Decorin is a small proteoglycan that is ubiquitous in the extracellular matrix of mammalian tissues. It has been extensively demonstrated that decorin inhibits tumor cell growth; however, no data have been reported on the effects of decorin in normal cells. Using nontransformed macrophages from bone marrow, results of this study showed that decorin inhibits macrophage colony-stimulating factor (M-CSF)–dependent proliferation by inducing blockage at the G1 phase of the cell cycle without affecting cell viability. In addition, decorin rescues macrophages from the induction of apoptosis after growth factor withdrawal. Decorin induces the expression of the cdk inhibitors p21Waf1 and p27Kip1. Using macrophages from mice where these genes have been disrupted, inhibition of proliferation mediated by decorin is related to p27Kip1 expression, whereas p21Waf1expression is necessary to protect macrophages from apoptosis. Decorin also inhibits M-CSF–dependent expression of MKP-1 and extends the kinetics of ERK activity, which is characteristic when macrophages become activated instead of proliferating. The effect of decorin on macrophages is not due to its interaction with epidermal growth factor or interferon-γ receptors. Furthermore, decorin increases macrophage adhesion to the extracellular matrix, and this may be partially responsible for the expression of p27Kip1 and the modification of ERK activity, but not for the increased cell survival.
Introduction
Stimulated monocytes and macrophages secrete a diverse set of mediators that influence cellular immune functions and inflammation. These mediators include proinflammatory and anti-inflammatory cytokines, prostaglandins, leukotrienes, and reactive oxygen metabolites.1 At the inflammatory sites, proteoglycans are both secreted by activated mononuclear leukocytes and released as a result of extracellular matrix (ECM) degradation. Thus, proteoglycans, which are major constituents of the ECM, are another class of molecules produced by monocytes and macrophages2 3 that are potential modulators of the immune response.
Decorin belongs to a family of small leucine-rich proteoglycans4,5 and is found in the ECM of several of tissues such as skin,6,7 cartilage,8,9 and bone.10 The biologic importance of these molecules is unclear. In vitro binding studies have shown that some of them interact with several types of collagen11,12 and act as important regulators of collagen fibrillogenesis. In support of this hypothesis, a decorin-deficient mouse was found to have fragile skin with an abnormal organization of collagen fibers.13 Decorin may also affect the production of other ECM components by regulating the activity of transforming growth factor-β (TGF-β).14,15Additionally, decorin can modulate the interactions of matrix molecules (eg, fibronectin) with cells.16-18 These observations suggest that decorin and perhaps other proteoglycans regulate the production and assembly of the ECM at several levels and hence the remodeling of connective tissue.
Different observations have revealed that decorin is involved in the control of cell proliferation. The forced expression of decorin in Chinese hamster ovary (CHO) cells leads to a decreased growth rate, lower saturation density, and altered morphology.19 It has been suggested that decorin causes these effects by sequestering TGF-β, an autocrine growth stimulator for these cells.15In addition, decorin is markedly up-regulated during quiescence in human diploid fibroblasts20,21 and its expression is strongly suppressed on viral transformation with SV40.20Recently, an antioncogenic role for decorin has been reported.22 Decorin is rarely expressed by malignant epithelial cells from a wide variety of human tumors, including colon, pancreas, prostate, and breast carcinomas.23 In human colon carcinoma cells, the de novo expression of decorin reverted the cells to a normal phenotype; the cells lost anchorage-independent growth, failed to generate tumors in severe combined immunodeficient mice, and became arrested in the G1 phase of the cell cycle.24 When decorin expression was abrogated by treatment with decorin-specific antisense oligodeoxynucleotides, the cell re-entered the cell cycle.24 This growth arrest induced by decorin was associated with a marked expression of p21Waf1. Moreover, decorin inhibits proliferation in tumor cells with different histogenetic backgrounds through the induction of p21Waf1, and it has been reported that decorin interacts with components of the epidermal growth factor (EGF) receptor family expressed by these cells.22 25
Besides the antioncogenic role of decorin, a protective role of decorin in fibrotic diseases has been observed.26,27 However, there are no data regarding the effects of decorin on normal cells. We have analyzed the role of decorin in the control of macrophage proliferation. We have used primary bone marrow-derived macrophage (BMDM) cultures, which provide a homogeneous population that responds to physiologic proliferative or activating stimuli.28Decorin inhibits macrophage colony-stimulating factor (M-CSF)–dependent proliferation of macrophages and induces the expression of p21Waf1 and, in contrast with other cellular models, also p27Kip1. Moreover, decorin increases both the adhesion of these cells and their resistance to die after withdrawal of growth factor. The effects of decorin in macrophages are not mediated through interaction with EGF or interferon-γ (IFN-γ) receptors.
Materials and methods
Reagents
Recombinant purified decorin was a generous gift from E. Ruoslahti (The Burnham Institute, La Jolla, CA). Histone H1 was obtained from Roche Molecular Biochemicals (Indianapolis, IN). Fibronectin, vitronectin, poly-2-hydroxyethyl methacrylate (PHM), collagen, and laminin were obtained from Sigma Chemical (St Louis, MO). [3H]-Thymidine was obtained from Amersham Pharmacia Biotech (Uppsala, Sweden). 4, 6-Diamidino-2-phenylindole (DAPI) and the RGD peptide were purchased from Calbiochem (La Jolla, CA). All the other products were of the best grade available and were purchased from Sigma. Deionized water further purified with a Millipore Milli-Q system (Bedford, MA) was used.
Cell culture
The BMDMs were isolated from 6-week-old balb/c mice (Charles River Laboratories, Wilmington, MA) as described.29 The cells were cultured in plastic tissue culture dishes (150 mm) in 40 mL Dulbecco modified Eagle medium (DMEM) containing 20% fetal bovine serum (FBS) and 30% L cell-conditioned media as a source of M-CSF. The cells were incubated at 37°C in a humidified 5% CO2 environment. After 7 days of culture a homogeneous population of adherent macrophages was obtained (> 99% Mac-1+). The purity of the culture was checked regularly by flow cytometry using anti–Mac-1 antibodies (BD Pharmingen, Heidelberg, Germany). To render the cells quiescent, when the macrophages were 80% confluent they were deprived of L cell-conditioned medium for 18 hours before carrying out the experiment. BMDMs from knock-out mice were isolated in the same conditions. The p21Waf1 and p27Kip1 knock-out mice were obtained from Dr J. Roberts (Howard Hughes Medical Institute (HHMI), Seattle, WA) and IFN-γ receptor knock-out mice were a kind gift by Dr M. Modolell (Max Plank Institute, Freiburg, Germany).
Antibodies and constructs
The analysis of p21Waf1 and p27Kip1expression by Western blotting was performed with monoclonal antimouse p21Waf1 and p27Kip1 antibodies (BD Pharmingen). Antibodies to cdk-4 and cyclin D1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Peroxidase-conjugated antimouse IgG (Cappel, Turnhout, Belgium) was used as secondary antibody. A primary antibody against mouse β-actin was used as loading control and purchased from Sigma. The antibody against cdk-2 (M-2) used for the analysis of cdk-2 activity and the polyclonal antimouse β1-integrin antibody were obtained from Santa Cruz Biotechnology.
The pMH117 plasmid corresponds to the mouse p21Waf1full-length complementary DNA (cDNA) cloned in pEx-lox and was kindly provided by Dr J. Massague (Sloan Kettering Institute, HHMI, New York, NY). The MKP-1 probe was obtained from Dr R. Bravo (Bristol-Myers Squibb, Princeton, NJ). The probe for the 18S ribosomal RNA (rRNA) was obtained as described.30
Proliferation assay
Cell proliferation was measured as previously described31 with minor modifications. The cells were deprived of M-CSF for 18 hours and then 105 macrophages were incubated for 24 hours in 24-well plates (3424 MARK II; Costar, Cambridge, MA) in 1 mL complete medium in the presence or absence of the indicated reagents. In some cases, the plates were precoated with the indicated ECM proteins. After this period, the media was removed and replaced with 0.5 mL media containing [3H]-thymidine (0.037 MBq). After 6 additional hours of incubation at 37°C, the media was removed and the cells were fixed in ice-cold 70% methanol. After 3 washes in ice-cold 10% trichloroacetic acid, the cells were solubilized in 1% sodium dodecyl sulfate (SDS), 0.3 N NaOH. Radioactivity was counted by liquid scintillation using a 1500 Tri-Carb Packard scintillation counter. Each point was performed in triplicate and the results were expressed as the mean ± SD.
In parallel experiments, 1 × 106 cells were plated in 35-mm cell culture dishes and after 24 to 48 hours of culture in the indicated conditions, the viable cells were collected and counted by trypan blue exclusion using a hemocytometer. Again, each experiment was performed 3 times and the results were expressed as the mean ± SD.
Apoptosis assay
Low molecular apoptotic DNA created by internucleosomal cleavage was measured as described,32 using an enzyme-linked immunosorbent assay (ELISA) technique based on the detection of histone-associated DNA fragments (Cell Death Detection ELISA Plus, Roche Diagnostics, Mannheim, Germany). Each point was performed in triplicate, and the result was expressed as the mean ± SD.
Analysis of DNA content with DAPI
Cells (106) were previously subjected to a specific treatment and then the DNA content was analyzed as described previously.33 Twelve thousand cells were counted for each histogram, and cell cycle distributions were analyzed with the Multicycle program (Phoenix Flow Systems, San Diego, CA)
Adhesion analysis
Cell adhesion to the substrate was analyzed by crystal violet staining. Flat-bottomed ELISA plates were precoated in 50 μL/well phosphate-buffered saline (PBS) containing the indicated amount of each matrix protein, or bovine serum albumin (BSA) as a control, overnight at 4°C or for 2 hours at 37°C. After coating, the wells were blocked with PBS and 1.5% BSA for 1 hour at 37°C. Then, 10 000 cells/well were cultured for only 30 to 60 minutes due to the high capacity of macrophages to attach themselves at any surface. The cells were washed twice in PBS and fixed with 4% paraformaldehyde for 30 minutes at room temperature. After 3 washes by immersion with twice-distilled water, the cells were stained in a solution of 0.1% crystal violet in twice-distilled water for 20 minutes. After 3 new washes, the plates were dried at 37°C, developed by adding 0.1 M HCl for 5 minutes, and quantified using an ELISA reader at 630 nm. Each sample was analyzed in triplicate and the results were represented as the mean ± SD.
Protein extraction and Western blot analysis
Western blot analysis was performed as previously described.32 Cell lysates (100 μg/lane) were loaded. The analysis of p21Waf1 and p27Kip1 expression was performed with monoclonal antimouse p21Waf1 and p27Kip1 antibodies (BD Pharmingen). Antibodies to cdk-4 and cyclin D1 were obtained from Santa Cruz Biotechnology. A primary antibody against mouse β-actin was used as loading control and purchased from Sigma. Peroxidase-conjugated antimouse IgG was used as secondary antibody. All antibody incubations were performed for 1 hour at room temperature.
Determination of ERK activity by in-gel kinase assay
Total protein (50 μg) was separated by 12.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in the presence of 0.1 mg/mL myelin basic protein (MBP) (Sigma) copolymerized in the gel. After electrophoresis, SDS was removed by washing the gel with 2 changes of 20% 2-propanol in 50 mM Tris-HCl (pH 8.0) for 1 hour at room temperature. The gel was then incubated with 50 mM Tris-HCl (pH 8.0) containing 5 mM β-mercaptoethanol (buffer A) for 1 hour at room temperature. The proteins were denatured by incubating the gel with 2 changes of 6 M guanidine-HCl for 1 hour at room temperature and then renatured by incubating with 5 changes of buffer A containing 0.04% Tween-20 for 16 hours at 4°C. To perform the phosphorylation assay, the gel was first equilibrated in 40 mM Hepes-NaOH (pH 7.4) containing 2 μM DTT, 0.1 mM EGTA, 15 mM MgCl2, 300 μM sodium orthovanadate for 30 minutes at 25°C and then incubated for 1 hour in the same solution containing 50 μM adenosine triphosphate (ATP) and 3.7 MBq 32P-γ-ATP (ICN Pharmaceuticals, Costa Mesa, CA). The reaction was stopped by washing the gel with 5% trichloroacetic acid containing 10 mM sodium pyrophosphate to inhibit phosphatase activity. The gel was dried, exposed to x-ray films (Kodak) and quantified using a Bio-Rad Molecular Analyst System (Bio-Rad Labs, Richmond, CA).
Northern blot analysis
Total cellular RNA (20 μg), extracted with the acid guanidinium thiocyanate-phenol-chloroform method was separated in 1% agarose with 5 mM MOPS (3-[N-morpholino]propanesulfonic acid), pH 7.0/1 M formaldehyde buffer, transferred overnight to a GeneScreen membrane (Life Science Products, Boston, MA) and fixed by UV irradiation (150 millijoule). All the probes were labeled with32P-α-dCTP (ICN Pharmaceuticals) using the oligolabeling kit method (Amersham Pharmacia). After incubating the membranes for 18 hours at 65°C in hybridization solution (20% formamide, 5 × Denhart, 5 × standard sodium citrate [SSC], 10 mM EDTA, 1% SDS, 25 mM Na2HPO4, 25 mM NaH2PO4, 0.2 mg/mL salmon sperm DNA, and 106 cpm/mL 32P-labeled probe), they were exposed to Kodak films.
Cdk-2 activity
Quiescent macrophages were cultured in 60-mm plates precoated with 10 μg/mL BSA or decorin and stimulated with 1000 U/mL M-CSF for the indicated times. The analysis of cdk-2 activity was performed as described elsewhere, without modifications.33
Results
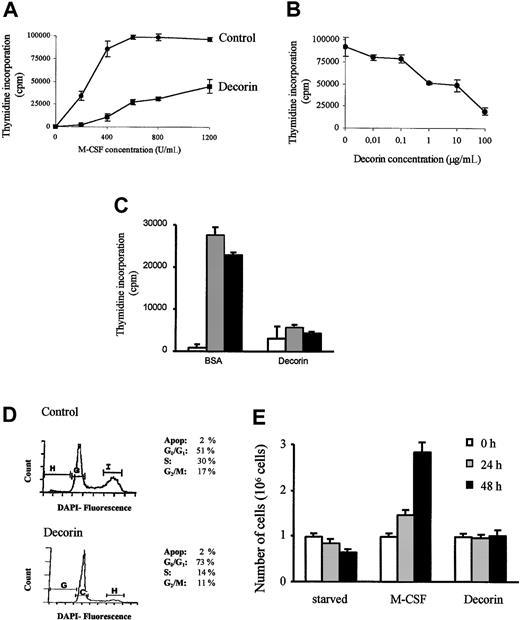
In the present work, we used macrophages obtained from bone marrow cultures, because they represent a homogeneous population of macrophages that require M-CSF to proliferate and survive. Under the effect of M-CSF, macrophages proliferate in a dose-dependent manner. When decorin (10 μg/mL) was precoated to the plates, macrophage proliferation was inhibited (Figure 1A). This effect was dose-dependent, and macrophage proliferation was completely inhibited at a concentration of 100 μg/mL decorin (Figure1B). It is important to note that the indicated concentrations of decorin correspond to the concentration of the precoating solution and that we are not able to quantify the amount of decorin adsorbed to the plate after precoating, but other proteins under the same conditions bound less than 10% to 20%. Decorin also inhibits macrophage proliferation in the presence of either recombinant M-CSF or granulocyte-macrophage colony-stimulating factor (GM-CSF) proteins (Figure 1C).
Decorin inhibits the M-CSF–dependent proliferation of BMDMs.
(A) BMDMs were obtained after 7 days of culture in the presence of M-CSF. Then, 105 macrophages were incubated in the presence of the indicated amounts of M-CSF in 24-well plates precoated with BSA (10 μg/mL; control) or with decorin (10 μg/mL). Proliferation was determined as described in “Materials and methods.” (B) Decorin inhibits macrophage proliferation in a dose-dependent manner. The 105 macrophages were incubated in 24-well plates precoated with the indicated amounts of decorin in the presence of 1000 U/mL M-CSF. For both panels A and B, each determination was made in triplicate and the values represented correspond to the mean ± SD of one representative of 3 independent experiments. (C) Decorin also blocks proliferation of macrophages induced by recombinant M-CSF (░, 2 ng/mL), GM-CSF (▪, 10 ng/mL), or control (■). (D) Decorin blocks the cell cycle at G1 phase. The 106 macrophages were cultured in the presence of 1000 U/mL M-CSF in 35-mm Petri dishes precoated with BSA or with 100 μg/mL decorin for 24 hours. DNA content was measured by DAPI staining and flow cytometry. Cell cycle distribution was analyzed using the Multicycle program (Phoenix Flow Systems). (E) Counting of viable cells cultured in 100 μg/mL decorin-precoated plates for 24 to 48 hours. The cells were counted by trypan blue exclusion using a hemocytometer. Each point was performed in triplicate, and the results were represented as the mean ± SD.
Decorin inhibits the M-CSF–dependent proliferation of BMDMs.
(A) BMDMs were obtained after 7 days of culture in the presence of M-CSF. Then, 105 macrophages were incubated in the presence of the indicated amounts of M-CSF in 24-well plates precoated with BSA (10 μg/mL; control) or with decorin (10 μg/mL). Proliferation was determined as described in “Materials and methods.” (B) Decorin inhibits macrophage proliferation in a dose-dependent manner. The 105 macrophages were incubated in 24-well plates precoated with the indicated amounts of decorin in the presence of 1000 U/mL M-CSF. For both panels A and B, each determination was made in triplicate and the values represented correspond to the mean ± SD of one representative of 3 independent experiments. (C) Decorin also blocks proliferation of macrophages induced by recombinant M-CSF (░, 2 ng/mL), GM-CSF (▪, 10 ng/mL), or control (■). (D) Decorin blocks the cell cycle at G1 phase. The 106 macrophages were cultured in the presence of 1000 U/mL M-CSF in 35-mm Petri dishes precoated with BSA or with 100 μg/mL decorin for 24 hours. DNA content was measured by DAPI staining and flow cytometry. Cell cycle distribution was analyzed using the Multicycle program (Phoenix Flow Systems). (E) Counting of viable cells cultured in 100 μg/mL decorin-precoated plates for 24 to 48 hours. The cells were counted by trypan blue exclusion using a hemocytometer. Each point was performed in triplicate, and the results were represented as the mean ± SD.
The inhibitory effect of decorin was confirmed by flow cytometry (Figure 1D) and by cell counting (Figure 1E). The distribution of the DNA content of cells stained with DAPI showed that macrophages treated with decorin were blocked mainly at the G1 phase of the cell cycle (73%), whereas macrophages growing in normal conditions showed a distribution corresponding to an active proliferating population (51% cells at G1 phase; Figure 1C). Moreover, the inhibition of proliferation was not due to a lower cell viability because we did not detect any subdiploid peak corresponding to apoptotic cells (Figure 1C) or a decrease in the cell number after 48 hours of culture in the presence of 100 μg/mL decorin (Figure 1E).
It has been reported that decorin inhibits proliferation through the expression of p21Waf1 in certain tumor cellular models.25,34 Therefore, we analyzed the expression of this cdk inhibitor in macrophages treated with decorin. Western blot analysis showed that decorin induced the expression of p21Waf1 in a time- and dose-dependent manner (Figure2A). However, and in contrast with other cellular models, decorin also induced in macrophages the expression of another cdk inhibitor, p27kip1 (Figure 2A). No differences were observed in the expression of cyclin D1, E, cdk-2, and cdk-4 protein analyzed by Western blot (data not shown). Moreover, the analysis of cdk-2 activity measured as in vitro phosphorylation of histone H1 showed that treatment of macrophages with decorin inhibits cdk-2 activity (Figure 2B). To characterize the involvement of each of these 2 molecules in the inhibitory effect of decorin, we used macrophages obtained from mice where these genes have been disrupted by homologous recombination. Decorin inhibited proliferation in macrophages from p21Waf1 knock-out mice but no effect was observed in macrophages from p27Kip1 knock-out mice (Figure2C). Thus, although decorin in macrophages induced the expression of both p21Waf1 and p27Kip1, only p27Kip1 was involved in the inhibition of proliferation by decorin, in contrast with what has been reported in tumor cells.34
Decorin inhibits macrophage proliferation through p27Kip1 expression.
(A) Decorin induces the expression of both p21Waf1 and p27Kip1. The expression of p21Waf1 and p27Kip1 after treatment with decorin was analyzed by Western blotting. Macrophages were cultured in 100 μg/mL decorin precoated surface for the indicated times or for 24 hours at the indicated concentrations of decorin. Then 100 μg total protein was loaded per lane. Expression of p21Waf1 and p27Kip1 was analyzed using monoclonal antibodies as described in “Materials and methods.” The expression of β-actin was used as a control of sample loading and transfer efficiency. This is representative of 4 independent experiments. (B) Decorin inhibits cdk-2 activity. The cdk-2 activity induced by M-CSF in macrophages cultured on plates precoated with BSA or decorin was measured as histone H1 phosphorylation in vitro at the indicated times after M-CSF stimulation. (C) Decorin did not inhibit M-CSF–dependent proliferation of BMDMs from p27Kip1 knock-out mice. After 7 days of culture, a total of 105 macrophages from wild-type, p27Kip1, or p21Waf1 knock-out mice were cultured for 24 hours in BSA or 10 μg/mL precoated plates in the presence of 1000 U/mL of M-CSF. Proliferation was determined by [3H]-thymidine incorporation. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments.
Decorin inhibits macrophage proliferation through p27Kip1 expression.
(A) Decorin induces the expression of both p21Waf1 and p27Kip1. The expression of p21Waf1 and p27Kip1 after treatment with decorin was analyzed by Western blotting. Macrophages were cultured in 100 μg/mL decorin precoated surface for the indicated times or for 24 hours at the indicated concentrations of decorin. Then 100 μg total protein was loaded per lane. Expression of p21Waf1 and p27Kip1 was analyzed using monoclonal antibodies as described in “Materials and methods.” The expression of β-actin was used as a control of sample loading and transfer efficiency. This is representative of 4 independent experiments. (B) Decorin inhibits cdk-2 activity. The cdk-2 activity induced by M-CSF in macrophages cultured on plates precoated with BSA or decorin was measured as histone H1 phosphorylation in vitro at the indicated times after M-CSF stimulation. (C) Decorin did not inhibit M-CSF–dependent proliferation of BMDMs from p27Kip1 knock-out mice. After 7 days of culture, a total of 105 macrophages from wild-type, p27Kip1, or p21Waf1 knock-out mice were cultured for 24 hours in BSA or 10 μg/mL precoated plates in the presence of 1000 U/mL of M-CSF. Proliferation was determined by [3H]-thymidine incorporation. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments.
Due to these differences between primary macrophages and tumor cells, and because it has been described that decorin inhibits tumor cell growth through its interaction with the EGF receptor,22,25the activation of the ERK pathway and the expression of p21Waf1, we analyzed the involvement of this pathway in our model. Both IFN-γ and decorin inhibit M-CSF–dependent macrophage proliferation (Figure 3A) and induce p21Waf1 messenger RNA (mRNA) expression (Figure 3B). However, no induction of p21Waf1 or inhibition of macrophage proliferation was observed after treatment with 20 mM EGF, thus suggesting that this growth factor and its receptor do not modulate macrophage proliferation. We also analyzed the effect of decorin and EGF on the activation of the ERK pathway. In-gel kinase assays showed that the treatment of macrophages with M-CSF induced the activation of ERK-1 and ERK-2 with a maximal activation after 5 minutes, but this activation decreased quickly and basal levels were reached after 15 to 30 minutes. In contrast, the treatment with decorin or EGF alone did not induce ERK activation. However, decorin, but not EGF, modified and extended the kinetics of ERK activity induced by M-CSF (Figure 3C). The extension of the ERK activity in response to decorin correlated with the inhibition of the MKP-1 expression (Figure3D), a phosphatase responsible for ERK dephosphorylation and inactivation.35
The inhibitory effect of decorin is independent of the EGF or IFN-γ receptors.
(A) Decorin and IFN-γ, but not EGF, inhibit macrophage proliferation. Macrophages were either treated with 20 nM EGF, 300 U/mL IFN-γ, or 10 μg/mL decorin or remained untreated for 24 hours in the presence of 1000 U/mL M-CSF, and proliferation was determined as indicated in “Materials and methods.” Each determination was made in triplicate, and the values represented correspond to the mean ± SD of one representative of 3 independent experiments. (B) Decorin and IFN-γ, but not EGF, induce the expression of p21Waf1 mRNA in BMDMs. Macrophages were treated for 3 hours with either 20 nM EGF, 300 U/mL IFN-γ, or 10 μg/mL decorin or remained untreated. Expression of p21Waf1 was determined by Northern blotting. (C) Decorin, but not EGF, elongates the M-CSF–induced activation of ERK. Quiescent macrophages were stimulated with one or a combination of the following: 1000 U/mL M-CSF, 20 nM EGF, or 10 μg/mL decorin for the indicated times. ERK activity was determined by in-gel kinase assay. (D) Decorin inhibits MKP-1 expression induced by M-CSF. Quiescent macrophages cultured on plates precoated with BSA or 10 μg/mL decorin were stimulated with M-CSF for 30 minutes. The expression of MKP-1 was analyzed by Northern blotting. The levels of the 18S rRNA transcript were used as a loading and transfer control. (E) Decorin inhibits the proliferation of macrophages from IFN-γ receptor knock-out mice. The 105 macrophages from control (░) and IFN-γ receptor knock-out (▪) mice were cultured in the presence of 1000 U/mL M-CSF and treated with 300 U/mL IFN-γ or 100 μg/mL decorin. Proliferation was determined after 24 hours by [3H]-thymidine uptake. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments.
The inhibitory effect of decorin is independent of the EGF or IFN-γ receptors.
(A) Decorin and IFN-γ, but not EGF, inhibit macrophage proliferation. Macrophages were either treated with 20 nM EGF, 300 U/mL IFN-γ, or 10 μg/mL decorin or remained untreated for 24 hours in the presence of 1000 U/mL M-CSF, and proliferation was determined as indicated in “Materials and methods.” Each determination was made in triplicate, and the values represented correspond to the mean ± SD of one representative of 3 independent experiments. (B) Decorin and IFN-γ, but not EGF, induce the expression of p21Waf1 mRNA in BMDMs. Macrophages were treated for 3 hours with either 20 nM EGF, 300 U/mL IFN-γ, or 10 μg/mL decorin or remained untreated. Expression of p21Waf1 was determined by Northern blotting. (C) Decorin, but not EGF, elongates the M-CSF–induced activation of ERK. Quiescent macrophages were stimulated with one or a combination of the following: 1000 U/mL M-CSF, 20 nM EGF, or 10 μg/mL decorin for the indicated times. ERK activity was determined by in-gel kinase assay. (D) Decorin inhibits MKP-1 expression induced by M-CSF. Quiescent macrophages cultured on plates precoated with BSA or 10 μg/mL decorin were stimulated with M-CSF for 30 minutes. The expression of MKP-1 was analyzed by Northern blotting. The levels of the 18S rRNA transcript were used as a loading and transfer control. (E) Decorin inhibits the proliferation of macrophages from IFN-γ receptor knock-out mice. The 105 macrophages from control (░) and IFN-γ receptor knock-out (▪) mice were cultured in the presence of 1000 U/mL M-CSF and treated with 300 U/mL IFN-γ or 100 μg/mL decorin. Proliferation was determined after 24 hours by [3H]-thymidine uptake. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments.
The differences observed between the treatment with decorin and EGF suggest that the inhibition of macrophage proliferation induced by decorin could not be mediated through its interaction with the EGF receptor, because this growth factor does not seem to modulate macrophage proliferation in BMDMs. However, IFN-γ mimics most of the effects of decorin; that is, it inhibits macrophage proliferation, induces the expression of p21Waf1, and also inhibits MKP-1 and extends ERK activity (manuscript in preparation). Therefore, we tested whether the action of decorin through the activation of the IFN-γ receptor could explain the inhibition of proliferation. As expected, IFN-γ does not inhibit the proliferation of macrophages obtained from IFN-γ receptor knock-out mice. However, decorin still inhibited the proliferation of macrophages from these mice (Figure 3E), thus showing that the effects of decorin are independent of the IFN-γ receptor.
Macrophages growing in vitro adhere to the surface of the plates, and it has been described in several cellular models that this adhesion is important for the proliferation and viability of the cells.36 37 Because decorin is a component of the ECM, we decided to test how this proteoglycan affects macrophage adhesion and if this was important in modulating proliferation and viability.
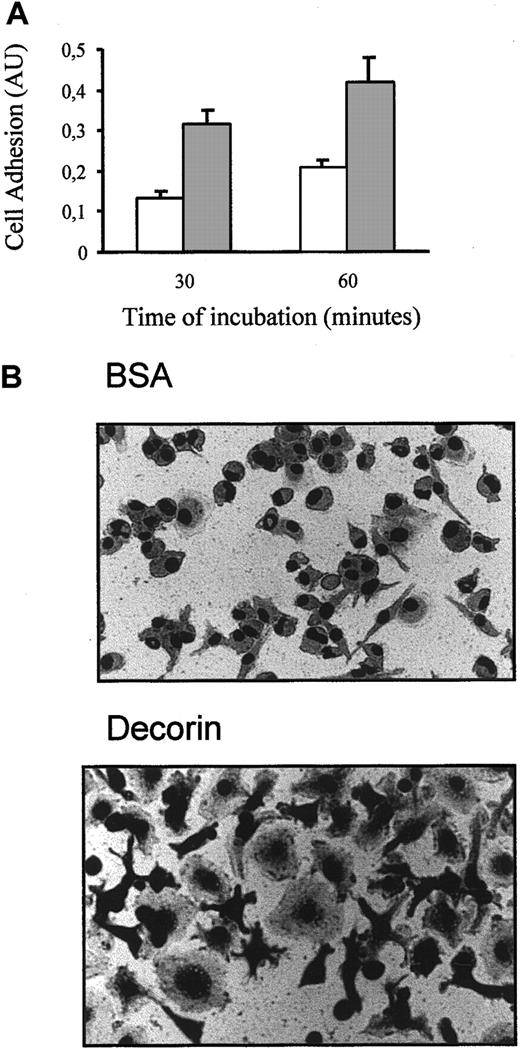
We observed that decorin enhanced the adhesion of macrophages to the surface of plastic dishes (Figure 4A). Moreover, macrophages growing in dishes precoated with decorin showed a higher degree of spreading than nontreated macrophages, and their ameboid morphology changed to a more complex morphology with pseudopoda ramification (Figure 4B).
Decorin increases macrophage adhesion.
(A) Cells (10 000) were cultured on plates precoated with BSA (■) or 10 μg/mL decorin (░) for only 30 and 60 minutes. After several washes, adhesion of macrophages was analyzed by crystal violet staining as indicated in “Materials and methods.” Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 5 independent experiments. (B) Photographs of BMDMs attached to BSA-coated (control) or decorin-coated surfaces using a phase contrast microscope with an objective of × 40.
Decorin increases macrophage adhesion.
(A) Cells (10 000) were cultured on plates precoated with BSA (■) or 10 μg/mL decorin (░) for only 30 and 60 minutes. After several washes, adhesion of macrophages was analyzed by crystal violet staining as indicated in “Materials and methods.” Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 5 independent experiments. (B) Photographs of BMDMs attached to BSA-coated (control) or decorin-coated surfaces using a phase contrast microscope with an objective of × 40.
Macrophages bind with different affinities to several proteins present in the ECM. We found that BMDMs showed a higher level of binding to plates precoated with fibronectin and vitronectin, whereas laminin reduced their adhesion (Figure 5A). We then tested how macrophage adhesion affected their proliferation. Using an inhibitor of cellular adhesion, P-OH-M, that blocked macrophage adherence to the dishes (Figure 5A), we showed that these cells must adhere to proliferate (Figure 5B). Surprisingly, a strong adhesion could also decrease macrophage proliferation. Macrophages growing on a fibronectin surface, to which they attach strongly, proliferate less than macrophages growing on a control BSA-precoated surface (Figure5B). Macrophages cultured on a surface to which they attach slightly (ie, laminin-coated surface) showed a higher level of proliferation than control cells. Similar results were obtained using vitronectin or collagen I (data not shown). We could not dismiss the fact that the different effects on proliferation were due to signaling through different integrin receptors. However, our results suggest that, although macrophage anchorage is necessary to proliferate, the proliferation of macrophages could be modulated by their degree of adhesion.
The adhesion of macrophages modulate their proliferation.
(A) Macrophages adhere preferably to fibronectin- or vitronectin-precoated surfaces. Cells (10 000) were cultured for 60 minutes on plates precoated with 10 μg/mL BSA (control), 10 μg/mL of the indicated components of the ECM, or 25 μg/mL P-OH-M, an inhibitor of cell adhesion. Macrophage adhesion was analyzed by crystal violet staining. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 3 independent experiments. (B) Adhesion modulates macrophage proliferation. Macrophages adhered to plates precoated with 10 μg/mL BSA, laminin or fibronectin, or 25 μg/mL P-OH-M were stimulated with the indicated concentrations of M-CSF and their proliferation was analyzed by [3H]-thymidine incorporation after 24 hours. Each point was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments.
The adhesion of macrophages modulate their proliferation.
(A) Macrophages adhere preferably to fibronectin- or vitronectin-precoated surfaces. Cells (10 000) were cultured for 60 minutes on plates precoated with 10 μg/mL BSA (control), 10 μg/mL of the indicated components of the ECM, or 25 μg/mL P-OH-M, an inhibitor of cell adhesion. Macrophage adhesion was analyzed by crystal violet staining. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 3 independent experiments. (B) Adhesion modulates macrophage proliferation. Macrophages adhered to plates precoated with 10 μg/mL BSA, laminin or fibronectin, or 25 μg/mL P-OH-M were stimulated with the indicated concentrations of M-CSF and their proliferation was analyzed by [3H]-thymidine incorporation after 24 hours. Each point was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments.
So far, our results showed that both decorin and fibronectin enhance macrophage adhesion and inhibit M-CSF–dependent proliferation. To determine if the effect of decorin on macrophage proliferation is mediated by its effect on adhesion, we checked the mechanism by which adhesion to fibronectin modulates macrophage proliferation.
When the expression of several components of the machinery that regulate progression through the G1 phase of the cell cycle was analyzed, we found that both decorin and fibronectin did not modify the expression of either cyclin D1, cdk4 (Figure6A), cdk-2, or cyclin E protein expression (data not shown). However, whereas decorin induced the expression of both cdk inhibitors p21Waf1 and p27Kip1, fibronectin only induced p27Kip1expression (Figure 6A). Similar to decorin, fibronectin also inhibited cdk-2 activity (data not shown). The adhesion of macrophages to a fibronectin-coated surface also extended ERK activity in response to M-CSF (Figure 6B) by inhibiting MKP-1 expression (Figure 6C). Also, the inhibitory effect of fibronectin on macrophage proliferation was abolished in macrophages from p27Kip1 knock-out mice but not in those from p21Waf1 knock-out mice (Figure 6D). This suggested that an increased macrophage adhesion probably inhibited macrophage proliferation, and this correlated with an extended ERK activity and the induction of p27Kip1.
Fibronectin also inhibits macrophage proliferation through p27Kip1 expression.
(A) Fibronectin induces p27Kip1 but not p21Waf1expression. The expression of these components of the cell cycle machinery was analyzed by Western blotting. Macrophages were cultured on plates precoated with 10 μg/mL decorin or fibronectin for 6 or 24 hours. Then, 100 μg total protein was loaded per lane. The expression of β-actin was used as a sample loading and transfer efficiency control. This is the result of 3 independent experiments. (B) Decorin and fibronectin elongate the activation of ERK induced by M-CSF. Quiescent macrophages were grown on plates precoated with 10 μg/mL fibronectin or 10 μg/mL decorin and were then treated with or without 1000 U/mL M-CSF for the indicated times. ERK activity was determined by in-gel kinase assay. (C) Fibronectin also inhibits MKP-1 expression induced by M-CSF. Quiescent macrophages cultured on plates precoated with BSA or 10 μg/mL fibronectin were stimulated with M-CSF for 30 minutes. The expression of MKP-1 was analyzed by Northern blotting. The levels of the 18S rRNA transcript were used as a loading and transfer control. (D) Fibronectin did not inhibit M-CSF–dependent proliferation of BMDMs from p27Kip1 knock-out mice. After 7 days of culture, a total of 105 macrophages from wild-type, p27Kip1, or p21Waf1 knock-out mice were cultured for 24 hours on plates precoated with BSA or 10 μg/mL fibronectin in the presence of 1000 U/mL M-CSF. Proliferation was determined by [3H]-thymidine incorporation. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments.
Fibronectin also inhibits macrophage proliferation through p27Kip1 expression.
(A) Fibronectin induces p27Kip1 but not p21Waf1expression. The expression of these components of the cell cycle machinery was analyzed by Western blotting. Macrophages were cultured on plates precoated with 10 μg/mL decorin or fibronectin for 6 or 24 hours. Then, 100 μg total protein was loaded per lane. The expression of β-actin was used as a sample loading and transfer efficiency control. This is the result of 3 independent experiments. (B) Decorin and fibronectin elongate the activation of ERK induced by M-CSF. Quiescent macrophages were grown on plates precoated with 10 μg/mL fibronectin or 10 μg/mL decorin and were then treated with or without 1000 U/mL M-CSF for the indicated times. ERK activity was determined by in-gel kinase assay. (C) Fibronectin also inhibits MKP-1 expression induced by M-CSF. Quiescent macrophages cultured on plates precoated with BSA or 10 μg/mL fibronectin were stimulated with M-CSF for 30 minutes. The expression of MKP-1 was analyzed by Northern blotting. The levels of the 18S rRNA transcript were used as a loading and transfer control. (D) Fibronectin did not inhibit M-CSF–dependent proliferation of BMDMs from p27Kip1 knock-out mice. After 7 days of culture, a total of 105 macrophages from wild-type, p27Kip1, or p21Waf1 knock-out mice were cultured for 24 hours on plates precoated with BSA or 10 μg/mL fibronectin in the presence of 1000 U/mL M-CSF. Proliferation was determined by [3H]-thymidine incorporation. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments.
Because fibronectin inhibited proliferation in a way similar to decorin, and macrophages could produce fibronectin, we checked whether the effects of decorin on macrophage adhesion and proliferation were only mediated by the release of fibronectin induced by decorin.
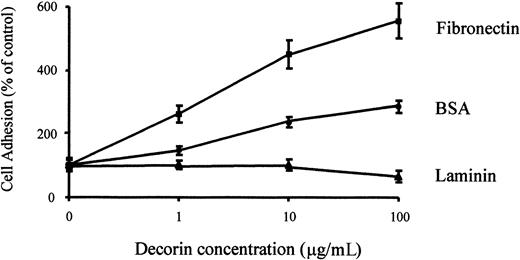
Decorin modulates the interactions of matrix molecules, such as fibronectin, with the cells.16 17 Therefore, we analyzed the effect of decorin on macrophage adhesion to fibronectin. The treatment of macrophages with decorin enhanced their adhesion to a fibronectin-coated surface but did not modify their adhesion to laminin-coated surfaces (Figure 7). Because adhesion analysis is performed for only 30 to 60 minutes, it is unlikely that the effect of decorin will be mediated through an increase of fibronectin secretion induced by decorin. Moreover, Northern blot analysis of fibronectin expression in macrophages demonstrated that decorin did not induce or increase fibronectin expression induced by M-CSF in macrophages (data not shown). This suggests that decorin binds to fibronectin through a different domain than the one recognized by macrophages and also that decorin and fibronectin are probably recognized by different receptors on macrophages.
Decorin enhances macrophage adhesion to fibronectin but not to laminin.
104 macrophages were cultured on plates precoated with 10 μg/mL fibronectin or laminin together with the indicated amounts of decorin. After 60 minutes, adhesion of BMDMs was determined by crystal violet staining as described in “Materials and methods.” Each determination was made in triplicate, and the values shown correspond to the mean ± SD of 5 independent experiments.
Decorin enhances macrophage adhesion to fibronectin but not to laminin.
104 macrophages were cultured on plates precoated with 10 μg/mL fibronectin or laminin together with the indicated amounts of decorin. After 60 minutes, adhesion of BMDMs was determined by crystal violet staining as described in “Materials and methods.” Each determination was made in triplicate, and the values shown correspond to the mean ± SD of 5 independent experiments.
To definitively discard that the effects of decorin could be mediated by the secretion of fibronectin or through fibronectin receptors, we blocked the fibronectin signal pathway using anti-β1–polyclonal antibodies. We observed that anti-β1 antibodies reduced fibronectin-induced adhesion, whereas no effect was observed on decorin adhesion (Figure8A). Adhesion of macrophages was almost completely blocked using an RGD peptide, which is a nonspecific integrin inhibitor. Interestingly, the effect of anti-β1antibodies on adhesion again correlated inversely with macrophage proliferation. Anti-β1 antibodies blocked the inhibitory effect of fibronectin on macrophage proliferation but had no effect on decorin inhibition (Figure 8B). In the presence of RGD peptides, macrophages do not proliferate.
Fibronectin, but not decorin, mediates its effects through β1-integrin receptors.
(A) Adhesion of macrophages to fibronectin is mediated by a β1-integrin. The 10 000 cells, previously stimulated with 1 mM RGD peptides or 5 μg/mL anti-β1 antibodies for 1 hour, were cultured for 60 minutes on plates precoated with 10 μg/mL BSA (control) or 10 μg/mL decorin or fibronectin. Macrophage adhesion was analyzed by crystal violet staining. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments. (B) The effect of fibronectin, but not that of decorin, on macrophage proliferation was inhibited by anti-β1 antibodies. Macrophages cultured as in panel A were stimulated with 1000 U/mL M-CSF and their proliferation was analyzed by [3H]-thymidine incorporation after 24 hours. Each point was made in triplicate, and the values represented correspond to the mean ± SD of 3 independent experiments.
Fibronectin, but not decorin, mediates its effects through β1-integrin receptors.
(A) Adhesion of macrophages to fibronectin is mediated by a β1-integrin. The 10 000 cells, previously stimulated with 1 mM RGD peptides or 5 μg/mL anti-β1 antibodies for 1 hour, were cultured for 60 minutes on plates precoated with 10 μg/mL BSA (control) or 10 μg/mL decorin or fibronectin. Macrophage adhesion was analyzed by crystal violet staining. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments. (B) The effect of fibronectin, but not that of decorin, on macrophage proliferation was inhibited by anti-β1 antibodies. Macrophages cultured as in panel A were stimulated with 1000 U/mL M-CSF and their proliferation was analyzed by [3H]-thymidine incorporation after 24 hours. Each point was made in triplicate, and the values represented correspond to the mean ± SD of 3 independent experiments.
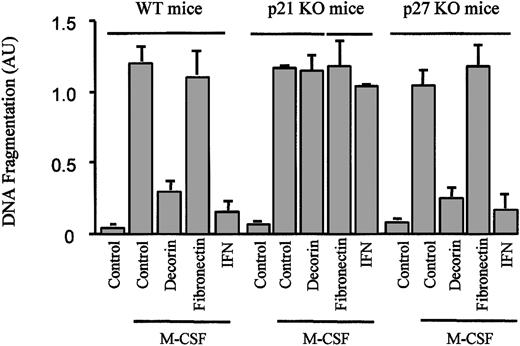
Also, attachment to the ECM may modulate cell viability.35Previously, we had reported that the expression of p21Waf1together with a blockage of the cell cycle protected macrophages from apoptosis.33 Because decorin increased macrophage adhesion to the ECM, induced p21Waf1 expression, and inhibited proliferation, we decided to further explore the role of decorin in the control of macrophage survival. From the experiments described in Figure 1, we concluded that decorin did not induce apoptosis in BMDMs. Instead, decorin protected macrophages from apoptosis induced by growth factor withdrawal (Figure 9). In contrast with its effect on macrophage proliferation, the increase in adhesion induced by decorin was not the mediator of this event, because the culture of macrophages on a fibronectin-coated surface did not protect macrophages from apoptosis induced by M-CSF starvation (Figure 9). Similar to our previous observations with IFN-γ, this effect was caused by the expression of p21Waf1. Decorin did not inhibit apoptosis in macrophages from p21Waf1 knock-out mice, whereas no differences were found in macrophages from p27Kip1 knock-out mice (Figure 9).
Decorin, but not fibronectin, protects macrophages from apoptosis induced by M-CSF starvation.
Macrophages from wild-type, p21Waf1 knock-out, and p27Kip1 knock-out mice cultured on plates precoated with BSA, decorin, or fibronectin were treated with 300 U/mL IFN-γ. The cells were then cultured in the absence of M-CSF for 24 hours. Apoptosis induced by M-CSF starvation was measured by ELISA, a method that detects fragmented histone-associated low-molecular-weight DNA. That is characteristic of the apoptotic process. Each point was made in triplicate, and the values are represented as the mean ± SD of 2 independent experiments.
Decorin, but not fibronectin, protects macrophages from apoptosis induced by M-CSF starvation.
Macrophages from wild-type, p21Waf1 knock-out, and p27Kip1 knock-out mice cultured on plates precoated with BSA, decorin, or fibronectin were treated with 300 U/mL IFN-γ. The cells were then cultured in the absence of M-CSF for 24 hours. Apoptosis induced by M-CSF starvation was measured by ELISA, a method that detects fragmented histone-associated low-molecular-weight DNA. That is characteristic of the apoptotic process. Each point was made in triplicate, and the values are represented as the mean ± SD of 2 independent experiments.
Discussion
Macrophages are derived from undifferentiated stem cells in the bone marrow and through the blood they reach the different tissues where, in most cases, they undergo apoptosis. In the presence of specific growth factors or cytokines, macrophages proliferate, become activated, or differentiate. To carry out their functional activities, macrophages must become activated. After interacting with IFN-γ, a cytokine released by activated T lymphocytes, macrophages undergo biochemical, morphologic, and functional modifications that allow them to perform their functional activity.38 IFN-γ also blocks their proliferation and protects them from apoptosis.33
At the inflammatory sites, different proteoglycans (such as decorin) are secreted by macrophages.39 Therefore, we wanted to know its effect on BMDMs, a homogeneous population of nontransformed cells.28 Decorin inhibits M-CSF–dependent proliferation of macrophages and inhibits apoptosis induced by growth factor withdrawal. After the treatment with decorin, we detected the induction of both cdk inhibitors (p21Waf1 and p27Kip1). Using mice with these genes disrupted by homologous recombination, we have found that p27Kip1 is responsible for the antiproliferative effect of decorin, whereas p21Waf1 is required to induce protection against apoptosis.
The inhibition of M-CSF–dependent proliferation in macrophages by decorin confirms previous observations in several tumor cells.34 However, the mechanism of inhibition is different from what has been described because in tumor cells the growth-suppressive properties of decorin require a functional cdk inhibitor p21Waf1.34 Our observation is in accordance with the fact that fibrillar collagen, a molecule that interacts closely with decorin both in vitro40 and in vivo,13 inhibits smooth muscle cell proliferation by inducing the expression of p27Kip1.41 In addition, we have observed that binding of macrophages to fibronectin, another protein of the ECM that binds to decorin, also inhibits macrophage proliferation through the induction of p27Kip1.
We have found other differences compared to tumor cells. It has also been reported that decorin causes a rapid phosphorylation of the EGF receptor and a concurrent activation of the ERK signal pathway, which leads to a protracted induction of endogenous p21Waf1 and, ultimately, cell cycle arrest.22,25 Although we did not detect a direct activation of the ERK pathway by decorin, the latter extended the ERK activity induced by M-CSF. In previous works we have described that this elongation is important for the regulation of macrophage proliferation and activation.42 43 Besides, we have observed that decorin does not interact with EGF or IFN-γ receptors to inhibit macrophage proliferation.
An increasing number of observations indicate that proteoglycans can regulate cell proliferation through interaction with various growth factors.44 The expression of decorin in CHO cells has a dramatic effect on their morphology and growth properties.19 This effect is partly caused by the ability of decorin to bind to TGF-β, which stimulates the growth of those cells.15 Current studies indicate that the nature of the local ECM can modulate cell responses to a given signal in several ways, such as by modifying the affinity of the ligand for its cognate receptor or by influencing on proteolytic processing and internalization.4 We believe these could not be the mechanisms that explain the antiproliferative effect of decorin. Decorin binds to TGF-β, and in previous studies we have shown that TGF-β increases the proliferation of macrophages.31However, the blockage of TGF-β signaling using antibodies specific for this cytokine abolishes the enhancement of proliferation induced by TGF-β without modifying the normal proliferative capacity of macrophages.31 Moreover, decorin does not seem to act by sequestering growth factors required by macrophages, such as M-CSF, because decorin also inhibits the proliferation of RAW 264.7, a macrophagic cell line that is growth independent of M-CSF (data not shown). Moreover, the fact that decorin also inhibits the proliferation of macrophages using recombinant M-CSF suggests that no other growth factors or mitogenic derivatives (ie, proteoglycan–M-CSF) present in the L cell-conditioned media should mediate the effects of decorin. Also, the inhibition of GM-CSF–induced proliferation by decorin suggests that the effect of decorin is a general mechanism of proliferation.
The growth of adherent cells such as fibroblasts or macrophages requires signals not only from growth factor receptors but also from integrins.36,45 46 That is also true for macrophages, because the total inhibition of their adhesion blocks macrophage proliferation. However, we thereby show for the first time that the level of this adhesion has a strong effect on the modulation of the level of macrophage proliferation. A strong attachment induced by decorin or fibronectin is enough to reduce macrophage proliferation, whereas a slight adhesion such as that induced by laminin increases proliferation. The use of anti-β1 antibodies and RGD peptides confirm this hypothesis. Macrophages adhere to plastic dishes mainly through integrin receptors, because RGD peptides almost totally block this adhesion. Moreover, macrophages need to adhere to proliferate; in this regard, RGD and P-OH-M inhibit both macrophage adhesion and proliferation. Besides, the studies using anti-β1 antibodies and RGD suggest that the effect of decorin is mediated through a non–β1-integrin receptor, whereas fibronectin uses a β1-integrin receptor. The fact that decorin has a synergic effect on macrophage adhesion to fibronectin also suggests that decorin uses a different receptor than fibronectin. However, more studies are necessary to determine which receptor is used by decorin in macrophages.
A few examples have been described for the G1 phase blockage mediated by cellular adhesion. This effect is caused by the increase of the expression of p27Kip1, which inhibits cyclin E-cdk2 kinase activity.41,47,48 Cell-to-cell contact induces the cells to stop proliferating during normal organ development. More recently it has become clear that such contact-mediated growth arrest is caused by the up-regulation of p27Kip1. This is shown in the p27Kip1 knock-out mice, which are generally bigger and have a significantly expanded hematopoietic progenitor pool.49 50 We have observed that the inhibitory effect on macrophage proliferation induced by adhesion to some ECM components (ie, decorin or fibronectin) is also mediated through the expression of p27Kip1.
In addition, the signals mediated by the ECM are important for cell survival. A laminin-rich ECM is a potential survival factor for differentiated mammary alveolar epithelial cells both in vivo and in vitro.51 It has been suggested that the laminin-rich ECM acts through α6β1-integrin receptors to affect cell survival, partly by stabilizing phosphoinositol-3 kinase-dependent survival responses to insulin (or insulinlike growth factor 1).52,53 Our results with decorin are very interesting and confirm previous results from our group.33Decorin protects macrophages from apoptosis induced by M-CSF starvation. In this case, the effect of decorin is not mediated by the increase in adhesion. As we have described elsewhere,33the induction of p21Waf1 and the blockage of the cell cycle induced by decorin (or IFN-γ) are responsible for the protective effect against apoptosis. The results obtained using p21Waf1 knock-out mice confirm this conclusion.
Our results could have physiologic relevance. Although the concentration of decorin precoating solution used in our studies (10-100 μg/mL) could seem slightly higher than that estimated to occur in collagenous matrices (5-12.5 μg/mL) found in vivo,22 the amount of decorin adsorbed to the plate should be significantly lower. Macrophages play a critical role during inflammation. From blood, macrophages reach the inflammatory foci and remain there until inflammation disappears.54 In the tissues, macrophages need to survive in the absence of growth factors. Whereas stimulated Th1 cells remain at the inflammatory loci and produce IFN-γ, macrophages are protected against apoptosis.33 In addition to IFN-γ, the elements that form the ECM, such as decorin, could also protect against apoptosis. An example of this situation may be found in the formation of granulomas that can appear in the course of certain inflammatory responses. The macrophages in these granulomas secrete decorin.55,56 Each granuloma can be viewed as a small spherical organ made of a variety of differentiated macrophages whose function is to limit the expansion of, and allow the eventual destruction of, extravascular bacteria.57 58 Decorin may favor macrophage survival and accumulation in granulomas, thus leading to bacterial elimination. Our observations on IFN-γ and decorin could provide an explanation for long-term living macrophages within the tissues.
We specially thank Dr E. Ruoslahti and Dr Richard Maki from The Burnham Institute, La Jolla, CA, for the kind gift of all the purified decorin used in this work as well as for all their support. We also thank Dr J. Roberts from HHMI, Seattle, WA, and Dr Modolell from the Max Plank Institute, Freiburg, Germany, for the p21Waf1/p27Kip1 and IFN-γR knock-out mice, respectively. We specially thank Dr Gabriel Gil, Institut Municipal d'Investigacions Biomèdiques, Barcelona, Spain, for his help with the p21Waf1 and p27Kip1 knock-out mice.
Supported by grants from Comisión Interministerial de Ciencia y Tecnologia (CICYT) (SAF98-102 and pm 98/0200) to A.C. J.X. and A.F.V. are recipients of fellowships from the ComissióInterdepartamental de Recerca i Innovació Tecnològica. M. Comalada is a recipient of a fellowship from the Fundació August Pi i Sunyer.
J.X., M.C., and M.C. are equal contributors to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Antonio Celada, Departament de Fisiologia, Facultat de Biologia, Av Diagonal 645, 08028 Barcelona, Spain; e-mail:acelada@bio.ub.es.

![Fig. 2. Decorin inhibits macrophage proliferation through p27Kip1 expression. / (A) Decorin induces the expression of both p21Waf1 and p27Kip1. The expression of p21Waf1 and p27Kip1 after treatment with decorin was analyzed by Western blotting. Macrophages were cultured in 100 μg/mL decorin precoated surface for the indicated times or for 24 hours at the indicated concentrations of decorin. Then 100 μg total protein was loaded per lane. Expression of p21Waf1 and p27Kip1 was analyzed using monoclonal antibodies as described in “Materials and methods.” The expression of β-actin was used as a control of sample loading and transfer efficiency. This is representative of 4 independent experiments. (B) Decorin inhibits cdk-2 activity. The cdk-2 activity induced by M-CSF in macrophages cultured on plates precoated with BSA or decorin was measured as histone H1 phosphorylation in vitro at the indicated times after M-CSF stimulation. (C) Decorin did not inhibit M-CSF–dependent proliferation of BMDMs from p27Kip1 knock-out mice. After 7 days of culture, a total of 105 macrophages from wild-type, p27Kip1, or p21Waf1 knock-out mice were cultured for 24 hours in BSA or 10 μg/mL precoated plates in the presence of 1000 U/mL of M-CSF. Proliferation was determined by [3H]-thymidine incorporation. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2124/5/m_h81911594002.jpeg?Expires=1763483795&Signature=wDMKHCRyvF2Fm3Mxy8fjykTiwPaw0m19sgGsjolI56HDC1-Qd0Wqe70gAd0x7CBMA~bvXRBB8CGVuMo5DbugBC1ff962SIIbRnfMvRW3nkkzCmyeOXg-CXsAlzPE-qrC6EBvXfS9C46TXUgCDAiBtGbsx9kwZmcf5HP3mQEfNXEXEvxwMmAL3gyJJY-YNPsMO6xUuqmrDkY-QLhmgkRzI2qCOoLancKuoejPpN4Uvh~VqZoZ6mSJm-gatoRfJr2iOLVss60eV3r67YbXYZKGVK9QNdpBwa0J8G95FCtSd7DkrG6cVj6x2V3aIVK84WStGHXahgoqUdh5RBGbYlETfg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. The inhibitory effect of decorin is independent of the EGF or IFN-γ receptors. / (A) Decorin and IFN-γ, but not EGF, inhibit macrophage proliferation. Macrophages were either treated with 20 nM EGF, 300 U/mL IFN-γ, or 10 μg/mL decorin or remained untreated for 24 hours in the presence of 1000 U/mL M-CSF, and proliferation was determined as indicated in “Materials and methods.” Each determination was made in triplicate, and the values represented correspond to the mean ± SD of one representative of 3 independent experiments. (B) Decorin and IFN-γ, but not EGF, induce the expression of p21Waf1 mRNA in BMDMs. Macrophages were treated for 3 hours with either 20 nM EGF, 300 U/mL IFN-γ, or 10 μg/mL decorin or remained untreated. Expression of p21Waf1 was determined by Northern blotting. (C) Decorin, but not EGF, elongates the M-CSF–induced activation of ERK. Quiescent macrophages were stimulated with one or a combination of the following: 1000 U/mL M-CSF, 20 nM EGF, or 10 μg/mL decorin for the indicated times. ERK activity was determined by in-gel kinase assay. (D) Decorin inhibits MKP-1 expression induced by M-CSF. Quiescent macrophages cultured on plates precoated with BSA or 10 μg/mL decorin were stimulated with M-CSF for 30 minutes. The expression of MKP-1 was analyzed by Northern blotting. The levels of the 18S rRNA transcript were used as a loading and transfer control. (E) Decorin inhibits the proliferation of macrophages from IFN-γ receptor knock-out mice. The 105 macrophages from control (░) and IFN-γ receptor knock-out (▪) mice were cultured in the presence of 1000 U/mL M-CSF and treated with 300 U/mL IFN-γ or 100 μg/mL decorin. Proliferation was determined after 24 hours by [3H]-thymidine uptake. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2124/5/m_h81911594003.jpeg?Expires=1763483795&Signature=DxoS3eIwp9PVyK0hq6qQKit9g2lKMUTHSyb8vLLU2BEbSg1TZOj9vCfg5ndfENfw8UKa9zgQQ8K9f5mSE0dypgZUUW2JDEFKgQUStxwYKgczHUeU~blfPhham-~roo83BtTtOYPuvmHAVNUrCoMsX-wFD~gPsd7nf65dww8dATa~ubmPSH1VN4fXQxFpb9kAgcuK2Yt16ScWlEo0kFBJ55GOq-JHR~I2CPADjvP6pzdETk0VLVHimZaNvFEdsm-hnRxd2JMh-ba0ASpGZvxzjCitpFMlVG-MK9cw~MghFL-8Fp8QLZjwu7e0O2udVuz4a9~ppd8PTmbvnM-VsuwA9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. The adhesion of macrophages modulate their proliferation. / (A) Macrophages adhere preferably to fibronectin- or vitronectin-precoated surfaces. Cells (10 000) were cultured for 60 minutes on plates precoated with 10 μg/mL BSA (control), 10 μg/mL of the indicated components of the ECM, or 25 μg/mL P-OH-M, an inhibitor of cell adhesion. Macrophage adhesion was analyzed by crystal violet staining. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 3 independent experiments. (B) Adhesion modulates macrophage proliferation. Macrophages adhered to plates precoated with 10 μg/mL BSA, laminin or fibronectin, or 25 μg/mL P-OH-M were stimulated with the indicated concentrations of M-CSF and their proliferation was analyzed by [3H]-thymidine incorporation after 24 hours. Each point was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2124/5/m_h81911594005.jpeg?Expires=1763483795&Signature=AqBsf6SiZHnKuYrgfH4~bfwB-wU4gSJFWsxMuIbS7td8WOWzjxLCAVGs78UqgnkrGIYrR1p~HRkuN9NP9undfLaAs5OjM4UIK5WvDCBPtEyz2B8JlcRAp5gX9N9KzJY-Yf79e8lB1QLokYcjTGwW8MAMEHRroy3DCDd9tZIHidd~yRfy6G8w8n4VURNo8PYMH7QqGEMJuT1sjLQ6eKhlJdA9ZA1M~wFzdoLS7yiqR1FypnNoeSUCgg5jdmnKNBNXH9yhOGMV8Mdrtq6fNMyJZzUl3-nZqgOMGWFF67d-XfEtSE0a4QBk8avh-oNs-AyzlDZEGACFPOHssgSMNjbPlw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Fibronectin also inhibits macrophage proliferation through p27Kip1 expression. / (A) Fibronectin induces p27Kip1 but not p21Waf1expression. The expression of these components of the cell cycle machinery was analyzed by Western blotting. Macrophages were cultured on plates precoated with 10 μg/mL decorin or fibronectin for 6 or 24 hours. Then, 100 μg total protein was loaded per lane. The expression of β-actin was used as a sample loading and transfer efficiency control. This is the result of 3 independent experiments. (B) Decorin and fibronectin elongate the activation of ERK induced by M-CSF. Quiescent macrophages were grown on plates precoated with 10 μg/mL fibronectin or 10 μg/mL decorin and were then treated with or without 1000 U/mL M-CSF for the indicated times. ERK activity was determined by in-gel kinase assay. (C) Fibronectin also inhibits MKP-1 expression induced by M-CSF. Quiescent macrophages cultured on plates precoated with BSA or 10 μg/mL fibronectin were stimulated with M-CSF for 30 minutes. The expression of MKP-1 was analyzed by Northern blotting. The levels of the 18S rRNA transcript were used as a loading and transfer control. (D) Fibronectin did not inhibit M-CSF–dependent proliferation of BMDMs from p27Kip1 knock-out mice. After 7 days of culture, a total of 105 macrophages from wild-type, p27Kip1, or p21Waf1 knock-out mice were cultured for 24 hours on plates precoated with BSA or 10 μg/mL fibronectin in the presence of 1000 U/mL M-CSF. Proliferation was determined by [3H]-thymidine incorporation. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2124/5/m_h81911594006.jpeg?Expires=1763483795&Signature=DmxW2aWr2gzlwaK1tiVnLAMVI8pl6~m6cd7uf~XhkjBcAPHlL6yGDUQKZUjKEZOLrqSuO2I2lHsRj0nj-u~zzWGUhPx-4R130-tMOaH7Q0NMS~xI1~i-grU-zbUgRXi87XPlhBSht42-iOCwU9nXhAWi-L87Ydz9UcSDMHTx6yOd36PPnGtZBvCRE2SPz~klwxEt4~K~sFwdC~Pg9RJcGyR0Yk6qjbbYew86PYrf4uNQ3v-4sKHkpzsE8lHeF1KjGYZ8plVe2iEM36TBgfWKFxOHSKHuvZnqrlxrDPC9yL-OW4OCaSVlussDYAIqZmQaG5ugCzHc8b1-lN~PHtcv0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Fibronectin, but not decorin, mediates its effects through β1-integrin receptors. / (A) Adhesion of macrophages to fibronectin is mediated by a β1-integrin. The 10 000 cells, previously stimulated with 1 mM RGD peptides or 5 μg/mL anti-β1 antibodies for 1 hour, were cultured for 60 minutes on plates precoated with 10 μg/mL BSA (control) or 10 μg/mL decorin or fibronectin. Macrophage adhesion was analyzed by crystal violet staining. Each determination was made in triplicate, and the values represented correspond to the mean ± SD of 2 independent experiments. (B) The effect of fibronectin, but not that of decorin, on macrophage proliferation was inhibited by anti-β1 antibodies. Macrophages cultured as in panel A were stimulated with 1000 U/mL M-CSF and their proliferation was analyzed by [3H]-thymidine incorporation after 24 hours. Each point was made in triplicate, and the values represented correspond to the mean ± SD of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2124/5/m_h81911594008.jpeg?Expires=1763483795&Signature=kRaaDLIY1dzcgl~pX51dlKR~R6HUjFTOHF6VYNokj-67TeqqqNR7VjAi8Hb~85mmViXslmmKx3L0rVWy3Qn1Sg2Quqm5AXlHP3~E7xuX59pdQQzYJvPOazKeVTEIsBs4edM9nmMeTrmbj~nCOxQ~lFRXpHLbj7vXVjZfUtOut1-0g8uq2cmY5-2fc17NtdLMelTjN-czllG3dbiUZPQ3v8jRGA4HOakHjRdiC7EcsT8sIOoJj6YFdSJ-VEbdXm-VkawLiVHA~NZDS2jEygJtZehu6p1~idIhwHqZl2W~Di6rQ7I0vklr70PxcXqJ1YBmfut0MaExo2i4DxqkAjwqmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal