALCAM−cells to hematopoietic cell lineage, 500 progenitors were cultured with OP9 cells in the presence of 100 ng/mL SCF, 100 ng/mL IL-6, 2 U/mL Epo, and 20 U/mL IL-7 (a gift from Dr T. Sudo) for 12 days. Nonadherent cells were obtained after passing through a G10 column, the number of cells was scored, and the frequency of c-Kit+ cells was examined by FACS. For morphologic examination, cytospins were stained with May-Grünwald-Giemsa solution.
Construct and production of human IgG fusion protein
Soluble forms of ALCAM-Fc and CD4-Fc were constructed by fusion of the extracellular domain of ALCAM or CD4 and the Fc part of human IgG. ALCAM-Fc– or CD4-Fc–inserted expression vector was transfected to COS7 cells and cultured in serum-free medium as previously reported.20 Conditioned medium were harvested and purified over protein A column (Affi-Gel, Bio-Rad, Hercules, CA). After dialysis, purity was examined by Coomassie brilliant blue staining of sodium dodecyl sulfate gels and enzyme-linked immnunosorbent assay.
Immunostaining of embryonic and adult endothelium
E9.5 or E10.5 embryos were dissected out, and samples were fixed with 4% paraformaldehyde for 4 to 6 hours at 4°C. The embryos or aorta were then embedded in polyester wax and sectioned at 5 to 7 μm. Dewaxed with ethanol, sections were treated with 0.3% hydrogen peroxide in methanol and stained with anti-ALCAM antibody (1-2 μg/mL), anti–PECAM-1 antibody, or isotype-matched rat IgG overnight at 4°C as reported previously.19 Staining for cardiac muscle or smooth muscle, antisarcomeric actin antibody (α-Sr-1) (Dako, Glostrup, Denmark), and antismooth muscle actin antibody (1A4) (Dako) were incubated with horseradish peroxidase–conjugated Envision polymer reagent (K1391) (Dako) at room temperature for 45 minutes and sections were incubated with these antibody-binding reagents for 30 minutes. Color reaction was performed using diaminobenzidine as described elsewhere.19
Binding assay
Mononuclear cells from mouse bone marrow were fractionated for ALCAM+ or ALCAM− cells, and 1 × 104 cells of each fraction were plated onto vector-transfected cells or GM2. After 4 or 5 hours of incubation at 37°C, cells were washed 3 times with phosphate-buffered saline and the remaining cells were harvested. Cells were then stained with PE-conjugated anti-CD45 antibody (Pharmingen), and the frequency of CD45+ cells was analyzed by FACS.
Analysis of endothelial tube formation in Matrigel
Analysis of capillary formation in Matrigel (Becton Dickinson) was performed as per the manufacturer's instructions; 200 μL Matrigel (No. 354234) was applied into a 4-well plate (Nalge Nunc, Rochester, NY) and incubated at 37°C for 30 minutes. YSCL-71 cells were trypsinized, and 5 × 104 cells were suspended with 400 μL Dulbecco modified Eagle medium/10% fetal bovine serum and plated onto Matrigel. Then 25 μg ALCAM-Fc or CD4-Fc was added to Matrigel culture, and it was incubated for 60 hours. Tube formation in Matrigel was observed under a microscope, and the number of capillary tubes in a random field from each of 4 wells was quantitated.
Statistical analysis
Statistical evaluations of data were conducted by using the Student t test for per-comparison analysis. The data are presented as means ± SD.
Results
ALCAM-transfected endothelial cell line (GM2) supports hematopoiesis
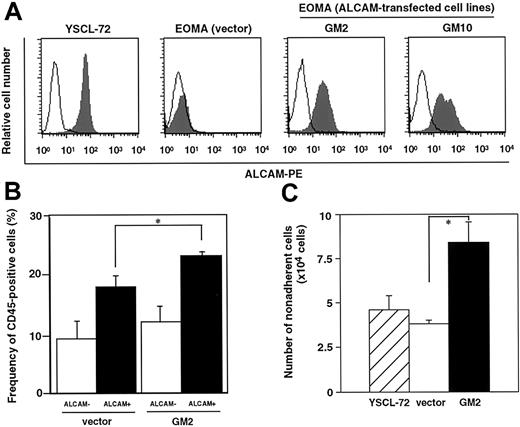
We hypothesized that ALCAM might be involved in expansion of hematopoietic stem or progenitor cells. To examine the potential role of ALCAM in hematopoiesis, a cDNA encoding this protein was transfected into the EOMA endothelial cell line derived from adult mouse aorta, and the ability of the resultant cells to support hematopoiesis was investigated. As seen in Figure 1A, ALCAM was highly expressed in both the yolk sac–derived endothelial cell line, YSCL-72, as well as in the established ALCAM-transfected cell lines, while ALCAM expression was not detected in vector-transfected EOMA cells. To analyze whether homophilic interactions via ALCAM between hematopoietic cells and stromal cells occur, ALCAM+mononuclear cells from mouse bone marrow were incubated with vector-transfected stromal cells and ALCAM-transfected stromal cells (GM2) (Figure 1B). ALCAM+ hematopoietic cells adhered to GM2 in higher frequency than ALCAM− stromal cells (22.9% ± 1.3% vs 17.8% ± 2.6%, P < 0.05). It was reported previously that YSCL-72 has the ability both to expand yolk sac–derived CD34+ hematopoietic progenitors7 and maintain hematopoietic stem cells.4 Fetal liver–derived hematopoietic stem cells (Lin−Sca-1+c-Kit+) were cocultured with either YSCL-72 or ALCAM-transfected cells (GM2), and expanded hematopoietic cells were analyzed (Figure 1C). This figure illustrates that the number of nonadherent cells were significantly greater in coculture with GM2 compared with coculture with vector-transfected cell. Similar data were obtained with the GM10, which is also an ALCAM-transfected EOMA cell line (data not shown). Morphologic analysis of cells stained with May-Grünwald-Giemsa revealed that blastlike cells were observed in ALCAM-transfected cocultures, whereas most cells expanded on vector-transfected cells were mature hematopoietic cells (data not shown). These data are consistent with the hypothesis that ALCAM-mediated adhesion is involved with the expansion of hematopoietic progenitor cells by endotheliallike stromal cell lines.
An ALCAM-transfected endothelial cell line supports hematopoiesis.
(A) EOMA was transfected with full-length ALCAM cDNA or vector alone. E11-derived yolk sac endothelial cell line, YSCL-72, and established ALCAM-transfected cell lines, GM2 and GM10, were examined for their expression of ALCAM by FACS. (B) ALCAM+ or ALCAM− bone marrow mononuclear cells were cultured with vector-transfected stromal cells or ALCAM-transfected stromal cells (GM2). After 4 or 5 hours of incubation, cells were washed several times, and the remaining cells were analyzed for the expression of CD45; *P < .05. (C) A total of 1000 fetal liver–derived stem cells (Lin−Sca-1+c-Kit+) were plated onto each stromal cell line and cultured for 6 days without cytokines. Nonadherent cells were harvested, and the cell number was scored; *P < .05.
An ALCAM-transfected endothelial cell line supports hematopoiesis.
(A) EOMA was transfected with full-length ALCAM cDNA or vector alone. E11-derived yolk sac endothelial cell line, YSCL-72, and established ALCAM-transfected cell lines, GM2 and GM10, were examined for their expression of ALCAM by FACS. (B) ALCAM+ or ALCAM− bone marrow mononuclear cells were cultured with vector-transfected stromal cells or ALCAM-transfected stromal cells (GM2). After 4 or 5 hours of incubation, cells were washed several times, and the remaining cells were analyzed for the expression of CD45; *P < .05. (C) A total of 1000 fetal liver–derived stem cells (Lin−Sca-1+c-Kit+) were plated onto each stromal cell line and cultured for 6 days without cytokines. Nonadherent cells were harvested, and the cell number was scored; *P < .05.
To examine whether ALCAM was involved in maintenance of hematopoietic stem cells, fetal liver–derived hematopoietic stem cells (Lin−Sca-1+c-Kit+) were cocultured with ALCAM- or vector-transfected cells, and the expanded hematopoietic cells were injected into lethally irradiated mice (Table1). Hematopoietic stem cells cocultured with each transfectant (GM2 or vector-transfected cell) for 7 days or 25 days were analyzed for their abilities to repopulate the recipient mice. Irradiated recipient mice were reconstituted by injection of hematopoietic cells cocultured with ALCAM-transfected endothelial cells for 7 days. On the other hand, no reconstitution was observed when hematopoietic cells were cultured with vector-transfected cells. Importantly, hematopoietic stem cells cocultured with GM2 cells for 25 days still possessed the ability to repopulate irradiated recipients, while repopulating activity was almost completely lost when progenitor cells were cultured on vector-transfected EOMA cells. These data suggested that ALCAM might be involved with the maintenance of hematopoietic stem cells or expansion of primitive hematopoietic progenitor cells. However, because we cannot rule out the possibility of involvement of ALCAM in maintenance of progenitor cells but not stem cells, secondary transplantation studies will be required to determine if ALCAM expressed on stromal cells mediates hematopoietic stem cells in an undifferentiated state.
Reconstitution analysis in lethally irradiated mice transplanted with cultured hematopoietic stem cells on ALCAM-transfected stromal cells
| Primary fetal liver stem cells . | Cultured cells . | Total % Ly5.1 cells with vector-transfected cells . | Total % Ly5.1 cells with ALCAM-transfected cells (GM2) . | ||
|---|---|---|---|---|---|
| 7 days . | 25 days . | 7 days . | 25 days . | ||
| 10.1% ± 5.8% (500 cells/mouse) | One sixth of culture | ND | 0.7% ± 0.2% | ND | 3.2% ± 2.1% |
| 18.2% ± 14.3% (1000 cells/mouse) | One third of culture | 1.5% ± 1.2% | 0.9% ± 0.2% | 9.4% ± 4.4% | 6.2% ± 0.9% |
| Primary fetal liver stem cells . | Cultured cells . | Total % Ly5.1 cells with vector-transfected cells . | Total % Ly5.1 cells with ALCAM-transfected cells (GM2) . | ||
|---|---|---|---|---|---|
| 7 days . | 25 days . | 7 days . | 25 days . | ||
| 10.1% ± 5.8% (500 cells/mouse) | One sixth of culture | ND | 0.7% ± 0.2% | ND | 3.2% ± 2.1% |
| 18.2% ± 14.3% (1000 cells/mouse) | One third of culture | 1.5% ± 1.2% | 0.9% ± 0.2% | 9.4% ± 4.4% | 6.2% ± 0.9% |
Day 14 fetal liver cells (Ly5.1) were purified for Lin−Sca-1+c-Kit+ cells, and 3000 stem cells were cultured with ALCAM- or vector-transfected cell lines for 7 days or 25 days. Cells were harvested, and one third of culture (corresponding to 1000 stem cells) or one sixth of culture (corresponding to 500 stem cells) was transplanted with 1 × 106 Ly5.2 competitor bone marrow cells into lethally irradiated Ly5.2 mice. The expression of the donor-type Ly5.1 marker on peripheral blood cells was examined 12 weeks after transplantation. Three independent experiments were performed, and representative data were obtained from one experiment.
ND indicates not done.
Immunostaining of embryonic and adult endothelium with anti-ALCAM antibody
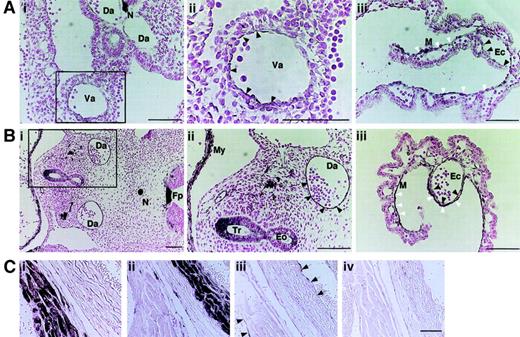
While the data described above are consistent with a role for ALCAM in the regulation of hematopoiesis, it was important to demonstrate the expression of this protein in the appropriate hematopoietic sites in vivo. Figure 2illustrates that endothelial cells in both dorsal aorta and vitelline artery were weakly positive for ALCAM in the E9.5 embryo, whereas endothelial cells surrounding yolk sac hematopoietic cells and mesenchymal cells differentiated from mesoderm were strongly positive for this protein. In E10.5 embryos, the expression of ALCAM was clearly observed in dorsal aorta endothelium (Figure 2Bi,ii) and, as with the staining observed in the E9.5 yolk sac, both endothelial cells and closely attached mesenchymal cells were strongly positive for ALCAM in the yolk sac (Figure 2Biii). These immunostaining data were consistent with the observation of highly expressed ALCAM in yolk sac–derived endothelial cell line, YSCL-72, by FACS (Figure 1A).
Immunohistochemical staining.
Transverse-sectioned E9.5- or E10.5-derived embryos and sagittal-sectioned adult hearts were stained with anti-ALCAM antibody. (A) The E9.5-derived dorsal aorta or vitelline artery was weakly positive for ALCAM (Ai). The vitelline artery in the square area is shown in high magnification (Aii). Black arrowheads indicate positively stained endotheliallike cells. In yolk sac, both mesenchymal cells (M) (white arrowheads) and endothelial cells (Ec) (black arrowheads) were ALCAM+ (Aiii). (B) In the E10.5 embryo dorsal aorta, endothelial cells were strongly positive for ALCAM (square area in panel Bi; high magnification of the square area: black arrowheads in panel Bii). Similarly, M (white arrowheads) and Ec (black arrowheads) were ALCAM+ in E10.5 yolk sac (Biii). (C) Adult mouse, heart, and aorta were examined for the expression of ALCAM. Antiheart muscle antibody (Ci), antismooth muscle antibody (Cii), and anti-CD31 antibody (Ciii) (black arrowheads indicate endocardium; black arrows, wall of aorta) were used for control, respectively. Note that ALCAM was negative for endocardium and endothelium of aorta (Civ). Scale bar, 200 μm. Da indicates dorsal aorta; N, notocord; Va, vitelline artery; Fp, floor plate; My, myocardium; Tr, trachea; Eo, esophagus.
Immunohistochemical staining.
Transverse-sectioned E9.5- or E10.5-derived embryos and sagittal-sectioned adult hearts were stained with anti-ALCAM antibody. (A) The E9.5-derived dorsal aorta or vitelline artery was weakly positive for ALCAM (Ai). The vitelline artery in the square area is shown in high magnification (Aii). Black arrowheads indicate positively stained endotheliallike cells. In yolk sac, both mesenchymal cells (M) (white arrowheads) and endothelial cells (Ec) (black arrowheads) were ALCAM+ (Aiii). (B) In the E10.5 embryo dorsal aorta, endothelial cells were strongly positive for ALCAM (square area in panel Bi; high magnification of the square area: black arrowheads in panel Bii). Similarly, M (white arrowheads) and Ec (black arrowheads) were ALCAM+ in E10.5 yolk sac (Biii). (C) Adult mouse, heart, and aorta were examined for the expression of ALCAM. Antiheart muscle antibody (Ci), antismooth muscle antibody (Cii), and anti-CD31 antibody (Ciii) (black arrowheads indicate endocardium; black arrows, wall of aorta) were used for control, respectively. Note that ALCAM was negative for endocardium and endothelium of aorta (Civ). Scale bar, 200 μm. Da indicates dorsal aorta; N, notocord; Va, vitelline artery; Fp, floor plate; My, myocardium; Tr, trachea; Eo, esophagus.
BEN/DM-GRASP/SC1, which is the avian homolog of ALCAM, is a marker in the developing nervous system,8-10 and E9.5- or E10.5-derived notochord and floor plate were intensely stained with anti-ALCAM antibody16 (Figure 2Ai,ii). In E10.5 embryo, the epithelium in trachea and esophagus was also positive for ALCAM (Figure 2Bii), and in heart, myocardium was reactive for anti-ALCAM antibody, although the endocardium was not labeled with anti-ALCAM antibody (Figure 2Bii). This latter observation indicates that the differential expression of ALCAM in endothelium of different organs existed at an early embryonic stage. Finally, expression of ALCAM in adult aorta and endocardium was examined (Figure 2C). In accordance with the absence of ALCAM expression in the adult aorta-derived cell, EOMA, neither endothelium of aorta nor endocardium in adult mice was positively stained with anti-ALCAM antibody (Figure 2Civ). These immunohistochemical data, together with previous studies,16 demonstrate that both the endothelium of hematopoietic sites as well hematopoietic progenitor cells express this homophilic adhesion molecule.
Hematopoietic cells derived from yolk sac and AGM were expanded on ALCAM-transfected endothelial cell
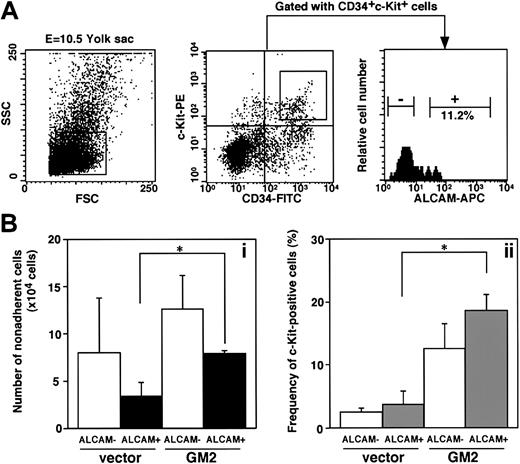
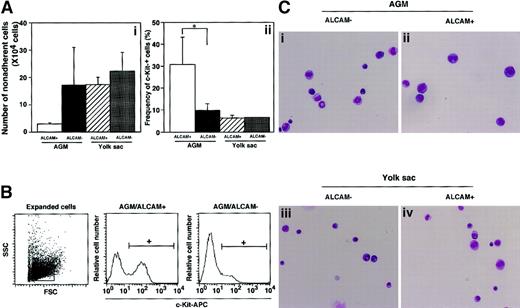
Because ALCAM appears to mediate a homophilic adhesion, we hypothesized that homophilic binding between hematopoietic cells and stromal cells might play an important role in expansion of hematopoietic cells in yolk sac. Because ALCAM appears to be expressed in yolk sac endothelium (Figure 2), we first investigated the expression of ALCAM on hematopoietic cells derived from the yolk sac. E10.5 yolk sac cells were sorted for CD34+c-Kit+ cells and further subdivided at the expression of ALCAM (Figure 3A). Approximately 11.2% of CD34+c-Kit+ cells derived from E10.5 yolk sac were ALCAM+ cells. CD34+c-Kit+ALCAM+ cells or CD34+c-Kit+ALCAM− cells were cocultured with ALCAM-transfected cells (GM2) or vector-transfected cells to examine the role of ALCAM (Figure 3B). GM2 expanded more nonadherent cells from CD34+c-Kit+ALCAM+ cells than vector alone (Figure 3Ba, black column), and the frequency of c-Kit+ cells within those nonadherent cells was higher in GM2 than vector-transfected cells (Figure 3Bb, black column). Although CD34+c-Kit+ALCAM− cells cultured on GM2 generated the most nonadherent cells (Figure 3Ba, white column), the frequency of c-Kit+ cells (Figure 3Bb, white column) was lower than that from ALCAM+ cells in GM2 (Figure 3Bb, gray column). These data were consistent with the suggestion that ALCAM might be involved in interaction between hematopoietic cells and stromal cells and that homophilic interaction between them would mediate yolk sac–derived hematopoietic cells in immature state.
Examination of ability to support yolk sac–derived hematopoiesis in an ALCAM-transfected endothelial cell line.
(A) E10.5-derived yolk sac cells were stained with anti-CD34 antibody, anti–c-Kit antibody, and anti-ALCAM antibody. CD34+c-Kit+ cells were subdivided on the basis of the expression of ALCAM. (B) A total of 1000 CD34+c-Kit+ALCAM− or CD34+c-Kit+ALCAM+ cells were cultured with GM2 cells (ALCAM-transfected cell line) or vector-transfected cells for 7 days. The number of nonadherent cell was scored (Bi), and expression of c-Kit was analyzed by FACS (Bii). *P < .05.
Examination of ability to support yolk sac–derived hematopoiesis in an ALCAM-transfected endothelial cell line.
(A) E10.5-derived yolk sac cells were stained with anti-CD34 antibody, anti–c-Kit antibody, and anti-ALCAM antibody. CD34+c-Kit+ cells were subdivided on the basis of the expression of ALCAM. (B) A total of 1000 CD34+c-Kit+ALCAM− or CD34+c-Kit+ALCAM+ cells were cultured with GM2 cells (ALCAM-transfected cell line) or vector-transfected cells for 7 days. The number of nonadherent cell was scored (Bi), and expression of c-Kit was analyzed by FACS (Bii). *P < .05.
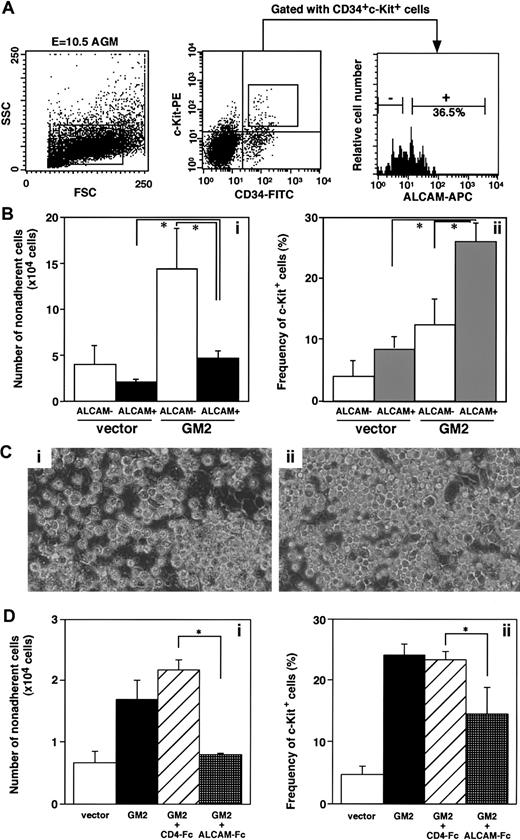
As shown in immunohistochemical staining in Figure 2B, endothelium in dorsal aorta was strongly positive for ALCAM, and it is therefore hypothesized that ALCAM also acts in hematopoiesis in E10.5 dorsal aorta. To examine a role of ALCAM on hematopoiesis in E10.5 AGM, the expression of ALCAM in AGM-derived hematopoietic progenitors was analyzed. Hematopoietic cells from the E10.5 AGM region were sorted for CD34+c-Kit+ cells, and this double-positive population was subdivided on the expression of ALCAM (Figure4A). Approximately 36.5% of the hematopoietic cells within the CD34+c-Kit+population were ALCAM+. As was found for yolk sac–derived hematopoietic progenitor cells (Figure 3B), GM2 supported more c-Kit+ nonadherent cells derived from AGM than vector-transfected cells (Figure 4Ba, black column; Figure 4Bb, gray column). The morphology of hematopoietic cells expanded on vector-transfected cells appears to be macrophagelike cells (Figure 4Ca); on the contrary, blastlike hematopoietic cells were expanded on GM2 (Figure 4Cb). Those expanded hematopoietic cells on vector-transfected cells and GM2 were subsequently cultured in methylcellulose medium containing SCF, IL-3, IL-6, and Epo for 7 days. No colonies were detected from cells expanded on vector-transfected cells; on the other hand, 24 ± 6 colonies (G/GM colony, 11 ± 4; M colony, 9 ± 2; E colony, 1 ± 1; GEM/GEMM colony, 2 ± 0) developed from cells expanded on GM2.
Examination of the ability to support AGM derived-hematopoiesis in an ALCAM-transfected endothelial cell line.
(A) E10.5-derived AGM cells were fractionated into CD34+c-Kit+ cells and further subdivided at the expression of ALCAM. Among CD34+c-Kit+ cells, 72.4% were CD45+ (data not shown). (B) A total of 1000 CD34+c-Kit+ALCAM+ cells were cultured with ALCAM-transfected GM2 cells or vector-transfected cells for 7 days. Nonadherent cell number was scored (Bi), and expression of c-Kit was examined by FACS (Bii). *P < .05. (C) Hematopoietic colony morphologies of CD34+c-Kit+ALCAM+ cells were cultured on vector-transfected cells (Ci) or ALCAM-transfected cells (Cii) for 7 days. (Ci) Macrophagelike cells that predominate in the culture (× 200). (Cii) Blastlike cells (× 200). (D) A total of 1000 CD34+c-Kit+ cells were cultured in the presence of ALCAM-Fc or control CD4-Fc for 7 days. Nonadherent cells were harvested, and the cell number was scored (Di). Expression of c-Kit was examined in each nonadherent cell by FACS (Dii). *P < .05.
Examination of the ability to support AGM derived-hematopoiesis in an ALCAM-transfected endothelial cell line.
(A) E10.5-derived AGM cells were fractionated into CD34+c-Kit+ cells and further subdivided at the expression of ALCAM. Among CD34+c-Kit+ cells, 72.4% were CD45+ (data not shown). (B) A total of 1000 CD34+c-Kit+ALCAM+ cells were cultured with ALCAM-transfected GM2 cells or vector-transfected cells for 7 days. Nonadherent cell number was scored (Bi), and expression of c-Kit was examined by FACS (Bii). *P < .05. (C) Hematopoietic colony morphologies of CD34+c-Kit+ALCAM+ cells were cultured on vector-transfected cells (Ci) or ALCAM-transfected cells (Cii) for 7 days. (Ci) Macrophagelike cells that predominate in the culture (× 200). (Cii) Blastlike cells (× 200). (D) A total of 1000 CD34+c-Kit+ cells were cultured in the presence of ALCAM-Fc or control CD4-Fc for 7 days. Nonadherent cells were harvested, and the cell number was scored (Di). Expression of c-Kit was examined in each nonadherent cell by FACS (Dii). *P < .05.
CD34+c-Kit+ALCAM− cells cultured on GM2 developed more hematopoietic cells (Figure 4Ba, white column) than did ALCAM+ cells on GM2, suggesting that ALCAM+ precursor cells as reported previously16 would differentiate from CD34+c-Kit+ALCAM− cells and might be expanded on GM2, possibly by homophilic interaction because GM2 does not express CD6 by reverse transcriptase–polymer chain reaction analysis (data not shown).
To examine the involvement of ALCAM in the expansion of hematopoietic cells in vitro, a soluble from of ALCAM was constructed as a chimera with the Fc region of human immunoglobulin (ALCAM-Fc). ALCAM-Fc was subsequently added to the coculture of ALCAM+ hematopoietic cells and GM2 cell, and the effects of soluble ALCAM on the expansion of c-Kit+ cells was investigated (Figure 4D). Addition of ALCAM-Fc in the coculture mediated the complete inhibition of expanded nonadherent cells (Figure 4Da), and ALCAM-Fc partially inhibited the frequency of c-Kit+ cells (Figure 4Db), suggesting that ALCAM may play an important role in hematopoiesis at the AGM region via a homophilic adhesive interaction between the endothelium and hematopoietic progenitor cells.
The role of ALCAM in development of endothelial precursor cells
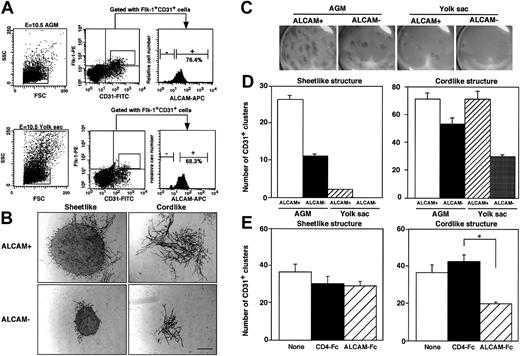
Because ALCAM was highly expressed at endothelium in dorsal aorta and yolk sac in E10.5 embryo (Figure 2B), we examined how ALCAM was involved in development of endothelial precursor cells in vitro. First, the frequency of ALCAM+ endothelial precursor cells was analyzed by FACS (Figure 5A). E10.5 AGM– or yolk sac–derived endothelial precursor cells were sorted for Flk-1+CD31+ cells and further subdivided on the basis of the expression of ALCAM. In agreement with the immunohistochemical experiments above (Figure 2), most of Flk-1+CD31+ endothelial progenitor cells were ALCAM+ in E10.5-derived AGM and yolk sac. To characterize the potential involvement of ALCAM in endothelial precursor cell development, ALCAM+ or ALCAM− cells were further fractionated from Flk-1+CD31+endothelial precursor cells and cultured in vitro on the OP9 cell in the presence of VEGF. After 9- or 10-day cocultivation, cultured cells were fixed and stained with anti-CD31 antibody to examine the differentiation of endothelial precursor cells. Two types of differentiated endothelial clusters, a tightly packed sheetlike cluster or slenderly elongated cordlike cluster, were observed (Figure 5B). It appeared that ALCAM+ precursor cells formed larger sheetlike and cordlike endothelial clusters than those from ALCAM− endothelial precursor cells. Macroscopic observation clearly demonstrated that largely extended CD31+ endothelial clusters were detected in AGM-derived endothelial precursor cells, particularly in ALCAM+endothelial precursor cells compared with endothelial clusters derived from yolk sac (Figure 5C). Quantitative analyses were done in both sheetlike and cordlike clusters (Figure 5D). The number of sheetlike clusters observed in AGM-derived endothelial precursor cells was higher than those from yolk sac, and ALCAM+ endothelial precursor cells differentiated to more sheetlike clusters than those from ALCAM− endothelial precursor cells in AGM and yolk sac (Figure 5D, left). As similarly observed for sheetlike clusters, more cordlike clusters were detected in ALCAM+ endothelial precursor cells than in ALCAM− endothelial precursor cells. However, the difference between the number of cordlike clusters between ALCAM+ and ALCAM− endothelial precursor cells in AGM or yolk sac was not significant (Figure 5D, right). These data strongly suggested that ALCAM+endothelial precursor cells have high potential to develop into more mature vascular structures on OP9 cells.
Analysis of ALCAM expression in endothelial precursor cells and examination of development of endothelial clusters in vitro.
(A) E10.5-derived AGM cells (upper column) or yolk sac cells (lower column) were fractionated into Flk-1+CD31+cells. Flk-1+CD31+ cells were subdivided at the expression of ALCAM, and ALCAM− or ALCAM+cells were sorted individually for further experiments. Among CD34+c-Kit+ cells in E10.5-derived AGM, 7.6% were Flk-1+; however, they were ALCAM− (data not shown). (B,C) A total of 750 AGM- or yolk sac–derived Flk-1+CD31+ALCAM+ or Flk-1+CD31+ALCAM− cells were cultured with OP9 cell in the presence of VEGF for 9 or 10 days. Culture was fixed and stained with anti-CD31 antibody. Two types of CD31+ clusters (sheetlike and cordlike structure) were observed under a microscope (B). Macroscopic observation clearly demonstrated that distribution of endothelial clusters developed on OP9 cell (magnification, ×6.3) (C). Note that the larger CD31+endothelial clusters were observed in both sheetlike and cordlike clusters when ALCAM+ endothelial precursor cells were cocultured with OP9 cell. Scale bar, 100 μm. (D) The number of CD31+ sheetlike (left) or cordlike (right) clusters derived from AGM and yolk sac was scored. (E) AGM-derived endothelial precursor cells (Flk-1+CD31+) were cultured with OP9 cell in the presence of ALCAM-Fc or control CD4-Fc, and the number of CD31+ endothelial clusters was scored (left, sheetlike cluster; right, cordlike cluster). *P < .05.
Analysis of ALCAM expression in endothelial precursor cells and examination of development of endothelial clusters in vitro.
(A) E10.5-derived AGM cells (upper column) or yolk sac cells (lower column) were fractionated into Flk-1+CD31+cells. Flk-1+CD31+ cells were subdivided at the expression of ALCAM, and ALCAM− or ALCAM+cells were sorted individually for further experiments. Among CD34+c-Kit+ cells in E10.5-derived AGM, 7.6% were Flk-1+; however, they were ALCAM− (data not shown). (B,C) A total of 750 AGM- or yolk sac–derived Flk-1+CD31+ALCAM+ or Flk-1+CD31+ALCAM− cells were cultured with OP9 cell in the presence of VEGF for 9 or 10 days. Culture was fixed and stained with anti-CD31 antibody. Two types of CD31+ clusters (sheetlike and cordlike structure) were observed under a microscope (B). Macroscopic observation clearly demonstrated that distribution of endothelial clusters developed on OP9 cell (magnification, ×6.3) (C). Note that the larger CD31+endothelial clusters were observed in both sheetlike and cordlike clusters when ALCAM+ endothelial precursor cells were cocultured with OP9 cell. Scale bar, 100 μm. (D) The number of CD31+ sheetlike (left) or cordlike (right) clusters derived from AGM and yolk sac was scored. (E) AGM-derived endothelial precursor cells (Flk-1+CD31+) were cultured with OP9 cell in the presence of ALCAM-Fc or control CD4-Fc, and the number of CD31+ endothelial clusters was scored (left, sheetlike cluster; right, cordlike cluster). *P < .05.
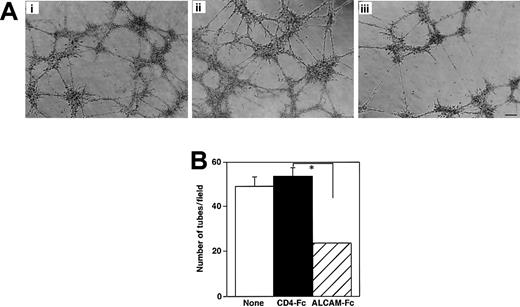
To examine how ALCAM was associated with the formation of endothelial clusters, ALCAM-Fc was added to the culture, and the number of 2 types of endothelial clusters derived from AGM was analyzed (Figure 5E). Interestingly, ALCAM-Fc inhibited the formation of cordlike clusters but not the formation of sheetlike clusters. These data clearly demonstrated that ALCAM was involved in the formation of elongated vessellike endothelial clusters. We next investigated whether ALCAM would affect tube formation by endothelial cells suspended in Matrigel. The ALCAM+ yolk sac–derived endothelial cell line, YSCL-71, was used for this assay. YSCL-71 cells were plated onto Matrigel in the presence of ALCAM-Fc or control CD4-Fc, and tube formation was analyzed after 60 hours. ALCAM-Fc mediated the inhibition of tube formation (Figure 6Aiii) compared with the control CD4-Fc (Figure 6Aii). Scoring the extent of tube formation in each well clearly demonstrated that ALCAM-Fc suppressed the endothelial tube formation in Matrigel, suggesting that ALCAM might be also involved in angiogenesis in vivo. These data, together with the results shown in Figure 5, are consistent with a role for ALCAM-mediated adhesion in endothelial cell development, particularly in the formation of vessellike structures.
Endothelial tube formation in Matrigel.
(A) Yolk sac–derived endothelial cells were plated in Matrigel and cultured for 60 hours without factor (Ai) or with CD4-Fc (Aii) or ALCAM-Fc (Aiii). Scale bar, 50 μm. (B) The number of tubes formed in Matrigel was counted in each field under a microscope, and mean ± SD was obtained from quadriplicate wells. *P < .05.
Endothelial tube formation in Matrigel.
(A) Yolk sac–derived endothelial cells were plated in Matrigel and cultured for 60 hours without factor (Ai) or with CD4-Fc (Aii) or ALCAM-Fc (Aiii). Scale bar, 50 μm. (B) The number of tubes formed in Matrigel was counted in each field under a microscope, and mean ± SD was obtained from quadriplicate wells. *P < .05.
Analysis of differentiation of hematopoietic cells from ALCAM+ endothelial precursor cells
Hemangioblast cells are the common precursor cells for hematopoietic and endothelial cells. However, cell surface markers to purify hemangioblast cells have not been reported yet. Because it has been demonstrated that differentiated embryonic stem cells expressing Flk-1 differentiate into both hematopoietic cells and endothelial cells in vitro,21 it has been speculated that hemangioblast cells might express Flk-1 in yolk sac or AGM. Because ALCAM is expressed on both embryonic hematopoietic as well as endothelial cells, we hypothesized that this protein might be a marker for hemangioblasts. Flk-1+CD31+ cells were analyzed to determine whether hematopoietic cells were differentiated from those endothelial precursor cells when coculture was done with OP9 cells in the presence of several kinds of cytokines (SCF, Epo, IL-6, IL-7) instead of VEGF (Figure 7). This experiment showed that although more hematopoietic cells were expanded from AGM-derived ALCAM− cells and yolk sac cells, the highest frequency of c-Kit+ cells was observed in hematopoietic cells derived from ALCAM+ Flk-1+CD31+ cells (Figure 7A,B). May-Grünwald-Giemsa staining revealed that immature hematopoietic cells were differentiated from ALCAM+ Flk-1+CD31+ cells (Figure 7Cii). In contrast, maturated hematopoietic cells were differentiated from ALCAM−Flk-1+CD31+ cells in AGM– or yolk sac–derived Flk-1+CD31+ cells (Figure 7Ci,iii,iv).
Differentiation of hematopoietic cells derived from endothelial progenitor cells.
(A,B) E10.5 AGM– or yolk sac–derived cells were fractionated into Flk-1+CD31+ cells and further subdivided on the basis of the expression of ALCAM. A total of 1000 Flk-1+CD31+ALCAM+ or Flk-1+CD31+ALCAM− cells were cultured with OP9 cell for 7 days in the presence of SCF, Epo, IL-6, and IL-7. After nonadherent cells were harvested, the number of cells was scored (Ai) and the frequency of c-Kit expression was analyzed by FACS (Aii,B). *P < .05. (C) Morphology in each nonadherent cell was examined by May-Grünwald-Giemsa staining; expanded hematopoietic cells in AGM-derived Flk-1+CD31+ ALCAM− cells (Ci), AGM-derived Flk-1+CD31+ALCAM+ cells (Cii), yolk sac–derived Flk-1+CD31+ALCAM− cells (Ciii), and yolk sac–derived Flk-1+CD31+ALCAM+ cells (Civ). Note that immature hematopoietic precursor cells were observed in AGM-derived Flk-1+CD31+ALCAM+cells (Cii).
Differentiation of hematopoietic cells derived from endothelial progenitor cells.
(A,B) E10.5 AGM– or yolk sac–derived cells were fractionated into Flk-1+CD31+ cells and further subdivided on the basis of the expression of ALCAM. A total of 1000 Flk-1+CD31+ALCAM+ or Flk-1+CD31+ALCAM− cells were cultured with OP9 cell for 7 days in the presence of SCF, Epo, IL-6, and IL-7. After nonadherent cells were harvested, the number of cells was scored (Ai) and the frequency of c-Kit expression was analyzed by FACS (Aii,B). *P < .05. (C) Morphology in each nonadherent cell was examined by May-Grünwald-Giemsa staining; expanded hematopoietic cells in AGM-derived Flk-1+CD31+ ALCAM− cells (Ci), AGM-derived Flk-1+CD31+ALCAM+ cells (Cii), yolk sac–derived Flk-1+CD31+ALCAM− cells (Ciii), and yolk sac–derived Flk-1+CD31+ALCAM+ cells (Civ). Note that immature hematopoietic precursor cells were observed in AGM-derived Flk-1+CD31+ALCAM+cells (Cii).
These findings clearly demonstrated that Flk-1+CD31+ cells derived from E10.5 AGM or yolk sac have the ability to differentiate into hematopoietic cell lineage in the presence of various cytokines on OP9 cells; in addition, differentiated hematopoietic cells from Flk-1+CD31+ALCAM+ cells in AGM were less mature than ALCAM− progenitors, suggesting that ALCAM might be expressed in AGM-derived hemangioblasts or primitive endothelial precursor cells. Thus, ALCAM, in combination with Flk-1, might be a good candidate for a cell surface marker to purify embryonic hemangioblasts.
Discussion
The proliferation and differentiation of hematopoietic stem cells is dependent on a close association with stromal cells, which generate a diversity of regulatory factors, including cytokines and growth factors, and maintain adhesive interactions essential to the survival and function of the hematopoietic cells. It appears that adhesive interactions are likely to be responsible for the retention of hematopoietic cells in the microenvironment. In addition, adhesive interactions themselves may serve as growth or survival signals, and adhesion itself may modulate cytokine- or growth factor–dependent signals.22 Accordingly, these cell-to-cell and cell-to-extracellular matrix interactions are responsible not only for the localization of hematopoietic cells but also play an important role in the regulation of hematopoiesis in the hematopoietic microenvironment. Thus, it is of great interest to analyze how an adhesion molecule such as ALCAM is involved in hematopoiesis in the early stage of embryos.
It has been reported that adhesion molecule ALCAM acts not only as a homophilic adhesion protein but also in heterophilic binding to CD6 in hematopoiesis, the immune response, and the nervous system.14,15,18,23 According to binding assays, the 3-membrane proximal constant immunoglobulinlike domains of human ALCAM have been shown to be involved in homophilic binding.14,24 In addition, it was demonstrated that the chicken homolog, BEN/ DM-GRASP/SC1, is required for formation of chicken myeloid colonies via homophilic binding.15 On the other hand, heterophilic interactions via CD6 were initially reported by Bowen et al to mediate hematopoietic cell binding to thymic epithelial cells.13
In this paper, to investigate a functional role of ALCAM in hematopoietic cell and endothelial cell development in vitro, we produced a soluble form of ALCAM to interfere with the function of this protein in coculture experiments. Uchida et al16hypothesized that HCA-HCA (human ALCAM) interactions would be involved in cobblestone formation, and its homophilic binding might be important for hematopoietic stem cells to remain undifferentiated. Although they have demonstrated that more cobblestones were observed in HCA+ progenitors than with HCA− progenitors, there was no effect of HCA-Fc on the outcome of in vitro assay of cobblestone-area formation by isolated HCA+ hematopoietic stem cells cultured on stromal cell.16
In contrast, our hypothesis is that ALCAM is involved in binding between stromal cell and hematopoietic cells, and ALCAM may support not only hematopoietic development but also maintain stem cells or progenitors undifferentiating. Indeed, we observed here an effect of ALCAM-Fc on primitive ALCAM+ hematopoietic cells development on ALCAM+ stromal cells (Figure 4). Because the inhibitory effect of ALCAM-Fc on expansion of c-Kit+hematopoietic progenitors was not complete, we speculate that the amount of ALCAM-Fc used in this experiment may not be sufficient or that other adhesion molecules may be involved in the development of progenitors.
Cortes et al17 also reported that HCA was expressed in primary bone marrow–derived stromal cells. Because they demonstrated that CD6 was expressed in a human stem cell population, they speculated that not only HCA but also CD6-expressing hematopoietic stem cell or progenitor might be expanded via homophilic and/or heterophilic binding.17 Although we clearly demonstrated that ALCAM acts to expand hematopoietic progenitor cells by the coculture of ALCAM+ hematopoietic cells and stromal cell in the presence of ALCAM-Fc, we cannot rule out the possibility that CD6 or other molecules are involved in hematopoiesis via heterophilic interaction with ALCAM.
Vasculogenesis is the process by which hemangioblasts or angioblasts proliferate and differentiate and form a primary vascular plexus. To examine the ability of endothelial progenitors to develop in vitro, we used endothelial progenitors and an OP9 coculture system as reported previously25 and observed 2 types of endothelial clusters, sheetlike or cordlike clusters, which developed from Flk-1+CD31+ endothelial progenitors in the AGM and yolk sac. Interestingly, higher numbers of both types of endothelial clusters were developed from ALCAM+ endothelial progenitors, indicating that ALCAM+ endothelial progenitors have the potential to develop extensively on OP9 cells compared with ALCAM− progenitors. Possibly, ALCAM-ALCAM homophilic interaction might act effectively to induce proliferation and/or survival signals for development of endothelial progenitor cells.
As far as effects on formation of endothelial clusters, addition of ALCAM-Fc resulted in impaired formation of cordlike structure, whereas suppression of sheetlike structures was not observed in the presence of ALCAM-Fc. It was previously demonstrated that Flk-1+ cells from embryonic stem cells differentiated to sheetlike endothelial clusters on OP9 cells.25 However, it is still unknown whether 2 types of clusters observed in our experiment were derived from same stage of endothelial progenitors, even though Flk-1+CD31+ cells were sorted for the coculture with OP9 cells.
Angiogenesis is the process by which new capillaries are formed from preexisting vessels by processes including protease induction, migration, proliferation, differentiation, and morphogenesis of the cells into tubelike structures.26 We examined a role of ALCAM on the tube formation using a yolk sac–derived endothelial cell line, which is known to express ALCAM. Addition of ALCAM-Fc in the Matrigel assay mediated the inhibition of formation of tubelike structures, strongly suggesting that ALCAM plays a role in the tube formation, although the mechanism underlying this observation is still unknown. Although we expect that homophilic binding via ALCAM-ALCAM interactions might be involved in the development of hematopoietic cells, endothelial clusters, and tube-formation in Matrigel, we still cannot rule out the possibility of a contribution of heterophilic interaction via CD6-ALCAM. To understand how homophilic binding (ALCAM-ALCAM) or heterophilic binding (ALCAM-CD6) is involved in development of hematopoietic stem cells and endothelial progenitors, expression of CD6 on hematopoietic or endothelial progenitors in early-stage embryos should be investigated, and addition of blocking CD6 antibody to coculture with OP9 cell might be required.
It will also be important to analyze the effects of in vivo loss of expression of ALCAM on hematopoietic and endothelial cell development in early-stage embryos. Finally, analysis of the effect of ALCAM on hematopoietic stem cell and endothelial progenitor development may provide an opportunity to dissect the hemangioblast development process during embryogenesis.
J.L., D.D., and L.A.L. are employed by Genentech, whose potential product was studied in the present work.
Submitted November 28, 2000; accepted June 8, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
O. Ohneda, Center for TARA and Instutute of Basic Medical Sciences, University of Tsukuba, Tsukuba, 305-8577, Japan; e-mail: oohneda@tara.tsukuba.ac.jp; or T. Suda, Department of Cell Differentiation, IMEG, Kumamoto University, 2-2-1, Honjo, Kumamoto, 860-0811, Japan; e-mail: sudato@gpo.kumamoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal